Abstract
Objective
To evaluate if molecular markers of eosinophilia in olfactory enriched mucosa are associated with olfactory dysfunction.
Study Design
Cross-sectional study of tissue biopsies from 99 patients, and a further 30 patients who underwent prospective olfactory testing prior to sinonasal procedures.
Methods
Tissue biopsies were processed for analysis of inflammatory markers using qRT-PCR. Ipsilateral olfactory performance was assessed using the Sniffin Sticks threshold component and the UPSIT and age-adjusted data was correlated with inflammatory marker expression and clinical measures of obstruction from CT and endoscopy.
Results
Gene expression of the eosinophil marker CLC (Charcot Leyden crystal protein) was elevated in superior turbinate (ST) tissue in CRS with nasal polyps (CRSwNP) compared to ST and inferior turbinate (IT) tissue in CRS without nasal polyps (CRSsNP) and control patients (all p < 0.001 respectively). CLC in ST tissue was correlated with IL-5 and eotaxin-1 expression (all p<0.001; r = 0.65 and 0.49 respectively). CLC expression was strongly correlated with eosinophilic cationic protein levels (p<0.001; r=0.-76) and ST CLC expression was inversely related to olfactory threshold (p = 0.002, r = −0.57) and discrimination scores (p = 0.05, r = −0.42). In multiple linear regression of CLC gene expression, polyp status, radiographic and endoscopic findings with olfactory threshold, CLC was the only significantly correlated variable (p<0.05).
Conclusions
Markers of eosinophils are elevated in the ST of patients with CRSwNP and correlate with olfactory loss. These findings support the hypothesis that olfactory dysfunction in CRS correlates local eosinophil influx into the olfactory cleft.
Keywords: eosinophils, olfactory dysfunction, chronic rhinosinusitis
Introduction
Chronic rhinosinusitis (CRS) is a common inflammatory condition that affects approximately 30 million Americans.1 This diagnosis includes both CRS with nasal polyps (CRSwNP) and CRS without nasal polyps (CRSsNP). Many CRS patients, especially those with CRSwNP, are affected by olfactory impairment.2 Traditionally, mucosal edema leading to nasal obstruction, and ultimately, decreased airflow to the olfactory cleft was advanced as the etiology of CRS-associated olfactory loss.3 This theory is challenged by data suggesting that the degree of anosmia is not correlated with the degree of resistance to nasal airflow, and that removal of polyps does not reliably improve olfaction despite improvement in airflow.4 In addition, histologic studies of olfactory mucosa in patients with CRS have demonstrated changes in the olfactory epithelium, including hyperplasia of goblet cells, squamous metaplasia, and olfactory epithelium erosion that are correlated with olfactory dysfunction.2,5 Due to these findings, there is qualitative evidence that olfactory dysfunction also has a sensorineural component. Since eosinophil degranulation products like ECP and EDN are known to directly modulate neuronal function,6,7 we hypothesized that molecular markers of eosinophilia would specifically be locally elevated in olfactory neuroepithelium bearing tissue, and would correlate with CRS-associated olfactory loss (Figure 1).
Figure 1.
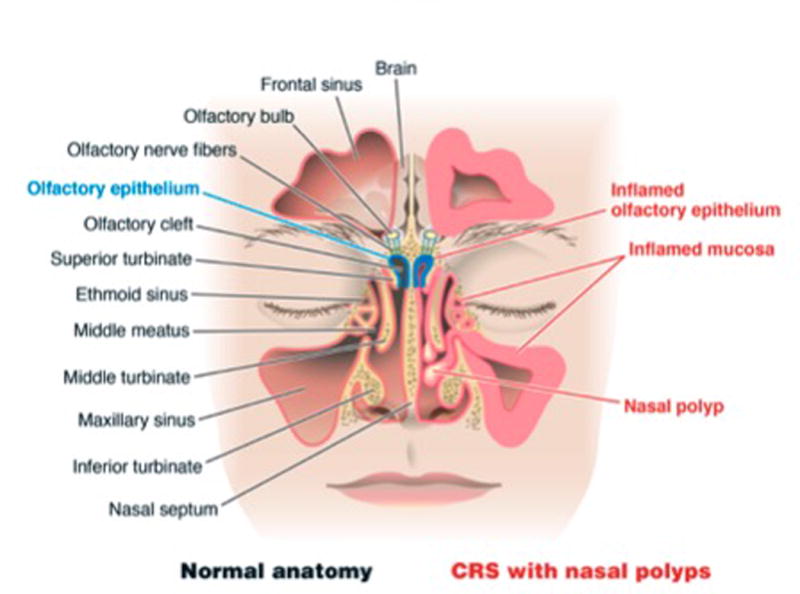
Anatomic depiction of the sinonasal cavity and olfactory tract. The left side of the image represents normal anatomy, and the right side of the image demonstrates inflammatory changes that occur in CRSwNP.
The human olfactory neuroepithelium (ON), is located in a region called the olfactory cleft primarily on the superior turbinate (ST), and more variably on regions of the upper middle turbinate and septum.8–11 Specific markers for olfactory neurons include olfactory marker protein (OMP) and growth associated protein 43 (GAP-43). The inferior turbinate (IT) is not known to have ON, but has respiratory epithelium.12 In this study, we characterize the nature of inflammation in the ST of patients with CRS and compare molecular biomarkers of eosinophilia with the performance on current clinical measures of olfactory function.
Materials and Methods
Patients
Specimens were first obtained from a tissue repository obtained from patients with CRS who underwent primary or revision endoscopic sinus surgery (ESS), and control patients without evidence of CRS undergoing septoplasty, or skull base tumor resection by participating Northwestern Medicine otolaryngologists. The anatomic location of assessed tissue biopsies included the IT, ST, and nasal polyps. In patients undergoing ESS/skull base tumor resections, superior turbinate biopsies were obtained in the course of routine sphenoidotomy as previously described.13,14 Biopsies of the superior turbinate from control patients not undergoing ESS were obtained with a pediatric through-cut forceps consistent with previously published studies involving olfactory biopsies.15 These studies have demonstrated that olfaction remains unaffected following these small biopsies. Collection of specimens from this subset of patients was single sided and corresponded to the side selected for olfactory testing. Patients were excluded if they had any history of trauma or tumor-associated anosmia. The Northwestern University Institutional Review Board (IRB) reviewed the study protocol and approved the study. Informed consent was obtained from all participants in the study.
