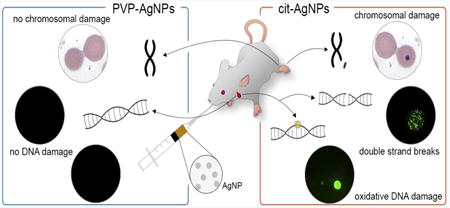
Abstract
Incorporation of silver nanoparticles (AgNPs) in toothpaste, food containers, dietary supplements and other consumer products can result in oral exposure to AgNPs and/or silver ions (Ag+) released from the surface of AgNPs. To examine whether ingestion of AgNPs or Ag+ results in genotoxic damage and whether AgNP coatings modulate the effect, we exposed mice orally to 20 nm citrate-coated AgNPs, polyvinylpyrrolidone (PVP)-coated AgNPs, silver acetate or respective vehicles at a 4 mg/kg dose (equivalent to 800x the EPA reference dose for Ag) for 7 days. Genotoxicity was examined in the systemic circulation and bone marrow at 1, 7, and 14 days post-exposure. We found that citrate-coated AgNPs induced chromosomal damage in bone marrow and oxidative DNA damage and double strand breaks in peripheral blood. These damages persisted for at least 14 days after exposure termination. Because oxidative DNA damage and strand breaks are repaired rapidly, their presence after exposure cessation indicates that citrate-coated AgNPs persist in the body. In contrast, PVP-coated AgNPs and silver acetate did not induce DNA or chromosomal damage at any time point measured. To determine whether coating-dependent genotoxicity is related to different AgNP changes in the gastrointestinal tract, we examined AgNP behavior and fate in an in vitro gastrointestinal digestion model using UV-visible spectroscopy and DLS. Citrate-coated AgNPs were more susceptible to agglomeration than PVP-coated AgNPs in digestive juices with or without proteins. In summary, AgNPs but not Ag+ are genotoxic following oral ingestion. Nanoparticle coatings modulate gastrointestinal transformation and genotoxicity of AgNPs, where higher agglomeration of AgNPs in gastrointestinal juices is associated with higher genotoxicity in tissues. Since genotoxicity is a strong indicator of cancer risk, further long-term studies focusing on cancer are warranted.
Keywords: Silver nanoparticles, oxidative DNA damage, DNA double strand breaks, micronucleus, cancer
Graphical abstract
1. Introduction
Silver nanoparticles (AgNPs) are the most commercialized engineered nanomaterial that is used in more than 30% of nanotechnology-enabled consumer products (PEN, 2015). Due to their unique antimicrobial and antifungal properties, AgNPs are used in personal care products, household items, food contact materials and textile fabrics (Hajipour et al., 2012; PEN, 2015; Tolaymat et al., 2010). Their unique optical properties are exploited in electronics, imaging, catalysis and biosensing (Tolaymat et al., 2010). There are numerous AgNP applications, including everyday items, which can lead to oral exposure to AgNPs and/or silver ions (Ag+) released from the surface of AgNPs through the process of oxidative dissolution. For example, consumer products, including food storage containers (Echegoyen and Nerin, 2013), socks (Benn and Westerhoff, 2008; Geranio et al., 2009; Lorenz et al., 2012) and children's items (Quadros et al., 2013) have been shown to leach Ag (AgNPs and/or Ag+) into water or food, drink and sweat simulating solutions. Incorporation of AgNPs in toothpaste and toothbrushes, food and beverage containers, kitchen utensils and dietary supplements can lead to ingestion of AgNPs and/or Ag+. In addition to direct exposures, the use of AgNPs in laundry detergents, textile fabrics and home appliances can lead to environmental contamination, accumulation in soil or water and unintentional ingestion via edible plants or food animals (Blaser et al., 2008). Thus, the likelihood of exposure to AgNPs is high and gastrointestinal route is a primary route of exposure (Bergin and Witzmann, 2013).
Physicochemical properties of nanomaterials determine their biological effects. Of particular interest are the effects of nanoparticle surface coating and size. Surface coatings are used to stabilize AgNPs in solution by preventing their agglomeration, oxidation and Ag+ release. The effects of AgNP coating and size have been examined on several toxicological parameters, in particular, cell death, oxidative stress and inflammation. Most studies were performed in vitro in cultured cells. In general, the smaller the AgNPs, the greater the effect was observed (Carlson et al., 2008; T. H. Kim et al., 2012; Liu et al., 2010; Miethling-Graff et al., 2014; Prasad et al., 2013). The effects of coatings have been investigated to a lesser extent. Citrate and polyvinylpyrrolidone (PVP) are two of the most commonly used coating agents (Huynh and Chen, 2011). Citrate provides electrostatic stabilization, is weakly bound and can be replaced by other molecules. In comparison, PVP stabilizes AgNPs by steric repulsion. It binds very strongly to metal surfaces and provides high AgNP stability in different solutions. Both coatings provide a negative surface charge to the nanoparticle. Studies that comparatively assessed citrate- and PVP-coated AgNPs found that coating, relative to size, has a small effect (Guo et al., 2016) or results were mixed (Prasad et al., 2013; Vecchio et al., 2014). Only a few studies examined the effect of coating in vivo in rodent animals. Anderson et al. reported that in rats exposed to AgNPs by intratracheal instillation, citrate-coated AgNPs resulted in a greater Ag retention in the lungs and a greater increase in lung macrophages at 21 days post-exposure compared to PVP-coated AgNPs (Anderson et al., 2015). Bergin et al. examined the effects of citrate- and PVP-coated AgNPs on body and organ weights, histopathology effects and fecal elimination kinetics in orally exposed mice, but did not observe changes in any of these parameters (Bergin et al., 2016). To date, the toxicological impact of nanoparticle coating is unclear.
While cytotoxic effects of AgNPs have been confirmed in many in vitro studies, understanding of AgNP-induced genotoxicity is limited. The importance of assessing genotoxicity is underscored by the fact that genotoxicity is a strong indicator of delayed i.e. long-term health effects, especially, cancer. Studies that utilized the Ames test measuring point mutations in bacterial stains of S. typhimurium and/or E. coli reported that AgNPs tested negative for mutagenicity in bacteria (Butler et al., 2015; Guo et al., 2016). The lack of mutagenicity was explained by the inability of bacteria to take up AgNPs (Butler et al., 2015; Guo et al., 2016). However, AgNPs induced mutagenic and clastogenic effects in mammalian cells as shown by mouse lymphoma and micronucleus assays, respectively (Butler et al., 2015; Guo et al., 2016; Vecchio et al., 2014). The effects of AgNPs were similar to those of soluble Ag salts (Ag acetate or Ag nitrate), indicating that both AgNPs and Ag+ can induce genotoxic effects in cultured cells. In contrast to in vitro studies, animal studies that examined micronucleus formation in response to AgNP exposure reported mixed results (Dobrzynska et al., 2014; J. S. Kim et al., 2011; Y. S. Kim et al., 2008; Kovvuru et al., 2015; Y. Li et al., 2014; Patlolla et al., 2015). These studies were performed using different routes of exposure (intravenous, oral and inhalation), treatment durations and AgNP doses. In addition, AgNPs with different sizes and surface coatings or without coatings were used. These differences are likely to contribute to different study results. Further studies linking physiochemical properties of AgNPs to a specific genotoxic outcome and route of exposure are needed to understand whether and what kind of AgNPs pose genotoxic and cancer risks.
Our previous study showed that oral exposure of mice at a high dose (500 mg/kg) of PVP-coated AgNPs resulted in DNA damage and genomic instability in multiple tissues (Kovvuru et al., 2015). The current studies were conducted to understand whether genotoxicity can be induced at a significantly lower dose (4 mg/kg) and whether nanoparticle coating modulates the effect. In addition, to understand whether Ag+ are genotoxic in vivo, we also examined the effect of silver acetate (soluble Ag salt that is used as a source of Ag+). The current studies were performed at a dose equivalent to 800× the EPA oral reference dose (RfD) for Ag (Varner et al., 2010). RfD refers to a daily chronic oral exposure dose in humans that is considered to be safe, while 800× RfD represents the upper range of potential Ag exposure in humans that is associated with development of argyria (permanent discoloration of the skin) after ingestion of colloidal Ag solutions (Chung et al., 2010; Wadhera and Fung, 2005). Little is known how much Ag can be received from the use of AgNP-containing consumer products. A study that characterized AgNPs in selected consumer products estimated that oral exposure to AgNPs when drinking milk formula from a sippy cup is 1.53 μg Ag/kg (Tulve et al., 2015). Significantly higher exposure levels can be anticipated from unregulated use of AgNP dietary supplements and could potentially reach 800× RfD. In addition, irrespective of whether or not human exposure data is available, dose selection in animal studies involves considerations of interspecies differences, in particular, higher susceptibility to toxicants, longer exposure durations and large inter-individual differences in humans versus laboratory rodents. These considerations imply that in order to identify a possible adverse health effect in a small group of animals and translate findings to human populations, doses that are several orders of magnitude higher than human exposure levels are used in animal studies.
