Abstract
The treatment of sepsis remains a significant challenge and is the cause of high mortality and morbidity. The pathophysiological alterations that are associated with sepsis can complicate drug dosing. Critical care patients often have capillary leak, increased cardiac output and altered protein levels which can have profound effects on the volume of distribution (Vd) and clearance (Cl) of antibacterial agents, both of which may affect the pharmacokinetics (PK) / pharmacodynamics (PD) of the drug. Along with antibacterial factors such as the hydrophilicity and its kill characteristics and the susceptibility and site of action of the microorganism, different dosing and administration strategies may be needed for the different drug classes. In conclusion, developing dosing and administration regimes of antibacterials that adhere to PK/PD principles increase antibacterial exposure. Tailoring therapy to the individual patient combined with TDM may contribute to improved clinical efficacy and contain the spread of resistance.
Keywords: Antibiotics, pharmacokinetics, critical care, sepsis, beta lactams
Antibacterial dose optimisation is a significant clinical challenge in the treatment of sepsis. This is due to the pathophysiological alterations that are associated with sepsis that alter the pharmacokinetics of the prescribed antibiotic and complicate dosing. The incidence of sepsis in intensive care units (ICUs) internationally has been shown to be as high as 51% with 71% of patients receiving an antibacterial during their ICU stay.1 With persisting high mortality and morbidity rates, optimal antimicrobial therapy needs therefore to be a priority for septic patients.
The prognosis of sepsis and septic shock remains poor despite advances in critical care medicine. Much research has been targeted at the inflammatory and coagulation cascade associated with sepsis; however, none of these interventions have been found to be as effective as optimising antibacterial therapy.2
Therapeutic drug monitoring (TDM) to guide antimicrobial dosing is only routinely available for a small number of antibiotics. Nevertheless, interest has grown in alternative antimicrobial dosing strategies that are better aligned with the antimicrobial’s pharmacokinetic (PK) and pharmacodynamic (PD) properties. Knowledge of PK and PD of commonly used antibiotics may help to select appropriate dosage regimens and schedule intervals that will contribute to therapeutic efficacy and improve clinical outcome. A recent large study that measured beta-lactam concentrations in critically ill patients showed that many patients did not achieve PK/PD targets and therefore may be less likely to achieve a positive clinical outcome.3 Sub-therapeutic dosing of antibiotics may lead to the development of antibiotic resistance and/or therapeutic failure if appropriate dose adjustments are not made.4 Low serum blood concentrations of tigecycline in two case reports have led to the development of resistant strains of Acinetobacter baumannii.5
The aim of this article is to review and highlight the factors (including patient, antibacterial and microorganism) that may affect dosing of antibacterials in critically ill patients.
Patient factors
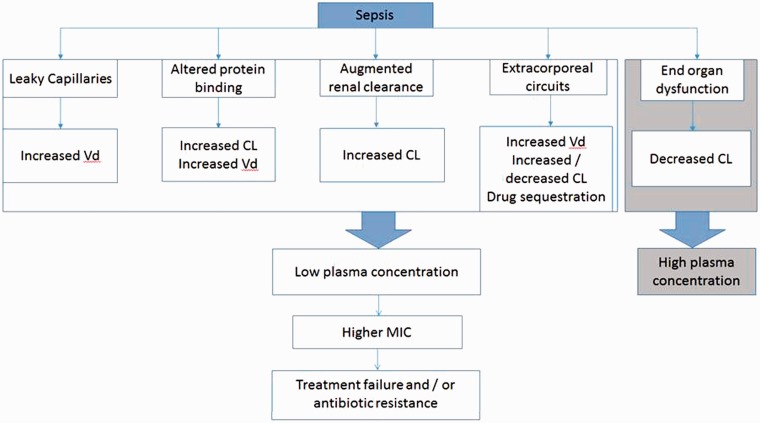
Critically ill septic patients often have capillary leak, increased cardiac output and/or modification of serum protein levels and binding. Antibiotic dosing is especially challenging in these patients due to increased volume of distribution (Vd) and changes in clearance (Cl) (see Table 1 for definitions). Additionally, increased renal and hepatic clearance or, on the contrary, organ dysfunction, are common and lead to significant pharmacokinetic changes (Figure 1).
Table 1.
