Abstract
Objective
Atherosclerosis developed during premenopausal years predicts postmenopausal atherosclerosis burden. Selective serotonin reuptake inhibitor (SSRI) antidepressants, recently approved for hot flushes, have been associated with increased ischemic stroke risk in several observational studies; however effects on carotid artery atherosclerosis, a strong predictor of future vascular events, are unknown.
Methods
The effects of chronic administration of a commonly prescribed SSRI, sertraline HCl, on atherosclerosis in the carotid artery was assessed in a placebo-controlled, longitudinal, randomized study of premeonopausal depressed and nondepressed cynomolgus monkeys (Macaca fascicularis; n=42). Physiologic and behavioral phenotypes were evaluated at baseline and after 18-months of oral sertraline (20 mg/kg, n=21) or placebo (n=21). Carotid artery atherosclerosis was measured post-mortem via histomorphometry.
Results
Atherosclerosis extent in the right common carotid artery, on average, was 60% greater in sertraline-treated depressed monkeys compared to all other groups (p=0.028). The results of linear regression analyses suggested that sertraline and depression effects on atherosclerosis were not mediated by their effects on behavioral and physiological risk factors.
Conclusions
These findings suggest that chronic SSRI treatment is associated with the progression of carotid artery atherosclerosis, which may increase the risk for future vascular events, particularly in depressed women. The underlying mechanism remains to be determined, but does not appear to be related to SSRI effects on traditional cardiovascular risk factors.
Keywords: Macaca fascicularis, selective serotonin reuptake inhibitor, carotid artery atherosclerosis, female, depression, ischemic stroke
Introduction
Cardiovascular diseases are the leading global cause of death. Coronary heart disease (CHD) is the single leading cause of dealth in women, taking more lives than all forms of cancer combined. Cerebrovascular events, or strokes, are the second-leading global cause of death behind heart disease as well as the leading preventable cause of disability. Compared to men, women have a higher lifetime risk of stroke and more likely to die of a result of stroke-related complications.1 Although both CHD and stroke are primarily diseases of individuals older than 55 years of age, disease risk has its origins in childhood and progresses with age. The multicenter Pathologic Determinants of Atherosclerosis in Youth study reported that by 35 years of age, about 70% of women have coronary artery fatty streaks.2 Changes to the carotid arteries, namely common carotid artery intimal medial thickening, have also been observed in younger individuals.3–6 Premenopausal cardiovascular risk factor measures are associated with subclinical post-menopausal carotid artery atherosclerosis,6,7 which is in turn is predictive of future cardiovascular events.8 Additionally, subclinical carotid artery atherosclerosis in younger patients has been found to predict vascular events suffered later in life.3 Taken together, it is increasingly clear that the premenopausal years are crucial determinants for postmenopausal atherosclerosis burden, CHD, and stroke risk.3, 6,9,10
Depression has recently emerged as a notable risk factor for both CHD11,12 and stroke.13–15 Depressive disorders are twice as likely in women as men16 and are the leading cause of disease burden for women worldwide.17 In the US depression is more prevalent females aged 40–59 than any other age or sex group.18 Depression often begins early in life and that the gender difference emerges with puberty,19 thus the life-long disease burden of depression isamplified in women.
Antidepressant drugs (ADs) are an important therapy for not only the treatment of depressive disorders, but they have recently been labled for the treatment of hot flushes. ADs are the third most commonly prescribed medication in America and women are 2.5 times more likely than men to take ADs. Prior Food and Drug Administration (FDA) approval of AD therapy for the treatment of hot flushes, 23% of women aged 40–59 were already taking ADs.20 In 2014, the FDA approved the use of paroxetine for menopausal hot flushes, which occur in as many as 75% of menopausal women.21 Paroxetine is in the most commonly prescribed class of antidepressants, selective serotonin reuptake inhibitors (SSRIs). SRRIs as a class have been associated with increases in ischemic stroke risk in several observational studies22–25 and may also increase cardiovascular morbidity and mortality.26 Because of their widespread use in women, knowledge of the multisystem effects of these medications has important implications for women’s health.
It is currently unknown to what extent SSRIs may influence cardiovascular disease risk, and whether SSRI use affects atherosclerosis progression, a precursor to vascular events27. One methodological limitation of studies of SSRI effects in people is the inability to control for treatment indication – often depression, which is a known risk factor for cardiovascular disease.11–15 Consequently, there is a need for longitudinal, controlled, randomized studies to determine effects of SSRIs on carotid artery atherosclerosis progression independent of depression; however, long-term randomized trials evaluating SSRI effects are not ethical in healthy human populations. The aim of this study was to determine the effects of chronic SSRI treatment and depression on atherosclerosis in several carotid artery anatomical sites using a translational nonhuman primate model of depression.
Laboratory-housed nonhuman primates develop naturally occurring behavioral depression which closely resembles human depression in physiological, neurobiological, and behavioral characteristics including reduced body mass, hypothalamic-pituitary-adrenal axis perturbations, autonomic dysfunction, increased cardiovascular disease risk, reduced hippocampal volume, altered serotonergic function, decreased activity levels, and increased mortality.28 Macaques are also a widely used animal model of atherosclerosis due to their high responsivity to atherogenic diets which results in an abnormal lipid profile the development of extensive atherosclerotic lesions of similar distribution and composition to human lesions.29 The physiological and neurobiological characteristics of female cynomolgus monkeys (Macaca fascicularis) that exhibit behavioral depression has been well characterized and includes dyslipidemia and exacerbated coronary artery atherosclerosis.30–33 Previously we have reported that sertraline HCl treated monkeys exhibited exacerbated coronary artery atherosclerosis, independent of depression, but did not experience adverse effects on traditional cardiovascular risk factors such as plasma lipids, adiposity, and insulin resistance.34 In this report we describe the effects of depression and SSRI on atherosclerosis extent in several anatomical sites within the carotid arteries.
Materials and Methods
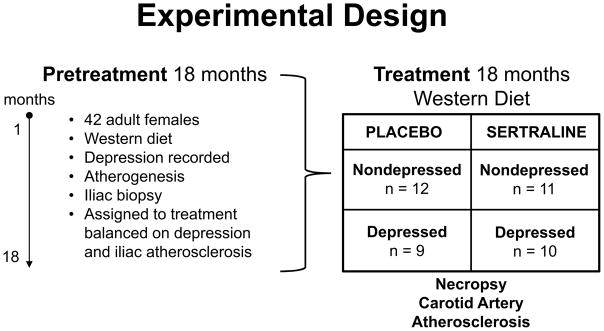
Details about the animals; diets and methods used to evaluate circulating sertraline and desmethylsertraline concentrations,35, cardiac function36 cardiovascular risk factors34,37, and coronary artery atherosclerosis34 have been published previously. Briefly, forty-five reproductively-aged female cynomolgus were imported from Indonesia (Institut, Pertanian Bogor, Bogor, Indonesia) and quarantined in single cages for a one-month. Following quarantine, monkeys were randomly assigned to social groups (n = 4–5) and housed in indoor pens (3.05m X 3.05m X 3.05m) with 12/12 light/dark and water ad libitum. Monkeys consumed an atherogenic diet (containing 44% of calories from fat and 0.29 mg/Cal cholesterol) during an 18-month pretreatment phase, during which behavior was recorded. Stratified randomization was used to assign the monkeys by social group to either oral placebo (n=21) or SSRI (n=21) treatment balanced on pretreatment rate of depressive behavior, body weight, and iliac artery atherosclerosis extent (Figure 1)34. Sertraline HCl (Zoloft®) was chosen due to the need to dose monkeys according to body weight and its lack of effect on body weight in macaques38. We have previously published that there were no differences between treatment groups in cardiac function36 cardiovascular risk factors34, 37, or illiac artery atherosclerosis34 prior to randomization. Monkeys were treated daily for 18 months. During the study, three animals died (prior to randomization) of causes unrelated to the experiment, resulting in a final sample size of 42. All animal manipulations were performed according to the guidelines of state and federal laws, the US Department of Health and Human Services, and the Animal Care and Use Committee of Wake Forest University School of Medicine.