In addition to oral exposure studies in whole animals, we examined the behavior and fate of citrate-coated AgNPs and PVP-coated AgNPs in an in vitro gastrointestinal digestion model (Brandon et al., 2006; Versantvoort et al., 2005; Walczak et al., 2013). We found that citrate-coated AgNPs were more susceptible to agglomeration than PVP-coated AgNPs in digestive juices, with or without proteins, and induced genotoxic effects in the systemic circulation and bone marrow. In contrast, ingestion of PVP-AgNPs or Ag+ did not result in genotoxicity.
2. Materials and methods
2.1. Nanoparticles and reagents
AgNPs manufactured by nanoComposix (San Diego, CA) were supplied by the National Institute of Environmental Health Sciences Centers for Nanotechnology Health Implications Research (NCNHIR) consortium. Particles were provided as 20 nm citrate- or PVP-stabilized aqueous 1 mg/ml dispersions (BioPure™). Citrate-coated AgNPs were in 2 mM sodium citrate (pH 7.0) and PVP-coated AgNPs were in water. Ag acetate (AgOAc) and sodium citrate were purchased from Sigma-Aldrich (St. Louis, MO). Chemicals used for the preparation of artificial gastrointestinal juices were obtained from Sigma-Aldrich.
2.2. AgNP Characterization
Physicochemical characterization of AgNPs was performed by the manufacturer (nanoComposix) and by the Nanotechnology Characterization Laboratory at the National Cancer Institute. AgNPs were also characterized in-house with dynamic light scattering (DLS) and UV-visible (UV-vis) spectroscopy prior to use. DLS was performed on samples diluted 1:100 in deionized water with a Zetasizer Nanoseries (Malvern Instruments, Westborough, MA) to characterize AgNP size distribution. UV-visible spectroscopy was performed on samples diluted 1:30 in deionized water with a NanoDrop1000 Spectrophotometer v3.8 (Thermo Fisher Scientific, Franklin, MA) to monitor AgNP colloidal stability.
2.3. Mice and treatments
C57BL/6J pun/pun mice (Jackson Laboratory, Bar Harbor, ME), congenic to C57BL/6J strain, were housed in the virus-free animal facility at the University at Albany Cancer Research Center under standard conditions. All procedures were approved by the institutional animal use and care committee. Seven to 10 week-old mice in equal proportions of males and females were used. There were 3 treatments that were performed at 3 different times: 1) citrate-coated AgNPs and vehicle control (2 mM sodium citrate), 2) PVP-coated AgNPs and vehicle control (water) and 3) AgOAc and vehicle control (water). There were a total of 6 groups with 10 mice each. Test agents were administered by oral gavage daily for 7 consecutive days. Dosing was performed between noon and 2 PM. AgNPs were administered at a daily dose of 4 mg/kg of body weight (i.e. 100 μl of 1 mg/ml AgNPs per 25 mg mouse). Since the actual amounts of Ag+ released from AgNPs (in consumer products, in gastrointestinal lumen or intracellularly) are unknown, mice were treated with AgOAc using a dose that is equivalent to Ag content in 4 mg/kg of AgNPs (i.e. 6 mg/kg or 100 μl of 1.5 mg/ml AgOAc per 25 mg mouse). At this concentration AgOAc results in complete dissolution to ions (solubility in water is 10 mg/ml). After treatment termination, peripheral blood was sampled on days 1, 7 and 14 post-treatment (day 1 represents 24 h after the last dose) and used immediately for analyses. Blood was drawn via sub-mandibular bleeding using a 5 mm lancet (Braintree Scientific, Braintree, MA) into EDTA coated microtubes (Braintree Scientific).
2.4. Immunofluorescence of γ-H2AX
Phosphorylated histone H2AX (γ-H2AX) foci were measured as a marker of DNA double strand breaks (DSBs). The assay was performed as in our previous study (Kovvuru et al., 2015). Briefly, 50 μl of peripheral blood was incubated with 10× (v/v) erythrocyte lysis buffer (8.5 g/L ammonium chloride in 0.01 M Tris-HCl buffer, pH 7.5) to eliminate red blood cells. The remaining cells were collected by centrifugation, suspended in 100 μl of phosphate buffered saline (PBS) and pipetted onto poly-D-lysine coated coverslips to allow cell attachment to the coverslips. Cells were then fixed with 4% paraformaldehyde, permeabilized with 0.5% Triton-X 100 and washed with PBS. After blocking, cells were incubated with mouse anti-phospho-Histone2AX (Ser139) antibody clone JBW301 (EMD Millipore, Billerica, MA, Catalog #05-636) at a 1:400 dilution followed by incubation with fluorescein (FITC)-conjugated anti-mouse immunoglobulin G (IgG) secondary antibody (Jackson Immunochemicals, West Grove, PA, Catalog #115-095-008) at a 1:200 dilution. Coverslips were mounted on microscopic slides with 7 μl of 4′,6-diamidino-2-phenylindole (DAPI)/Vectashield solution (Vector Laboratories, Burlingame, CA) to counterstain nuclei. Slides were visualized under a 100X objective on a Nikon Eclipse TS100 microscope. A minimum of 100 cells were analyzed in a blinded fashion and cells with more than 4 distinct foci in the nucleus were considered positive for γ-H2AX foci formation.
2.5. Immunofluorescence of 8-oxoG
7,8-dihydro-8-oxoguanine (8-oxoG) was measured as a marker of oxidative DNA damage. Fifty til of peripheral blood was incubated with 10× erythrocyte lysis buffer to lyse red blood cells. The remaining cells were centrifuged, suspended in 100 μl of PBS and pipetted onto poly-D-lysine coated coverslips. Cells were fixed, permeabilized, washed and blocked in a same fashion as for the γ-H2AX staining with the exception that different primary antibodies were used. Mouse anti-8-oxoG antibody clone 438.15 (EMD Millipore # MAB3560) at a dilution of 1:500 was used. Slides were examined in a blinded fashion using a 100X objective on a Nikon Eclipse TS100 microscope. A minimum of 100 cells were analyzed and nuclei that were clearly observable under FITC filter and had their presence confirmed under DAPI filter were considered positive for 8-oxoG damage.
2.6. Micronucleus assay
Micronuclei were measured as a marker of chromosomal damage, which includes chromosomal breaks and aneuploid changes. Three μl of peripheral blood was smeared onto microscopic glass slides and stained with Giemsa (Sigma-Aldrich). Micronuclei were examined under a 100X objective on a Nikon Eclipse TS100 microscope. At least 2000 erythrocytes were scored in a blinded fashion and the frequency of micronuclei was calculated as the number of micronucleated erythrocytes per 2000 erythrocytes.
2.7. Characterization of AgNPs in gastrointestinal juices
An in vitro gastrointestinal digestion model (the “fed” model variant) mimicking AgNP transformation in saliva, stomach and intestines was used (Brandon et al., 2006; Versantvoort et al., 2005). The model takes into consideration salt and protein composition, pH differences and transit times in different gastrointestinal compartments. Gastrointestinal digestive juices were prepared and AgNPs were incubated, essentially as described in Walczak et al (Walczak et al., 2013). Constituents and concentrations of gastrointestinal digestive juices are listed in Table 1. AgNP samples were characterized with UV-vis spectroscopy and DLS at each step of the digestion. There were 3 steps of digestion: saliva, gastric and intestinal digestion. Volumes of digestive juices were calculated to achieve a final AgNP concentration of 29 μg/ml. To determine the effect of saliva, 24 μl of AgNPs with a concentration of 1 mg/ml was mixed with 200 μl of water, then 600 μl of saliva (pH 6.8) was added and the mixture was incubated for 5 min at 37°C. To determine the effect of subsequent gastric digestion, 30 μl of AgNPs with a concentration of 1 mg/ml was mixed with 100 μl of water, then 300 μl of saliva (pH 6.8) was added and incubated for 5 min at 37°C, followed by the addition of 600 μl of gastric juice (pH 1.3). This resulting mixture was incubated for 1 h at 37°C. To determine the effect of intestinal digestion, 30 μl of AgNPs with a concentration of 1 mg/ml was mixed with 62 μl of water, 188 μl of saliva (pH 6.8) and incubated for 5 min at 37°C, then 375 μl of gastric juice (pH 1.3) was added and incubated for 1 h at 370C, and finally 375 μl of intestinal juice (pH 8.1) was added, followed by an additional 2 h incubation at 37°C. Samples were mixed by inversion every 15 min.