Definitions of PK and PD terms.
| Pharmacokinetics refers to the study of concentration changes of a drug over a period of time. Parameters of importance to antibacterials include: • Volume of distribution (Vd) – is calculated as the ratio of the dose present in the body and its plasma concentration when the distribution of the drug between the tissues and the plasma is at equilibrium. • Clearance (Cl) – represents the volume of blood, serum or plasma completely cleared of drug per unit of time. • Elimination half –life (t1/2)– The time it takes for the concentration of the drug to fall to 50%. • Cmax– peak serum drug concentration achieved by a single dose. • Cmin– minimum serum drug concentration during a dosing period. • AUC – area under the serum concentration curve. Pharmacodynamics relates pharmacokinetic parameters and pharmacological effect. Parameters of importance to antibacterials include: • T >MIC – time for which the serum concentration of a drug remains above the MIC during a dosing period. • Cmax/MIC – ratio of the antibacterial Cmax to MIC. • AUC/MIC – ratio of the AUC during a dosing interval to MIC. Minimum inhibitory concentrations (MICs) are defined as the lowest concentration of an antimicrobial that will inhibit the visible growth of a microorganism after overnight incubation. |
Figure 1.
Pathophysiological changes that occur during sepsis and effects on pharmacokinetics. Vd: volume of distribution; CL: clearance; MIC: minimum inhibitory concentration.
Changes in Vd
Antibacterials that distribute essentially in the extracellular fluid (mainly hydrophilic) have a low Vd, whilst those that have rapid cellular uptake (lipophilic) have high Vd. Sepsis can lead to the development of endothelial damage and increased capillary permeability. This capillary leak syndrome results in fluid shifts from the intravascular compartment to the interstitial space. This leads to an increase in Vd of hydrophilic drugs and a decrease in the plasma concentration. Administration of intravenous fluids during the initial phase of Systemic Inflammatory Response Syndrome (SIRS) and in sepsis, the presence of mechanical ventilation, extracorporeal circuits (e.g. plasma exchange, cardiopulmonary bypass, extracorporeal membrane oxygenator), postsurgical drains, or in patients with significant (>20%) burn injuries6 may also increase the Vd of hydrophilic drugs.
Changes in clearance
Critically ill patients with sepsis receive administration of intravenous fluids as standard initial management.6 When hypotension persists, vasopressor agents are prescribed which leads to higher than normal cardiac indices. In the absence of significant organ dysfunction, there is often an increased renal perfusion and consequently increased creatinine clearance (often referred to as augmented renal clearance (CrCL >130 ml/min)) and increased elimination of hydrophilic antibiotics.
However, a significant number of patients in critical care will present with multi-organ failure including acute kidney injury (AKI). Sepsis-induced AKI is not only associated with decreased glomerular filtration but also with impairment of tubular secretion and reabsorption.7 This will result in decreased antibiotic clearance of hydrophilic antibiotics (such as beta-lactams and aminoglycosides), prolonged half-life and potential toxicity from elevated antibiotic plasma concentrations and accumulations of metabolites. When AKI is present or the patient needs renal replacement therapy, there is a need for individualised therapy and dose adjustments to be made to reflect these changes.
Changes in protein binding
Hypoalbuminaemia is a frequently occurring condition in patients with sepsis as a consequence of increased capillary permeability. In one study in septic patients, the mean serum albumin was <28 g/L.8 There is an increased albumin escape rate through the leaky endothelium which will result in loss of oncotic pressure and loss of fluid into the interstitial space. This may influence the Vd and CL of many antibiotics. The PK for highly protein bound drugs (e.g. flucloxacillin9 ertrapenem,10 teicoplanin11) is markedly altered in sepsis and can result in higher unbound concentrations that are subject to a greater clearance.
Antibacterial factors
Antibacterials can be classed in terms of their propensity to partition into either fat (lipophilic) or water (hydrophilic).
Hydrophilic versus lipophilic
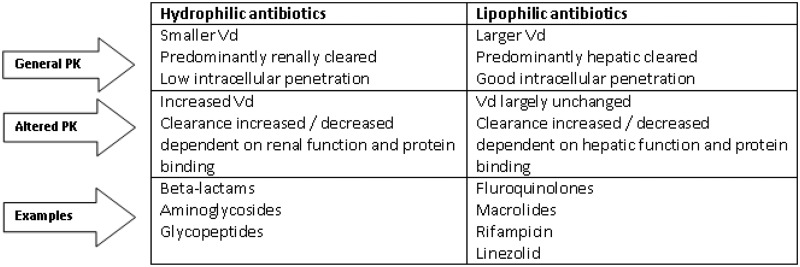
Hydrophilic antibiotics will have a smaller Vd, lower protein binding and are more likely to be excreted unchanged via the kidney. Lipophilic antibiotics tend to have a much larger Vd, a greater degree of protein binding and are more likely to be metabolised by the liver. Examples are shown in Figure 2.
