Abstract
Objective:
To examine potential disease-modifying effects of statin drugs, we conducted a 12-month randomized, placebo-controlled clinical trial of simvastatin in cognitively normal adults using change in CSF Alzheimer disease biomarkers as primary outcome measure.
Methods:
Participants were 45–64 years old and statin-naive with normal cognition and normal or mildly elevated cholesterol. Forty-six participants completed the 1-year study per protocol (25 in the simvastatin and 21 in the placebo group). Simvastatin was titrated to 40 mg/d. CSF Aβ42, total tau, and p-tau181 were measured at baseline and after 12 months of treatment using the INNO-BIA AlzBio3 assay. We used analysis of covariance to assess differences in biomarker change from baseline between treatment groups, adjusting for age, sex, and APOE ε4 status.
Results:
Changes from baseline did not differ significantly between treatment groups for any CSF biomarker, with p values of 0.53, 0.36, and 0.25 for CSF Aβ42, total tau, and p-tau181, respectively. There was no significant modifying effect of sex, APOE ε4, or baseline high-density lipoprotein or triglycerides on treatment group for any of the biomarkers (all p > 0.18). However, a significant interaction between treatment group and baseline low-density lipoprotein (LDL) was observed for p-tau181 (p = 0.003), where greater decreases from baseline in CSF p-tau181 concentrations were associated with higher baseline LDL level for the simvastatin group.
Conclusions:
Simvastatin-related reductions in CSF p-tau181 concentrations may be modulated by LDL cholesterol. The potential disease-modifying effects of simvastatin on CSF phospho-tau should be further investigated in persons with hypercholesterolemia.
Elevated cholesterol levels and genetic variants in HMG-CoA reductase (the rate-limiting enzyme in cholesterol synthesis) are associated with altered risk of Alzheimer disease (AD).1,2 Treatment with cholesterol-lowering statin drugs is associated with decreased AD risk and neurofibrillary tangle burden.1,3 However, clinical efficacy trials of statins in patients with AD have been negative,4 possibly because statins cannot reverse neuronal damage associated with progression to symptomatic AD. CSF biomarkers of AD (i.e., Aβ42, total tau, and p-tau181) change long before the onset of clinical dementia and may serve as surrogate markers of underlying disease processes. We previously demonstrated that 14 weeks of treatment with simvastatin (which penetrates the blood–brain barrier [BBB]), but not pravastatin (which does not penetrate BBB), was associated with a significant reduction of CSF p-tau181 in cognitively normal adults with hypercholesterolemia.5 In an attempt to confirm potential disease-modifying effects of simvastatin, we conducted a randomized, placebo-controlled trial in cognitively normal participants using change in CSF AD biomarkers as the primary outcome measure. To address concerns of potential adverse effect of simvastatin on cognitive function, neuropsychological tests that are sensitive to subtle cognitive changes (e.g., attention, memory, learning, psychomotor speed, fine motor coordination) were performed.
METHODS
Study design.
Participants were 45–64 years of age, were statin-naive, and had normal cognition based on history, clinical examination, and neuropsychological testing. Persons with unstable medical or psychiatric conditions, head injury, or substance abuse were excluded, as were those whose cholesterol levels were sufficiently high as to require statin treatment (given the ethical concern of withholding statin treatment in those randomized to placebo).
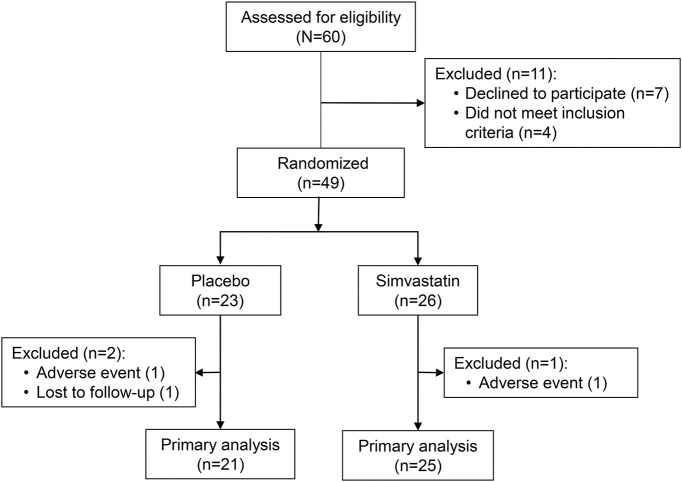
Sixty potential participants were screened and 49 were randomized to treatment with simvastatin (n = 26) or placebo (n = 23). Twenty-five participants in the simvastatin group and 21 in the placebo group completed the 1-year study per protocol and were included in our primary analysis (figure 1). One participant in each group discontinued drug due to an adverse event, and one participant in the placebo group was lost to follow-up.
