Abstract
Previous work (Brown et al., 2003a,b) has shown that limb position drifts when individuals make repetitive movements in the absence of visual feedback. The purpose of this study was to examine whether limb position drift might reflect a misalignment in visual and proprioceptive maps by examining the nature of information used to specify new movements from a drifted limb position. In a virtual reality (VR) environment, participants made continuous movements with their dominant right hand between two targets positioned 15 cm apart, paced by a 0.625-Hz metronome. After 5 cycles, cursor feedback of the hand was removed for the next 44 cycles, which induced an average drift in hand position of roughly 5 cm. On the 50th cycle, participants were required to move to one of 6 new targets from the drifted hand position. Kinematic analysis indicated that movement direction was unambiguously determined by the visual input marked by the original start position, or the last-seen hand position. Forward dynamics analysis revealed that current limb configuration was used to inform joint torques to produce this parallel direction. For new movement specification, accurate proprioceptive information about the drifted limb position was used, even though it was apparently not available for detecting drift in the first place. Movement distance varied directly with the extent of limb drift, although the differentiation of visual and proprioceptive control of distance could not be analyzed, as our control conditions were not significantly different for this measure. We suggest that movement drift, in the absence of visual feedback during cyclic repetitive movements, reflects a misalignment between largely accurate visual and proprioceptive maps, rather than a weighted fusion of the two modalities. Key words: multisensory integration, proprioception, limb position drift, sensory integration, reaching movement.
INTRODUCTION
Most common everyday activities, like picking up a glass of water, are smoothly performed without much thought or apparent effort. Even simple movements, however, require complex perceptual motor processing incorporating multiple sensory modalities to accurately inform control processes. In reaching for a glass of water, vision and proprioception (sense of position and movement of body parts) inform of the position of the hand, both in relation to the body and to the target (Graziano, 1999; van Beers et al., 1998, 1999). The question of how these two sensory modalities are used to perform visual-motor tasks has been addressed by studies that examine damage to multi-sensory processing centers, such as posterior parietal cortical areas (Andersen and Zipser, 1988; Kertzman et al., 1997; Andersen and Buneo, 2002; Mutha et al., 2011) and other structures in dorsal stream networks (Tanne et al., 1995; Culham et al., 2003; Fridman et al., 2005). Visual and proprioceptive integration has also been studied in individuals without CNS damage by inducing discrepancies in visual and proprioceptive feedback about hand position, either by distorting proprioceptive information via vibration, or by distorting visual feedback (Harris, 1965; Welch and Warren, 1980; Sittig et al., 1985; Gilhodes et al., 1986; Lateiner and Sainburg, 2003; Sainburg et al., 2003). In studies designed to determine how conflicting information from vision and proprioception is used to program and execute targeted reaching movements, both Lateiner et al. (2003), Sainburg et al. (2003) and Sober and Sabes (2003) concluded that visual information predominates in planning an intended movement vector, while proprioceptive information is critical for specifying muscle activations necessary to achieve this action from the conflicting proprioceptive and visual information about hand position. In these studies, neither movement planning nor motor command generation appeared to be distorted by the discrepancy between modalities.
The individual contribution of vision and proprioception to motor planning and control has also been examined in studies in which visual feedback is removed, or when proprioception is unavailable to due to peripheral neuropathy. Patients who lack proprioception due to specific large fiber sensory neuropathy demonstrate deficits in movement that reflect the inability to predict mechanical effects of one’s own body, such as configuration-dependent variations in limb inertia (Ghez et al., 1990) and intersegmental dynamics (Ghez and Sainburg, 1995; Sainburg et al., 1995). Interestingly, visual feedback can partially compensate these deficits, emphasizing the important role of vision in motor planning and control. In fact, when healthy participants are instructed to a maintain posture or repetitive movements of the hand in the absence of visual feedback, they tend to show substantial drift in the hand position (Rothwell et al., 1982; Brown et al., 2003a,b). Participants limb positions drift substantially, even when they are instructed to return the hand to the last-seen hand location after each movement. Thus, in the case of limb drift, a discrepancy exists between the currently available proprioceptive information about limb configuration and the participant’s visual memory of their undrifted hand position.
Brown et al. (2003a,b) examined limb position drift during cyclic out and back movements between two visual targets, following removal of visual feedback. Whereas limb position drifted roughly 8 cm during the course of repetitive movements, the direction and the distance of the individual movements were maintained. Inverse dynamics analysis and forward dynamic simulations confirmed that joint muscle torques were progressively modified in order to maintain the direction and distance of motion as limb configuration changed. This finding appeared paradoxical because it indicates that proprioceptive information was accurate for modulating muscle activations with drift-dependent changes in limb configuration, but this detailed information about limb configuration was not used in order to prevent limb position drift. In a related study, Zelaznick and Lantero (1996) showed that when subjects repetitively moved their hand in a continuous circular motion in the absence of visual feedback, the average limb position drifted while the shape of the hand path was maintained. While that study did not analyze inverse dynamics, one would expect that in order to maintain the shape of the hand path while the limb drifted would require modifications to the muscle activations in accord with drift in limb configuration. In both of these studies, limb position drift appeared to dissociate the instructed position of the limb from the actual position. While it is not clear what might produce the drift, proprioceptive information appears to remain intact during drift in order to specify the joint torques and limb configurations needed to maintain repetitive motions of similar directions and amplitudes (Brown et al., 2003a,b), and repetitive circular motions with similar shapes (Zelaznick and Lantero, 1996), in the face of changing limb configurations.
Two findings are very clear from previous studies of limb position drift: 1) In the absence of vision, the limb drifts, but participants retain accurate proprioceptive information about limb configuration that is used to adjust motor commands to the progressive drift in limb configuration. 2) Participants do not use this information to prevent or correct position drift, suggesting that the movement planning process does not account for the drifted limb configurations. However, in previous studies participants made repetitive movements that may not have required the planning of new movements but rather the adjusting of the existing movement plan to the changes in limb configuration. We now examine how participants plan new movements, following drift induced by performing repetitive movements in the absence of visual feedback.
We now attempt to resolve the apparent paradox between the use of proprioception to modify motor commands in accord with drifted limb configurations, and the failure to use this information to prevent drift in hand position. When faced with the problem of planning a movement to a new target following drift, we ask whether participants use proprioceptive information about the position of the hand to plan a new movement vector. We hypothesize that, just as updated proprioceptive information is used to maintain distance and direction from varying positions during repetitive movements, planning new movements should be able to rely on this information to update new movement plans in accord with the drifted hand positions. We tested this hypothesis by inducing limb drift during performance of a series of repetitive out and back movements and then asking participants to make new movements toward one of six different targets from their drifted limb positions. In a control session, subjects returned to the laboratory another day and were given start circles that matched the positions to which they had previously drifted in order to provide baseline movements that reflected veridical proprioceptive and visual information about those start positions. We then compared the direction and distance of movements made from the drifted positions to movements made to the same six targets when participants had accurate visual and proprioceptive information about these same initial positions.
