Abstract
The concept of lipoprotein mimetics was developed and extensively tested in the last three decades. Most lipoprotein mimetics were designed to recreate one or several functions of high-density lipoprotein in context of cardiovascular disease, however, the application of this approach are much broader. Lipoprotein mimetics should not just be seen as a set of compounds aimed at replenishing a deficiency or dysfunctionality of individual elements of lipoprotein metabolism but rather as a designer concept with remarkable flexibility and numerous applications in medicine and biology. In this review, we discuss the fundamental design principles used to create lipoprotein mimetics, mechanisms of their action, medical indications and efficacy in animal models and human studies.
Keywords: Lipoprotein metabolism, apolipoproteins, cholesterol metabolism, atherosclerosis, peptides
Introduction
Lipoproteins are macromolecular complexes of various lipids and proteins found in most organisms. In vertebrates, the principal function of lipoproteins is the systemic transport of lipids, which otherwise would be insoluble in aqueous solutions. Impaired lipoprotein metabolism plays a key role in pathogenesis of many diseases. A classical example is familial hypercholesterolemia, a condition causing severe atherosclerosis at early age; however, disturbances in lipid and lipoprotein metabolism are key elements in pathogenesis of a diverse range of diseases from diabetes, obesity to cancer and infectious diseases. Hence, a concept has emerged to correct what’s missing or broken by designing mimetics of individual components of the various lipoprotein metabolism pathways. However, with time this concept progressed well beyond simple replenishment of a defective element of lipoprotein metabolism.
In humans, lipoprotein metabolism is comprised of two interconnected, but distinctive arms. Apolipoprotein B (apoB) containing lipoproteins (chylomicrons, very low density lipoprotein (VLDL) and low density lipoprotein (LDL)) deliver lipids, mainly triglycerides (TG) and cholesterol, from intestine and liver to other tissues, predominantly to supply TG-derived fatty acids as a source of energy. The current paradigm is that, at least in context of cardiovascular disease, excess of apoB-containing lipoproteins leads deposition of cholesterol in the vessel wall and development of atherosclerosis, thus, potential mimetics should have a capacity to reduce levels of apoB-containing lipoproteins by e.g. enhancing their catabolism. In contrast, apolipoprotein A-I (apoA-I) containing lipoprotein (high-density lipoprotein (HDL)), removes excessive lipids, predominantly cholesterol, from most tissues delivering it to the liver, the only organ capable of catabolism and excretion of cholesterol. Because a high level of apoA-I containing lipoproteins is considered beneficial, potential mimetics based on HDL should have a capacity to substitute, supplement or functionally improve apoA-I containing lipoproteins. Conceptually, adding and supplementing is an easier task and the majority of current lipoprotein mimetics are based on apoA-I-containing lipoproteins.
In addition to playing a central role in reverse cholesterol transport (RCT), HDL has several other functions (for review see [1, 2]). The best investigated function is anti-inflammatory property of HDL, affecting both local inflammation (such as expression of adhesion molecules on endothelium) and systemic inflammation (such as expression of adhesion molecules and cytokine secretion by monocytes). Furthermore, HDL displays anti-oxidant, anti-thrombotic and anti-apoptotic functions, improves vascular function and prevents aggregation of LDL. Some of these properties are most likely a consequence of the ability of HDL to prevent cholesterol accumulation in the cells, i.e. depend on its role in RCT, but others are not. It is important to recognize that HDL is not a simple complex of apolipoproteins and lipids, but also carries a large number of enzymes, biologically active proteins and miRNAs [3, 4] that regulate various aspects of cellular and systemic metabolism independently from reverse cholesterol transport. Consequently, as will be discussed below, the concept of lipoprotein mimetics is now much broader with applications beyond atherosclerosis.
Lipoproteins are natural constituents of human plasma; isolating lipoproteins and separating their elements can be easily achieved using routine methodology. CSL, owing its unique access to large quantities of human plasma and appropriate technology, uses apoA-I purified from human plasma to create HDL mimetics (CSL111 and CSL112). However, most of the mimetics are fully synthetic for the following reasons:
Cost and access to human plasma makes feasibility of large scale production of a mimetic-based drug problematic
The requirements for consistency and ensuring that human plasma product is infection- and endotoxin- free are difficult and/or costly to fulfil
Synthetic analogues provide considerable flexibility in modifying the structure of a mimetic, allowing for e.g. adaptation for oral administration or targeting a specific function of a lipoprotein
Use of natural products creates complexity in IP protection
Lipoprotein mimetics have been used for numerous potential indications (Table 1). Majority of studies describe use of lipoprotein mimetics in context of cardiovascular disease. The second most frequent indication is cancer. HDL has remarkable anti-cancer properties, which were reproduced with HDL mimetics [5–7]. ApoA-I mimetics have also been used to reduce obesity and diabetes [8]. ApoA-I and apoE mimetic peptides were shown to reduce amyloid burden in animal and cellular models of Alzheimer’s disease [9, 10]. In context of infectious diseases, apoA-I mimetic peptides reduced inflammation caused by an infection [11] and provided considerable multiple organ protection in sepsis [12]. Anti-inflammatory and anti-oxidation properties of HDL were behind the ability of apoA-I mimetics to reduce rejection after heart transplantation [13], alleviate asthma [14] and treat experimental arthritis [15]. ApoA-I mimetics were also effective in preventing cardiac ischemic/reperfusion injury [16] and were able to rescue pulmonary hypertension [17]. Finally, lipoprotein mimetics were used as carriers for targeted delivery of drugs [18] and contrast agents [19] to atherosclerotic plaques and cancer cells [20]. It must be recognized however, that most of these findings were generated in cellular and animal models and have not yet been translated into clinical trials.
Table 1.
Indications and mechanisms of action of lipoprotein mimetics
| Indications | Mimetic type | Mimetic name | Mechanisms | References |
|---|---|---|---|---|
|
| ||||
| Atherosclerosis | • HDL mimetic • apoA-I mimetic • apo E mimetic • apoC-II mimetic • Cyclodextrin • PL vesicles • Nanoparticles |
ETC-216, CSL111, CSL112, CER-001, 4F, 5A, ELKs ATI-5261, fmHDL | • Enhancing RCT by supplementing or modifying HDL | [47, 52, 53, 55, 57] |
| • Anti-inflammatory | [12, 47, 66] | |||
| • Anti-oxidant | [47, 70–72] | |||
| • Anti-apoptosis | [73] | |||
| • Stimulation NO production | [74, 75, 77] | |||
| • Anti-thrombotic | [78] | |||
| • Inhibition of LDL aggregation | [79] | |||
| • Activation LPL and reducing TG | [37] | |||
| • Binding of oxidized lipids in intestine | [80] | |||
|
| ||||
| Cancer | • HDL mimetic | HDL-NPs | Starving of cholesterol | [6] |
| • HDL mimetic | Drug delivery | [20] | ||
| • apoA-I mimetic | L-4F | Scavenging lysophosphatidic acid | [7] | |
|
| ||||
| Diabetes | apoA-I mimetic | L-4F | Phosphorylation of insulin receptor | [8] |
|
| ||||
| Obesity | apoA-I mimetic | L-4F | Upregulation of heme oxygenase-1 | [8] |
|
| ||||
| Alzheimer’s | • apoA-I mimetic | D-4F | Anti-inflammatory | [9] |
| • apoE mimetic | Ac-hE18A-NH2 | Anti-oxidation | ||
|
| ||||
| Infection | apoA-I mimetic | D-4F | Anti-inflammatory | [11, 12] |
|
| ||||
| Transplantation | apoA-I mimetic | D-4F | Anti-inflammatory (upregulation of heme oxygenase-1) | [13] |
|
| ||||
| Astma | apoA-I mimetic | D-4F | Anti-inflammatory, anti-oxidation | [14] |
|
| ||||
| Arthritis | apoA-I mimetic | D-4F | Anti-inflammatory | [15] |
|
| ||||
| Sepsis | apoA-I mimetic | 4F | Anti-inflammatory | [68] |
|
| ||||
| Cardioprotection | apoA-I mimetic | 37pA | Anti-inflammatory, anti-oxidation | [16] |
|
| ||||
| Pulmonary hypertension | apoA-I mimetic | D-4F | Restoration of miR193 expression | [17] |
|
| ||||
| Drug delivery | HDL-mimetics | HDL-NPs | Targeted drug delivery to macrophages/plaques | [18, 19] |
General design principles for lipoprotein mimetics
Several design principles for lipoprotein mimetics were proposed over the years (Table 2). A straight forward concept to design a lipoprotein mimetic is to recreate structural and functional properties of the native particle in its entirety or at least as close as possible, a supplementing/replacement therapy approach. Reconstituted HDL (rHDL) is the most advanced example of such approach. RHDL consists of full-length wild type mature human apoA-I complexed to phospholipids forming a discoidal particle. Composition and physical properties of rHDL are similar to that of nascent HDL; therefore, they are expected to enter HDL metabolism cycle recreating most of the biological properties of endogenous HDL. Currently, two rHDL mimetics are undergoing trials: CSL112 [21], like its predecessor CSL111[22], incorporates apoA-I isolated from human plasma, while CER-001 incorporates recombinant apoA-I [23].
Table 2.