Real Time PCR
RNA extraction from nasal and sinus tissue was performed using QIAzol (Qiagen, Valencia, CA) as previously described.16 All material was treated with DNAse to eliminate genomic DNA contamination. RNA quality was determined using the 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA) and the Taqman method for semiquantitative RT-PCR (Applied Biosystems, Grand Island, NY) was performed. Specimens were initially analyzed using a panel of gene expression markers for eosinophils (CLC: Hs01055743_m1), neutrophils (CXCR1: Hs01921207_s1), mast cells (tryptase: Hs02576518_gH), T-cells (CD3: Hs99999153_m1) and leukocytes (CD45: Hs04189704_m1). However, analysis suggested the eosinophil marker CLC was most relevant and subsequent specimens were only analyzed for CLC, the cytokine IL-5 (Hs01548712_g1), the chemokine eotaxin-1/CCL11 (Hs00237013_m1), and the olfactory neural markers OMP (Hs01087269_s1) and GAP-43 (Hs00967138_m1). All cycle threshold (Ct) values were normalized to the β-glucuronidase (GUSB) housekeeping gene (Hs00939627_m1) using the delta Ct method as this was previously found to be the most consistently expressed housekeeping gene.17 Samples where the gene of interest remained undetectable after 40 cycles assigned a Ct value of 40. A specimen was considered to contain olfactory neuroepithelium if the ΔCt was less than the lower limit of the 95% confidence interval of the mean for inferior turbinate specimens.
ECP measurement in sinonasal tissue
In specimens where sufficient tissue sample was available for protein analysis in addition to qRT-PCR analysis, homogenates were prepared and analyzed for eosinophilic cationic protein (ECP) using ELISA (MBL, Nagoya, Japan) according to manufacturer’s instructions as previously described.18 Per laboratory protocol, ECP levels were normalized to total protein and only samples achieving a total protein of 0.5mg/ml were analyzed.
Olfactory Function Test
After preliminary data was obtained using qRT-PCR analysis of repository specimens, consecutive patients were recruited in the second phase of the study to undergo olfactory testing. Subjects were administered two separate tests prior to surgery and specimen retrieval: one of olfactory threshold and one of olfactory discrimination. Olfactory threshold was determined by one of two trained personnel on the 1–16 point score by the Sniffin Sticks (Burghart, Wedel, Germany) n-butanol threshold test according to manufacturer instructions19,20. A modification to designate a score of “0” to those unable to smell the most concentrated marker was added. Olfactory discrimination was determined by the UPSIT test (Sensonics, Haddon Heights, NJ) that is a 40-item forced choice test.21 Prior to initiating either test, a wetted Merocel sponge was placed in one nostril to isolate a single side for testing. The left nostril was selected for testing in all patients unless anatomical nasal obstruction prevented passage of air on that side. The side selected was communicated to the surgical team so all biopsy specimens were obtained from the side where olfactory testing was performed. At the completion of testing, the patient’s raw score was recorded and compared to the published data on the age and sex-matched median scores.9,22 To account for published, non-linear age and sex specific performance on olfactory testing, we subtracted the subject’s score from the age and gender-matched mean. Using this metric, a score close to 0 represents normal olfaction while more negative scores denote worse olfactory function. In addition to objective testing, patients completed the SNOT-22 questionnaire of patient perceived symptoms in sinusitis.23 The SNOT-22 score is scored between 0–110 with average reported SNOT-22 scores in normal controls being 9.24
CT and endoscopic analysis
Preoperative CT scans were also evaluated for all patients with CRS who underwent olfactory testing. Coronal images of both the anterior olfactory cleft, defined by the first image containing the heads of both middle turbinates, and the posterior olfactory cleft, defined by the first image containing the heads of both superior turbinates, were extracted and placed into a separate document. In these images, all other sinuses were cropped out to so all that was visible was the olfactory cleft on the test side. A third party removed all identifying information and assigned a random number to each pair of images prior to analysis. The images were then analyzed by two separate physicians and scored in both the anterior and posterior cleft. Anterior cleft scoring was on a scale of 1–4 with 1 (<25% opacification), 2 (25–50% opacification), 3 (51–75% opacification), and 4 (>75% opacification) similar to the methods of a published study.25 Posterior cleft was scored on a scale of 1–4 with 1 (both surfaces of superior turbinate visible), 2 (one surface of superior turbinate visible), 3 (neither surface of superior turbinate visible, incomplete opacification) and 4, (neither surface of superior turbinate visible, complete opacification).
At the time of surgery, endoscopic images were obtained from the middle meatus and the olfactory cleft. Olfactory cleft images were obtained by superiorly directing the endoscope medial to the middle turbinate. As with CT images, a third party removed all identifying information and assigned a random number to each pair of images prior to analysis. Two separate physicians used the same grading scale as the CT images scored the images of the olfactory cleft.
Statistical Analysis
Analysis was performed using Prism (GraphPad, La Jolla, CA). 1-way ANOVA analysis was used for analyses comparing multiple specimen/disease groups, with post-hoc pair-wise Tukey adjustment when significant results were found on ANOVA. Pearson correlation coefficients were determined with r and p values reported. Univariate and multiple linear regression was performed using SPSS (IBM Corp, Armonk NY). In the multiple linear regression model, all clinical factors with p<0.2 were included in models for age/sex adjusted olfactory threshold and discrimination scores. Colinearity diagnostics were performed using the variance inflation factor. A value of p < 0.05 was deemed to be statistically significant.
Results
Demographic information
Sinonasal and polyp tissues were obtained from 26 controls, 37 patients with CRSsNP and 36 patients with CRSwNP and were studied using qRT-PCR. Tests of olfaction were performed on 7 controls, 10 patients with CRSsNP and 13 patients with CRSwNP prior to collection of specimens. Due to the small size of the biopsies, particularly from ST tissue, simultaneous protein analysis was available in 50 of the studied specimens. Subject characteristics are shown in Table 1. Of the 30 patients who had prospective olfactory testing, 29 completed the UPSIT testing while 25 completed the Sniffin Sticks test. Complete olfactory testing data was not always available as some patients declined further testing particularly due to the more time and concentration intensive nature of threshold testing.
