Abstract
This study was conducted to evaluate the biochemical effects of grape seed extract against dexamethasone-induced hepatic and renal dysfunction in a female albino rat. Twenty-eight adult female rats were divided randomly into four equal groups: Group 1: animals were injected subcutaneously with saline and consider as normal control one. Group 2: animals were injected subcutaneously with dexamethasone in a dose of 0.1 mg/kg body weight. Group 3: animals were injected subcutaneously with 0.1 mg/kg body weight of dexamethasone, and then treated with a grape seed extract in a dose of 200 mg/kg body weight by oral gavage. Group 4: animals were injected subcutaneously with 0.1 mg/kg body weight of dexamethasone, and then treated with a grape seed extract in a dose of 400 mg/kg body weight by oral gavage. After 4 weeks, serum alanine aminotransferase (ALT), aspartate aminotransferase (AST) activities, albumin, uric acid, creatinine, and glucose levels were assayed. Hepatic reduced glutathione (GSH), total protein content, and catalase and glucose-6-phosphate dehydrogenase activities were also assayed. Dexamethasone administration caused elevation of serum levels of glucose, uric acid, creatinine, ALT, AST activities, and a decrease in other parameters such as hepatic glutathione, total protein levels, and catalase enzyme activity. Treatment with Vitis vinifera L. seed extract showed a significant increase in the body weight of rats in the group treated with Vitis vinifera L. seed extract orally compared with the dexamethasone control group. An increase in GSH and catalase activity in response to oral treatment with Vitis vinifera L. seed extract was observed after treatment. Grape seed extract positively affects glucocorticoid-induced hepatic and renal alteration in albino rats.
Keywords: alteration, hepatic, dexamethasone, grape seed, catalase, glucose-6-phosphate dehydrogenase, GSH
1. Introduction
Recently, the trends in traditional and complementary medicines for patients are becoming more interested in medicinal plants [1]. Some medicinal plants may exert promising pharmacological properties and improve the effectiveness of conventional medications as complementary agents [2].
Vitis vinifera (Grape) is one of the most consumed fruits globally. V. vinifera possesses a wide range of pharmacological activities due to its rich polyphenol ingredients, most of which are contained in its seeds [3]. Grape seed extract comprises flavonoids such as proanthocyanidins, which are potent antioxidants and stimulate many health-promoting effects [3]. Grape seed extract has a wide range of pharmacological and remedial impacts such as antioxidative, anti-inflammatory, and additionally having cardioprotective, hepatoprotective, and neuroprotective effects [4].
Previous studies have also shown that grape seed extracts repress enzymes responsible for free radical formation and also have antimutagenic and anticarcinogenic properties [5,6,7].
Glucocorticoids are among the most utilized medications around the world, and they are effective medications for a multitude of inflammatory and immunological disorders. Despite its therapeutic activity, high doses and long-term utilization of these medications are concomitant with serious side effects, such as diabetes mellitus, liver disorder, cardiovascular events, hypertension, dyslipidemia, and osteoarthritis [8,9].
Dexamethasone is a potent synthetic glucocorticoid medication for multitudinous inflammatory and immunological disorders [9]. Similar to other glucocorticoids, high doses and long-term utilization of dexamethasone are associated with negative side effects [10].
In the current study, we hypothesized that V. vinifera seed extract was capable of preserving activity levels of metabolizing enzymes and preventing glucocorticoid side effects. We further hypothesized that the seed extract was able to reduce free radicals and enhance antioxidant activity.
2. Materials and Methods
2.1. Grape Seed Extract Preparation
Grape seeds were removed from fresh fruits purchased from a local market in Hail City, KSA and thoroughly washed, and were then dried. The dried seed were ground to powder form. The powder was soaked for 24 h in an appropriate volume of Millipore distilled water to obtain 200 mg/mL solution. The solution was filtered by using 0.22 µm sterile filters with the help of a vacuum pump, then stored in a brown bottle at 4 °C until use.
2.2. Drugs and Chemicals
Dexamethasone (4 mg/mL, EIPICO, 10th of Ramadan City, Egypt) was used in the study. Before administration, dexamethasone was diluted with normal saline. All chemicals used in our study were of analytical or reagent grade.