General design principles of lipoprotein mimetics.
| Design principle | Type of mimetic | Name | What is mimicked | References |
|---|---|---|---|---|
|
| ||||
| Recreate native LP particle | rHDL | ETC-216, CSL111, CSL112, CER-001 | All properties of nascent HDL | [21–23] |
|
| ||||
| Recreate “improved” LP particle | • ApoA-IMilano | ApoA-IMilano | All “improved” properties of mutated HDL | [24] |
| • Trimerized apoA-I | TripA | Preserving properties of HDL, but retarding catabolism leading to higher HDL levels | [26–28] | |
| • Pegylated HDL | PegHDL | [29] | ||
|
| ||||
| Recreate primary structure of an apolipoprotein | • apoA-I peptide | FAMP5 | Lipid-binding domain of apoA-I | [30] |
| • apoA-I peptide | S1A10, S2A10 | ABCA1-interacting domain, stapled | [32] | |
| • apoE peptide | Ac-hE-18A-NH2, L-mR18L | Receptor-binding & lipid binding domains of apoE and apoA-I | [34, 35] | |
| • apoE peptide | ATI-5261 | ABCA1-interacting domain of apoE | [31] | |
| • apoE peptide | EpK | Receptor-binding & lipid binding domains of apoE and apoA-I | [36] | |
| • apoC-II peptide | C-II-a | LPL activation domain of apoC-II | [37] | |
|
| ||||
| Recreate secondary structure of an apolipoprotein | Mono- and bi-helical peptides containing amphypatic αhelix | 18A, 37pA, D-4F, L-4F, 5A, 5A-CH, ETC-642, ELK-2A2K2E, ELKs | Ability of amphipathic proteins to bind lipoproteins, to promote cholesterol efflux from cells, anti-oxidant and anti-inflammatory properties. | [39–42, 44, 45, 47, 90] |
|
| ||||
| Recreate a function | Cyclodextrins | MBC | Ability of HDL to promote cholesterol efflux | [48] |
| Phospholipid vesicles | SUV, LUV | [49] | ||
|
| ||||
| Recreating LP size and vector properties | Lipid nanoparticles | TPP-HDL-apoA-I-QD NP, | Ability of HDL to promote cholesterol efflux and to deliver hydrophobic substances into the cells | [19] |
| Gold nanoparticles | fmHDL, HDL Au NP, HDL-NP | [6, 50, 51] | ||
Recreation of the lipoprotein structure in vitro creates opportunities to improve functional properties of mimetics over natural product. The first attempt was apoA-IMilano –based rHDL (also known as ETC-216). ApoA-IMilano is a naturally occurring mutant of apoA-I carrying a substitution of Cys for Arg173; apoA-IMilano molecules form dimers [24] and may have better antioxidant properties [25]. Carriers of apoA-IMilano have low levels of HDL due to enhanced HDL catabolism; however, this was not accompanied by higher risk of CVD, leading to an assumption that apoA-IMilano based HDL has enhanced atheroprotective functionality. Despite intensive work this assumption, it has not been convincingly proven and reasons why carriers of apoA-IMilano have low cardiovascular risk are still not entirely clear.
Another approach to improve properties of rHDL was to base it on recombinant trimer of apoA-I creating rHDL with functional properties similar to WT apoA-I-based rHDL, but with reduced plasma clearance rate [26, 27]. Trimeric-rHDL was more effective than rHDL in slowing development of atherosclerosis in animal model [28]. In another study, pegylation of rHDL also retarded its catabolism without adversely affecting functional properties, leading to a greater reduction in development of atherosclerosis and plaque stabilization in animal model [29].
The most common approach for the design for lipoprotein mimetics involves the use of synthetic peptides. Two design approaches have been widely used, namely peptides mimicking primary structure of an apolipoprotein and peptides mimicking its secondary structure without homology to the primary structure. Uehara et al described a single helix peptide (FAMP5) with high degree of homology to C-terminus of apoA-I [30], while Bielicki et al described a peptide (ATI-5261) homologous to the C-terminus of apoE [31]. Both peptides mimicked the lipid-binding domains of the corresponding apolipoproteins, aiming to recreating their cholesterol efflux properties and indeed both peptides were potent in cholesterol efflux assay. Sviridov et al further developed this design by describing two bi-helical peptides (S1A10 and S2A10) based on homology to C-terminus of apoA-I with hydrocarbon “staples” introduced to connect turns of the helices. “Stapling” resulted in a dramatic enhancement of the ability of the peptides to promote cholesterol efflux and made them resistant to proteases [32]. Zhao et al attached three peptides mimicking C-terminus of apoA-I to a small organic scaffold; this complex remained active after both intraperitoneal and oral administration, indicating protease resistance [33]. Similar design principle was used in studies where a mimetic combined several different elements: a peptide capable of stimulating cholesterol efflux and/or anchoring to lipoproteins and another peptide facilitating a subsequent stage of lipoprotein metabolism. The objective of such design was to create a mimetic capable of advancing its cargo along the pathway of lipoprotein metabolism. Anantharamaiah et al described conjugates of apoA-I mimetic peptide, active in promoting cholesterol efflux from cells, with a mimetic peptide reproducing the sequence of the receptor-binding domain of apoE, active in delivering cholesterol to the liver (Ac-hE-18A-NH2 and L-mR18L) [34, 35]. Similar approach was used by Zhao et al, who described a peptide (EpK) containing lipid binding domain and LDL-receptor binding domain [36]. Amar et al described a peptide (C-II-a) which is conjugate of an apoA-I mimetic, to anchor peptide to a lipoprotein particle, and a apoC-II mimetic, an activator of lipoprotein lipase (LPL) [37].
Many lipoprotein mimetic peptides, however, do not carry homology to the primary structure of apolipoproteins, instead just recreating its secondary structure, amphipathic α-helix. Most apolipoproteins contain such helices in their structure, which allows them to bind to the hydrophobic lipid core of lipoprotein and at the same time form hydrophilic surface of the particle. It was originally suggested by Segrest et al that this structural property of apolipoproteins is fundamental to many of their biological functions, including cholesterol efflux, and that peptides carrying the same structural elements would have similar biological properties [38]. This design principle laid foundation for the first apoA-I mimetic peptide, 18A (also known as 2F) [39]. Many peptides were created using this principle with modifications to their physiochemical properties. Most modifications aimed at further improving lipid-binding properties, and while significant variations in physiochemical properties of the peptides exist, strong lipid-binding property is not always beneficial. Subsequent developments of the peptide design included:
Introducing into the structure of a peptide several hydrophobic amino acids increasing their ability to bind lipids and to promote cholesterol efflux. This includes the most studied apolipoprotein mimetic, namely peptide 4F [40], which is an 18A based peptide with four Phe residues on the hydrophobic face, or ETC-642 [41].
Longer peptides with two amphipathic α-helixes connected by a proline residue to recreate secondary structure together with elements of tertiary structure of apoA-I, such as 37pA [42]. Bi-helical peptides were generally more potent than single helix peptides [43].
Bi-helical peptides with two helices of different hydrophobicity; this reduced damage peptides cause to the plasma membrane due their detergent properties. An example of this approach is peptide 5A [44].
Synthesizing peptides from D-amino acids to enable oral administration, such as D-4F [45]. Other reported methods to make peptides resistant to proteases allowing oral delivery are stapling of the peptides [32] and administering peptides in complex with niclosamide [46].
Introducing cysteine and histidine to boost anti-oxidant properties of the peptides (5A-CH) [47].
Changes in hydrophobicity, charge and connection angle between the two helices to boost lipid-binding and anti-inflammatory properties (ELKs) [47].
A different approach to mimetic design was to focus on function, rather than the structure. Cyclodextrins [48] and large and small unilamellar phospholipid vesicles (LUV and SUV) [49] are water soluble and are capable of removing cholesterol from cells, although mostly through non-specific mechanisms. The overall capacity of these mimetics to promote cholesterol efflux exceeds that of HDL and apoA-I, however, the non-specific (i.e. independent of any cholesterol transporters) nature of their activity and inability to advance their cargo along the lipoprotein metabolic pathways or pass it to other lipoproteins are severe limitations.
A recent development in lipoprotein mimetic design is nanoparticle mimetics. Gold nanoparticles covered with phospholipids and apoA-I were used to recreate the structure of HDL and promoted cholesterol efflux [50, 51] or as carriers for targeted drug delivery [6]. Nanoparticles consisting of lipid core resembling HDL and a vector (e.g. apoA-I mimetic peptide) were used as carriers for delivery of drugs and contrast agents, as well as to promote cholesterol efflux [19, 20]. Advantage of this design is that it allows considerable flexibility in adjusting the size of the particle, which is an important determinant of lipoprotein functionality, and offers scaffolding on which several functional elements can be mounted in different arrangements and combinations.
Mechanism of action
Lipoproteins perform many functions and synthetic mimetics were designed to recreate many of them, as well as to contrive new functions not performed by the native lipoproteins (Table 1).
Reverse cholesterol transport
The main intended mechanism of action of many lipoprotein mimetics, at least of HDL mimetics, is that they would enhance reverse cholesterol transport (RCT). That is, mimetics will take up excessive cholesterol from peripheral cells and deliver it to the liver either directly or after transferring it to other lipoproteins. There is evidence that this does occur with rHDL – in humans infusion of CSL111 and CSL112 stimulates the capacity of plasma to efflux cholesterol from cells in vitro and this precedes the rise in HDL level [52, 53]. Removal of apoB-containing lipoproteins from plasma eliminated improvements in cholesterol efflux after rHDL infusion, indicating that other elements of lipoprotein metabolism are required for this enhancement to work [52]. In rabbits, CSL112 increased the capacity of plasma to promote cholesterol efflux and stimulated cholesterol esterification [21]. One possible mechanism supported by these findings is that small rHDL particles assume role of a “shuttle” taking cholesterol from cells and delivering it to the “sink”, a pool of large lipoprotein particles taking up cholesterol from small rHDL and proceeding along RCT pathway [52]. However, two other mechanisms cannot be ruled out. First is that administered rHDL particles fuse with HDL particles of the recipient, leading to their remodelling and release of lipid-free or lipid-poor apoA-I, the latter being the main specific cholesterol acceptor. This mechanism is supported by significant increase of preβ1-HDL/free apoA-I level after infusions of rHDL [52, 53]. Second mechanism also involves fusion of rHDL with the recipient plasma HDL delivering phospholipids to these particles and thus improving their ability to accept cellular cholesterol. Overall, it is unlikely, however, that rHDL recreates a complete RCT pathway removing cholesterol from cells and delivering cholesterol to the liver on its own; most likely it stimulates few initial steps of the RCT then feeding cholesterol into the pathway of recipient.
Mimetic peptides, lipid-free or complexed with phospholipids, also successfully recreated the ability of HDL and apoA-I to promote cholesterol efflux in vitro [30–32, 54, 55]. However, a requirement to feed cholesterol taken up from cells into RCT may be a key limitation of compounds mimicking cholesterol efflux only. While apoA-I mimetic peptides can fuse with homologous HDL delivering to it their cargo, mimetics like gold nanoparticles [56], cyclodextrin [48] and phospholipid vesicles [49] cannot, thus possibly limiting their utility in vivo. Peptides combining lipid-binding domain coupled with receptor-binding domain, such as Ac-hE-18A-NH2 [34, 35] and EpK [36], have an advantage of being capable, at least theoretically, of recreating both early and late steps of RCT. ApoE mimetic ATI-5261 promoted cholesterol efflux and the generated particles capable to undergo further metabolism similar to HDL, including the ability to be taken up by SR-B1 [57]. In most cases, however, it is unlikely that peptides are capable of recreating the full RCT picking up cellular cholesterol and transporting it to the liver without first fusing with HDL particles of the recipient. It is more likely that they deliver cellular cholesterol to HDL supplementing a rate-limiting step of RCT, rather than generate a parallel pathway.
