Summary
Root exudation is a key component of nutrient and carbon dynamics in terrestrial ecosystems. Exudation rates vary widely by plant species and environmental conditions, but our understanding of how root exudates affect soil functioning is incomplete, in part because there are few viable methods to manipulate root exudates in situ. To address this, we devised the Automated Root Exudate System (ARES), which simulates increased root exudation by applying small amounts of labile solutes at regular intervals in the field.
The ARES is a gravity‐fed drip irrigation system comprising a reservoir bottle connected via a timer to a micro‐hose irrigation grid covering c. 1 m2; 24 drip‐tips are inserted into the soil to 4‐cm depth to apply solutions into the rooting zone. We installed two ARES subplots within existing litter removal and control plots in a temperate deciduous woodland. We applied either an artificial root exudate solution (RE) or a procedural control solution (CP) to each subplot for 1 min day−1 during two growing seasons. To investigate the influence of root exudation on soil carbon dynamics, we measured soil respiration monthly and soil microbial biomass at the end of each growing season.
The ARES applied the solutions at a rate of c. 2 L m−2 week−1 without significantly increasing soil water content. The application of RE solution had a clear effect on soil carbon dynamics, but the response varied by litter treatment. Across two growing seasons, soil respiration was 25% higher in RE compared to CP subplots in the litter removal treatment, but not in the control plots. By contrast, we observed a significant increase in microbial biomass carbon (33%) and nitrogen (26%) in RE subplots in the control litter treatment.
The ARES is an effective, low‐cost method to apply experimental solutions directly into the rooting zone in the field. The installation of the systems entails minimal disturbance to the soil and little maintenance is required. Although we used ARES to apply root exudate solution, the method can be used to apply many other treatments involving solute inputs at regular intervals in a wide range of ecosystems.
Keywords: forest, litter manipulation, microbial biomass, micro‐irrigation, rhizodeposition, soil carbon dynamics, timed application
Introduction
The release of organic compounds into the rhizosphere by fine roots is a ubiquitous process and a key component of ecosystem carbon and nutrient cycling (Grayston, Vaughan & Jones 1996; Jones, Hodge & Kuzyakov 2004). Root exudates contain many different organic compounds and associated ions, which influence nutrient availability, fuel microbial growth and activity, and stimulate the mineralisation or immobilisation of soil organic matter (Jones, Hodge & Kuzyakov 2004). The composition of root exudates can be highly species specific and also varies with plant physiological state (Smith 1976; Bais et al. 2006; Vranova et al. 2013). The main components (sugars, organic acids and amino acids) are a valuable metabolic resource for soil microbes, and organic acids can also liberate soil organic solutes from their mineral protection, which in turn promote the microbial mineralisation of soil organic matter and the release of inorganic nutrients into the rhizosphere for uptake by plants (Jones, Hodge & Kuzyakov 2004; Drake et al. 2011; Keiluweit et al. 2015). A number of studies have highlighted the importance of root exudates in promoting ‘rhizosphere priming’ in which soil microbial activity is stimulated, resulting in the mineralisation of soil organic matter (De Graaff et al. 2010; Cheng et al. 2014; Sulman et al. 2014).
Studies in forest ecosystems suggest that root exudates from trees can represent anything from 1 to 10% of the total carbon (C) assimilated during photosynthesis (Jones, Hodge & Kuzyakov 2004; Phillips et al. 2008; Qiao et al. 2014; Yin, Wheeler & Phillips 2014). Although the amount of C released to the soil by exudation is relatively small compared to total ecosystem C fluxes, the C entering the soil food web in temperate forests is predominantly root derived (Pollierer et al. 2007, 2012) and root exudates may have a strong influence on soil C and nutrient dynamics under global change (Phillips, Finzi & Bernhardt 2011; Fransson 2012). Experiments in the laboratory and field demonstrate that exudation rates increase markedly in response to elevated CO2 (Phillips, Finzi & Bernhardt 2011; Cheng et al. 2014), nutrient deficiency (Grayston, Vaughan & Jones 1996; Phillips, Finzi & Bernhardt 2011), moderate drought stress (Preece & Peñuelas 2016) and herbivory (Holland, Cheng & Crossley 1996) and are also influenced by changes in temperature and soil water content (Grayston, Vaughan & Jones 1996; Dijkstra & Cheng 2007; Yin et al. 2013).
Despite the importance of root exudates in ecosystem functioning, we still know very little about how changes in exudation rates will affect soil carbon and nutrient dynamics, mainly because there are few viable methods to experimentally manipulate inputs of root exudates at the field scale. Decreased root exudation in wooded ecosystems can be achieved experimentally by tree girdling (i.e. stripping stem bark to the depth of the current xylem to terminate the supply of photosynthates to roots; Weintraub et al. 2007; Högberg et al. 2009), but measurements can only be made for a limited time because girdling can severely damage or kill trees and their fine root systems (Kaiser et al. 2010). Although free‐air CO2 enrichment (FACE) increases root exudation (Phillips, Finzi & Bernhardt 2011; Fransson 2012), it can also alter plant growth (Norby & Zak 2011), soil water uptake (Hungate et al. 1997; Warren et al. 2011) and the quality and quantity of litter inputs (Norby et al. 2001; De Graaff et al. 2006); FACE experiments are also expensive and logistically problematic in many ecosystems.
An alternative approach is to use an artificial root exudate solution, which has been successfully applied to soils in laboratory microcosms (e.g. Baudoin, Benizri & Guckert 2003; De Graaff et al. 2010). Scaling up this type of experiment for application in the field is challenging because plants continuously release extremely small amounts of exudates throughout the rooting zone during the growing season (Kuzyakov & Cheng 2001). Experiments applying artificial root exudate solution in the field would need to mimic this to a certain extent: firstly, because root exudates contain many compounds that are readily available to soil microbes (Kuzyakov & Cheng 2001; Van Hees et al. 2005), and a single large application of artificial root exudates to the soil is likely to have a very different effect than when exudates are released slowly throughout the day (Qiao et al. 2014). Secondly, root exudates affect a number of soil properties as well as mediating important microbial processes (Grayston, Vaughan & Jones 1996; Hinsinger et al. 2003), and hence the effects are likely to differ depending on whether the solution is applied to the soil surface or released within the rooting zone.
We wanted to investigate changes in forest soil carbon dynamics in response to increased plant inputs both above‐ and belowground. Whereas manipulating above‐ground litter inputs in forest ecosystems is fairly straightforward (Sayer 2006), we needed to find a viable way of experimentally increasing the input of root exudates to the soil. Our objectives were therefore to: (i) design a low‐cost system that releases small quantities of liquid gradually into the rooting zone in the field; (ii) use the system to apply an artificial root exudate solution to subplots within an existing litter manipulation experiment to assess the effects of changes in above‐ and belowground plant inputs and (iii) assess whether the daily application of small quantities of root exudate solution has a detectable effect on soil carbon dynamics by measuring soil respiration and microbial biomass.