Our results show that participants plan movement direction to the new targets from a location consistent with the original start position, or the last seen position occupied by their hand, and not from their actual hand positions. Simulation results indicate that they also use current proprioceptive information to adjust their neural commands to muscles in order to account for the altered dynamic requirements of the drifted limb configurations. These findings support the idea that visual information about hand position, in this case visual memory of the last-seen hand position, also marked by the start position, predominates in planning movement vectors, while proprioceptive information is used to adjust movement commands to take account of precise variations in real limb configuration. These two sources of information are discrepant in the current paradigm. However, we find no evidence that this discrepancy results in a fusion of the positions reflected by each modality. Instead, each representation appears to contribute unbiased information for different aspects of motor planning.
EXPERIMENTAL PROCEDURES
Participants
Eleven volunteers, four males and seven females ages 20–23, participated in the experiment. According to the Edinburgh Handedness Questionnaire (Oldfield 1971), all participants were right-handed. Each had normal or corrected-to-normal vision and no history of neurological disorder. All volunteers gave informed consent before participating and were compensated at minimum wage. All the work in this manuscript received formal approval to conduct the experiments from the human subjects’ institutional review board of Penn State University and can be provided upon request.
Apparatus
Each participant sat at a horizontal surface leveled just below shoulder height (see Fig. 1). The seat was secured with the participant’s trunk touching the edge of the table. A mirror was suspended 21 cm above the horizontal table and a 55″ high-definition digital monitor (Sony Bravia) was suspended an additional 21 cm above the mirror. When the participants looked at the mirror, a computer-generated visual display of targets against a white background appeared to be originating on the horizontal table surface. The mirror blocked view of the participant’s arm and hand, but a visual cursor could be displayed that reflected the tip of the outstretched index finger.
Fig. 1.

Experimental setup. Participants sat at a virtual reality system with their arms supported by air sleds. Vision of the hand was blocked as participants made repetitive movements between two targets and new movements toward varying targets.
The participant’s dominant right forearm was placed on air-jet sleds that allowed the arm to move over the surface of the table with minimal friction. A splint within the air-jet sled immobilized the fingers, hand, and wrist to restrict motions to the shoulder and elbow joints. Arm movements were tracked by a Trackstar (Northern Digital Instruments) magnetic motion recording system connected to a Macintosh computer. A 6-DOF sensor was tightly secured to the center of the back of the hand with a wrap and medical tape. A second 6-DOF was also tightly secured with underwrap (ACE™ Brand) and tape to the side of the upper right arm. The sensors collected positional information, calibrated for each participant’s dimensions, and were digitized at 103 Hz. Customized software (REAL Software, In., Austin, TX, USA) synchronized the presentation of stimuli and data collection.
Experimental design and task
The purpose of this experiment was to examine how participants’ plan and execute movements to new targets after drifting during repetitive movements made in the absence of visual feedback. Each participant engaged in two sessions on separate days.
Session I
In session I, a start location (2.3 cm diameter) was customized to each participant, defined at a shoulder angle of 50° and an elbow angle of 90°. A target circle (4 cm diameter) was located 15 cm away from the start location in a direction 120° counterclockwise from the 0° vector extending to the right of the start circle. A new set of six target circles were located 5 cm and either 60°, 80°, 100°, 140°, 160°, or 180° from the start circle.
The participants’ task was to make reaching movements out to the target and back to the start circle in time with a metronome. Participants were given the instructions to move quickly and accurately and to time their outward movements synchronously with the metronome tone.
After two practice blocks of 20 trials with full vision to have participants gain familiarity with the set-up, participants performed a block of 49 repetitive movements between the original start circle and original target circle. The first movement of each block was initiated when the cursor marking the hand was placed within the start circle, triggering an auditory ‘go’ signal that initiated a metronome cue that played every 1.6 s, regardless of hand location. For the first five movement trials of each block, the participant could track the location of their hand through a 1 cm diameter cursor that marked the position of the extended index finger. After the fifth trial, vision of the cursor disappeared and subjects were required to continue the movements at a regular, metronome-cued frequency.
After 49 repetitive movement trials, both the original start circle and the original target circle disappeared and one of the six new targets was presented, without the presentation of a start circle. The participant viewed a white background with one new target. Participants were instructed to move quickly and accurately to the new target circle, concurrent with the next (50th) metronome tone. This same block was repeated six times, such that each of the six new targets was presented.
Fig. 2a shows the arrangement of the new targets in relation to the initial start and end location of the 15 cm 120° target used during the drift task. The new target was presented at the same delay as the ‘go’ signal during the drift trials. As seen in this figure, a movement planned from the current position of the hand in the original hand position would yield distinct differences in direction. Planning a movement from the original start position to target 1 (T1) would result in a movement direction of about 60°, whereas one planned from the current hand position would result in a movement direction of about 140°, relative to a horizontal (left to right) axis. Thus, for most targets and most drift positions, the distinction between these alternatives was unambiguous. It should be stressed that each participant performed six drift sessions, and was presented with one of six of the targets shown in Fig. 2a, after each one. The targets were presented in a random, counterbalanced order between participants.
Fig. 2.
Targets and hand paths. (A) Display of Targets. The repetitive movement axis is between the Original Hand Position and Original Target (T0). New targets that appear individually are T1–T6. Following drift, Current Hand Position is shown. (B) Control session display of new targets from both the Original Start Position, or visual control, or the Drifted Start Positions, or proprioceptive control.
Session II
Participants returned for a second session to record baseline movements in the absence of drift. In session II, subjects moved to each of the six targets (from session I) from a start location that reflected the position that each subject had drifted to at the end of each block from session I. The six target circles were the same as the six target circles used in session I. Movements from the original start circle to the original target circle (15 cm, 120°) were also included. Under veridical conditions in which a start circle was placed at the previously determined drifted position, participants placed their hand in this start circle and moved toward the target, at an audiovisual ‘go’ signal, when the cursor was removed.
In order to familiarize participants with the set-up, each session began with five vision trials moving between the original start circle and original target circle with vision of the cursor. Following this, the participant was shown a single start circle and target circle. They held the cursor within the start circle for 300 ms, when a single ‘go’ tone sounded. The cursor was then blanked and the participants made a rapid movement toward the target. These trials provided the control conditions for the movements made from the drifted positions in session I. In session II, participants had consistent visual and proprioceptive information about start position, but made movements in the absence of visual feedback.