Figure 2.
Change in pharmacokinetic parameters for antimicrobials according to their solubility in patients with sepsis. Vd: volume of distribution.
Kill characteristics
Pharmacodynamically, different antibacterial classes appear to demonstrate different kill characteristics on bacteria which describe the pharmacokinetic measurements that represent optimal bactericidal activity. The kill characteristics of common antibiotic classes are illustrated in Figure 3.
Figure 3.
Pharmacokinetic and pharmacodynamic parameters of antibiotics on a concentration versus time curve. Adapted from Roberts and Lipman with permission from Lippincott Williams & Wilkins.6 Cmax: peak serum drug concentration achieved by a single dose; Cmin: minimum serum drug concentration during a dosing period; AUC: area under the serum concentration curve; T > MIC: time for which the serum concentration of a drug remains above the minimum inhibitory concentration (MIC) during a dosing period; Cmax/MIC: ratio of the antibacterial Cmax to MIC; AUC/MIC: ratio of the area under curve (AUC) to MIC during a dosing interval.
Concentration dependent
With concentration dependent antibiotics, such as aminoglycosides, a high initial concentration is required to ensure maximum bacterial kill. The efficacy of these agents is related to the achievement of a high Cmax/MIC (minimum inhibitory concentration) ratio. This high initial concentration may also aid tissue penetration.12
Time dependent
For time-dependent antibiotics, such as beta-lactams and glycopeptides, optimal bacterial kill is achieved by maximising the time of the concentration over the MIC (T >MIC). The maximum effect is achieved in septic patients when this time is approaching 100% of the dosing interval.
Area under curve/MIC
For area under curve (AUC)/MIC antibiotics such as fluoroquinolones, the ratio of the area under the curve during a 24-h time period to MIC is important to achieve adequate plasma concentrations.
Microorganism factors
Minimum inhibitory concentration
MIC of a target organism is the denominator in the PK/PD relationship and therefore a central component in guiding dose selection. The MIC and antibiotic combination is not usually available upon initiation of therapy and may not become available for at least 24–48 h after specimens have been identified at the microbiology laboratory.
Susceptibility breakpoints have been classified by the European Committee on Antimicrobial Susceptibility Testing (EUCAST; available at http://www.eucast.org/clinical_breakpoints)13 which provide useful epidemiological susceptibility data for dose optimisation when local laboratory antibiograms are absent.
Less susceptible pathogens, with higher MIC values are frequently isolated in the ICU and therefore conventional dosing strategies are unlikely to achieve the required antibiotic exposure.14
Tissue penetration
For antimicrobial agents to be effective, they must reach their site of infection, which may be within an isolated tissue or organ system. Tissue penetration of antibiotics is governed by passive diffusion, transport mechanisms, lipid solubility and protein binding.
As a general rule, hydrophilic antimicrobials, as opposed to lipophilic ones, diffuse slowly and partially in deep-seated infection sites. Overall, this supports the view that higher dosages or improved administration schedules for hydrophilic antimicrobials are needed to treat deep-seated infections (such as pneumonia and intra-abdominal infections) to ensure optimal pharmacodynamic exposure at the infection site, compared to treatment of ‘easily accessible’ infections such as bacteraemia.
General dosing considerations
To maximise microorganism eradication, several dosing methods that exploit the antimicrobial PK/PD properties have been investigated. These include administration of time-dependent antimicrobials via extended (for example over a period of 3–4 h) or continuous (over 24 h) infusion as compared with traditional intermittent infusions (for example, over a period of 30 min), altering doses based on both patient-specific pharmacokinetic parameters and the MIC of the target organism.