We constructed our Automated Root Exudate System (ARES) and tested it during two growing seasons in an existing litter manipulation experiment in temperate deciduous woodland.
Materials and methods
Study sites
Our experimental site was located in a patch of old (c. 120 years) mixed deciduous temperate woodland within Wytham Woods, Oxfordshire, UK (51°46′42′′N, 1°19′42′′W; mean slope c. 6%). The forest canopy is dominated by a mixture of sycamore (Acer pseudoplatanus L.), ash (Fraxinus excelsior L.) and occasionally pedunculate oak (Quercus robur L.), with hawthorn (Crataegus monogyna L.) and common hazel (Corylus avellana L.) scattered in the sub‐canopy. This area of the woodland has had minimal intervention and soil disturbance, with no silvicultural management for at least 40 years (Fenn et al. 2015). The soil is a base‐rich clay loam classified as stagni‐vertic cambisol (FAO/WRB classification; Beard 1993; IUSS Working Group WRB 2006), with c. 4·5% total organic C, c. 0·4% total nitrogen (N) content and 1·0 g cm−3 bulk density at 0–10 cm depth. Mean annual precipitation is 714 ± 29 mm and mean air temperature is 10·0 ± 0·1 °C, ranging from 4·2 ± 0·4 °C in December to 16·6 ± 0·3 °C in July (data from 1993 to 2011; Fenn et al. 2015).
In summer 2013, we established 15 plots, measuring 25 m × 25 m each, in five replicate blocks. The borders of the plots were trenched to 0·5‐m depth, lined with plastic and then backfilled to limit water and nutrient transfer. All subsequent samples and measurements were taken within the inner 15 m × 15 m of each plot to avoid edge effects. Starting in December 2013, one of three litter manipulation treatments was randomly assigned to each plot per block: in the five litter removal plots, we raked up the litter twice a year (November and January) and spread it as evenly as possible on the corresponding litter addition plots, leaving five undisturbed control plots.
In February 2015, we established two subplots (1·6 m × 1·4 m) in each of the control and litter removal plots. To minimise variation due to tree species composition or tree size, we placed the subplots close to each other but at least 1·5 m apart and at least 2·5 m from the nearest large tree. The subplots were fenced with wire mesh to avoid disturbance by badgers and deer (Fig. 1a,b). Within each of these subplots, we installed a drip irrigation system to apply either an artificial root exudate solution (RE) or a procedural control solution (CP). We completed the installation of the systems in March 2015 and started applying treatments to the subplots 1 month later in April 2015.
Figure 1.

Set up of subplots for the application of root exudate solution (RE) or a procedural control solution (CP) within litter treatment plots at Wytham Woods (Oxfordshire), showing (a) the litter removal treatment; (b) adjacent RE and CP subplots; (c) detail of the three ‘H’ segments of the irrigation grid, with sheaths and drip‐tips and (d) the location of drip‐tips marked in red around the 20‐cm diameter collar for soil respiration measurements.
Automated Root Exudate System: components, installation and operation
The ARES is a low‐cost, gravity‐fed drip irrigation system that applies precise quantities of solutions to field plots at regular intervals: briefly, the solution is contained in 2·5‐L aspirator bottles suspended from a pole with the aperture located c. 1·2 m above the soil surface. An irrigation timer controls the flow of solution through a polyethylene micro‐hose connected to a drip‐irrigation system. A cross joint connects the main hose to three pieces of tubing, which each feed an H‐shaped irrigation grid (60 cm × 20 cm) on the soil surface (Fig. 1c,d). Each H‐grid consists of 10 pieces of tubing and eight drip‐tips coupled by T‐pieces (intersections) or elbows (ends; Fig. 2). The drip‐tips are placed 20 cm apart and each tip is inserted 4 cm into the soil. To avoid blockage with soil particles, each drip‐tip is slotted into a polypropylene cylindrical sheath (0·5‐cm inner diameter) sunk into the soil to c. 4·5‐cm depth (Fig. 2). Once the tips are fitted inside the sheaths, the H‐grids are anchored to the ground using clip stakes (Figs 1c and 2). Thus, each ARES has 24 drip‐tips covering a total area of 0·6 m × 1 m (Fig. 2). Apart from the aspirator bottle, pipette tips and sheaths, all the ARES components are common gardening supplies (Appendix S1, Supporting Information).
Figure 2.
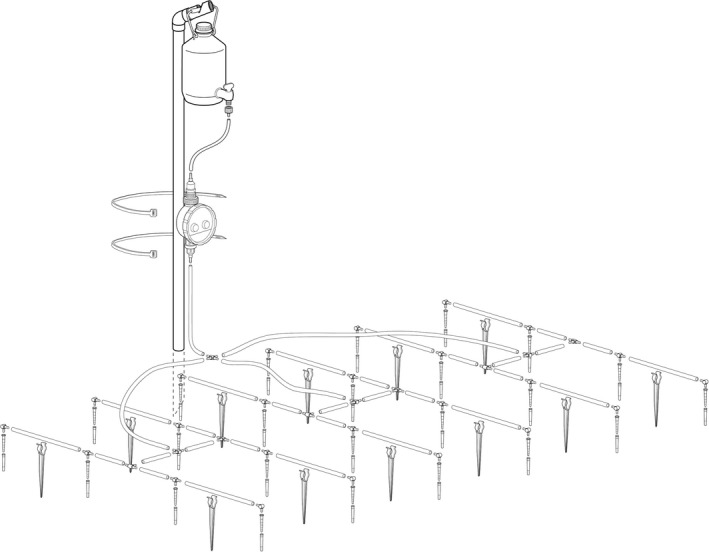
Schematic diagram showing the individual parts and set up of the Automated Root Exudate System.
We carried out preliminary tests of the system to determine the optimum height of the aspirator bottles to achieve a constant daily flow rate using a 1‐week supply of solution, and to test for consistent dispersion of the solution around the tips (Appendix S2). Assuming a maximum radius of influence of 10 cm around each tip in field conditions, the treatment area for each ARES is c. 0·8 m × 1·2 m (0·96 m2). The total volume of the tubing (6·4–6·5 m) is c. 26 mL, which is <10% of the total volume of solution applied each day (see below).