TABLE 1.
Subject characteristics
| Control | CRSsNP | CRSwNP | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Total number of subjects (M/F) | 26 (9/17) | 37 (16/21) | 36 (17/19) | ||||||
| Age, yrs, mean (range) | 43.9 (24–73) | 48.8 (21–66) | 51.2 (28–74) | ||||||
| Y | N | U | Y | N | U | Y | N | U | |
| Atopy, number | 4 | 17 | 5 | 20 | 14 | 3 | 24 | 7 | 5 |
| Asthma, number | 0 | 24 | 2 | 9 | 26 | 2 | 15 | 18 | 3 |
| Tissue Type | IT | ST | P | IT | ST | P | IT | ST | P |
| Real-time PCR, number | 19 | 11 | 0 | 27 | 29 | 0 | 21 | 32 | 24 |
| ELISA, number | 6 | 3 | 0 | 2 | 7 | 0 | 3 | 15 | 14 |
| Subjects with olfactory function testing, number (M/F) | 7 (4/3) | 10 (3/7) | 13 (7/6) | ||||||
| Age, yr, mean (range) | 52.1(26–73) | 43.3 (25–61) | 58.6 (37–71) | ||||||
| SNOT-22 questionnaire | 5 | 9 | 12 | ||||||
| CT scan images | 2 | 10 | 12 | ||||||
| Endoscopic images | 5 | 10 | 10 | ||||||
CRSsNP, chronic rhinosinusitis without nasal polyps; CRSwNP, chronic rhinosinusitis with nasal polyps; M, male; F, female; Y, yes; N, no; U, unknown; IT, inferior turbinate; ST, superior turbinate; P, polyp; PCR, polymerase chain reaction; CT, computed tomography
qRT-PCR characterization of markers of inflammation, Th2 cytokines
To compare the association between markers of inflammation in ST tissue from patients with CRS and controls, qRT-PCR analysis of markers for eosinophils (CLC), neutrophils (CXCR1), T-cells (CD-3), and mast cells (tryptase) and leukocytes (CD45) were completed on an initial set of specimens. These demonstrated that only CLC showed significant elevation in CRSwNP or CRSsNP ST tissues compared to control ST tissue and further analysis on the other markers were discontinued (Data not shown). We then assessed expression of the eosinophil associated molecules CLC, IL-5, and Eotaxin-1 on specimens from a total of 99 subjects. CLC expression in ST tissue from CRSwNP patients was significantly elevated compared with ST and IT tissue in CRSsNP and control patients (all p < 0.001) (Figure 2 A). Polyp tissue had higher expression of CLC compared to ST (p<0.001) and data are provided for reference. IL-5 expression was elevated in ST tissue of both CRSsNP and CRSwNP relative to both IT and ST tissue in controls (p= 0.01 – 0.0001) (Figure 2B). Interestingly, eotaxin-1 was not elevated in ST tissue relative to other sinonasal tissue, however it was elevated in polyps (Figure 2C). There was a significant moderate positive correlation between IL-5 and CLC gene expression in the ST tissue from all patient groups (p < 0.001, r = 0.65) (Figure 3A). Despite insignificant elevations of eotaxin-1 in ST in CRSwNP, there was a weak but significant correlation with CLC expression in ST tissue (p< 0.0001, r = 0.49) (Figure 3B). Interestingly, these same correlations were not significant in polyp tissue (data not shown).
Figure 2.
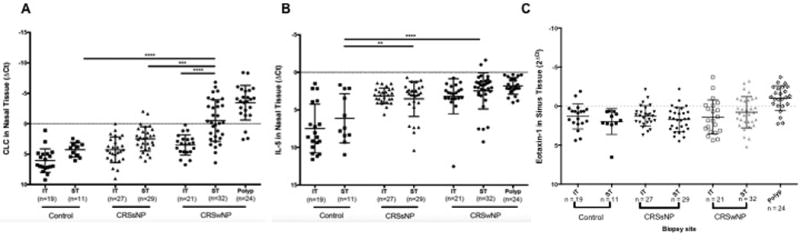
Relative expression of RNA markers of A) CLC- a marker for eosinophils, B) IL-5, and C) Eotaxin-1 in superior and inferior turbinate tissue in normal controls, patients with CRSsNP and with CRSwNP. In this representation, more negative numbers signify higher concentrations of CLC. For reference, relative expression of these markers is also shown in polyp tissue. ** p <0.01, *** p < 0.001, ****p <0.0001.
Figure 3.
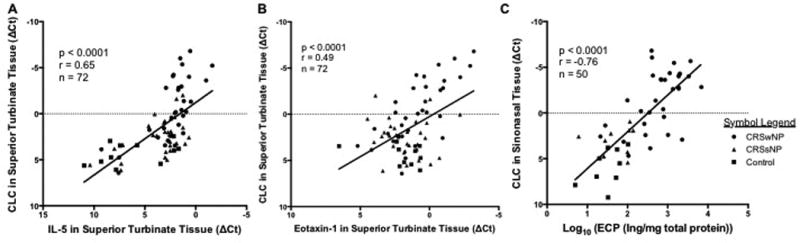
Correlation between the relative expression of CLC and the relative expression of the pro-eosinophil cytokine A) IL-5, and chemokine B) Eotaxin-1 in ST tissue. C) Correlation between CLC gene expression and ECP protein in sinonasal tissue.
ECP analysis and correlation with CLC gene expression
ECP levels were studied on available homogenates primarily to validate CLC gene expression as a biomarker of eosinophils. We found significantly higher ECP levels in CRSwNP ST compared to CRSsNP ST (p<0.01) and control IT (p<0.05). There was a strong and highly significant correlation between CLC gene expression and ECP protein levels (p<0.001, r=−0.76) (Figure 3C).
