Abstract
In this perspective we summarize current knowledge of the effect of monosialoganglioside GM1 on the membrane-mediated aggregation of the β-amyloid (Aβ) peptide. GM1 has been suggested to be actively involved in the development of Alzheimer’s disease due to its ability to seed the aggregation of Aβ. However, GM1 is known to be neuroprotective against Aβ-induced toxicity. Here we suggest that the two scenarios are not mutually exclusive but rather complementary, and might depend on the organization of GM1 in membranes. Improving our understanding of the molecular details behind the role of gangliosides in neurodegenerative amyloidoses might help in developing disease-modifying treatments.
Main Text
Aberrant misfolding and aggregation of amyloidogenic proteins is implicated in the onset and progression of devastating diseases including the neurodegenerative Alzheimer’s disease (AD). While the exact molecular factors responsible for the incurable neurodegenerative amyloidoses are largely unknown, AD pathogenesis seems to be linked to the oligomerization of the β-amyloid (Aβ) peptide. Certainly many factors will contribute to AD development; nonetheless, the amyloid hypothesis continues to accumulate support and validation (1).
Currently, oligomers of the Aβ peptide are viewed as the cytotoxic species mainly responsible for the initial biochemical alterations that culminate in the development of AD (2). The most common forms of Aβ found in the human body are the Aβ40 and Aβ42 peptides (3). The generation of Aβ peptides in cells is the terminal stage of a rather complex proteolytic processing of the amyloid precursor protein (APP) by β- and γ-secretases (4). It is now generally accepted that this process takes place in intracellular membrane compartments (endosomes) after which Aβ is released to the extracellular medium (5, 6). Oligomerization of Aβ occurs spontaneously at high (micromolar) concentrations (7, 8). However, the oligomerization of nanomolar concentrations of Aβ in the brain is very likely to be mediated by membranes (9, 10).
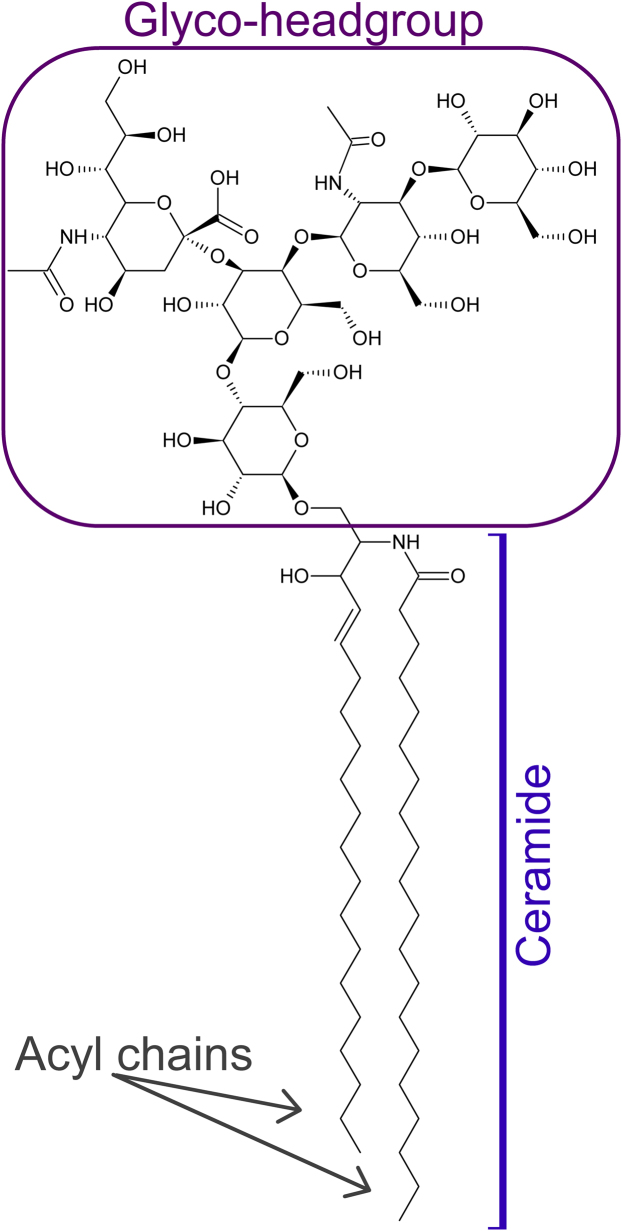
Over the last few decades, efforts have been made to identify specific neuronal receptors for the Aβ peptide. In this pursuit, gangliosides have been found to be primary targets and modulators of Aβ aggregation (11, 12). Gangliosides are sialic acid-containing glycosphingolipids present in membranes of all vertebrate cells, being particularly abundant in the nervous system. The monosialoganglioside GM1 (Fig. 1) is one of the most abundant gangliosides in the brain (in general) (13) which, combined with the availability of analytical tools (e.g., antibodies), leads to the majority of studies being performed on Aβ/GM1 interactions.
Figure 1.
Chemical structure of the monosialoganglioside GM1. To see this figure in color, go online.