Figure 1.
Forty-two adult female monkeys consumed an atherogenic diet for an 18-month pretreatment phase, during which depressive behavior and physiological risk factors were assessed. At the end of the pretreatment phase, the iliac artery was biopsied and atherosclerosis extent measured. Monkey social groups were assigned to the placebo or sertraline treatment groups balanced on mean pretreatment rates of depression, iliac artery atherosclerosis extent, and body weight. The monkeys continued to consume the atherogenic diet during the 18-month treatment phase, during which behavioral and physiological risk factors were assessed. The animals were then necropsied and carotid artery atherosclerosis was measured.
Behavior Observations
Depressive behavior was recorded during 10 minute focal animal observations, 6–8 times per month, counterbalanced for time of day, for 12 months during both the pretreatment and treatment phases (an average of 33.3 hours/monkey total), using a previously described technique39,40. Depressive behavior was defined as: 1) a slumped or collapsed body posture; accompanied by 2) a lack of responsiveness to environmental stimuli to which other monkeys are attending; and 3) open eyes to distinguish this behavior from resting or sleeping32. This depressive behavior is easily recognizable, and inter-observer reliability, determined biannually, was r ≥ 0.92 throughout the experiment30. The average frequency/hour that the monkeys exhibited depressive behavior was calculated from these observations. Monkeys with pretreatment depressive behavior rates below the mean depression rate were classified as nondepressed (n=23), and those with depressive behavior rates above the mean were classified as depressed (n=19). Sertraline had no effect on depressive behavior34. During focal sampling other behaviors recorded included aggressive behaviors (the summed frequency of open mouth threat, stare threat, yawn threat, chase, bite, slap/grab, displace, and cage shake display), submissive behaviors (the summed frequency of fear grimace, lip smack, move away, crouch, flee, present hindquarters, scream, and scream threat), and percent of time spent in body contact (body contact, close, or alone)39,40.
Physiological Risk Factors
Anthropometrics and body composition, lipid metabolism, ovarian function, heart rate,34 cardiac structure/function36, carbohydrate metabolism, adiponectin, and physical activity37 were assessed using previously described methods. Briefly, body weight (BW), body mass index (BMI), body composition, fasting insulin and glucose, and adipocytokines were measured prior to and at the end of the treatment phase.37 Transthoracic echocardiography was performed 14 months after the onset of the treatment phase, following ketamine (15mg/kg) sedation.36 Fasting total plasma cholesterol (TPC), high-density lipoprotein cholesterol (HDLC), triglycerides concentrations were measured after 0, 3, 10, and 17 months of treatment, the ratio of TPC/HDLC was determined at each timepoint, and averaged across the treatment phase. Ovarian function was determined over the course of one-year during the pretreatment and treatment phases by vaginal swabbing to detect menses. Progesterone concentrations measured from three times/week by femoral venipuncture.34 Heart rate (HR) and activity were measured over a 24 hours period prior to and at the end of the treatment phase using portable telemetry and actigraphy units.34,37
We also explored relationships with hemodynamic factors, indices of physical inactivity, and circulating leukocyte counts – all have been identified as correlates of atherosclerosis extent in humans.41–43 Aortic velocity time integral (AO-VTI) and eft ventricular end internal diastolic dimension (LV-IDD) was measured by Doppler echocardiography at the end of the treatment phase.36 Indirect blood pressure (BP) was measured at the end of the treatment phase using High Definition Oscillometry (Vetline LLC, Saint Kitts and Nevis). Average BP (systolic and diastolic) were averaged from three measurements, taken 40-seconds apart an average of 12.5 minutes post- ketamine sedation. Physical inactivity was measured prior to and at the end of the treatment phase and was defined by the absense of recorded activity during 60-second epochs (daytime sedentary bouts), which were summed over the daytime period (500–1900h) and were correlated with rate of depressive behavior (r=0.41; p=0.008). Finally, complete blood counts were evaluated during routine clinical exams prior to and following 15 months of treatment and were measured at a commercial laboratory using a VetAutoread Hematology Analyzer (Idexx Laboratories, Westbrook, Maine).39
Necropsy and Measurement of Carotid Artery Atherosclerosis
Animals were anesthetized deeply with pentobarbital (60 mg/kg) at the time of necropsy. The circulatory system was flushed with lactated Ringer’s solution and the following left and right carotid artery sites were dissected free and removed: common carotid arteries (L/RCC), carotid bifurcations (L/RCB), and t internal carotid arteries (L/RIC). Following dissection, the common carotid arteries were opened longitudinally and segments measuring 3mm long proximal to the bifurcation was collected from each artery. One segment was collected from the LCB, RCB, and both internal carotid arteries. Due to the delicate nature of dissecting the carotid arteries, 3 RCBs, 2 LICs, and 4 RICs were not collected.
All arteries were immersion-fixed in 10% neutral-buffered formalin, individually embedded in paraffin blocks, and 5-μm sections were cut and stained with Verhoeff-van Gieson’s stain.39 Images of stained sections were captured using a Hammamatsu NanoZoomer digital slide scanner. Intimal area (IA; mm2) and maximum medial thickness (MXMT; mm) were measured as described previously44 using Image-Pro Plus version 9.1 imaging software (Media Cybernetics Inc, Bethesda, MD). For consistency one investigator (MGSM) measured all arteries, blinded to treatment. A random subset of 10% of the arteries was remeasured and the mean intraobserver coefficient of variation was less than 3%.39
Statistical Analysis
The main outcome for this study was atherosclerosis extent (IA). Square root transformations were used to reduce skewness and heterogeneity of variance in the data. The pretreatment ratio of total plasma to high-density lipoprotein cholesterol (TPC/HDLC), a significant predictor of carotid artery atherosclerosis,45 was used as a covariate to adjust for pretreatment variation in atherosclerosis extent. We used 2 (placebo, sertraline) X 2 (nondepressed, depressed) analyses of covariance (ANCOVAs) to determine the effects of sertraline and depression on the individual arteries (LCC, RCC, LCB, RCB, LIC, and RIC). Fisher’s protected least significant difference test was used for post-hoc comparisons.39 MXMT was analyzed by 2 (placebo, sertraline) X 2 (nondepressed, depressed) analyses of variance (ANOVAs).
