Abstract
Nonadherence to immunosuppressant medications is a leading cause of poor long-term outcomes in transplant recipients. The Medication Level Variability Index (MLVI) provides a vehicle for transplant outcome risk-stratification through continuous assessment of adherence. The MALT (Medication Adherence in children who had a Liver Transplant) prospective multi-site study evaluated whether MLVI predicts Late Acute Rejection (LAR). 400 pediatric (1–17 year old) liver transplant recipients were enrolled and followed for 2 years. The a-priori hypothesis was that a higher MLVI predicts LAR. Pre-defined secondary analyses evaluated other outcomes such as liver enzyme levels, and sensitivity analyses compared adolescent to pre-adolescents. In the primary analysis sample of 379 participants, a higher pre-rejection MLVI predicted LAR [mean pre-rejection MLVI with LAR: 2.4 (3.6 SD) vs. without LAR, 1.6 (1.1); p=0.026]. 53% of the adolescents with MLVI>2 in year 1 had LAR by the end of year 2, as compared with 6% of those with year 1 MLVI≤2. A higher MLVI was significantly associated with all secondary outcomes. MLVI, a marker of medication adherence that uses clinically-derived information, predicts LAR in pediatric liver transplant recipients.
Introduction
Liver transplantation is a life-saving, costly procedure that requires the use of a limited resource (the transplanted organ). To preserve this scarce resource, it is important to identify predictors of poor outcomes in recipients and, if possible, focus efforts to mitigate the increased risk. In pediatric liver transplant recipients, nonadherence to medications contributes to Late Acute Rejection (LAR) in up to 90% of cases1–11, and is associated with increased mortality5, making it perhaps the most important factor associated with poor outcomes in long-term survivors1. Indeed, research and clinical guidelines recommend a focus on adherence as a modifiable risk in liver transplant recipients.12–14 Therefore, it would seem logical to evaluate adherence as a risk-stratification tool. However, to our knowledge, continuous assessment of adherence is rarely, if ever, incorporated into practice.
There is no “gold standard” adherence measurement technique.15–17 Subjective methods such as patient reports are unreliable2,16 and are considered to be inferior to objective methods.18 On the other hand, objective methods, such as pill counts or electronic monitoring, frequently impose additional burden on the patient, and may require significant logistic support.19,20 Increased patient burden matters because nonadherent patients are not likely to cooperate with a procedure that increases their burden. A patient who finds it hard to take the medications as prescribed, will probably also find it hard to bring the pill bottle to clinic, or use an electronic monitor.19,20 Resource-allocation barriers as well as difficulties in engaging the very segment of the population that should be monitored,20 limits the use a robust adherence monitoring plan.18, 21, 22 There is particular interest in identifying simple objective methods allowing efficient targeting of at-risk patients via algorithms built into standard care.
We previously described an innovative method to detect nonadherence to medications using existing clinical data without additional patient burden: computing the standard deviation (SD) of consecutive blood levels of a medication over time. The resulting variable, the Medication Level Variability Index (MLVI,) reflects the degree of fluctuation between the levels. A higher MLVI means less consistent medication adherence4. Variability seems to be a more robust indicator of persistent (and clinically relevant) nonadherence as compared with one “out-of-range” level.23–25
MLVI has been described in retrospective single-center pediatric and adult transplant analyses1–11, 26–29, and has been used in pilot intervention studies27,28. The measure’s value in predicting LAR and other poor outcomes in a representative sample, an important attribute if quality of care is to be improved,30 has not been adequately investigated.
We report the primary results from MALT (Medication Adherence in children who had a Liver Transplant; ClinicalTrials.Gov registration # NCT01154075), a prospective pediatric multi-site study in 5 centers across the United States. Participants were followed for two years. The pre-defined primary hypothesis was that participants with a high MLVI would have a higher likelihood of subsequent LAR, assessed in a central masked review of liver biopsies. Sensitivity analyses sought to determine potential cutoffs, using Receiver-Operator Characteristic (ROC) curve analyses in adolescents as compared with all children, and to evaluate the association between MLVI values and biochemical markers of liver injury. In practice, the MLVI would be continuously re-calculated every time a blood level is obtained (quarterly). To evaluate whether the MLVI would provide advance warning before LAR is experienced, we examined how much time took from the time the MLVI threshold was met at the end of year 1 until patients experienced a rejection in year 2 of the study.