2.3. Animals
The twenty-eight female albino rats of the Wistar Strain weighing 175–205 g used for this study were obtained from the animal house of the college of pharmacy, King Saud University, Riyadh, KSA. They were housed at 26 ± 2 °C, 12 h:12 h light:dark cycle. Animals were provided with a standard diet and water ad libitum. Rats were allowed to acclimate to laboratory conditions for 7 days prior to dosing. The experimental work on female rats was carried out in accordance with the Institutional Scientific and Research Ethics Committee, College of medicine, Hail University, KSA (EC Ref No.: EC-014, 15 December 2016).
2.4. Animal Groups
After the acclimation period, 28 female albino rats were randomly divided into four groups of seven each, as follows: Rats of group I were injected subcutaneously with normal saline only and considered intact control. Animals of group II served as the dexamethasone-control and were injected subcutaneously with dexamethasone (0.1 mg/kg/day) [11]. Rats of group III were injected with dexamethasone and treated with grape seed extract (200 mg/kg BW) by oral gavage [12]. Animals of group IV were injected with dexamethasone and treated with grape seed extract (400 mg/kg BW) by oral gavage [12].
All experimental animals were treated three times per week for four consecutive weeks, and the administration with dexamethasone and grape seed extract were done between 7.30 a.m. and 9.00 a.m.
After the end of experimental duration (30 days), rats were fasted for 12 h and sacrificed under light diethyl ether anaesthesia. Blood samples were collected from each rat; serum was drawn after centrifugation at 3500 rpm for 10 min at 4 °C. The sera were kept in a deep freezer (−20 °C) for further biochemical assays.
During the experimental period, body weight changes of female rats were recorded weekly. Liver and kidney of rats from each group were quickly removed, cleaned, weighed, and used for biochemical studies. Then, relative weight was calculated.
2.5. Biochemical Examination
The glucose level in serum was assayed using the glucose oxidase method [13]. Serum activities of aspartate aminotransferase (AST), and alanine aminotransferase (ALT) were assayed according to Reitman and Frankel [14] using reagent kits purchased from Kashef diagnostic company (Jeddah, Saudi Arabia). Serum albumin level was assayed by Bromo cresol green (Kashef diagnostic company, Jeddah, Saudi Arabia). Serum levels of creatinine and uric acid were assayed according to the methods of [15,16], respectively.
Hepatic glucose-6-phosphate dehydrogenase activity (G6PDH) was assayed according to [17], based on the conversion of NADP+ to NADPH and followed by monitoring the change in absorbance at 340 nm.
The hepatic catalase activity was assayed according to Cohen et al. [18], based on the ability of catalase to induce the H2O2 decomposition.
The hepatic content of reduced glutathione (GSH) was assayed by the spectrophotometric technique according to Sedlack and Lindsay [19].
2.6. Statistical Analysis
The data analysis was carried out using statistical software SPSS 18.0 (SPSS Inc., Chicago, IL, USA). Data are presented as a mean ± standard deviation. Statistical analyses were performed using one-way ANOVA, and means were compared using Duncan’s multiple range test as a post hoc test at the 5% probability level. A p-value <0.05 was considered statistically significant.
3. Results
Table 1 shows the effect of dexamethasone administration on the body weight gain of female albino rats and relative liver weight. One way ANOVA test showed a highly significant effect of treatment on the body weight gain in the different animal groups (F = 61.88, p < 0.001). The control group showed a mean weight gain of 32.64 ± 10.21 g. Dexamethasone administrations decreased this value significantly to −60.87 ± 17.40 g. Treatment with 200 mg/kg BW grape seed extract after dexamethasone administration significantly decreased the weight gain to −9.86 ± 16.01 g. Treatment with 400 mg/kg BW GSE after dexamethasone administration increased the weight gain to 7.30 ± 6.57 g. Additionally, the relative liver weight of animals in the four groups was significantly different, as shown in Table 1.
Table 1.