Root exudate solution
We wanted to use ARES to apply a treatment that was broadly representative of increased tree root exudation and we therefore developed our root exudate solution (henceforth RE solution) with the following considerations: (i) the chemical composition of root exudates is highly diverse (Smith 1976; Hütsch, Augustin & Merbach 2002; Bais et al. 2006) and our experiment required large quantities of solution, so we would need to simplify the formula to make it cost effective while maintaining its functionality; (ii) roots continuously produce exudates during photosynthesis, so we aimed to apply the RE solution at a constant daily rate during the growing season and (iii) the treatments should represent a twofold increase in root exudation, and hence our RE solution should provide a total carbon input equivalent to 4–5% net primary productivity (NPP), which lies in the mid‐range of current estimates for root exudation by trees (Jones, Hodge & Kuzyakov 2004; Qiao et al. 2014; Yin, Wheeler & Phillips 2014).
We used published data on the composition of root exudates and relative amounts of individual compounds (Table S4.1) to determine the basic formula for our RE solution. Despite the huge variation among studies, plant species and growing conditions, root exudates contain three principal organic components: carbohydrates (CHO), organic acids (OA) and amino acids (AA). For our RE solution, we used two main sources of carbohydrate (glucose and sucrose), five different organic acids (oxalate, acetate, succinate, citrate and fumaric acid) and three amino acids (glutamate, proline and serine; Table 1). The choice of sucrose and glutamate adds complexity to the RE solution because the former is a disaccharide that hydrolyses into glucose and fructose, and the latter can be directly or indirectly involved in the synthesis of many other amino acids (glutamine, arginine, ornithine, aspartate, methionine, threonine, leucine, lysine). We also added sodium as the most relevant ion in natural tree root exudates, and ammonium as the main source of N (Smith 1976; Table 1). Our final root exudate solution had a CHO : OA : AA mass ratio of 60 : 35 : 5, a C : N ratio of 10·0 and a pH of 5·3 (Table 1), all of which are well within the ranges reported in the literature (Grayston, Vaughan & Jones 1996; Vranova et al. 2013; Table S4.1). The final carbon concentration in the RE solution was 646·5 mg C L−1, which represents 4–5% of the NPP at the study site (700 g C m−2 year−1; Fenn et al. 2015) when applied at a rate of c. 2 L m−2 week−1 during the growing season.
Table 1.
Chemical composition of the root exudate solution applied to subplots in the field using an Automated Root Exudate System, showing the inputs of carbon (C), nitrogen (N) and sodium (Na) per litre of solution as prepared following the protocol in Appendix S3
| Name | Formula | mg L−1 | % mass | % mol | mg C L−1 | mg N L−1 | mg Na L−1 |
|---|---|---|---|---|---|---|---|
| Carbohydrates | |||||||
| D‐glucose | C6H12O6 | 544 | 30·0 | 29·5 | 217·6 | 0 | 0 |
| Sucrose | C12H22O11 | 544 | 30·0 | 15·5 | 229·0 | 0 | 0 |
| Total carbohydrates | 60·0 | 45·0 | 446·6 | 0 | 0 | ||
| Organic acids | |||||||
| Ammonium oxalate | C2H10O5N2 | 228 | 12·6 | 15·7 | 38·5 | 45·0 | 0 |
| Sodium acetate | C2H3O2Na | 153 | 8·4 | 18·2 | 44·8 | 0 | 42·9 |
| Disodium succinate | C4H4O4Na2 | 110 | 6·1 | 6·6 | 32·6 | 0 | 31·2 |
| Ammonium citrate | C6H14O7N2 | 88 | 4·9 | 3·8 | 28·0 | 10·9 | 0 |
| Fumaric acid | C4H4O4 | 55 | 3·0 | 4·6 | 22·8 | 0 | 0 |
| Total organic acids | 35·0 | 49·0 | 166·7 | 55·9 | 74·1 | ||
| Amino acids | |||||||
| L‐glutamic acid | C5H10O5NNa | 57 | 3·1 | 3·0 | 18·3 | 4·3 | 7·0 |
| L‐proline | C5H9O2N | 18 | 1·0 | 1·5 | 9·4 | 2·2 | 0 |
| L‐serine | C3H7O3N | 16 | 0·9 | 1·5 | 5·5 | 2·1 | 0 |
| Total amino acids | 5·0 | 6·0 | 33·2 | 8·6 | 7·0 | ||
| Total | 1813 | 100·0 | 100·0 | 646·5 | 64·4 | 81·1 | |
The root exudate solution was prepared weekly within 24 h of application in the field. Briefly, sucrose, D‐glucose and fumaric acid were dissolved in 2‐L deionised water (dH2O). The solution was mixed for 5 min, after which the remaining substrates were added in the following order: ammonium oxalate, ammonium citrate, sodium acetate, disodium succinate, L‐glutamic acid, L‐proline and L‐serine (Table 1; Appendix S3). The flask was then filled to 5 L with dH2O and the solution mixed for another 1 min before being transferred into a 50‐L aspirator bottle and topped up with dH2O to make 45 L of solution (Appendix S3). Each aspirator bottle for use in the field had been previously sterilised with ethanol (96%) and allowed to dry before being filled with solution. If the solution was prepared the day before application, the bottles were stored at 5 °C overnight. Analysis of the prepared solution showed negligible changes in total organic C concentrations over the course of a week (Appendix S2).
Application of RE solution using the ARES
In each of the main control and litter removal plots, we randomly assigned one subplot to receive the RE solution (RE subplots). The other subplot was used as a procedural control (CP subplots) and received an equivalent volume of CaCl2 solution (15 mg L−1), which provides an ionic strength similar to rain water and maintains soil structure (Lopez‐Sangil, Rovira & Casals 2013). We applied the RE and CP solutions daily from 16 April to 7 October 2015 (25 weeks) and 14 April to 14 September 2016 (22 weeks), which corresponds to the main period of plant growth at the site (Fenn, Malhi & Morecroft 2010). The irrigation timers were set to open the valve for 1 min every 24 h between 17.00 and 19.00 h local time, and we aimed to apply the solutions at a flow rate of 2 L week−1. To limit microbial growth and mineralisation of the root exudate solution, we replaced and sterilised the empty aspirator bottles every week. We also checked whether there was any solution remaining in the bottles, recorded a visual estimate of the remaining proportion and re‐adjusted the bottle height to increase the flow rate if necessary (Appendix S1). At the end of the first growing season, we dismantled the ARES and visual inspection revealed the presence of microbial biofilms in some of the drip‐tips, but no apparent microbial growth inside the tubing after 175 days of operation. We cleaned the ARES tubing and drip‐tips with ethanol before re‐installation in the following growing season.