Table 1.
Composition of gastrointestinal juices of the “fed” in vitro gastrointestinal digestion model (for 1000ml of juice).
| Saliva (pH 6.8± 0.1) | Gastric juice (pH 1.3± 0.1) | Intestinal juice (pH 8.1± 0.1) |
|---|---|---|
| 896 mg KCl | 2752 mg NaCl | 7012 mg NaCl |
| 200 mg KSCN | 306 mg NaH2PO4-H2O | 3388 mg NaHCO3 |
| 1021 mg NaH2PO4-H2O | 824 mg KCl | 80 mg KH2PO4 |
| 570 mg Na2SO4 | 302 mg CaCl2 | 564 mg KCl |
| 298 mg NaCl | 306 mg NH4Cl | 50 mg MgCl2.6H2O |
| 1694 mg NaHCO3 | 6.5 ml 37% HCl | 180 μl 37% HCl |
| 200 mg urea | 650 mg glucose | 100 mg urea |
| 290 mg amylase * | 20 mg glucuronic acid | 151 mg CaCl |
| 15 mg uric acid | 85 mg urea | 1 g BSA * |
| 25 mg mucin * | 330 mg glucoseaminehydrochloride | 9 g pancreatin * |
| Milli-Q water | 1 g BSA * | Milli-Q water |
| 2.5 g pepsin * | ||
| 3 g mucin * | ||
| Milli-Q water |
Proteins included in the incubation with proteins but not in the incubation without proteins.
2.8. Statistical analysis
Data was analyzed by two-way analysis of variance (ANOVA) followed by Tukey post-hoc test using Statistical Analysis Systems (SAS) studio software with treatment and time post-exposure as the two variables. Since background levels in immunofluorescent assays (γ-H2AX and 8-oxoG assays) vary significantly from experiment to experiment, comparisons between different treatment groups were made after corrections in the background levels. P-values less than 0.05 were considered statistically significant. The error bars represent standard error of the mean.
3. Results
3.1. AgNP characterization
The AgNPs were supplied by the NCNHIR consortium and their physicochemical properties were extensively characterized (Wang et al., 2014). We performed UV-vis spectroscopy and DLS analyses to characterize AgNPs prior to administration in animals. UV-vis spectroscopy measurements showed that AgNP dispersions in deionized water had Surface Plasmon Resonance (SPR) peaks at 400 nm, characteristic of AgNPs with an approximate size of 20 nm (NanoComposix, 2016) (Fig. 1A, B). DLS measurements showed that hydrodynamic diameter of AgNPs was 28 ± 0.2 nm for citrate-coated AgNPs and 32 ± 0.7 nm for PVP-coated AgNPs (Fig. 1C, D). The polydispersity index, a measure of the variance in mean hydrodynamic diameter measurements, was 0.15 ± 0.01 for citrate-coated AgNPs and 0.2 ± 0.01 for PVP-coated AgNPs. These analyses indicated AgNPs of the expected size and a narrow size distribution, consistent with other studies using the same AgNPs (Anderson et al., 2015; Wang et al., 2014).
Fig. 1. Characterization of AgNPs by UV-vis spectroscopy and DLS.
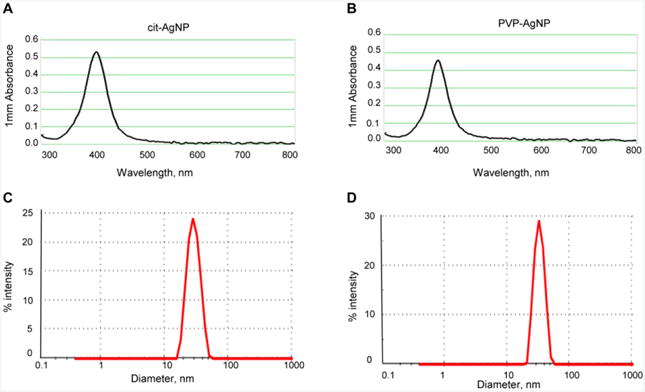
(A) UV-vis absorbance spectra for citrate-coated AgNPs. (B) UV-vis absorbance spectra for PVP-coated AgNPs. (C) Size distribution of citrate-coated AgNPs measured by DLS (D) Size distribution of PVP-coated AgNPs measured by DLS.
3.2. Effect of ingested AgNPs and Ag+ on oxidative DNA damage
Oxidative DNA damage was determined by measuring 8-oxoG levels. 8-oxoG is a pro-mutagenic lesion that can result in C:G to A:T transversion mutations, which are one of the most common somatic mutations in human cancers (Greenman et al., 2007; Hirano, 2008). The levels of 8-oxoG were determined in peripheral blood leukocytes of mice exposed orally to citrate-coated AgNPs, PVP-coated AgNPs or AgOAC. Mice were treated with one kind of AgNPs (or AgOAc) and respective vehicle at a given time and samples were analyzed immediately after blood collection. We found that intake of citrate-coated AgNPs resulted in significantly increased 8-oxoG levels at day 1 post-exposure and 8-oxoG levels were significantly elevated at 7 and 14 days post-exposure (Fig. 2A). The magnitude of DNA damage was similar (not statically significant) at 1, 7 and 14 days post-exposure. Since 8-oxoG is repaired within a short period of time (Ohno et al., 2009), the presence of elevated 8-oxoG at 7 and 14 days after exposure termination implies that citrate-coated AgNPs persist in the body. In contrast, PVP-coated AgNPs did not increase the levels of 8-oxoG beyond background levels (Fig. 2B). Because recent in vitro studies reported that genotoxicity of AgNPs is comparable to that of soluble Ag salts, suggesting that Ag+ is genotoxic (Butler et al., 2015; Guo et al., 2016), we determined 8-oxoG levels in mice exposed to AgOAc. The levels of 8-oxoG were unaltered in AgOAc treated mice compared to their respective controls at 1, 7 and 14 days post-exposure (Fig. 2C). Statistical data analyses of different treatment groups showed a significant difference between citrate-coated AgNPs and PVP-coated AgNPs as well as between citrate-coated AgNPs and AgOAc. There were no statistically significant differences between PVP-coated AgNP and AgOAc treatments. These data show that the capability of AgNPs to induce oxidative DNA damage is modulated by surface coating and that Ag+ are not genotoxic after oral exposure.
Fig. 2. Induction of oxidative DNA damage by AgNPs and Ag+ at 1 day, 1 week and 2 weeks after treatment.

(A) Levels of 8-oxoG in mice treated with citrate-coated AgNPs. (B) Levels of 8-oxoG in mice treated with PVP-coated AgNPs. (C) Levels of 8-oxoG in mice treated with AgOAc. Filled bars show treated mice and open bars show respective controls. Mean ± SEM is shown, n = 10 mice/group, *p ≤ 0.05, **p ≤ 0.01 compared to controls.
3.3. Effect of ingested AgNPs and Ag+ on DNA double strand breaks
DSBs are highly recombinogenic lesions that can result in large-scale genome rearrangements including chromosomal deletions, duplications and translocations that may affect multiple genes (Helleday et al., 2007; van Gent et al., 2001). γ-H2AX foci formation occurs rapidly after DSB induction, serving as a sensitive marker of DSBs (Rogakou et al., 1999). We analyzed γ-H2AX foci in peripheral blood leukocytes of mice treated with citrate-coated AgNPs, PVP-coated AgNPs or AgOAC and compared the γ-H2AX foci levels to their respective vehicle controls. We found that ingestion of citrate-coated AgNPs resulted in increased levels of γ-H2AX foci and that γ-H2AX foci remained similarly elevated for at least 14 days post-exposure (Fig. 3A). In comparison, neither PVP-AgNPs nor AgOAc increased γ-H2AX foci formation after exposure termination (24 h after last dose) or within a 2-week follow-up period (Fig. 3B, C). The levels of γ-H2AX foci were significantly higher in mice treated with citrate-coated AgNPs compared to mice treated with PVP-coated AgNPs or AgOAC. There were no significant differences between treatments with PVP-coated AgNPs and AgOAC. Thus, oral exposure to AgNPs but not Ag+ results in DSB formation in peripheral blood. Citrate coating is an important determinant of the DSB induction by AgNPs.
Fig. 3. Induction of DSBs by AgNPs and Ag+ at 1 day, 1 week and 2 weeks after treatment.