Kinematic analysis
Custom-designed software developed with IgorPro (Wavemetrics, Inc., Lake Oswego, OR, USA) was used to analyze the data. Before beginning the experiment, the 3-D positions of each participant’s fingertip, elbow, and shoulder were measured using a calibration stylus. Stylus position relative to the 6-DOF sensors on the hand and upper arm was used to calculate joint position and orientation. A 3rd-order dual-pass Butterworth filter with a cutoff frequency of 8 Hz was used to calculate linear velocity and acceleration of each joint. Movement initiation and termination were calculated with respect to a 12% peak velocity cutoff threshold, with initiation as the last minimum that fell below the threshold before peak velocity and termination as the first minimum that fell below the threshold after peak velocity. These marker selections were visually confirmed.
Cumulative hand drift was measured over the 44 repetitive movement trials without vision of the hand. It was measured as the Euclidean distance between the position of the hand at the beginning of the first trial without vision (trial 6) and the position of the hand at the beginning of each subsequent movement cycle (trials 7–49). Movement distance was measured for each outward phase of movement. The outward phase was demarcated by minima in velocity as described above. Finally, movement direction was measured at peak velocity of the outward phase of movement and again at movement end.
Inverse dynamics analysis
Joint torques were calculated for shoulder and elbow using the equations detailed below. For the sake of this analysis, we assumed that the upper extremity was two interconnected rigid links (upper arm and forearm) with frictionless joints at the shoulder and elbow. The shoulder was allowed to move freely, and the torques resulting from linear accelerations of the shoulder were included in the equations of motion for each joint. The equations of motion are shown below:
Simulations
We used a simulation to estimate what the movement trajectory would look like if participants were to apply the same torque strategy that they used under control conditions, when they made movements toward targets, following drift. We solved the equations of motion for a two segment, two frictionless pin-joint systems, and then forward integrated using a fixed 1-ms time step. Inputs to each simulation were initial shoulder and elbow angles, participants’ limb dimensions and inertial values, and the joint torque histories calculated from a recorded movement trial. Thus we were able to predict the effects of an ideal open-loop controller using the muscle torques computed for a movement made from a given initial position to drive the simulation originating from a new initial position. We calculated the forward integration error by comparing a simulated hand path to that of the actual trial, beginning with the same initial conditions. The maximum error was 0.61 mm (see Sainburg et al., 1999; Brown et al., 2003a,b).
Statistical analysis
Both repetitive movement trials and new movement trials without vision underwent statistical analysis for cumulative drift, distance, and direction. In order to assess changes across the drift phase of session I, we separated each drift block into 6 epochs of outward movements, including the first 5 movements made with visual feedback and last 44 movement made without visual feedback. The first two epochs were comprised of 5 trials each, the next 4 epochs were comprised of 10 trials, and the last epoch was comprised of 9 trials. We employed a 1-way repeated measures ANOVA design, using epoch as the within-subject factor with 6 levels to test for changes in drift, distance, and direction of movement. For movements made following drift to each of the 6 new targets, we employed a 3 condition (experimental, visual control, and proprioceptive control) X 6 target repeated measures ANOVA design. The experimental (E) condition included the movements toward each of the 6 targets following drift in session I. The visual control (VC) condition included the movements to each of the 6 targets made from the single original start position after the participants were able to place the cursor in this position prior to each movement in session II. The proprioceptive control (PC) condition included the movements made in session II from start circles that reflected the positions that the subject had drifted to in each of the trial blocks from session I. In this control session, however, a start circle was displayed along with the target, and the participant was able to place the cursor within the start circle prior to each movement.
RESULTS
Limb position drift
The purpose of this study was to determine how participants plan and execute new movements after their limb position has drifted. To this end, participants were first exposed to a series of repetitive movements made in the absence of vision in order to induce drift of limb position. Following each drift trial, they were then required to make a movement to one of six new targets. By analyzing the distance and direction of movements toward new targets, we were able to determine whether the new movement plan was specified with respect to the current position of the hand (specified by proprioception). We will first describe participants’ drift sessions.
Participants performed repetitive movements between two specified positions with the dominant arm. After 5 trials with veridical visual feedback of the cursor, representing the position of the tip of the dominant index finger, visual feedback was removed, and participants continued to move another 44 cycles (out and back motions). Fig. 3a shows a typical set of outward hand paths. The paths show substantial drift in start position, but the paths remain parallel in direction and consistent with respect to distance. These trends are similar to that reported by Brown et al. (2003a,b).
Fig. 3.
Limb position drift. (A) The first half of each cycle of repetitive movements toward a target. The lighter the color of the line, the higher the trial number. The first trial without vision (Start), the trial midway through (Mid), and the final trial for each block (End) are marked as bold lines. (B) Average drift extent and direction for each participant, originating from the initial start circle. (C) Average limb position drift from the initial start position for each subject. The gray horizontal line marks 2.5 cm.
Fig. 3c shows the average drift in initial cycle location, across the trials for all 11 participants. Whereas participant 11 drifted on average 8.54 cm, participants 1 and 2 drifted less than 2.5 cm. The average drift across all participants was 4.89 cm. The variety of drift extent and direction across participants is displayed in Fig. 3b, with most participants drifting to the right of the initial start position. Because the study depends on participants achieving substantial drift in initial hand position, we restricted our analysis to those subjects who drifted more than 2.5 cm, which was the diameter of the initial start circle. As a result, participants 1 and 2 were removed from the analysis, which was performed on data from the 9 remaining participants.
Fig. 4a shows the time course of drift in initial cycle location across the block of 49 repetitive movements. The first 5 cycles were performed with vision (VIS), and removed (No Vision; NV) for the rest of the cycles (Epochs NV1-NV5). The asymptotic profile in Fig. 4 reflects the time course of drift, which was highest in the early phase (NV1-NV2). The rate of drift became lower between NV3 and NV4, and initial cycle position did not show drift between NV3, NV4, and NV5. We conducted a 1-way repeated measures ANOVA with drift distance as the dependent variable and epoch as the within subjects’ factor. We found a significant main effect of trial block [F(1,10)=31.393, p<0.01]. Post-hoc analysis (t-test) showed significant drift between epochs NV1 and NV2 (t=4.716, p<0.01), NV2 and NV3 (t=2.493, p<0.05), but not between NV3 and NV4 (t (7) 0.787, n.s.) nor NV4 and NV5 (t(7)=0.1188, n.s.). Across subjects, hand position at the initiation of each cycle of movement drifted on average 4.89±0.0236 cm (Mean±SE).
Fig. 4.

Measures of limb drift. (A) Cumulative Drift. Positional difference from original start location to current hand position. The first five trials were with vision (VIS) and the next 45 trials without vision (NV). No vision trials are split up into 5 blocks (NV1, NV2, NV3, NV4, NV5). Standard error bars are shown. (B) Repetitive movement distance and (C) direction. Measurements from trials of cumulative drift with error bars representing inter-subject SE.