Independent of the capacity of mimetic peptides to be an acceptor of cellular cholesterol, a likely important contributing factor is their ability to stabilize ABCA1 on the cell surface [58]. ApoA-I mimetic peptides are also capable of binding to HDL improving its functionality and/or displacing apoA-I from the particles with the formation of preβ1-HDL/apoA-I [59], the mechanisms discussed above in relation to rHDL.
Clearance of plasma lipoproteins
A novel mechanism of clearance of plasma lipoproteins mimicking elements of forward cholesterol transport was proposed for apoE mimetics (for recent review see [60]). Peptide Ac-hE-18A-NH2 effectively binds to LDL and VLDL through its lipid-binding domain and dramatically enhances their clearance by liver significantly reducing hyperlipidemia. Interestingly, the increased uptake of apoB-containing lipoproteins in the liver after peptide administration was mediated not by classical LDL/VLDL receptors, but rather through binding of lipoproteins to heparin sulfate proteoglycane (HSPG). This approach was effective in reducing hyperlipidemia and mouse and rabbit models.
Inflammation
Anti-inflammatory properties have also been demonstrated for many mimetic peptides. Multiple mechanisms were suggested: (i) inhibition of VCAM expression [41, 47, 61, 62], (ii) reduction of cytokine expression [12, 61–63]; (iii) suppression of chemotaxis [64]; (iv) reduction of pro-inflammatory properties of modified LDL [65]; (v) inhibition of proinflammatory gene expression through altering the assembly of TLR–ligand complexes in cell membranes [66]. Which of these pathways is central to the anti-inflammatory ability of mimetic peptides is currently not known but is an active area of investigation. Most of the anti-inflammatory peptides were tested in context of atherosclerosis; however, several other inflammatory diseases are also potential indications. For example, peptides D- 4F and L-4F were used to reduce oxidation and inflammation after transplantation [13], in experimental obesity [8] and diabetes [67] by upregulation of heme oxygenase-1 [13]. Although mechanistically less defined, the anti-inflammatory properties of 4F peptides were probably also behind its therapeutic utility in asthma [14], arthritis [15], infection [11, 12] and sepsis [68].
Oxidation
Many mimetic peptides have anti-oxidant properties, especially if their sequence includes anti-oxidant amino-acids His and Cys [47, 69]. Other mechanisms contributing to anti-oxidant capacity are stabilization and enhancing activity of PON [70], binding and sequestration of oxidized lipids [71] or facilitating transfer of oxidized lipids from LDL to HDL [72].
Apoptosis
Lipoprotein mimetics displayed anti-apoptotic properties by reducing endoplasmic reticulum stress in macrophages through inhibition of CD-36-mediated uptake of oxLDL [73].
Vascular function
Several mimetics were able to protect or improve vascular function by increasing NO production [74, 75], reducing formation of superoxide anions, which are scavengers of NO [34, 76], and improving eNOS-depednent vasodilatation [77].
Thrombogenesis
Mimetics were able to reduce platelet aggregation through lowering levels of thromboxane A2, and prostaglandins D2 and E2 [78].
Triglyceride lowering
One feature of apoA-I mimetic peptides is that their administration in animal models often results in hypertriglyceridemia; possibly due to the displacement of apoC-II, an important cofactor for LPL. To counteract this effect, and perhaps as a new tool to control hypertriglyceridemia, Amar et al synthesized a bi-functional peptide, consisting of lipid-binding sequence anchoring peptide to a lipoprotein particle coupled with a fragment of apoC-II known to activate LPL [37]. C-II-a peptide effectively reduced plasma triglyceride levels caused by the 5A peptide when both peptides were co-administered in mice; when administered by itself it also stimulated lipolysis in apoE-KO mice [37].
LDL aggregation
Modified and especially aggregated LDL are pro-atherogenic and apoA-I mimetic peptide L-4F was able to block sphingomyelinase-induced LDL aggregation [79].
Regulation of intestinal uptake of oxidized lipids
Recently Navab et al suggested that the main target of apoA-I mimetic peptides may not be systemic lipoprotein metabolism or atherosclerotic plaque, but rather lipid metabolism in the small intestine [80]. They found that following oral or subcutaneous administration of D-4F into LDLR−/− mice, intestinal and liver levels of metabolites of arachidonic and linoleic acids, as well plasma levels of amyloid A and TG were reduced; the effect was independent of systemic concentration of the peptide. The interpretation of this finding was that the true target in the anti-inflammatory effects of the peptide is the small intestine and that concentration of the peptide in the intestine, rather than in plasma, is of primary importance. The hypothesis was that various oxidized lipids are produced in the intestine, presumably by microbiota, in quantities sufficient to contribute to systemic inflammation and the peptide is able to bind these oxidized lipids mitigating their inflammatory effects [81].
As outlined above, mimetic peptides were able to recreate many “non-RCT” functions of HDL. As with HDL, however, it is not clear which of the “non-RTC” properties of the mimetics are truly independent from their ability to promote cholesterol efflux and reduce cellular cholesterol content. For example, several anti-inflammatory properties were mechanistically explained by depleting cells of cholesterol, which disrupts lipid rafts playing a key role in inflammation [66]. However, some properties of the peptides are clearly independent of their ability to modify cellular cholesterol metabolism, such as rescue of pulmonary hypertension through regulation of microRNA-193 [17].
Finally, nanoparticles conjugated with mimetic peptides were used as carriers for targeted drug delivery directing lipophilic drugs, including statins, or contrast substances to atherosclerotic plaques [19, 82] or cancer cells [5, 6, 20].
Efficacy of the lipoprotein mimetics in animal models and clinical trials
Atherosclerosis
Many lipoproteins mimetics were investigated in animal models of atherosclerosis and in early stage clinical trials. Much of the early animal work assessing development of atherosclerosis after administration of rHDL has been done with apoA-IMilano-based rHDL (ETC-216). Chronic intravenous administration of apoA-IMilano-based rHDL in apoE−/− mice almost completely halted development of atherosclerosis [83] and even a single injection reduced plaque cholesterol and macrophage content [84] in this model. In rabbit model of injury-induced atherosclerosis, a single infusion of apoA-IMilano-based rHDL reduced lipid content and size of atherosclerotic plaques in carotid arteries [85]. When tested side-by-side, apoA-IMilano-based rHDL was more effective than WT apoA-I-based rHDL in reducing atherosclerosis and especially in reducing inflammation in the plaque [86]. CER-001 (rHDL) was also effective in stimulating reverse cholesterol transport and reducing development of atherosclerosis in LDLR−/− mice [87]. CSL111 in unchanged form and as pegylated HDL was only mildly effective in reducing burden of atherosclerosis when administered to apoE−/− mice [29].
While some of the early apoA-I mimetic peptides did not substantially affect the development of atherosclerosis, most of the re-developed peptides did; variants of the peptide 4F are most investigated in this regard. Oral administration of D-4F (a peptide synthesized from D-amino acids and therefore resistant to proteolysis in the gut) suppressed development of atherosclerosis by 79% in LDLR−/− mice [45] and by 50% in diabetic apoE−/− mice [88]. A slightly modified version of D-4F, 6F, expressed in transgenic tomatoes and added to Western diet reduced atherosclerosis in LDLR−/− mice by about 40% [89]. Smaller reductions of 10–30% were found after intraperitoneal or retro-orbital administration of L-4F in apoE−/− mice. The peptide 5A administered intraperitoneally or intravenously reduced atherosclerosis in apoE−/−mice by 30–50% [55]. The peptides ELK-2A2K2E [90], ATI-5261 [31] and FAMP [30] administered intraperitoneally reduced atherosclerosis by 16–30% in apoE−/− mice.
Apo E mimetic peptide Ac-hE18A-NH2 was effective in reducing hyperlipidemia in mouse [91] and rabbit [92] models and was also effective in reducing atherosclerosis in mouse model [93]. Nanoparticles coupled with monomeric or trimeric apoA-I mimetic peptide reduced atherosclerosis in LDLR−/− mice by 50–70% after intraperitoneal or oral (trimeric only) administration [33].
In humans, chronic infusion of apoA-IMilano-based rHDL reduced the size of coronary atheroma assessed by IVUS [94]. Sort term infusion of WT apoA-I based rHDL, CSL111, significantly reduced coronary atheroma volume at high dose, but unfortunately this dose had unacceptable level of toxicity, possibly due to excess cholic acid used in the reconstitution process. Low dose CSL111 was well tolerated, but the effect on atheroma volume was not statistically significant [22]. New formulation of the CSL product, CSL112, was well tolerated [95] and in an early stage clinical trial appears to stimulate markers of cholesterol efflux [53]. In a small clinical trial of patients with peripheral vascular disease, a single infusion of CSL111 led to significant reductions in cholesterol content and inflammation in atherosclerotic plaques [96]. However, CER-001 failed to reduce atheroma volume in patients with acute coronary syndrome [23], but mildly decreased carotid mean vessel wall area in patients with familial hypoalphalipoproteinemia [97].
Oral administration of the peptide D-4F in humans was well tolerated and reduced inflammatory markers despite low bioavailability [98]. Intravenous and subcutaneous infusions of L-4F were also well tolerated, the peptide had good bioavailability, but had limited effect on HDL level and or functionality and did not improve any inflammatory markers [99].
Very few lipoprotein mimetics were tested side-by-side and the variability in doses, route of administration, animal models, patient populations and other conditions make comparisons of the various mimetics difficult. Generally, most mimetics were active in reducing atherosclerosis in vivo in animal studies; however, it is difficult to establish a definitive connection between properties of the mimetics in vitro and in vivo and their efficacy in reducing atherosclerosis. It is not clear which element of pathogenesis of atherosclerosis is the main target of the mimetics, e.g. removal of excessive cholesterol by RCT or inflammation or both, and where the main action is, locally or systemically.
Other diseases
The therapeutic potential of lipoprotein mimetics in conditions other than atherosclerosis was also extensively tested. The peptide L-4F protected vascular function in animal models of hypercholesterolemia and sickle cell disease [100]. D-4F decreased myocardial inflammation, improved vascular function and restored angiogenic potential of the hearts in mouse model of systemic sclerosis [101]; it also reduced inflammation in animal model of asthma [14]. D-4F in combination with statin reduced severity of arthritis in animal model [15]. The same combination of D-F4 and statin, as well as apoE mimetic peptide Ac-hE18A-NH2, reduced amyloid deposition and improved cognitive function in animal models of Alzheimer’s disease [9, 10]. L-4F reduced insulin resistance and inflammation in animal models of obesity [8]. L-4F also improved vascular function and inflammation in two animal models of sepsis [68, 75] and inhibited growth of colon cancer in vivo [7]. As discussed above, D-4F rescued pulmonary hypetrtension through an unusual mechanism involving a miR193 [17].
