Abstract
We report a case of a 75-year-old patient who presented with severe acute kidney injury due to Goodpasture’s syndrome. It is an uncommon autoimmune condition, requiring treatment with immunosuppressive drugs and plasma exchange. Prognosis depends largely on early diagnosis, so it is important to be aware of these rarer causes whenever anyone presents with acute kidney injury. She had two cardiac arrests in the emergency department, had a long stay in the intensive care unit and went on to develop pulmonary haemorrhage which improved with treatment. However, she developed end-stage renal failure for which she is on life-long dialysis.
Keywords: Goodpasture’s syndrome, anti-glomerular basement membrane disease, acute kidney injury, hyperkalemia, hyperkalaemia, uremia, uraemia, highest urea, highest blood urea nitrogen
Case report
A 75-year-old English Caucasian lady was referred to the emergency department by her general practitioner with a three-week history of diarrhoea, lethargy and poor appetite on the background of a presumed chest infection which gave her symptoms of dyspnoea and cough. There was no history of haemoptysis. She had been treated with courses of antibiotics and prednisolone. Her medical history included hypertension, asthma, hypothyroidism, paroxysmal atrial fibrillation and a single functioning kidney since birth. She was a non-smoker and functionally independent.
While in the emergency department, she collapsed and developed a pulseless ventricular tachycardia cardiac arrest. She was cardioverted and received three cycles of cardiopulmonary resuscitation (CPR) before return of spontaneous circulation (ROSC). An arterial blood gas (ABG) taken during the arrest showed a serum potassium of 9.7 mmol/l, pH 6.97, base excess −21.6 mmol/l and lactate 2.4 mmol/l. The hyperkalaemia was treated and she was given a loading dose of amiodarone. Approximately 30 min after ROSC she became bradycardic and descended into a pulseless electrical activity cardiac arrest, which resulted in ROSC after 1 mg of adrenaline and one cycle of CPR. An adrenaline infusion was started. A repeat ABG was similar to the previous except for an improved potassium level of 7.3 mmol/l.
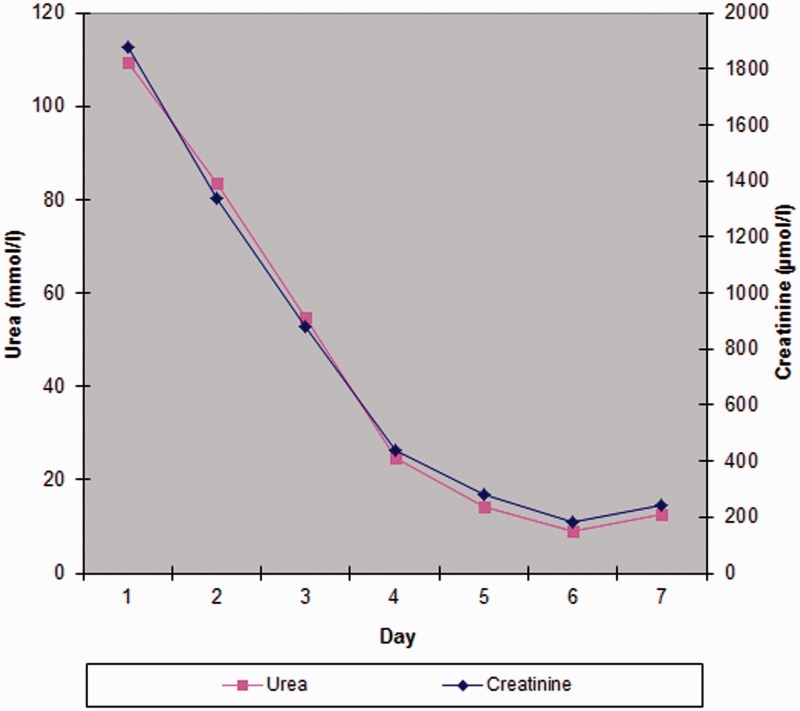
Her Glasgow Coma Score would not improve further than 5, so she was intubated uneventfully using a modified rapid sequence induction technique. She was transferred to the intensive care unit (ICU), after which her blood results became available. Her urea was 109.5 mmol/l (657.7 mg/dl) (Blood urea nitrogen (BUN) 306.7 mg/dl), creatinine 1877 µmol/l (21.2 mg/dl) and, unexpectedly, her haemoglobin concentration was 5.3 g/dl. Levels of her potassium, urea and creatinine were 4.7 mmol/l, 7.5 mmol/l and 83 µmol/l, respectively three weeks prior and her calculated estimated glomerular filtration rate had varied between 58 and 76 ml/min over the previous year. Her previous measured haemoglobin was 10.3 g/dl a year and a half earlier. A vascath was inserted and standard-volume haemofiltration at 35 ml/kg/h was commenced, improving her metabolic acidaemia. She was given four units of packed red cells. The renal team advised to keep the urea over 80 mmol/l during the first 24 h, to avoid disequilibrium syndrome due to excessive osmolar fluid shifts. Over the ensuing days, haemofiltration was performed at intervals such that the urea decreased gradually (see Figures 1 and 2).