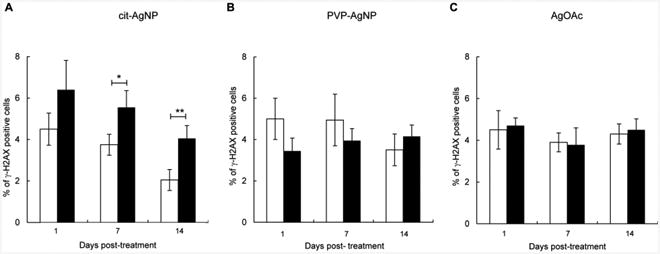
(A) Levels of γ-H2AX foci in mice treated with citrate-coated AgNPs. (B) Levels of γ-H2AX foci in mice treated with PVP-coated AgNPs. (C) Levels of γ-H2AX foci in mice treated with AgOAc. Filled bars show treated mice and open bars show respective controls. Mean ± SEM is shown, n = 10 mice/group, *p ≤ 0.05, **p ≤ 0.01 compared to controls.
3.4. Effect of ingested AgNPs and Ag+ on micronucleus formation
Micronuclei are the result of irreversible chromosomal damage including chromosome breaks and mitotic spindle abnormalities that occur in dividing erythroblasts in the bone marrow. In mice, micronuclei can be scored in peripheral blood since damaged erythroblasts complete division and maturation in the bone marrow and subsequently enter the systemic circulation (Hayashi et al., 2000). We analyzed micronuclei in peripheral blood erythrocytes of mice treated with citrate-coated AgNPs, PVP-coated AgNPs, AgOAC or their respective vehicles. Ingestion of citrate-coated AgNPs resulted in significantly increased numbers of micronucleated cells at 1, 7 and 14 days post-exposure and the magnitude of chromosomal damage was similar at all time points measured (Fig. 4A). In contrast, exposure to PVP-coated AgNPs did not increase the frequency of micronucleated cells (Fig. 4B). Likewise, exposure to AgOAC did not induce micronucleus formation (Fig. 4C). The frequency of micronuclei was significantly higher in mice treated with citrate-coated AgNPs than in mice treated with PVP-coated AgNPs or in mice treated with AgOAC. No difference was observed between PVP-coated AgNP and AgOAC treatments. Thus, only citrate-coated AgNPs induced chromosomal damage in dividing erythroblasts. These data are in agreement with genotoxicity data shown above and support our conclusion that citrate coating is an important determinant AgNP-induced genotoxicity after oral route of exposure.
Fig. 4. Induction of permanent chromosomal damage by AgNPs and Ag+ at 1 day, 1 week and 2 weeks after treatment.
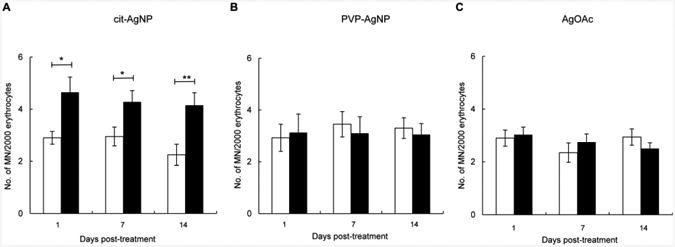
(A) Frequency of micronuclei in mice treated with citrate-coated AgNPs. (B) Frequency of micronuclei in mice treated with PVP-coated AgNPs. (C) Frequency of micronuclei in mice treated with AgOAc. Filled bars show treated mice and open bars show respective controls. Mean ± SEM is shown, n = 10 mice/group, *p ≤ 0.05, **p ≤ 0.01 compared to controls.
3.5. AgNP behavior in gastrointestinal juices
To determine whether differential genotoxicity of citrate-coated AgNPs and PVP-coated AgNPs can be explained by different changes in the gastrointestinal tract, we examined AgNP behavior and fate in an in vitro gastrointestinal digestion model using UV-vis spectroscopy and DLS.
Measuring absorbance of AgNP dispersions within 300 - 800 nm UV-vis spectra can be used to monitor AgNP stability and predict agglomeration in different media (Mwilu et al., 2013). AgNPs with an approximate size of 20 nm dispersed in water display the SPR peak at 400 nm (NanoComposix, 2016). Both citrate-coated AgNPs and PVP-coated AgNPs dispersed in deionized water exhibited the SPR peaks at 400 nm, indicating that AgNPs were about 20 nm (Fig. 5). The absorbance spectra did not change for at least 3 h, which indicates that AgNPs are stable in water (data not shown). However, the absorbance spectra changed markedly after incubation in digestive juices (Table 2 and Fig. 5). The effect was more pronounced for citrate-coated AgNPs. For example, in digestive juices without proteins, the SPR peak for citrate-coated AgNPs sharply decreased after incubation in saliva, disappeared after subsequent incubation in gastric juice and no further changes were induced by intestinal juices (Fig. 5A). In contrast, only a small gradual decrease in the SRP peak was observed for PVP-coated AgNPs after each step of the digestion (Fig. 5B). A sharp decrease in the SPR peak for citrate-coated AgNPs suggested that AgNPs agglomerated to clusters larger than 100 nm, while PVP-coated AgNPs were relatively stable (X. Li et al., 2010). In digestive juices with proteins, AgNP transformation was somewhat different than in digestive juices without proteins. Although citrate-coated AgNPs exhibited a similar loss in the SRP peak after incubation in saliva and gastric juice, the SPR peak at 400 nm reemerged after subsequent incubation in intestinal juice (Fig. 5C). Compared to citrate-coated AgNPs, PVP-coated AgNPs exhibited only a small change in saliva (Fig. 5D). However, the SPR peak decreased and shifted to a larger wave length region after incubation in gastric juice (Fig. 5D) and, similarly to citrate-coated AgNPs, the SPR peak reemerged after incubation in intestinal juice (Fig. 5D). These data suggested that both kinds of AgNPs agglomerate in gastric juices followed by a partial reversal in intestinal juices. This reversal was only observed in the presence of proteins, indicating that intestinal proteins play a role in this process. We also examined whether AgOAc digestion results in the appearance of the SPR peak at about 400 nm, given the reported formation of 20 - 30 nm AgNPs from intestinal digestion of Ag nitrate (Walczak et al., 2013). However, no SPR peaks were observed within 300 - 800 nm UV-vis spectra after AgOAc incubation in digestive juices, suggesting the lack of AgNP formation from AgOAc (data not shown).
Fig. 5. UV-vis spectroscopy characterization of AgNPs in digestive juices with or without protein supplementation.
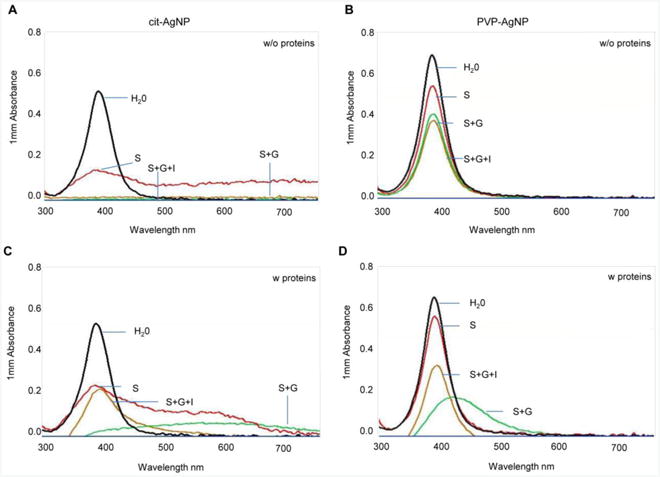
(A) UV-vis absorbance spectra for citrate-coated AgNPs incubated in digestive juices without proteins. (B) UV-vis absorbance spectra for PVP-coated AgNPs incubated in digestive juices without proteins. (C) UV-vis absorbance spectra for citrate-coated AgNPs incubated in digestive juices with proteins (D) UV-vis absorbance spectra for PVP-coated AgNPs incubated in digestive juices with proteins. One representative determination of two is shown. S, saliva; G, gastric juice; I intestinal juice.
Table 2.
Change in UV-Vis absorbance due to AgNP incubation in digestive juices.
| Digestion | Cit-AgNPs | PVP-AgNPs | Cit-AgNPs | PVP-AgNPs |
|---|---|---|---|---|
| Control (water only) | Without proteins 100 | Without proteins 100 | With proteins 100 | With proteins 100 |
| Saliva | 27 | 77 | 45 | 85 |
| Saliva + gastric | 0.6 | 59 | 4.6 | 22 |
| Saliva + gastric + intestinal | 2.9 | 54 | 42 | 51 |
% of SPR peak at 400 nm relative to control is shown. The SRP peak in control is set to be 100. Means of two independent determinations are shown.
