Abstract
Objective:
Sleep disturbance is common in dementia, although it is unclear whether differences in sleep architecture precede dementia onset. We examined the associations between sleep architecture and the prospective risk of incident dementia in the community-based Framingham Heart Study (FHS).
Methods:
Our sample comprised a subset of 321 FHS Offspring participants who participated in the Sleep Heart Health Study between 1995 and 1998 and who were aged over 60 years at the time of sleep assessment (mean age 67 ± 5 years, 50% male). Stages of sleep were quantified using home-based polysomnography. Participants were followed for a maximum of 19 years for incident dementia (mean follow-up 12 ± 5 years).
Results:
We observed 32 cases of incident dementia; 24 were consistent with Alzheimer disease dementia. After adjustments for age and sex, lower REM sleep percentage and longer REM sleep latency were both associated with a higher risk of incident dementia. Each percentage reduction in REM sleep was associated with approximately a 9% increase in the risk of incident dementia (hazard ratio 0.91; 95% confidence interval 0.86, 0.97). The magnitude of association between REM sleep percentage and dementia was similar following adjustments for multiple covariates including vascular risk factors, depressive symptoms, and medication use, following exclusions for persons with mild cognitive impairment at baseline and following exclusions for early converters to dementia. Stages of non-REM sleep were not associated with dementia risk.
Conclusions:
Despite contemporary interest in slow-wave sleep and dementia pathology, our findings implicate REM sleep mechanisms as predictors of clinical dementia.
Disturbed sleep is a common feature of dementia, including dementia due to Alzheimer disease (AD).1 Cross-sectional studies demonstrate that aspects of sleep architecture differ in patients with dementia as compared to controls.2,3 Different stages of sleep may differentially affect key prodromal features of AD pathophysiology, including the generation4 and clearance of β-amyloid (Aβ),4 which aggregates into oligomers and plaques in AD.5 Sleep is associated with a marked increase in the clearance of extracellular Aβ, likely reflecting increased glymphatic flow.6 However, the temporal sequence between alterations in sleep architecture and dementia onset remains unclear. Clarifying the role of sleep in the onset of dementia may have important clinical applications, from the development of biomarkers to generating potential avenues for intervention. Accordingly, we examined whether aspects of sleep architecture were associated with the incidence of all-cause dementia and AD dementia in the community-based Framingham Heart Study over a maximum of 19 years of follow-up.
METHODS
This study involved the Framingham Heart Study Offspring cohort,7 which commenced in 1971. Participants have been studied across 9 examination cycles approximately every 4 years, with the latest cycle concluding in 2014. During the sixth examination cycle (1995–1998), 699 Offspring cohort participants and 298 Omni 1 cohort participants (aged ≥40 years, 50% male) completed an overnight polysomnography (PSG) as part of the multicenter Sleep Heart Health Study.8 The present study reports on the Offspring study participants only for whom we have data for incident dementia. Of the 699 Offspring participants with PSG, we excluded 370 participants aged younger than 60 years at the time of the PSG, 2 with prevalent dementia, 5 who were without follow-up for dementia, and 1 who was missing PSG data. Thus, our final sample comprised 321 participants.
Standard protocol approvals, registrations, and patient consents.
All participants provided written informed consent. The study was approved by the institutional review board of Boston University Medical Center.
Assessment of PSG.
Participants completed home-based PSG. The methods, including scoring guidelines and reliability, have been published previously.8–10 Briefly, sleep stages were scored in 30-second epochs by the criteria outlined by Rechtschaffen and Kales.11 Exposures for the present study included the percentage of time in stage 1, stage 2, slow-wave sleep (SWS; stages 3 and 4 combined), and REM sleep. Measures of sleep continuity, fragmentation, and sleep-disordered breathing were examined as additional exposures. These exposures included total sleep time, sleep onset latency, REM sleep latency, sleep efficiency (percentage of total sleep time/total time in bed), wake after sleep onset, and the apnea-hypopnea index (AHI) accompanied by a 4% or greater oxygen desaturation.
Ascertainment of incident dementia.
The Framingham Heart Study (FHS) monitors incident dementia through continuous surveillance of the cohort.12 Cognitive screening is performed at each FHS examination cycle using the Mini-Mental State Examination (MMSE)13 supplemented with extensive neuropsychological testing at selected examination cycles. Participants are flagged with suspected cognitive impairment using the MMSE if (1) performance falls below education-based cutoff scores,14 (2) a decline of 3 or more points is observed between consecutive examinations, or (3) a decrease of 5 or more points is observed from the participant's highest past MMSE score. Participants are also flagged following referrals or concern expressed by the participant, their family, or primary care physician. Once flagged with suspected cognitive impairment, participants complete annual neuropsychological and neurologic evaluations until they develop dementia or are adjudicated to be normal. Evaluations indicative of possible dementia are followed by referral to our study dementia review committee, comprising a neurologist and neuropsychologist. The committee adjudicates a diagnosis of dementia in line with the DSM-IV.15 A diagnosis of AD dementia is based on the criteria of the National Institute of Neurologic and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association for definite, probable, or possible AD.16
Statistical analysis.