Several behavioral and physiological risk factors were evaluated as predictors of carotid artery atherosclerosis. Sertraline and depression effects on these risk factors were analyzed by 2 (placebo, sertraline) X 2 (nondepressed, depressed) analyses of variance (ANOVA). All variables had some degree of heterogeneity thus analyses were originally run on square root transformed data. Analyses were repeated using untransformed data. Transformation had no effect on p-values; therefore significance tests are reported for the untransformed adjusted means ± standard error of the means. 39
The associations between carotid artery atherosclerosis and behavioral and physiological outcomes were determined by Pearson correlation coefficients (r); linearity was verified by visual assessment of each scatterplot. Depressive behavior and variables that were correlated with IA in at least one arterial site (p<0.1) were selected for multiple regression models and included: rate of depressive behavior, rate of body contact, TPC/HDLC, visceral abdomincal fat volume (VAF), triglycerides, HR, number of daytime sedentary bouts, systolic BP, monocyte counts, LV-IDD, and AO-VTI (Table 1). Behavioral and physiological variables were identified with backward regression models for each artery (list-wise exclusion was used to account for missing variables) and were used to identify subsets of predictors for further evaluation using subsequent stepwise analysis. Separate stepwise, multiple-regression models were performed to explain the variance (coefficient of determination; R2) in atherosclerosis extent for each artery. Multicollinearity for the explanatory variables was verified by variance inflation factor (VIF). Treatment group and interaction terms were entered into final regression models to determine whether sertraline or sertraline × depression interaction modified the relationship between risk factors and atherosclerosis extent. All ANOVA/ANCOVA analyses were performed using STATISTICA 12.0 for Windows (StatSoft Inc, Tulsa, OK). Linear regression analysis was performed with SPSS software (v22; IBM, Somers, NY). All tests were two sided, and the level of significance was set at p=0.05.39
Table 1.
Behavioral and physiological predictors of carotid artery atherosclerosis extent
| Dep Beh | Body Cont | TPC | HDLC | TPC/HDLC | VAF | TG | HR | Day Sed | SBP | Monos | LV-IDD | AO-VTI | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LCC | 0.02 | 0.10 | 0.55 | −0.38 | 0.54 | 0.16 | 0.20 | 0.19 | 0.17 | 0.28 | 0.24 | −0.31 | −0.16 |
| LCB | 0.08 | 0.09 | 0.27 | −0.37 | 0.34 | 0.35 | 0.26 | 0.26 | 0.32 | 0.14 | 0.31 | −0.13 | −0.25 |
| LIC | −0.03 | −0.03 | 0.17 | −0.16 | 0.19 | 0.21 | 0.24 | −0.17 | 0.10 | 0.10 | −0.02 | −0.38 | −0.36 |
| RCC | 0.12 | 0.21 | 0.55 | −0.49 | 0.61 | −0.06 | 0.18 | 0.29 | 0.15 | 0.17 | 0.25 | −0.07 | −0.40 |
| RCB | 0.14 | 0.24 | 0.30 | −0.44 | 0.45 | −0.07 | 0.19 | 0.29 | 0.39 | 0.24 | 0.30 | −0.14 | −0.39 |
| RIC | −0.04 | −0.09 | 0.25 | −0.38 | 0.40 | 0.10 | 0.25 | −0.03 | 0.30 | −0.03 | −0.01 | −0.12 | −0.22 |
Significant (2-tailed, p<0.05) Pearson correlations (r) are shown in bold. Borderline (p<0.1) r’s are shown in italics. L/RCC=left/right common carotid; L/RCB=left/right carotid bifurcation; L/RIC=left/right internal carotid; Dep Beh=depressive behavior; Body Cont=body contact; TPC=total plasma cholesterol, HDLC=high-density lipoprotein cholesterol; VAF=visceral abdominal fat; TG=triglycerides; 24h HR=average heart rate over 24 hours; Day Sed=number of 60-sec bouts of inactivity from 500–1900; SBP=systolic blood pressure; Monos=monocyte count; LV-IDD=left ventricular end diastolic dimension; AO-VTI=aortic doppler velocity tracing.
Results
Behavioral and Physiological Risk Factors
Prior to treatment, sertraline and placebo groups were matched on behavior, body weight (BW), body mass index (BMI), plasma lipids and lipoproteins, body composition, insulin resistance, adiponectin, heart rate (HR), and ovarian function.34,37 Sertraline and depression effects on cardiovascular risk factors are summarized in Table S1, Supplemental Digital Content. Some of these have been reported previously and are so noted in Table S1.34,36,37 Novel findings include no differences between the placebo and sertraline groups in rates of body contact and aggression, daytime sedentary behavior, BP, and AO-VTI. There were significant main effects of sertraline and depression on submissions sent. Placebo-treated depressed monkeys sent greater than twice the number of submissions sent by all other groups; although there was not a significant treatment by depression interaction effect. Sertraline-treated monkeys and depressed monkeys had reduced numbers of circulating monocytes compared to placebo-treated and non-depressed monkeys, respectively. During the treatment phase, depressed monkeys were more likely to engage in body contact with another monkey and displayed more daytime sedentary bouts than did their nondepressed counterparts. There were no significant treatment by depression interaction effects.39
Sertraline and Depression Effects on Carotid Artery Atherosclerosis
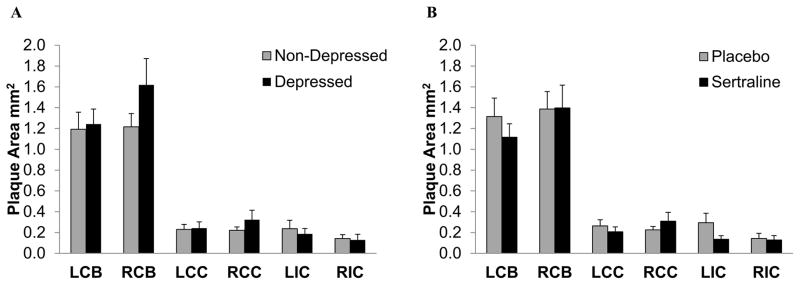
Figure 2 depicts carotid artery extent expressed as raw means and standard errors of the mean, in depressed versus nondepressed and sertraline versus placebo groups. Visually, depressed monkeys appeared to have greater IAs, on average, than nondepressed monkeys, particularly in the right common carotid artery and bifurcation (Figure 2A). Average IA did not appear to differ across carotid artery locations in sertraline compared with placebo-treated monkeys (Figure 2B).
Figure 2.
Carotid artery atherosclerosis extent expressed as raw means and SEMs in the left and right carotid artery bifurcations (LCB, RCB), the left and right common carotid arteries (LCC, RCC), and the left and right internal carotid arteries (LIC, RIC) by depressed versus nondepressed (A), and sertraline versus placebo (B). SEMs=standard error of the means.
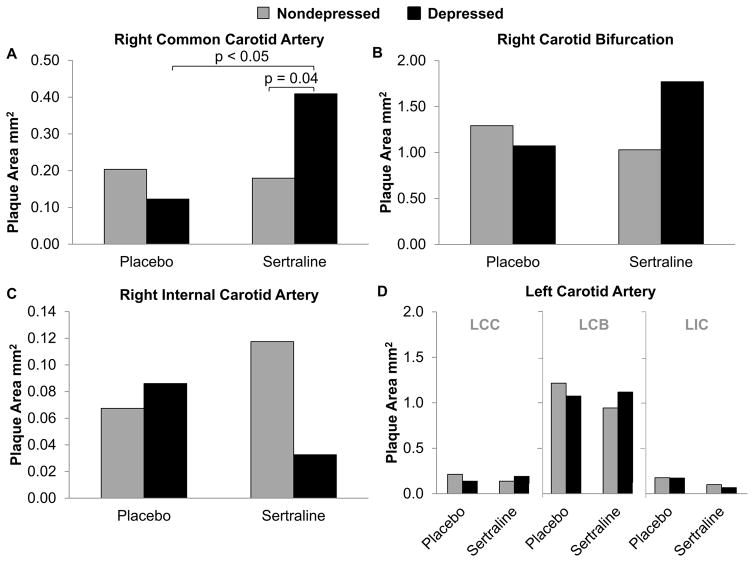
Atherosclerosis extent in the individual arteries, expressed as adjusted means of the distributions of transformed data converted to original units, is depicted in Figure 3. There were no main effects of depression or sertraline treatment (p’s > 0.05). There was a significant interaction between sertraline treatment and depression on atherosclerosis in the RCC (p = 0.028; Figure 3A). IA was 60% greater, on average, in sertraline-treated depressed monkeys compared to all other groups (Figure 4). Post-hoc comparisons suggested depressed monkeys treated with sertraline had significantly more atherosclerosis than those treated with placebo (p = 0.039). A similar pattern was observed in the right carotid bifurcation but did not reach significance (RCB p = 0.057; Figure 3B). Sertraline × depression did not significantly affect MXMT in either artery (RCC: p = 0.24; RCB: p = 0.089). There were no significant effects of sertraline or depression in the right internal or left carotid arteries (Figures 3C, D).