Figure 1.
Urea and creatinine over first week.
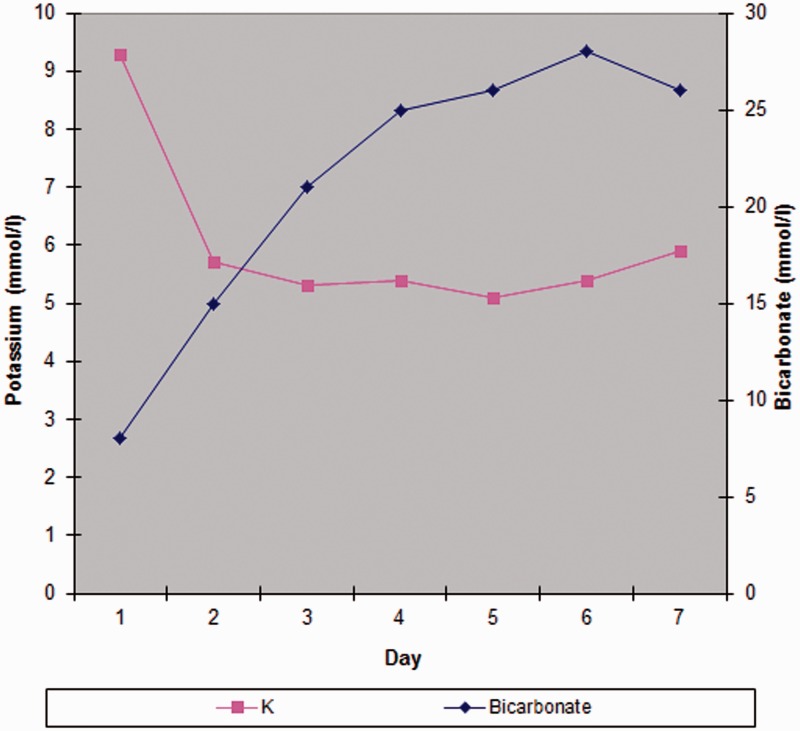
Figure 2.
Potassium and bicarbonate over first week.
She was extubated on day three but developed a hospital-acquired pneumonia and the next day required re-intubation due to respiratory distress and copious secretions. On day five, she had another failed extubation and was re-intubated. On day six her urea and creatinine had fallen to 8.9 mmol/l and 181 µmol/l, respectively and haemofiltration was discontinued. Over the next two days they slowly rose again and by the morning of day eight her potassium level had crept up to 6.3 mmol/l, so renal dialysis was commenced and continued daily. A percutaneous tracheostomy was performed on day nine after which her propofol infusion was stopped. A small amount of remifentanil was continued until the next day due to agitation. She soon became alert and orientated. She was slow to wean from respiratory support and intermittently she suffered periods of what was thought to be pulmonary oedema. She produced pink blood-stained sputum and her chest X-rays at the time showed small bilateral pleural effusions, prominent central pulmonary vascular markings consistent with a degree of congestion, bibasal atelectasis and resolving right lower lobe consolidation. She continued to receive daily haemodialysis, mainly for fluid removal to prevent significantly positive fluid balance. After day 15, this was reduced to alternate days. During the first three weeks of her ICU stay she needed two further transfusions of packed red cells due to gradually decreasing haemoglobin concentrations, which would drift down to levels below 7 g/dl.