After confirming the proportionality of hazards assumption, we used SAS software V9.4 (SAS Institute, Cary, NC) to estimate Cox proportional hazards regression models. The sleep exposures at baseline were related prospectively to the incidence of dementia. Surveillance for dementia commenced from examination cycle 6 (baseline) to the time of incident event over a maximum of 19 years. Non-events were censored at death or until the last date they were known to be dementia-free, also up to 19 years. Hazard ratios (HR) are presented accompanied by 95% confidence intervals (CIs). A square root transformation was applied to SWS. Log transformations were applied to sleep latency, wake after sleep onset, and the AHI to restore normality. All other sleep variables were left as continuous and untransformed variables except sleep efficiency and REM sleep latency, which could not be normalized through transformation. These variables were analyzed according to tertiles, using the highest tertile as the reference group.
Analyses were performed according to 2 statistical models. Model 1 included adjustments for age and sex. Model 2 included additional adjustments for body mass index, education (dichotomized at the level of college degree attainment), positivity for an APOE ε4 allele, systolic blood pressure, current smoking, prevalent diabetes, prevalent heart disease, depressive symptoms (Center for Epidemiologic Study Depression scores ≥16), use of antidepressants (monoamine oxidase inhibitors [MAOIs], nontricyclic antidepressants other than MAOIs, and tricyclic antidepressants), anxiolytics (benzodiazepines), antihypertensive medications, and medications to aid sleeping (participant self-reporting use of pills or medications at least once a month to help them sleep). Persons with missing data were excluded from analysis.
Sensitivity analysis.
We examined the associations between absolute time in each sleep stage (rather than percentage of time) and the risks of incident all-cause and AD dementia. We also examined for interactions between APOE ε4 allele status and each sleep stage. We then explored for factors that may explain the observed association between REM sleep percentage and dementia risk. We examined the associations between REM sleep percentage and incident all-cause and AD dementia after excluding (1) persons with mild cognitive impairment (MCI) at baseline, (2) the first 3 years of follow-up (thereby excluding early converters to dementia), (3, 4) persons with a very early or late chronotype (bottom 5% and top 5% of mid-sleep time [<1 am and >4 am, respectively]), (5) persons with a high number of arousals from REM sleep (top 10% [>30.64 arousals/h]), (6) persons with a high number of arousals from REM sleep due to hypopnea irrespective of desaturation (top 10% [>14.77 arousals/h]), and (7) persons with a high number of arousals from REM sleep due to obstructive apnea irrespective of desaturation (top 10% [>10.00 arousals/h]). Sensitivity analyses were adjusted for model 1 covariates. Results were considered significant if p < 0.05, except tests of interaction, which were considered significant if p < 0.10.
RESULTS
Sample overview.
Sample demographics and sleep measures at baseline are presented in tables 1 and 2, respectively. Over a mean of 12 (SD 5) years of follow-up, we observed 32 cases of incident dementia, 24 of which were due to AD dementia. Of the incident all-cause dementia cases, 25% occurred within the first 6.6 years, and 50% occurred between 6.6 and 11.8 years of follow-up, respectively.
Table 1.