In humans, rHDL (CSL111) had profound anti-diabetic effect increasing insulin secretion and reducing insulin resistance [102]. It also reduced platelet aggregation and thrombus formation ex vivo [103]. Despite findings being fragmented and many of them are yet to be confirmed, it is becoming clear that utility of lipoprotein mimetics is not limited to atherosclerosis and cardiovascular disease.
Limitations of lipoprotein mimetics
Utility of lipoprotein mimetics has several important limitations. Cost of synthesis, purification and testing of apoA-I, as well as cost of phospholipids to produce rHDL are high; cost of synthesis of long peptides is also considerable. This is one reason why rHDL preparations are currently being marketed for short-term use in acute cardiac events – to rapidly alleviate burden of the ruptured plaque and to stabilize unstable plaques, with other types of therapy to follow in long-term. Another limitation is that most of lipoprotein mimetics require intravenous administration essentially precluding long-term use of these mimetics e.g. as a preventative strategy. This limitation, however, was addressed by several means. Peptides synthesized from D-amino acids are resistant to proteolysis in the gut and are adsorbed without significant modification. This strategy however has important limitations. Firstly, while resistant to gut proteases, D-peptides are also incompatible with intestinal transport systems and bioavailability of D-peptides is low [80]. Secondly, these peptides are resistant not only to gut proteases, but to proteases in general; the route of their catabolism is uncertain and they potentially may accumulate in liver or kidney. Several other strategies were suggested to enable oral administration of the peptides such as stapling of α-helices [32], or use as a complex with niclosamide [46]. Nanoparticles, although containing peptides made of L-amino acids, were also resistant to proteolysis in the gut [33]. These approaches not only potentially allow for oral administration of the mimetics, but prolong usually very rapid turnover. Other approaches to improve pharmacokinetic properties include reformulation of lipid content of rHDL [95], pegylation of rHDL [29], or use of trimeric apoA-I based rHDL [28].
HDL carries a number of biologically active molecules affecting many aspects of cellular metabolism, which may play an important role in determining properties of HDL. This aspect is yet to be incorporated into concept of lipoprotein mimetics and most available mimetics were not designed or tested for their capacity to selectively deliver biologically active proteins and miRNAs.
However, one of the main obstacle for successful translation of an experimental compound into an effective medication is the uncertainly about mechanisms. Although many mimetics effectively target specific facets of atherosclerosis in vitro and reduce atherosclerosis in vivo, there is little evidence that in vitro properties of these mimetics are reproduced in vivo, and especially in human studies. Multiple targets, RCT, inflammation, oxidation etc, uncertainty of whether mimetics act locally on systemically hold back development of effective lipoprotein mimetics. Even with intended target of mimetics, systemic lipoprotein metabolism, it is uncertain whether mimetics bind and modify existing lipoproteins or deliver cholesterol from cells to HDL or recreate several steps of RCT. Combined with poor understanding of complex pathogenesis of atherosclerosis, e.g. uncertainty about the relative contribution of cholesterol accumulation, inflammation, oxidation and other facets of pathogenesis, as well as of different cell types in the vessel wall to overall severity of the disease, makes it difficult to predict what an ideal lipoprotein mimetic should mechanistically do and how it should look like.
An ideal lipoprotein mimetic
Despite these uncertainties, we speculate in the following section on the potential “ideal” features of a lipoprotein mimetic (Fig. 1). We restricted our attempts to an HDL mimetic, as there is a greater knowledge base in this area. We based our proposal on two reasonable but not fully tested assumptions: first, that the main objective of a mimetic is recreation or enhancement of RCT, and second, that the mimetic should promote as many stages of RCT as possible.
Figure 1.
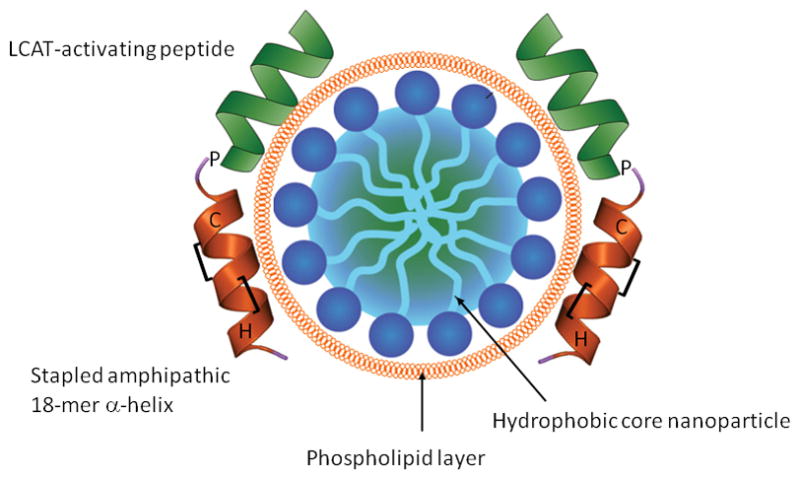
The proposed structure of an “ideal” lipoprotein mimetic.
RCT pathway principally consists of three steps: (1) removing free cholesterol from the cells, (2) esterifying cholesterol preventing it from flowing back to cells and (3) delivering cholesteryl esters to the liver. Thus, the first essential step that must be performed by a mimetic is to effect specific cholesterol efflux from cells. Amphipathic α-helical peptides of 18–37 amino acids seem to be adequate for this activity. Bi-helical peptides where two helices connected by a proline residue are more active and more specific, but the role of the second helix is most likely in proper positioning of the first helix, and this function can possibly be fulfilled by another structure. It may be beneficial to include into the structure of the peptide histidine and/or cysteine residues boosting anti-oxidant properties of the mimetic without affecting its secondary structure and hydrophobicity. Peptide L-4F or hydrophobic helix of the peptide 5A-CH1 may provide template for the first element of the ideal lipoprotein mimetic.
The second element of an ideal mimetic should facilitate cholesterol esterification and to properly position the “efflux” peptide. A peptide with homology to the 7th helix of apoA-I (residues 143–164) would be a suitable candidate for this role when connected to the “efflux” peptide through a proline residue. The 7th helix of apoA-I is involved in LCAT activation [104], it is less hydrophobic than the “efflux” peptide and, like second helix in peptide 5A, it would prevent the structure from “sinking” into the cell membrane, the latter seems to be a cause of non-specific efflux and toxicity.
The requirement for the third element of an ideal mimetic is to have a capacity to hold and carry a cargo of cholesteryl esters. That could be best fulfilled by an organic nanoparticle that has a micellar-like structure with hydrophobic core covered by phospholipid. It is expected that the newly formed cholesteryl esters would move into hydrophobic core and the particle would acquire additional phospholipids for the expanding surface from cells and/or other lipoproteins. Similar micellar particles have been described [105]; however, there is an uncertainly of whether it would be best for peptide construct(s) to hold on the particle by the hydrophobic face of amphipathic α-helices or to be covalently linked to the particle to prevent dissociation. Delivery of cholesteryl esters to the liver occurs via two pathways: after exchange of cholesteryl esters for triglycerides through the action of cholesteryl ester transfer protein (CETP) and through extraction of cholesteryl esters from HDL by SR-B1 in the liver. The former pathway is predominant in humans, however, structural requirements for a particle to be able to interact with CETP are uncertain leaving little basis for generating a mimetic recreating this feature. Interaction with SR-B1 is known to require presence of amphipathic α-helices and is influenced by the particle size, providing a good opportunity for empirical modification of these parameters to facilitate the interaction with SR-B1 and release of cholesteryl esters. An advantage of this approach is that, theoretically, the particle would recycle, speeding up RCT and benefiting pharmacokinetic properties of the mimetic. An alternative approach would be to explore receptor binding domains of apoB or apoE, directing mimetic holoparticle to the liver. Advantage of apoE in this context is that its receptor binding domain is an amphipathic α-helix of suitable size (16 residues); therefore, it may be adapted to substitute the “efflux” peptide combining the two functions. Ideally, a mimetic should permit oral administration. The best way to achieve this may be to either “staple” the peptide adjusting stoichiometry for the peptide to cover most of the micelle surface protecting lipids from the intestinal lipases, or to complex it with niclosamid. Thus, the hypothesised structure of an ideal mimetic is a micellar nanoparticle with hydrophobic core covered with phospholipids and coupled to a bi-helical stapled peptide consisting of one hydrophobic amphipathic α-helix and LCAT-activating domain of apoA-I (Fig. 1).
Conclusions
Recent failures of the clinical trials with CETP inhibitors and the outcomes of the Mendelian randomization analysis has put the idea of “HDL therapy” out of favour and on a wrong foot [106]. However, most of the approaches tested so far aimed at raising plasma HDL-cholesterol levels by inhibiting catabolic steps of RCT, perhaps not the ideal way to improve cholesterol flow through the pathway. Lipoprotein mimetics offer a different approach; they stimulate one or several steps of RCT and improve its functionality without necessarily raising plasma HDL levels. They offer remarkable flexibility of structural and functional properties allowing adjusting them to almost any requirement. Their potential use is not limited to cardiovascular disease; numerous pathologies where cholesterol metabolism is a part of their pathogenesis are also potential indications for this type of therapy. Therefore, in summary, lipoprotein mimetics offer incredible opportunities, but also many challenges in translating these interesting new agents into therapies. The most important challenge is to better understand the mechanisms behind many beneficial biological properties of lipoprotein mimetics, thus allowing for a more rational drug design.
Acknowledgments
Funding
Research by DS was supported by a Program grant from the National Health and Medical Research Council of Australia (NHMRC) (GNT1036352). DS is a fellow of the NHMRC. Research by AR is supported by intramural research funds from the National Heart, Lung and Blood Institute.
Abbreviations
- ABCA1
ATP binding cassette transporter type A1
- apo
apolipoprotein
- CETP
cholesteryl ester transfer protein
- HDL
high-density lipoprotein
- HSPG
heparin sulfate proteoglycane
- LDL
low-density lipoprotein
- LDLR
low-density lipoprotein receptor
- LPL
lipoprotein lipase
- RCT
reverse cholesterol transport
- rHDL
reconstituted HDL
- SR-B1
scavenger receptor type B1
- TG
triglycerides
- VLDL
very low density lipoprotein
Footnotes
Declaration of conflict of interests
AR is an inventor on a patent for the 5A peptide. DS declares no conflict of interests in relation to this manuscript.