Effect of grape seed extract on body weight (BW) gain and relative liver and kidney weight in albino rats injected with dexamethasone (n = 7).
| Groups | Body Weight Gain (g) | Relative Liver Weight (g/100 BW) | Relative Kidney Weight (g/100 BW) |
|---|---|---|---|
| Group (1) | 32.64 d ± 10.21 | 3.84 b ± 0.27 | 0.68 ± 0.05 |
| Group (2) | −60.87 a ± 17.40 | 4.68 c ± 1.23 | 1.09 ± 0.19 |
| Group (3) | −9.86 b ± 16.01 | 2.99 a ± 0.41 | 0.94 ± 0.15 |
| Group (4) | 7.30 c ± 6.57 | 3.36 a,b ± 0.36 | 0.81 ± 0.08 |
| F-ratio | 61.88 | 7.88 | 12.34 |
| p-value | 0.00 | 0.00 | 0.00 |
The different letters indicate statistically different means according to Duncan multiple range test.
Table 2 shows the effect of the administration of dexamethasone on the liver function tests. For ALT, one-way ANOVA test showed a highly significant effect of treatment in the different animal groups (F = 102.94, p < 0.001). The control group showed a mean ALT enzyme activity of 55.57 ± 8.92 U/L. Administration of dexamethasone elevated this value significantly to 124.29 ± 6.52 U/L. Treatment with 200 mg/kg BW GSE significantly decreased the enzyme activity to 79.43 ± 4.50 U/L, whereas with 400 mg/kg BW GSE, ALT activity reduced to 69.71 ± 9.86 U/L. For AST, a very similar effect was shown (F = 167.17, p < 0.001). For albumin, one-way ANOVA test showed a highly significant effect of administration in the different animal groups (F = 55.87, p < 0.001). The control group showed a mean albumin level of 28.29 ± 2.98 g/L. Administration of dexamethasone decreased this value significantly to 11.34 ± 1.76 g/L. Treatment with 200 mg/kg BW GSE significantly elevated the albumin level to 18.07 ± 1.88 g/L, whereas with 400 mg/kg BW GSE, albumin level increased to 24.17 ± 3.44 g/L, as shown in Table 2.
Table 2.
Effect of grape seed extract on liver function tests in albino rats injected with dexamethasone (n = 7).
| Groups | ALT (U/L) | AST (U/L) | Albumin (g/L) |
|---|---|---|---|
| Group (1) | 55.57 a ± 8.92 | 80.14 a ± 4.85 | 28.29 d ± 2.98 |
| Group (2) | 124.29 d ± 6.52 | 175.29 c ± 12.26 | 11.34 a ±1.76 |
| Group (3) | 79.43 c ± 4.50 | 123 b ± 6.68 | 18.07 b ± 1.88 |
| Group (4) | 69.71 b ± 9.86 | 114.86 b ± 6.36 | 24.14 c ± 3.44 |
| F-ratio | 102.94 | 167.17 | 55.87 |
| p-value | 0.00 | 0.00 | 0.00 |
The different letters indicate statistically different means according to Duncan multiple range test. ALT is alanine aminotransferase; AST is aspartate aminotransferase.
Table 3 shows the effect of administration of dexamethasone on the fasting glucose, uric acid, and creatinine levels. For glucose, one-way ANOVA test showed a highly significant effect of treatment in the different animal groups (F = 72.01, p < 0.001). The control group showed a mean glucose level of 4.79 ± 0.53 mmol/L. Administration of dexamethasone elevated this value significantly to 9.37 ± 0.98 mmol/L. Treatment with 200 mg/kg BW GSE significantly decreased the glucose level to 6.64 ± 0.25 mmol/L, whereas with 400 mg/kg BW GSE, glucose level reduced to 5.71 ± 0.47 mmol/L. For uric acid and creatinine level, a very similar effect was shown (Table 3).
Table 3.
