Abstract
The AdaptResponse trial is designed to test the hypothesis that preferential adaptive left ventricular‐only pacing with the AdaptivCRT® algorithm reduces the incidence of the combined endpoint of all‐cause mortality and intervention for heart failure (HF) decompensation, compared with conventional cardiac resynchronization therapy (CRT), among patients with a CRT indication, left bundle branch block (LBBB) and normal atrioventricular (AV) conduction. The AdaptResponse study is a prospective, randomized, controlled, single‐blinded, multicentre, clinical trial (ClinicalTrials.gov Identifier: NCT02205359), conducted at up to 200 centres worldwide. Following enrolment and baseline assessment, eligible subjects will be implanted with a CRT system containing the AdaptivCRT algorithm, and randomized in a 1:1 fashion to either a treatment (‘AdaptivCRT’) or control (‘Conventional CRT’) group. The study is designed to observe a primary endpoint in 1100 patients (‘event‐driven’) and approximately 3000 patients will be randomized. The primary endpoint is the composite of all‐cause mortality and intervention for HF decompensation; secondary endpoints include all‐cause mortality, intervention for HF decompensation, clinical composite score (CCS) at 6 months, atrial fibrillation, quality of life measured by the Kansas City Cardiomyopathy Questionnaire (KCCQ), health outcome measured by the EQ‐5D instrument, all‐cause readmission after a HF admission, and cost‐effectiveness. The AdaptResponse clinical trial is powered to assess clinical endpoints and is expected to provide definitive evidence on the incremental utility of AdaptivCRT‐enhanced CRT systems.
Keywords: Cardiac resynchronization therapy, Left ventricular pacing, Optimization, Atrioventricular conduction, Left bundle branch block, Clinical outcome, Heart failure
Introduction
Cardiac resynchronization therapy (CRT) is recommended by current guidelines for the treatment of patients with symptomatic heart failure (HF), impaired left ventricular (LV) systolic function, and an electrocardiogram (ECG) which displays evidence of electrical dyssynchrony,1, 2 with established effects on morbidity and mortality.3, 4 However, in spite of the overall beneficial effects of CRT, no early clinical improvement is observed in approximately 30% of CRT recipients.3, 5
While CRT is most commonly delivered by using biventricular (BiV) pacing, it has been suggested by meta‐analysis6 that LV‐only pacing can be at least as efficacious as BiV pacing, with no observed differences in mortality.7, 8 In patients with sinus rhythm and normal atrioventricular (AV) conduction, pacing only the left ventricle with appropriate AV delays [i.e. synchronized to the right ventricle to produce fusion of left and right ventricular (RV) activation] can result in superior LV9 and RV10 function compared to standard BiV pacing. Optimization of the AV and interventricular (VV) intervals during BiV pacing is another option to maximize the positive effects of CRT.11, 12 Optimization is usually accomplished by using echocardiography or other in‐office modalities. However, these methods have not consistently shown benefit,13 can be resource‐intensive, often need patient–physician contact, and only a minority of clinicians routinely optimize AV and VV delays. Optimization using a proprietary peak endocardial acceleration sensor on the atrial lead recently showed promising results.14, 15
The AdaptivCRT® (Medtronic plc) algorithm16, 17 has been developed to provide RV‐synchronized LV‐only fusion pacing (i.e. to produce fusion of left‐ and right‐sided ventricular activation) when intrinsic AV conduction is normal or, alternatively, BiV pacing, when required. Preliminary studies have suggested that AdaptivCRT‐optimized resynchronization therapy results in improved clinical outcomes.18, 19, 20
The present report describes the rationale and design of the AdaptResponse trial, which we designed to test the hypothesis that AdaptivCRT reduces the incidence of the combined endpoint of all‐cause mortality and intervention for HF decompensation, compared with conventional CRT, among patients with a CRT indication, left bundle branch block (LBBB), and normal AV conduction.
Algorithm
Adaptive LV‐only pacing makes use of the patient's intrinsic conduction by pre‐pacing the left ventricle to synchronize with intrinsic RV activation and establish fusion. When the patient's heart rate increases or AV conduction is prolonged, the pacing mode switches automatically to adaptive BiV pacing. Unlike programmer‐based algorithms, adaptive BiV pacing provides continuous optimization of AV/VV timing settings based on periodic automatic evaluation of the patient's intrinsic conduction intervals and activity level. Adaptive BiV is aimed at maximizing the CRT benefit by optimizing ventricular filling and ejection, and eliminating the need for manual echocardiographic optimization. The algorithm is intended to provide continuous ambulatory CRT optimization, and to allow for more physiological ventricular activation and greater device longevity in patients with normal AV conduction by reducing unnecessary RV pacing. A schematic representation of the AdaptivCRT algorithm can be found in Figure 1.
Figure 1.