Soil respiration measurements
To determine if the application of RE solution affected soil respiration, we measured soil CO2 efflux over four soil collars in each main treatment plot and one soil collar per subplot, placed between adjacent segments of irrigation tubing (Fig. 1d). The collars were made of polypropylene tubes (20‐cm inner diameter, 12‐cm height) sunk into the soil to 3‐cm depth. We measured soil respiration monthly from April 2015 to September 2016 using an infrared gas analyser with a 20‐cm diameter survey chamber (Li‐8100; LiCor BioSciences, Lincoln, NE, USA). Measurements were made 18.00–22.00 h after the previous day's application of RE or CP solution; each measurement was taken during 120 s with an initial 15‐s dead‐band to eliminate the effects of turbulence from chamber closure and we recorded soil temperature and soil water content (Thetaprobe; Delta‐T Devices, Cambridge, UK) within c. 0·5 m of the collars and within the irrigation grids. All soil collars were kept free of live vegetation, and dead organic material inside the collars was carefully removed before each measurement and replaced once measurements were completed.
To determine the short‐term effects of applying the RE solution, we also measured soil respiration at regular intervals during 8 h after the application of the solutions to the subplots in June 2015 (control litter treatment only). Soil respiration was measured immediately before solutions were applied, and then again after 15, 125, 280 and 460 min.
Soil sampling and analyses
To assess the influence of RE solution on soil pH and microbial biomass, we collected two soil samples (0–10 cm depth and 5‐cm distance from a drip‐tip) per subplot at the end of the each growing season (September 2015 and 2016) using a 3·3‐cm diameter punch corer. Individual soil cores were kept intact and refrigerated during transport, mixed thoroughly upon return to the laboratory to give one composite sample per subplot, and all soil analyses were completed within 36 h. Gravimetric soil water content was determined by drying subsamples at 105 °C for 48 h and pH was measured on a 1 : 3 ratio of soil to dH2O. Microbial biomass C and N were determined on paired 8‐g subsamples of fresh soil by the fumigation‐extraction method following Vance, Brookes & Jenkinson (1987) with modifications by Jones & Willett (2006). Briefly, one subsample per pair was fumigated for 24 h with ethanol‐free chloroform and all samples were extracted in 40‐mL 0·5‐M K2SO4, followed by centrifugation and filtration. Total organic C (TOC) and total N in the extracts were analysed on a TOC‐L combustion analyser coupled with a TNM‐L unit (Shimadzu Corp, Kyoto, Japan); microbial biomass was calculated as the difference between fumigated and unfumigated samples (without correction for extraction efficiency).
To account for potential changes in fine root (diameter <2 mm) biomass, which could influence soil respiration, we collected two additional soil cores from each RE and CP subplot in July 2016. We separated the roots from the soil using a modified version of the method described in Upson, Burgess & Morison (2016), in which soil cores were agitated in water overnight in 250‐mL bottles filled to 80% capacity. Roots were separated from the resulting soil slurry by sieving and washing, sorted by diameter (<2, 2–5 and >5 mm), dried at 105 °C and weighed.
Statistical analyses
All statistical analyses were conducted in R version 3.3.1. (R Core Team 2016) and soil respiration data were log‐transformed to achieve normality. Treatment effects on soil respiration and soil properties (microbial biomass C and N, soil pH) were assessed using linear mixed effects models (lmer function in the lme4 package; Bates et al. 2015), with litter treatment, subplot treatment and their interaction as fixed effects, and block and time as random effects. Given the strong influence of temperature on soil respiration, we included soil temperature in all models as a covariate. The significance of each term was determined by comparing nested models using likelihood ratio tests. Models were simplified by sequentially dropping terms until a minimum adequate model was reached, using AICs and P values to check for model improvement (Pinheiro & Bates 2000), and the fit of the final model was inspected using diagnostic plots. Preliminary comparisons of soil respiration rates revealed significant differences between CP subplots and the main litter treatment plots, and we therefore used the CP subplots as the controls to assess the effects of RE application. If the final model included a significant interaction between litter treatments and RE application, we subsequently tested the effects of RE application for each litter manipulation treatment separately. Statistics are given for the comparison between the best‐fit model and the corresponding null model.
We tested the effects of litter treatments and RE application on root biomass using nested linear models, with experimental block included as an error term. We simplified the models to reach a minimal adequate model as described above.
Results
Despite the additional input of c. 2‐L m−2 water in RE and CP subplots, soil water content did not differ between subplots and main litter treatments or between RE and CP subplots (Fig. S4.1). Respiration measurements during the first 8 h after application of the RE and CP solutions showed no significant short‐term changes in soil respiration relative to the main control plots (Fig. 3).
Figure 3.
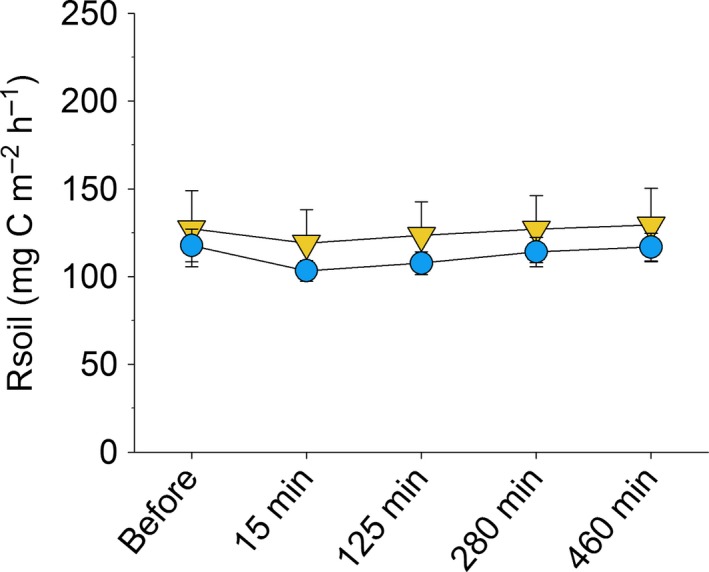
Short‐term soil respiration (Rsoil) in Automated Root Exudate System (ARES) subplots within main control plots, showing no immediate increase in Rsoil in subplots with root exudate solution (RE, yellow triangles) compared to procedural controls (CP, blue circles); measurements were taken immediately before and 15, 125, 280 and 460 min after the daily application of RE and CP solutions; means ± SE for n = 5 are given.
Although the additional C input from our RE solution only corresponded to c. 4% NPP at the site, we observed a clear effect of RE application on soil carbon dynamics during the growing season but the effect varied depending on litter treatment. For monthly measurements of soil respiration, the final model included main litter treatment, subplot treatment and their interaction (χ2 = 50·6; P < 0·001). Soil respiration in the control plots was not affected by the application of RE solution. By contrast, soil respiration in the litter removal plots was significantly higher in the RE compared to the CP subplots within 2 months of the start of ARES inputs (Fig. 4), after which soil respiration rates in the litter removal treatment were consistently higher in the RE compared to the CP subplots (χ2 = 31·1; P < 0·001; Fig. 4). Across both years, mean soil respiration during the treatment period (May to October) was 25% higher in RE subplots (194 ± 16 mg C m−2 h−1) than CP subplots (155 ± 13 mg C m−2 h−1; Table S4.2).