References
- 1.Rye KA, Barter PJ. Cardioprotective functions of HDLs. J Lipid Res. 2014;55:168–179. doi: 10.1194/jlr.R039297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sviridov D, Mukhamedova N, Remaley AT, Chin-Dusting J, Nestel P. Antiatherogenic Functionality of High Density Lipoprotein: How Much versus How Good. J Atheroscler Thromb. 2008;15:52–62. doi: 10.5551/jat.e571. [DOI] [PubMed] [Google Scholar]
- 3.Vaisar T, Pennathur S, Green PS, Gharib SA, Hoofnagle AN, Cheung MC, Byun J, Vuletic S, Kassim S, Singh P, Chea H, Knopp RH, Brunzell J, Geary R, Chait A, Zhao XQ, Elkon K, Marcovina S, Ridker P, Oram JF, Heinecke JW. Shotgun proteomics implicates protease inhibition and complement activation in the antiinflammatory properties of HDL. J Clin Invest. 2007;117:746–756. doi: 10.1172/JCI26206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vickers KC, Palmisano BT, Shoucri BM, Shamburek RD, Remaley AT. MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nat Cell Biol. 2011;13:423–433. doi: 10.1038/ncb2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McMahon KM, Foit L, Angeloni NL, Giles FJ, Gordon LI, Thaxton CS. Synthetic high-density lipoprotein-like nanoparticles as cancer therapy. Cancer Treat Res. 2015;166:129–150. doi: 10.1007/978-3-319-16555-4_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang S, Damiano MG, Zhang H, Tripathy S, Luthi AJ, Rink JS, Ugolkov AV, Singh TKA, Dave SS, Gordon LI, Thaxton CS. Biomimetic, synthetic HDL nanostructures for lymphoma. Proc Nat Acad Sci USA. 2013;110:2511–2516. doi: 10.1073/pnas.1213657110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Su F, Grijalva V, Navab K, Ganapathy E, Meriwether D, Imaizumi S, Navab M, Fogelman AM, Reddy ST, Farias-Eisner R. HDL Mimetics Inhibit Tumor Development in Both Induced and Spontaneous Mouse Models of Colon Cancer. Mol Cancer Therap. 2012;11:1311–1319. doi: 10.1158/1535-7163.MCT-11-0905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peterson SJ, Kim DH, Li M, Positano V, Vanella L, Rodella LF, Piccolomini F, Puri N, Gastaldelli A, Kusmic C, L’Abbate A, Abraham NG. The L-4F mimetic peptide prevents insulin resistance through increased levels of HO-1, pAMPK, and pAKT in obese mice. J Lipid Res. 2009;50:1293–1304. doi: 10.1194/jlr.M800610-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Handattu SP, Garber DW, Monroe CE, van Groen T, Kadish I, Nayyar G, Cao D, Palgunachari MN, Li L, Anantharamaiah GM. Oral apolipoprotein A-I mimetic peptide improves cognitive function and reduces amyloid burden in a mouse model of Alzheimer’s disease. Neurobiol Dis. 2009;34:525–534. doi: 10.1016/j.nbd.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Handattu SP, Monroe CE, Nayyar G, Palgunachari MN, Kadish I, van Groen T, Anantharamaiah GM, Garber DW. In vivo and in vitro effects of an apolipoprotein e mimetic peptide on amyloid-beta pathology. J Alzheimers Dis. 2013;36:335–347. doi: 10.3233/JAD-122377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van Lenten BJ, Wagner AC, Navab M, Anantharamaiah GM, Hui EKW, Nayak DP, Fogelman AM. D-4F, an Apolipoprotein A-I Mimetic Peptide, Inhibits the Inflammatory Response Induced by Influenza A Infection of Human Type II Pneumocytes. Circulation. 2004;110:3252–3258. doi: 10.1161/01.CIR.0000147232.75456.B3. [DOI] [PubMed] [Google Scholar]
- 12.Moreira RS, Irigoyen M, Sanches TR, Volpini RA, Camara NO, Malheiros DM, Shimizu MH, Seguro AC, Andrade L. Apolipoprotein A-I mimetic peptide 4F attenuates kidney injury, heart injury, and endothelial dysfunction in sepsis. Am J Physiol Regul Integr Comp Physiol. 2014;307:R514–524. doi: 10.1152/ajpregu.00445.2013. [DOI] [PubMed] [Google Scholar]
- 13.Hsieh GR, Schnickel GT, Garcia C, Shefizadeh A, Fishbein MC, Ardehali A. Inflammation/oxidation in chronic rejection: apolipoprotein a-i mimetic peptide reduces chronic rejection of transplanted hearts. Transplantation. 2007;84:238–243. doi: 10.1097/01.tp.0000268509.60200.ea. [DOI] [PubMed] [Google Scholar]
- 14.Nandedkar SD, Weihrauch D, Xu H, Shi Y, Feroah T, Hutchins W, Rickaby DA, Duzgunes N, Hillery CA, Konduri KS, Pritchard KA. D-4F, an apoA-1 mimetic, decreases airway hyperresponsiveness, inflammation, and oxidative stress in a murine model of asthma. J Lipid Res. 2011;52:499–508. doi: 10.1194/jlr.M012724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Charles-Schoeman C, Banquerigo ML, Hama S, Navab M, Park GS, Van Lenten BJ, Wagner AC, Fogelman AM, Brahn E. Treatment with an apolipoprotein A-1 mimetic peptide in combination with pravastatin inhibits collagen-induced arthritis. Clin Immunol. 2008;127:234–244. doi: 10.1016/j.clim.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 16.Gomaraschi M, Calabresi L, Rossoni G, Iametti S, Franceschini G, Stonik JA, Remaley AT. Anti-inflammatory and cardioprotective activities of synthetic high-density lipoprotein containing apolipoprotein A-I mimetic peptides. J Pharmacol Exp Ther. 2008;324:776–783. doi: 10.1124/jpet.107.129411. [DOI] [PubMed] [Google Scholar]
- 17.Sharma S, Umar S, Potus F, Iorga A, Wong G, Meriwether D, Breuils-Bonnet S, Mai D, Navab K, Ross D, Navab M, Provencher S, Fogelman AM, Bonnet S, Reddy ST, Eghbali M. Apolipoprotein A-I Mimetic Peptide 4F Rescues Pulmonary Hypertension by Inducing MicroRNA-193–3p. Circulation. 2014;130:776–785. doi: 10.1161/CIRCULATIONAHA.114.007405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sanchez-Gaytan BL, Fay F, Lobatto ME, Tang J, Ouimet M, Kim Y, van der Staay SE, van Rijs SM, Priem B, Zhang L, Fisher EA, Moore KJ, Langer R, Fayad ZA, Mulder WJ. HDL-mimetic PLGA nanoparticle to target atherosclerosis plaque macrophages. Bioconjug Chem. 2015;26:443–451. doi: 10.1021/bc500517k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marrache S, Dhar S. Biodegradable synthetic high-density lipoprotein nanoparticles for atherosclerosis. Proc Nat Acad Sci USA. 2013;110:9445–9450. doi: 10.1073/pnas.1301929110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Z, Chen J, Ding L, Jin H, Lovell JF, Corbin IR, Cao W, Lo PC, Yang M, Tsao MS, Luo Q, Zheng G. HDL-mimicking peptide-lipid nanoparticles with improved tumor targeting. Small. 2010;6:430–437. doi: 10.1002/smll.200901515. [DOI] [PubMed] [Google Scholar]
- 21.Diditchenko S, Gille A, Pragst I, Stadler D, Waelchli M, Hamilton R, Leis A, Wright SD. Novel Formulation of a Reconstituted High-Density Lipoprotein (CSL112) Dramatically Enhances ABCA1-Dependent Cholesterol Efflux. Arterioscler Thromb Vasc Biol. 2013;33:2202–2211. doi: 10.1161/ATVBAHA.113.301981. [DOI] [PubMed] [Google Scholar]
- 22.Tardif JC, Gregoire J, L’Allier PL, Ibrahim R, Lesperance J, Heinonen TM, Kouz S, Berry C, Basser R, Lavoie MA, Guertin MC, Rodes-Cabau J. Effects of Reconstituted High-Density Lipoprotein Infusions on Coronary Atherosclerosis: A Randomized Controlled Trial. JAMA. 2007;297:1675–1682. doi: 10.1001/jama.297.15.jpc70004. [DOI] [PubMed] [Google Scholar]
- 23.Tardif JC, Ballantyne CM, Barter P, Dasseux JL, Fayad ZA, Guertin MC, Kastelein JJ, Keyserling C, Klepp H, Koenig W, L’Allier PL, Lesperance J, Luscher TF, Paolini JF, Tawakol A, Waters DD, Can HDL. Effects of the high-density lipoprotein mimetic agent CER-001 on coronary atherosclerosis in patients with acute coronary syndromes: a randomized trial. Eur Heart J. 2014;35:3277–3286. doi: 10.1093/eurheartj/ehu171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roma P, Gregg RE, Meng MS, Ronan R, Zech LA, Franceschini G, Sirtori CR, Brewer HJ. In vivo metabolism of a mutant form of apolipoprotein A-I, apo A-IMilano, associated with familial hypoalphalipoproteinemia. J Clin Invest. 1993;91:1445–1452. doi: 10.1172/JCI116349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jia Z, Natarajan P, Forte TM, Bielicki JK. Thiol-bearing synthetic peptides retain the antioxidant activity of apolipoproteinA-I(Milano) Biochem Biophys Res Commun. 2002;297:206–213. doi: 10.1016/s0006-291x(02)02143-5. [DOI] [PubMed] [Google Scholar]
- 26.Ohnsorg PM, Mary JL, Rohrer L, Pech M, Fingerle, von Eckardstein A. Trimerized apolipoprotein A-I (TripA) forms lipoproteins, activates lecithin:cholesterol acyltransferase, elicits lipid efflux, and is transported through aortic endothelial cells. Biochim Biophys Acta. 2011;1811:1115–1123. doi: 10.1016/j.bbalip.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 27.Murphy AJ, Hoang A, Aprico A, Sviridov D, Chin-Dusting J. Anti-Inflammatory Functions of Apolipoprotein A-I and High-Density Lipoprotein Are Preserved in Trimeric Apolipoprotein A-I. J Pharmacol Exp Ther. 2013;344:41–49. doi: 10.1124/jpet.112.199257. [DOI] [PubMed] [Google Scholar]
