Abstract
Serotonin syndrome is a potentially life-threatening side-effect of agents that enhance synaptic serotonin levels. With the increasing use of serotoninergic agents greater awareness of serotonin syndrome is necessary, including the potential for drug interactions with serotoninergic agents to produce serotonin syndrome. Clinical presentation ranges from mild reactions to a severe toxic state involving generalized tonic-clonic seizures, fevers exceeding 40℃ or coma. This case highlights the necessity of considering serotonin syndrome alongside other differential diagnoses: neuroleptic malignant syndrome, infectious cause, atypical seizures and delirium tremens, in the setting of altered mental state with abnormal neurology. It highlights the potential diagnostic use of electroencephalography in supporting a diagnosis of serotonin syndrome.
Keywords: Serotonin syndrome, quetiapine, venlafaxine, intensive care, adverse drug reaction
Clinical case report
A 44-year-old male with a complex psychiatric background presented to the emergency department (ED) after several weeks of feeling unwell, with abnormal movements of his arms and slurred speech. Two months prior to his admission, his venlafaxine dose had been increased to 300 mg OD and a new antipsychotic added: quetiapine at 50 mg BD. The quetiapine was increased to 100 mg BD the following week and then a further 125 mg BD, two weeks prior to his collapse. In the days preceding his admission, he presented to the GP with myoclonic jerks and was prescribed procyclidine. His procyclidine was stopped following his first dose as it was thought to cause him diarrhoea. Two days prior to admission, quetiapine dose was further increased to 150 mg for his evening dose and then the following day, he took 150 mg BD. The next morning he collapsed and was brought into the ED. At the time of admission, his medication included venlafaxine MR 300 mg OD (dose increased two months ago), quetiapine 150 mg BD, clomethiozole 384 mg OD, zomorph 20 mg BD, pregabalin 300 mg BD, procyclidine 2.5 mg TDS, and diazepam 2 mg TDS.
In ED, he had a tonic-clonic seizure lasting five min which self-terminated. He awoke but was combative at times, and had no recollection of the seizure. There was no headache or overt signs of infection. He was slightly hypertensive at 133/85. On examination, he had no photophobia and no neck stiffness. He was diaphoretic. Neurologically, he had normal power in all four limbs, but had markedly abnormal oro-facial movements, odd posturing, increased tone on his left side, and seven beats of clonus on his left foot. He had a systemic inflammatory response syndrome (SIRS)—he was tachycardic, tachypnoiec, febrile (38.1°C) and had a leukocytosis (22.4). Following his seizure in ED, he was found to have a lactic acidosis (17 mmol/L), which rapidly resolved. His ECG and CXR showed no abnormalities. Working diagnosis at this point included tardive dyskinesia, side effect of anti-psychotics, acute psychosis, meningo-encephalitis, an epilepsy syndrome, or intracranial bleed. It appears that venlafaxine was not considered as a possible cause for his clinical picture in the first few hours of his admission. He was treated empirically with acyclovir and ceftriaxone for a possible central nervous system (CNS) infection; phenytoin for seizures and intravenous crystalloid. Haloperidol 5 mg and diazepam 2 mg were given on the ward to ensure his safety overnight.
The following day, he was referred to ICU to facilitate a computed tomography (CT) head scan in his agitated state. His contrast CT head showed no abnormalities. Magnetic resonance imaging (MRI) was requested to rule out limbic encephalitis. Lumbar puncture was not performed initially as it was thought he would not tolerate this without general anesthesia. Neuroleptic malignant syndrome (NMS) was considered by the ICU team as part of the differential diagnosis, and therefore no further antipsychotics, such as haloperidol, were administered.