Movement distance and direction remain constant during drift
Regardless of substantial drift in hand position during the course of repetitive movements made in the absence of visual feedback, the distance and direction of movements remained fairly constant throughout the session (see Fig. 3a). This is reflected in Fig. 4b, c, which show the average initial direction (4b) and distance (4c) of each outward path for each epoch of the session. We conducted a 1-way repeated measures ANOVA with initial movement direction as the dependent variable and epoch as the within subjects’ factor. We found no effect of trial block on initial direction, regardless of epoch [F(1,10)=0.955, n.s.]. With regard to distance, the epoch performed with visual feedback was, on average, 2 cm shorter than the epoch performed with vision. This difference was significant (t=5.82, p<0.05). This is consistent with reported tendencies to move greater distances during planar reaching movements in the absence of vision (Gordon et al., 1994; Sainburg et al., 1995). We conducted a 1-way repeated measures ANOVA on the epochs performed without vision (NV1-NV5) with distance as the dependent variable and epoch as the within subjects’ factor. We found no significant main effect of trial block on distance [F(1,10)=3.180, n.s.].
Movements to new targets following drift
The previous section confirmed that participants drifted their hand position while making repetitive movements in the absence of visual feedback, and maintained constant directions and distances during these repetitive movements. These findings are consistent with those reported earlier (Brown et al., 2003a,b), and were necessary to complete this study. During drift, a discrepancy is introduced between the participants’ visual memory of the last seen hand position and the currently available proprioceptive information. We ask whether participants plan movement vectors in accord with their current hand position, or in accord with the last seen hand position, which corresponded to the original start location.
Movement directions for both phases of the experiment are shown in Fig. 5. Three conditions are shown. Visual control (VC) movements were session II movements made to each target from the original start position. Proprioceptive control (PC) movements were session II movements made to each target from each drifted start position. Experimental condition (E) movements were movements made to each new target following each of six drift sessions during session I.
Fig. 5.

(A) Movement Direction Condition Separated by Target. Filled circles mark subject’s movements from an unknown drifted position toward a new target in session I (E). Hollow squares mark subjects’ baseline movements from the initial position, or visual control (VC), to the new target in session II. Hollow circles mark subjects’ baseline movements from the hand position, or proprioceptive control (PC), to the new target. (B) Movement direction at onset (Vmax) and completion (End) of movement. (C) Movement Distance by Condition. Markers follow those presented in (A).
We conducted a 2-way repeated measures ANOVA, with experimental condition (PC, VC, E) and target as factors. Our results indicated a significant main effect of condition [F(2,16)=6.251, p<0.05], as well as target [F(5,40)=46.576, p<0.01] and a significant interaction between condition and target [F(10,80) =2.154, p<0.05]. While the main effect of target on movement direction is expected by the task, the main effect of condition directly addressed our hypotheses. As reflected by the data in Fig. 5a, post hoc analysis revealed that our two control conditions (VC, PC) yielded significantly different movement directions across targets (t(7)= −2.857, p<0.05). While the experimental condition (E) was significantly different than the proprioceptive control (PC) condition (t(7) = −3.232, p<0.01), post hoc analysis revealed no significant difference between the experimental condition (E) and the visual control (VC) condition (t(7)= −0.376, n.s.). These results indicate that following drift, movement direction was planned and executed in accord with the initial position (marked by start circle), or last seen visual location of the hand, and did not appear to be influenced by the current proprioceptive information about the drifted location of the hand.
The statistics presented above are based on movement direction measured at the end of the movement. However, because movements were fairly straight, direction measured at the end of movement was not significantly different than that measured at the peak velocity. Fig. 5b shows movement direction measured at peak tangential hand velocity and at the end of movement. We subjected movement direction to 2-way repeated measures ANOVA with target and time measured (maximum velocity, movement end) as factors. This ANOVA revealed a main effect for target, as expected, [F(5,40)=48.89, p<0.001], but no main effect of time [F(1,8)=0.593, n.s.]. Thus, both planned movement direction and final movement direction reflected the last seen visual location of the hand, and not the drifted hand positions.
Movement distance following drift
Movement distances under the three conditions (VC, PC, E) are shown across targets in Fig. 5c. As evident in this figure, the two control conditions are very similar to one another and both are close to the target distance of 0.05 meters. However, for the first 4 targets, the experimental condition (drifted movements) was close to twice the distance of both control conditions. Our 2-way (target and condition factors) repeated measures ANOVA for movement distance showed a significant main effect of target [F(5,40)=33.82, p<0.0001], a main effect of condition [F(2,16)=10.303, p<0.01], and a significant interaction between both factors [F (10,80)=4.089, p<0.001]. Most importantly, post-hoc analysis did not show a significant difference between conditions VC and PC. [t(7)=0.686, n.s.]. Therefore, it is impossible to determine whether movement distance was determined in accord with the last seen position of the hand at the original start circle (VC) or with the current drifted position of the hand (PC) during the experimental trials following drift.
However, the large overshoot of the experimental drift condition compared with the control conditions suggested that the movement drift did influence distance control. Fig. 6 shows the relationship between distance moved and extent of drift per subject. There was a tendency for subjects with greater drift to move greater distances. In fact, 64.5% (r2) of the variance in distance could be explained by variance in drift. We conclude that movement distance was affected by the drifted hand location.
Fig. 6.

Movement distance in relation to the extent of drift. Each point represents a participant’s average drift and movement distance.
If movement dynamics had not been adapted to the drifted hand position, movement direction would not have been preserved
We next asked how the movement trajectory would have changed if participants had not modified their muscle torque strategies to the drifted hand positions. We implemented a simple two-segment rigid body simulation (see Bagesteiro and Sainburg, 2002). The muscle torques calculated from a baseline trial, made from the original start position during session II, were used as inputs to the forward dynamic equations of motion. The forward simulation was performed with the inertial values and geometric parameters of the participant, but was initiated from the new hand position that reflected the drifted start position. In effect, this simulation predicted what would have happened to movement direction if the torques used to move from the original undrafted start position were used to make a movement from the drifted position.