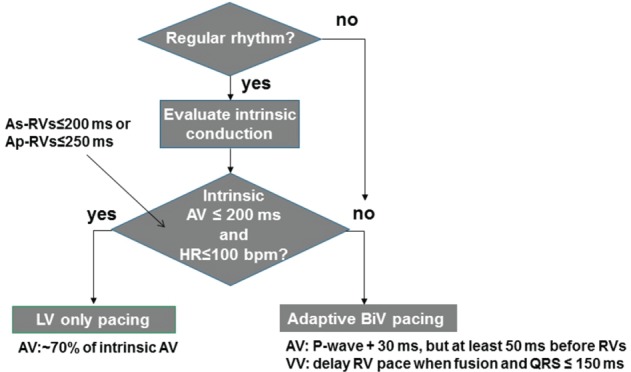
AdaptivCRT algorithm. The AdaptivCRT algorithm continuously and dynamically optimizes the cardiac resynchronization therapy pacing method and atrioventricular/interventricular delays depending on the patient's activity levels and conduction status. Adaptive left ventricular pacing makes use of the patient's intrinsic conduction by pre‐pacing the left ventricle to synchronize with intrinsic right ventricular activation and establish fusion. When the patient's heart rate increases or atrioventricular conduction is prolonged, the pacing mode switches automatically to adaptive biventricular pacing. During adaptive biventricular pacing, the atrioventricular delays are updated every minute based on atrioventricular interval and P wave width measurements. Intrinsic atrioventricular intervals are measured every minute, and P wave and QRS widths are measured every 16 h. The atrioventricular delay is adjusted to pace about 30 ms after the end of the P wave but at least 50 ms before the onset of the intrinsic QRS. This provides enough time for atrial contraction, while ensuring biventricular pacing, prior to intrinsic conduction to the ventricles. In addition, the ventricular pacing configuration (right ventricle → left ventricle, left ventricle → right ventricle) and interventricular pace delay are updated every minute based on the atrioventricular interval and QRS width measurements. In patients with normal atrioventricular conduction, as measured intracardially by the device, the AdaptivCRT algorithm will primarily provide adaptive left ventricular pacing. During this pacing operation, the timing of the left ventricular pace is automatically adjusted based on the intrinsic atrioventricular interval measurement that occurs every 60 s. After the left ventricular pace occurs, the intrinsic right ventricular contraction completes the biventricular activation. Every minute, the atrioventricular delays are updated to ensure optimal cardiac resynchronization therapy delivery. When programmed to adaptive biventricular and left ventricular pacing, the device employs adaptive left ventricular‐only pacing when the patient's heart rate is 100 b.p.m. or below, when atrioventricular conduction is normal, and left ventricular capture is confirmed. Normal atrioventricular intervals are defined as less than 200 ms for atrial‐sensed intervals and less than 250 ms for atrial‐paced intervals.16 AV, atrioventricular; BiV, biventricular; HR, heart rate; LV, left ventricular; RV, right ventricular; VV, interventricular; As‐RVs, atrial sensed atrioventricular interval; Ap‐RVs, atrial paced atrioventricular interval.
Study design
The AdaptResponse study is a prospective, randomized, parallel, controlled, single‐blinded, multicentre, post‐market, global cardiac resynchronization clinical trial (ClinicalTrials.gov; Identifier: NCT02205359). This study is being conducted at up to 200 centres in Australia, Canada, Europe, India, Japan, Korea, Latin America, the Middle East, Taiwan, and the USA, and approximately 3000 subjects will be randomized. After study enrolment and baseline assessment, the eligible patients will be implanted with a CRT device containing the AdaptivCRT algorithm. Within 7 days of completing a successful implant procedure (system consisting of a CRT device and right atrial, RV and LV leads), the subjects will be randomized in a 1:1 fashion to either treatment (‘AdaptivCRT’) or control (‘Conventional CRT’). The randomization schedule will be stratified by centre and by New York Heart Association (NYHA) class, using permuted blocks with random block sizes.
The study will be single‐blinded (i.e. patients are blinded to the randomization assignment) to reduce bias effects. All study enrollees will be followed until the required number of 1100 endpoint events is reached (‘event‐driven’ design), or until the pre‐specified stopping boundary is crossed at interim analysis. The expected total study duration will approximately be 5.5 years, representing 3 years of patient enrolment and 2.5 years of study follow‐up. The data monitoring committee (DMC) will review interim analysis results and advise on study continuation. The DMC is also responsible for regular review of adverse event data summaries to address any potential safety issues, and to monitor the overall study conduct. In addition, the DMC will be unblinded to the patient's treatment assignments; however, the endpoint adjudication committee (EAC) will be blinded to the treatment designation when reviewing case files, wherever reasonably achievable. To further minimize any potential sources of bias, the following measures will also be taken: (i) an ECG core laboratory will be used to confirm the ECG inclusion criteria by validating the presence of LBBB and normal AV conduction after enrolment, (ii) subject characteristics will be collected at baseline and differences between randomized groups that may affect primary endpoints will be identified, (iii) all medical personnel responsible for the device implants must be experienced, (iv) data collection requirements and study procedures will be standardized across all centres and geographies, (v) monitoring visits will be conducted to safeguard adherence to the protocol and to verify the collected data against the source data, (vi) an independent DMC will review endpoint and other data to monitor the overall integrity of the study, (vii) the Steering Committee members will not have any influence over HF treatment decisions by centre investigators during the trial except for approval for crossover, and (viii) the analysis will be intent‐to‐treat, following predefined statistical methods specified in the statistical analysis plan (SAP). More detailed information on the DMC, EAC, and the Steering Committee can be found in Appendix 1.
Study population and enrolment criteria
The patients will be screened to ensure they meet all of the inclusion and none of the exclusion criteria prior to study enrolment. The subjects will have to meet the following inclusion criteria to be eligible to participate in the study: (i) indication for a CRT device according to the international scientific guidelines, (ii) sinus rhythm at time of enrolment, (iii) LBBB according to the Strauss criteria as determined by the physician,21 and (iv) normal AV conduction per ECG (PR interval ≤200 ms). More information regarding the inclusion criteria and a complete overview of the exclusion criteria are reported in Table 1.21
Table 1.