Despite numerous investigations, the role of GM1 in the pathology of AD remains controversial. On the one hand, it has been suggested that GM1 can seed the aggregation of Aβ and, in this way, be actively involved in the development of AD (12, 14, 15). On the other hand, reports have evidenced that GM1 can have neuroprotective and neuroregenerative effects (16, 17, 18, 19, 20). In this perspective, we summarize current knowledge of the effect of GM1 on the aggregation of Aβ and suggest that the two scenarios mentioned above are not mutually exclusive, but rather complementary. This view is supported by a multifaceted function and distribution of GM1 in cells and its complex structural organization in model membranes is described in the following section.
GM1 Organization on Model and Cellular Membranes
In model membranes, the percolation threshold of GM1 has been calculated to be ∼22 mol % (21), meaning that at this concentration, and above, GM1 forms an interconnected network. Thus, when at high densities, GM1 generates a platform whose surface is fully covered by the glycoheadgroups of the ganglioside. Below the percolation threshold, GM1 exists in the form of clusters whose size and properties depend on the lipid composition of the membrane and the concentration of GM1 (22, 23, 24, 25). In membranes with so-called raft composition (sphingomyelin/cholesterol/DOPC 1:1:1), micrometer size clusters (0.40–1.50 μm) have been observed in the liquid-ordered phase with as low as 1 mol % of GM1 (22). On the other hand, nanometer clusters (7–50 nm in diameter, depending on sphingomyelin and cholesterol content) have been detected in fluid, liquid-disordered membranes with physiological levels of GM1 (∼0.1–5 mol %) (23, 24, 25). These nanoclusters are highly fluid and do not exhibit raftlike properties (24, 25). Such clusters probably encompass well-accessible and highly mobile GM1 molecules, which contrasts with the platforms of clustered GM1 in liquid-ordered phases. Importantly, the association of GM1 with its ligands (e.g., cholera toxin subunit B; CTxB) can modulate the structure and organization of the ganglioside in membranes (24, 26).
In cells, GM1 regulates a plethora of events (27, 28, 29). All of its structural elements (acyl chains, ceramides, and sugar moieties; Fig. 1) are essential for this purpose (30). To analyze the distribution and organization of GM1 in cellular membranes, the most used methods are specific antibodies and ligands of endogenous GM1. In the plasma membrane of cultured fibroblasts, GM1 has been found to exist in small domains (∼50 nm; Fig. 2) (31, 32), the labeling density of which does not exceed 2000 events/μm2 (31). This indicates that, in these cells, either GM1 forms low-density clusters or the detection of GM1 was incomplete (33). Similar GM1 domains were found in cultured lymphoid cells (34). These GM1 domains are reminiscent of the fluid, low-density nanoclusters observed in model membranes containing low (physiological) concentration of GM1 (25).
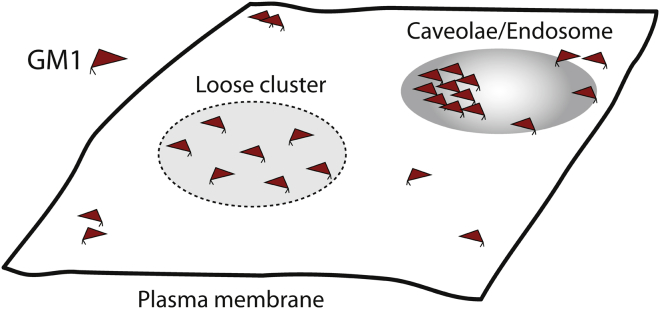
Figure 2.
Schematic illustration of GM1 organization on the plasma membrane of mammalian cells. Accumulation of the ganglioside in loose clusters is indicated by the dotted ellipse. Putative formation of densely packed GM1 platform is visualized at the edge of a caveolae/endocytic cavity. To see this figure in color, go online.
Using a similar approach, Parton (35) found GM1 accumulated in plasma membrane caveolae and endosomes of the human epidermoid carcinoma cell line A431. In these structures, dense labeling has been observed indicating a high local concentration of ganglioside (35, 36), and indicating that ligands are capable of detecting areas dense in GM1 molecules. Both caveolae and endosomes have been reported to have raftlike membrane properties (37) suggesting that, in these structures, GM1 might be organized in rigid, high-density platforms (Fig. 2). On the other hand, caveolae and endosomes/lysosomes are different endocytic pathways for GM1 in cells expressing high or low concentrations of the ganglioside, respectively (38). In addition, lipid headgroup packing in the caveolae-dependent pathway is less constrained compared to endosomes and lysosomes (39). This could indicate that distinct nanoscopic GM1 organization might regulate its internalization via different endocytic pathways.