Loading dose (LD)
The LD of any drug is calculated from the Vd and the required serum concentration (Css) using the formula LD = Vd × Css. As renal function plays no role in this calculation, the LD should not be adjusted for creatinine clearance. In septic patients, there is a larger than predicted Vd of hydrophilic antibacterials and therefore a larger required LD. For lipophilic antibacterials that penetrate deep into fatty tissues, the concentration in extravascular space is less pronounced. There is limited evidence to guide dosing in obesity in the critically ill; however, published studies and case reports suggest higher doses of lipophilic antibacterials are required in this setting.15,16
Reduced bacterial susceptibility
For organisms with high MIC values, application of PK/PD models, increasing the total daily dose of the antibacterial with TDM (where applicable) should be considered.
Augmented renal clearance
A measured CrCl >130 ml/min/1.73 m2 in critically ill patients receiving beta-lactams has been associated with sub-therapeutic dosing.17 Measuring creatinine clearance by use of 24 h urine collection may be used to optimise antibiotic dosing by increasing the total daily dose, shortening the dose interval or use of extended/continuous infusions should be considered.
Altered protein binding
Hypoalbuminaemia is likely to affect highly protein bound drugs that are predominantly renally eliminated. To optimise antibacterial dosing, larger loading doses should be given and shortening the dose interval or use of extended/continuous infusions should be considered. This should be guided by TDM.
TDM
TDM has traditionally been used to guide dosing of aminoglycosides and glycopeptides in the main to monitor for and avoid toxicity. With this in mind, assays are only widely available and used for a very small number of narrow therapeutic index drugs e.g. vancomycin and gentamicin. Where TDM has been done for beta-lactams, an increase in PK/PD target attainment has been shown when compared to conventional dosing.18 The ability to do TDM on commonly used beta-lactams is not currently widely available in the United Kingdom.
Individual drug classes
The dosing strategies for individual antibiotic classes are discussed below.
Aminoglycosides
Aminoglycosides are hydrophilic, accumulate in the extracellular fluid, are poorly bound to proteins and therefore susceptible to PK changes occurring in the critically ill patient. The kill characteristic of the aminoglycosides is concentration dependent; with a significant postantibiotic effect (PAE) that can prevent bacterial re-growth for prolonged periods should drug concentrations fall below the MIC.19 The PAE increases with the ratio between peak concentration and MIC which supports once daily administration as opposed to small, multiple doses.
With aminoglycosides, optimal antibacterial activity is achieved when the peak is eight to 10 times greater than the MIC.19–21 It has been indicated that therapy should usually target problematic pathogens in ICU patients such as Pseudomonas aeruginosa. The clinical MIC breakpoint for this pathogen is 8 µg/ml13 indicating that peak drug concentrations for amikacin should reach >64 µg/ml in order to optimise antibacterial activity. Even with high amikacin doses (such as 25 mg/kg doses), the increased Vd of critically ill patients may therefore preclude the achievement of a high peak:MIC ratio.22 A low Cmin below target should be obtained to minimise aminoglycoside toxicity.
Beta-lactams (including carbapenems)
For beta-lactam antibiotics, higher drug concentrations do not result in significantly greater bacterial kill. Beta-lactams have shown a slow continuous kill that is almost entirely related to T > MIC and if antibiotic concentrations fall below the MIC, bacteria proliferate almost immediately. As a minimum standard for carbapenems, the percentage of the dosing interval that free drug concentration remains above the MIC should be maintained at 40%. However, patients with severe bacterial infections T >MIC of 100% have shown to display significantly greater cure and bacterial eradication than patients with T <100%.23
Studies in critically ill patients have demonstrated that administration of piperacillin-tazobactam,24 meropenem25 and ceftazidime26 via extended intervals or continuous infusion maximise time of bacteria exposure to adequate drug concentrations and may improve clinical cure rates particularly with pathogens with low susceptibily. Despite clinical trials failing to show a mortality benefit from this strategy,27 there are theoretical arguments and case reports that support the efficacy and safety of prolonged or continuous infusions. In severe infections caused by less susceptible microorganisms in critical ill patients, where the risk of underdosing is higher, continuous or extended infusions of beta-lactams have proven to be safe, with comparable therapeutic efficacy.
Fluoroquinolones
Fluoroquinolones are lipophilic and have a high Vd. They have extensive distribution characteristics and achieve good extracellular and intracellular concentrations. The Vd of most drugs in this class is minimally affected in the critically ill patient. They exhibit concentration-dependent PK, and a peak:MIC ratio of 10 predicts bacterial eradication.28 However, this requires high doses, which has raised concerns about neurotoxicity and therefore precludes its clinical use. Therefore, the AUC:MIC is the parameter that is usually associated with dosing.