Olfactory gene expression in ST tissue
We next used qRT-PCR to verify the enrichment of olfactory tissue in our samples; IT samples (not anticipated to contain olfactory tissue) were tested to determine the mean and 95% CI of gene expression in non-olfactory tissue. Using this 95% confidence interval, we then assessed expression of ON markers in ST samples. The presence of ON was defined as expression of GAP-43 and OMP expression above that of the upper limit of the 95% CI of IT biopsies. We found that 29/49 (59%) of ST samples contained ON based on their expression of the immature olfactory neuronal marker GAP-43 and the mature olfactory neuron marker OMP 21/49 (43%) of ST samples (Figure 4).
Figure 4.
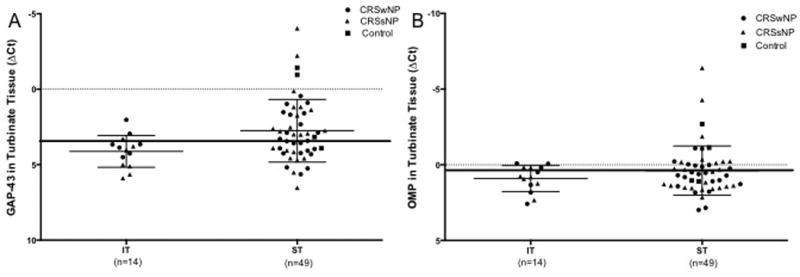
Relative expression of neural markers A) GAP-43, and B) OMP from a representative sample of IT and ST biopsies. A specimen was deemed to contain olfactory neuroepithelium if the relative expression was outside the lower limit of the 95% CI for IT biopsies (solid line). From this data, 59% and 43% of samples were deemed to have ON by GAP-43 and OMP analysis respectively.
Olfactory testing results
Analysis of olfactory threshold performance using Sniffin Sticks, revealed the age/sex-adjusted olfactory threshold mean was −5.69, −4.27, and −0.91 for CRSwNP, CRSsNP, and controls respectively (p<0.01). Similar to olfactory threshold, the average age/sex–adjusted mean for olfactory discrimination as determined by the UPSIT was −14.5, −6.36, and −1.89 for CRSwNP, CRSsNP and controls respectively (p<0.01). There was a significant modest correlation between the severity of the “smell/taste loss” item on the patient-reported SNOT-22 questionnaire and age-adjusted olfactory discrimination and olfactory threshold scores (r=0.73 p<0.01 for both correlations, data not shown).
Comparison of preoperative olfactory testing to tissue CLC expression and clinical measures of obstruction
When the age-adjusted Sniffin Sticks olfactory threshold scores were compared to the ΔCt CLC in ST, a statistically significant moderate correlation was found (p = 0.002, r = 0.57) with worse performance on the test being associated with higher CLC levels (Figure 5A). Similarly, there was a positive correlation between UPSIT performance and ΔCt CLC in ST (p = 0.02, r = 0.42) (Figure 5B). We then performed univariate and multiple linear regression of atopy status, polyp status, endoscopic observations of obstruction, CT opacification and CLC. On univariate analysis, multiple factors were significantly correlated with olfactory threshold and discrimination (Table 2a- threshold, 2b- discrimination). On multivariate regression, both models significantly modeled olfactory performance (r=0.83 and r=0.80 for threshold and discrimination respectively). However, only CLC expression was significantly correlated with olfactory threshold after adjusting for the other clinical factors (Table 2a), none of the individual items was significantly correlated with discrimination. The variance inflation factor did not raise significant concerns of collinearity.
Figure 5.
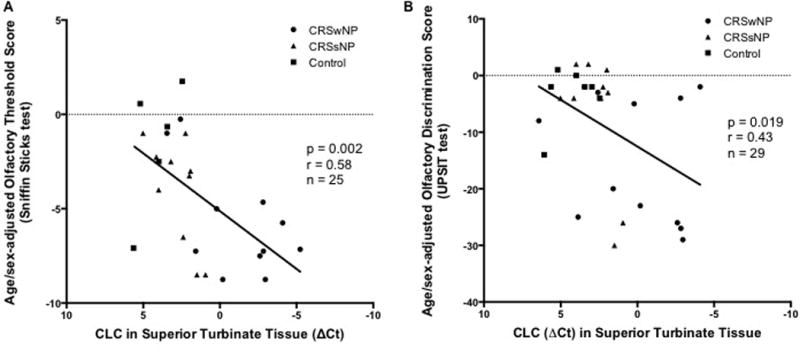
Correlation between performance on tests of A) olfactory threshold (Sniffin Sticks deviation from the age/sex-matched mean), and B) olfactory discrimination (UPSIT deviation from the age/sex-matched mean), and relative expression of markers for eosinophilia in tissue biopsies of the superior turbinate in normal controls, and patients with CRSsNP and CRSwNP.
Table 2.