To verify the observed changes, we analyzed AgNP size distributions by DLS, which measures hydrodynamic diameter including particle core, coating agents and layers of solvent molecules associated with the individual particle or particle agglomerates (Mwilu et al., 2013). DLS analyses were performed in digestive juices with proteins, as these conditions better mimic the environment of gastrointestinal tract. Both kinds of AgNPs dispersed in water showed mono-modal narrow size range distributions. An average hydrodynamic diameter in water was 28 nm for citrate-coated AgNPs (Fig. 6A) and 31 nm for PVP-coated AgNPs (Fig. 6E). After incubation in saliva, a small population of citrate-coated AgNPs (11% of total DLS intensity) was about 20 nm (their original size), while the remaining nanoparticles were about 70 nm, suggesting protein corona formation and/or slight agglomeration (Fig. 6B). Unlike citrate-coated AgNPs, PVP-coated AgNPs showed only a very small increase in saliva (from 31 nm to 36 nm), although a small peak at ∼200 nm indicates that some AgNPs agglomerated (Fig. 6F). Both kinds of AgNPs showed extensive agglomeration in gastric juice, but the average diameter of citrate-coated AgNPs was 4-times lower than the average diameter of PVP-coated AgNPs (433 nm versus 1753 nm) (Fig. 6C, 6G). After incubation in intestinal juice, AgNPs segregated into two populations, a small population at the nanoscale range and a large population at a higher size range (Fig. 6D, 6H). The average particle size of the small population was 120 nm for citrate-coated AgNPs and 50 nm for PVP-coated AgNPs. The size of the large population was about 1000 nm for citrate-coated AgNPs and 400 nm for PVP-coated AgNPs. In summary, in digestive juices supplemented with proteins, citrate-coated AgNPs were less susceptible to agglomeration in gastric juice but more susceptible to agglomeration in intestinal juice than PVP-coated AgNPs. Some of the agglomerates that formed in gastric juice disintegrated in intestinal juice, in agreement with UV-vis spectroscopy measurements.
Fig. 6. DLS characterization of AgNPs in digestive juices supplemented with proteins.
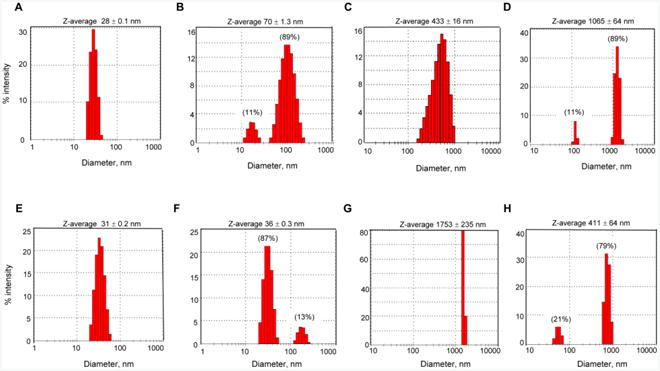
(A) citrate-coated AgNPs in water. (B) citrate-coated AgNPs incubated in saliva. (C) citrate-coated AgNPs incubated in saliva and then gastric juice. (D) citrate-coated AgNPs incubated in saliva, gastric juice and intestinal juice. (E) PVP-coated AgNPs in water. (F) PVP-coated AgNPs incubated in saliva. (G) PVP-coated AgNPs incubated in saliva and then gastric juice. (H) PVP-coated AgNPs incubated in saliva, gastric juice and intestinal juice. Percentage values in brackets above peak 1 and peak 2 in panels B, D, F and H show % of the scattered light intensity in individual peaks. Z-average shown above each panel is a mean hydrodynamic diameter ± SD of three determinations.
4. Discussion
Studies that examined genotoxic potential of AgNPs in vivo in rodents yielded mixed results as to whether AgNPs are genotoxic when inhaled, ingested or following intravenous injection (Dobrzynska et al., 2014; J. S. Kim et al., 2011; Y. S. Kim et al., 2008; Kovvuru et al., 2015; Y. Li et al., 2014; Patlolla et al., 2015). These studies however did not directly address the effect of physicochemical properties of AgNPs. In the current study, we show that surface coating is an important determinant of AgNP-induced genotoxicity. In particular, we demonstrate that citrate-coated AgNPs induced oxidative DNA damage, DSBs and chromosomal damage after oral exposure. In contrast, PVP-coated AgNPs were not genotoxic at the same dose. In addition, citrate-coated AgNP-induced genome lesions remained elevated during a 2-week follow up period. Oxidative DNA damage and DSBs are transient DNA lesions that are repaired rapidly or, alternatively, result in permanent rearrangements or cell death. Therefore, increased oxidative DNA damage and DSBs at 7 and 14 days post-exposure suggest that citrate-coated AgNPs persist in the body and cause DNA damage even after exposure cessation.
The possible mechanisms of AgNP-induced genotoxicity have been discussed in the literature and include oxidative stress resulting from mitochondrial damage, glutathione depletion and activation of immune cells or from direct damage to chromosomal DNA during cell division (Butler et al., 2015; McShan et al., 2014; Volker et al., 2013). In addition, in vivo studies conducted by us and in vitro studies performed by other investigators showed that AgNPs downregulate DNA repair genes involved in the repair of oxidative DNA damage (Chatterjee et al., 2014; Kovvuru et al., 2015; Piao et al., 2011). Thus, induction of oxidative stress-mediated or direct damage to DNA and aberrant expression of relevant DNA repair genes are possible mechanisms of genotoxicity of AgNPs.
However, the role of surface coatings is unclear. Few studies have compared genotoxic effects of AgNPs coated with citrate or PVP, and none of them were performed in vivo in rodent animals. In vitro studies have showed that citrate-coated AgNPs were more genotoxic than PVP-coated AgNPs in a micronucleus assay and/or mouse lymphoma assay (Guo et al., 2016; Vecchio et al., 2014). Given these findings in cultured cells and our current observations in a mouse model, it is likely that AgNPs, which are electrostatically stabilized by small molecules such as citrate, pose higher genotoxic risk compared to AgNPs stabilized by polymers providing steric hindrance such as PVP. The reasons for coating-dependent genotoxicity of AgNPs are currently unknown, but differences in AgNP uptake and retention in tissues and gastrointestinal transformation could be possible explanations for this phenomenon.
There was no observed difference in the uptake or intracellular localization of AgNPs coated with citrate or PVP in BEAS 2B cells, despite different agglomeration patterns in cell culture medium (Gliga et al., 2014). Likewise, Ag levels were not significantly different in the lungs of rats at 1 day after intratracheal instillation of citrate- and PVP-coated AgNPs (Anderson et al., 2015). However, Ag levels at 21 days post-exposure were 3-fold higher in rats exposed to citrate-coated AgNPs than PVP-coated AgNPs (Anderson et al., 2015). This observation suggested that citrate-coated AgNPs persist in the lung longer than PVP-coated AgNPs. Other studies did not address the effect of different coatings, but measured Ag tissue retention in animals exposed to bare AgNPs or both bare AgNPs and PVP-coated AgNPs. A study that examined a time-dependent tissue distribution of bare AgNPs injected intravenously reported high Ag levels in all examined organs (liver, spleen, kidneys, lung and brain) after 28 days of exposure (Dziendzikowska et al., 2012). Also, higher Ag levels were found in kidney and spleen of rats exposed orally to bare AgNPs than PVP-coated AgNPs at 1, 7 and 55 days post-exposure (van der Zande et al., 2012). These studies suggested that bare AgNPs or citrate- coated AgNPs can result in higher tissue accumulation and longer retention than PVP-coated AgNPs.