Effect of High-density GM1 Platforms in Aβ Oligomerization
The influence of highly dense GM1 membrane clusters in the oligomerization of the Aβ peptide has been studied for over 20 years now. Ex vivo and in vitro studies have revealed binding of Aβ to GM1 in so-called raftlike membrane environments (12, 14, 15). Membranes with characteristics of ordered phases (or lipid rafts) were at the center of these studies (40, 41, 42, 43, 44). In model systems of large and small unilamellar vesicles it has been shown that Aβ (Aβ40) adsorbs to ganglioside-rich (>20 mol %) membranes with a conformational transition resulting in β-sheet structure (45). The formation of oligomers is responsible for the transition to β-sheets and depends on peptide density on the ganglioside-rich membranes, occurring only at Aβ to GM1 ratios of 1:22 or higher (40, 42). Furthermore, fibrillation studies (using lipid vesicles composed of ganglioside/cholesterol/sphingomyelin 2:4:4), demonstrated that the rate of fibril formation was proportional to the solution concentration of Aβ. In this work, GM1 exhibited the strongest fibril seeding potential compared to gangliosides with a different glycan structure (e.g., GD1a or GT1a). Based on the above findings, a model emerged, suggesting that Aβ first adsorbs to GM1 platforms (high GM1 density clusters) in raftlike membranes. This locally concentrates Aβ and promotes the formation of oligomers. Such membrane-bound, β-sheet-containing, Aβ oligomers can act as a seed for the further aggregation of Aβ available in solution and lead to fibril formation (40, 42, 43, 44).
In the brain, high solution concentrations of Aβ are not achieved, and rapid fibrillation is unlikely to occur. However, under certain circumstances, the relatively low levels of Aβ present in the brain could be locally concentrated in endosomes (46) and transformed into toxic oligomeric species via the catalytic effect of the localized high-density platforms of GM1. Such events could also take place in presynaptic neuritic termini as suggested by the observation of Aβ fibril formation in synaptosomes containing high-density clusters of GM1 (44, 47). Notably, the fibrillation was inhibited by pretreatment with GM1 ligand (CTxB), demonstrating that GM1 is involved in this process (44).
Effect of Low-density GM1 Nanoclusters in Aβ Oligomerization
In contrast to high-density GM1 platforms in ordered membranes, the relation between Aβ and gangliosides in low-density nanoclusters has been investigated in model membranes only very recently (25). The study used several model membranes (giant unilamellar vesicles) in the liquid-disordered phase with physiological amounts of GM1 (2–4 mol %) that self-organized into low-density nanoclusters (24, 25). The fluid and dynamic GM1 nanoclusters failed to catalyze the oligomerization of nanomolar (physiological) solutions of Aβ (Aβ40). The peptide to GM1 ratio used in this oligomerization study (25) was estimated to be, at maximum, ≈1:80. Thus, the results are seemingly in accordance with the studies on high-density platforms of GM1 where oligomer formation was detected only at Aβ/GM1 ratios above 1:22.
The most interesting finding in the study, however, was the inhibitory effect of GM1 on the oligomerization of the amyloid peptide and the molecular insight into specific interactions of Aβ with membranes (25). The work showed that sphingomyelin, but not phosphatidylcholine with long and saturated acyl chains (DSPC), is able to trigger the membrane-mediated oligomerization of Aβ (at nanomolar concentration in solution). This indicates that the catalytic effect is specific and does not require an ordered phase membrane. Intriguingly, when GM1 was present in the membranes containing sphingomyelin, the oligomerization process was prevented. Under the tested conditions, the ganglioside counteracted the effect of sphingomyelin, thus inhibiting the oligomerization of Aβ. Molecular dynamics simulations demonstrated binding between the peptide and GM1, which involved the β-sheet-forming residues (25). Such data are in agreement with previous in silico studies (48, 49) and experimental results (14, 50, 51, 52). Thus, it was proposed that the inhibitory effect of GM1 resides in its capacity to bind and sequester the Aβ peptide.
To the best of our knowledge, the study (25) presents the first molecular evidence for GM1 acting as an inhibitor of the oligomerization of Aβ, supporting GM1 as potentially beneficial. The findings provide rationalization for the neuroprotective effect of GM1 against Aβ toxicity and AD progression observed in vivo (17, 18, 19, 20, 52). Interestingly, recently it has been shown in vivo that GM1 has the power to disrupt Aβ dimers (52).
Two Sides of the Same Coin: Multifaceted GM1 in the Brain
It is known that surfaces in general (9, 53, 54, 55, 56, 57) and lipid membranes in particular (58) can modulate the aggregation of proteins. Adsorption of proteins onto the surface of lipid membranes can result in a form of heterogeneous catalysis of aggregation (as a general term). The phenomenon can cause a local increase in protein concentration on the confined two-dimensional surface, induce crowding (40, 43), and/or induce a misfolded state of the protein (43, 54). All these factors (alone or in tandem) increase the probability of surface-mediated oligomerization that can nucleate the formation of amyloid fibrils.