Effect of grape seed extract on fasting blood glucose, uric acid, and creatinine levels in albino rats injected with dexamethasone (n = 7).
| Groups | Fasting Blood Glucose (mmol/L) | Uric Acid (µmol/L) | Creatinine (µmol/L) |
|---|---|---|---|
| Group (1) | 4.79 a ± 0.53 | 116.14 a ± 13.26 | 50 a ± 11.49 |
| Group (2) | 9.37 d ± 0.98 | 176.43 b ± 11.28 | 128.57 c ± 5.47 |
| Group (3) | 6.64 c ± 0.25 | 124 a ± 6.22 | 76.86 b ± 7.40 |
| Group (4) | 5.71 b ± 0.47 | 122.14 a ± 5.64 | 57.43 a ± 5.62 |
| F-ratio | 72.01 | 58.91 | 142.04 |
| p-value | 0.00 | 0.00 | 0.00 |
The different letters indicate statistically different means according to Duncan multiple range test.
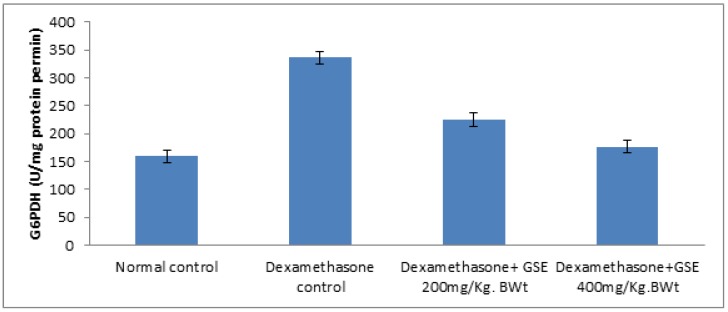
Regarding hepatic activity of glucose-6-phosphate dehydrogenase, our findings revealed that the grape seed extract administration to female albino rats produced profound and significant reduction in the hepatic glucose-6-phosphate dehydrogenase, as shown in Figure 1.
Figure 1.
Effect of grape seed extract (GSE) on hepatic activity of glucose-6-phosphate dehydrogenase (G6PDH) in albino rats injected with dexamethasone (n = 7).
With respect to the oxidative stress markers, hepatic catalase enzyme, GSH, and total protein content were studied. For hepatic catalase enzyme activity, one-way ANOVA test showed a highly significant difference between the different animal groups (F = 111.01, p < 0.001). The control group showed enzyme activity of 0.88 ± 0.05 UI/mg protein; enzyme activity reduced to 0.42 ± 0.04 UI/mg proteins after dexamethasone administration. Treating the animals with 200 mg/kg GSE increased the catalase enzyme activity to 0.54 ± 0.03 UI/mg protein, whereas treatment with 400 mg/kg GSE increased the enzyme activity to 0.70 ± 0.07 UI/mg protein. Hepatic GSH and total protein content showed the same trend as catalase enzyme activity (Table 4).
Table 4.
Effect of grape seed extract on hepatic catalase activity, GSH, and total protein content in albino rats injected with dexamethasone (n = 7).
| Groups | Catalase (UI/mg Protein) | GSH (μg/mg Protein) | Total Protein (g/dL) |
|---|---|---|---|
| Group (1) | 0.88 d ± 0.05 | 3.77 d ± 0.37 | 2.78 d ± 0.11 |
| Group (2) | 0.42 a ± 0.04 | 1.03 a ± 0.19 | 1.78 a ± 0.13 |
| Group (3) | 0.54 b ± 0.03 | 1.66 b ± 0.18 | 2.11 b ± 0.10 |
| Group (4) | 0.70 c ± 0.07 | 2.44 c ± 0.18 | 2.44 c ± 0.12 |
| F-ratio | 111.01 | 166.31 | 97.77 |
| p-value | 0.00 | 0.00 | 0.00 |
The different letters indicate statistically different means according to Duncan multiple range test. GSH is reduced form of glutathione.
4. Discussion
Dexamethasone is a synthetic glucocorticoid indicated for treating inflammatory and autoimmune syndromes. However, dexamethasone therapy is associated with a variety of side effects, including liver and kidney dysfunction. The current study reveals the protection and the dose–response effect conferred by grape seed extract (Vitis vinifera) against dexamethasone-induced oxidative stress in experimental female albino rats.