Methods
Participants and follow-up
Children and adolescents were prospectively recruited from 5 pediatric liver transplant centers in the United States (Cincinnati Children’s Hospital Medical Center, Cincinnati OH; Mattell Children’s Hospital, UCLA, Los Angeles, CA; Lurie Children’s Hospital, Chicago, IL; Children’s Hospital of Pittsburgh of UPMC, Pittsburgh, PA; and Mount Sinai Medical Center, New York, NY). Each participant was followed for 2 years. Adherence studies frequently suffer from selection bias21. To mitigate bias, the investigative team compared the rate of rejection in MALT with the rate of rejection reported in a national database of transplant centers: Studies in Pediatric Liver Transplantation, SPLIT,31 during and after enrollment. SPLIT collects data on children undergoing liver transplant in 44 transplant centers in the US and Canada. We aimed to identify a similar rejection rate in MALT as compared to SPLIT. In addition, since outcome clinical data were gathered during usual care, patient burden was minimized, reducing the likelihood of attrition and selection bias. To evaluate selection bias in the resulting sample, in addition to the SPLIT comparison, key characteristics of subjects who were not approached or approached and declined to consent were compared to those who consented.
Inclusion/exclusion criteria
The study was approved by the respective IRB’s of all participating institutions and involved guardian’s consent and child assent when relevant. Inclusion criteria were:
Age 1–17 years old at enrollment,
Receipt of a liver transplant at least one year prior to enrollment (we excluded recipients in the first year since transplant since variability in levels in this period is more likely to be affected by graft dysfunction and by variable prescription practices, not by patient adherence),
Tacrolimus is prescribed.
Participants had to be seen at the enrolling center at least once in the two years prior to enrollment, to ensure completeness of data.
Exclusion criteria were:
More than one transplant (including bone marrow replacement),
Biopsy-proven rejection within the past six months from enrollment (to ensure that pre-existing rejection is not the immediate reason for fluctuation in medication levels),
Hepatitis C (as hepatitis C infection in transplant recipients might affect tacrolimus prescription practices),
Instructed by a physician not to obtain tacrolimus levels for at least one year,
Participants who were seen only for consultation (with most or all of the child’s routine care is provided at another center), to ensure that follow-up is occurring at the center of record,
Medically unstable/hospitalized at the time of enrollment (because of concerns about inability to provide informed consent/assent),
Participant or guardian who were actively psychotic or severely disoriented due to any cause, including hepatic encephalopathy (temporary exclusion) or had been diagnosed with moderate or severe mental retardation as defined in the Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV).
The Medication level Variability Index (MLVI)
MLVI was calculated as the Standard Deviation of a set of at least 3 tacrolimus trough blood levels for each participant, as described elsewhere.29 Tacrolimus trough levels are routinely obtained, at least quarterly, as standard care in all participating centers. Centers prospectively reported conditions that might lead to changed drug absorption or metabolism. At the outset of the study, we compiled a list of medications that might interact with tacrolimus to change its level (supplement 1), and centers reported whenever patients were prescribed those medications.
Primary outcome measure
The primary outcome measure was LAR, as determined by two independent readings in a central pathology lab at Children’s Hospital of Pittsburgh, using Banff consensus criteria.32 All for cause biopsies performed during the study period on study participants were reviewed by the central pathologists, who were masked to the clinical course of the participant. If the central pathologists disagreed, the case was adjudicated by the senior study pathologist. For each participant, if there was at least one biopsy-proven episode of rejection during the study period, it was entered as a “positive rejection” in the primary analysis.
Secondary outcome measures
The predefined secondary outcomes included the following:
Biopsy-read rejection as determined at the site.
Increase in immunosuppressant prescription dose in response to suspected rejection in absence of a biopsy.