- 28.Graversen JH, Laurberg JM, Andersen MH, Falk E, Nieland J, Christensen J, Etzerodt M, Thogersen HC, Moestrup SK. Trimerization of Apolipoprotein A-I Retards Plasma Clearance and Preserves Antiatherosclerotic Properties. J Cardiovasc Pharmacol. 2008;51:170–177. doi: 10.1097/FJC.0b013e31815ed0b9. [DOI] [PubMed] [Google Scholar]
- 29.Murphy AJ, Funt S, Gorman D, Tall AR, Wang N. Pegylation of High-Density Lipoprotein Decreases Plasma Clearance and Enhances Antiatherogenic Activity. Circ Res. 2013;113:e1–e9. doi: 10.1161/CIRCRESAHA.113.301112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Uehara Y, Ando S, Yahiro E, Oniki K, Ayaori M, Abe S, Kawachi E, Zhang B, Shioi S, Tanigawa H, Imaizumi S, Miura S, Saku K. FAMP, a Novel ApoA-I Mimetic Peptide, Suppresses Aortic Plaque Formation Through Promotion of Biological HDL Function in ApoE-Deficient Mice. J Am Heart Assoc. 2013;2:e000048. doi: 10.1161/JAHA.113.000048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bielicki JK, Zhang H, Cortez Y, Zheng Y, Narayanaswami V, Patel A, Johansson J, Azhar S. A new HDL mimetic peptide that stimulates cellular cholesterol efflux with high efficiency greatly reduces atherosclerosis in mice. J Lipid Res. 2010;51:1496–1503. doi: 10.1194/jlr.M003665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sviridov DO, Ikpot IZ, Stonik J, Drake SK, Amar M, Osei-Hwedieh DO, Piszczek G, Turner S, Remaley AT. Helix stabilization of amphipathic peptides by hydrocarbon stapling increases cholesterol efflux by the ABCA1 transporter. Biochem Biophys Res Comm. 2011;410:446–451. doi: 10.1016/j.bbrc.2011.05.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao Y, Black AS, Bonnet DJ, Maryanoff BE, Curtiss LK, Leman LJ, Ghadiri MR. In vivo efficacy of HDL-like nanolipid particles containing multivalent peptide mimetics of apolipoprotein A-I. J Lipid Res. 2014;55:2053–2063. doi: 10.1194/jlr.M049262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gupta H, White CR, Handattu S, Garber DW, Datta G, Chaddha M, Dai L, Gianturco SH, Bradley WA, Anantharamaiah GM. Apolipoprotein E Mimetic Peptide Dramatically Lowers Plasma Cholesterol and Restores Endothelial Function in Watanabe Heritable Hyperlipidemic Rabbits. Circulation. 2005;111:3112–3118. doi: 10.1161/CIRCULATIONAHA.104.497107. [DOI] [PubMed] [Google Scholar]
- 35.Handattu SP, Datta G, Epand RM, Epand RF, Palgunachari MN, Mishra VK, Monroe CE, Keenum TD, Chaddha M, Anantharamaiah GM, Garber DW. Oral administration of L-mR18L, a single domain cationic amphipathic helical peptide, inhibits lesion formation in ApoE null mice. J Lipid Res. 2010;51:3491–3499. doi: 10.1194/jlr.M006916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao W, Du F, Zhang M, Sun S, Yu H, Fan D. A new recombinant human apolipoprotein E mimetic peptide with high-density lipoprotein binding and function enhancing activity. Exp Biol Med (Maywood) 2011;236:1468–1476. doi: 10.1258/ebm.2011.011169. [DOI] [PubMed] [Google Scholar]
- 37.Amar MJ, Sakurai T, Sakurai-Ikuta A, Sviridov D, Freeman L, Ahsan L, Remaley AT. A novel apolipoprotein C-II mimetic peptide that activates lipoprotein lipase and decreases serum triglycerides in apolipoprotein E-knockout mice. J Pharmacol Exp Ther. 2015;352:227–235. doi: 10.1124/jpet.114.220418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chung BH, Anatharamaiah GM, Brouillette CG, Nishida T, Segrest JP. Studies of synthetic peptide analogs of the amphipathic helix. Correlation of structure with function. J Biol Chem. 1985;260:10256–10262. [PubMed] [Google Scholar]
- 39.Mendez AJ, Anantharamaiah GM, Segrest JP, Oram JF. Synthetic amphipathic helical peptides that mimic apolipoprotein A-I in clearing cellular cholesterol. J Clin Invest. 1994;94:1698–1705. doi: 10.1172/JCI117515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Datta G, Chaddha M, Hama S, Navab M, Fogelman AM, Garber DW, Mishra VK, Epand RM, Epand RF, Lund-Katz S, Phillips MC, Segrest JP, Anantharamaiah GM. Effects of increasing hydrophobicity on the physical-chemical and biological properties of a class A amphipathic helical peptide. J Lipid Res. 2001;42:1096–1104. [PubMed] [Google Scholar]
- 41.Di Bartolo BA, Nicholls SJ, Bao S, Rye KA, Heather AK, Barter PJ, Bursill C. The apolipoprotein A-I mimetic peptide ETC-642 exhibits anti-inflammatory properties that are comparable to high density lipoproteins. Atherosclerosis. 2011;217:395–400. doi: 10.1016/j.atherosclerosis.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 42.Mishra VK, Palgunachari MN, Lund-Katz S, Phillips MC, Segrest JP, Anantharamaiah GM. Effect of the arrangement of tandem repeating units of class A amphipathic alpha-helixes on lipid interaction. J Biol Chem. 1995;270:1602–1611. doi: 10.1074/jbc.270.4.1602. [DOI] [PubMed] [Google Scholar]
- 43.Wool GD, Vaisar T, Reardon CA, Getz GS. An apoA-I mimetic peptide containing a proline residue has greater in vivo HDL binding and anti-inflammatory ability than the 4F peptide. J Lipid Res. 2009;50:1889–1900. doi: 10.1194/jlr.M900151-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sethi AA, Stonik JA, Thomas F, Demosky SJ, Amar M, Neufeld E, Brewer HB, Davidson WS, D’Souza W, Sviridov D, Remaley AT. Asymmetry in the Lipid Affinity of Bihelical Amphipathic Peptides: A STRUCTURAL DETERMINANT FOR THE SPECIFICITY OF ABCA1-DEPENDENT CHOLESTEROL EFFLUX BY PEPTIDES. J Biol Chem. 2008;283:32273–32282. doi: 10.1074/jbc.M804461200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Navab M, Anantharamaiah GM, Hama S, Garber DW, Chaddha M, Hough G, Lallone R, Fogelman AM. Oral Administration of an Apo A-I Mimetic Peptide Synthesized From D-Amino Acids Dramatically Reduces Atherosclerosis in Mice Independent of Plasma Cholesterol. Circulation. 2002;105:290–292. doi: 10.1161/hc0302.103711. [DOI] [PubMed] [Google Scholar]
- 46.Navab M, Ruchala P, Waring AJ, Lehrer RI, Hama S, Hough G, Palgunachari MN, Anantharamaiah GM, Fogelman AM. A novel method for oral delivery of apolipoprotein mimetic peptides synthesized from all L-amino acids. J Lipid Res. 2009;50:1538–1547. doi: 10.1194/jlr.M800539-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.D’Souza W, Stonik JA, Murphy A, Demosky SJ, Sethi AA, Moore XL, Chin-Dusting J, Remaley AT, Sviridov D. Structure/Function Relationships of Apolipoprotein A-I Mimetic Peptides: Implications for Antiatherogenic Activities of High-Density Lipoprotein. Circ Res. 2010;107:217–227. doi: 10.1161/CIRCRESAHA.110.216507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Atger VM, de la Llera Moya M, Stoudt GW, Rodrigueza WV, Phillips MC, Rothblat GH. Cyclodextrins as catalysts for the removal of cholesterol from macrophage foam cells. J Clin Invest. 1997;99:773–780. doi: 10.1172/JCI119223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rodrigueza WV, Mazany KD, Essenburg AD, Pape ME, Rea TJ, Bisgaier CL, Williams KJ. Large versus small unilamellar vesicles mediate reverse cholesterol transport in vivo into two distinct hepatic metabolic pools. Implications for the treatment of atherosclerosis. Arterioscler Thromb Vasc Biol. 1997;17:2132–2139. doi: 10.1161/01.atv.17.10.2132. [DOI] [PubMed] [Google Scholar]
- 50.Thaxton CS, Daniel WL, Giljohann DA, Thomas AD, Mirkin CA. Templated Spherical High Density Lipoprotein Nanoparticles. J Am Chem Soc. 2009;131:1384–1385. doi: 10.1021/ja808856z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Luthi AJ, Zhang H, Kim D, Giljohann DA, Mirkin CA, Thaxton CS. Tailoring of biomimetic high-density lipoprotein nanostructures changes cholesterol binding and efflux. ACS Nano. 2012;6:276–285. doi: 10.1021/nn2035457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hoang A, Drew BG, Low H, Remaley AT, Nestel P, Kingwell BA, Sviridov D. Mechanism of cholesterol efflux in humans after infusion of reconstituted high-density lipoprotein. Eur Heart J. 2012;33:657–665. doi: 10.1093/eurheartj/ehr103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gille A, Easton R, D’Andrea D, Wright SD, Shear CL. CSL112 Enhances Biomarkers of Reverse Cholesterol Transport After Single and Multiple Infusions in Healthy Subjects. Arterioscler Thromb Vasc Biol. 2014;34:2106–2114. doi: 10.1161/ATVBAHA.114.303720. [DOI] [PubMed] [Google Scholar]
- 54.Iwata A, Miura S-i, Zhang B, Imaizumi S, Uehara Y, Shiomi M, Saku K. Antiatherogenic effects of newly developed apolipoprotein A-I mimetic peptide/phospholipid complexes against aortic plaque burden in Watanabe-heritable hyperlipidemic rabbits. Atherosclerosis. 2011;218:300–307. doi: 10.1016/j.atherosclerosis.2011.05.029. [DOI] [PubMed] [Google Scholar]