Figure 1. Study design.
Interventions.
Simvastatin was started at 20 mg/d and titrated over 2 weeks to a final dose of 40 mg/d. Placebo dosing was similarly titrated over 2 weeks.
Standard protocol approvals, registrations, and patient consents.
The trial was registered on clinicaltrials.gov (NCT01142336), was approved by the University of Washington and VA Puget Sound Health Care System (VAPSHCS) institutional review boards, and all participants provided written informed consent prior to any study procedures.
Study outcomes.
CSF and blood samples were collected in the morning after overnight fasting. CSF Aβ42, total tau, and p-tau181 were measured by the Knight Alzheimer's Disease Research Center at Washington University in St. Louis using the INNO-BIA AlzBio3 assay (Fujirebio, formerly Innogenetics, Ghent, Belgium), with longitudinal samples run on the same assay plate. Fasting serum lipids were analyzed in the VAPSHCS clinical laboratory. APOE (GenBank, M12529) genotype was determined by restriction digest methodology. Neuropsychological tests were performed at baseline and at end of study as safety measures.
Statistical analysis.
Analysis of covariance (ANCOVA) was used to assess biomarker change from baseline, with concentration at end of study as the response variable. Predictor variables included concentration at baseline, treatment assignment, age, sex, and APOE ε4 status (presence/absence of ε4). Models for neuropsychological tests included education and Wechsler Test of Adult Reading scores as covariates. As a sensitivity analysis, we used linear mixed-effects models to include all participants who were randomized and had a baseline visit. To examine the potential modifying effect of sex, APOE ε4 status, and baseline cholesterol levels, we included an interaction term for treatment assignment by each of these factors in the ANCOVA model for each biomarker.
RESULTS
Demographics, APOE ε4 status, and baseline serum analyte and CSF biomarker values were similar between participants randomized to the placebo and simvastatin groups (table e-1 at Neurology.org), except that baseline serum high-density lipoprotein (HDL) was slightly lower in the simvastatin group. A similar pattern was seen in the primary analysis sample (data not shown).
Effect of simvastatin on CSF AD biomarkers.
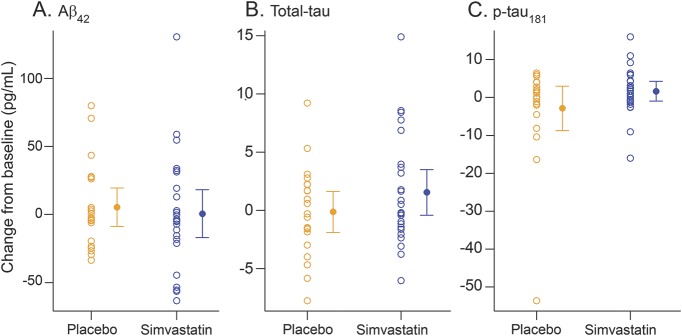
Change from baseline did not differ significantly between treatment groups for any of the CSF biomarkers by ANCOVA, after adjusting for age, sex, and presence of APOE ε4 allele (figure 2), with p values of 0.53, 0.36, and 0.25 for CSF Aβ42, total tau, and p-tau181, respectively. Results based on the linear mixed effects models that included all study participants were similar (data not shown).
Figure 2. CSF Alzheimer disease biomarker changes in placebo and simvastatin-treated groups.
(A–C) Change from baseline is concentration at 1 year minus concentration at baseline.
Modifying effect of sex, APOE genotype, and baseline serum cholesterol.