Glycopeptides
The optimal PK/PD properties of glycopeptides have not yet been completely elucidated. For example, vancomycin (like beta-lactam antibiotics) exhibits slow and time-dependent killing during in vitro experiments. However, it has a moderately long PAE (unlike beta-lactams) and therefore T > MIC becomes less important. There is little consensus on whether T > MIC or Cmax:MIC should be used in optimising dosing regimens. Studies examining continuous infusions have provided mixed results. Due to its nephrotoxic effects, empirical dosing based on creatinine clearance with subsequent TDM of Cmin plasma concentrations is recommended.29
Conclusion
Critically ill patients are unique in that they undergo pathophysiological changes which can complicate dosing of antibiotics. Developing dosing and administration regimens that adhere to pharmacodynamic and pharmacokinetic principles in critically ill patients and maximise antibiotic exposure are being shown to be of increasing importance for achieving clinical cure and containing the spread of resistance. Ideally these strategies should be used in conjunction with MIC measurements and TDM to measure their potential success and guide the clinician in tailoring the delivery of antibiotic to suit an individual patient’s needs.
Declaration of Conflicting Interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Vincent JL, Rello J, Marshall J, et al. International study of the prevalence and outcomes of infection in intensive care units. JAMA 2009; 302: 2323–2329. [DOI] [PubMed] [Google Scholar]
- 2.Rice T, Bernard G. Therapeutic intervention and targets for sepsis. Annu Rev Med 2005; 56: 225–248. [DOI] [PubMed] [Google Scholar]
- 3.Roberts J, Paul S, Akova M, et al. DALI: defining antibiotic levels in intensive care patients: are current β-lactam antibiotic doses sufficient for critically ill patients. Clin Inf Dis 2014; 58: 1072–1083. [DOI] [PubMed] [Google Scholar]
- 4.Roberts J, Kruger P, Paterson D, et al. Antibiotic resistance – what’s dosing got to do with it? Crit Care Med 2008; 36: 2433–2440. [DOI] [PubMed] [Google Scholar]
- 5.Peleg A, Potoski B, Rea R, et al. Acinetobacter baumannii bloodstream infection while receiving tigecycline: a cautionary report. J Antimicrob Chemother 2007; 59: 128–131. [DOI] [PubMed] [Google Scholar]
- 6.Roberts J, Lipman J. Pharmacokinetic issues for antibiotics in the critically ill patient. Crit Care Med 2009; 37: 840–851. [DOI] [PubMed] [Google Scholar]
- 7.Sun H, Frassetto L, Benet L. Effects of renal failure on drug transport and metabolism. Pharmacol Ther 2006; 109: 1–11. [DOI] [PubMed] [Google Scholar]
- 8.Finfer S, Bellomo R, Boyce N. A comparison of albumin and saline for fluid resuscitation in the intensive care unit, the SAFE study investigators. N Engl J Med 2004; 350: 2247–2256. [DOI] [PubMed] [Google Scholar]
- 9.Ulldemolins M, Roberts JA, Wallis SC, et al. Flucloxacillin dosing in critically ill patients with hypoalbuminaemia: special emphasis on unbound pharmacokinetics. J Antimicrob Chemother 2010; 65: 1771–1778. [DOI] [PubMed] [Google Scholar]
- 10.Burkhardt O, Kumar V, Katterwe D, et al. Ertapenem in critically ill patients with early-onsetventilator-associated pneumonia: pharmacokinetics with special consideration of free-drug concentration. J Antimicrob Chemother 2007; 59: 277–284. [DOI] [PubMed] [Google Scholar]
- 11.Barbot A, Venisse N, Rayeh F, et al. Pharmacokinetics and pharmacodynamics of sequential intravenous and subcutaneous teicoplanin in critically ill patients without vasopressors. Intensive Care Med 2003; 29: 1528–1534. [DOI] [PubMed] [Google Scholar]
- 12.Panidis D, Markantonis SL, Boutzouka E, et al. Penetration of gentamicin into the alveolar lining fluid of critically ill patients with ventilator-associated pneumonia. Chest 2005; 128: 545–552. [DOI] [PubMed] [Google Scholar]
- 13.EUCAST. Aminoglycosides: EUCAST clinical MIC breakpoints, http://www.eucast.org/clinical_breakpoints/ (accessed 21 July 2014).