Figure 3.
The effects of sertraline on atherosclerosis in the individual territories of the carotid arteries in depressed and nondepressed monkeys. The data were analyzed by 2 (placebo, sertraline) X 2 (nondepressed, depressed) analyses of covariance (ANCOVAs) adjusting for baseline total plasma cholesterol/high-density lipoprotein cholesterol. A, Atherosclerosis extent in the right common carotid artery. There were no main effects of sertraline (p = 0.070) or depression (p = 0.410), but a significant interaction effect was observed (p=0.028). B, Atherosclerosis extent in the right carotid bifurcation. There were no main effects of sertraline (p = 0.428) or depression (p = 0.318), but a suggestive interaction effect was observed (p=0.057). C, Atherosclerosis extent in the right internal carotid artery. There were no effects of sertraline, depression, or their interaction (p’s all > 0.05). D, Atherosclerosis extent in the left carotid artery (LCC, LCB, and LIC). There were no effects of sertraline, depression, or their interaction (p’s all > 0.05). Adjusted means of the distributions of transformed data converted to original units are depicted in all graphs. LCC=left common carotid; LCB=left carotid bifurcation; LCC=left common carotid.
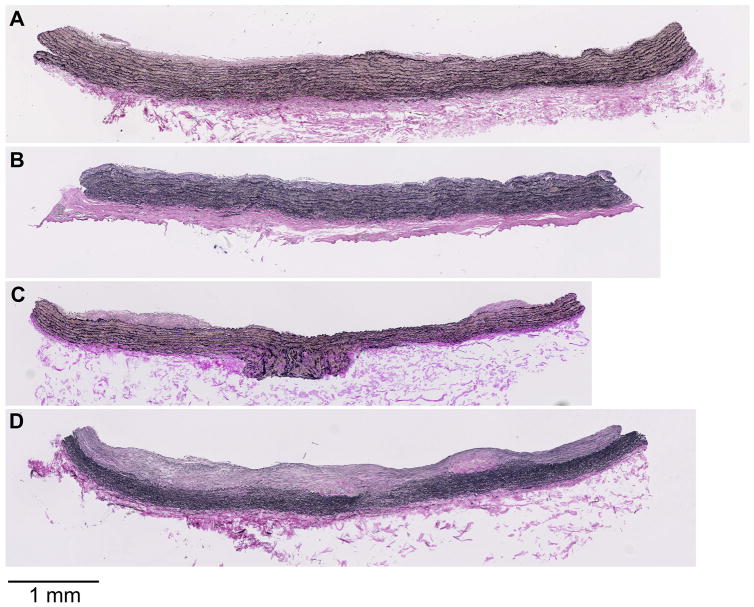
Figure 4.
Representative photomicrographs of right common carotid artery atherosclerosis by treatment group. A, Placebo-treated, non-depressed representative lesion (intimal area = 0.208 mm2). B, Placebo-treated, depressed representative lesion (intimal area = 0.241 mm2). C, Sertraline-treated, non-depressed representative lesion (intimal area = 0.205 mm2). D, Sertraline-treated, depressed representative lesion (intimal area = 0.851 mm2).
Behavioral and Physiological Predictors of Carotid Artery Atherosclerosis
Pearson correlations of behavioral and physiological phenotypes with carotid artery atherosclerosis are presented in Table 1. Across all groups, behavior (depressive, aggressive, submissive, and body contact), BW, BMI, adiponectin, insulin resistance, 24h activity, HR, ovarian function, and BP were not significantly associated with IA in any artery (p’s > 0.05). TPC/HDLC was positively associated and HDLC negatively associated with IA in the RCC, RBC, RIC, LCC, and LBC. VAF and circulating monocytes were positively associated with LCB IA. The number of daytime sedentary bouts was associated with IA at both bifurcations. LV-IDD) was associated with LIC IA and AO-VTI associated with LIC, RCC, and RCB IA. Finally, IA was significantly correlated across carotid artery sites (Table S2, Supplemental Digital Content).
Backward regression models for each artery are detailed in Table S3, Supplemental Digital Content. The variance in LCC atherosclerosis extent (R2 = 0.49, p = 0.001, maximum VIF =1.42) was significantly explained by rate of depressive behavior, TPC/HDLC, triglycerides, monocyte count, and daytime sedentary bouts. Variance in LCB atherosclerosis extent was explained by TPC/HDLC, VAF, triglycerides, monocyte count, daytime sedentary bouts, and LV-IDD (R2 = 0.59, p < 0.001, maximum VIF=1.55). A significant proportion of the variance in LIC atherosclerosis extent was explained by TPC/HDLC, VAF, HR, systolic BP, monocyte count, LV-IDD, and AO-VTI (R2 = 0.51, p = 0.006, maximum VIF = 1.71). The variance in RCC atherosclerosis extent was explained by TPC/HDLC, triglycerides, monocyte count, and LV-IDD (R2 = 0.49, p = 0.001, maximum VIF = 1.40). Variance in RCB atherosclerosis extent (R2 = 0.57, p = 0.002, maximum VIF = 3.61) was explained by rate of depressive behavior, rate of body contact, TPC/HDLC, triglycerides, daytime sedentary bouts, monocyte count, and AO-VTI. Finally, variables that significantly explained variance in RIC atherosclerosis extent included TPC/HDLC, VAF, triglycerides, and HR (R2 = 0.42, p = 0.005, maximum VIF = 1.46).
Backwards models helped to refine the list of predictive variables from a rich premortem database, however not all predictors identified by these models were significant. Subsequently, using the variables identified with the backward regression model, stepwise multiple-regression models were performed to explain the variance (R2) in atherosclerosis extent (Table 2). Sertraline treatment and treatment × depression (depression was already included as a continuous predictor) were then sequentially added as covariates (Table S4, Supplemental Digital Content). LCC IA was significantly explained by TPC/HDLC (R2 = 0.26, p = 0.001). The strongest explanatory variables for LCB IA were TPC/HDLC, VAF, and triglycerides (R2 = 0.47, p < 0.001, maximum VIF = 1.06). LV-IDD predicted LIC IA (R2 = 0.18, p = 0.014). For the right carotid, TPC/HDLC alone predicted RCC (R2 = 0.33, p < 0.001), RCB (R2 = 0.21, p = 0.007), and RIC (R2 = 0.19, p = 0.012) intimal areas. Adding treatment group as a covariate did not alter the relationships between IA and predictors for any artery, nor did it affect the overall significance of the model. Similarly, covarying for the interaction between sertraline treatment and depression did not alter the relationship between significant predictors and IA but did it affect overall model significance in the LIC and RIC.
Table 2.