- 55.Amar MJA, D’Souza W, Turner S, Demosky S, Sviridov D, Stonik J, Luchoomun J, Voogt J, Hellerstein M, Sviridov D, Remaley AT. 5A Apolipoprotein Mimetic Peptide Promotes Cholesterol Efflux and Reduces Atherosclerosis in Mice. J Pharmacol Experiment Ther. 2010;334:634–641. doi: 10.1124/jpet.110.167890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Luthi AJ, Lyssenko NN, Quach D, McMahon KM, Millar JS, Vickers KC, Rader DJ, Phillips MC, Mirkin CA, Thaxton CS. Robust passive and active efflux of cellular cholesterol to a designer functional mimic of high density lipoprotein. J Lipid Res. 2015;56:972–985. doi: 10.1194/jlr.M054635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hafiane A, Bielicki JK, Johansson JO, Genest J. Apolipoprotein E derived HDL mimetic peptide ATI-5261 promotes nascent HDL formation and reverse cholesterol transport in vitro. Biochim Biophys Acta. 2014;1841:1498–1512. doi: 10.1016/j.bbalip.2014.07.018. [DOI] [PubMed] [Google Scholar]
- 58.Tang C, Vaughan AM, Anantharamaiah GM, Oram JF. Janus kinase 2 modulates the lipid-removing but not protein-stabilizing interactions of amphipathic helices with ABCA1. J Lipid Res. 2006;47:107–114. doi: 10.1194/jlr.M500240-JLR200. [DOI] [PubMed] [Google Scholar]
- 59.Navab M, Anantharamaiah GM, Reddy ST, Hama S, Hough G, Grijalva VR, Wagner AC, Frank JS, Datta G, Garber D, Fogelman AM. Oral D-4F Causes Formation of Pre-{beta} High-Density Lipoprotein and Improves High-Density Lipoprotein-Mediated Cholesterol Efflux and Reverse Cholesterol Transport From Macrophages in Apolipoprotein E-Null Mice. Circulation. 2004;109:3215–3220. doi: 10.1161/01.CIR.0000134275.90823.87. [DOI] [PubMed] [Google Scholar]
- 60.Sharifov OF, Nayyar G, Garber DW, Handattu SP, Mishra VK, Goldberg D, Anantharamaiah GM, Gupta H. Apolipoprotein E mimetics and cholesterol-lowering properties. Am J Cardiovasc Drugs. 2011;11:371–381. doi: 10.2165/11594190-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 61.Tabet F, Remaley AT, Segaliny AI, Millet J, Yan L, Nakhla S, Barter PJ, Rye KA, Lambert G. The 5A Apolipoprotein A-I Mimetic Peptide Displays Antiinflammatory and Antioxidant Properties In Vivo and In Vitro. Arterioscler Thromb Vasc Biol. 2010;30:246–252. doi: 10.1161/ATVBAHA.109.200196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Datta G, White CR, Dashti N, Chaddha M, Palgunachari MN, Gupta H, Handattu SP, Garber DW, Anantharamaiah GM. Anti-inflammatory and recycling properties of an apolipoprotein mimetic peptide, Ac-hE18A-NH2. Atherosclerosis. 2010;208:134–141. doi: 10.1016/j.atherosclerosis.2009.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Smythies LE, White CR, Maheshwari A, Palgunachari MN, Anantharamaiah GM, Chaddha M, Kurundkar AR, Datta G. The apolipoprotein A-I mimetic, 4F, alters the function of human monocyte-derived macrophages. Am J Physiol Cell Physiol. 2010;298:1538–1548. doi: 10.1152/ajpcell.00467.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Madenspacher JH, Azzam KM, Gong W, Gowdy KM, Vitek MP, Laskowitz DT, Remaley AT, Wang JM, Fessler MB. Apolipoproteins and Apolipoprotein Mimetic Peptides Modulate Phagocyte Trafficking through Chemotactic Activity. J Biol Chem. 2012;287:43730–43740. doi: 10.1074/jbc.M112.377192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vaziri ND, Moradi H, Pahl MV, Fogelman AM, Navab M. In vitro stimulation of HDL anti-inflammatory activity and inhibition of LDL pro-inflammatory activity in the plasma of patients with end-stage renal disease by an apoA-1 mimetic peptide. Kidney Int. 2009;76:437–444. doi: 10.1038/ki.2009.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.White CR, Smythies LE, Crossman DK, Palgunachari MN, Anantharamaiah GM, Datta G. Regulation of Pattern Recognition Receptors by the Apolipoprotein A-I Mimetic Peptide 4F. Arterioscler Thromb Vasc Biol. 2012;32:2631–2639. doi: 10.1161/ATVBAHA.112.300167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kruger AL, Peterson S, Turkseven S, Kaminski PM, Zhang FF, Quan S, Wolin MS, Abraham NG. D-4F Induces Heme Oxygenase-1 and Extracellular Superoxide Dismutase, Decreases Endothelial Cell Sloughing, and Improves Vascular Reactivity in Rat Model of Diabetes. Circulation. 2005;111:3126–3134. doi: 10.1161/CIRCULATIONAHA.104.517102. [DOI] [PubMed] [Google Scholar]
- 68.Zhang Z, Datta G, Zhang Y, Miller AP, Mochon P, Chen YF, Chatham J, Anantharamaiah GM, White CR. Apolipoprotein A-I mimetic peptide treatment inhibits inflammatory responses and improves survival in septic rats. Am J Physiol Heart Circ Physiol. 2009;297:H866–873. doi: 10.1152/ajpheart.01232.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nguyen SD, Jeong TS, Sok DE. Apolipoprotein A-I-mimetic peptides with antioxidant actions. Arch Biochem Biophys. 2006;451:34–42. doi: 10.1016/j.abb.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 70.Mishra VK, Palgunachari MN, McPherson DT, Anantharamaiah GM. Lipid complex of apolipoprotein A-I mimetic peptide 4F is a novel platform for paraoxonase-1 binding and enhancing its activity and stability. Biochem Biophys Res Comm. 2013;430:975–980. doi: 10.1016/j.bbrc.2012.11.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Van Lenten BJ, Wagner AC, Jung C-L, Ruchala P, Waring AJ, Lehrer RI, Watson AD, Hama S, Navab M, Anantharamaiah GM, Fogelman AM. Anti-inflammatory apoA-I-mimetic peptides bind oxidized lipids with much higher affinity than human apoA-I. J Lipid Res. 2008;49:2302–2311. doi: 10.1194/jlr.M800075-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Meriwether D, Imaizumi S, Grijalva V, Hough G, Vakili L, Anantharamaiah GM, Farias-Eisner R, Navab M, Fogelman AM, Reddy ST, Shechter I. Enhancement by LDL of transfer of L-4F and oxidized lipids to HDL in C57BL/6J mice and human plasma. J Lipid Res. 2011;52:1795–1809. doi: 10.1194/jlr.M016741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yao S, Tian H, Miao C, Zhang DW, Zhao L, Li Y, Yang N, Jiao P, Sang H, Guo S, Wang Y, Qin S. D4F alleviates macrophage-derived foam cell apoptosis by inhibiting CD36 expression and ER stress-CHOP pathway. J Lipid Res. 2015;56:836–847. doi: 10.1194/jlr.M055400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang Z, Qun J, Cao C, Wang J, Li W, Wu Y, Du L, Zhao P, Gong K. Apolipoprotein A-I mimetic peptide D-4F promotes human endothelial progenitor cell proliferation, migration, adhesion though eNOS/NO pathway. Mol Biol Rep. 2012;39:4445–4454. doi: 10.1007/s11033-011-1233-0. [DOI] [PubMed] [Google Scholar]
- 75.Dai L, Datta G, Zhang Z, Gupta H, Patel R, Honavar J, Modi S, Wyss JM, Palgunachari M, Anantharamaiah GM, White CR. The apolipoprotein A-I mimetic peptide 4F prevents defects in vascular function in endotoxemic rats. J Lipid Res. 2010;51:2695–2705. doi: 10.1194/jlr.M008086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ou Z, Ou J, Ackerman AW, Oldham KT, Pritchard KA., Jr L-4F, an Apolipoprotein A-1 Mimetic, Restores Nitric Oxide and Superoxide Anion Balance in Low-Density Lipoprotein-Treated Endothelial Cells. Circulation. 2003;107:1520–1524. doi: 10.1161/01.cir.0000061949.17174.b6. [DOI] [PubMed] [Google Scholar]
- 77.Ou J, Wang J, Xu H, Ou Z, Sorci-Thomas MG, Jones DW, Signorino P, Densmore JC, Kaul S, Oldham KT, Pritchard KA., Jr Effects of D-4F on Vasodilation and Vessel Wall Thickness in Hypercholesterolemic LDL Receptor-Null and LDL Receptor/Apolipoprotein A-I Double-Knockout Mice on Western Diet. Circ Res. 2005;97:1190–1197. doi: 10.1161/01.RES.0000190634.60042.cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Buga GM, Navab M, Imaizumi S, Reddy ST, Yekta B, Hough G, Chanslor S, Anantharamaiah GM, Fogelman AM. L-4F Alters Hyperlipidemic (But Not Healthy) Mouse Plasma to Reduce Platelet Aggregation. Arterioscler Thromb Vasc Biol. 2010;30:283–289. doi: 10.1161/ATVBAHA.109.200162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nguyen SD, Javanainen M, Rissanen S, Zhao H, Huusko J, Kivelä AM, Ylä-Herttuala S, Navab M, Fogelman AM, Vattulainen I, Kovanen PT, Öörni K. Apolipoprotein A-I mimetic peptide 4F blocks sphingomyelinase-induced LDL aggregation. J Lipid Res. 2015;56:1206–1221. doi: 10.1194/jlr.M059485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Navab M, Reddy ST, Anantharamaiah GM, Imaizumi S, Hough G, Hama S, Fogelman AM. Intestine may be a major site of action for the apoA-I mimetic peptide 4F whether administered subcutaneously or orally. J Lipid Res. 2011;52:1200–1210. doi: 10.1194/jlr.M013144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Navab M, Reddy ST, Van Lenten BJ, Buga GM, Hough G, Wagner AC, Fogelman AM. High-Density Lipoprotein and 4F Peptide Reduce Systemic Inflammation by Modulating Intestinal Oxidized Lipid Metabolism. Arterioscler Thromb Vasc Biol. 2012;32:2553–2560. doi: 10.1161/ATVBAHA.112.300282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Duivenvoorden R, Tang J, Cormode DP, Mieszawska AJ, Izquierdo-Garcia D, Ozcan C, Otten MJ, Zaidi N, Lobatto ME, van Rijs SM, Priem B, Kuan EL, Martel C, Hewing B, Sager H, Nahrendorf M, Randolph GJ, Stroes ES, Fuster V, Fisher EA, Fayad ZA, Mulder WJ. A statin-loaded reconstituted high-density lipoprotein nanoparticle inhibits atherosclerotic plaque inflammation. Nat Commun. 2014;5:3065. doi: 10.1038/ncomms4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shah PK, Nilsson J, Kaul S, Fishbein MC, Ageland H, Hamsten A, Johansson J, Karpe F, Cercek B. Effects of recombinant apolipoprotein A-I(Milano) on aortic atherosclerosis in apolipoprotein E-deficient mice. Circulation. 1998;97:780–785. doi: 10.1161/01.cir.97.8.780. [DOI] [PubMed] [Google Scholar]