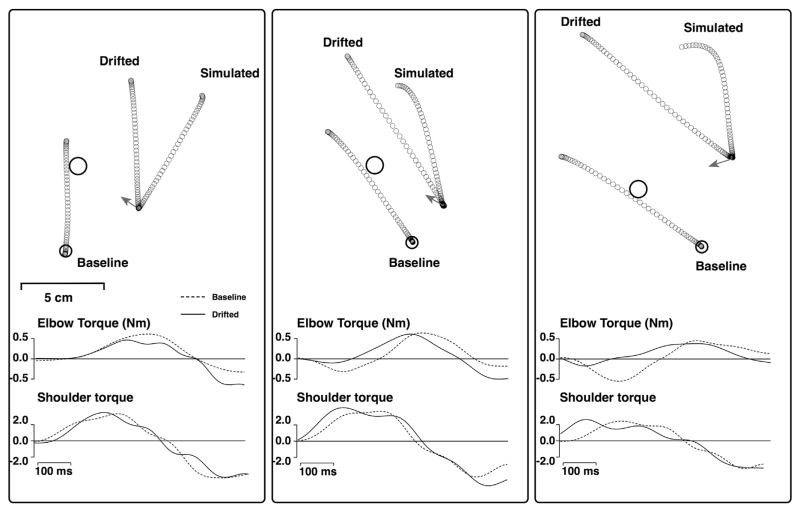
Fig. 7 shows our simulation results for representative 80° (left panel), 110° (middle panel) and 130° (right panel) targets. Baseline movements were movements from session II, made from the baseline start position to the target. The cursor was available to position the hand at the baseline start position, but was removed prior to movement initiation. For this participant, baseline movements tended to be directed slightly counterclockwise to the target and overshot the target. The ‘drifted’ hand paths reflect the movements, from session I, made to the same target, after the subjects’ hand had drifted. Each drift location was slightly different, because in session I only one movement toward a novel target was made following each drift trial. The drifted paths also overshot the baseline path length, but were made in parallel directions. This indicates that participants used the last seen (baseline) start position to specify the movement direction, rather than the current position of the hand. The small arrows indicate the direction that the movement would have taken if participants had used the current hand position to specify the movement to the target. As detailed above, participants made movements in accord with the baseline start position, not the current, drifted, position of the hand. The elbow and shoulder joint torques for the baseline (dashed) and drifted (solid) movements are shown below. For all three movements, the torques change substantially between the two starting limb configurations. The simulated paths show the movement path that would have occurred had the baseline muscle torque profiles been used at the drifted movement start location. It is clear from these results that 1) Movement directions following drift were specified as though the hand were at the initial, non-drifted, start position, and 2) Joint torques were modified through a transformation process that accounted for the actual, drifted limb configuration in producing the movement. These two processes resulted in hand paths from the drifted positions that were parallel to those made from the baseline positions.
Fig. 7.
Simulation results for representative 80° (left) and 110° (middle) and 130° targets. Baseline movements were movements from session II, made from the baseline start position to the target. The cursor was available to position the hand at the baseline start position, but was removed prior to movement initiation. For this participant, baseline movements tended to be directed slightly counterclockwise and tended to significantly overshoot the target. The ‘drifted’ hand paths reflect the movements from session I, made to the same target, after the subjects’ hand had drifted. Each drift location is slightly different. The drifted paths slightly overshoot the baseline path length, but are made in a parallel direction. The elbow and shoulder joint torques for the baseline (dashed) and drifted (solid) movements are shown below. For all three movements, the torques change substantially between the two starting limb configurations. The simulated paths show the movement path that would have been performed if the baseline muscle torque profiles had been used at the drifted movement start location.
DISCUSSION
In this study, we examined the use of vision and proprioception for new movement specification following limb position drift to investigate the nature of information used to plan new movements from the drifted position. Limb position drift was induced by having participants perform repetitive out and back movements between two targets in the absence of visual feedback of their dominant right hand, in the horizontal plane. Following 49 repetitive movement cycles, participants made the 50th movement toward one of six new targets without pause and without vision. Participants returned for a control session in which they made movements toward the same six targets that they encountered in session I, but from the following start positions: for the visual control, participants moved from their initial start position from session I, and for the proprioceptive control, participants moved from the 6 positions that matched their previously drifted start positions from session I. The control movements in session II were performed with veridical visual and proprioceptive information about initial start position.
Our results confirmed substantial limb position drift, following repetitive movements in the absence of visual feedback. We found that drift plateaued after 30 trials, which is consistent with the work of Brown and coworkers (Brown et al., 2003a,b). The reasoning for this plateau is still unknown, and an area for future study, although the possibility of an attraction to a specific location of maximal comfort, control, or stability has been ruled out by previous studies (Brown et al., 2003a,b). The direction and extent of drift was also unique to each participant, with most participants drifting to the right of the target. Previous studies (Brown et al., 2003a,b) have shown that drift accumulation and direction depends on many factors, including uncompensated inertial interactions that vary with the size and configuration of the participants’ limbs, and thus differences in initial limb configuration and inertial parameters could account for differences in drift accumulation across participants. In addition, movement distance and direction were maintained throughout drift, demonstrating that the participants were able to accurately update their control strategies to account for drift-dependent variations in limb configuration (Brown et al., 2003a). In summary, the limb drift results in this study were consistent with past reports.
Independence of movement direction and drift
Examining the specification of direction and distance of movements to new targets following drift revealed differential uses of visual memory of previously seen limb position and proprioceptive information about the current limb position. Our results indicate that, following drift, movement direction is determined almost exclusively by the last-seen visual location of the cursor that indicated the position of the hand. This cursor was last seen when the participant’s hand was located at the initial start circle at the beginning of repetition 6 of the series of repetitive movements used to induce drift. The cursor of the hand’s position disappeared, but the original start circle remained until the new target was presented. Thus, participants’ visual memory for hand location should have coincided with the initial start circle, even as the actual position of the hand drifted. From the drifted position, the new movement direction consistently followed a path parallel to the path from the initial start circle to the new target. For this plan to be implemented correctly, actual limb configuration must have been accounted for in the neural commands to muscles. We reasoned that proprioception must have provided accurate information about the drifted limb configuration, even though this information was apparently not available for detecting and preventing drift in the first place.
In a similar planar reaching paradigm, previous research (Brown et al., 2003a,b) has shown that as limb position progressively drifted, movement direction and distance were maintained. One study performed both inverse dynamics analysis and forward dynamic simulations to demonstrate that muscle torques were progressively and substantially modified in order to maintain movement parameters during drift, and that had this not occurred, substantial directional errors would have resulted (Brown et al., 2003a). We performed similar simulations in the current study, showing that joint torques were modified according to the limb configuration at the drifted position. Had the new movement trajectory reflected the joint torques from the initial position, the new movement directions would have been substantially different. Therefore, while new movement directions were specified from visual memory of the initial hand position, joint torques to execute those directions accounted for the drifted limb configurations, presumably informed by proprioception. This finding supports the work of Sober and Sabes (2003), who assessed subjects’ responses to discrepancies between visual cursor location and actual hand position. They used a control based simulation with two modules, one that specified the planned movement kinematics, and one that transformed this plan into neural commands that accounted for limb dynamics. This study found that the position estimate used for movement vector planning relied almost exclusively on visual input, whereas the estimate used to compute the joint-based motor command relied on proprioceptive signals. In a related set of studies, Sainburg and Co-workers (Lateiner and Sainburg, 2003; Sainburg et al., 2003) showed that when visual and proprioceptive feedback of initial hand position was dissociated using a virtual reality environment, specification of direction depended almost entirely on visual information. However, when they performed inverse dynamic analysis, they showed that subjects adjusted joint torque strategies to the actual configuration of the limb to achieve these visually specified directions. Thus, these two lines of evidence are consistent with our current findings indicating that when visual and proprioceptive information about hand position is misaligned, participants will use accurate visual information for planning the movement trajectory and accurate proprioceptive information to specify joint coordination to achieve the planned trajectory. The misalignment in the current study is presumably produced by the discrepancy between visual input of the last seen hand position, also marked by the start circle, and proprioceptive information about the drifted limb position.