Regression models demonstrating relations between carotid artery atherosclerosis and physiological risk factors
| Artery | Independent variables | β | p |
|---|---|---|---|
| LCC | TPC/HDLC | 0.26 | 0.001 |
| LCB | TPC/HDLC | 0.32 | 0.001 |
| VAF | 0.10 | 0.002 | |
| Triglycerides | 0.027 | 0.043 | |
| LIC | LV-IDD | −1.12 | 0.014 |
| RCC | TPC/HDLC | 0.27 | <0.001 |
| RCB | TPC/HDLC | 0.31 | 0.0037 |
| RIC | TPC/HDLC | 0.20 | 0.012 |
L/RCC=left/right common carotid; L/RCB=left/right carotid bifurcation; L/RIC=left/right internal carotid; TPC/HDLC=total plasma cholesterol/high-density lipoprotein cholesterol; VAF=visceral abdominal fat; LV-IDD=left ventricular end diastolic dimension.
Discussion
This is the first published study examining the effects of long-term SSRI treatment on carotid artery atherosclerosis in a placebo-controlled, longitudinal, randomized pre-clinical trial. Sertraline appeared to exacerbate atherosclerosis in the carotid arteries of depressed relative to nondepressed monkeys. This finding is consistent with a previous report in this cohort demonstrating that coronary artery atherosclerosis extent (IA) was approximately five times greater in sertraline-treated depressed monkeys compared to all other groups34. Neither sertraline nor the sertraline × depression interaction was found to modify the relationship between behavioral and physiological risk factors and carotid artery atherosclerosis, suggesting that SSRI effects on carotid artery atherosclerosis are not mediated by effects on traditional cardiovascular risk factors.39
This study used a stratified randomization design to assign individuals to treatment groups while controlling for pretreatment atherosclerosis extent and depressive behavior, thus avoiding many confounders in clinical trials. This is an important strength, as sertraline and other SSRIs are prescribed for a variety of non-depressive disorders which could bias the outcomes of clinical trials.39 This study design also allowed for separation of treatment effects from pretreatment differences in the groups which was accomplished through balancing the treatment groups on pretreatment atherosclerosis extent34. Sertraline dosing was similar to patient dosing in terms of route of administration, plasma concentrations, and cerebrospinal fluid metabolite changes allowing these findings to be translated to patient populations35,37,46.
This trial used a well-established model of depression that shares many physiological, behavioral, and neurobiological characteristics of depression with people.28 Depressed monkeys are generally dyslipidemic, have decreased BWs and BMI, and develop greater coronary artery atherosclerosis than non-depressed monkeys34 Carotid artery atherosclerosis pathogenesis has been described in this model45,47 but the carotid artery atherosclerosis phenotype associated with depressive symptomology has not been previously studied. Use of this model provides for the unique opportunity to characterize comorbid depression and carotid artery atherosclerosis while simultaneously conducting a placebo-controlled long-term randomized trial in model well-suited for clinical translation.39
We found no main effects of depression or sertraline treatment on carotid artery atherosclerosis. Epidemiological studies have suggested that SSRIs may increase ischemic stroke risk in patient populations;22–25 however the mechanism is currently unknown. One possible mechanism is via exacerbated carotid artery atherosclerosis. To date there is only one report of a relationship between SSRIs and carotid artery atherosclerosis. Shah et al. reported at the 2011 American College of Cardiology Scientific Sessions that SSRI treatment increased carotid intimal-medial thickening in a study of twins discordant for SSRI use. The majority of studies have focused on SSRI effects on ischemic stroke, a sequela to carotid artery atherosclerosis. Two epidemiologic studies have focused on middle-aged to older women and found non-significant increases in ischemic stroke risk among SSRI-users.48,49 In one of these studies, it was found that while AD use as a whole was not associated with a significantly increased risk for all-cause stroke in patients that were depressed at baseline, AD use was significantly associated with stroke risk in patients depressed at their first follow-up visit.49 This discrepancy highlights the need to separate out drug effects from depression effects, especially to assess risk in patients taking SSRI for non-depressive disorders, such as hot flushes.
We found that SSRI treatment alone was not associated with carotid artery atherosclerosis extent, but that there was a significant sertraline × depression interaction effect. Present studies examining the relationship between SSRIs and ischemic stroke have all been observational. This adds a level of complexity in interpreting results because SSRI users may differ from non-users across a range of factors that can affect cardiovascular health, including depressive symptoms. As a result, investigators have made adjustments for severity of depression and/or exclusively selected patients with depression. Similar to our findings, when Trifiro et al.23 restricted analysis to depressed patients only he found a 99% increase in ischemic stroke risk among SSRI-users, but those taking SSRIs for any other indication were not at an increased risk.
The finding of no main effects of depression or sertraline treatment on carotid artery atherosclerosis despite having previously reported such effects on coronary artery atherosclerosis in these animals is also interesting. Additionally, within the carotid arteries we found that the sertraline × depression interaction effect was only significant in the RCC, and suggestive in the RCB. This indicates that effects of depression and treatment on atherosclerosis progression may be site-specific. While it is commonly accepted that coronary and carotid artery atherosclerosis are related, subtle differences in disease characteristics, including pharmacotherapy effects, have been observed.50 Within the carotid arteries there are reports of site-specific plaque progression rates51,52 and subsequent clinical events.53 Furthermore, the right and left carotid arteries have different anatomic origins which may affect local hemodynamics forces, a known contributor to carotid artery plaque localization and progression.27 Although left-to-right comparisons of hemodynamic measures have not been studied directly, side-specific relationships between hemodynamic factors and carotid artery atherosclerosis have been documented cardiovascular disease-free patients.54
The results of our trial suggest that sertraline may increase carotid artery atherosclerosis in depressed individuals in susceptible arterial beds. Sertraline did not significantly alter lipids34 and had beneficial effects on body composition and carbohydrate metabolism.37 Because none of the traditional cardiovascular risk factors examined were worsened by sertraline, we sought to identify unique predictors of carotid artery atherosclerosis at each arterial location and then examine whether these relationships were mediated by SSRI treatment. Pearson correlation coefficients were used to select variables for the multiple regression models from a rich premortem database. Although corrections for multiple comparisons were not made, we observed several novel relationships between behavioral and physiological risk factors and carotid artery atherosclerosis extent. In agreement with previous studies,45,47 circulating cholesterol was the strongest predictor of atherosclerosis in all but the LIC. Instead LIC atherosclerosis extent was most strongly associated with echocardiographic measures, LV-IDD and AO-VTI. Although absolute flow may have been underestimated in cases where the Doppler beam was not perfectly parallel to blood flow, these findings indicate a potential relationship between cardiac hemodynamics and downstream effects in the carotid arteries.39
Daytime sedentary behavior was associated with atherosclerosis extent at both bifurcations and circulating monocytes uniquely predicted IA in the LCB. Physical inactivity42 and elevated monocyte count43 have been identified as correlates of atherosclerosis extent in humans, but this is the first study to directly correlate these measures with carotid artery atherosclerosis in a nonhuman primate model. Monocytes were increased with depression and decreased with sertraline treatment. Depression has been linked to inflammation while SSRIs have anti-inflammatory properties including decreasing endothelial adhesion molecule expression55. Sertraline-treated, depressed animals had the greatest atherosclerosis extent but did not have the highest level of monocytes, likely do the opposing effects of SSRI treatment and depression. Interestingly neither depression nor sertraline modified the relationship between monocytes and atherosclerosis. Specific analysis of cytokines and chemokines would be useful to better understand these relationships and develop targeted therapy.