Study inclusion and exclusion criteria checked by the physician at enrolment
| Inclusion criteria | Exclusion criteria |
|---|---|
|
|
Study conduct
This study conduct is guided by ISO‐14155 and by good clinical practice (GCP), in accordance with the Declaration of Helsinki and the laws and regulations in the countries. Written approval from the Institutional Review Board and/or Medical Ethics Committee is required for participation and each patient must provide written informed consent. The sponsor ensured training of all involved study personnel with regard to programming and interpretation of data. All devices used in this investigation are market released in all countries and geographies participating in the clinical study (Australia, Canada, Europe, India, Japan, Korea, Latin America, the Middle East, Taiwan, and the USA), and used within the approved labelling. Case report form completion and handling will be performed electronically using an electronic data management system for clinical studies. Data will be stored in a secure, password‐protected database which will be backed up nightly.
Study flow
The sequence of enrolment, device implantation, randomization, and planned study visits is illustrated in Figure 2. The study will be conducted using market‐released CRT systems with pacing‐only (CRT‐P) or pacing and defibrillation (CRT‐D) capabilities, containing the AdaptivCRT algorithm, a Medtronic market‐released LV lead, and any market‐released RA and RV leads. The programming requirements, applicable to the study subjects according to their respective randomization assignment, are summarized in Table 2. The only meaningful difference between both groups is either the activation or deactivation of the AdaptivCRT feature.
Figure 2.
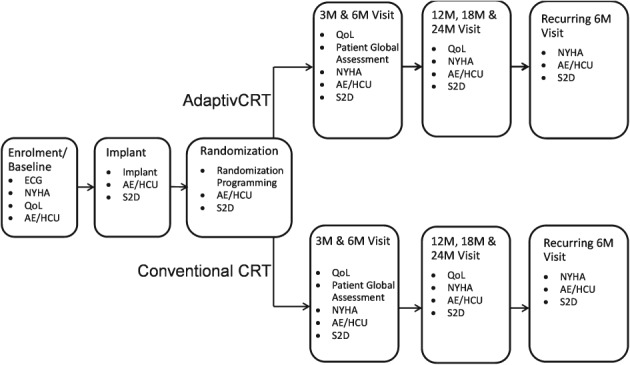
Study flow from enrolment to planned study visits. AE, adverse event; CRT, cardiac resynchronization therapy; ECG, electrocardiogram; HCU, health care service utilization; M, month; NYHA, New York Heart Association class; QoL, quality of life; S2D, device data.
Table 2.
Device programming requirements according to patient assignment
| Parameter | AdaptivCRT group | Conventional CRT group |
|---|---|---|
| AdaptivCRT® | AdaptivCRT required (adaptive BiV and LV) | Non‐adaptive CRT, BiV required |
| Mode (NASPE/BPEG pacing codes) | DDD required, with DDDR only if clinically needed (this would need to be documented) | DDD required, with DDDR only if clinically needed (this would need to be documented) |
| Ventricular blanking post VP | ≥200 ms | No requirement |
| Sensed AV interval, paced AV interval, VV delay | No requirement | Programming with or without optimization per physician's discretion |
| Ventricular pacing | LV → RV or RV → LV | Per preferred in‐office/physician method |
| LV capture management | On | On |
| Lower rate |
≤60 b.p.m. (nominal setting) If programmed otherwise a documented rationale for alternative programming must be provided |
≤60 b.p.m. (nominal setting) If programmed otherwise a documented rationale for alternative programming must be provided |
| Upper tracking rate | ≤140 b.p.m. | ≤140 b.p.m. |
| If programmed otherwise a documented rationale for alternative programming must be provided | If programmed otherwise a documented rationale for alternative programming must be provided | |
| Upper sensor rate | ≤140 b.p.m. | ≤140 b.p.m. |
| Ventricular sense response | On | On |
| Conducted AF response | On | On |
| Lead polarity for Attain Performa LV leads | No requirements. Lead polarity information and changes will be collected | No requirements. Lead polarity information and changes will be collected |
AV, atrioventricular; BiV, biventricular; CRT, cardiac resynchronization therapy; LV, left ventricular; RV, right ventricular; VP, ventricular pace; VV, interventricular.
Study endpoints
The primary study endpoint is the composite of all‐cause death and any intervention for HF decompensation as adjudicated by the independent blinded EAC. Intervention for HF decompensation is defined in the EAC charter as an event that (i) occurred primarily because of new or worsening signs and/or symptoms of HF, or biomarker or imaging evidence of HF, and (ii) received additional or increased pharmacological or mechanical intervention to treat HF. In case the patient is not hospitalized, the treatment is required to be intravenous or invasive. The EAC adjudicates according to a charter that provides more detailed definitions.22, 23 The different centres may adhere to their own standard practice pertaining to diagnosing HF, but are required to report all diagnostic assessments, tests, and procedures done with supporting material as appropriate to allow the EAC to adjudicate.
Secondary endpoints will include (i) all‐cause mortality, (ii) intervention for HF decompensation, (iii) improved clinical composite score (CCS)24 at 6 months, (iv) incidence of atrial fibrillation (AF) defined as the first occurrence of ≥6 h of device‐detected AF in a day, (v) quality of life measured by the Kansas City Cardiomyopathy Questionnaire (KCCQ),25 (vi) health outcome measured by the EQ‐5D instrument, (vii) incidence of all‐cause readmissions within 30 days after a HF admission, and (viii) cost‐effectiveness.