Figure 4.
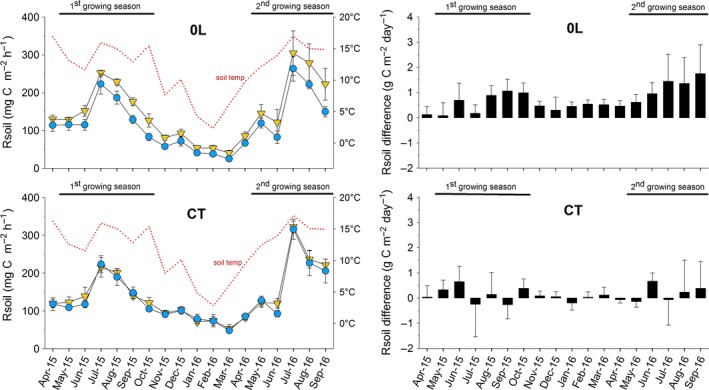
Soil respiration (Rsoil, left‐hand panels) measured monthly in subplots with daily applications of root exudate solution (RE, yellow triangles) or a procedural control solution (CP, blue circles); and barplots showing the significant increase in Rsoil in RE compared to CP subplots (right‐hand panels) within litter removal plots (0L) but not control plots (CT); means ± SE are given for n = 5. The Automated Root Exudate System (ARES) treatment periods corresponding to the main growing seasons in 2015 and 2016 are indicated with black horizontal lines; the dotted red line (left) indicates the mean soil temperature at 0–10 cm depth.
For microbial biomass C and N, the final models also included main litter treatment, subplot treatment and their interaction (microbial biomass C: χ2 = 15·9; P = 0·001; microbial biomass N: χ2 = 9·6; P = 0·023). Mean microbial biomass C was 33 and 24% higher in the RE subplots compared to CP subplots in control and litter removal plots, respectively (Fig. 5; Table S4.2), whereas microbial biomass N was 26% higher in RE subplots compared to CP subplots in the control litter treatments (χ2 = 11·9; P < 0·001), but not in the litter removal plots (Table S4.2). There was no effect of litter treatments or RE application on fine root biomass (Fig. S4.2) or soil pH (data not shown).
Figure 5.
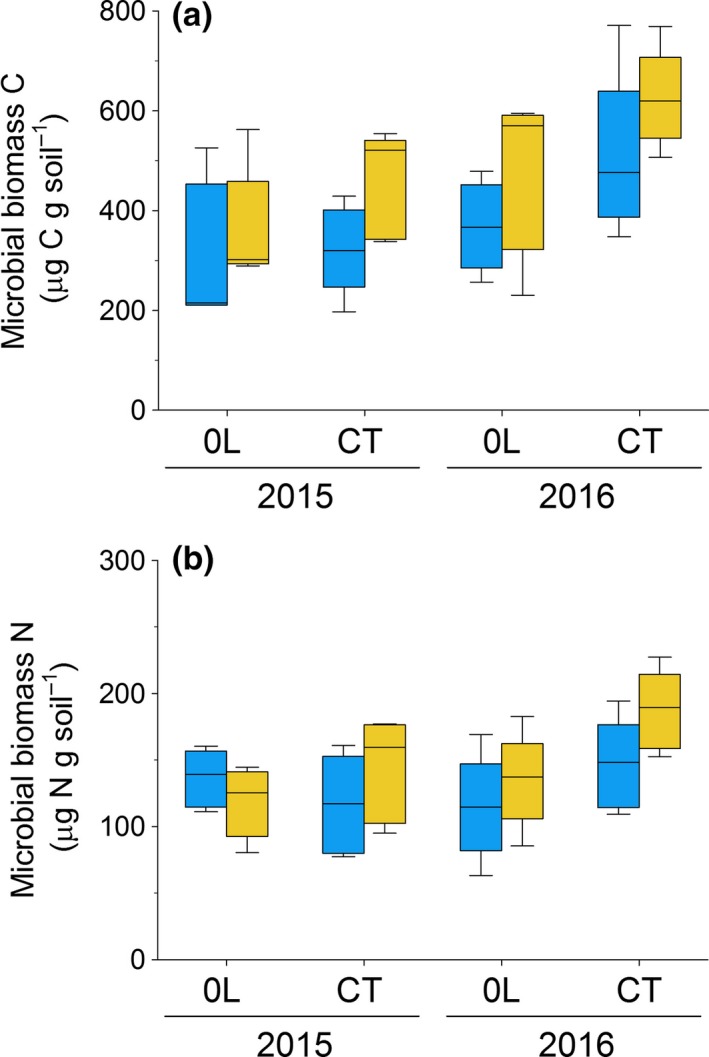
(a) Soil microbial biomass carbon (C) and (b) soil microbial biomass nitrogen (N) at the end of the growing season (September 2015 and 2016) in subplots with daily applications of root exudate solution (yellow boxplots) or a procedural control solution (blue boxplots) within litter removal (0L) and control plots (CT); the boxes indicate medians and upper and lower quartiles, whereas whiskers indicate 90% minimum and maximum values for n = 5.
Discussion
We designed the ARES to simulate increased root exudation and assess the effects of this increase on soil C dynamics in the field. Using our automated system, we were able to apply precise amounts of RE solution to field plots at regular intervals. Despite very low C inputs, we detected clear effects of RE application on soil respiration and microbial biomass, which were already apparent within the first few months of application and increased during the second year (Figs 4 and 5).
Our system offers new opportunities to assess the effects of increased root exudation rates on soil processes in the field. Although micro‐lysimeters have previously been used to apply root exudate solution directly into the rooting zone in the field (Drake et al. (2013), the range of application (within c. 5 mm of the lysimeters) precludes measurements of many key soil processes. Laboratory studies have provided valuable information to improve our understanding of root exudation, but the results may not be representative of changes in soil processes in situ (Qiao et al. 2014) because root exudate solutions are often applied in a single large pulse and at rates ranging from 50 to 1500 μg C g soil−1 day−1 or higher (De Graaff et al. 2010; Table S4.1). In contrast, we used the ARES to apply RE solution representing organic carbon inputs of c. 4% NPP or 1·7 μg C g soil−1 day−1 (in top 10‐cm depth and assuming 10‐cm radius of influence around the tips; Appendix S2), which is orders of magnitude lower than the majority of laboratory studies, but in the mid‐range of in situ estimates for tree root exudation rates (Smith 1976; Phillips et al. 2008; Phillips, Finzi & Bernhardt 2011; Brzostek et al. 2013; Yin, Wheeler & Phillips 2014). Thus, we believe that the ARES can be used to provide a more realistic assessment of the effects of increased root exudation in the field.