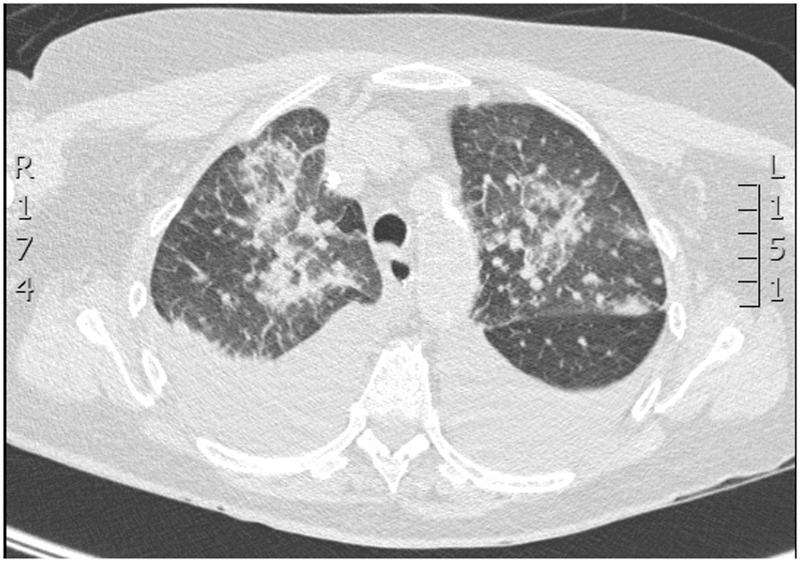
On day 24 her anti-glomerular basement membrane (anti-GBM) antibody titre was found to be elevated (8 RU/ml) (reference range 0–0.9 RU/ml). Antineutrophil cytoplasmic antibodies (ANCA) were found to be present using the indirect immunofluorescence technique. Antinuclear antibodies (ANA) were not detected on antigen specific assay and complement C3 and C4 levels were normal. The numerous episodes of ‘pulmonary oedema’ were re-evaluated and the possibility of pulmonary haemorrhage due to Goodpasture’s syndrome was considered. A high-resolution computed tomography (CT) scan on day 25 showed pulmonary haemorrhage (see Figure 3). The radiologist’s report stated ‘There are multiple patchy areas of consolidation throughout both upper lobes. In view of their aspect on the imaging these are likely to be in keeping with pulmonary haemorrhage rather than pneumonia.’ A renal biopsy was not deemed necessary and the patient was started on a course of steroids with three days of high dose methylprednisolone 500 mg daily followed by prednisolone 60 mg daily. Plasmaphoresis was commenced on day 26 and continued on alternate days and on the same day ultrafiltration was added to her alternate day dialysis for increased fluid removal. She was also started on cyclophosphamide.
Figure 3.
High-resolution CT scan.
On day 34, her tracheostomy was removed and she was discharged from the high dependency unit to the ward, where she continued to receive plasma exchange and dialysis on alternate days. On day 43, a long-term dialysis catheter was inserted. She remained anuric throughout her admission. She was discharged from hospital on day 56 on daily prednisone 30 mg and she received her third dose of cyclophosphamide at her follow up appointment two weeks later. Her prednisolone was tapered down to 10 mg daily over the next four and a half months and then continued life-long. Her haemodialysis regime was three 4-h sessions per week.
She remained dialysis-dependent and three months after discharge she underwent AV fistula formation surgery.
Discussion
American pathologist Ernest Goodpasture first described this disease in 1919. Goodpasture’s syndrome is a rare autoimmune disease characterised by autoantibodies targeted at the alpha3 subunits of type IV collagen in the alveolar and GBM, leading to pulmonary haemorrhage and renal failure. It has an incidence of approximately 0.5–1.0 per million population. It affects Caucasian people more frequently than black people, but is even more common in the Maori people of New Zealand. It has a strong association with certain human leucocyte antigen alleles. Its incidence peaks in spring and early summer and some clustering of the disease suggest that a pathogen or environmental factor may also be involved.1 Its onset is bimodal and peaks between the ages of 20 to 30 and 60 to 70 years, the former usually being males, while the latter being females.2
Substantial variation exists in the clinical manifestations of anti-GBM disease; 60–80% of patients have pulmonary and renal disease, 20–40% renal disease alone and less than 10% pulmonary disease alone.3 The differential diagnosis includes community-acquired pneumonia, pneumocystis jiroveci pneumonia, acute glomerulonephritis, Churg–Strauss syndrome, granulomatosis with polyangiitis, microscopic polyangiitis, rheumatoid arthritis, systemic lupus erythematosus, undifferentiated connective tissue disease, essential mixed cryoglobulinaemia and drug-induced vasculitides.