Multiple regression models confirmed that circulating cholesterol contributed significantly to predicting carotid artery atherosclerosis extent in all but the LIC, which was significantly predicted by LV-IDD. Covarying for sertraline and sertraline × depression did not change these relationships. Since most population-based studies looking at these relationships are conducted in modern Western societies, we fed our monkeys an atherogenic diet that would mimic the typical Western diet rich in animal protein, saturated fat, and cholesterol. There is a suggested link between diet, depression, and physiological risk factors – including inflammation which is a key component in depression and atherosclerosis56. We recognize that not all women consume such a diet. Additional research is needed to examine these relationships which may differ among those who eat a more traditional versus Western diet.
Despite having beneficial effects on several stroke risk factors, sertraline appears to exacerbate both coronary and carotid artery atherosclerosis, particularly in depressed subjects. This study suggests that sertraline and depression effects on atherosclerosis occur independently of effects on traditionally measured cardiovascular risk factors. From these results we cannot identify a mechanism of action for SSRI exacerbation of atherosclerosis, which is needed to better understand patient risk. However, atherosclerosis extent in several carotid arteries was associated with echocardiography measures and in the LIC atherosclerosis extent was best predicted by echocardiographic measures related to diastolic filling and aortic flow velocity. Hemodynamic factors are known to significantly influence atherosclerosis progression in the carotid arteries27 and, in humans, systolic hypertension is one of the best predictors of carotid artery atherosclerosis 57,58. This study is limited in that only anesthetized blood pressures were obtained as indirect, awake blood pressures are not feasible in nonhuman primates. Future studies aimed at determining SSRI and depression effects on serotoninergic modulation of arterial tone on atherosclerosis are indicated.
Conclusions
This study focused on atherosclerosis development during the late premenopausal years in which there is a high rate depression and SSRI use by women. The results of this nonhuman primate study suggest that long-term SSRI treatment may promote carotid artery atherosclerosis, which may increase ischemic stroke risk. This is especially concerning given the widespread use of SSRI drugs and evidence that the premenopausal years are crucial determinants for postmenopausal atherosclerosis burden, CHD, and stroke risk.3, 6,9,10 These results are compelling because they match the finding of additive sertraline and depression effects in the coronary arteries. However, unlike in the coronary arteries, main effects of SSRI treatment and depression were not observed, indicating site specific effects on atherosclerosis progression. Covarying for sertraline and sertraline × depression did not change the relationship between atherosclerosis extent and predictive risk factors indicating that sertraline and depression effects on atherosclerosis extent are not mediated via effects on established behavioral and physiological risk factors. The underlying mechanism remains to be elucidated, but does not appear to be related to SSRI effects on traditional behavioral and physiological risk factors.
Supplementary Material
Table SI: Behavioral and physiological phenotypes by depression status and treatment group - word document
Table S2: Associations of atherosclerotic plaque size (mm2) across carotid artery anatomical locations – word document
Table S3: Backward egression models demonstrating relations between carotid artery atherosclerosis and behavioral and physiological risk factors – word document
Table S4: Step-wise regression models demonstrating relations between carotid artery atherosclerosis, behavioral and physiological risk factors, and sertraline treatment – word document
Potential Clinical Value.
This study suggests that long-term SSRI treatment may promote carotid artery atherosclerosis, particularly in depressed women, thereby potentially increasing ischemic stroke risk. SSRI use is presently widespread, additionally an SSRI drug has been recently approved for the treatment of hot flushes, suggesting that a significant portion of women are exposed long-term treatment with this drug class. While replication is needed to understand risk levels in defined patient populations, these data suggest that long-term SSRI therapy should be prescribed judiciously and that further study is critical given the broad use of these medications.
Acknowledgments
We acknowledge the following people for their technical support: Beth Uberseder, JD Bottoms, Edison Floyd, and Maryanne Post. We would also like to acknowledge Dr. Thomas B. Clarkson, DVM (deceased) who was instrumental in the inception and analysis component of this study.
Footnotes
Portions of this work were presented and published in thesis form in fulfillment of the requirements for the PhD for MGSM from Wake Forest University
Disclosures: We have no conflicts of interest to disclose. This work was supported in part by NIH grants ROIHL87103, R21MH86731, T32OD10957, and the Pepper Older Americans for Independence Center (P30 AG21332).
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Judd SE, Kissela BM, Lackland DT, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Matchar DB, McGuire DK, Mohler ER, 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Willey JZ, Woo D, Yeh RW, Turner MB. Heart disease and stroke statistics--2015 update: a report from the American Heart Association. Circulation. 2015;131(4):e29–322. doi: 10.1161/cir.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 2.McGill HC, McMahan CA, Zieske AW, Sloop GD, Walcott JV, Troxclair DA, Malcom GT, Tracy RE, Oalmann MC, Strong JP. Associations of coronary heart disease risk factors with the intermediate lesion of atherosclerosis in youth. Arterioscler Thromb Vasc Biol. 2000;20(8):1998–2004. doi: 10.1161/01.atv.20.8.1998. [DOI] [PubMed] [Google Scholar]
- 3.Lorenz MW, von Kegler S, Steinmetz H, Markus HS, Sitzer M. Carotid intima-media thickening indicates a higher vascular risk across a wide age range prospective data from the Carotid Atherosclerosis Progression Study (CAPS) Stroke. 2006;37(1):87–92. doi: 10.1161/01.STR.0000196964.24024.ea. [DOI] [PubMed] [Google Scholar]
- 4.Meyer AA, Kundt G, Steiner M, Schuff-Werner P, Kienast W. Impaired flow-mediated vasodilation, carotid artery intima-media thickening, and elevated endothelial plasma markers in obese children: the impact of cardiovascular risk factors. Pediatrics. 2006;117(5):1560–1567. doi: 10.1542/peds.2005-2140. [DOI] [PubMed] [Google Scholar]
- 5.Sorof JM, Alexandrov AV, Cardwell G, Portman RJ. Carotid artery intimal-medial thickness and left ventricular hypertrophy in children with elevated blood pressure. Pediatrics. 2003;111(1):61–66. doi: 10.1542/peds.111.1.61. [DOI] [PubMed] [Google Scholar]
- 6.Sutton-Tyrrell K, Lassila HC, Meilahn E, Bunker C, Matthews KA, Kuller LH. Carotid atherosclerosis in premenopausal and postmenopausal women and its association with risk factors measured after menopause. Stroke. 1998;29(6):1116–1121. doi: 10.1161/01.str.29.6.1116. [DOI] [PubMed] [Google Scholar]
- 7.Matthews KA, Kuller LH, Sutton-Tyrrell K, Chang Y-F. Changes in cardiovascular risk factors during the perimenopause and postmenopause and carotid artery atherosclerosis in healthy women. Stroke. 2001;32(5):1104–1111. doi: 10.1161/01.str.32.5.1104. [DOI] [PubMed] [Google Scholar]
- 8.Bots ML, Hoes AW, Koudstaal PJ, Hofman A, Grobbee DE. Common Carotid Intima-Media Thickness and Risk of Stroke and Myocardial Infarction: The Rotterdam Study. Circulation. 1997;96(5):1432–1437. doi: 10.1161/01.cir.96.5.1432. [DOI] [PubMed] [Google Scholar]