The administration of dexamethasone significantly decreased the body weight gain and relative liver and kidney weights at the end of the experimental period. These results were in accordance with the findings of [20,21]. The adverse effects of glucocorticoid treatment are well-known, including the induction of insulin resistance and some metabolic disorders like hyperleptinemia, loss of appetite, and weight loss with a higher level of blood glucose and triglycerides [22,23].
Grape seed extract treatment repressed dexamethasone-induced weight loss and showed a conversely marginal increase in body weight. The improvement of body weight gain after grape seed extract may be due to the increase in the sensitivity to insulin and the resultant increase in the glucose uptake [24].
The liver is structurally heterogeneous, and therefore executes various important functions, such as detoxification. Liver enzymes such as AST and ALT are among the marker enzymes for liver function and integrity [25,26] which are usually elevated in the manifestation of acute hepatotoxicity or mild hepatocellular injury. In our study, administration of dexamethasone led to a significant elevation in AST and ALT activities, and conversely to a decrease in albumin level. These results are in accordance with [27,28].
Elevation of AST and ALT enzyme is attributed to the damaged structural integrity of the liver [25]. Additionally, the decreased level of albumin after dexamethasone administration may be due to its damaging effect on DNA and RNA. Treatment with grape extract to dexamethasone-exposed rats produced significant improvement in liver functions, indicating the beneficial role of grapes to counteract the dexamethasone-induced liver dysfunctions. These results are in accordance with [29,30].
In the current study, the dexamethasone-administered rats showed a significant elevation in blood glucose level, which agrees with previous studies [21]. A dose-dependent decrease in glucose levels was observed in grape seed extract-treated rats compared to the dexamethasone control.
Many factors clarify hyperglycemia after dexamethasone administration, such as decreased insulin sensitivity, attenuated pancreatic α- and β-cell functions, and augmented hepatic gluconeogenesis [31].
It was also observed that rats treated with dexamethasone showed a significant elevation in serum uric acid and creatinine levels. Creatinine is thought to be a dependable indicator of how well the kidneys are filtering out toxins. Treatment of rats with grape seed extract resulted in a significant improvement in uric acid and creatinine levels. These findings agree with [32], who showed the ameliorative effects of grape seed on serum kidney functions of paracetamol-induced hepatotoxicity in rats.
In the current study, dexamethasone induced increased oxidative stress, which is presented by a significantly decreased hepatic GSH content and decreased catalase activity. Oxidative stress has been stated as a major cause of dexamethasone-induced liver injury and extreme production of free radicals. These findings agree with results reported by [21,33].
The protection of grape seed extract against oxidative stress was measured by detecting glutathione and catalase (in liver tissues). Moreover, GSE (200 and 400 mg/kg) significantly increased hepatic GSH content and catalase activity in a dose-dependent manner to near the normal levels. Accordingly, this is because grape seeds have antioxidant and free radical scavenging activities, therefore protecting against oxidative stress and replenishing the reduced glutathione content. These findings agree with results reported by [34,35], who reported that oral administration of grape seed extract ameliorated and enhanced the antioxidant defense against Ehrlich solid tumor-induced oxidative stress in mice.
The potential beneficial role of grape seed extract is in preventing oxidative stress-mediated damage and strengthening antioxidant defense mechanisms, with an increase in the antioxidant status of animals. These actions are due to the content of phytochemicals like polyphenolic compounds such as procyanidins and proanthocyanidins, which have a powerful free radical scavenging effect by inhibiting advanced glycation end product (AGE) formation, which helps reverse the effects on lipid peroxidation level and the activities of antioxidant enzymes.
Glucose-6-phosphate dehydrogenase is a crucial enzyme of the pentose phosphate pathway that catalyses key steps in the regulation of redox balance and in anabolic processes [36]. Our results demonstrate that the hepatic activity of glucose-6-phosphate dehydrogenase was significantly elevated after administration with dexamethasone in rats. Our findings are consistent with other authors [37]. Overexpression and up-regulation of glucose-6-phosphate dehydrogenase in liver tissue may promote oxidative stress, which is a major cause of dexamethasone-induced liver injury. Similarly, authors in [38,39] reported that up-regulation of glucose-6-phosphate dehydrogenase activity increases oxidative stress, which leads to functional defects in the tissues. The ameliorative effects of grape seed extract significantly decrease hepatic glucose-6-phosphate dehydrogenase activity in a dose-dependent manner to near-normal levels. This is because grape seeds have antioxidant and free radical scavenging activities.