Serum levels of Alanine Aminotransferase (ALT) and Gamma-Glutamyl Transferase (GGT), operationalized as continuous variables (mean per participant) and as the highest value during the study period.
Organ failure or death.
Analysis
Sample size calculations, using extensive pre-trial data, confirmed that a rejection rate of 10% in a sample size of 300 participants would be required to detect a difference in MLVI scores as small as 0.81 with 80% power and type I error rate of 5% using a two-sided t-test. We assumed a 25% attrition rate and therefore aimed at a sample size of 400. Our statistical analysis plan, which was locked before the analyses, is provided as a supplement. For the primary analysis, only tacrolimus levels obtained before the index rejection were entered, to ensure that the study examines the MLVI as predictor of rejection (rather than a general association); non-parametric Wilcoxon rank Sum test was used to compare the distribution of MLVI between centrally-read rejection vs no rejection groups. To evaluate the predefined MLVI cut-off of 2.5 as a predictor of rejection, Fisher’s exact test was used. The primary “prediction” analysis, by design, used only pre-rejection levels to calculate MLVI, but in exploratory analyses we also used all available levels to determine whether a higher MLVI is associated with rejection if all levels are used. To look at the effect of time, we describe MLVI when calculated on a “rolling” basis (recalculated for each participant every quarter), and also present the time that elapsed before the first year’s MLVI reached a threshold and subsequent (second year) rejections. In sensitivity analyses, MLVI as predictor of rejection was evaluated using the area under the curve (AUC). Multivariate logistic regression analysis was performed to evaluate the effect of additional covariates on the relationship between MLVI (dichotomous score using 2.5 as the cut-off) and central pathologist confirmed rejection. Evaluated baseline factors included primary diagnosis, age at baseline (enrollment in MALT), race, time from transplant to MALT enrollment, primary insurance (state/federal funded vs traditional private insurance), and center. The backward elimination method was used to develop the final multivariate model. To evaluate the possibility that the presence of “undetectable” blood levels could substitute for the MLVI as predictor, we repeated the primary analysis while substituting the MLVI with at least one “undetectable” level pre-rejection as the predictor of LAR.
To evaluate the possibility of selection bias, we compared the rate of rejection in MALT to that rate in SPLIT patients. 3,152 patients were enrolled into SPLIT until June 2009. In the first step we matched patients for age at transplant, gender, and primary liver disease. Next, we matched those patients on days since transplant at MALT enrollment. This resulted in 951 unique SPLIT participants whose data served as the comparison group.
Undetectable levels