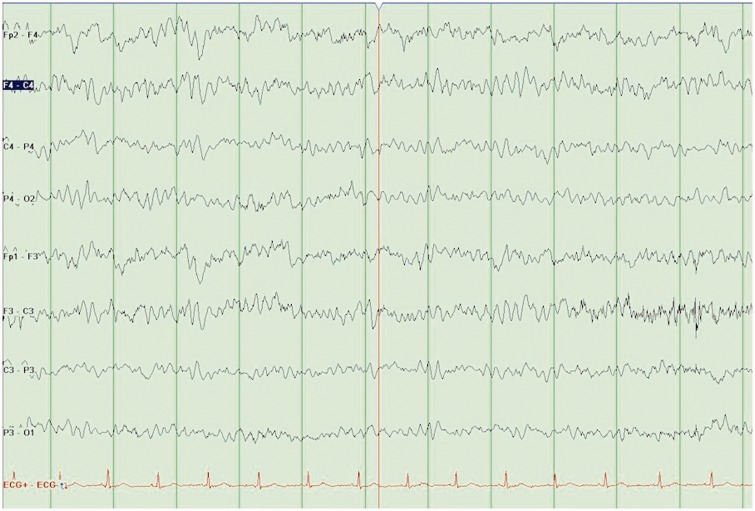
In ICU (third day of admission), he continued to show signs of ongoing SIRS response and persistently abnormal neurology. He had ongoing loose stools, which began days before his admission. Bloods showed a rising creatine kinase (CK) (77 → 1397 → 3381). Despite ongoing concerns about possible meningo-encephalitis, a diagnosis of serotonin syndrome (SS) was considered the most likely clinical scenario. An electroencephalography (EEG) was performed (Figure 1), which was grossly abnormal. It showed persistent rhythmic slow (theta) wave activity throughout the recording, which did not vary with state. Occasional alpha waves were present—but no clear posterior dominant alpha rhythm. There were no overt discharges or slow wave (delta) changes. This EEG result supported a diagnosis of SS. Quetiapine and venlafaxine had been withheld since admission.
Figure 1.
EEG 1: Abnormal EEG.
EEG: electroencephalography.
Over the next two days, his abnormal neurology, SIRS response, and raised CK resolved. By the fifth day, he was more alert and talking—questioning what had happened in his current admission. He was still muddled and not yet to his baseline, but had started to eat and drink when he was discharged to the wards with a plan to discontinue antimicrobials as there were no features to suggest CNS infection. On return to the ward, his awaited MRI scan was cancelled, as it was felt it would not add further to the patient's management. The resolution of his clinical features over three to four days confirmed the initial suspicion of SS. Two months following his admission into hospital with SS, a second EEG was performed, which was normal.
Discussion
SS is a potentially life-threatening adverse drug reaction resulting from an excess of serotonin agonist activity in the nervous system, usually as a result of polypharmacy or drug to drug interactions involving serotonin agonist drugs.1 SS presents clinically as a triad of symptoms and signs:
Mental status changes (agitation)
Autonomic hyperactivity (diaphoresis, diarrhoea, shivering, hyperthermia)
Neuromuscular abnormalities (myoclonus, hyperreflexia, tremor, ataxia)1
SS may manifest as a mild state involving tremor and diarrhoea, but has the potential to present as a life-threatening toxic state, which include coma, generalized tonic-clonic seizures, and/or fever that may exceed 40℃.2
In this case, the patient was experiencing myoclonic jerks five days prior to admission, and was seen by his GP who prescribed procyclidine. This was discontinued as it was thought to be causing him diarrhoea. In hindsight, the diarrhoea was a manifestation of SS toxicity, as were the myoclonic jerks. In retrospect, early recognition and termination of the offending agent could have avoided the progression of SS to its toxic form.
SS is most commonly caused by taking two or more proserotoninergic agents. This includes a range of drug classes in addition to the typical agents selective serotonin reuptake inhibitor (SSRI): serotonin norepinephrine reuptake inhibitors (SNRI), monoamine oxidase inhibitors, tricyclic antidepressant, analgesics, over-the-counter cough medicines, antibiotics, weight-reduction agents, antiemetics, antimigraine agents, drugs of abuse, dietary supplements, and herbal products.3 The underlying mechanism by which these drugs cause SS is by the agonist acting at the 5HT1A receptor.4 Venlafaxine is a SNRI, which increases synaptic availability of serotonin and norepinephrine. Quetiapine is a second-generation atypical antipsychotic, which acts as an antagonist at both serotonin and dopamine receptors. There have been case reports of venlafaxine and quetiapine combination induced NMS—the proposed underlying mechanism being a serotoninergic inhibition of central dopaminergic activity, which increases the likelihood of NMS.5 This same drug interaction can also cause SS via a different mechanism. Quetiapine acts as an antagonist at 5HT2A receptor, which causes hyperactivation of 5HT1A receptor—it is by this mechanism that the combination of serotoninergic agents and atypical antipsychotics causes SS.6,7 It is at higher doses of quetiapine that there is greater 5HT2A antagonism and therefore further hyperactivation of 5HT1A receptor. It is likely that the gradual increase in the quetiapine dose in this case lead to both the development of SS and its progression from a mild to a severe toxic state.