The distinct effects of RE application on soil C dynamics between litter treatments are intriguing because differences in the mineralisation of C or its incorporation into the microbial biomass are likely to result from changes in resource availability or microbial community composition, which determine the efficiency and extent of substrate utilisation (De Graaff et al. 2010; Kaiser et al. 2010; Brzostek et al. 2013; Yin, Wheeler & Phillips 2014). Soil respiration increased with RE application in the litter removal plots but not in the controls, whereas microbial biomass N increased with RE application in control plots but not in litter removal plots; this suggests that differences in C and N inputs from leaf litter played a role in determining whether the additional C from the RE application was respired or incorporated into microbial biomass (Grayston, Vaughan & Jones 1996; Drake et al. 2013). A previous microcosm study investigated interactions between litter inputs and root exudates by adding labelled compounds, which are typically present in root exudates, to soils from long‐term litter manipulation plots (Brandt, Sulzman & Myrold 2006). The incorporation of oxalate and glutamate into the microbial biomass was lower in soils from plots with no litter inputs, but soil C release by priming effects in response to the added substrates was much higher. Such differences in soil C priming and substrate use efficiency between control and litter removal treatments would explain the distinct responses to RE addition in our study.
Microbial turnover of root exudates is rapid (Nguyen et al. 1999; Kuzyakov & Cheng 2001). Many compounds have a half‐life of only 0·5–6 h, but this can be greatly increased by incorporation of root exudate C into the microbial biomass, which has a much slower estimated turnover of 30–90 days (Jones, Hodge & Kuzyakov 2004). In our study, we saw negligible short‐term changes in soil respiration during 8 h after RE application, but a significant increase in soil respiration over the growing season, which suggests a persistent effect of RE application on soil C dynamics via changes in microbial growth and substrate utilisation, rather than a transient short‐term increase in microbial activity.
The initial comparisons of soil respiration rates showed significant differences between main treatment plots and procedural controls (CP subplots). Although these differences potentially indicate an effect of the CaCl2 solution on soil respiration, the subplot treatments did not affect soil water content and none of the measured soil properties differed between the main plots and CP subplots. It is conceivable that the differences are simply a result of the high spatial heterogeneity in soil respiration in forests. However, it is also possible that the drip‐tips and their sheaths, which are inserted into the soil, create conduits for gas exchange and influence CO2 efflux; we therefore believe that the procedural control is necessary for accurate comparisons. Nonetheless, exploratory data analyses revealed that the increase in soil respiration in RE subplots was significant regardless of whether procedural controls or true controls (main plots) were used for the comparison (data not shown), indicating a clear and consistent effect of RE application on soil respiration rates in the litter removal treatment.
The size of the ARES treatment area is largely determined by the minimum pressure required to distribute the solution evenly throughout the irrigation grid and the duration of the irrigation period. However, larger subplots could be established by installing several adjacent systems. Optimising the height of the aspirator bottles after installation was essential to achieve the target flow rates and the even distribution of the solution throughout the irrigation grid of each ARES (Appendix S2) and as some of our plots were installed on a gentle slope, we initially adjusted the height of the aspirator bottles by up to 10 cm to account for additional gravitational pull by downhill flow. We do not recommend installing the ARES on slopes greater than 10% without additional tests and adjustments. Regular checks and light maintenance of the ARES were required during the treatment period (Appendix S2) because occasional malfunctioning of timers or blocked drip‐tips caused variation in the total volume of solutions applied to the subplots each week. However, the clear and consistent increase in soil respiration rates and microbial biomass with RE application indicate that the treatment was nonetheless highly effective.
Conclusions
Our results demonstrate that the ARES is an effective method to apply artificial root exudates in the field and is therefore a valuable tool for manipulative experiments. Although this study used a simplified root exudate solution and a consistent flow rate, the type of solutes and the application rates can be easily adjusted to allow a range of qualitative and quantitative manipulations in a variety of ecosystems. Our method also has several practical advantages: firstly, apart from the initial subplot set‐up and the installation of the sheaths for the drip‐tips (Figs 1 and 2), the installation of the ARES involves minimal disturbance to the subplot area, and the irrigation grid is easily removed for cleaning without causing further soil disturbance. Secondly, the systems do not require much maintenance – in our study, the bottles were refilled once a week, the batteries used to power the irrigation timers were replaced only once every growing season and the tubing only needed cleaning at the end of each treatment period (Appendix S1). Finally, although we designed the ARES to apply artificial root exudates, the system can be used for other experimental treatments that require the application of small amounts of solutes to the soil at regular intervals (e.g. additions of nutrients, pollutants and many other specific compounds) to improve our understanding of biogeochemical processes in situ.
Authors’ contributions
E.J.S. conceived the study; L.L.S. and E.E.G. designed the methodology; L.L.S., C.G., E.M.B., C.B. and A.B. collected the data; L.L.S., A.B. and C.B. performed laboratory analyses; E.J.S. and L.L.S. analysed the data and wrote the manuscript. All authors provided input and comments on manuscript drafts and approved the manuscript for submission.
Data accessibility
The full datasets used in this study are deposited in the Dryad repository https://doi.org/10.5061/dryad.dt4jq (Lopez‐Sangil et al. 2017).
Supporting information
Appendix S1. ARES assembly and installation protocol (contains Figs S1.1, S1.2 and Table S1.1).
Appendix S2. ARES design criteria and preliminary tests for validation (contains Figs S2.1 and S2.2).
Appendix S3. Protocol and recipe for root exudate solution (contains Table S3.1).
Appendix S4. Supplementary figures (S4.1 and S4.2) and tables (S4.1 and S4.2).
Acknowledgements
This work was carried out with approval from the Wytham Woods Research and Management Committee, to whom we are very grateful, and we thank Nigel Fisher in particular for his friendly advice and support. We are also grateful to Joe Sayer for the excellent technical diagram, Annette Ryan for managing the plant–soil laboratory, Davey Jones for advice on the root exudate recipe, the field team at the Centre for Ecology and Hydrology and everyone who helped rake litter. The research leading to these results has received funding from the European Research Council under the European Union's Seventh Framework Programme (FP/2007‐2013)/ERC Grant Agreement No. 307888.