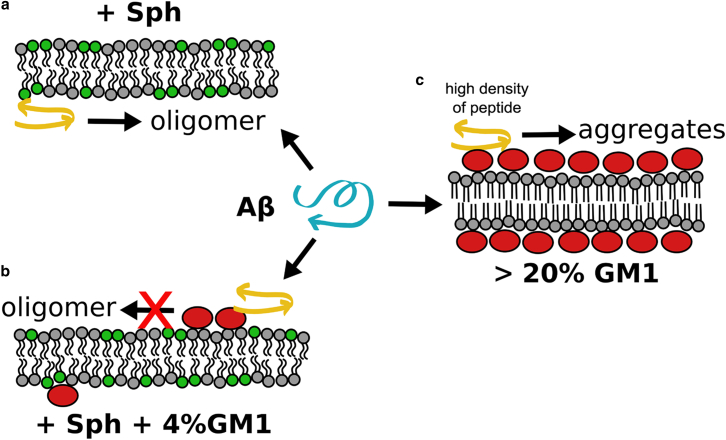
In the particular case of Aβ, the formation of toxic oligomers can be differently influenced by different local GM1 organization (Fig. 3), although the key event is the affinity of Aβ toward the ganglioside. The static high-density GM1 platforms can act as a surface that catalyzes oligomerization due to the enhanced adsorption of Aβ. On the contrary, loose and dynamic low-density GM1 nanoclusters can inhibit the formation of oligomers by sequestering the peptide at the surface of lipid membranes via a specific molecular interaction between Aβ and the ganglioside. Seemingly, Aβ binding to GM1 is more favorable than Aβ binding with itself. This is supported by the observation that GM1 might even disrupt preformed dimers (52). Thus, well-accessible and dynamic GM1 molecules act as an inhibitor of membrane-mediated Aβ oligomerization, at least at low (nanomolar) local concentrations of the peptide.
Figure 3.
Formation of toxic Aβ oligomers can be differently influenced by different local GM1 organization. Proposed model: (a) and (b) depict the inhibitory effect of low concentrations of GM1, organized in low-density and dynamic nanoclusters, on the membrane-mediated oligomerization of Aβ (membrane-bound peptide is symbolized by the yellow arrows). (a) Membrane-mediated oligomerization of low nanomolar solution concentrations of Aβ (Aβ/total lipid ratios of 1:4000, at maximum) is triggered by the presence of sphingomyelin (green lipids; print version: light gray) (25). (b) Binding of Aβ to the headgroup of GM1 (red ellipses) sequesters the peptide and prevents it from oligomerizing (25). (c) High concentrations of GM1, organized in high-density platforms of slow dynamics, create a surface that facilitates the adsorption and local concentration of Aβ. At high Aβ densities, oligomerization of the peptide is promoted (42, 43), which is a generic effect also observed for other surfaces (54, 55). Thus, in the low GM1 concentration regime (also low local Aβ concentration) the ganglioside acts as an inhibitor of membrane-mediated oligomerization. In the high GM1 concentration regime—where the membrane is covered by GM1 (21)—the ganglioside can become a catalyst of oligomerization, depending on the amount of Aβ on the surface of the GM1 platform. To see this figure in color, go online.
In cells, GM1 was detected in small low-density clusters in the flat areas of the plasma membrane (31, 59). These may represent the loose and dynamic nanoclusters in which GM1 interacts with Aβ to prevent its oligomerization. Plasma membrane invaginations and intracellular vesicles exhibit important heterogeneity in local GM1 concentration, as observed by indirect labeling (CTxB or antibodies) (35, 36). Among those are densely labeled invaginations and vesicles (see Figs. 1, 3, 4, 5, and 6 in (35) or Fig. 4 in (36)) that could contain the static, high-density GM1 platforms, which have the potential to promote Aβ aggregation.
Conclusion
In the previous sections we summarized current knowledge on the effect of different GM1 organization on Aβ oligomerization and, probably, development of AD. The data obtained using model membranes, cultured cells, and in vivo studies indicate that GM1 can have, at least, two alternative effects on the oligomerization of the amyloid peptide: catalytic and inhibitory. The prevalent function is probably regulated by the local organization of the ganglioside in the plasma membrane, or intracellular membrane compartments, and its expression in neuronal cells.
Several studies suggest that changes in the metabolism of gangliosides are associated with aging and AD (60, 61, 62, 63), with GM1 and GD1a seemingly being the most affected species (62, 63). In addition, Aβ peptides were shown to modulate the function of enzymes involved in lipid biosynthesis and degradation (64). Such changes can influence GM1 levels and organization, and affect the capacity of cells to prevent the oligomerization of Aβ. The finding that GM1 has an inhibitory effect on the formation of Aβ oligomers and the fact that GM1 concentration was shown to decrease in aged brains and AD patients suggests that decreasing GM1 levels (or its relocalization) could lead to reduced protection against the formation of toxic Aβ oligomers and thus contribute to the onset of AD.
Gangliosides also interact with several membrane proteins (e.g., integrins (28)) and are recognized by a variety of ligands, including surface molecules of pathogens (e.g., SV40 virus envelope or cholera toxin). Such events frequently lead to the internalization of gangliosides (38), which could induce the accumulation of GM1 in endosomes and the formation of GM1 platforms to promote Aβ oligomerization (46). To date, these processes were extensively characterized using biochemical and basic imaging approaches.