Sample characteristics at the time of polysomnography
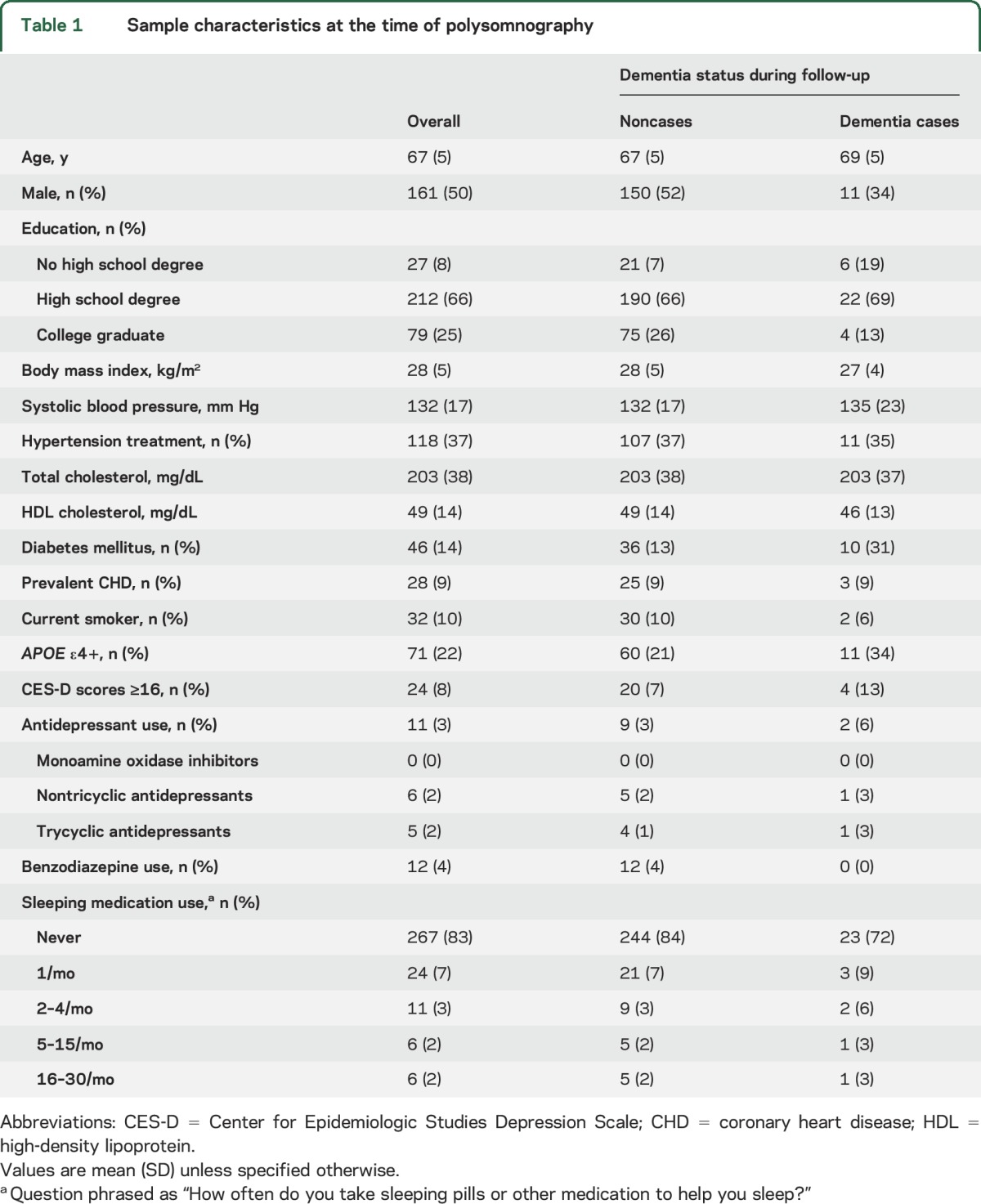
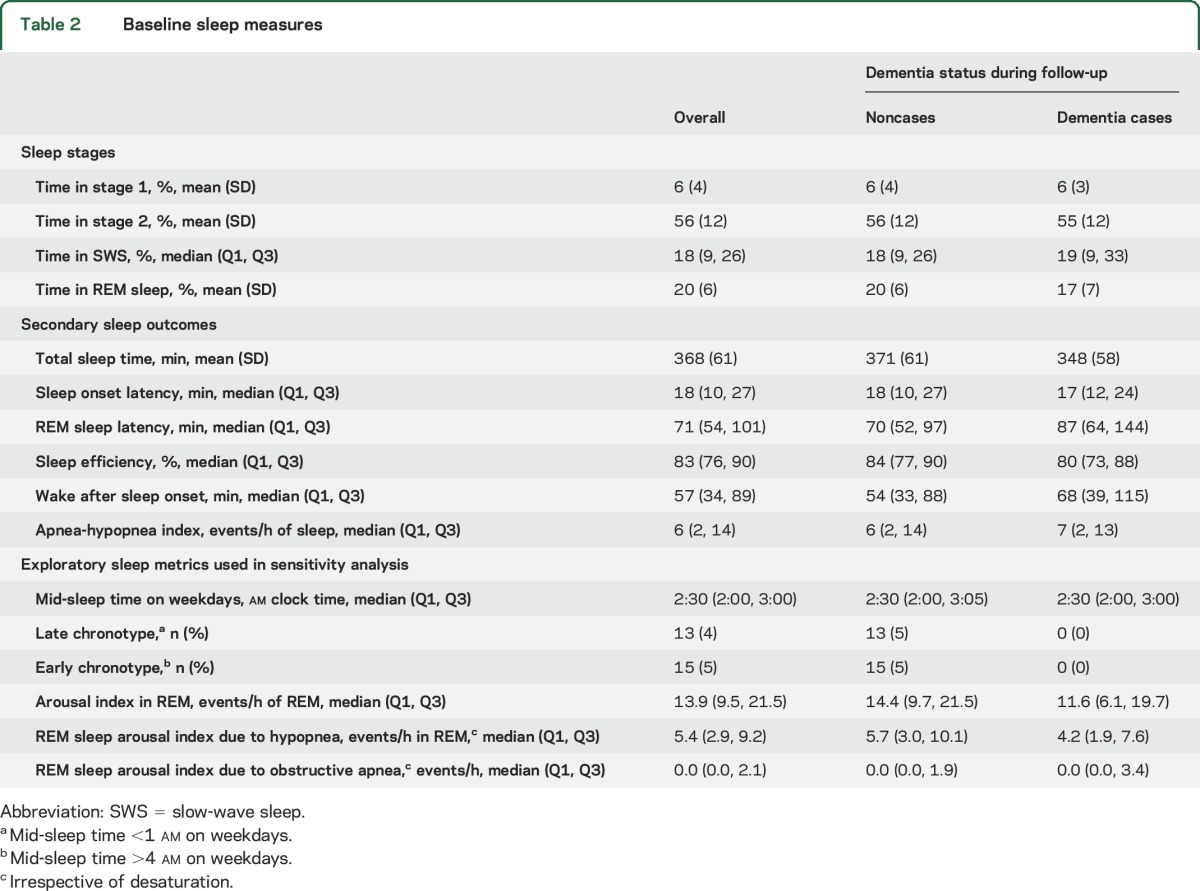
Table 2.