| a. | |||||
|---|---|---|---|---|---|
| Olfactory Threshold Test (age/sex-adjusted Sniffin Sticks score) | |||||
| Univariate regression | Multiple regressiona | Collinearity Statistics |
|||
| Variables | Unadjusted β (95% CI) |
P | Adjusted β (95% CI) |
P | VIF |
| Asthma | −0.90 (−3.74 – 1.95) | 0.52 | Not modeled | - | |
| Atopy | −1.94 (−4.29 – 0.40) | 0.10 | 0.36 (−2.14 – 2.85) | 0.76 | 1.29 |
| ΔCt CLC | 0.61 (0.25 –0.98) | 0.00 | 0.73 (0.06 – 1.40) | 0.04 | 3.22 |
| Polyp status | −3.07(−5.32 – −0.81) | 0.01 | 3.12 (−0.10 – 6.34) | 0.06 | 2.30 |
| CT score (Ant.) | −0.94 (−2.06 – 0.18) | 0.09 | −0.43 (−1.97 – 1.12) | 0.55 | 2.62 |
| CT score (Post.) | −1.15 (−2.27 – −0.04) | 0.04 | −1.32 (−3.14 – 0.47) | 0.13 | 2.92 |
| Endoscopic score (MM) | −1.58 (−2.74 – −0.42) | 0.01 | −0.17 (−1.69 – 1.34) | 0.81 | 2.21 |
| Endoscopic score (OC) | −1.68 (−2.97 – −0.38) | 0.01 | −0.24 (−2.43 – 1.96) | 0.82 | 3.76 |
| b. | |||||
|---|---|---|---|---|---|
| Olfactory Discrimination Test (age/sex-adjusted UPSIT score) | |||||
| Univariate regression | Multiple regressiona | Collinearity Statistics |
|||
| Variables | Unadjusted β (95% CI) |
P | Adjusted β (95% CI) |
P | VIF |
| Asthma | −5.19 (−13.85 – 3.47) | 0.23 | Not modeled | - | - |
| Atopy | −4.36 (−11.85 – 3.12) | 0.24 | Not modeled | - | - |
| ΔCt CLC | 1.64 (0.29 – 2.99) | 0.02 | 0.91 (−1.79 – 3.60) | 0.48 | 2.51 |
| Polyp status | −11.42 (−18.21 – −0.62) | 0.00 | 7.98 (−4.48 – 20.44) | 0.24 | 2.09 |
| CT score (Ant) | −5.77 (−9.59 – −1.96) | 0.01 | −4.34(−10.80 – 2.13) | 0.17 | 2.80 |
| CT score (Post) | −6.59 (−10.24 – −2.93) | 0.00 | −6.08 (−13.27 – 1.10) | 0.09 | 2.90 |
| Endoscopic score (MM) | −4.37 (−8.72 – −0.02) | 0.05 | 3.51 (−3.05 – 10.07) | 0.26 | 2.18 |
| Endoscopic score (OC) | −4.89 (−9.71 – −0.05) | 0.05 | −2.66 (−11.15 – 5.83) | 0.51 | 2.83 |
CI, confidence interval; VIF, variance inflation factor; CLC, Charcot Leyden Crystal Protein; CT, computed tomography; MM, middle meatus; OC, olfactory cleft
R of this model is 0.83 and adjusted R of this model is 0.70.
UPSIT, University of Pennsylvania Smell Identification Test; CI, confidence interval; VIF, variance inflation factor; CLC, Charcot Leyden Crystal Protein; CT, computed tomography; MM, middle meatus; OC, olfactory cleft
R of this model is 0.80 and adjusted R of this model is 0.67.
Discussion
Chronic rhinosinusitis, especially CRSwNP, has been associated with olfactory impairment. To date, the exact etiology of the alteration in smell threshold and discrimination in CRS in humans is unknown, and is unlikely to be only related to a loss of airflow.4,26 In mouse models by Lane et. al, TNF-α induced olfactory inflammation impaired olfactory function but the role of this cytokine in CRS-associated olfactory loss in vivo has not been confirmed.27,28 In our study, we found that eosinophilic infiltration, as measured by gene expression of CLC, was elevated in biopsy specimens of ST tissue in patients with CRSwNP compared to those from CRSsNP and non-CRS controls (Figure 2). The selection of CLC as a marker was supported by its highly reliable correlation with eosinophilic asthma, celiac disease and CRS with published correlations with cytokines associated with eosinophilic inflammation in sinonasal tissue.29–32 There was striking regional variation in CLC expression in the nasal passages of patient with CRSwNP. Unlike NP tissue which was mostly eosinophilic, ST had a large range of eosinophilia but was still significantly higher than the ST of control and CRSsNP patiens as well as IT biopsies from patients with CRSwNP, CRSsNP and controls. This is in contrast to markers for neutrophils (CXCR1), T-cells (CD3), mast cells (tryptase) and leukocytes (CD45) that were not elevated in ST tissue of CRSwNP patients compared to ST from control and CRSsNP patients. We further found that CLC gene expression correlated with the cytokine IL-5 and the chemokine eotaxin-1(CCL11) suggesting CLC is reflective of type 2 eosinophilic inflammation (Figure 3). CLC gene expression was also strongly correlated with ECP protein levels, a more widely used eosinophil granule protein, thus validating its utility for measuring eosinophilia using gene expression. Given these findings, we tested the hypothesis that local CLC gene expression in ST tissue would be related to the olfactory impairment most commonly found in CRSwNP patients. We found several clinical, radiographic and endoscopic measures as well as CLC gene expression were significantly correlated with olfactory threshold on univariate analysis but only CLC expression remained significant on multivariate testing (Table 2, Figure 5).
While ON is variably distributed in the superior aspects of the middle turbinate and upper septum, the ST was selected as an anatomically standardized biopsy site due to its high concentration of ON and because it is partially resected in the course routine endoscopic sinus surgery.8,10,11,33 Indeed, our study finds elevated expression of olfactory neuronal markers including GAP-43 and OMP, at rates similar to those previously described in the literature using histological methods (Figure 4).2,5,9 Previous studies of olfactory tissue in CRS by Yee et al. used histopathology specimens collected from the ON rich upper septum to demonstrate that squamous metaplasia and mucosal erosion were more common and severe in patients with CRS when compared to normal patients.2 A separate study by Kern using histopathology specimens from the same region of the nasal cavity demonstrated that patients with abnormal olfactory discrimination (UPSIT <35) had increased frequency of inflammatory changes, including edema in the lamina propria, and increased presence of lymphocytes, macrophages, and eosinophils.5 A study by Zhao et. al, histopathology was utilized to correlate olfaction with erosion of the olfactory cleft, however this analysis only explained approximately 20% of olfactory variance.34 These studies are limited by the semiquantitative nature of histologic-based studies and for this reason, our analysis used qRT-PCR over histopathologic analysis.
In other studies of non-olfactory mucosal tissue from CRS patients, increased mucosal eosinophil counts were associated with increased hyposmia and anosmia compared to those with normosmia although not after adjusting for the presence of nasal polyps.35,36 These prior studies had not examined eosinophilia in tissue in the olfactory cleft and hypothesized that their results would correlate better if olfactory tissue were directly examined. Very recently, Schlosser found that olfactory cleft mucus IL-5 was correlated with olfactory performance, particularly identification and discrimination, in CRS patients.27 Similarly, eosinophilia, as measured by ECP in nasal secretions, has been associated with air flow independent reductions in olfactory thresholds and identification in patients with grass pollen allergies upon season onset.37 As shown in this paper, and published research from our laboratory, IL-5, IL-13, the eotaxins, and eosinophilia are intercorrelated and are reflective of a type 2 inflammatory environment in CRSsNP and CRSwNP.18,29 While the study by Schlosser suggested olfactory identification, rather than threshold correlates best with type 2 inflammation, we interpret these studies as complementary evidence that type 2 inflammation has specific effects on olfactory function independent of airflow (Figure 1).