We observed that citrate- and PVP-coated AgNPs undergo different transformation in gastrointestinal juices. In the absence of proteins, citrate-coated AgNPs agglomerated extensively in saliva, gastric and intestinal juices, relative to PVP-coated AgNPs. High ionic strength in all digestive juices and low pH in gastric juice is responsible for agglomeration of citrate-coated AgNPs (El Badawy et al., 2010; Rogers et al., 2012). However, in the presence of proteins, both kinds of AgNPs agglomerated in gastric and intestinal juices. It is interesting that some agglomerates that formed in gastric juice partially reversed in intestinal juice. Proteins were essential for this reversal because it did not occur without proteins. This observation and findings by Walczak et al provide evidence that under physiological conditions agglomerates that are formed in the stomach can be partially reversed to nanoscale particles in the intestine (Walczak et al., 2013). Nanoscale particles however comprised only a small population, whereas the majority of AgNPs were larger than 400 nm. Given that oral bioavailability of AgNPs is low (1 - 4%) and that larger than 300 nm AgNPs are not absorbed through gastrointestinal tract, it is possible than only this small population of AgNPs is bioavailable to organs and tissues (Park et al., 2010; Park et al., 2011). The average particle size of this population was 120 nm for citrate-coated AgNPs and 50 nm for PVP-coated AgNPs. This difference in hydrodynamic diameter may be due to differences in protein corona formation or agglomeration in digestive juices. Citrate-coated AgNPs have a higher affinity to proteins than PVP-coated AgNPs, suggesting they could absorb more proteins on their surface (Ahlberg et al., 2014). However, the amount of protein (serum albumin) absorbed on AgNPs was similar in citrate- and PVP-coated AgNPs (Pang et al., 2016). In addition, protein corona composition was similar for citrate- and PVP-coated AgNPs after AgNP incubation in cell culture medium supplemented with fetal bovine serum (Shannahan et al., 2013). It is therefore unlikely that citrate- and PVP-coated AgNPs obtain a different protein corona in physiological environments. A difference in AgNP size is probably due to differences in agglomeration. Larger size AgNPs and agglomerates may be retained in the body longer than smaller AgNPs. Thus, genotoxic effects of citrate-coated AgNPs that we observed for at least two weeks after exposure termination may be associated with their longer retention in tissues.
It has been debated whether biological effects of AgNPs are mediated by particulate form or released Ag+ (McShan et al., 2014; Volker et al., 2013). Ag+ are bioactive due to their ability to complex protein thiol groups, interfere with Na+/Cl- transport and contribute to generation of reactive oxygen species (Wang et al., 2014). Ag+ release from AgNPs can occur in different environments. For example, Ag+ can be released in food or beverage from food and drink containers, in gastrointestinal lumen during AgNP transit through gastrointestinal tract, in blood after gastrointestinal absorption and in sub-cellular compartments after AgNP uptake in tissues. To discriminate between the effects of particulate and ionic forms of AgNPs, research studies often include soluble Ag salts as Ag+ control. In the current study, we examined the effect of Ag+ and failed to observe DNA and chromosomal damage. Thus, our study results suggest that Ag+ are non-genotoxic. This is in contrast to recent in vitro studies reporting that Ag+ (soluble Ag salts) are mutagenic in cultured cells as shown by mouse lymphoma and micronucleus assays (Butler et al., 2015; Guo et al., 2016). The capability of Ag salts to induce genotoxic effects in cultured cells has often been interpreted as Ag+ being responsible for genotoxicity of AgNPs (a ‘Trojan horse’ effect). Our observation that Ag+ are not genotoxic after oral ingestion can be explained that, in high salt and acidic environment within the gastrointestinal tract, Ag+ transform into insoluble salts (AgCl), resulting in the loss of Ag+ activity. After absorption from gastrointestinal tract into blood and tissues, AgNPs may release Ag+ intracellularly and act by a ‘Trojan horse’ mechanism. However complexation of Ag+ with intracellular thiols and association with anions, such as Cl-, PO43- S2- and SO42-, and formation of Ag precipitates would strongly reduce bioavailability of Ag+. Moreover, intracellular Ag+ release in AgNP exposed cells is unknown. This is perhaps due to the lack of analytic techniques that can distinguish Ag+ from other Ag species in complex and compartmentalized environments such as live cells. To mimic intracellular Ag+ release AgNPs were mixed with cell culture media or other solutions and Ag levels were measured after removal of AgNPs. AgNPs shed relatively low levels of Ag+ in cell culture media. For example, 20 nm AgNPs regardless of coating released about 4% of Ag+ into BEGM medium, and larger AgNPs shed lesser amounts of Ag+ (Wang et al., 2014). Release of Ag+ from 5 nm PVP-coated AgNPs into RPMI medium was even lower (0.5%) (Y. Li et al., 2016). Ag+ were neither cytotoxic nor genotoxic at this concentration (Y. Li et al., 2016). These studies suggested that the contribution of Ag+ to genotoxicity of AgNPs is only minimal. Thus, a particulate form of AgNPs seems to be a major contributor of genotoxic effects of AgNPs.
5. Conclusions
This study demonstrated that genotoxicity of orally ingested AgNPs depends on the nanoparticle surface coating material. Ingestion of citrate-coated AgNPs in mice resulted in genotoxic effects (8-oxoG, DSBs and micronuclei) in the systemic circulation and bone marrow, while PVP-coated AgNPs were not genotoxic at the same dose used. In addition, citrate-coated AgNP-induced 8-oxoG and DSBs remained elevated for at least two weeks following exposure termination. Given that this kind of DNA damage can be repaired within short periods of time, their persistence post-exposure suggests that citrate-coated AgNPs persist in the body. This study also shows that Ag+ did not induce systemic or bone marrow genotoxicity following oral exposure. Therefore, AgNPs are the likely cause of genotoxic effects in vivo. Citrate-coated AgNPs were more susceptible to agglomeration than PVP-coated AgNPs in digestive juices and were more genotoxic in tissues. Despite extensive agglomeration in gastric juice, a proportion of AgNPs reverted to nanoparticles following incubation in intestinal juice. This reversal occurred only in juices supplemented with proteins, but not in juices without proteins. Thus, under physiological conditions, both nanoscale and larger particles seem to arrive at the intestine. Our findings that citrate-coated AgNPs produced genotoxic effects after short-term exposure warrant further investigations. Since genotoxicity is a precursor for carcinogenicity, studies focusing on cancer are recommended.
Highlights.
Citrate-coated AgNPs but not PVP-coated AgNPs are genotoxic after oral exposure
Citrate-coated AgNPs induce persistent DNA damage
Silver ions are not genotoxic after oral exposure
Acknowledgments
This study was supported by the National Institute of Environmental Health Sciences (R56ESO24123) and the Dominic Ferraioli Foundation. Silver nanoparticles used in this study were procured, characterized and provided by the NCNHIR consortium.
Abbreviations
- AgNPs
silver nanoparticles
- cit
citrate
- PVP
polyvinylpyrrolidone
- AgOAc
silver acetate
- Ag+
silver ions
- 8-oxoG
7,8-dihydro-8-oxoguanine
- γ-H2AX
phosphorylated histone 2AX
- DSBs
double strand breaks, MN, micronuclei
- SPR
surface plasmon resonance
- DLS
dynamic light scattering
- UV-vis
UV-visible spectroscopy
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahlberg S, Antonopulos A, Diendorf J, Dringen R, Epple M, Flock R, Goedecke W, Graf C, Haberl N, Helmlinger J, et al. PVP-coated, negatively charged silver nanoparticles: A multi-center study of their physicochemical characteristics, cell culture and in vivo experiments. Beilstein J Nanotechnol. 2014;5:1944–1965. doi: 10.3762/bjnano.5.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson DS, Silva RM, Lee D, Edwards PC, Sharmah A, Guo T, Pinkerton KE, Van Winkle LS. Persistence of silver nanoparticles in the rat lung: Influence of dose, size, and chemical composition. Nanotoxicology. 2015;9(5):591–602. doi: 10.3109/17435390.2014.958116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benn TM, Westerhoff P. Nanoparticle silver released into water from commercially available sock fabrics. Environ Sci Technol. 2008;42(11):4133–4139. doi: 10.1021/es7032718. [DOI] [PubMed] [Google Scholar]