Statistical considerations
The primary analysis will follow the intent‐to‐treat principle. All randomized patients will be included in the analysis. The AdaptResponse trial is event‐driven. A total of 1100 patients experiencing a primary endpoint will generate 90% statistical power to demonstrate a significant reduction in the incidence of the primary endpoint, accounting for three equally spaced interim analyses (α = 0.05) and assuming a true intent‐to‐treat hazard ratio (HR) of 0.82 for ‘AdaptivCRT’ compared with ‘Conventional CRT’. With randomization of 3000 patients enrolled over 3 years and followed for 2.5 years, 1100 events are expected when the true control arm event‐free rate is 75% at 2 years (which is consistent with results from MADIT‐CRT,26 REVERSE,27 RAFT,28 Cleland's CRT meta‐analysis,29 and the adaptive CRT study18, 19).
The primary objective of this study will be to test the hypothesis that AdaptivCRT reduces the incidence of the composite primary endpoint, i.e. all‐cause mortality and intervention for HF decompensation, compared with conventional CRT, in CRT‐indicated patients with LBBB and normal AV conduction. This hypothesis will be tested using a Cox proportional hazards regression model with a random centre effect, and stratified by NYHA class at enrolment. Two further analyses of the primary endpoint are planned. A multivariable Cox regression model will be developed in two steps. The first step will consider baseline demographic and disease characteristics that may be predictive of endpoints, such as HF aetiology and NYHA class. Significant predictors will be determined through backward variable selection. The second step will assess treatment effect controlling for individual patient risk as measured by the linear predictor function from the first step. The main analysis, which will include all randomized patients, will be repeated excluding the patients for whom the ECG core laboratory did not confirm LBBB.
The three interim analyses will follow a symmetric group sequential design using the alpha‐spending methodology of Lan and DeMets30 with O'Brien–Fleming‐type boundaries,31 after 275, 550, and 825 patients have experienced a primary endpoint event, respectively. The DMC will review interim analysis results and will advise on continuation of enrolment and patient follow‐up.
The secondary objectives will be analysed when the study has stopped after an interim analysis or the final analysis. A Hommel procedure32 will be applied to the secondary objectives (excluding the cost‐effectiveness objective) using an overall α‐level as determined from a Pocock‐type alpha spending function. Secondary objectives for which the hypothesis is rejected under the adjusted significance level of the Hommel procedure will be reported as significant with strictly controlled familywise type I error.
Discussion
Despite the overall efficacy of CRT in reducing morbidity and mortality endpoints in patients with HF with systolic dysfunction and ventricular dyssynchrony, its effect remains largely heterogeneous, with patients showing a varying degree of clinical benefit.1, 2, 3, 4, 5 In this context, optimization of the device settings is a logical priority of current device‐related research activity.
The application of the AdaptivCRT mode provides a novel pacing algorithm specifically designed for preferential LV‐only RV‐synchronized pacing with conduction time‐adaptive AV delay, to maximize fusion of RV and LV activation and achieve optimized LV and BiV pacing.17 The AdaptivCRT pre‐market approval study has demonstrated that AdaptivCRT‐optimized CRT is at least as effective as echo‐optimized BiV pacing determined by the CCS24 (73.6% improved in the AdaptivCRT arm vs. 72.5% in the echo‐optimized arm, P < 0.001 for non‐inferiority with a non‐inferiority margin of 12%18). Furthermore, in a post hoc sub‐analysis of this study, in patients with sinus rhythm, device‐determined normal AV conduction and presence of LBBB per medical history, more AdaptivCRT patients improved in their CCS compared with the echo arm (80.7% vs. 68.4%, P = 0.041 for superiority).19 In this subgroup, the patients in the AdaptivCRT arm received LV‐only pacing 64 ± 32.8% of the time.19 This meant that RV pacing was minimized, which might be desirable in such patients from both haemodynamic as well as from energy‐efficiency perspectives. In the subgroup with normal AV conduction, there was a lower risk of death or HF hospitalization [HR 0.52; 95% confidence interval (CI) 0.27–0.98, P = 0.044] with the AdaptivCRT algorithm.19 Moreover, with longer‐term follow‐up (20.2 ± 5.9 months) the AdaptivCRT algorithm has been shown to reduce the risk of 48 consecutive hours in AF (HR 0.54; 95% CI 0.31–0.93, P = 0.03) and AdaptivCRT patients without history of AF tended to be less likely to develop persistent AF (HR 0.44; 95% CI 0.19–1.03, P = 0.05).33 The AdaptResponse study was designed to confirm these post hoc findings and differs from the earlier study in that it is powered for a mortality/morbidity endpoint and enrols a subgroup of patients who were eligible for the earlier study.