5. Conclusions
Our findings showed that dexamethasone is capable of triggering marked oxidative stress. Supplementation with grape seed exerted undeniable ameliorative and therapeutic action against dexamethasone-induced oxidative stress.
Author Contributions
N.A.H. and M.Q.A. conceived and designed the experiments; A.A.A., T.Z.A. and A.M.A. performed the experiments; N.A.H. and A.M.A. analyzed the data; A.A.A., T.Z.A., and M.Q.A. contributed reagents/materials/analysis tools; N.A.H. wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest
References
- 1.Tovchiga O.V. The influence of goutweed (Aegopodium podagraria L.) tincture and metformin on the carbohydrate and lipid metabolism in dexamethasone-treated rats. BMC Complement. Altern. Med. 2016;16:235. doi: 10.1186/s12906-016-1221-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yuan H.D., Ma Q.Q., Ye L., Piao G.C. The traditional medicine and modern medicine from natural products. Molecules. 2016;21:559. doi: 10.3390/molecules21050559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Georgiev V., Ananga A., Tsolova V. Recent advances and uses of grape flavonoids as nutraceuticals. Nutrients. 2014;6:391–415. doi: 10.3390/nu6010391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patel A.K., Davis A., Rodriguez M.E., Agron S., Hackam A.S. Protective effects of a grape-supplemented diet in a mouse model of retinal degeneration. Nutrition. 2016;32:384–390. doi: 10.1016/j.nut.2015.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ali K., Maltese F., Choi Y.H., Verpoorte R. Metabolic constituents of grapevine and grape-derived products. Phytochem. Rev. 2010;9:357–378. doi: 10.1007/s11101-009-9158-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saxena A., Kaur K., Hegde S., Kalekhan F.M., Baliga M.S., Fayad R. Dietary agents and phytochemicals in the prevention and treatment of experimental ulcerative colitis. J. Tradit. Complement. Med. 2014;4:203–217. doi: 10.4103/2225-4110.139111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yen C.-Y., Hou M.-F., Yang Z.-W., Tang J.-Y., Li K.-T., Huang H.-W., Huang Y.-H., Lee S.Y., Fu T.F., Hsieh C.Y., et al. Concentration effects of grape seed extracts in anti-oral cancer cells involving differential apoptosis, oxidative stress, and DNA damage. BMC Complement. Altern. Med. 2015;15:94. doi: 10.1186/s12906-015-0621-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu D., Ahmet A., Ward L., Krishnamoorthy P., Mandelcorn E.D., Leigh R., Brown J.P., Cohen A., Kim H. A practical guide to the monitoring and management of the complications of systemic corticosteroid therapy. Allergy Asthma Clin. Immunol. 2013;9:30. doi: 10.1186/1710-1492-9-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hapgood J.P., Avenant C., Moliki J.M. Glucocorticoid-independent modulation of GR activity: Implications for immunotherapy. Pharmacol. Ther. 2016;165:93–113. doi: 10.1016/j.pharmthera.2016.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luskin A.T., Antonova E.N., Broder M.S., Chang E.Y., Omachi T.A., Ledford D.K. Health care resource use and costs associated with possible side effects of high oral corticosteroid use in asthma: a claims-based analysis. ClinicoEconomics Outcomes Res. 2016;8:641–648. doi: 10.2147/CEOR.S115025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen Z., Xue J., Shen T., Mu S., Fu Q. Curcumin alleviates glucocorticoid-induced osteoporosis through the regulation of the Wnt signaling pathway. Int. J. Mol. Med. 2016;37:329–338. doi: 10.3892/ijmm.2015.2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li S.G., Ding Y.S., Niu Q., Xu S.Z., Pang L.J., Ma R.L., Jing M.X., Feng G.L., Liu J.