- Bergin IL, Wilding LA, Morishita M, Walacavage K, Ault AP, Axson JL, Stark DI, Hashway SA, Capracotta SS, Leroueil PR, et al. Effects of particle size and coating on toxicologic parameters, fecal elimination kinetics and tissue distribution of acutely ingested silver nanoparticles in a mouse model. Nanotoxicology. 2016;10(3):352–360. doi: 10.3109/17435390.2015.1072588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergin IL, Witzmann FA. Nanoparticle toxicity by the gastrointestinal route: evidence and knowledge gaps. Int J Biomed Nanosci Nanotechnol. 2013;3(1-2) doi: 10.1504/IJBNN.2013.054515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaser SA, Scheringer M, Macleod M, Hungerbuhler K. Estimation of cumulative aquatic exposure and risk due to silver: contribution of nano-functionalized plastics and textiles. Sci Total Environ. 2008;390(2-3):396–409. doi: 10.1016/j.scitotenv.2007.10.010. [DOI] [PubMed] [Google Scholar]
- Brandon EF, Oomen AG, Rompelberg CJ, Versantvoort CH, van Engelen JG, Sips AJ. Consumer product in vitro digestion model: Bioaccessibility of contaminants and its application in risk assessment. Regul Toxicol Pharmacol. 2006;44(2):161–171. doi: 10.1016/j.yrtph.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Butler KS, Peeler DJ, Casey BJ, Dair BJ, Elespuru RK. Silver nanoparticles: correlating nanoparticle size and cellular uptake with genotoxicity. Mutagenesis. 2015;30(4):577–591. doi: 10.1093/mutage/gev020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson C, Hussain SM, Schrand AM, Braydich-Stolle LK, Hess KL, Jones RL, Schlager JJ. Unique cellular interaction of silver nanoparticles: size-dependent generation of reactive oxygen species. J Phys Chem B. 2008;112(43):13608–13619. doi: 10.1021/jp712087m. [DOI] [PubMed] [Google Scholar]
- Chatterjee N, Eom HJ, Choi J. Effects of silver nanoparticles on oxidative DNA damage-repair as a function of p38 MAPK status: a comparative approach using human Jurkat T cells and the nematode Caenorhabditis elegans. Environ Mol Mutagen. 2014;55(2):122–133. doi: 10.1002/em.21844. [DOI] [PubMed] [Google Scholar]
- Chung IS, Lee MY, Shin DH, Jung HR. Three systemic argyria cases after ingestion of colloidal silver solution. Int J Dermatol. 2010;49(10):1175–1177. doi: 10.1111/j.1365-4632.2009.04380.x. [DOI] [PubMed] [Google Scholar]
- Dobrzynska MM, Gajowik A, Radzikowska J, Lankoff A, Dusinska M, Kruszewski M. Genotoxicity of silver and titanium dioxide nanoparticles in bone marrow cells of rats in vivo. Toxicology. 2014;315:86–91. doi: 10.1016/j.tox.2013.11.012. [DOI] [PubMed] [Google Scholar]
- Dziendzikowska K, Gromadzka-Ostrowska J, Lankoff A, Oczkowski M, Krawczynska A, Chwastowska J, Sadowska-Bratek M, Chajduk E, Wojewodzka M, Dusinska M, et al. Time-dependent biodistribution and excretion of silver nanoparticles in male Wistar rats. J Appl Toxicol. 2012;32(11):920–928. doi: 10.1002/jat.2758. [DOI] [PubMed] [Google Scholar]
- Echegoyen Y, Nerin C. Nanoparticle release from nano-silver antimicrobial food containers. Food Chem Toxicol. 2013;62:16–22. doi: 10.1016/j.fct.2013.08.014. [DOI] [PubMed] [Google Scholar]
- El Badawy AM, Luxton TP, Silva RG, Scheckel KG, Suidan MT, Tolaymat TM. Impact of environmental conditions (pH, ionic strength, and electrolyte type) on the surface charge and aggregation of silver nanoparticles suspensions. Environ Sci Technol. 2010;44(4):1260–1266. doi: 10.1021/es902240k. [DOI] [PubMed] [Google Scholar]
- Geranio L, Heuberger M, Nowack B. The behavior of silver nanotextiles during washing. Environ Sci Technol. 2009;43(21):8113–8118. doi: 10.1021/es9018332. [DOI] [PubMed] [Google Scholar]
- Gliga AR, Skoglund S, Wallinder IO, Fadeel B, Karlsson HL. Size-dependent cytotoxicity of silver nanoparticles in human lung cells: the role of cellular uptake, agglomeration and Ag release. Part Fibre Toxicol. 2014;11:11. doi: 10.1186/1743-8977-11-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenman C, Stephens P, Smith R, Dalgliesh GL, Hunter C, Bignell G, Davies H, Teague J, Butler A, Stevens C, et al. Patterns of somatic mutation in human cancer genomes. Nature. 2007;446(7132):153–158. doi: 10.1038/nature05610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X, Li Y, Yan J, Ingle T, Jones MY, Mei N, Boudreau MD, Cunningham CK, Abbas M, Paredes AM, et al. Size- and coating-dependent cytotoxicity and genotoxicity of silver nanoparticles evaluated using in vitro standard assays. Nanotoxicology. 2016;10(9):1373–1384. doi: 10.1080/17435390.2016.1214764. [DOI] [PubMed] [Google Scholar]
- Hajipour MJ, Fromm KM, Ashkarran AA, Jimenez de Aberasturi D, de Larramendi IR, Rojo T, Serpooshan V, Parak WJ, Mahmoudi M. Antibacterial properties of nanoparticles. Trends Biotechnol. 2012;30(10):499–511. doi: 10.1016/j.tibtech.2012.06.004. [DOI] [PubMed] [Google Scholar]
- Hayashi M, MacGregor JT, Gatehouse DG, Adler ID, Blakey DH, Dertinger SD, Krishna G, Morita T, Russo A, Sutou S. In vivo rodent erythrocyte micronucleus assay. II. Some aspects of protocol design including repeated treatments, integration with toxicity testing, and automated scoring. Environ Mol Mutagen. 2000;35(3):234–252. [PubMed] [Google Scholar]
- Helleday T, Lo J, van Gent DC, Engelward BP. DNA double-strand break repair: from mechanistic understanding to cancer treatment. DNA Repair (Amst) 2007;6(7):923–935. doi: 10.1016/j.dnarep.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Hirano T. Repair system of 7, 8-dihydro-8-oxoguanine as a defense line against carcinogenesis. J Radiat Res. 2008;49(4):329–340. doi: 10.1269/jrr.08049. [DOI] [PubMed] [Google Scholar]
- Huynh KA, Chen KL. Aggregation kinetics of citrate and polyvinylpyrrolidone coated silver nanoparticles in monovalent and divalent electrolyte solutions. Environ Sci Technol. 2011;45(13):5564–5571. doi: 10.1021/es200157h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JS, Sung JH, Ji JH, Song KS, Lee JH, Kang CS, Yu IJ. In vivo Genotoxicity of Silver Nanoparticles after 90-day Silver Nanoparticle Inhalation Exposure. Saf Health Work. 2011;2(1):34–38. doi: 10.5491/SHAW.2011.2.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TH, Kim M, Park HS, Shin US, Gong MS, Kim HW. Size-dependent cellular toxicity of silver nanoparticles. J Biomed Mater Res A. 2012;100(4):1033–1043. doi: 10.1002/jbm.a.34053. [DOI] [PubMed] [Google Scholar]
- Kim YS, Kim JS, Cho HS, Rha DS, Kim JM, Park JD, Choi BS, Lim R, Chang HK, Chung YH, et al. Twenty-eight-day oral toxicity, genotoxicity, and gender-related tissue distribution of silver nanoparticles in Sprague-Dawley rats. Inhal Toxicol. 2008;20(6):575–583. doi: 10.1080/08958370701874663. [DOI] [PubMed] [Google Scholar]
- Kovvuru P, Mancilla PE, Shirode AB, Murray TM, Begley TJ, Reliene R. Oral ingestion of silver nanoparticles induces genomic instability and DNA damage in multiple tissues. Nanotoxicology. 2015;9(2):162–171. doi: 10.3109/17435390.2014.902520. [DOI] [PubMed] [Google Scholar]
- Li X, Lenhart JJ, Walker HW. Dissolution-accompanied aggregation kinetics of silver nanoparticles. Langmuir. 2010;26(22):16690–16698. doi: 10.1021/la101768n. [DOI] [PubMed] [Google Scholar]
- Li Y, Bhalli JA, Ding W, Yan J, Pearce MG, Sadiq R, Cunningham CK, Jones MY, Monroe WA, Howard PC, et al. Cytotoxicity and genotoxicity assessment of silver nanoparticles in mouse. Nanotoxicology. 2014;8(Suppl 1):36–45. doi: 10.3109/17435390.2013.855827. [DOI] [PubMed] [Google Scholar]
- Li Y, Qin T, Ingle T, Yan J, He W, Yin JJ, Chen T. Differential genotoxicity mechanisms of silver nanoparticles and silver ions. Arch Toxicol. 2016 doi: 10.1007/s00204-016-1730-y. [DOI] [PubMed] [Google Scholar]
- Liu W, Wu Y, Wang C, Li HC, Wang T, Liao CY, Cui L, Zhou QF, Yan B, Jiang GB. Impact of silver nanoparticles on human cells: effect of particle size. Nanotoxicology. 2010;4(3):319–330. doi: 10.3109/17435390.2010.483745. [DOI] [PubMed] [Google Scholar]
- Lorenz C, Windler L, von Goetz N, Lehmann RP, Schuppler M, Hungerbuhler K, Heuberger M, Nowack B. Characterization of silver release from commercially available functional (nano)textiles. Chemosphere. 2012;89(7):817–824. doi: 10.1016/j.chemosphere.2012.04.063. [DOI] [PubMed] [Google Scholar]
- McShan D, Ray PC, Yu H. Molecular toxicity mechanism of nanosilver. J Food Drug Anal. 2014;22(1):116–127. doi: 10.1016/j.jfda.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miethling-Graff R, Rumpker R, Richter M, Verano-Braga T, Kjeldsen F, Brewer J, Hoyland J, Rubahn HG, Erdmann H. Exposure to silver nanoparticles induces size-and dose-dependent oxidative stress and cytotoxicity in human colon carcinoma cells. Toxicol In Vitro. 2014;28(7):1280–1289. doi: 10.1016/j.tiv.2014.06.005. [DOI] [PubMed] [Google Scholar]
- Mwilu SK, El Badawy AM, Bradham K, Nelson C, Thomas D, Scheckel KG, Tolaymat T, Ma L, Rogers KR. Changes in silver nanoparticles exposed to human synthetic stomach fluid: effects of particle size and surface chemistry. Sci Total Environ. 2013;447:90–98. doi: 10.1016/j.scitotenv.2012.12.036. [DOI] [PubMed] [Google Scholar]
- NanoComposix. 2016 http://nanocomposix.com/collections/silver.