- 9.Speroff L. Premenopausal atherosclerosis: Setting the stage for later clinical disease-Considering the amount of experimental evidence to show that adequate amounts of endogenous premenopausal estrogen protect a woman from coronary artery disease, there’s reason to believe that the hormone should still play an important role in protecting women after menopause. Contemp OBGYN. 2007:84. [Google Scholar]
- 10.Kaplan J, Manuck S, Anthony M, Clarkson T. Premenopausal social status and hormone exposure predict postmenopausal atherosclerosis in female monkeys. Obstet Gynecol. 2002;99(3):381–388. doi: 10.1016/s0029-7844(01)01659-3. [DOI] [PubMed] [Google Scholar]
- 11.Hemingway H, Marmot M. Evidence based cardiology-Psychosocial factors in the aetiology and prognosis of coronary heart disease: systematic review of prospective cohort studies. Bmj. 1999;318(7196):1460–1467. doi: 10.1136/bmj.318.7196.1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rugulies R. Depression as a predictor for coronary heart disease: a review and meta-analysis1 1The full text of this article is available via AJPM Online at www.ajpm-online.net. Am J Prev Med. 2002;23(1):51–61. doi: 10.1016/s0749-3797(02)00439-7. [DOI] [PubMed] [Google Scholar]
- 13.Simonsick EM, Wallace RB, Blazer DG, Berkman LF. Depressive symptomatology and hypertension-associated morbidity and mortality in older adults. Psychosom Med. 1995;57(5):427–435. doi: 10.1097/00006842-199509000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Wassertheil-Smoller S, Applegate WB, Berge K, Chang CJ, Davis BR, Grimm R, Kostis J, Pressel S, Schron E. Change in depression as a precursor of cardiovascular events. Arch Intern Med. 1996;156(5):553–561. [PubMed] [Google Scholar]
- 15.Salaycik KJ, Kelly-Hayes M, Beiser A, Nguyen AH, Brady SM, Kase CS, Wolf PA. Depressive symptoms and risk of stroke: the Framingham Study. Stroke. 2007;38(1):16–21. doi: 10.1161/01.STR.0000251695.39877.ca. [DOI] [PubMed] [Google Scholar]
- 16.Kessler RC, Bromet EJ. The epidemiology of depression across cultures. Annu Rev Public Health. 2013;34:119–138. doi: 10.1146/annurev-publhealth-031912-114409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mathers C, Fat DM, Boerma JT. The Global Burden of Disease: 2004 Update. World Health Organization; 2008. [Google Scholar]
- 18.Pratt L, Brody DJ. Depression in the US household population, 2009–2012. NCHS Data Brief. 2014;(172):1–8. [PubMed] [Google Scholar]
- 19.Kessler RC. Epidemiology of women and depression. J Affect Disord. 2003;74(1):5–13. doi: 10.1016/s0165-0327(02)00426-3. [DOI] [PubMed] [Google Scholar]
- 20.Pratt LAB, DJ . Depression in the U.S. Household Population, 2009–2012. NCHS Data Brief, No. 172. Hyattsville, MD: National Center for Health Statistics; 2014. [PubMed] [Google Scholar]
- 21.Orleans RJ, Li L, Kim M-J, Guo J, Sobhan M, Soule L, Joffe HV. FDA approval of paroxetine for menopausal hot flushes. N Engl J Med. 2014;370(19):1777–1779. doi: 10.1056/NEJMp1402080. [DOI] [PubMed] [Google Scholar]
- 22.Shin D, Oh YH, Eom CS, Park SM. Use of selective serotonin reuptake inhibitors and risk of stroke: a systematic review and meta-analysis. J Neurol. 2014;261(4):686–695. doi: 10.1007/s00415-014-7251-9. [DOI] [PubMed] [Google Scholar]
- 23.Trifiro G, Dieleman J, Sen EF, Gambassi G, Sturkenboom MC. Risk of ischemic stroke associated with antidepressant drug use in elderly persons. J Clin Psychopharmacol. 2010;30(3):252–258. doi: 10.1097/JCP.0b013e3181dca10a. [DOI] [PubMed] [Google Scholar]
- 24.Chen Y, Guo JJ, Li H, Wulsin L, Patel NC. Risk of cerebrovascular events associated with antidepressant use in patients with depression: A population-based, nested case–control study. Ann Pharmacother. 2008;42(2):177–184. doi: 10.1345/aph.1K369. [DOI] [PubMed] [Google Scholar]
- 25.Bak S, Tsiropoulos I, Kjærsgaard JO, Andersen M, Mellerup E, Hallas J, García Rodríguez LA, Christensen K, Gaist D. Selective Serotonin Reuptake Inhibitors and the Risk of Stroke: A Population-Based Case-Control Study. Stroke. 2002;33(6):1465–1473. doi: 10.1161/01.str.0000018589.56991.ba. [DOI] [PubMed] [Google Scholar]
- 26.Rieckmann N, Kronish IM, Shapiro PA, Whang W, Davidson KW. Serotonin reuptake inhibitor use, depression, and long-term outcomes after an acute coronary syndrome: a prospective cohort study. JAMA Intern Med. 2013;173(12):1150–1151. doi: 10.1001/jamainternmed.2013.910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hall HA, Bassiouny HS. Ultrasound and Carotid Bifurcation Atherosclerosis. Springer; 2012. Pathophysiology of carotid atherosclerosis; pp. 27–39. [Google Scholar]
- 28.Shively CA, Willard SL. Behavioral and neurobiological characteristics of social stress versus depression in nonhuman primates. Exp Neurol. 2012;233(1):87–94. doi: 10.1016/j.expneurol.2011.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xiangdong L, Yuanwu L, Hua Z, Liming R, Qiuyan L, Ning L. Animal models for the atherosclerosis research: a review. Protein Cell. 2011;2(3):189–201. doi: 10.1007/s13238-011-1016-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shively CA, Musselman DL, Willard SL. Stress, depression, and coronary artery disease: modeling comorbidity in female primates. Neurosci Biobehav Rev. 2009;33(2):133–144. doi: 10.1016/j.neubiorev.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shively CA, Register TC, Adams MR, Golden DL, Willard SL, Clarkson TB. Depressive behavior and coronary artery atherogenesis in adult female cynomolgus monkeys. Psychosom Med. 2008;70(6):637–645. doi: 10.1097/PSY.0b013e31817eaf0b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shively CA, Register TC, Friedman DP, Morgan TM, Thompson J, Lanier T. Social stress-associated depression in adult female cynomolgus monkeys (Macaca fascicularis) Biol Psychol. 2005;69(1):67–84. doi: 10.1016/j.biopsycho.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 33.Shively CA, Laber-Laird K, Anton RF. Behavior and physiology of social stress and depression in female cynomolgus monkeys. Biol Psychiatry. 1997;41(8):871–882. doi: 10.1016/S0006-3223(96)00185-0. [DOI] [PubMed] [Google Scholar]
- 34.Shively CA, Register TC, Appt SE, Clarkson TB. Effects of long-term sertraline treatment and depression on coronary artery atherosclerosis in premenopausal female primates. Psychosom Med. 2015;77(3):267–278. doi: 10.1097/PSY.0000000000000163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shively CA, Register TC, Higley JD, Willard SL. Sertraline effects on cerebrospinal fluid monoamines and species-typical socioemotional behavior of female cynomolgus monkeys. Psychopharmacology (Berl) 2014;231(7):1409–1416. doi: 10.1007/s00213-013-3329-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Groban L, Kitzman DW, Register TC, Shively CA. Effect of Depression and Sertraline Treatment on Cardiac Function in Female Nonhuman Primates. Psychosom Med. 2014;76(2):137–146. doi: 10.1097/Psy.0000000000000036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Silverstein-Metzler MG, Shively CA, Clarkson TB, Appt SE, Carr JJ, Kritchevsky SB, Jones SR, Register TC. Sertraline inhibits increases in body fat and carbohydrate dysregulation in adult female cynomolgus monkeys. Psychoneuroendocrinology. 2016;68:29–38. doi: 10.1016/j.psyneuen.2016.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Higley J, Hasert M, Suomi S, Linnoila M. The serotonin reuptake inhibitor sertraline reduces excessive alcohol consumption in nonhuman primates: effect of stress. Neuropsychopharmacology. 1998;18(6):431–443. doi: 10.1016/s0893-133x(97)00180-2. [DOI] [PubMed] [Google Scholar]
- 39.Silverstein-Metzler MG. [Accessed February 10, 2017];Effects of Chronic Selective Serotonin Reuptake Inhibitor Use on Carotid Artery Atherosclerosis. 2016 https://wakespace.lib.wfu.edu/handle/10339/59263.