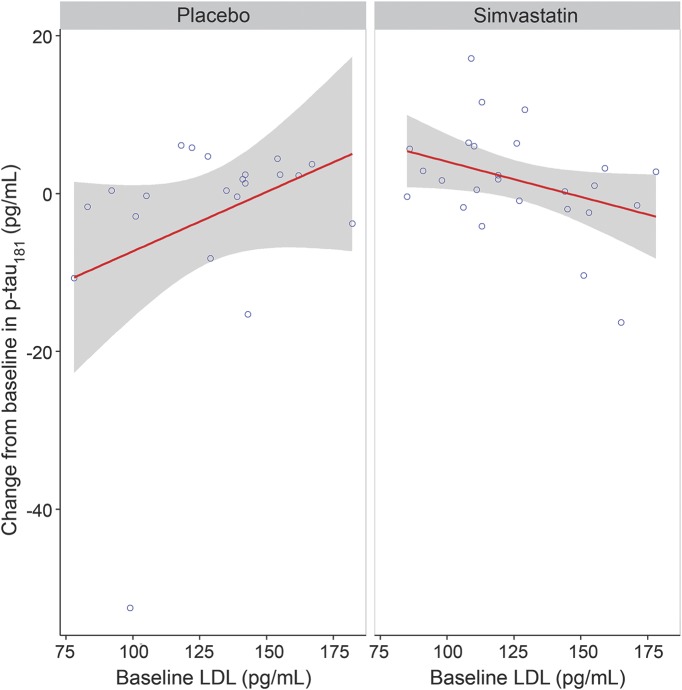
There were no significant modifying effects of sex, APOE ε4, baseline low-density lipoprotein (LDL), baseline HDL, or triglycerides by treatment group on any of the CSF AD biomarkers (all p > 0.05), except for a significant interaction between treatment group and baseline LDL for p-tau181 (p = 0.003). Greater decreases from baseline in CSF p-tau181 concentrations were associated with higher baseline LDL for the simvastatin group, whereas the opposite was true for the placebo group (figure 3). The direction and significance of this interaction were unchanged after exclusion of a single high-leverage case (large CSF p-tau181 decrease) in the placebo group (p = 0.01). Decreases from baseline in CSF p-tau181 were also correlated with decreases from baseline in LDL levels in the simvastatin group (r = 0.40, p = 0.047).
Figure 3. Observed change from baseline in CSF p-tau181 vs baseline low-density lipoprotein (LDL) by treatment group.
Gray area represents the 95% confidence interval for the slope.
Safety.
There were no increased reports of memory problems, anxiety, or depression (p > 0.05) or elevations of serum creatine phosphokinase or liver enzymes (all p > 0.05) in the simvastatin group compared to the placebo group (data not shown). There were no significant differences by treatment group in 12-month change for any of the 12 neuropsychological test scores after covariate adjustment (table e-2).
DISCUSSION
Twelve months of treatment with simvastatin 40 mg/d did not change CSF Aβ42, total tau, or p-tau181 concentrations in cognitively normal participants with normal or mildly elevated cholesterol levels and did not impair cognitive function. We did not replicate our previous finding that 3 months of simvastatin treatment reduced CSF p-tau181 in cognitively normal adults with hypercholesterolemia, even though the current study was 2 times larger, of longer duration (12 vs 3 months), and used placebo (rather than a non-brain-penetrant statin) as the control intervention.
The lack of effect of simvastatin on p-tau181 in the current study may reflect the relatively low serum cholesterol levels in our study participants, as decreases of CSF p-tau181 were associated with both baseline LDL levels and decreases of serum LDL. Although the mechanism by which statins reduce tau phosphorylation is unclear, studies have shown that cholesterol modulates tau phosphorylation in cultured neurons6; elevated serum cholesterol levels are associated with enhanced neuronal tau pathology in P301L human tau mice7; and atorvastatin reverses hypercholesterolemia-induced increases in rat brain p-tau production.8 Furthermore, high CSF cholesterol levels in humans are associated with elevated CSF p-tau181.9 Consistent with our earlier study5 and others,10 simvastatin did not alter CSF concentrations of Aβ42 or total tau.
A major limitation of this study is the small sample size; the null findings could be due to lack of power. This study was originally powered to detect an effect size of 0.6 with 80% power based on our pilot study,5 assuming 50 participants per group would complete the 1-year study. Another limitation is that women are overrepresented in our sample. Nevertheless, that high serum LDL predicts simvastatin-related reductions in CSF p-tau181 suggests that potential disease-modifying effects of simvastatin on CSF phospho-tau should be further investigated in a larger sample of persons with clinically significant hypercholesterolemia.