Similar to other studies, we find that olfaction has several intercorrelated dimensions including olfactory threshold and suprathreshold discrimination.38 However, we find threshold testing correlates with CLC better than discrimination and it remained significantly correlated on multiple regression (Figure 5, Table 2). In examining the distribution of the data, it appeared that discrimination testing yielded more binary results with participants demonstrating either near normal or significantly impaired olfactory discrimination while olfactory threshold was more linearly correlated with CLC gene expression in the ST. This finding is supported by Whitcroft et al, who recently published a large retrospective series evaluating tests of discrimination, threshold, and identification in patients with different etiologies of olfactory loss.39 Their study further identified that patients with sinonasal inflammatory disease (mix of allergic rhinitis and CRS patients) were particularly impaired in olfactory threshold supporting our findings.
Together, our data suggests that eosinophils have a more direct and specific role in CRS associated olfactory dysfunction than commonly recognized. Importantly, studies of eosinophilic inflammation in other disease states suggest that eosinophils are frequently associated with neuropathology. For example, Costello et al. found perineural eosinophila was found in autopsy specimens of patients who died of status asthmaticus, and that the concentration of eosinophils clustered around nerve fibers was inversely correlated with M2 muscarinic receptor function in antigen challenged guinea pigs.40 In other studies, major basic protein, which is released from activated eosinophils, was shown to act as an antagonist to the M2 receptor.41 Peri-neural eosinophilia has further been implicated in peripheral neuropathies in Churg-Strauss syndrome.42,43
There is also increasing evidence that treatments targeting mediators of type 2 eosinophilic inflammation have dramatic effects on improving inflammation in CRS patients.44–46 In placebo controlled randomized control trials of corticosteroids, mepolizumab and dupilumab for CRSwNP, olfactory improvements were highly significant. Interestingly, in the mepolizumab study, the authors commented that olfaction durably improved despite recurrence of all other CRS symptoms. Currently, the only approved medications for CRSwNP is topical mometasone but this mode of delivery does not effectively deliver drugs to the olfactory cleft even in the absence of inflammatory changes and olfactory loss frequently relapses following cessation of systemic corticosteroid therapy.47,48 Our study highlights the profound eosinophilia of structures surrounding the olfactory cleft and illustrates the challenges for topical drug delivery to effectively treat this relatively inaccessible region of nasal anatomy.
Conclusion
Our study demonstrates that superior turbinates of CRSwNP patients had significantly increased eosinophilic inflammation. Furthermore, olfactory threshold deficits were significantly associated with superior turbinate eosinophilia even after controlling for nasal polyp status. This work provides direct evidence that CRS is a symptom of olfactory mucosal eosinophilia and developmental therapeutics targeting type 2 eosinophilic inflammation are important for improving the treatment of CRS-associated olfactory dysfunction.
Acknowledgments
This work was supported by NIH grants K23DC012067 and the Triological Society/American College of Surgeons (B.K.T), the Chronic Rhinosinusitis Integrative Studies Program (CRISP) U19 AI106683 (B.K.T, R.C.K, A.K, K.E.H and R.P.S); R01AI104733 (A.K); R01HL068546, R01HL078860, R01 AI072570 as well as the Ernest S. Bazley Trust (R.P.S).
Footnotes
Disclosure of potential conflict of interest: The authors have declared that they have no conflict of interest.
Portions of this work were previously presented by Lavin et al at the 2013 AAAAI annual meeting in San Antonio, TX
Contribution Statement
Study conception: Lavin, Kern, Schleimer, Tan
Design of study: Lavin, Kato, Mahdavinia, Hulse, Schleimer, Tan
Acquisition of data: Lavin, Lidder, Huang, Lam, Meen, Norton, Suh, Conley, Chandra, Shintani-Smith, Kern, Tan
Interpretation of data: Lavin, Min, Kato, Chmiel, Tan
Drafting of article and critical review: All authors
Final approval: All authors
References
- 1.Pleis JR, Ward BW, Lucas JW. Summary health statistics for U.S. adults: National Health Interview Survey, 2009. Vital and health statistics Series 10, Data from the National Health Survey. 2010:1–207. [PubMed] [Google Scholar]
- 2.Yee KK, Pribitkin EA, Cowart BJ, et al. Neuropathology of the olfactory mucosa in chronic rhinosinusitis. Am J Rhinol Allergy. 2010;24:110–120. doi: 10.2500/ajra.2010.24.3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baroody FN R. Allergic Rhinitis. In: Smell and Taste in Health and Disease. Cetchell T, Bartoshuk LM, Doty RL, Snow J, editors. New York: Raven Press; 1991. pp. 731–740. [Google Scholar]
- 4.Downey LL, Jacobs JB, Lebowitz RA. Anosmia and chronic sinus disease. Otolaryngol Head Neck Surg. 1996;115:24–28. doi: 10.1016/S0194-5998(96)70131-6. [DOI] [PubMed] [Google Scholar]