Randomization is done after successful implant to ensure that the start of CRT can be taken as the starting point for analysis. Attempting randomization prior to implant could result in a subgroup of patients where initiation of CRT was delayed due to implant complications. A double‐blinded study design has been considered; however, it would not have been possible to blind site personnel interacting with the device, and a set‐up with blinded and unblinded hospital staff was considered to increase study complexity. The worsening HF event definition in the AdaptResponse trial is broader than the more traditional HF hospitalization, adding outpatient treatment with i.v. diuretics. The reason for this choice is that the incidence rate of HF hospitalizations has decreased over the years due to advances in the treatment of HF, and the fact that there are geographic differences in the treatment of HF and the definition of hospitalization that led to different rates of HF hospitalization.34, 35, 36 The broader definition is intended to ensure that the event rate is high enough to have an achievable sample size and to accommodate geographic differences due to differing health care systems. Heart failure hospitalizations underestimate HF worsening and its serious implications. Recent trials have shown that the risk of death is similar in outpatient intensification of HF therapy, emergency department visit, or HF hospitalization.23
As the LBBB inclusion criteria refers to the Strauss LBBB criteria,21 both males with an intrinsic QRS duration ≥140 ms and females with a QRS duration of ≥130 ms can be enrolled. This might reduce a bias in the regular criteria favouring men. Also the requirement of normal AV conduction ≤200 ms at the time of enrolment may help to increase enrolment of females19, 37 and collection of evidence as they are normally under‐represented in CRT trials for HF.
The above‐outlined preliminary evidence suggests that the AdaptivCRT algorithm allows for more physiological ventricular activation and increased device longevity,16 and may result in improved clinical outcomes, especially in patients with normal AV conduction and LBBB in sinus rhythm. The AdaptResponse clinical trial is powered to assess clinical endpoints and is expected to provide definitive data on the potential clinical utility of AdaptivCRT‐enhanced CRT systems.
Funding
The AdaptResponse study is sponsored in its entirety by Medtronic plc. Medtronic initiated the study and investigators receive reimbursement for collected data. An independent Steering Committee, Data Monitoring Committee, and Endpoint Adjudication Committee have been installed. The trial has been designed by the Steering Committee together with the sponsor. Medtronic is responsible for trial management and data analysis.
Conflict of interest: G.F. participated in Committees of trials sponsored by Bayer, Novartis, Servier, Vifor, and Medtronic. B.L.W. participated in Physician Advisory Committees of Medtronic, St. Jude Medical, and Spectranetics, and reports honoraria from Medtronic, St. Jude Medical, Spectranetics, Boston Scientific, and Convatec. C.L. participated in a Medtronic advisory board and reports honoraria received from Medtronic, Biotronik, Liva Nova, Boston Scientific, and St. Jude Medical. D.B. is a mid‐career investigator supported by the Heart and Stroke Foundation of Ontario, and by a University of Ottawa Chair in Electrophysiology Research. He has received major research funding from Medtronic, Boston Scientific, Boehringer Ingelheim, Bayer, Biotronik, Pfizer, and Bristol Myers Squibb. K.K., W.M., A.H., and M.R.G. have no conflicts of interest to disclose. B.G. and S.J. are employees of Medtronic.
Acknowledgements
The authors gratefully and specially acknowledge the early contributions of Dr William Little, from the University of Mississippi Medical Centre (Jackson, MS, USA), as a member of the Steering Committee, who was unfortunately not able to make further contributions as he passed away on 9 July 2015. The authors would also like to acknowledge the contributions of Koen J.P. Verhees, PhD, an employee of Medtronic, for his editorial and logistical support, and the critical appraisal of this manuscript, and Dr George Giannopoulos and Professor Spyridon G. Deftereos for their useful suggestions.
AdaptResponse Steering, Data Monitoring and Endpoint Adjudication Committees, and ECG Core Laboratory
AdaptResponse Steering Committee
Bruce L. Wilkoff, MD, Chair, Cleveland Clinic, Cleveland, OH, USA; Gerasimos Filippatos, MD, Co‐chair, National and Kapodistrian University of Athens, Athens, Greece; David Birnie, MD, University of Ottawa Heart Institute, Ottawa, Ontario, Canada; Michael R. Gold, MD, Medical University of South Carolina, Charleston, SC, USA; Ahmad Hersi, MD, King Saud University, College of Medicine, Department of Cardiac Sciences, Riyadh, Saudi Arabia; Kengo Kusano, MD, National Cerebral and Cardiovascular Center, Osaka, Japan; Christophe Leclercq, MD, University Hospital Rennes, University of Rennes, Rennes, France; William Little, MD†, University of Mississippi, Jackson, MS, USA, †deceased 9 July 2015; Wilfried Mullens, MD, Ziekenhuis Oost‐Limburg, Genk, Belgium.
AdaptResponse Data Monitoring Committee
John Cleland, MD, Chair, Magdi Yacoub Institute–Heart Science Centre National Heart & Lung Institute Harefield Hospital, Harefield, UK; Kenneth Dickstein, MD, Stavanger Universitetssykehus, Stavanger, Norway; Kerry Lee, PhD, Professor of Biostatistics, Duke Clinical Research Institute, Durham, NC, USA; Jonathan Steinberg, MD, Summit Medical Group Arrhythmia Institute, University of Rochester School of Medicine, Short Hills, NJ, USA.
AdaptResponse Endpoint Adjudication Committee
Michael Felker, MD, Chair, Duke University School of Medicine, Duke University West Campus, Durham, NC, USA; Piotr Ponikowski, MD, Medical University, Military Hospital, Department of Cardiology, Wroclaw, Poland; Frieder Braunschweig, MD, Karolinska University Hospital, Solna, Department of Cardiology, Stockholm, Sweden; Daniel Lustgarten, MD, Medicine Cardiovascular Medicine, University of Vermont Medical Center, Cardiology Burlington, VT, USA; John Teerlink, MD, San Francisco VA Medical Center Cardiology, San Francisco, CA, USA; John Lekakis, MD, Cardiology, Athens University Medical School, University Hospital ATTIKON, Athens, Greece.