Movement distance depends on drift
While the specification of movement direction clearly relied on visual memory, movement distance specification adapted to neither visual memory nor current proprioceptive input. Instead, movement distance tended to overshoot the targets, which was not the case for the baseline repetitive movements made during the drift session. The amount of movement overshoot increased with the discrepancy between visual and proprioceptive inputs, coinciding with greater extent of drift. This implies that distance control may depend on merged information, such as that shown for declarative reports of hand position in previous studies (van Beers et al., 1996). However, if this were the case, then the merged hand position would be expected to fall somewhere between the last seen initial position (visual) and the drifted (proprioceptive) position. Because both positions fell about the same distance from the new target, such fusion would be expected to yield a similar distance movement. New movements did not reflect this distance. Instead, it seems that the discrepancy itself induced the overshoot in movement distance. This finding might be explained by previous research, which has demonstrated that when making horizontal plane centerout reaching movements in a range of directions from a central starting location, participants show variations in initial acceleration that reflect direction-dependent anisotropy in limb inertia (Ghez et al., 1990). This variation in acceleration is normally compensated by direction-dependent variations in limb deceleration that result in fairly accurate movement distances in all directions. Patients with proprioceptive deafferentation show similar variations in initial acceleration that are not compensated by variations in deceleration, which leads to large variations in movement distance across directions (Gordon et al., 1995). Thus, proprioception appears critical in controlling movement distance through feedback mechanisms. We propose that this process is achieved by specifying stiffness around a target limb configuration (Scheidt and Ghez, 2007; Yadav and Sainburg, 2014) that corresponds to a planned visual target. This process should require alignment between the visual and proprioceptive maps, which might explain why movement distance varied with the extent of misalignment between the visual memory of initial hand positions and the actual hand positions, in the current study.
Movement specification reflects dissociation in sensory modalities
Regardless of the process, it appears that predictive specification of movement direction depends on visual information, regardless of drift, while online control of movement distance depends on accurate alignment between the two modality representations. These differences in how direction and distance are controlled are consistent with previous research indicating differential specification of movement distance and direction (Sainburg et al., 2003; Lateiner, 2003; Rosenbaum, 1980; Gordon et al., 1994). The current findings emphasize the importance of alignment between these two modalities for distance control. The finding that movement direction specification reflects visual memory, while achieving this direction requires current proprioceptive input of the drifted limb configurations demonstrates that these two sensory modalities remain intact with drift. In addition, our finding that visual memory positional information centers at the initial start position while proprioceptive positional information centers at the drifted position demonstrates that these two sensory modalities become misaligned with limb position drift. This misalignment results in new movements that miss the target because direction is specified from the initial position but originates from the drifted hand position. These findings suggest that limb position drift results from a misalignment between intact sensory modalities, rather than from a distortion that represents a weighted fusion of the inputs into a single visual-proprioceptive map (Desmurget et al., 1995; Rossetti et al., 1995; van Beers et al., 1996) or from vision attracting proprioception when the two are misaligned (Rock, 1966; Pick et al., 1969; Welch, 1996; Mon-Williams et al., 1997).
These classic hypotheses are largely based on experimental paradigms in which visual feedback of limb position is distorted, and a participant is asked to identify the position in space of the occluded limb, which assesses perception. By testing movements toward new targets, our study is inherently different in that it assesses action. This distinction is supported by research suggesting that two different pathways might exist to mediate sensory processing for perception and for action (Goodale et al., 1992; Jeannerod and Rossetti, 1993; Schwartz et al., 2004). In the visual system, an analogous differentiation between pathways that mediate perception and action has been well documented (Goodale and Milner, 1992; Goodale and Westwood, 2004; Schenk and McIntosh, 2010). In the proprioceptive system, the dorsal column pathway and the spinocerebellar pathways might reflect the distinction between perception and action, respectively (Jansen et al., 1967; Ross et al., 1979; Kaas et al., 2008). We propose that the integration of vision, or visual memory, with current proprioceptive information is carried out differently for the purposes of perception versus action and that limb configuration information for action depends on the alignment of two independent, but accurate representations.
Neural representation of visual-proprioceptive alignment
This idea of the alignment of independent representations of limb position derived from vision and proprioception is supported by the findings of Mutha et al. (2014). They showed that focal lesions of left hemisphere posterior parietal cortex specifically prevented the ability to adapt to visuomotor rotations. This adaptation paradigm has received a great deal of attention over the last few decades as a method for studying multisensory integration for motor adaptation (Flanagan and Banks, 2002; Wang and Sainburg, 2005; Seidler et al., 2006). Participants make pointing movements toward a range of targets, usually on a horizontal surface, from a central starting location. A rotation of the cursor relative to the start location is introduced, such that the cursor moves in a different direction than the hand. Participants readily adapt to this type of visual distortion, and they generalize this adaptation to different regions of space as a vectorial representation of their motion (Wang and Sainburg, 2005). The idea that this adaptation reflects a realignment of movement vectors is emphasized by the finding that, once adapted to a visuomotor rotation, participants specify rotated vectors from various new locations in space. Interestingly, Mutha et al. (2014) showed that specific lesions to posterior parietal cortex can disrupt this adaptation, without altering the ability to make accurate movements to visual targets, under veridical conditions. This supports the idea that visual specification of movement direction, as well as the process of specifying configuration-dependent movement dynamics, each remains intact. This, in turn, emphasizes that the representation of position within each modality also remains intact. However, the process of aligning two independent representations is specifically disrupted by this lesion. There is substantial evidence that distinct regions of posterior parietal cortex mediate the alignment visual and proprioceptive representations of space (Ghilardi et al., 2000; Gregoriou and Savaki, 2003; Mutha et al., 2011). We now suggest that limb position drift demonstrates that these independent representations of visual and proprioceptive space require constant recalibration and that without this realignment, such as in the absence of visual feedback while relying on visual memory of the hand position marked by the initial start circle, along with current proprioception, new movement direction and distance are differentially specified using accurate yet dissociated sensory maps (Wann and Ibrahim, 1993; Ghilardi et al., 1995; Rossetti et al., 1995). Our current findings on limb position drift support this point of view.