The presence of anti-GBM antibodies is pathognomonic and distingishes this disease from the other causes of the pulmonary-renal syndrome. Goodpasture’s syndrome can be diagnosed based on the clinical picture and raised anti-GBM titre as in our case, but conclusive diagnosis can only be achieved via a renal or lung biopsy. The false-negative rate for direct enzyme-linked immunosorbent assay (ELISA) detection of anti-GBM antibodies is less than 5% and may occur in patients with low anti-GBM antibody titres or in some patients with Alport syndrome who develop anti-GBM disease after transplantation. A false-positive rate of less than 1% is attributable to the detection of antibodies directed against other chains of type IV collagen.4 The question of whether or not a biopsy is needed after positive serological testing is a recurring one. ‘A well-read kidney biopsy can tell the nephrologist what the patient has and whether there is a reasonable chance of recovery with successful therapy. There is no serologic test that can impart that degree of information.’5 Uncertainty can have implications regarding future treatment decisions. However, our nephrologists felt the clinical features in this case did not necessitate a biopsy.
The disease may progress to permanent lung and kidney damage and in the past usually led to death. The use of immunosuppressive agents and plasma exchange has improved the prognosis. With treatment the patient can often recover fully from the lung damage but the kidneys are less able to heal and so life-long dialysis is usually required, as in the case of our patient, or transplantation. Prognosis depends on the degree of renal failure at presentation as there is evidence of some recovery of renal function if the presenting creatinine is below 5 mg/dl (442 µmol/l)6 and 600 µmol/l.2 Diagnosis of the condition early in its natural history is required to improve this and is also important in preventing fulminant pulmonary haemorrhage, which can be fatal. ANCA is detectable in up to 30% of patients with anti-GBM disease and titres of ANCA and anti-GBM antibodies tend to be inversely related. The detection of ANCA is clinically relevant in anti-GBM disease because these patients are more likely to respond to therapy, and so has prognostic value.4 With treatment Levy et al.7 showed a 5-year survival of 63 to 94%, while Shah and Hugghins8 reported that 20% of their patients had normal recovery, 39% were receiving maintenance hemodialysis, 12% received transplants and were doing well, 5% were awaiting transplants, and 24% died.
In the setting of acute kidney injury higher creatinine levels have been reported9 in the past, but there does not seem to be any report of serum urea/BUN as high as in our patient. The highest non-fatal serum potassium level reported is 14.0 mmol/l,10 but this was not due to acute kidney injury and was thought to be due to massive potassium efflux due to cell damage, due to myocardial infarction and subsequent six episodes of external defibrillation due to cardiac arrest. Our patient did not suffer a myocardial infarction, but did receive two episodes of external defibrillation before the initial ABG was taken. In the literature there does not appear to be any report of an individual patient with a combination of such a high serum creatinine, urea/BUN and potassium due to acute kidney injury.
Consent
Written consent to publish this article was obtained from the patient.
Declaration of Conflicting Interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Alenzi FQ, Salem ML, Alenazi FA, et al. Cellular and molecular aspects of Goodpasture syndrome. Iran J Kidney Dis 2012; 6: 1–8. [PubMed] [Google Scholar]
- 2.Savage CO, Pusey CD, Bowman C, et al. Antiglomerular basement membrane antibody mediated disease in the British Isles 1980-4. Br Med J (Clin Res Ed) 1986; 292: 301–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kathuria P, Sanghera P, Stevenson FT, et al. Goodpasture syndrome clinical presentation, http://emedicine.medscape.com/article/240556-clinical#showall (accessed 30 May 2015).
- 4.Swiatecka-Urban A and Devarajan P. Anti-GBM antibody disease workup, http://emedicine.medscape.com/article/981258-workup (accessed 30 May 2015).
- 5.Bomback AS. Anti-glomerular basement membrane nephritis: why we still ‘need’ the kidney biopsy. Clin Kidney J 2012; 5: 496–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Daly C, Conlon PJ, Medwar W, et al. Characteristics and outcome of anti-glomerular basement membrane disease: a single-center experience. Ren Fail 1996; 18: 105–112. [DOI] [PubMed] [Google Scholar]
- 7.Levy JB, Turner AN, Rees AJ, et al. Long term outcomes of anti-glomerular basement membrane antibody disease treated with plasma exchange and immunosuppression. Ann Intern Med 2001; 134: 1033–1042. [DOI] [PubMed] [Google Scholar]
- 8.Shah MK, Hugghins SY. Characteristics and outcomes of patients with Goodpasture's syndrome. South Med J 2002; 95: 1411–1418. [PubMed] [Google Scholar]
- 9.Abuhasna SD. Highest serum creatinine ever reported. Hemodial Int 2013; 17: 137–138. [DOI] [PubMed] [Google Scholar]
- 10.Tran H. Extreme hyperkalemia. South Med J 2005; 98: 729–732. [DOI] [PubMed] [Google Scholar]