Supplementary Material
ACKNOWLEDGMENT
The authors thank Dr. David Gruenwald at Veterans Affairs Puget Sound Health Care System, Seattle, Washington, and Dr. William Hazzard at Wake Forest School of Medicine, Winston-Salem, North Carolina, for serving on the Data Safety Monitoring Board for the clinical trial, and all the volunteers who participated in this study.
GLOSSARY
- AD
Alzheimer disease
- ANCOVA
analysis of covariance
- BBB
blood–brain barrier
- HDL
high-density lipoprotein
- LDL
low-density lipoprotein
- VAPSHCS
VA Puget Sound Health Care System
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Ge Li: study concept and design, interpretation of data, and drafting manuscript. Cynthia Mayer: acquisition and interpretation of data. Daniel Morelli: acquisition and interpretation of data. Steven P. Millard: study design, statistical analysis, drafting manuscript, and interpretation of data. Wendy H. Raskind: acquisition and interpretation of data. Eric C. Petrie: study design, drafting manuscript, and interpretation of data. Monique Cherrier: study design and interpretation of data. Anne M. Fagan: acquisition and interpretation of data. Murray A. Raskind: study concept and design and critical revision of manuscript for intellectual content. Elaine R. Peskind: study concept and design, data acquisition, and interpretation of data.
STUDY FUNDING
Supported by the NIH (AG033693 and AG05136), the Department of Veterans Affairs, and an anonymous foundation. None of the funding organizations or sponsors was involved in the design and conduct of the study, the collection, management analysis, and interpretation of the data, or the preparation, review, and approval of the manuscript.
DISCLOSURE
G. Li, C. Mayer, D. Morelli, S. Millard, W. Raskind, E. Petrie, and M. Cherrier report no disclosures relevant to the manuscript. A. Fagan is on the Scientific Advisory Boards for Roche, IBL International, and AbbVie and consults for Biogen, DiamiR, and LabCorp. M. Raskind and E. Peskind report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Shepardson NE, Shankar GM, Selkoe DJ. Cholesterol level and statin use in Alzheimer disease: I: review of epidemiological and preclinical studies. Arch Neurol 2011;68:1239–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leduc V, De Beaumont L, Theroux L, et al. HMGCR is a genetic modifier for risk, age of onset and MCI conversion to Alzheimer's disease in a three cohorts study. Mol Psychiatry 2015;20:867–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li G, Larson EB, Sonnen JA, et al. Statin therapy is associated with reduced neuropathologic changes of Alzheimer disease. Neurology 2007;69:878–885. [DOI] [PubMed] [Google Scholar]
- 4.Sano M, Bell KL, Galasko D, et al. A randomized, double-blind, placebo-controlled trial of simvastatin to treat Alzheimer disease. Neurology 2011;77:556–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Riekse RG, Li G, Petrie EC, et al. Effect of statins on Alzheimer's disease biomarkers in cerebrospinal fluid. J Alzheimers Dis 2006;10:399–406. [DOI] [PubMed] [Google Scholar]
- 6.Fan QW, Yu W, Senda T, Yanagisawa K, Michikawa M. Cholesterol-dependent modulation of tau phosphorylation in cultured neurons. J Neurochem 2001;76:391–400. [DOI] [PubMed] [Google Scholar]
- 7.Glockner F, Meske V, Lutjohann D, Ohm TG. Dietary cholesterol and its effect on tau protein: a study in apolipoprotein E-deficient and P301L human tau mice. J Neuropathol Exp Neurol 2011;70:292–301. [DOI] [PubMed] [Google Scholar]
- 8.Lu F, Li X, Suo AQ, Zhang JW. Inhibition of tau hyperphosphorylation and beta amyloid production in rat brain by oral administration of atorvastatin. Chin Med J (Engl) 2010;123:1864–1870. [PubMed] [Google Scholar]
- 9.Popp J, Meichsner S, Kolsch H, et al. Cerebral and extracerebral cholesterol metabolism and CSF markers of Alzheimer's disease. Biochem Pharmacol 2013;86:37–42. [DOI] [PubMed] [Google Scholar]
- 10.Carlsson CM, Gleason CE, Hess TM, et al. Effects of simvastatin on cerebrospinal fluid biomarkers and cognition in middle-aged adults at risk for Alzheimer's disease. J Alzheimers Dis 2008;13:187–197. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.