AdaptResponse ECG Core Laboratory
Christophe Leclercq, MD, Centre Hospitalier Universitaire and Centre d'Investigation Clinique–Innovations Technologiques, Cardiology Department, CIC‐IT 804, Pontchaillou Hospital, Rennes, France.
The copyright line for this article was changed on 21 July 2017 after original online publication.
References
- 1. Brignole M, Auricchio A, Baron‐Esquivias G, Bordachar P, Boriani G, Breithardt OA, Cleland J, Deharo JC, Delgado V, Elliott PM, Gorenek B, Israel CW, Leclercq C, Linde C, Mont L, Padeletti L, Sutton R, Vardas PE; ESC Committee for Practice Guidelines (CPG) , Zamorano JL, Achenbach S, Baumgartner H, Bax JJ, Bueno H, Dean V, Deaton C, Erol C, Fagard R, Ferrari R, Hasdai D, Hoes AW, Kirchhof P, Knuuti J, Kolh P, Lancellotti P, Linhart A, Nihoyannopoulos P, Piepoli MF, Ponikowski P, Sirnes PA, Tamargo JL, Tendera M, Torbicki A, Wijns W, Windecker S; Document Reviewers , Kirchhof P, Blomstrom‐Lundqvist C, Badano LP, Aliyev F, Bansch D, Baumgartner H, Bsata W, Buser P, Charron P, Daubert JC, Dobreanu D, Faerestrand S, Hasdai D, Hoes AW, Le Heuzey JY, Mavrakis H, McDonagh T, Merino JL, Nawar MM, Nielsen JC, Pieske B, Poposka L, Ruschitzka F, Tendera M, Van Gelder IC, Wilson CM. 2013 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy: the Task Force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC). Developed in collaboration with the European Heart Rhythm Association (EHRA). Eur Heart J 2013;34:2281–2329. [DOI] [PubMed] [Google Scholar]
- 2. Epstein AE, DiMarco JP, Ellenbogen KA, Estes NA 3rd, Freedman RA, Gettes LS, Gillinov AM, Gregoratos G, Hammill SC, Hayes DL, Hlatky MA, Newby LK, Page RL, Schoenfeld MH, Silka MJ, Stevenson LW, Sweeney MO; American College of Cardiology Foundation ; American Heart Association Task Force on Practice Guidelines; Heart Rhythm Society. 2012 ACCF/AHA/HRS focused update incorporated into the ACCF/AHA/HRS 2008 guidelines for device‐based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Circulation 2013;127:e283–352. [DOI] [PubMed] [Google Scholar]
- 3. Abraham WT, Fisher WG, Smith AL, Delurgio DB, Leon AR, Loh E, Kocovic DZ, Packer M, Clavell AL, Hayes DL, Ellestad M, Trupp RJ, Underwood J, Pickering F, Truex C, McAtee P, Messenger J; MIRACLE Study Group . Cardiac resynchronization in chronic heart failure. N Engl J Med 2002;346:1845–1853. [DOI] [PubMed] [Google Scholar]
- 4. Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L, Tavazzi L; Cardiac Resynchronization–Heart Failure (CARE‐HF) Study Investigators . The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med 2005;352:1539–1549. [DOI] [PubMed] [Google Scholar]
- 5. Chung ES, Leon AR, Tavazzi L, Sun JP, Nihoyannopoulos P, Merlino J, Abraham WT, Ghio S, Leclercq C, Bax JJ, Yu CM, Gorcsan J 3rd, St John Sutton M, De Sutter J, Murillo J. Results of the Predictors of Response to CRT (PROSPECT) trial. Circulation 2008;117:2608–2616. [DOI] [PubMed] [Google Scholar]
- 6. Boriani G, Gardini B, Diemberger I, Bacchi Reggiani ML, Biffi M, Martignani C, Ziacchi M, Valzania C, Gasparini M, Padeletti L, Branzi A. Meta‐analysis of randomized controlled trials evaluating left ventricular vs. biventricular pacing in heart failure: effect on all‐cause mortality and hospitalizations. Eur J Heart Fail 2012;14:652–660. [DOI] [PubMed] [Google Scholar]
- 7. Thibault B, Ducharme A, Harel F, White M, O'Meara E, Guertin MC, Lavoie J, Frasure‐Smith N, Dubuc M, Guerra P, Macle L, Rivard L, Roy D, Talajic M, Khairy P; Evaluation of Resynchronization Therapy for Heart Failure (GREATER‐EARTH) Investigators . Left ventricular versus simultaneous biventricular pacing in patients with heart failure and a QRS complex ≥120 milliseconds. Circulation 2011;124:2874–2881. [DOI] [PubMed] [Google Scholar]
- 8. Boriani G, Kranig W, Donal E, Calo L, Casella M, Delarche N, Lozano IF, Ansalone G, Biffi M, Boulogne E, Leclercq C; B‐LEFT HF study group. A randomized double‐blind comparison of biventricular versus left ventricular stimulation for cardiac resynchronization therapy: the Biventricular versus Left Univentricular Pacing with ICD Back‐up in Heart Failure Patients (B‐LEFT HF) trial. Am Heart J 2010;159:1052–1058.e1. [DOI] [PubMed] [Google Scholar]
- 9. van Gelder BM, Bracke FA, Meijer A, Pijls NH. The hemodynamic effect of intrinsic conduction during left ventricular pacing as compared to biventricular pacing. J Am Coll Cardiol 2005;46:2305–2310. [DOI] [PubMed] [Google Scholar]