For a better understanding of the dual role of GM1, high-resolution imaging and dynamic analysis focused on GM1 and Aβ in neuronal cells will be required. An updated description of the distribution of GM1 in neuronal cells using state-of-the-art technology is necessary to understand better the connection between the ganglioside(s) and disease progression. In addition, model membranes and molecular dynamics simulations will remain valuable tools to dissect the role of GM1, and other lipids, in Aβ oligomerization under controlled conditions.
Even though multiple factors are likely to participate in AD, the involvement of gangliosides has been documented since the early 1980s. The efforts to understand the molecular details behind the role of gangliosides in neurodegenerative amyloidoses are a crucial task on the road to developing disease-modifying treatments. For example, currently the GM1-Aβ affinity is being positively explored to create a GM1-based therapy to clear Aβ from brain (65, 66).
Author Contributions
M.C., M.H., and M.A. have contributed to the writing of the article.
Acknowledgments
The authors acknowledge financial support from the Czech Science Foundation (grant No. 17-03160S). M.H. acknowledges the Academy of Sciences for the Praemium Academie award and M.C. acknowledges the Purkyne Fellowship.
Editor: Anne Kenworthy.
Contributor Information
Martin Hof, Email: hof@jh-inst.cas.cz.
Mariana Amaro, Email: amaro@jh-inst.cas.cz.
References
- 1.Selkoe D.J., Hardy J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol. Med. 2016;8:595–608. doi: 10.15252/emmm.201606210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Viola K.L., Klein W.L. Amyloid β oligomers in Alzheimer’s disease pathogenesis, treatment, and diagnosis. Acta Neuropathol. 2015;129:183–206. doi: 10.1007/s00401-015-1386-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Selkoe D.J. Cell biology of protein misfolding: the examples of Alzheimer’s and Parkinson’s diseases. Nat. Cell Biol. 2004;6:1054–1061. doi: 10.1038/ncb1104-1054. [DOI] [PubMed] [Google Scholar]
- 4.Zhang Y.-J., Shi J.-M., Ji S.-R. Intra-membrane oligomerization and extra-membrane oligomerization of amyloid-β peptide are competing processes as a result of distinct patterns of motif interplay. J. Biol. Chem. 2012;287:748–756. doi: 10.1074/jbc.M111.281295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koo E.H., Squazzo S.L. Evidence that production and release of amyloid beta-protein involves the endocytic pathway. J. Biol. Chem. 1994;269:17386–17389. [PubMed] [Google Scholar]
- 6.Cirrito J.R., Kang J.-E., Holtzman D.M. Endocytosis is required for synaptic activity-dependent release of amyloid-β in vivo. Neuron. 2008;58:42–51. doi: 10.1016/j.neuron.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amaro M., Birch D.J.S., Rolinski O.J. β-amyloid oligomerisation monitored by intrinsic tyrosine fluorescence. Phys. Chem. Chem. Phys. 2011;13:6434–6441. doi: 10.1039/c0cp02652b. [DOI] [PubMed] [Google Scholar]
- 8.Narayan P., Orte A., Klenerman D. The extracellular chaperone clusterin sequesters oligomeric forms of the amyloid-β (1-40) peptide. Nat. Struct. Mol. Biol. 2011;19:79–83. doi: 10.1038/nsmb.2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vácha R., Linse S., Lund M. Surface effects on aggregation kinetics of amyloidogenic peptides. J. Am. Chem. Soc. 2014;136:11776–11782. doi: 10.1021/ja505502e. [DOI] [PubMed] [Google Scholar]
- 10.Zhu D., Bungart B.L., Askarova S. Role of membrane biophysics in Alzheimer’s-related cell pathways. Front. Neurosci. 2015;9:186. doi: 10.3389/fnins.2015.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ariga T., McDonald M.P., Yu R.K. Role of ganglioside metabolism in the pathogenesis of Alzheimer’s disease—a review. J. Lipid Res. 2008;49:1157–1175. doi: 10.1194/jlr.R800007-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yanagisawa K. GM1 ganglioside and Alzheimer’s disease. Glycoconj. J. 2015;32:87–91. doi: 10.1007/s10719-015-9579-5. [DOI] [PubMed] [Google Scholar]
- 13.Tettamanti G., Anastasia L. Chemistry, tissue and cellular distribution, and developmental profiles of neural sphingolipids. In: Lajtha A., Tettamanti G., Goracci G., editors. Handbook of Neurochemistry and Molecular Neurobiology. Springer; Boston, MA: 2010. pp. 99–171. [Google Scholar]