-M., Guo S.X. Grape seed proanthocyanidin extract alleviates arsenic-induced oxidative reproductive toxicity in male mice. Biomed. Environ. Sci. 2015;28:272–280. doi: 10.3967/bes2015.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barham D., Trinder P. An improved colour reagent for the determination of blood glucose by the oxidase system. Analyst. 1972;97:142–145. doi: 10.1039/an9729700142. [DOI] [PubMed] [Google Scholar]
- 14.Reitman S., Frankel S. The colorimetric method for determination of serum glutamic oxaloacetic transaminase and serum glutamic pyruvic transaminase. Am. J. Clin. Pathol. 1957;28:56. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- 15.Faulkner N.R., King J.W. Fundamental of Clinical Chemistry. 2nd Edition. Saunders; Philadelphia, PA, USA: 1976. pp. 994–998. [Google Scholar]
- 16.Barham D., Trinder P. A colorimetric method for the determination of serum uric acid. Analyst. 1972;97:142. doi: 10.1039/an9729700142. [DOI] [PubMed] [Google Scholar]
- 17.Langdon R.G. Wood WA. Volume 9. Academic Press; New York, NY, USA: 1966. Glucose-6-phosphate dehydrogenase from erythrocytes; pp. 126–131. [Google Scholar]
- 18.Cohen G., Dembiec D., Marcus J. Measurement of catalase activity in tissue. Anal. Biochem. 1970;34:30–38. doi: 10.1016/0003-2697(70)90083-7. [DOI] [PubMed] [Google Scholar]
- 19.Sedlack J., Lindsay R.H. Estimation of total protein bound and non-protein sulfhydryl groups in tissues with Ellman’s reagent. Anal. Biochem. 1968;25:192–205. doi: 10.1016/0003-2697(68)90092-4. [DOI] [PubMed] [Google Scholar]
- 20.Eason J.M., Dodd S.L., Powers S.K., Martin A.D. Detrimental effects of short-term glucocorticoid use on the rat diaphragm. Phys. Ther. 2000;80:160–167. [PubMed] [Google Scholar]
- 21.Prashant M. Effect of psoralea corylifolia on dexamethasone-induced insulin resistance in mice. J. King Saud Univ. Sci. 2012;24:251–255. [Google Scholar]
- 22.Kim D.S., Kim T.W., Park I.K., Kang J.S., Om A.S. Effect of chromium picolinate supplementation on insulin sensitivity, serum lipid, and body weight in dexamethasone-treated rats. Metabolism. 2002;51:589–594. doi: 10.1053/meta.2002.31985. [DOI] [PubMed] [Google Scholar]
- 23.Shalam M.D., Harish M.S., Farhana S.A. Prevention of dexamethasone and fructose-induced insulin resistance in rats by SH-01D, a herbal preparation. Indian J. Pharmacol. 2006;38:419–422. doi: 10.4103/0253-7613.28209. [DOI] [Google Scholar]
- 24.Schmidt T.J., Miller-Diener A., Litwack G. Lapachone, a specific competitive inhibitor of ligand binding to the glucocorticoid receptor. J. Biol. Chem. 1984;259:9536–9543. [PubMed] [Google Scholar]
- 25.Bastway M., Hasona N., Selemain H. Protective effects of extract from dates (Phoenix Dactylifera. L.) and ascorbic acid on thioacetamide-induced hepatotoxicity in rats. Iran. J. Pharm. Res. 2008;7:193–201. [Google Scholar]
- 26.Hasona N.A., Amer O.H., Morsi A., Raef A. Comparative biochemical, parasitological, and histopathological studies on cystic echinococcosis in infected sheep. Comp. Clin. Pathol. 2017 doi: 10.1007/s00580-017-2450-2. [DOI] [Google Scholar]
- 27.Nkono B.L.N.Y., Sokeng S.D., Désiré D.D.P., Kamtchouing P. Antihyperglycemic and antioxydant properties of alstonia boonei De wild. (Apocynaceae) stem bark aqueous extract in dexamethasone-induced hyperglycemic rats. Int. J. Diabetes Res. 2014;3:27–35. [Google Scholar]