- 14.Roberts J, Lipman J. Optimal doripenem dosing simulations in critically ill nosocomial pneumonia patients with obesity, augmented renal clearance, and decreased bacterial susceptibility. Crit Care Med 2013; 41: 489–459. [DOI] [PubMed] [Google Scholar]
- 15.Muzevich K, Lee K. Subtherapeutic linezolid concentrations in a patient with morbid obesity and methicillin-resistant Staphylococcus aureus pneumonia: case report and review of the literature. Ann Pharmacother 2013; 47: e25. [DOI] [PubMed] [Google Scholar]
- 16.Erstad B. Dosing of medications in morbidly obese patients in the intensive care unit setting. Intensive Care Med 2004; 30: 18–32. [DOI] [PubMed] [Google Scholar]
- 17.Udy AA, Varghese JM, Altukroni M, et al. Subtherapeutic initial beta-lactam concentrations in select critically ill patients: association between augmented renal clearance and low trough drug concentrations. Chest 2012; 142: 30–39. [DOI] [PubMed] [Google Scholar]
- 18.Waele J, Carrette S, Carlier M, et al. Therapeutic drug monitoring-based dose optimisation of piperacillin and meropenem: a randomised controlled trial. Intensive Care Med 2014; 40: 380–387. [DOI] [PubMed] [Google Scholar]
- 19.Moore RD, Lietman P, Smith C. Clinical response to aminoglycoside therapy: importance of the ratio of peak concentration to minimal inhibitory concentration. J Infec Dis 1987; 155: 93–99. [DOI] [PubMed] [Google Scholar]
- 20.Deziel-Evans LM, Murphy JE, Job ML. Correlation of pharmacokinetic indices with therapeutic outcome in patients receiving aminoglycosides. Clin Pharm 1986; 5: 319–324. [PubMed] [Google Scholar]
- 21.Pinder M, Bellomo R, Lipman J. Pharmacological principles of antibiotic prescription in the critically ill. Anaesth Intensive Care 2002; 30: 134–144. [DOI] [PubMed] [Google Scholar]
- 22.Taccone F, Laterre P, Spapen H, et al. Revisiting the loading dose of amikacin for patients with severe sepsis and septic shock. Crit Care 2010; 14: R53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McKinnon P, Paladino J, Schentag J. Evaluation of area under the inhibitory curve (AUIC) and time above the minimum inhibitory concentration (T >MIC) as predictors of outcomes for cefepime and ceftazidime in serious bacterial infections. Int J Antimicrob Agents 2008; 31: 345–351. [DOI] [PubMed] [Google Scholar]
- 24.Lorente L, Jimenez A, Martin MM, et al. Clinical cure of ventilator associated pneumonia treated with piperacillin/tazobactam administered by continuous or intermittent infusion. Int J Antimicrob Agents 2009; 33: 464–468. [DOI] [PubMed] [Google Scholar]
- 25.Lorente L, Lorenzo L, Martin MM, et al. Meropenem by continuous versus intermittent infusion in ventilator associated pneumonia due to gram negative bacilli. Ann Pharmacother 2006; 40: 219–223. [DOI] [PubMed] [Google Scholar]
- 26.Lorente L, Jimenez A, Palmero S, et al. Comparison of clinical cure rates in adults with ventilator-associated pneumonia treated with intravenous ceftazidime administered by continuous or intermittent infusion: a retrospective, nonrandomized, open label, historical chart review. Clin Ther 2007; 29: 2433–2439. [DOI] [PubMed] [Google Scholar]
- 27.Chant C, Leung A, Friedrich J. Optimal dosing of antibiotics in critically ill patients by using continuous/extended infusions: a systematic review and meta-analysis. Critical Care 2013; 17: R279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van Zanten A, Polder-Man K, Van Geijlswijk I, et al. Ciprofloxacin pharmacokinetics in critically ill patients: a prospective cohort study. J Crit Care 2008; 23: 422–430. [DOI] [PubMed] [Google Scholar]
- 29.Llopis-Salvia P, Jimenez-Torres N. Population pharmacokinetics parameters of vancomycin in critically ill patients. J Clin Pharm Ther 2006; 31: 447–454. [DOI] [PubMed] [Google Scholar]