- 5.Kern RC. Chronic sinusitis and anosmia: pathologic changes in the olfactory mucosa. Laryngoscope. 2000;110:1071–1077. doi: 10.1097/00005537-200007000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Durack DT, Ackerman SJ, Loegering DA, Gleich GJ. Purification of human eosinophil-derived neurotoxin. Proceedings of the National Academy of Sciences of the United States of America. 1981;78:5165–5169. doi: 10.1073/pnas.78.8.5165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fredens K, Dahl R, Venge P. The Gordon phenomenon induced by the eosinophil cationic protein and eosinophil protein X. The Journal of allergy and clinical immunology. 1982;70:361–366. doi: 10.1016/0091-6749(82)90025-2. [DOI] [PubMed] [Google Scholar]
- 8.Morrison EE, Costanzo RM. Morphology of the human olfactory epithelium. J Comp Neurol. 1990;297:1–13. doi: 10.1002/cne.902970102. [DOI] [PubMed] [Google Scholar]
- 9.Paik SI, Lehman MN, Seiden AM, Duncan HJ, Smith DV. Human olfactory biopsy. The influence of age and receptor distribution. Arch Otolaryngol Head Neck Surg. 1992;118:731–738. doi: 10.1001/archotol.1992.01880070061012. [DOI] [PubMed] [Google Scholar]
- 10.Leopold DA, Hummel T, Schwob JE, Hong SC, Knecht M, Kobal G. Anterior distribution of human olfactory epithelium. Laryngoscope. 2000;110:417–421. doi: 10.1097/00005537-200003000-00016. [DOI] [PubMed] [Google Scholar]
- 11.Feron F, Perry C, McGrath JJ, Mackay-Sim A. New techniques for biopsy and culture of human olfactory epithelial neurons. Arch Otolaryngol Head Neck Surg. 1998;124:861–866. doi: 10.1001/archotol.124.8.861. [DOI] [PubMed] [Google Scholar]
- 12.Flegel C, Manteniotis S, Osthold S, Hatt H, Gisselmann G. Expression profile of ectopic olfactory receptors determined by deep sequencing. PloS one. 2013;8:e55368. doi: 10.1371/journal.pone.0055368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Orlandi RR, Lanza DC, Bolger WE, Clerico DM, Kennedy DW. The forgotten turbinate: the role of the superior turbinate in endoscopic sinus surgery. Am J Rhinol. 1999;13:251–259. doi: 10.2500/105065899782102908. [DOI] [PubMed] [Google Scholar]
- 14.Say P, Leopold D, Cochran G, Smith L, Greiner T. Resection of the inferior superior turbinate: does it affect olfactory ability or contain olfactory neuronal tissue? Am J Rhinol. 2004;18:157–160. [PubMed] [Google Scholar]
- 15.Lanza DC, Deems DA, Doty RL, et al. The effect of human olfactory biopsy on olfaction: a preliminary report. Laryngoscope. 1994;104:837–840. doi: 10.1288/00005537-199407000-00010. [DOI] [PubMed] [Google Scholar]
- 16.Takabayashi T, Kato A, Peters AT, et al. Glandular mast cells with distinct phenotype are highly elevated in chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2012;130:410–420. e415. doi: 10.1016/j.jaci.2012.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kato A, Xiao H, Chustz RT, Liu MC, Schleimer RP. Local release of B cell-activating factor of the TNF family after segmental allergen challenge of allergic subjects. J Allergy Clin Immunol. 2009;123:369–375. doi: 10.1016/j.jaci.2008.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Min JY, Ocampo CJ, Stevens WW, et al. Proton pump inhibitors decrease eotaxin-3/CCL26 expression in patients with chronic rhinosinusitis with nasal polyps: Possible role of the nongastric H,K-ATPase. J Allergy Clin Immunol. 2016 doi: 10.1016/j.jaci.2016.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wolfensberger M, Schnieper I, Welge-Lussen A. Sniffin'Sticks: a new olfactory test battery. Acta oto-laryngologica. 2000;120:303–306. doi: 10.1080/000164800750001134. [DOI] [PubMed] [Google Scholar]
- 20.Hummel T, Sekinger B, Wolf SR, Pauli E, Kobal G. 'Sniffin' sticks': olfactory performance assessed by the combined testing of odor identification, odor discrimination and olfactory threshold. Chem Senses. 1997;22:39–52. doi: 10.1093/chemse/22.1.39. [DOI] [PubMed] [Google Scholar]
- 21.Doty RL, Shaman P, Kimmelman CP, Dann MS. University of Pennsylvania Smell Identification Test: a rapid quantitative olfactory function test for the clinic. Laryngoscope. 1984;94:176–178. doi: 10.1288/00005537-198402000-00004. [DOI] [PubMed] [Google Scholar]
- 22.Hummel T, Kobal G, Gudziol H, Mackay-Sim A. Normative data for the "Sniffin' Sticks" including tests of odor identification, odor discrimination, and olfactory thresholds: an upgrade based on a group of more than 3,000 subjects. Eur Arch Otorhinolaryngol. 2007;264:237–243. doi: 10.1007/s00405-006-0173-0. [DOI] [PubMed] [Google Scholar]
- 23.Morley AD, Sharp HR. A review of sinonasal outcome scoring systems - which is best? Clinical otolaryngology : official journal of ENT-UK ; official journal of Netherlands Society for Oto-Rhino-Laryngology & Cervico-Facial Surgery. 2006;31:103–109. doi: 10.1111/j.1749-4486.2006.01155.x. [DOI] [PubMed] [Google Scholar]
- 24.Hopkins C, Gillett S, Slack R, Lund VJ, Browne JP. Psychometric validity of the 22-item Sinonasal Outcome Test. Clinical otolaryngology : official journal of ENT-UK ; official journal of Netherlands Society for Oto-Rhino-Laryngology & Cervico-Facial Surgery. 2009;34:447–454. doi: 10.1111/j.1749-4486.2009.01995.x. [DOI] [PubMed] [Google Scholar]
- 25.Kim DW, Kim JY, Jeon SY. The status of the olfactory cleft may predict postoperative olfactory function in chronic rhinosinusitis with nasal polyposis. Am J Rhinol Allergy. 2011;25:e90–94. doi: 10.2500/ajra.2011.25.3617. [DOI] [PubMed] [Google Scholar]
- 26.Doty RL, Frye R. Influence of nasal obstruction on smell function. Otolaryngologic clinics of North America. 1989;22:397–411. [PubMed] [Google Scholar]