- Ohno M, Oka S, Nakabeppu Y. Quantitative analysis of oxidized guanine, 8-oxoguanine, in mitochondrial DNA by immunofluorescence method. Methods Mol Biol. 2009;554:199–212. doi: 10.1007/978-1-59745-521-3_13. [DOI] [PubMed] [Google Scholar]
- Pang C, Brunelli A, Zhu C, Hristozov D, Liu Y, Semenzin E, Wang W, Tao W, Liang J, Marcomini A, et al. Demonstrating approaches to chemically modify the surface of Ag nanoparticles in order to influence their cytotoxicity and biodistribution after single dose acute intravenous administration. Nanotoxicology. 2016;10(2):129–139. doi: 10.3109/17435390.2015.1024295. [DOI] [PubMed] [Google Scholar]
- Park EJ, Bae E, Yi J, Kim Y, Choi K, Lee SH, Yoon J, Lee BC, Park K. Repeated-dose toxicity and inflammatory responses in mice by oral administration of silver nanoparticles. Environ Toxicol Pharmacol. 2010;30(2):162–168. doi: 10.1016/j.etap.2010.05.004. [DOI] [PubMed] [Google Scholar]
- Park K, Park EJ, Chun IK, Choi K, Lee SH, Yoon J, Lee BC. Bioavailability and toxicokinetics of citrate-coated silver nanoparticles in rats. Arch Pharm Res. 2011;34(1):153–158. doi: 10.1007/s12272-011-0118-z. [DOI] [PubMed] [Google Scholar]
- Patlolla AK, Hackett D, Tchounwou PB. Genotoxicity study of silver nanoparticles in bone marrow cells of Sprague-Dawley rats. Food and Chemical Toxicology. 2015;85:52–60. doi: 10.1016/j.fct.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PEN, C.P.I. [10 March 2016date last accessed)];Consumer Products Inventory The project on emerging nanotechnologies (PEN) 2015 http://www.nanotechproject.org/inventories/consumer/
- Piao MJ, Kim KC, Choi JY, Choi J, Hyun JW. Silver nanoparticles down-regulate Nrf2-mediated 8-oxoguanine DNA glycosylase 1 through inactivation of extracellular regulated kinase and protein kinase B in human Chang liver cells. Toxicol Lett. 2011;207(2):143–148. doi: 10.1016/j.toxlet.2011.09.002. [DOI] [PubMed] [Google Scholar]
- Prasad RY, McGee JK, Killius MG, Suarez DA, Blackman CF, DeMarini DM, Simmons SO. Investigating oxidative stress and inflammatory responses elicited by silver nanoparticles using high-throughput reporter genes in HepG2 cells: effect of size, surface coating, and intracellular uptake. Toxicol In Vitro. 2013;27(6):2013–2021. doi: 10.1016/j.tiv.2013.07.005. [DOI] [PubMed] [Google Scholar]
- Quadros ME, Pierson RT, Tulve NS, Willis R, Rogers K, Thomas TA, Marr LC. Release of silver from nanotechnology-based consumer products for children. Environ Sci Technol. 2013;47(15):8894–8901. doi: 10.1021/es4015844. [DOI] [PubMed] [Google Scholar]
- Rogakou EP, Boon C, Redon C, Bonner WM. Megabase chromatin domains involved in DNA double-strand breaks in vivo. J Cell Biol. 1999;146(5):905–916. doi: 10.1083/jcb.146.5.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers KR, Bradham K, Tolaymat T, Thomas DJ, Hartmann T, Ma L, Williams A. Alterations in physical state of silver nanoparticles exposed to synthetic human stomach fluid. Sci Total Environ. 2012;420:334–339. doi: 10.1016/j.scitotenv.2012.01.044. [DOI] [PubMed] [Google Scholar]
- Shannahan JH, Lai X, Ke PC, Podila R, Brown JM, Witzmann FA. Silver nanoparticle protein corona composition in cell culture media. PLoS One. 2013;8(9):e74001. doi: 10.1371/journal.pone.0074001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolaymat TM, El Badawy AM, Genaidy A, Scheckel KG, Luxton TP, Suidan M. An evidence-based environmental perspective of manufactured silver nanoparticle in syntheses and applications: a systematic review and critical appraisal of peer-reviewed scientific papers. Sci Total Environ. 2010;408(5):999–1006. doi: 10.1016/j.scitotenv.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Tulve NS, Stefaniak AB, Vance ME, Rogers K, Mwilu S, LeBouf RF, Schwegler-Berry D, Willis R, Thomas TA, Marr LC. Characterization of silver nanoparticles in selected consumer products and its relevance for predicting children's potential exposures. Int J Hyg Environ Health. 2015;218(3):345–357. doi: 10.1016/j.ijheh.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Zande M, Vandebriel RJ, Van Doren E, Kramer E, Herrera Rivera Z, Serrano-Rojero CS, Gremmer ER, Mast J, Peters RJ, Hollman PC, et al. Distribution, elimination, and toxicity of silver nanoparticles and silver ions in rats after 28-day oral exposure. ACS Nano. 2012;6(8):7427–7442. doi: 10.1021/nn302649p. [DOI] [PubMed] [Google Scholar]
- van Gent DC, Hoeijmakers JH, Kanaar R. Chromosomal stability and the DNA double-stranded break connection. Nat Rev Genet. 2001;2(3):196–206. doi: 10.1038/35056049. [DOI] [PubMed] [Google Scholar]
- Varner KE, el-Badawy A, Feldhake D, Venkatapathy R. State-of-the-science review:everything nanosilver and more EPA/600/R-10/084. Washington, DC: U.S. Environmental Protection Agency; 2010. [Google Scholar]
- Vecchio G, Fenech M, Pompa PP, Voelcker NH. Lab-on-a-chip-based high-throughput screening of the genotoxicity of engineered nanomaterials. Small. 2014;10(13):2721–2734. doi: 10.1002/smll.201303359. [DOI] [PubMed] [Google Scholar]
- Versantvoort CH, Oomen AG, Van de Kamp E, Rompelberg CJ, Sips AJ. Applicability of an in vitro digestion model in assessing the bioaccessibility of mycotoxins from food. Food Chem Toxicol. 2005;43(1):31–40. doi: 10.1016/j.fct.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Volker C, Oetken M, Oehlmann J. The biological effects and possible modes of action of nanosilver. Rev Environ Contam Toxicol. 2013;223:81–106. doi: 10.1007/978-1-4614-5577-6_4. [DOI] [PubMed] [Google Scholar]
- Wadhera A, Fung M. Systemic argyria associated with ingestion of colloidal silver. Dermatol Online J. 2005;11(1):12. [PubMed] [Google Scholar]
- Walczak AP, Fokkink R, Peters R, Tromp P, Rivera ZEH, Rietjens IMCM, Hendriksen PJM, Bouwmeester H. Behaviour of silver nanoparticles and silver ions in an in vitro human gastrointestinal digestion model. Nanotoxicology. 2013;7(7):1198–1210. doi: 10.3109/17435390.2012.726382. [DOI] [PubMed] [Google Scholar]
- Wang X, Ji Z, Chang CH, Zhang H, Wang M, Liao YP, Lin S, Meng H, Li R, Sun B, et al. Use of coated silver nanoparticles to understand the relationship of particle dissolution and bioavailability to cell and lung toxicological potential. Small. 2014;10(2):385–398. doi: 10.1002/smll.201301597. [DOI] [PMC free article] [PubMed] [Google Scholar]