- 14.Matsuzaki K., Kato K., Yanagisawa K. Aβ polymerization through interaction with membrane gangliosides. Biochim. Biophys. Acta. 2010;1801:868–877. doi: 10.1016/j.bbalip.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 15.Matsuzaki K. How do membranes initiate Alzheimer’s disease? Formation of toxic amyloid fibrils by the amyloid β-protein on ganglioside clusters. Acc. Chem. Res. 2014;47:2397–2404. doi: 10.1021/ar500127z. [DOI] [PubMed] [Google Scholar]
- 16.Mocchetti I. Exogenous gangliosides, neuronal plasticity and repair, and the neurotrophins. Cell. Mol. Life Sci. 2005;62:2283–2294. doi: 10.1007/s00018-005-5188-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kreutz F., Frozza R.L., Trindade V.M.T. Amyloid-β induced toxicity involves ganglioside expression and is sensitive to GM1 neuroprotective action. Neurochem. Int. 2011;59:648–655. doi: 10.1016/j.neuint.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 18.Kreutz F., Scherer E.B., Trindade V.M.T. Alterations on Na+,K+-ATPase and acetylcholinesterase activities induced by amyloid-β peptide in rat brain and GM1 ganglioside neuroprotective action. Neurochem. Res. 2013;38:2342–2350. doi: 10.1007/s11064-013-1145-6. [DOI] [PubMed] [Google Scholar]
- 19.Sokolova T.V., Zakharova I.O., Avrova N.F. Neuroprotective effect of ganglioside GM1 on the cytotoxic action of hydrogen peroxide and amyloid β-peptide in PC12 cells. Neurochem. Res. 2007;32:1302–1313. doi: 10.1007/s11064-007-9304-2. [DOI] [PubMed] [Google Scholar]
- 20.Yang R., Wang Q., Liu X. Monosialoanglioside improves memory deficits and relieves oxidative stress in the hippocampus of rat model of Alzheimer’s disease. Neurol. Sci. 2013;34:1447–1451. doi: 10.1007/s10072-012-1263-y. [DOI] [PubMed] [Google Scholar]
- 21.Sagle L.B., Ruvuna L.K., Van Duyne R.P. Single plasmonic nanoparticle tracking studies of solid supported bilayers with ganglioside lipids. J. Am. Chem. Soc. 2012;134:15832–15839. doi: 10.1021/ja3054095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yuan C., Furlong J., Johnston L.J. The size of lipid rafts: an atomic force microscopy study of ganglioside GM1 domains in sphingomyelin/DOPC/cholesterol membranes. Biophys. J. 2002;82:2526–2535. doi: 10.1016/S0006-3495(02)75596-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shi J., Yang T., Cremer P.S. GM1 clustering inhibits cholera toxin binding in supported phospholipid membranes. J. Am. Chem. Soc. 2007;129:5954–5961. doi: 10.1021/ja069375w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sachl R., Amaro M., Hof M. On multivalent receptor activity of GM1 in cholesterol containing membranes. Biochim. Biophys. Acta. 2015;1853:850–857. doi: 10.1016/j.bbamcr.2014.07.016. [DOI] [PubMed] [Google Scholar]
- 25.Amaro M., Šachl R., Hof M. GM1 ganglioside inhibits β-amyloid oligomerization induced by sphingomyelin. Angew. Chem. Int. Ed. Engl. 2016;55:9411–9415. doi: 10.1002/anie.201603178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Štefl M., Šachl R., Hof M. Dynamics and size of cross-linking-induced lipid nanodomains in model membranes. Biophys. J. 2012;102:2104–2113. doi: 10.1016/j.bpj.2012.03.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kopitz J., von Reitzenstein C., Gabius H.-J. Galectin-1 is a major receptor for ganglioside GM1, a product of the growth-controlling activity of a cell surface ganglioside sialidase, on human neuroblastoma cells in culture. J. Biol. Chem. 1998;273:11205–11211. doi: 10.1074/jbc.273.18.11205. [DOI] [PubMed] [Google Scholar]
- 28.Wu G., Lu Z.-H., Ledeen R.W. Induction of calcium influx through TRPC5 channels by cross-linking of GM1 ganglioside associated with α5β1 integrin initiates neurite outgrowth. J. Neurosci. 2007;27:7447–7458. doi: 10.1523/JNEUROSCI.4266-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ichikawa N., Iwabuchi K., Arikawa-Hirasawa E. Binding of laminin-1 to monosialoganglioside GM1 in lipid rafts is crucial for neurite outgrowth. J. Cell Sci. 2009;122:289–299. doi: 10.1242/jcs.030338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ewers H., Römer W., Johannes L. GM1 structure determines SV40-induced membrane invagination and infection. Nat. Cell Biol. 2010;12:11–18. doi: 10.1038/ncb1999. 1–12. [DOI] [PubMed] [Google Scholar]