- 28.Jackson E.R., Kilroy C., Joslin D.L., Schomaker S.J., Pruimboom-Brees I., Amacher D.E. The early effects of short-term dexamethasone administration on hepatic and serum alanine aminotransferase in the rat. Drug Chem. Toxicol. 2008;31:427–445. doi: 10.1080/01480540802390247. [DOI] [PubMed] [Google Scholar]
- 29.Giribabu N., Eswar Kumar K., Swapna Rekha S., Muniandy S., Salleh N. Vitis. vinifera (muscat variety) seed ethanolic extract preserves activity levels of enzymes and histology of the liver in adult male rats with diabetes. Evid. Based Complement. Alternat. Med. 2015;542026:1–8. doi: 10.1155/2015/542026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mansouri E., Khorsandi L., Abedi H.A. Antioxidant effects of proanthocyanidin from grape seed on hepatic tissue injury in diabetic rats. Iran. J. Basic Med. Sci. 2014;17:460–464. [PMC free article] [PubMed] [Google Scholar]
- 31.Bhujbal S.S., Providencia C.A., Nanda R.K., Hadawale S.S., Yeola R.R. Effect of Woodfordia. fruticosa on dexamethasone induced insulin resistance in mice. Rev. Bras. Farmacogn. 2012;22:611–616. doi: 10.1590/S0102-695X2012005000020. [DOI] [Google Scholar]
- 32.Madi Almajwal A., Farouk Elsadek M. Lipid-lowering and hepatoprotective effects of Vitis. vinifera dried seeds on paracetamol-induced hepatotoxicity in rats. Nutr. Res. Pract. 2015;9:37–42. doi: 10.4162/nrp.2015.9.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lingaiah H.B., Thamaraiselvan R., Periyasamy B. Dexamethasone induced alterations in lipid peroxidation, antioxidants; membrane bound ATPase in wistar albino rats. Int. J. Pharm. Pharm. Sci. 2012;4:497–499. [Google Scholar]
- 34.Al-Sowayan N.S., Kishore U. Prophylactic efficacy of a combination of proanthocyanidin and vitamin E on hepatotoxicity induced by doxorubicin in rats. Int. Res. J. Pharm. 2012;2:161–169. [Google Scholar]
- 35.Ali D.A., El-Din N.A.B., Abou-El-magd R.F. Antioxidant and hepatoprotective activities of grape seeds and skin against Ehrlich solid tumor induced oxidative stress in mice. Egypt. J. Basic Appl. Sci. 2015;2:98–109. doi: 10.1016/j.ejbas.2015.02.003. [DOI] [Google Scholar]
- 36.Hasona A.N., Qumani M.A., Alghassab T.A., Alghassab M.A., Alghabban A.A. Ameliorative properties of iranian trigonella foenum-graecum L. seeds and punica granatum L. peel extracts in streptozotocin-induced experimental diabetic guinea pigs. Asian Pac. J. Trop. Biomed. 2017;7:234–239. doi: 10.1016/j.apjtb.2016.12.004. [DOI] [Google Scholar]
- 37.Stumpo D.J., Prostko C.R., Kletzien R.F. Ethanol-glucocorticoid regulation of hepatic glucose-6-phosphate dehydrogenase. Alcohol. 1985;2:173–176. doi: 10.1016/0741-8329(85)90037-0. [DOI] [PubMed] [Google Scholar]
- 38.Gupte R.S., Floyd B.C., Kozicky M., George S., Ungvari Z.I., Neito V., Wolin M.S., Gupte S.A. Synergistic activation of glucose-6-phosphate dehydrogenase and NAD(P)H oxidase by Src kinase elevates superoxide in type 2 diabetic, Zucker fa/fa, rat liver. Free Radic. Biol. Med. 2009;47:219–228. doi: 10.1016/j.freeradbiomed.2009.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee J.W., Choi A.H., Ham M., Kim J.W., Choe S.S., Park J., Lee G.Y., Yoon K.H., Kim J.B. G6PD up-regulation promotes pancreatic beta-cell dysfunction. Endocrinology. 2011;152:793–803. doi: 10.1210/en.2010-0606. [DOI] [PubMed] [Google Scholar]