“Undetectable levels” were assigned the local laboratory’s lower limit of detection. When those limits were not available, we treated the data as “missing”. Missing data were not imputed, based on a detailed analysis of a different cohort.29
Results
Characteristics of the cohort
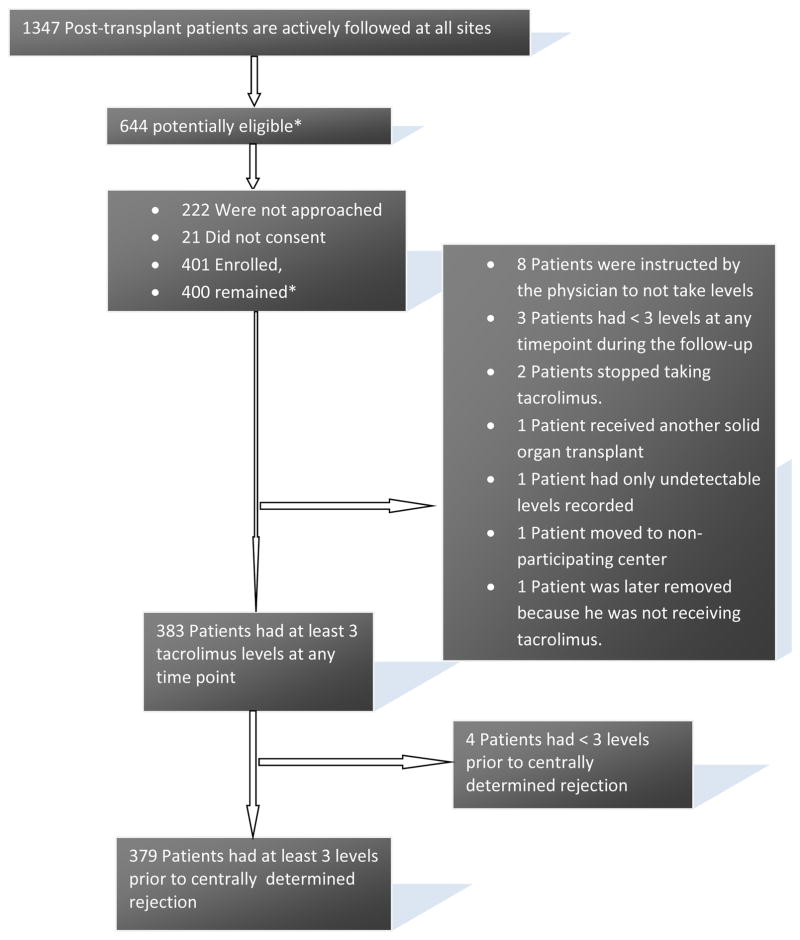
Chart review identified 644 potentially eligible patients at the five centers. Few patients refused to participate (CONSORT Figure 1), and therefore it was unnecessary to approach all of the potentially eligible patients to reach the pre-defined target (400 enrolled participants, 80 per site). Attrition was lower than anticipated; 379 evaluable participants were available for the primary analysis. Select participant characteristics are presented in Table 1. A comparison between key characteristics of patients who were approached, consented or not, and those who were not approached is presented in table s1. The groups were similar regarding patients’ race, primary diagnosis, and gender. Patients who consented were transplanted at an average age of 2.9 years, whereas those who were approached but did not consent were transplanted at an average age of 3.2 years. At the conclusion of the study, the number of biopsy-read rejections per participant months of follow-up in MALT (0.005 rejection episodes/participant month of follow up) was identical to the number obtained from the SPLIT database for matched recipients (0.005). Center-level distribution of MLVI scores is presented in figure s1. The primary analysis evaluated a total of 4479 tacrolimus levels obtained for 379 participants during the course of the study. There were only 2 “undetectable” levels for which the laboratory did not report the lower limit of detection.
FIGURE 1. Consort Diagram.
* As of October 2013. One patient turned out to be ineligible because he had a previously undocumented transplant; this patient was not included in analyses.
Table 1.
sample characteristics
| Characteristics | Central Pathologist Assigned Rejection | Total (N=400) | |
|---|---|---|---|
|
| |||
| Yes (N=43) | No (N=357) | ||
| Total participants | 43 (100.0%) | 357 (100.0%) | 400 (100.0%) |
| Primary diagnosis | |||
| Biliary Atresia | 18 (41.9%) | 175 (49.0%) | 193 (48.3%) |
| Other Cholestatic | 5 (11.6%) | 30 (8.4%) | 35 (8.8%) |
| Acute Liver Failure | 7 (16.3%) | 34 (9.5%) | 41 (10.3%) |
| Metabolic | 7 (16.3%) | 53 (14.8%) | 60 (15.0%) |
| Tumor | 3 (7.0%) | 31 (8.7%) | 34 (8.5%) |
| Autoimmune Hepatitis | 1 (2.3%) | 2 (0.6%) | 3 (0.8%) |
| Other | 2 (4.7%) | 32 (9.0%) | 34 (8.5%) |
| Age at transplant | |||
| < 1 year | 13 (30.2%) | 156 (43.7%) | 169 (42.3%) |
| 1–4 years | 15 (34.9%) | 130 (36.4%) | 145 (36.3%) |
| 5–12 years | 11 (25.6%) | 67 (18.8%) | 78 (19.5%) |
| 13–17 years | 4 (9.3%) | 4 (1.1%) | 8 (2.0%) |
| Age at baseline (years) | |||
| N | 43 | 357 | 400 |
| Mean (SD) | 10.7 (5.0) | 9.5 (4.4) | 9.6 (4.5) |
| Median (Min, Max) | 12.3 (1.9, 17.8) | 9.5 (1.4, 18.0) | 9.7 (1.4, 18.0) |
| Gender | |||
| Male | 12 (27.9%) | 177 (49.6%) | 189 (47.3%) |
| Female | 31 (72.1%) | 180 (50.4%) | 211 (52.8%) |
| Race | |||