Baseline sleep measures
Sleep stages and risk of dementia.
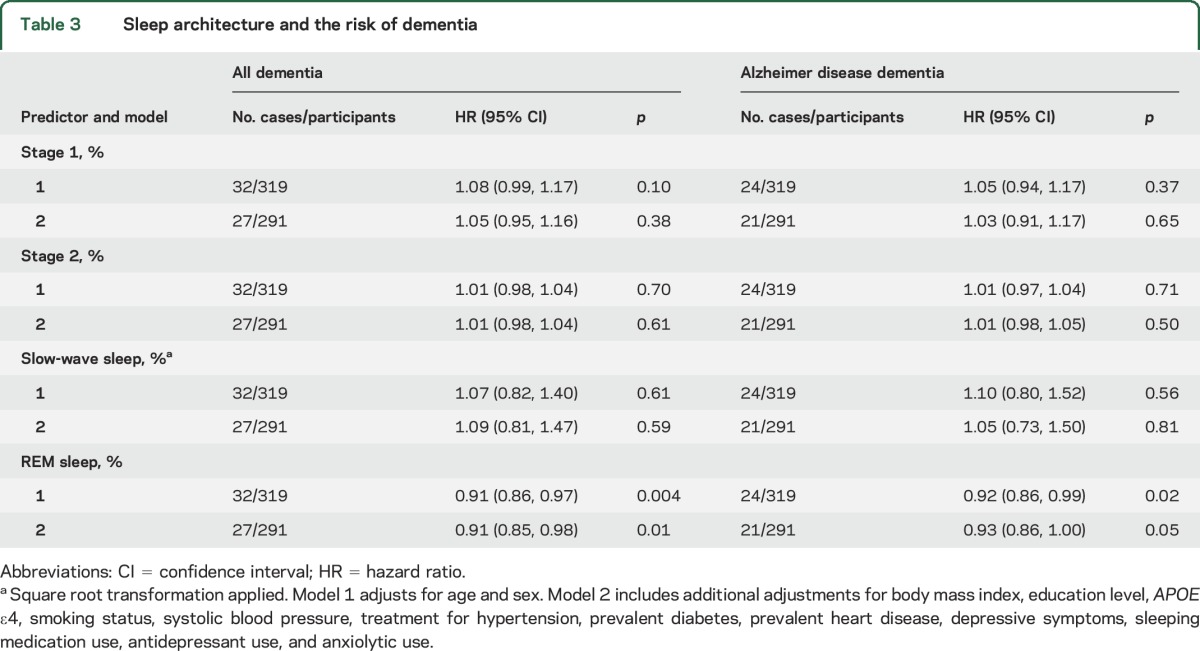
Lower REM sleep percentage was associated with an increased risk of all-cause dementia across both statistical models (table 3). After adjustment for age and sex, each percentage decrease in REM sleep was associated with approximately a 9% increase in the risk of incident dementia. The effect sizes were similar for AD dementia. Stages 1, 2, and SWS were not significantly associated with dementia risk.
Table 3.
Sleep architecture and the risk of dementia
Secondary sleep exposures and risk of dementia.
The lowest vs highest tertile of REM sleep latency was associated with a lower risk of all-cause dementia in model 1 (hazard ratio [HR] 0.37; 95% confidence interval [CI] 0.14–0.97) and model 2 (HR 0.26; 95% CI 0.08–0.85). Higher wake after sleep onset was associated with an increased risk of incident all-cause dementia in model 2 (HR 2.08; 95% CI 1.02–4.23) but not model 1 (HR 1.58; 95% CI 0.88–2.83). Although higher total sleep time was associated with a lower risk of all-cause dementia (HR 0.994; 95% CI 0.989–0.999) and AD dementia (HR 0.993; 95% CI 0.987–0.999) in model 1, the effect sizes were small. Sleep onset latency, sleep efficiency, and the AHI were not related to dementia incidence in our sample (table e-1 at Neurology.org).