Acknowledgments
Funding: This work was supported by a grant from the National Institutes of Health (R01HD059783) to R.L.S. and NSERC to L.E.B.
Abbreviations
- PC
proprioceptive control
- T1
target 1
- VC
visual control
- VR
virtual reality
References
- Andersen RA, Buneo CA. Intentional maps in posterior parietal cortex. Annu Rev Neuro. 2002;25:189–220. doi: 10.1146/annurev.neuro.25.112701.142922. [DOI] [PubMed] [Google Scholar]
- Andersen RA, Zipser D. The role of the posterior parietal cortex in coordinate transformations for visual-motor integration. Can J Physiol Pharmacol. 1988;66:488–501. doi: 10.1139/y88-078. [DOI] [PubMed] [Google Scholar]
- Bagesteiro LB, Sainburg RL. Handedness: dominant arm advantages in control of limb dynamics. J Neurophysiol. 2002;88:2408–2421. doi: 10.1152/jn.00901.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown LE, Rosenbaum DA, Sainburg RL. Movement speed effects on limb position drift. Exp Brain Res. 2003a;153:266–274. doi: 10.1007/s00221-003-1601-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown LE, Rosenbaum DA, Sainburg LA. Limb position drift: implications for control of posture and movement. J Neurophysiol. 2003b;90:3105–3118. doi: 10.1152/jn.00013.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culham JC, Danckert SL, DeSouza JFX, Gati JS, Menon RS, Goodale MA. Visually guided grasping produces fMRI activation in dorsal but not ventral stream brain areas. Exp Brain Res. 2003;153:180–189. doi: 10.1007/s00221-003-1591-5. [DOI] [PubMed] [Google Scholar]
- Desmurget M, Rossetti Y, Prablanc C, Stelmach GE, Jeannerod M. Representation of hand position prior to movement and motor variability. Can J Physiol Pharmacol. 1995;73:262–272. doi: 10.1139/y95-037. [DOI] [PubMed] [Google Scholar]
- Flanagan JR, Banks MS. Humans integrate visual and haptic information in a statistically optimal fashion. Nature. 2002;415:429–433. doi: 10.1038/415429a. [DOI] [PubMed] [Google Scholar]
- Fridman EA, Immisch I, Hanakawa T, Bohlhalter S, Waldvogel D, Kansaku K, Wheaton L, Wu T, Hallett M. The role of the dorsal stream for gesture production. Neuroimage. 2005;29:417–428. doi: 10.1016/j.neuroimage.2005.07.026. [DOI] [PubMed] [Google Scholar]
- Ghez C, Sainburg R. Proprioceptive control of interjoint coordination. Can J Physiol Pharmacol. 1995;73:273–284. doi: 10.1139/y95-038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghez C, Gordon J, Ghilardi MF, Christakos CN, Cooper SE. Roles of proprioceptive input in the programming of arm trajectories. Cold Spring Harb Symp Quant Biol. 1990;55:837–847. doi: 10.1101/sqb.1990.055.01.079. [DOI] [PubMed] [Google Scholar]
- Ghilardi MF, Gordon J, Ghez C. Learning a visuo-motor transformation in a local area of work space produces directional biases in other areas. J Neurophysiol. 1995;73:2535–2539. doi: 10.1152/jn.1995.73.6.2535. [DOI] [PubMed] [Google Scholar]
- Ghilardi MF, Ghez C, Dhawan V, Moeller J, Mentis M, Nakamura T, Antonini A, Eidelberg D. Patterns of regional brain activation associated with different forms of motor learning. Brain Res. 2000;871:127–145. doi: 10.1016/s0006-8993(00)02365-9. [DOI] [PubMed] [Google Scholar]
- Gilhodes JC, Roll JP, Tardy-Gervet MF. Pereceptual and motor effects of agonist-antagonist muscle vibration in man. Exp Brain Res. 1986;61:395–402. doi: 10.1007/BF00239528. [DOI] [PubMed] [Google Scholar]
- Goodale MA, Milner AD. Separate visual pathways for perception and action. Trends Neurosci. 1992;15:20–25. doi: 10.1016/0166-2236(92)90344-8. [DOI] [PubMed] [Google Scholar]
- Goodale MA, Westwood DA. An evolving view of duplex vision: separate but interacting cortical pathways for perception and action. Curr Opin Neurobiol. 2004;14:203–211. doi: 10.1016/j.conb.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Gordon J, Ghilardi MF, Ghez C. Accuracy of planar reaching movements. 1. Independence of direction and extent variability. Exp Brain Res. 1994;99:97–111. doi: 10.1007/BF00241415. [DOI] [PubMed] [Google Scholar]
- Gordon J, Ghilardi MF, Ghez C. Impairments of reaching movements in patients without proprioception. I. Spatial errors. J Neurosci. 1995;73:347–360. doi: 10.1152/jn.1995.73.1.347. [DOI] [PubMed] [Google Scholar]
- Graziano MSA. Where is my arm? The relative role of vision and proprioception in the neuronal representation of limb position. Proc Natl Acad Sci USA. 1999;96:10418–10421. doi: 10.1073/pnas.96.18.10418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregoriou GG, Savaki HE. When vision guides movement: a functional imaging study of the monkey brain. Neuroimage. 2003;19:959–967. doi: 10.1016/s1053-8119(03)00176-9. [DOI] [PubMed] [Google Scholar]
- Harris CS. Perceptual adaptation to inverted, reversed, and displaced vision. Psychol Rev. 1965;72:419–444. doi: 10.1037/h0022616. [DOI] [PubMed] [Google Scholar]
- Jansen JKS, Poppele RE, Terzuolo CA. Transmission of proprioceptive information via the dorsal spinocerebellar tract. Brain Res. 1967;6:382–384. doi: 10.1016/0006-8993(67)90206-5. [DOI] [PubMed] [Google Scholar]
- Jeannerod M, Rossetti Y. Visuomotor coordination as a dissociable visual function: experimental and clinical evidence. Bailliere Clin Neur. 1993;2:439–460. [PubMed] [Google Scholar]
- Kaas JH, Qi HX, Burish MJ, Gharbawie OA, Onifer SM, Massey JM. Cortical and subcortical plasticity in the brains of humans, primates, and rats after damage to sensory afferents in the dorsal columns of the spinal cord. Exp Neurol. 2008;209:407–416. doi: 10.1016/j.expneurol.2007.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kertzman C, Schwarz U, Zeffiro TA, Hallett M. The role of posterior parietal cortex in visually guided reaching movements in humans. Exp Brain Res. 1997;114:170–183. doi: 10.1007/pl00005617. [DOI] [PubMed] [Google Scholar]