| American Indian or Alaska Native | 0 | 1 (0.3%) | 1 (0.3%) |
| Asian | 4 (9.3%) | 21 (5.9%) | 25 (6.3%) |
| Black or African American | 11 (25.6%) | 45 (12.6%) | 56 (14.0%) |
| Native Hawaiian or Pacific Islander | 1 (2.3%) | 1 (0.3%) | 2 (0.5%) |
| White or Caucasian | 22 (51.2%) | 246 (68.9%) | 268 (67.0%) |
| Other | 1 (2.3%) | 24 (6.7%) | 25 (6.3%) |
| More than one race | 1 (2.3%) | 2 (0.6%) | 3 (0.8%) |
| Not reported | 3 (7.0%) | 17 (4.8%) | 20 (5.0%) |
| Donor organ type | |||
| Deceased whole liver | 22 (51.2%) | 159 (44.5%) | 181 (45.3%) |
| Deceased technical variant (reduced and split) | 11 (25.6%) | 117 (32.8%) | 128 (32.0%) |
| Living | 10 (23.3%) | 81 (22.7%) | 91 (22.8%) |
| Surgical procedure | |||
| Orthotopic | 43 (100.0%) | 355 (99.4%) | 398 (99.5%) |
| Heterotopic | 0 | 2 (0.6%) | 2 (0.5%) |
| Primary insurance | |||
| Medicaid or equivalent and/or state funded children’s services | 21 (48.8%) | 135 (37.8%) | 156 (39.0%) |
| HMO/managed care | 12 (27.9%) | 89 (24.9%) | 101 (25.3%) |
| Traditional private insurance | 8 (18.6%) | 86 (24.1%) | 94 (23.5%) |
| Champus (military) | 0 | 4 (1.1%) | 4 (1.0%) |
| None: self pay | 0 | 28 (7.8%) | 28 (7.0%) |
| None: no funding | 1 (2.3%) | 3 (0.8%) | 4 (1.0%) |
| Other | 1 (2.3%) | 12 (3.4%) | 13 (3.3%) |
| Primary caregiver | |||
| Mother | 40 (93.0%) | 311 (87.1%) | 351 (87.8%) |
| Father | 3 (7.0%) | 37 (10.4%) | 40 (10.0%) |
| Guardian | 0 | 5 (1.4%) | 5 (1.3%) |
| Other | 0 | 4 (1.1%) | 4 (1.0%) |
| Primary caregiver’s marital status | |||
| Missing | 0 | 3 (0.8%) | 3 (0.8%) |
| Single-parent household | 10 (23.3%) | 70 (19.6%) | 80 (20.0%) |
| Two-parent household | 33 (76.7%) | 284 (79.6%) | 317 (79.3%) |
| Primary caregiver’s highest level of education | |||
| Missing | 0 | 22 (6.2%) | 22 (5.5%) |
| Some high school or less | 5 (11.6%) | 38 (10.6%) | 43 (10.8%) |
| High school degree/GED | 16 (37.2%) | 81 (22.7%) | 97 (24.3%) |
| Vocational school or some college | 7 (16.3%) | 70 (19.6%) | 77 (19.3%) |
| College degree | 12 (27.9%) | 103 (28.9%) | 115 (28.8%) |
| Professional or graduate degree | 3 (7.0%) | 43 (12.0%) | 46 (11.5%) |
| Time since transplant at enrollment into MALT (years) | |||
| N | 43 | 357 | 400 |
| Mean (SD) | 5.7 (4.7) | 6.9 (4.3) | 6.8 (4.4) |
| Median (Min, Max) | 3.4 (1.0, 16.7) | 6.0 (1.0, 17.4) | 5.9 (1.0, 17.4) |
Effect of concomitant medications, intercurrent illness, absorption or altered metabolism on MLVI
Centers prospectively reported clinically significant conditions that may have impacted drug absorption or metabolism in 8 participants (table s2). The mean MLVI for those participants was nearly identical to the MALT cohort mean (1.78 vs 1.70). One participant who had a high MLVI value (3.2) was confirmed as nonadherent based on a detailed pharmacy record review. Concomitant medications did not affect the MLVI. A detailed review of the clinical history of all cases with very high (2 SD above cohort mean) MLVI’s, conducted per protocol, did not identify clinical issues, including medication absorption or metabolism, that would explain the high MLVIs.