SS is diagnosed by the presenting symptoms and signs and there are no routine diagnostic tests. Various diagnostic criteria have been set to aid diagnosis. Boyer’s SS review recommended a diagnostic criteria adapted from Dunkley8—this is one of the simpler, sensitive, and specific criteria compared with others.3 According to this criteria, a diagnosis of SS is possible if they have been administered a serotoninergic agent in the past five weeks and have one or more of the following clinical features:
Tremor and hyperreflexia
Spontaneous clonus
Muscle rigidity, temp > 38℃, either ocular clonus or inducible clonus
Ocular clonus and either agitation or diaphoresis
Inducible clonus and either agitation or diaphoresis
However, other pathologies can be wrongly classified as SS, and milder forms of SS may go undiagnosed.
Derangements in laboratory blood results have been documented in previous case reports of SS—raised CK, leukocyte count, transaminase levels, or lowered bicarbonate levels have been reported.2 Raised CK and leucocytosis was noted in our patient.
Case reports of SS have shown the following EEG abnormalities: delta range activity, slow waves, spike and waves, polyspike and waves and triphasic waves.9 The pattern seen in the first EEG is not typically expected in NMS or tardive dyskinesia. However, a similar EEG was seen in a single case report “Venlafaxine induced SS”10—which showed slow wave activity and the absence of normal alpha rhythm. As in our case, a repeat EEG was carried out following resolution of SS, showing the resolution of the abnormal EEG. This reversibility is in keeping with the EEG in the previous case report of SS.10 Hence, EEG was a useful adjunct in confirming SS.
The management of the patient involved removal of the offending agent and supportive therapy including intravenous fluids, controlling agitation with benzodiazepine and controlling hyperthermia. In more severe cases, the use of cyproheptadine (a histamine and serotonin antagonist) may need to be considered.1 Management may also involve treating for other differential diagnoses, i.e. empirically for CNS infection or possible epilepsy syndromes. NMS as a potential diagnosis should also have been considered earlier in the patient's admission, and potential trigger agents, such as haloperidol, avoided thereafter.
Data from the 2013 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (AAPCC-NPDS) showed 2.2 million total toxic exposures with 1.0 million having serious outcomes.11 SSRIs were involved in 47,366 of those exposures (47,115 cases documented in 2012)12 and 2080 of the serious outcomes in 2013.11 SNRIs were involved in 8112 of those exposures (only 10 cases documented in 20121,2) and 378 of the serious outcomes in 2013.11 The true incidence, however, is thought to be much greater—due to the syndrome being under diagnosed for a number of reasons, primarily the lack of awareness of the syndromes existence. The rising incidence is felt to parallel the increasing use of proserotoninergic agents.3 Given the increasing number of individuals on serotonergic agents13 and the rise in polypharmacy—it will be no surprise that we will be seeing an ongoing rise in SS. This is particularly important for professionals in ICU, as drugs commonly used in ICU, such as fentanyl14 or linezolid,15 can interact with the patient's regular SSRI medication leading to the development of SS.
SS must be recognized early to prevent further progression to a severe toxic state. More than 85% of physicians are unaware that the syndrome exists.3 This case also highlights the potential for drug to drug interactions in causing SS, with a serotoninergic drug with a wide range of other drug groups. It is important for SS to be recognized early and for the drug to be withdrawn.