Sensitivity analysis.
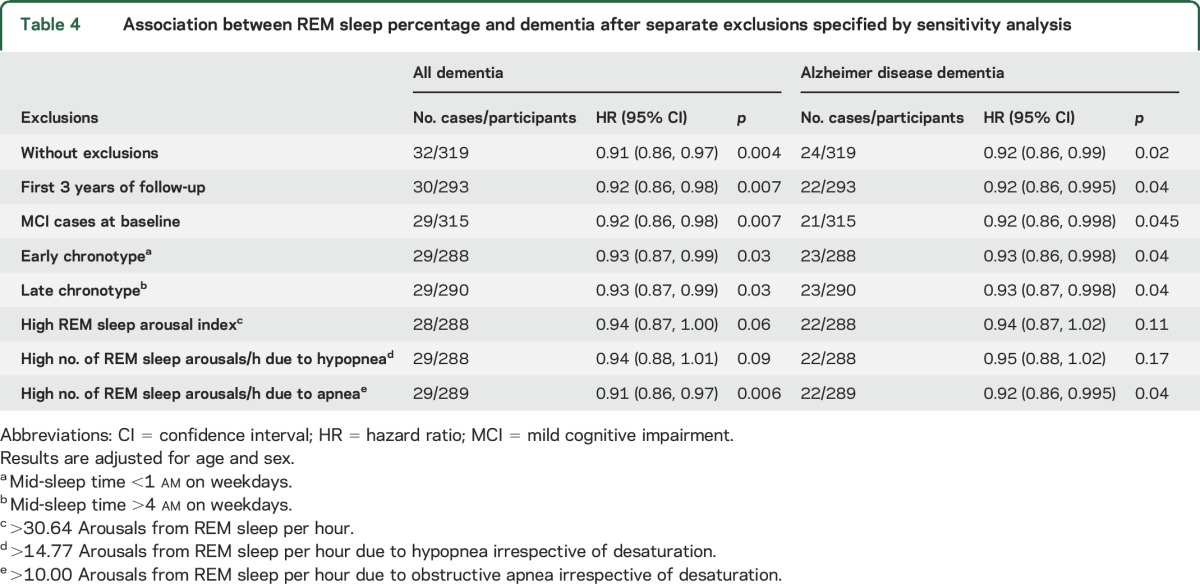
Results for absolute time in each sleep stage were consistent with our primary models, although effect sizes for time in REM sleep and dementia were less marked (table e-2). If we consider only those persons who developed new-onset dementia during follow-up, persons who spent a greater proportion of their time in REM sleep took longer to develop dementia (r = 0.37, p = 0.04). A lower proportion of time in REM sleep could be a marker of early dementia, but this seems less likely since excluding persons with MCI and persons developing dementia within 3 years of follow-up did not abolish the observed associations between REM sleep and incident dementia (table 4).
Table 4.
Association between REM sleep percentage and dementia after separate exclusions specified by sensitivity analysis
We found an APOE ε4 allele status by REM sleep percentage interaction when predicting incident AD dementia (p = 0.05). After stratifying the results, lower REM sleep percentage was associated with a higher risk of incident AD dementia in ε4 noncarriers (HR 0.88, 95% CI 0.80–0.96, p = 0.006, events/n = 14/245) but not carriers (HR 0.99, 95% CI 0.90–1.08, p = 0.75, events/n = 10/71). These results should be interpreted cautiously because of the overlapping confidence intervals, the small number of incident events, and the fact that no such APOE ε4 by REM sleep percentage interaction was identified when predicting all-cause dementia. No additional interactions were observed.
Sensitivity analysis: Exploring the mechanisms linking REM sleep percentage to dementia risk.
Separate exclusions for those with an early or late chronotype resulted in minimal change to the observed association between REM sleep percentage and incident dementia (table 4). The exclusion of persons with a high number of arousals from REM sleep attenuated the HR linking REM sleep percentage to incident dementia; this attenuation appeared to be due to arousals resulting from hypopnea rather than obstructive apnea.
DISCUSSION
This prospective study of a population-based sample reveals that shorter REM sleep percentage and longer latency to REM sleep were both independently associated with a higher risk of incident dementia. Greater wake after sleep onset, a measure of difficulty maintaining sleep, was also associated with an increased risk of dementia, but only in our fully adjusted statistical model. Stages of non-REM sleep were not associated with dementia incidence in our sample.