- 84.Shah PK, Yano J, Reyes O, Chyu KY, Kaul S, Bisgaier CL, Drake S, Cercek B. High-Dose Recombinant Apolipoprotein A-IMilano Mobilizes Tissue Cholesterol and Rapidly Reduces Plaque Lipid and Macrophage Content in Apolipoprotein E-Deficient Mice : Potential Implications for Acute Plaque Stabilization. Circulation. 2001;103:3047–3050. doi: 10.1161/hc2501.092494. [DOI] [PubMed] [Google Scholar]
- 85.Chiesa G, Monteggia E, Marchesi M, Lorenzon P, Laucello M, Lorusso V, Di Mario C, Karvouni E, Newton RS, Bisgaier CL, Franceschini G, Sirtori CR. Recombinant Apolipoprotein A-IMilano Infusion Into Rabbit Carotid Artery Rapidly Removes Lipid From Fatty Streaks. Circ Res. 2002;90:974–980. doi: 10.1161/01.res.0000018422.31717.ee. [DOI] [PubMed] [Google Scholar]
- 86.Ibanez B, Giannarelli C, Cimmino G, Santos-Gallego CG, Alique M, Pinero A, Vilahur G, Fuster V, Badimon L, Badimon JJ. Recombinant HDLMilano exerts greater anti-inflammatory and plaque stabilizing properties than HDLwild-type. Atherosclerosis. 2012;220:72–77. doi: 10.1016/j.atherosclerosis.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 87.Tardy C, Goffinet M, Boubekeur N, Ackermann R, Sy G, Bluteau A, Cholez G, Keyserling C, Lalwani N, Paolini JF, Dasseux JL, Barbaras R, Baron R. CER-001, a HDL-mimetic, stimulates the reverse lipid transport and atherosclerosis regression in high cholesterol diet-fed LDL-receptor deficient mice. Atherosclerosis. 2014;232:110–118. doi: 10.1016/j.atherosclerosis.2013.10.018. [DOI] [PubMed] [Google Scholar]
- 88.Morgantini C, Imaizumi S, Grijalva V, Navab M, Fogelman AM, Reddy ST. Apolipoprotein A-I mimetic peptides prevent atherosclerosis development and reduce plaque inflammation in a murine model of diabetes. Diabetes. 2010;59:3223–3228. doi: 10.2337/db10-0844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chattopadhyay A, Navab M, Hough G, Gao F, Meriwether D, Grijalva V, Springstead JR, Palgnachari MN, Namiri-Kalantari R, Su F, Van Lenten BJ, Wagner AC, Anantharamaiah GM, Farias-Eisener R, Reddy ST, Fogelman AM. A novel approach to oral apoA-I mimetic therapy. J Lipid Res. 2013;54:995–1010. doi: 10.1194/jlr.M033555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ditiatkovski M, D’Souza W, Kesani R, Chin-Dusting J, de Haan JB, Remaley A, Sviridov D. An Apolipoprotein A-I Mimetic Peptide Designed with a Reductionist Approach Stimulates Reverse Cholesterol Transport and Reduces Atherosclerosis in Mice. PLoS ONE. 2013;8:e68802. doi: 10.1371/journal.pone.0068802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Handattu SP, Nayyar G, Garber DW, Palgunachari MN, Monroe CE, Keenum TD, Mishra VK, Datta G, Anantharamaiah GM. Two apolipoprotein E mimetic peptides with similar cholesterol reducing properties exhibit differential atheroprotective effects in LDL-R null mice. Atherosclerosis. 2013;227:58–64. doi: 10.1016/j.atherosclerosis.2012.10.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gupta H, White CR, Handattu S, Garber DW, Datta G, Chaddha M, Dai L, Gianturco SH, Bradley WA, Anantharamaiah GM. Apolipoprotein E mimetic Peptide dramatically lowers plasma cholesterol and restores endothelial function in watanabe heritable hyperlipidemic rabbits. Circulation. 2005;111:3112–3118. doi: 10.1161/CIRCULATIONAHA.104.497107. [DOI] [PubMed] [Google Scholar]
- 93.Nayyar G, Garber DW, Palgunachari MN, Monroe CE, Keenum TD, Handattu SP, Mishra VK, Anantharamaiah GM. Apolipoprotein E mimetic is more effective than apolipoprotein A-I mimetic in reducing lesion formation in older female apo E null mice. Atherosclerosis. 2012;224:326–331. doi: 10.1016/j.atherosclerosis.2012.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nissen SE, Tsunoda T, Tuzcu EM, Schoenhagen P, Cooper CJ, Yasin M, Eaton GM, Lauer MA, Sheldon WS, Grines CL, Halpern S, Crowe T, Blankenship JC, Kerensky R. Effect of recombinant ApoA-I Milano on coronary atherosclerosis in patients with acute coronary syndromes: a randomized controlled trial. JAMA. 2003;290:2292–2300. doi: 10.1001/jama.290.17.2292. [DOI] [PubMed] [Google Scholar]
- 95.Easton R, Gille A, D’Andrea D, Davis R, Wright SD, Shear C. A multiple ascending dose study of CSL112, an infused formulation of ApoA-I. J Clin Pharmacol. 2014;54:301–310. doi: 10.1002/jcph.194. [DOI] [PubMed] [Google Scholar]
- 96.Shaw JA, Bobik A, Murphy A, Kanellakis P, Blombery P, Mukhamedova N, Woollard K, Lyon S, Sviridov D, Dart AM. Infusion of reconstituted high-density lipoprotein leads to acute changes in human atherosclerotic plaque. Circ Res. 2008;103:1084–1091. doi: 10.1161/CIRCRESAHA.108.182063. [DOI] [PubMed] [Google Scholar]
- 97.Kootte RS, Smits LP, van der Valk FM, Dasseux JL, Keyserling CH, Barbaras R, Paolini JF, Santos RD, van Dijk TH, Dallinga-van Thie GM, Nederveen AJ, Mulder WJM, Hovingh GK, Kastelein JJP, Groen AK, Stroes ES. Effect of open-label infusion of an apoA-I-containing particle (CER-001) on RCT and artery wall thickness in patients with FHA. J Lipid Res. 2015;56:703–712. doi: 10.1194/jlr.M055665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bloedon LT, Dunbar R, Duffy D, Pinell-Salles P, Norris R, DeGroot BJ, Movva R, Navab M, Fogelman AM, Rader DJ. Safety, pharmacokinetics, and pharmacodynamics of oral apoA-I mimetic peptide D-4F in high-risk cardiovascular patients. J Lipid Res. 2008;49:1344–1352. doi: 10.1194/jlr.P800003-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Watson CE, Weissbach N, Kjems L, Ayalasomayajula S, Zhang Y, Chang I, Navab M, Hama S, Hough G, Reddy ST, Soffer D, Rader DJ, Fogelman AM, Schecter A. Treatment of patients with cardiovascular disease with L-4F, an apo-A1 mimetic, did not improve select biomarkers of HDL function. J Lipid Res. 2011;52:361–373. doi: 10.1194/jlr.M011098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ou J, Ou Z, Jones DW, Holzhauer S, Hatoum OA, Ackerman AW, Weihrauch DW, Gutterman DD, Guice K, Oldham KT, Hillery CA, Pritchard KA., Jr L-4F, an Apolipoprotein A-1 Mimetic, Dramatically Improves Vasodilation in Hypercholesterolemia and Sickle Cell Disease. Circulation. 2003;107:2337–2341. doi: 10.1161/01.CIR.0000070589.61860.A9. [DOI] [PubMed] [Google Scholar]
- 101.Weihrauch D, Xu H, Shi Y, Wang J, Brien J, Jones DW, Kaul S, Komorowski RA, Csuka ME, Oldham KT, Pritchard KA. Effects of D-4F on vasodilation, oxidative stress, angiostatin, myocardial inflammation, and angiogenic potential in tight-skin mice. Am J Physiol Heart Circ Physiol. 2007;293:H1432–1441. doi: 10.1152/ajpheart.00038.2007. [DOI] [PubMed] [Google Scholar]
- 102.Drew BG, Duffy SJ, Formosa MF, Natoli AK, Henstridge DC, Penfold SA, Thomas WG, Mukhamedova N, de Courten B, Forbes JM, Yap FY, Kaye DM, van Hall G, Febbraio MA, Kemp BE, Sviridov D, Steinberg GR, Kingwell BA. High-density lipoprotein modulates glucose metabolism in patients with type 2 diabetes mellitus. Circulation. 2009;119:2103–2111. doi: 10.1161/CIRCULATIONAHA.108.843219. [DOI] [PubMed] [Google Scholar]
- 103.Calkin AC, Drew BG, Ono A, Duffy SJ, Gordon MV, Schoenwaelder SM, Sviridov D, Cooper ME, Kingwell BA, Jackson SP. Reconstituted High-Density Lipoprotein Attenuates Platelet Function in Individuals With Type 2 Diabetes Mellitus by Promoting Cholesterol Efflux. Circulation. 2009;120:2095–2104. doi: 10.1161/CIRCULATIONAHA.109.870709. [DOI] [PubMed] [Google Scholar]
- 104.Sviridov D, Hoang A, Sawyer W, Fidge N. Identification of a sequence of apolipoprotein A-I associated with activation of lecithin:cholesterol acyltransferase. J Biol Chem. 2000;275:19707–19712. doi: 10.1074/jbc.M000962200. [DOI] [PubMed] [Google Scholar]
- 105.Silva RAGD, Huang R, Morris J, Fang J, Gracheva EO, Ren G, Kontush A, Jerome WG, Rye KA, Davidson WS. Structure of apolipoprotein A-I in spherical high density lipoproteins of different sizes. Proc Nat Acad Sci USA. 2008;105:12176–12181. doi: 10.1073/pnas.0803626105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sviridov D. High density lipoprotein – a hero, a mirage or a witness? Front Cardiovasc Med. 2014;1:1–4. doi: 10.3389/fcvm.2014.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]