- 10. Lee KL, Burnes JE, Mullen TJ, Hettrick DA, Tse HF, Lau CP. Avoidance of right ventricular pacing in cardiac resynchronization therapy improves right ventricular hemodynamics in heart failure patients. J Cardiovasc Electrophysiol 2007;18:497–504. [DOI] [PubMed] [Google Scholar]
- 11. Bertini M, Delgado V, Bax JJ, Van de Veire NR. Why, how and when do we need to optimize the setting of cardiac resynchronization therapy? Europace 2009;11 (Suppl 5):v46–57. [DOI] [PubMed] [Google Scholar]
- 12. Vidal B, Sitges M, Marigliano A, Delgado V, Diaz‐Infante E, Azqueta M, Tamborero D, Tolosana JM, Berruezo A, Perez‐Villa F, Pare C, Mont L, Brugada J. Optimizing the programation of cardiac resynchronization therapy devices in patients with heart failure and left bundle branch block. Am J Cardiol 2007;100:1002–1006. [DOI] [PubMed] [Google Scholar]
- 13. Ellenbogen KA, Gold MR, Meyer TE, Fernndez Lozano I, Mittal S, Waggoner AD, Lemke B, Singh JP, Spinale FG, Van Eyk JE, Whitehill J, Weiner S, Bedi M, Rapkin J, Stein KM. Primary results from the SmartDelay determined AV optimization: a comparison to other AV delay methods used in cardiac resynchronization therapy (SMART‐AV) trial: a randomized trial comparing empirical, echocardiography‐guided, and algorithmic atrioventricular delay programming in cardiac resynchronization therapy. Circulation 2010;122:2660–2668. [DOI] [PubMed] [Google Scholar]
- 14. Brugada Terradellas J, Delnoy PP, Brachmann J, Reynolds DW, Padeletti L, Noelker G, Kantipudi C, Borri‐Brunetto A, Verhees L, Ritter P, Singh JP; RESPOND‐CRT Investigators . Clinical response to cardiac resynchronization therapy with the SonR hemodynamic sensor: the RESPOND‐CRT Randomized Trial. LBCT01‐040 [Abstract]. Heart Rhythm 2016;16:1369–1372. [Google Scholar]
- 15. Ritter P, Delnoy PP, Padeletti L, Lunati M, Naegele H, Borri‐Brunetto A, Silvestre J. A randomized pilot study of optimization of cardiac resynchronization therapy in sinus rhythm patients using a peak endocardial acceleration sensor vs. standard methods. Europace 2012;14:1324–1333. [DOI] [PubMed] [Google Scholar]
- 16. Krum H, Lemke B, Birnie D, Lee KL, Aonuma K, Starling RC, Gasparini M, Gorcsan J, Rogers T, Sambelashvili A, Kalmes A, Martin D. A novel algorithm for individualized cardiac resynchronization therapy: rationale and design of the adaptive cardiac resynchronization therapy trial. Am Heart J 2012;163:747–752.e1. [DOI] [PubMed] [Google Scholar]
- 17. Daoud GE, Houmsse M. Cardiac resynchronization therapy pacemaker: critical appraisal of the adaptive CRT‐P device. Med Devices (Auckl) 2016;9:19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Martin DO, Lemke B, Birnie D, Krum H, Lee KL, Aonuma K, Gasparini M, Starling RC, Milasinovic G, Rogers T, Sambelashvili A, Gorcsan J 3rd, Houmsse M; Adaptive CRT Study Investigators . Investigation of a novel algorithm for synchronized left‐ventricular pacing and ambulatory optimization of cardiac resynchronization therapy: results of the adaptive CRT trial. Heart Rhythm 2012;9:1807–1814. [DOI] [PubMed] [Google Scholar]
- 19. Birnie D, Lemke B, Aonuma K, Krum H, Lee KL, Gasparini M, Starling RC, Milasinovic G, Gorcsan J 3rd, Houmsse M, Abeyratne A, Sambelashvili A, Martin DO. Clinical outcomes with synchronized left ventricular pacing: analysis of the adaptive CRT trial. Heart Rhythm 2013;10:1368–1374. [DOI] [PubMed] [Google Scholar]
- 20. Singh JP, Abraham WT, Chung ES, Rogers T, Sambelashvili A, Coles JA Jr, Martin DO. Clinical response with adaptive CRT algorithm compared with CRT with echocardiography‐optimized atrioventricular delay: a retrospective analysis of multicentre trials. Europace 2013;15:1622–1628. [DOI] [PubMed] [Google Scholar]
- 21. Strauss DG, Selvester RH, Wagner GS. Defining left bundle branch block in the era of cardiac resynchronization therapy. Am J Cardiol 2011;107:927–934. [DOI] [PubMed] [Google Scholar]
- 22. Gheorghiade M, Zannad F, Sopko G, Klein L, Pina IL, Konstam MA, Massie BM, Roland E, Targum S, Collins SP, Filippatos G, Tavazzi L; International Working Group on Acute Heart Failure Syndromes . Acute heart failure syndromes: current state and framework for future research. Circulation 2005;112:3958–3968. [DOI] [PubMed] [Google Scholar]