Previous prospective studies have linked objective sleep disturbance to the risk of clinical cognitive impairment. In a substudy of the Study of Osteoporotic Fractures, sleep-disordered breathing (defined as an AHI ≥15) was associated with the risk of incident clinical cognitive impairment and dementia (as a combined outcome) over a median of 5 years follow-up.17 In the Rush Memory and Aging Project, 10 consecutive days of actigraphy-assessed sleep fragmentation was associated with an increased risk of AD over a mean of 3 years follow-up.18 Despite these insights, prospective population-based studies have been of short duration and have not examined the association between sleep stages and the risk of dementia.
Current research on sleep and AD is mostly focused on SWS.4,19,20 However, our findings implicate less REM sleep, and not SWS, with the risk of dementia. In support of our findings, patients with dementia display less REM sleep on average as compared to controls without dementia.2,3 A population-based study using PSG recently demonstrated that time in REM sleep is reduced in those with as compared to without cognitive impairment, perhaps as a result of sleep-disordered breathing.21 In the Osteoporotic Fractures in Men Study, men with the lowest (vs highest) quartile of REM sleep percentage experienced more than twice the annual rate of decline in modified MMSE scores.22 REM sleep latency is also longer in patients with AD dementia as compared to controls with latency appearing to increase across categories of AD severity.3 In the same study, REM sleep percentage was positively correlated with CSF levels of Aβ1-42 in 48 patients with AD. Our results add to these studies by demonstrating that less REM sleep is associated prospectively with an increased risk of incident dementia among community-dwelling individuals. The observed effect size was large, with each percentage reduction in REM sleep associated with an approximate 9% increase in dementia risk. Overall, our findings suggest that future research should consider the role of REM sleep in the pathophysiology of dementia.
The mechanisms linking REM sleep to incident dementia are yet to be determined. In our study, the association between REM sleep percentage and dementia was independent of numerous confounders such as pharmacologic intervention for mood disorders, which adversely interfere with REM sleep.23 REM sleep behavior disorder has been associated with dementia and is clinically diagnostic of dementia due to the synucleinopathies.24 Cases of dementia due to the synucleinopathies did not drive our results given that effect sizes were similar when examining incident all-cause dementia (which included 6 cases of dementia with Lewy bodies) and AD dementia (no cases of mixed AD with Lewy bodies, Parkinson disease, or multiple system atrophy).
Cholinergic neurons are important determinants of REM sleep,25,26 with cholinergic activity low during SWS and high during REM sleep.27 AD is well-known to be associated with loss of cholinergic function, from the degeneration of cholinergic projections in the basal forebrain and changes in acetylcholine release, high-affinity choline uptake, and acetylcholine receptor expression.28,29 Thus, loss of cholinergic function may underpin the association between reduced REM sleep and dementia risk, i.e., a healthy cholinergic system maintains REM sleep metrics better than a slowly degenerating cholinergic system. However, in our study, the association between REM sleep percentage and incident dementia was not driven by persons with MCI at the time of polysomnography or those who converted to dementia within the first 3 years of follow-up. Such findings suggest that the association between REM sleep and dementia was not simply the result of neurologic changes that occurred in the late prodromal phase of dementia.
The association between REM sleep percentage and dementia was not driven by persons with early or late chronotypes. However, without information on shift work, we were unable to ascertain whether a late or early chronotype resulted in sleep outside of a physiologically preferred sleep period. The absence of this information prevented us from determining circadian rhythm sleep disorders. The effect between REM sleep percentage and dementia was attenuated after excluding persons with a high number of arousals from REM sleep due to hypopneas, suggesting a possible contributing role of sleep-disordered breathing. However, even after excluding persons with a high number of arousals from REM sleep, each percentage reduction in REM sleep percentage was associated a non–statistically significant 6% increase in all-cause dementia risk, suggesting contributions from other unmeasured factors.
It is possible that reduced REM sleep may be a marker of increased responsiveness to stress,30 with heightened anxiety also implicated as a risk factor for dementia.31 On the other hand, REM sleep is thought to promote synaptic consolidation and to upregulate the activity of immediate early genes implicated in synaptic plasticity.27,32 Thus, REM sleep may also buffer against synaptic loss and cognitive decline by assisting in the production of new connections and networks. Though there are biologically plausible mechanisms to explain our results, more studies are needed to explore the mechanisms linking REM sleep to AD pathology and incident dementia.
Strengths of the current study include the population-based sample, rigorous surveillance for incident dementia, long duration of follow-up, and use of PSG to measure sleep architecture. Limitations of the current study include the small sample size and a small number of incident events. Consequently, we had limited power to detect weaker but potentially important associations with other sleep-related exposures and dementia. For example, an AHI ≥15 has been associated with clinical cognitive impairment,17 yet we did not have the power to examine this threshold in the present study. Second, as our sample was of Caucasian decent, it is unclear how our results generalize to other ethnic groups. Future research should confirm our findings and determine the mechanisms linking REM sleep to incident dementia.