References
- Bais, H.P. , Weir, T.L. , Perry, L.G. , Gilroy, S. & Vivanco, J.M. (2006) The role of root exudates in rhizosphere interactions with plants and other organisms. Annual Review of Plant Biology, 57, 233–266. [DOI] [PubMed] [Google Scholar]
- Bates, D. , Machler, M. , Bolker, B.M. & Walker, S.C. (2015) Fitting linear mixed‐effects models using lme4. Journal of Statistical Software, 67, 1–48. [Google Scholar]
- Baudoin, E. , Benizri, E. & Guckert, A. (2003) Impact of artificial root exudates on the bacterial community structure in bulk soil and maize rhizosphere. Soil Biology & Biochemistry, 35, 1183–1192. [Google Scholar]
- Beard, G.R. (1993) The Soils of Oxford University Field Station, Wytham. Soil Survey and Land Research Centre, Silsoe, UK. (now National Soil Resources Institute, Cranfield University). [Google Scholar]
- Brandt, J.B. , Sulzman, E.W. & Myrold, D.D. (2006) Microbial community utilization of added carbon substrates in response to long‐term carbon input manipulation. Soil Biology & Biochemistry, 38, 2219–2232. [Google Scholar]
- Brzostek, E.R. , Greco, A. , Drake, J.E. & Finzi, A.C. (2013) Root carbon inputs to the rhizosphere stimulate extracellular enzyme activity and increase nitrogen availability in temperate forest soils. Biogeochemistry, 115, 65–76. [Google Scholar]
- Cheng, W. , Parton, W.J. , Gonzalez‐Meler, M.A. , Phillips, R. , Asao, S. , McNickle, G.G. , Brzostek, E. & Jastrow, J.D. (2014) Synthesis and modelling perspectives of rhizosphere priming. New Phytologist, 201, 31–44. [DOI] [PubMed] [Google Scholar]
- De Graaff, M.A. , Van Groenigen, K.J. , Six, J. , Hungate, B. & Van Kessel, C. (2006) Interactions between plant growth and soil nutrient cycling under elevated CO2: a meta‐analysis. Global Change Biology, 12, 2077–2091. [Google Scholar]
- De Graaff, M.A. , Classen, A.T. , Castro, H.F. & Schadt, C.W. (2010) Labile soil carbon inputs mediate the soil microbial community composition and plant residue decomposition rates. New Phytologist, 188, 1055–1064. [DOI] [PubMed] [Google Scholar]
- Dijkstra, F.A. & Cheng, W. (2007) Moisture modulates rhizosphere effects on C decomposition in two different soil types. Soil Biology & Biochemistry, 39, 2264–2274. [Google Scholar]
- Drake, J.E. , Gallet‐Budynek, A. , Hofmockel, K.S. et al (2011) Increases in the flux of carbon belowground stimulate nitrogen uptake and sustain the long‐term enhancement of forest productivity under elevated CO2 . Ecology Letters, 14, 349–357. [DOI] [PubMed] [Google Scholar]
- Drake, J.E. , Darby, B.A. , Giasson, M.‐A. , Kramer, M.A. , Phillips, R.P. & Finzi, A.C. (2013) Stoichiometry constrains microbial response to root exudation – insights from a model and a field experiment in a temperate forest. Biogeosciences, 10, 821–838. [Google Scholar]
- Fenn, K. , Malhi, Y. & Morecroft, M.D. (2010) Soil CO2 efflux in a temperate deciduous forest: environmental drivers and component contributions. Soil Biology & Biochemistry, 42, 1685–1693. [Google Scholar]
- Fenn, K. , Malhi, Y. , Morecroft, M. , Lloyd, C. & Thomas, M. (2015) The carbon cycle of a maritime ancient temperate broadleaved woodland at seasonal and annual scales. Ecosystems, 18, 1–15. [Google Scholar]
- Fransson, P. (2012) Elevated CO2 impacts ectomycorrhizal‐mediated forest soil carbon flow: fungal biomass production, respiration and exudation. Fungal Ecology, 5, 85–98. [Google Scholar]
- Grayston, S.J. , Vaughan, D. & Jones, D. (1996) Rhizosphere carbon flow in trees, in comparison with annual plants: the importance of root exudation and its impact on microbial activity and nutrient availability. Applied Soil Ecology, 5, 29–56. [Google Scholar]
- Hinsinger, P. , Plassard, C. , Tang, C. & Jaillard, B. (2003) Origins of root‐mediated pH changes in the rhizosphere and their responses to environmental constraints: a review. Plant and Soil, 248, 43–59. [Google Scholar]
- Högberg, P. , Singh, B. , Löfvenius, M.O. & Nordgren, A. (2009) Partitioning of soil respiration into its autotrophic and heterotrophic components by means of tree‐girdling in old boreal spruce forest. Forest Ecology and Management, 257, 1764–1767. [Google Scholar]
- Holland, J.N. , Cheng, W. & Crossley Jr, D.A. (1996) Herbivore‐induced changes in plant carbon allocation: assessment of below‐ground C fluxes using carbon‐14. Oecologia, 107, 87–94. [DOI] [PubMed] [Google Scholar]
- Hungate, B.A. , Chapin, F.S. , Zhong, H. , Holland, E.A. & Field, C.B. (1997) Stimulation of grassland nitrogen cycling under carbon dioxide enrichment. Oecologia, 109, 149–153. [DOI] [PubMed] [Google Scholar]
- Hütsch, B.W. , Augustin, J. & Merbach, W. (2002) Plant rhizodeposition – an important source for carbon turnover in soils. Journal of Plant Nutrition and Soil Science, 165, 397–407. [Google Scholar]
- IUSS Working Group WRB (2006) World reference base for soil resources 2006, 2nd edn. World Soil Resources Reports No. 103. FAO, Rome, Italy. [Google Scholar]
- Jones, D.L. , Hodge, A. & Kuzyakov, Y. (2004) Plant and mycorrhizal regulation of rhizodeposition. New Phytologist, 163, 459–480. [DOI] [PubMed] [Google Scholar]
- Jones, D.L. & Willett, V.B. (2006) Experimental evaluation of methods to quantify dissolved organic nitrogen (DON) and dissolved organic carbon (DOC) in soil. Soil Biology & Biochemistry, 38, 991–999. [Google Scholar]
- Kaiser, C. , Koranda, M. , Kitzler, B. et al (2010) Belowground carbon allocation by trees drives seasonal patterns of extracellular enzyme activities by altering microbial community composition in a beech forest soil. New Phytology, 187, 843–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keiluweit, M. , Bougoure, J.J. , Nico, P.S. , Pett‐Ridge, J. , Weber, P.K. & Kleber, M. (2015) Mineral protection of soil carbon counteracted by root exudates. Nature Climate Change, 5, 588–595. [Google Scholar]