- Lateiner JE, Sainburg RL. Differential contributions of vision and proprioception to movement accuracy. Exp Brain Res. 2003;151:446–454. doi: 10.1007/s00221-003-1503-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mon-Williams M, Wann JP, Jenkinson M, Rushton K. Synesthesia in the normal limb. Proc Biol Sci. 1997;264:1007–1010. doi: 10.1098/rspb.1997.0139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutha PK, Sainburg RL, Haaland KY. Left parietal regions are critical for adaptive visuomotor control. J Neurosci. 2011;31:6972–6981. doi: 10.1523/JNEUROSCI.6432-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutha PK, Stapp LH, Sainburg RL, Haaland KY. Frontal and parietal cortex contributions to action modifications. Cortex. 2014;57:38–50. doi: 10.1016/j.cortex.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pick HL, Warren DH, Hay JC. Sensory conflict in judgments of spatial direction. Percept Psychophys. 1969;6:203–205. [Google Scholar]
- Rock I. The nature of visual perception. New York: Basic; 1966. [Google Scholar]
- Rosenbaum DA. Human movement initiation: specification of arm, direction, and extent. J Exp Psychol Gen. 1980;109:444–474. doi: 10.1037//0096-3445.109.4.444. [DOI] [PubMed] [Google Scholar]
- Ross ED, Kirkpatrick JB, Lastimosa ACB. Position and vibration sensations: functions of the dorsal spinocerebellar tracts? Ann Neurol. 1979;5:171–176. doi: 10.1002/ana.410050211. [DOI] [PubMed] [Google Scholar]
- Rossetti Y, Desmurget M, Prablanc C. Vectorial coding of movement: vision, proprioception or both? J Neurophysiol. 1995;74:457–463. doi: 10.1152/jn.1995.74.1.457. [DOI] [PubMed] [Google Scholar]
- Rothwell JL, Traub MM, Day BL, Obeso JA, Thomas PK, Marsden CD. Manual motor performance in a deafferented man. Brain. 1982;105:515–542. doi: 10.1093/brain/105.3.515. [DOI] [PubMed] [Google Scholar]
- Sainburg RL, Ghilardi MF, Poizner H, Ghez C. Control of limb dynamics in normal subjects and patients without proprioception. J Neurophysiol. 1995;73:820–835. doi: 10.1152/jn.1995.73.2.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainburg RL, Ghez C, Kalakanis D. Intersegmental dynamics are controlled by sequential anticipatory, error correction, and positional control mechanisms. J Neurophysiol. 1999;81:1045–1056. doi: 10.1152/jn.1999.81.3.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainburg RL, Lateiner JE, Latash ML, Bagesteiro LB. Effects of altering initial position on movement direction and extent. J Neurophysiol. 2003;89:401–415. doi: 10.1152/jn.00243.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheidt R, Ghez C. Separate adaptive mechanisms for controlling trajectory and final position in reaching. J Neurophys. 2007;98:3600–3613. doi: 10.1152/jn.00121.2007. [DOI] [PubMed] [Google Scholar]
- Schenk T, McIntosh RD. Do we have independent visual streams for perception and action? Cogn Neurosci. 2010;1:52–62. doi: 10.1080/17588920903388950. [DOI] [PubMed] [Google Scholar]
- Schwartz AB, Moran DW, Reina GA. Differential representation of perception and action in the frontal cortex. Science. 2004;303:380–383. doi: 10.1126/science.1087788. [DOI] [PubMed] [Google Scholar]
- Seidler RD, Noll DC, Chintalapati P. Bilateral basal ganglia activation associated with sensorimotor adaptation. Exp Brain Res. 2006;175:544–555. doi: 10.1007/s00221-006-0571-y. [DOI] [PubMed] [Google Scholar]
- Sittig AC, Denier Van Der Gon JJ, Gielen CCAM, Van Wijk AJM. The attainment of target position during step-tracking movements despite a shift of initial position. Exp Brain Res. 1985;60:407–410. doi: 10.1007/BF00235937. [DOI] [PubMed] [Google Scholar]
- Sober SJ, Sabes PN. Multisensory integration during motor planning. J Neurosci. 2003;23:6982–6992. doi: 10.1523/JNEUROSCI.23-18-06982.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanne J, Boussaoud D, Boyer-Zeller N, Rouiller EM. Direct visual pathways for reaching movements in the macaque monkey. Neuroreport. 1995;7:267–272. [PubMed] [Google Scholar]
- Van Beers RJ, Sittig AC, Denier van der Gon JJ. How humans combine simultaneous proprioceptive and visual position information. Exp Brain Res. 1996;111:253–261. doi: 10.1007/BF00227302. [DOI] [PubMed] [Google Scholar]
- Van Beers RJ, Sittig AC, Denier von der Gon JJ. The precision of proprioceptive position sense. Exp Brain Res. 1998;122:367–377. doi: 10.1007/s002210050525. [DOI] [PubMed] [Google Scholar]
- Van Beers RJ, Sittig AC, Denier von der Gon JJ. Integration of proprioceptive and visual position information: an experimentally supported model. J Neurophysiol. 1999;81:1355–1364. doi: 10.1152/jn.1999.81.3.1355. [DOI] [PubMed] [Google Scholar]
- Wang J, Sainburg RL. Adaptation to visuomotor rotations remaps movement vectors, not final positions. J Neurosci. 2005;25:4024–4030. doi: 10.1523/JNEUROSCI.5000-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wann JP, Ibrahim SF. Does limb proprioception drift? Exp Brain Res. 1993;91:162–166. doi: 10.1007/BF00230024. [DOI] [PubMed] [Google Scholar]
- Welch RB. Adaptation of space and perception. In: Boff KR, Kaufman L, Thomas JP, editors. Handbook of Perception and Human Performance, Sensory Processes and Perception. New York: Wiley; 1996. pp. 24.1–24.45. [Google Scholar]
- Welch RB, Warren DH. Immediate perceptual response to intersensory discrepancy. Psychol Bull. 1980;88:633–667. [PubMed] [Google Scholar]
- Yadav V, Sainburg RL. Limb dominance results from asymmetries in predictive and impedance control mechanisms. PLoS ONE. 2014:9. doi: 10.1371/journal.pone.0093892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelaznick HN, Lantero D. The role of vision in repetitive circle drawing. Acta Psychol. 1996;92:105–118. doi: 10.1016/0001-6918(95)00007-0. [DOI] [PubMed] [Google Scholar]