- 40.Shively CA, Kaplan JR, Adams MR. Effects of ovariectomy, social instability and social status on female Macaca fascicularis social behavior. Physiol Behav. 1986;36(6):1147–1153. doi: 10.1016/0031-9384(86)90492-0. [DOI] [PubMed] [Google Scholar]
- 41.Glagov S, Bassiouny H, Masawa N, Sakaguchi Y, Giddens D, Zarins C. Syndr Atheroscler Correl Clin Imaging Pathol Armonk NY Futura. 1996. Cerebrovascular disease: a pathologist’s view; pp. 161–179. [Google Scholar]
- 42.Folsom AR, Eckfeldt JH, Weitzman S, Ma J, Chambless LE, Barnes RW, Cram KB, Hutchinson RG. Relation of carotid artery wall thickness to diabetes mellitus, fasting glucose and insulin, body size, and physical activity. Atherosclerosis Risk in Communities (ARIC) Study Investigators. Stroke. 1994;25(1):66–73. doi: 10.1161/01.str.25.1.66. [DOI] [PubMed] [Google Scholar]
- 43.Chapman CM, Beilby JP, McQuillan BM, Thompson PL, Hung J. Monocyte count, but not C-reactive protein or interleukin-6, is an independent risk marker for subclinical carotid atherosclerosis. Stroke. 2004;35(7):1619–1624. doi: 10.1161/01.STR.0000130857.19423.ad. [DOI] [PubMed] [Google Scholar]
- 44.Clarkson TB, Anthony MS, Mikkola TS, St Clair RW. Comparison of Tibolone and Conjugated Equine Estrogens Effects on Carotid Artery Atherosclerosis of Postmenopausal Monkeys. Stroke. 2002;33(11):2700–2703. doi: 10.1161/01.str.0000033130.82164.24. [DOI] [PubMed] [Google Scholar]
- 45.Kaplan JR, Clarkson TB, Manuck SB. Pathogenesis of carotid bifurcation atherosclerosis in cynomolgus monkeys. Stroke. 1984;15(6):994–1000. doi: 10.1161/01.str.15.6.994. [DOI] [PubMed] [Google Scholar]
- 46.Sheline Y, Bardgett ME, Csernansky JG. Correlated reductions in cerebrospinal fluid 5-HIAA and MHPG concentrations after treatment with selective serotonin reuptake inhibitors. J Clin Psychopharmacol. 1997;17(1):11–14. doi: 10.1097/00004714-199702000-00003. [DOI] [PubMed] [Google Scholar]
- 47.Shively CA, Kaplan JR, Clarkson TB. Carotid artery atherosclerosis in cholesterol-fed female cynomolgus monkeys. Effects of oral contraceptive treatment, social factors, and regional adiposity. Arterioscler Thromb Vasc Biol. 1990;10(3):358–366. doi: 10.1161/01.atv.10.3.358. [DOI] [PubMed] [Google Scholar]
- 48.Pan A, Okereke OI, Sun Q, Logroscino G, Manson JE, Willett WC, Ascherio A, Hu FB, Rexrode KM. Depression and incident stroke in women. Stroke. 2011;42(10):2770–2775. doi: 10.1161/STROKEAHA.111.617043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smoller JW, Allison M, Cochrane BB, Curb JD, Perlis RH, Robinson JG, Rosal MC, Wenger NK, Wassertheil-Smoller S. Antidepressant use and risk of incident cardiovascular morbidity and mortality among postmenopausal women in the Women’s Health Initiative study. Arch Intern Med. 2009;169(22):2128–2139. doi: 10.1001/archinternmed.2009.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jashari F, Ibrahimi P, Nicoll R, Bajraktari G, Wester P, Henein MY. Coronary and carotid atherosclerosis: Similarities and differences. Atherosclerosis. 227(2):193–200. doi: 10.1016/j.atherosclerosis.2012.11.008.. [DOI] [PubMed] [Google Scholar]
- 51.Mackinnon AD, Jerrard-Dunne P, Sitzer M, Buehler A, von Kegler S, Markus HS. Rates and determinants of site-specific progression of carotid artery intima-media thickness: the carotid atherosclerosis progression study. Stroke. 2004;35(9):2150–2154. doi: 10.1161/01.STR.0000136720.21095.f3. [DOI] [PubMed] [Google Scholar]
- 52.Selwaness M, van den Bouwhuijsen Q, van Onkelen RS, Hofman A, Franco OH, van der Lugt A, Wentzel JJ, Vernooij M. Atherosclerotic plaque in the left carotid artery is more vulnerable than in the right. Stroke. 2014;45(11):3226–3230. doi: 10.1161/strokeaha.114.005202. [DOI] [PubMed] [Google Scholar]
- 53.Romero JR, Beiser A, Seshadri S, Benjamin EJ, Polak JF, Vasan RS, Au R, DeCarli C, Wolf PA. Carotid artery atherosclerosis, MRI indices of brain ischemia, aging, and cognitive impairment: the Framingham study. Stroke. 2009;40(5):1590–1596. doi: 10.1161/strokeaha.108.535245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Luo X, Yang Y, Cao T, Li Z. Differences in left and right carotid intima-media thickness and the associated risk factors. Clin Radiol. 2011;66(5):393–398. doi: 10.1016/j.crad.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 55.Lekakis J, Ikonomidis I, Papoutsi Z, Moutsatsou P, Nikolaou M, Parissis J, Kremastinos DT. Selective serotonin re-uptake inhibitors decrease the cytokine-induced endothelial adhesion molecule expression, the endothelial adhesiveness to monocytes and the circulating levels of vascular adhesion molecules. Int J Cardiol. 2010;139(2):150–158. doi: 10.1016/j.ijcard.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 56.Shelton RC, Miller AH. Eating ourselves to death (and despair): The contribution of adiposity and inflammation to depression. Prog Neurobiol. 2010;91(4):275–299. doi: 10.1016/j.pneurobio.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Solberg LA, Strong JP. Risk factors and atherosclerotic lesions. A review of autopsy studies. Arterioscler Thromb Vasc Biol. 1983;3(3):187–198. doi: 10.1161/01.atv.3.3.187. [DOI] [PubMed] [Google Scholar]
- 58.Crouse JR, Toole JF, McKinney WM, Dignan MB, Howard G, Kahl FR, McMahan MR, Harpold GH. Risk factors for extracranial carotid artery atherosclerosis. Stroke. 1987;18(6):990–996. doi: 10.1161/01.str.18.6.990. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table SI: Behavioral and physiological phenotypes by depression status and treatment group - word document
Table S2: Associations of atherosclerotic plaque size (mm2) across carotid artery anatomical locations – word document
Table S3: Backward egression models demonstrating relations between carotid artery atherosclerosis and behavioral and physiological risk factors – word document
Table S4: Step-wise regression models demonstrating relations between carotid artery atherosclerosis, behavioral and physiological risk factors, and sertraline treatment – word document