- Kuzyakov, Y. & Cheng, W. (2001) Photosynthesis controls of rhizosphere respiration and organic matter decomposition. Soil Biology & Biochemistry, 33, 1915–1925. [Google Scholar]
- Lopez‐Sangil, L. , Rovira, P. & Casals, P. (2013) Decay and vertical reallocation of organic C, and its incorporation into carbonates, in agricultural soil horizons at two different depths and rewetting frequencies. Soil Biology & Biochemistry, 61, 33–44. [Google Scholar]
- Lopez‐Sangil, L. , George, C. , Medina‐Barcenas, E. , Birkett, A.J. , Baxendale, C. , Brechet, L.M. , Estradera‐Gumbau, E. & Sayer, E.J. (2017) Data from: The Automated Root Exudate System (ARES): a method to apply solutes at regular intervals to soils in the field. Dryad Digital Repository, https://doi.org/10.5061/dryad.dt4jq [DOI] [PMC free article] [PubMed]
- Nguyen, C. , Todorovic, C. , Robin, C. , Christophe, A. & Guckert, A. (1999) Continuous monitoring of rhizosphere respiration after labelling of plant shoots with 14CO2 . Plant and Soil, 212, 189–199. [Google Scholar]
- Norby, R.J. & Zak, D.R. (2011) Ecological lessons from free‐air CO2 enrichment (FACE) experiments. Annual Reviews of Ecology, Evolution and Systematics, 42, 181–203. [Google Scholar]
- Norby, R.J. , Cotrufo, M.F. , Ineson, P. , O'Neill, E.G. & Canadell, J.G. (2001) Elevated CO2, litter chemistry, and decomposition: a synthesis. Oecologia, 127, 153–165. [DOI] [PubMed] [Google Scholar]
- Phillips, R.P. , Finzi, A.C. & Bernhardt, E.S. (2011) Enhanced root exudation induces microbial feedbacks to N cycling in a pine forest under long‐term CO2 fumigation. Ecology Letters, 14, 187–194. [DOI] [PubMed] [Google Scholar]
- Phillips, R.P. , Erlitz, Y. , Bier, R. & Bernhardt, E.S. (2008) New approach for capturing soluble root exudates in forest soils. Functional Ecology, 22, 990–999. [Google Scholar]
- Pinheiro, J.C. & Bates, D.M. (2000) Mixed‐Effects Models in S and S‐PLUS. Springer, New York City, NY, USA. [Google Scholar]
- Pollierer, M.M. , Langel, R. , Körner, C. , Maraun, M. & Scheu, S. (2007) The underestimated importance of belowground carbon inputs for forest soil animal food webs. Ecology Letters, 10, 729–736. [DOI] [PubMed] [Google Scholar]
- Pollierer, M.M. , Dyckmans, J. , Scheu, S. & Haubert, D. (2012) Carbon flux through fungi and bacteria into the forest soil animal food web as indicated by compound‐specific 13C fatty acid analysis. Functional Ecology, 26, 978–990. [Google Scholar]
- Preece, C. & Peñuelas, J. (2016) Rhizodeposition under drought and consequences for soil communities and ecosystem resilience. Plant and Soil, 409, 1–17. [Google Scholar]
- Qiao, N. , Schaefer, D. , Blagodatskaya, E. , Zou, X. , Xu, X. & Kuzyakov, Y. (2014) Labile carbon retention compensates for CO2 released by priming in forest soils. Global Change Biology, 20, 1943–1954. [DOI] [PubMed] [Google Scholar]
- R Core Team (2016) R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- Sayer, E. (2006) Using experimental manipulation to assess the roles of leaf litter in the functioning of forest ecosystems. Biological Reviews, 81, 1–31. [DOI] [PubMed] [Google Scholar]
- Smith, W.H. (1976) Character and significance of forest tree root exudates. Ecology, 57, 324–331. [Google Scholar]
- Sulman, B.N. , Phillips, R.P. , Christopher Oishi, A. , Shevliakova, E. & Pacala, S.W. (2014) Microbe‐driven turnover offsets mineral‐mediated storage of soil carbon under elevated CO2 . Nature Climate Change, 4, 1099–1102. [Google Scholar]
- Upson, M.A. , Burgess, P.J. & Morison, J.I.L. (2016) Soil carbon changes after establishing woodland and agroforestry trees in a grazed pasture. Geoderma, 283, 10–20. [Google Scholar]
- Van Hees, P.A.W. , Jones, D.L. , Finlay, R. , Godbold, D.L. & Lundström, U.S. (2005) The carbon we do not see – the impact of low molecular weight compounds on carbon dynamics and respiration in forest soils: a review. Soil Biology & Biochemistry, 37, 1–13. [Google Scholar]
- Vance, E.D. , Brookes, P.C. & Jenkinson, D.S. (1987) An extraction method for measuring soil microbial biomass C. Soil Biology & Biochemistry, 19, 703–707. [Google Scholar]
- Vranova, V. , Rejsek, K. , Skene, K.R. , Janous, D. & Formanek, P. (2013) Methods of collection of plant root exudates in relation to plant metabolism and purpose: a review. Journal of Plant Nutrition and Soil Science, 176, 175–199. [Google Scholar]
- Warren, J.M. , Pötzelsberger, E. , Wullschleger, S.D. , Hasenauer, H. , Thornton, P.E. & Norby, R.J. (2011) Ecohydrological impact of reduced stomatal conductance in forests exposed to elevated CO2 . Ecohydrology, 4, 196–210. [Google Scholar]
- Weintraub, M.N. , Scott‐Denton, L.E. , Schmidt, S.K. & Monson, R.K. (2007) The effects of tree rhizodeposition on soil exoenzyme activity, dissolved organic carbon, and nutrient availability in a subalpine forest ecosystem. Oecologia, 154, 327–338. [DOI] [PubMed] [Google Scholar]
- Yin, H. , Wheeler, E. & Phillips, R.P. (2014) Root‐induced changes in nutrient cycling in forests depend on exudation rates. Soil Biology & Biochemistry, 78, 213–221. [Google Scholar]
- Yin, H. , Li, Y. , Xiao, J. , Xu, Z. , Cheng, X. & Liu, Q. (2013) Enhanced root exudation stimulates soil nitrogen transformations in a subalpine coniferous forest under experimental warming. Global Change Biology, 19, 2158–2167. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. ARES assembly and installation protocol (contains Figs S1.1, S1.2 and Table S1.1).
Appendix S2. ARES design criteria and preliminary tests for validation (contains Figs S2.1 and S2.2).
Appendix S3. Protocol and recipe for root exudate solution (contains Table S3.1).
Appendix S4. Supplementary figures (S4.1 and S4.2) and tables (S4.1 and S4.2).
Data Availability Statement
The full datasets used in this study are deposited in the Dryad repository https://doi.org/10.5061/dryad.dt4jq (Lopez‐Sangil et al. 2017).