Supplementary Material
ACKNOWLEDGMENT
The authors thank the Framingham Heart Study participants for their commitment and dedication.
GLOSSARY
- Aβ
β-amyloid
- AD
Alzheimer disease
- AHI
apnea-hypopnea index
- CI
confidence interval
- DSM-IV
Diagnostic and Statistical Manual of Mental Disorders, 4th edition
- FHS
Framingham Heart Study
- HR
hazard ratio
- MAOI
monoamine oxidase inhibitor
- MCI
mild cognitive impairment
- MMSE
Mini-Mental State Examination
- PSG
polysomnography
- SWS
slow-wave sleep
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Drs. Pase, Himali, Grima, Beiser, Gottlieb, and Seshadri: study concept and design; Drs. Auerbach and Seshadri: acquisition of data; Drs. Pase, Himali, Beiser, Gottlieb, and Seshadri: analysis/interpretation of data; Dr. Pase: draft of the manuscript; Drs. Pase, Himali, Grima, Beiser, Satizabal, Aparicio, Thomas, Gottlieb, Auerbach, and Seshadri: critical revision of the manuscript for important intellectual content; Drs. Gottlieb and Seshadri: obtained funding; Drs. Gottlieb, Auerbach, and Seshadri: administrative, technical, or material support; Drs. Beiser and Seshadri: supervision.
STUDY FUNDING
M.P.P. is funded by an Australian National Health and Medical Research Council Early Career Fellowship (APP1089698). The Framingham Heart Study is supported by the National Heart, Lung, and Blood Institute (contract no. N01-HC-25195 and no. HHSN268201500001I) and by grants from the National Institute on Aging (R01 AG054076, R01 AG049607, R01 AG033193, U01 AG049505, U01 AG052409) and the National Institute of Neurologic Disorders and Stroke (NS0 17950 and UH2 NS100605). H.J.A. is supported by grants from the National Institute on Aging (T32-AG036697) and the American Heart Association (15GPSPG23770000). The Sleep Heart Health Study was supported by National Heart, Lung and Blood Institute cooperative agreements, including U01 HL53941 for the Framingham Heart Study site and U01 HL63463 for the Sleep Reading Center.
DISCLOSURE
M. Pase, J. Himali, N. Grima, A. Beiser, C. Satizabal, and H. Aparicio report no disclosures relevant to the manuscript. R. Thomas reports (1) a patent for a device to regulate CO2 in the positive airway pressure circuit for treatment of central/complex apnea; (2) a patent and license for an ECG-based method to phenotype sleep quality and sleep apnea (to MyCardio, LLV, through Beth Israel Deaconess Medical Center); (3) patent, past consultant—DeVilbiss-Drive, CPAP auto-titrating algorithm; (4) GLG Councils—general sleep medicine consulting. D. Gottlieb, S. Auerbach, and S. Seshadri report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Bliwise DL. Sleep disorders in Alzheimer's disease and other dementias. Clin Cornerstone 2004;6(suppl 1A):S16–S28. [DOI] [PubMed] [Google Scholar]
- 2.Prinz PN, Vitaliano PP, Vitiello MV, et al. Sleep, EEG and mental function changes in senile dementia of the Alzheimer's type. Neurobiol Aging 1982;3:361–370. [DOI] [PubMed] [Google Scholar]
- 3.Liguori C, Romigi A, Nuccetelli M, et al. Orexinergic system dysregulation, sleep impairment, and cognitive decline in Alzheimer disease. JAMA Neurol 2014;71:1498–1505. [DOI] [PubMed] [Google Scholar]
- 4.Ju Y-ES, Lucey BP, Holtzman DM. Sleep and Alzheimer disease pathology: a bidirectional relationship. Nat Rev Neurol 2014;10:115–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haass C, Selkoe DJ. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer's amyloid [beta]-peptide. Nat Rev Mol Cell Biol 2007;8:101–112. [DOI] [PubMed] [Google Scholar]
- 6.Xie L, Kang H, Xu Q, et al. Sleep drives metabolite clearance from the adult brain. Science 2013;342:373–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feinleib M, Kannel WB, Garrison RJ, McNamara PM, Castelli WP. The Framingham Offspring Study: design and preliminary data. Prev Med 1975;4:518–525. [DOI] [PubMed] [Google Scholar]
- 8.Quan SF, Howard BV, Iber C, et al. The Sleep Heart Health Study: design, rationale, and methods. Sleep 1997;20:1077–1085. [PubMed] [Google Scholar]
- 9.Whitney CW, Gottlieb DJ, Redline S, et al. Reliability of scoring respiratory disturbance indices and sleep staging. Sleep 1998;21:749–757. [DOI] [PubMed] [Google Scholar]
- 10.Redline S, Sanders MH, Lind BK, et al. Methods for obtaining and analyzing unattended polysomnography data for a multicenter study: Sleep Heart Health Research Group. Sleep 1998;21:759–767. [PubMed] [Google Scholar]