- 23. Okumura N, Jhund PS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Swedberg K, Zile MR, Solomon SD, Packer M, McMurray JJ; PARADIGM Investigators and Committees . Importance of clinical worsening of heart failure treated in the outpatient setting: evidence from the Prospective Comparison of ARNI With ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure Trial (PARADIGM‐HF). Circulation 2016;133:2254–2262. [DOI] [PubMed] [Google Scholar]
- 24. Packer M. Proposal for a new clinical end point to evaluate the efficacy of drugs and devices in the treatment of chronic heart failure. J Card Fail 2001;7:176–182. [DOI] [PubMed] [Google Scholar]
- 25. Green CP, Porter CB, Bresnahan DR, Spertus JA. Development and evaluation of the Kansas City Cardiomyopathy Questionnaire: a new health status measure for heart failure. J Am Coll Cardiol 2000;35:1245–1255. [DOI] [PubMed] [Google Scholar]
- 26. Moss AJ, Hall WJ, Cannom DS, Klein H, Brown MW, Daubert JP, Estes NA 3rd, Foster E, Greenberg H, Higgins SL, Pfeffer MA, Solomon SD, Wilber D, Zareba W; MADIT‐CRT Trial Investigators . Cardiac‐resynchronization therapy for the prevention of heart‐failure events. N Engl J Med 2009;361:1329–1338. [DOI] [PubMed] [Google Scholar]
- 27. Linde C, Abraham WT, Gold MR, St John Sutton M, Ghio S, Daubert C; REVERSE (REsynchronization reVErses Remodeling in Systolic left vEntricular dysfunction) Study Group . Randomized trial of cardiac resynchronization in mildly symptomatic heart failure patients and in asymptomatic patients with left ventricular dysfunction and previous heart failure symptoms. J Am Coll Cardiol 2008;52:1834–1843. [DOI] [PubMed] [Google Scholar]
- 28. Tang AS, Wells GA, Talajic M, Arnold MO, Sheldon R, Connolly S, Hohnloser SH, Nichol G, Birnie DH, Sapp JL, Yee R, Healey JS, Rouleau JL; Resynchronization‐Defibrillation for Ambulatory Heart Failure Trial Investigators . Cardiac‐resynchronization therapy for mild‐to‐moderate heart failure. N Engl J Med 2010;363:2385–2395. [DOI] [PubMed] [Google Scholar]
- 29. Cleland JG, Abraham WT, Linde C, Gold MR, Young JB, Claude Daubert J, Sherfesee L, Wells GA, Tang AS. An individual patient meta‐analysis of five randomized trials assessing the effects of cardiac resynchronization therapy on morbidity and mortality in patients with symptomatic heart failure. Eur Heart J 2013;34:3547–3556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lan KKG, DeMets DL. Discrete sequential boundaries for clinical trials. Biometrika 1983;70:659–663. [Google Scholar]
- 31. O'Brien PC, Fleming TR. A multiple testing procedure for clinical trials. Biometrics 1979;35:549–556. [PubMed] [Google Scholar]
- 32. Hommel G. A stagewise rejective multiple test procedure based on a modified Bonferroni test. Biometrika 1988;75:383–386. [Google Scholar]
- 33. Martin D, Lemke B, Aonuma K, Krum H, Lai‐Fun Lee K, Gasparini M, Starling R, Gorcsan J, Rogers T, Sambelashvili A, Hudnall J, Birnie D. Clinical outcomes with adaptive cardiac resynchronization therapy: long‐term outcomes of the adaptive CRT trial. HFSA Late Breakers [Abstract]. 23 September 2013. [Google Scholar]
- 34. Filippatos G, Anker SD, Bohm M, Gheorghiade M, Kober L, Krum H, Maggioni AP, Ponikowski P, Voors AA, Zannad F, Kim SY, Nowack C, Palombo G, Kolkhof P, Kimmeskamp‐Kirschbaum N, Pieper A, Pitt B. A randomized controlled study of finerenone vs. eplerenone in patients with worsening chronic heart failure and diabetes mellitus and/or chronic kidney disease. Eur Heart J 2016;37:2105–2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gheorghiade M, Pang PS, Ambrosy AP, Lan G, Schmidt P, Filippatos G, Konstam M, Swedberg K, Cook T, Traver B, Maggioni A, Burnett J, Grinfeld L, Udelson J, Zannad F. A comprehensive, longitudinal description of the in‐hospital and post‐discharge clinical, laboratory, and neurohormonal course of patients with heart failure who die or are re‐hospitalized within 90 days: analysis from the EVEREST trial. Heart Fail Rev 2012;17:485–509. [DOI] [PubMed] [Google Scholar]
- 36. Butler J, Gheorghiade M, Kelkar A, Fonarow GC, Anker S, Greene SJ, Papadimitriou L, Collins S, Ruschitzka F, Yancy CW, Teerlink JR, Adams K, Cotter G, Ponikowski P, Felker GM, Metra M, Filippatos G. In‐hospital worsening heart failure. Eur J Heart Fail 2015;17:1104–1113. [DOI] [PubMed] [Google Scholar]
- 37. Stockburger M, Moss AJ, Klein HU, Zareba W, Goldenberg I, Biton Y, McNitt S, Kutyifa V. Sustained clinical benefit of cardiac resynchronization therapy in non‐LBBB patients with prolonged PR‐interval: MADIT‐CRT long‐term follow‐up. Clin Res Cardiol 2016;105:944–952. [DOI] [PubMed] [Google Scholar]