We believe that the above case adds to the existing literature of SS. First, we describe a case of SS in association with venlafaxine and quetiapine, which highlights the importance of considering SS among the differential in patients presenting with altered mental states, especially while on medication. Second, it highlights the potential usefulness of EEG in supporting a diagnosis of SS, that is, otherwise entirely clinically based.
Conclusion
The incidence of SS is rising due to the increasing use of serotoninergic agents in the community. Various drug interactions are also implicated in inducing SS. Knowledge among physicians regarding SS remains poor—therefore, increased awareness is needed especially among primary care, ED, and ICU. Diagnosis of SS remains predominantly a clinical diagnosis; however, an EEG may be useful in differentiating SS from other neuropsychiatric reactions.
Acknowledgments
Patient consent was received for publication of this case report.
Declaration of conflicting interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Perry PJ, Wilborn CA. Serotonin syndrome vs neuroleptic malignant syndrome: a contrast of causes, diagnoses, and management. Ann Clin Psychiatry 2012; 24: 155–162. [PubMed] [Google Scholar]
- 2.Birmes P, Coppin D, Schmitt L, et al. Serotonin syndrome: a brief review. CMAJ 2003; 168: 1439–1442. [PMC free article] [PubMed] [Google Scholar]
- 3.Boyer EW, Shannon M. The serotonin syndrome. N Engl J Med 2005; 352: 1112–1120. [DOI] [PubMed] [Google Scholar]
- 4.Osei-Owusu P, James A, Crane J, et al. 5-Hydroxytryptamine 1A receptors in the paraventricular nucleus of the hypothalamus mediate oxytocin and adrenocorticotropin hormone release and some behavioral components of the serotonin syndrome. J Pharmacol Exp Ther 2005; 313: 1324–1330. [DOI] [PubMed] [Google Scholar]
- 5.Woods G, Taggart C, Boggs R, et al. Neuroleptic malignant syndrome associated with quetiapine and venlafaxine use: a case report and discussion. Ther Adv Psychopharmacol 2013; 3: 53–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duggal HS, Fetchko J. Serotonin syndrome and atypical antipsychotics. Am J Psychiatry 2002; 159: 672–673. [DOI] [PubMed] [Google Scholar]
- 7.Marlowe K, Schirgel D. Quetiapine and citalopram: aetiological significances in serotonin syndrome. N Z Med J 2006; 119: U2058. [PubMed] [Google Scholar]
- 8.Dunkley EJ, Isbister GK, Sibbritt D, et al. The Hunter Serotonin Toxicity Criteria: simple and accurate diagnostic decision rules for serotonin toxicity. QJM 2003; 96: 635–642. [DOI] [PubMed] [Google Scholar]
- 9.Dike GL. Triphasic waves in serotonin syndrome. J Neurol Neurosurg Psychiatry 1997; 62: 200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perry NK. Venlafaxine-induced serotonin syndrome with relapse following amitriptyline. Postgrad Med J 2000; 76: 254–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mowry JB, Spyker DA, Cantilena LR, Jr, et al. 2013 Annual report of the American Association of Poison Control Centers' National Poison Data System (NPDS): 31st annual report. Clin Toxicol (Phila) 2014; 52: 1032–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mowry JB, Spyker DA, Cantilena LR Jr, Jr, et al. 2012 Annual report of the American Association of Poison Control Centers' National Poison Data System (NPDS): 30th annual report. Clin Toxicol (Phila) 2013; 51: 949–1229. [DOI] [PubMed] [Google Scholar]
- 13.Easton M. Is England a nation on anti-depressants? http://www.bbc.co.uk/news/uk-23553897 (2013, accessed 6 February 2015).
- 14.Greenier E, Lukyanova V, Reede L. Serotonin syndrome: fentanyl and selective serotonin reuptake inhibitor interactions. AANA J 2014; 82: 340–345. [PubMed] [Google Scholar]
- 15.Quinn DK, Stern TA. Linezolid and serotonin syndrome. Prim Care Companion J Clin Psychiatry 2009; 11: 353–356. [DOI] [PMC free article] [PubMed] [Google Scholar]