- 31.Fujita A., Cheng J., Fujimoto T. Gangliosides GM1 and GM3 in the living cell membrane form clusters susceptible to cholesterol depletion and chilling. Mol. Biol. Cell. 2007;18:2112–2122. doi: 10.1091/mbc.E07-01-0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fujita A., Cheng J., Fujimoto T. Segregation of GM1 and GM3 clusters in the cell membrane depends on the intact actin cytoskeleton. Biochim. Biophys. Acta. 2009;1791:388–396. doi: 10.1016/j.bbalip.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 33.Mahfoud R., Manis A., Lingwood C.A. A major fraction of glycosphingolipids in model and cellular cholesterol-containing membranes is undetectable by their binding proteins. J. Biol. Chem. 2010;285:36049–36059. doi: 10.1074/jbc.M110.110189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kiyokawa E., Baba T., Kobayashi T. Spatial and functional heterogeneity of sphingolipid-rich membrane domains. J. Biol. Chem. 2005;280:24072–24084. doi: 10.1074/jbc.M502244200. [DOI] [PubMed] [Google Scholar]
- 35.Parton R.G. Ultrastructural localization of gangliosides; GM1 is concentrated in caveolae. J. Histochem. Cytochem. 1994;42:155–166. doi: 10.1177/42.2.8288861. [DOI] [PubMed] [Google Scholar]
- 36.Möbius W., Herzog V., Schwarzmann G. Intracellular distribution of a biotin-labeled ganglioside, GM1, by immunoelectron microscopy after endocytosis in fibroblasts. J. Histochem. Cytochem. 1999;47:1005–1014. doi: 10.1177/002215549904700804. [DOI] [PubMed] [Google Scholar]
- 37.Rajendran L., Simons K. Lipid rafts and membrane dynamics. J. Cell Sci. 2005;118:1099–1102. doi: 10.1242/jcs.01681. [DOI] [PubMed] [Google Scholar]
- 38.Pang H., Le P.U., Nabi I.R. Ganglioside GM1 levels are a determinant of the extent of caveolae/raft-dependent endocytosis of cholera toxin to the Golgi apparatus. J. Cell Sci. 2004;117:1421–1430. doi: 10.1242/jcs.01009. [DOI] [PubMed] [Google Scholar]
- 39.Waschuk S.A., Elton E.A., McLaurin J.A. Cellular membrane composition defines Aβ-lipid interactions. J. Biol. Chem. 2001;276:33561–33568. doi: 10.1074/jbc.M103598200. [DOI] [PubMed] [Google Scholar]
- 40.Kakio A., Nishimoto S., Matsuzaki K. Interactions of amyloid β-protein with various gangliosides in raft-like membranes: importance of GM1 ganglioside-bound form as an endogenous seed for Alzheimer amyloid. Biochemistry. 2002;41:7385–7390. doi: 10.1021/bi0255874. [DOI] [PubMed] [Google Scholar]
- 41.Kim S.-I., Yi J.-S., Ko Y.-G. Amyloid β oligomerization is induced by brain lipid rafts. J. Cell. Biochem. 2006;99:878–889. doi: 10.1002/jcb.20978. [DOI] [PubMed] [Google Scholar]
- 42.Ogawa M., Tsukuda M., Matsuzaki K. Ganglioside-mediated aggregation of amyloid β-proteins (Aβ): comparison between Aβ-(1-42) and Aβ-(1-40) J. Neurochem. 2011;116:851–857. doi: 10.1111/j.1471-4159.2010.06997.x. [DOI] [PubMed] [Google Scholar]
- 43.Ikeda K., Yamaguchi T., Matsuzaki K. Mechanism of amyloid β-protein aggregation mediated by GM1 ganglioside clusters. Biochemistry. 2011;50:6433–6440. doi: 10.1021/bi200771m. [DOI] [PubMed] [Google Scholar]
- 44.Yamamoto N., Matsubara T., Yanagisawa K. Age-dependent high-density clustering of GM1 ganglioside at presynaptic neuritic terminals promotes amyloid β-protein fibrillogenesis. Biochim. Biophys. Acta. 2008;1778:2717–2726. doi: 10.1016/j.bbamem.2008.07.028. [DOI] [PubMed] [Google Scholar]
- 45.Matsuzaki K., Horikiri C. Interactions of amyloid β-peptide (1-40) with ganglioside-containing membranes. Biochemistry. 1999;38:4137–4142. doi: 10.1021/bi982345o. [DOI] [PubMed] [Google Scholar]
- 46.Hu X., Crick S.L., Lee J.-M. Amyloid seeds formed by cellular uptake, concentration, and aggregation of the amyloid-β peptide. Proc. Natl. Acad. Sci. USA. 2009;106:20324–20329. doi: 10.1073/pnas.0911281106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gylys K.H., Fein J.A., Cole G.M. Increased cholesterol in Aβ-positive nerve terminals from Alzheimer’s disease cortex. Neurobiol. Aging. 2007;28:8–17. doi: 10.1016/j.neurobiolaging.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 48.Manna M., Mukhopadhyay C. Binding, conformational transition and dimerization of amyloid-β peptide on GM1-containing ternary membrane: insights from molecular dynamics simulation. PLoS One. 2013;8:e71308. doi: 10.1371/journal.pone.0071308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Devarajan S., Sharmila J.S. Molecular dynamics study of GM1 ganglioside complex with amyloid β peptide (Aβ42) in lipid membrane. J. Mol. Liq. 2014;195:59–64. [Google Scholar]