- 11.Rechtschaffen A, Kales A. A Manual of Standardized Terminology, Techniques, and Scoring System for Sleep Stages of Human Subjects. Washington, DC: US Government Printing Office; 1968. [Google Scholar]
- 12.Satizabal CL, Beiser AS, Chouraki V, Chêne G, Dufouil C, Seshadri S. Incidence of dementia over three decades in the Framingham Heart Study. N Engl J Med 2016;374:523–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Folstein MF, Folstein SE, McHugh PR. “Mini mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–198. [DOI] [PubMed] [Google Scholar]
- 14.Bachman DL, Wolf PA, Linn R, et al. Prevalence of dementia and probable senile dementia of the Alzheimer type in the Framingham Study. Neurology 1992;42:115–119. [DOI] [PubMed] [Google Scholar]
- 15.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th ed, text revision. Arlington, VA: American Psychiatric Association; 2000. [Google Scholar]
- 16.McKhann G, Drachman D, Folstein M. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA work group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology 1984;34:939–944. [DOI] [PubMed] [Google Scholar]
- 17.Yaffe K, Laffan AM, Harrison S, et al. Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. JAMA 2011;306:613–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lim AS, Kowgier M, Yu L, Buchman AS, Bennett DA. Sleep fragmentation and the risk of incident Alzheimer's disease and cognitive decline in older persons. Sleep 2013;36:1027–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lim MM, Gerstner JR, Holtzman DM. The sleep-wake cycle and Alzheimer's disease: what do we know? Neurodegener Dis Manag 2014;4:351–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mander BA, Winer JR, Jagust WJ, Walker MP. Sleep: a novel mechanistic pathway, biomarker, and treatment target in the pathology of Alzheimer's disease? Trends Neurosci 2016;39:552–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haba-Rubio J, Marti-Soler H, Tobback N, et al. Sleep characteristics and cognitive impairment in the general population: the HypnoLaus study. Neurology 2017;88:463–469. [DOI] [PubMed] [Google Scholar]
- 22.Song Y, Blackwell T, Yaffe K, Ancoli-Israel S, Redline S, Stone KL. Relationships between sleep stages and changes in cognitive function in older men: the MrOS Sleep Study. Sleep 2015;38:411–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilson S, Argyropoulos S. Antidepressants and sleep. Drugs 2005;65:927–947. [DOI] [PubMed] [Google Scholar]
- 24.Boeve BF, Silber MH, Ferman TJ, Lucas JA, Parisi JE. Association of REM sleep behavior disorder and neurodegenerative disease may reflect an underlying synucleinopathy. Mov Disord 2001;16:622–630. [DOI] [PubMed] [Google Scholar]
- 25.Vazquez J, Baghdoyan HA. Basal forebrain acetylcholine release during REM sleep is significantly greater than during waking. Am J Physiol Regul Integr Comp Physiol 2001;280:R598–R601. [DOI] [PubMed] [Google Scholar]
- 26.Saper CB, Fuller PM, Pedersen NP, Lu J, Scammell TE. Sleep state switching. Neuron 2010;68:1023–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Diekelmann S, Born J. The memory function of sleep. Nat Rev Neurosci 2010;11:114–126. [DOI] [PubMed] [Google Scholar]
- 28.Schmitt K, Holsboer-Trachsler E, Eckert A. BDNF in sleep, insomnia, and sleep deprivation. Ann Med 2016;16:42–51. [DOI] [PubMed] [Google Scholar]
- 29.Mesulam M, Shaw P, Mash D, Weintraub S. Cholinergic nucleus basalis tauopathy emerges early in the aging-MCI-AD continuum. Ann Neurol 2004;55:815–828. [DOI] [PubMed] [Google Scholar]
- 30.Petersen H, Kecklund G, D'Onofrio P, Nilsson J, Akerstedt T. Stress vulnerability and the effects of moderate daily stress on sleep polysomnography and subjective sleepiness. J Sleep Res 2013;22:50–57. [DOI] [PubMed] [Google Scholar]
- 31.Gulpers B, Ramakers I, Hamel R, Kohler S, Oude Voshaar R, Verhey F. Anxiety as a predictor for cognitive decline and dementia: a systematic review and meta-analysis. Am J Geriatr Psychiatry 2016;24:823–842. [DOI] [PubMed] [Google Scholar]
- 32.Ribeiro S, Mello CV, Velho T, Gardner TJ, Jarvis ED, Pavlides C. Induction of hippocampal long-term potentiation during waking leads to increased extrahippocampal zif-268 expression during ensuing rapid-eye-movement sleep. J Neurosci 2002;22:10914–10923. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