- 27.Schlosser RJ, Mulligan JK, Hyer JM, Karnezis TT, Gudis DA, Soler ZM. Mucous Cytokine Levels in Chronic Rhinosinusitis-Associated Olfactory Loss. JAMA Otolaryngol Head Neck Surg. 2016;142:731–737. doi: 10.1001/jamaoto.2016.0927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lane AP, Turner J, May L, Reed R. A genetic model of chronic rhinosinusitis-associated olfactory inflammation reveals reversible functional impairment and dramatic neuroepithelial reorganization. J Neurosci. 2010;30:2324–2329. doi: 10.1523/JNEUROSCI.4507-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tan BK, Klingler AI, Stevens WW, et al. Heterogenous inflammatory patterns in chronic rhinosinusitis without nasal polyps in Chicago, Illinois. J Allergy Clin Immunol. 2016 doi: 10.1016/j.jaci.2016.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baines KJ, Simpson JL, Wood LG, et al. Sputum gene expression signature of 6 biomarkers discriminates asthma inflammatory phenotypes. The Journal of allergy and clinical immunology. 2014;133:997–1007. doi: 10.1016/j.jaci.2013.12.1091. [DOI] [PubMed] [Google Scholar]
- 31.Chua JC, Douglass JA, Gillman A, O'Hehir RE, Meeusen EN. Galectin-10, a potential biomarker of eosinophilic airway inflammation. PloS one. 2012;7:e42549. doi: 10.1371/journal.pone.0042549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Re V, Simula MP, Cannizzaro R, et al. Galectin-10, eosinophils, and celiac disease. Ann N Y Acad Sci. 2009;1173:357–364. doi: 10.1111/j.1749-6632.2009.04627.x. [DOI] [PubMed] [Google Scholar]
- 33.Rawson NE, Gomez G, Cowart B, et al. Selectivity and response characteristics of human olfactory neurons. Journal of neurophysiology. 1997;77:1606–1613. doi: 10.1152/jn.1997.77.3.1606. [DOI] [PubMed] [Google Scholar]
- 34.Zhao K, Jiang J, Pribitkin EA, et al. Conductive olfactory losses in chronic rhinosinusitis? A computational fluid dynamics study of 29 patients. Int Forum Allergy Rhinol. 2014;4:298–308. doi: 10.1002/alr.21272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Soler ZM, Sauer DA, Mace JC, Smith TL. Ethmoid histopathology does not predict olfactory outcomes after endoscopic sinus surgery. Am J Rhinol Allergy. 2010;24:281–285. doi: 10.2500/ajra.2010.24.3477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mori E, Matsuwaki Y, Mitsuyama C, Okushi T, Nakajima T, Moriyama H. Risk factors for olfactory dysfunction in chronic rhinosinusitis. Auris Nasus Larynx. 2013;40:465–469. doi: 10.1016/j.anl.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 37.Klimek L, Eggers G. Olfactory dysfunction in allergic rhinitis is related to nasal eosinophilic inflammation. The Journal of allergy and clinical immunology. 1997;100:158–164. doi: 10.1016/s0091-6749(97)70218-5. [DOI] [PubMed] [Google Scholar]
- 38.Doty RL, Smith R, McKeown DA, Raj J. Tests of human olfactory function: principal components analysis suggests that most measure a common source of variance. Perception & psychophysics. 1994;56:701–707. doi: 10.3758/bf03208363. [DOI] [PubMed] [Google Scholar]
- 39.Whitcroft KL, Cuevas M, Haehner A, Hummel T. Patterns of olfactory impairment reflect underlying disease etiology. Laryngoscope. 2016 doi: 10.1002/lary.26229. [DOI] [PubMed] [Google Scholar]
- 40.Costello RW, Schofield BH, Kephart GM, Gleich GJ, Jacoby DB, Fryer AD. Localization of eosinophils to airway nerves and effect on neuronal M2 muscarinic receptor function. The American journal of physiology. 1997;273:L93–103. doi: 10.1152/ajplung.1997.273.1.L93. [DOI] [PubMed] [Google Scholar]
- 41.Jacoby DB, Gleich GJ, Fryer AD. Human eosinophil major basic protein is an endogenous allosteric antagonist at the inhibitory muscarinic M2 receptor. The Journal of clinical investigation. 1993;91:1314–1318. doi: 10.1172/JCI116331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nagashima T, Cao B, Takeuchi N, et al. Clinicopathological studies of peripheral neuropathy in Churg-Strauss syndrome. Neuropathology : official journal of the Japanese Society of Neuropathology. 2002;22:299–307. doi: 10.1046/j.1440-1789.2002.00454.x. [DOI] [PubMed] [Google Scholar]
- 43.Oka N, Kawasaki T, Matsui M, Shigematsu K, Unuma T, Sugiyama H. Two subtypes of Churg-Strauss syndrome with neuropathy: the roles of eosinophils and ANCA. Modern rheumatology / the Japan Rheumatism Association. 2011;21:290–295. doi: 10.1007/s10165-010-0400-9. [DOI] [PubMed] [Google Scholar]
- 44.Gevaert P, Van Bruaene N, Cattaert T, et al. Mepolizumab, a humanized anti-IL-5 mAb, as a treatment option for severe nasal polyposis. The Journal of allergy and clinical immunology. 2011;128:989–995. e981–988. doi: 10.1016/j.jaci.2011.07.056. [DOI] [PubMed] [Google Scholar]
- 45.Van Zele T, Gevaert P, Holtappels G, et al. Oral steroids and doxycycline: two different approaches to treat nasal polyps. J Allergy Clin Immunol. 2010;125:1069–1076. e1064. doi: 10.1016/j.jaci.2010.02.020. [DOI] [PubMed] [Google Scholar]
- 46.Bachert C, Mannent L, Naclerio RM, et al. Effect of Subcutaneous Dupilumab on Nasal Polyp Burden in Patients With Chronic Sinusitis and Nasal Polyposis: A Randomized Clinical Trial. JAMA : the journal of the American Medical Association. 2016;315:469–479. doi: 10.1001/jama.2015.19330. [DOI] [PubMed] [Google Scholar]
- 47.Vaidyanathan S, Barnes M, Williamson P, Hopkinson P, Donnan PT, Lipworth B. Treatment of chronic rhinosinusitis with nasal polyposis with oral steroids followed by topical steroids: a randomized trial. Annals of internal medicine. 2011;154:293–302. doi: 10.7326/0003-4819-154-5-201103010-00003. [DOI] [PubMed] [Google Scholar]
- 48.Lam K, Tan BK, Lavin JM, Meen E, Conley DB. Comparison of nasal sprays and irrigations in the delivery of topical agents to the olfactory mucosa. Laryngoscope. 2013 doi: 10.1002/lary.24239. [DOI] [PubMed] [Google Scholar]