- 50.Valdes-Gonzalez T., Inagawa J., Ido T. Neuropeptides interact with glycolipid receptors: a surface plasmon resonance study. Peptides. 2001;22:1099–1106. doi: 10.1016/s0196-9781(01)00432-6. [DOI] [PubMed] [Google Scholar]
- 51.Mandal P.K., Pettegrew J.W. Alzheimer’s disease: NMR studies of asialo (GM1) and trisialo (GT1b) ganglioside interactions with Aβ (1-40) peptide in a membrane mimic environment. Neurochem. Res. 2004;29:447–453. doi: 10.1023/b:nere.0000013750.80925.25. [DOI] [PubMed] [Google Scholar]
- 52.Hong S., Ostaszewski B.L., Selkoe D.J. Soluble Aβ oligomers are rapidly sequestered from brain ISF in vivo and bind GM1 ganglioside on cellular membranes. Neuron. 2014;82:308–319. doi: 10.1016/j.neuron.2014.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Linse S., Cabaleiro-Lago C., Dawson K.A. Nucleation of protein fibrillation by nanoparticles. Proc. Natl. Acad. Sci. USA. 2007;104:8691–8696. doi: 10.1073/pnas.0701250104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Giacomelli C.E., Norde W. Conformational changes of the amyloid β-peptide (1-40) adsorbed on solid surfaces. Macromol. Biosci. 2005;5:401–407. doi: 10.1002/mabi.200400189. [DOI] [PubMed] [Google Scholar]
- 55.Ryu J., Joung H.A., Park C.B. Surface plasmon resonance analysis of Alzheimer’s β-amyloid aggregation on a solid surface: from monomers to fully-grown fibrils. Anal. Chem. 2008;80:2400–2407. doi: 10.1021/ac7019514. [DOI] [PubMed] [Google Scholar]
- 56.Minton A.P. Effects of excluded surface area and adsorbate clustering on surface adsorption of proteins I. Equilibrium models. Biophys. Chem. 2000;86:239–247. doi: 10.1016/s0301-4622(00)00151-4. [DOI] [PubMed] [Google Scholar]
- 57.Minton A.P. Effects of excluded surface area and adsorbate clustering on surface adsorption of proteins. II. Kinetic models. Biophys. J. 2001;80:1641–1648. doi: 10.1016/S0006-3495(01)76136-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Byström R., Aisenbrey C., Gröbner G. Disordered proteins: biological membranes as two-dimensional aggregation matrices. Cell Biochem. Biophys. 2008;52:175–189. doi: 10.1007/s12013-008-9033-4. [DOI] [PubMed] [Google Scholar]
- 59.Janich P., Corbeil D. GM1 and GM3 gangliosides highlight distinct lipid microdomains within the apical domain of epithelial cells. FEBS Lett. 2007;581:1783–1787. doi: 10.1016/j.febslet.2007.03.065. [DOI] [PubMed] [Google Scholar]
- 60.Crino P.B., Ullman M.D., Volicer L. Brain gangliosides in dementia of the Alzheimer type. Arch. Neurol. 1989;46:398–401. doi: 10.1001/archneur.1989.00520400054019. [DOI] [PubMed] [Google Scholar]
- 61.Kracun I., Rosner H., Lauc G. Human brain gangliosides in development, aging and disease. Int. J. Dev. Biol. 1991;35:289–295. [PubMed] [Google Scholar]
- 62.Svennerholm L., Boström K., Olsson L. Membrane lipids of adult human brain: lipid composition of frontal and temporal lobe in subjects of age 20 to 100 years. J. Neurochem. 1994;63:1802–1811. doi: 10.1046/j.1471-4159.1994.63051802.x. [DOI] [PubMed] [Google Scholar]
- 63.Svennerholm L., Gottfries C.-G. Membrane lipids, selectively diminished in Alzheimer brains, suggest synapse loss as a primary event in early-onset form (type I) and demyelination in late-onset form (type II) J. Neurochem. 1994;62:1039–1047. doi: 10.1046/j.1471-4159.1994.62031039.x. [DOI] [PubMed] [Google Scholar]
- 64.Grimm M.O.W., Grimm H.S., Hartmann T. Regulation of cholesterol and sphingomyelin metabolism by amyloid-β and presenilin. Nat. Cell Biol. 2005;7:1118–1123. doi: 10.1038/ncb1313. [DOI] [PubMed] [Google Scholar]
- 65.Huang M., Hu M., Gao X. GM1-modified lipoprotein-like nanoparticle: multifunctional nanoplatform for the combination therapy of Alzheimer’s disease. ACS Nano. 2015;9:10801–10816. doi: 10.1021/acsnano.5b03124. [DOI] [PubMed] [Google Scholar]
- 66.Yuyama K., Sun H., Igarashi Y. Decreased amyloid-β pathologies by intracerebral loading of glycosphingolipid-enriched exosomes in Alzheimer model mice. J. Biol. Chem. 2014;289:24488–24498. doi: 10.1074/jbc.M114.577213. [DOI] [PMC free article] [PubMed] [Google Scholar]