Primary and secondary analyses
In Table 2, the MLVI is used as a continuous variable or as a pre-defined dichotomy. Mean pre-rejection MLVI score among participants with LAR was 2.4 (SD 3.6), compared with 1.6 (SD 1.1) in participants without LAR (p=0.026). When used either as a continuum or as the pre-defined threshold (MLVI>2.5), the MLVI was a significant predictor of all of the predefined (primary and secondary) rejection outcomes: central pathologist-read rejection, site-read rejection, and modification in immunosuppression because of suspicion of rejection. Table 3 shows a significant association between a high MLVI and a higher mean level of ALT or GGT or higher maximal level of ALT or GGT during the study period. There were no deaths or organ failure (re-transplantation) during the study period. Table s3 shows that the presence of undetectable levels in and of itself was not predictive of rejection. Table 4 presents significant findings from the multivariate analyses; age at baseline and center were significant covariates in the final model. MLVI remained a significant predictor (OR 2.54 (1.17, 5.54), P=0.02). Table s4 presents patient characteristics by MLVI status.
TABLE 2.
Primary (centrally read rejection), and secondary (site biopsy results, and modified immunosuppressive therapy because of suspicion of rejection) outcomes.
MLVI = Medication Level Variability Index *”n”‘s differ slightly because MLVI’s have been computed as the standard deviation of at least 3 blood levels obtained prior to rejection; depending on the method rejection was determined, a few cases might not have reached the “3 levels” threshold before the rejection and were therefore not included in the analysis.
| Central Pathologist Assigned Rejection | Total (N=379) | P Value | ||
|---|---|---|---|---|
|
| ||||
| Yes (N=39) | No (N=340) | |||
| PRIMARY: MLVI as continuous variable | ||||
| Mean (SD) | 2.4 (3.6) | 1.6 (1.1) | 1.7 (1.6) | 0.026 |
| Median (Min, Max) | 1.8 (0.0, 23.1) | 1.3 (0.1, 10.0) | 1.3 (0.0, 23.1) | |
| MLVI > 2.5 | 12 (30.8%) | 58 (17.1%) | 70 (18.5%) | 0.048 |
| Number of Tacrolimus Levels per Participant | ||||
| Mean (SD) | 10.4 (6.6) | 12.0 (6.6) | 11.8 (6.6) | 0.072 |
| Median (Min, Max) | 9.0 (3.0, 27.0) | 10.0 (3.0, 53.0) | 10.0 (3.0, 53.0) | |
| Duration (Days from enrollment) to Rejection | ||||
| Mean (SD) | 379.9 (203.4) | |||
|
| ||||
| Median (Min, Max) | 382.0 (64.0, 709.0) | |||
| Participants With Intensified Immunosuppressive Therapy Whether Or Not Rejection Was Diagnosed Via Biopsy | Total (N=382) | P Value | ||
|---|---|---|---|---|
|
| ||||
| Yes (N=42) | No (N=340) | |||
| MLVI | ||||
| Mean (SD) | 2.5 (3.5) | 1.6 (1.1) | 1.7 (1.6) | 0.0040 |
| Median (Min, Max) | 2.1 (0.0, 23.1) | 1.3 (0.1, 10.0) | 1.3 (0.0, 23.1) | |
| MLVI > 2.5 | 16 (38.1%) | 54 (15.9%) | 70 (18.3%) | 0.0012 |
| Number of Tacrolimus Levels per Participant | ||||
|
| ||||
| Mean (SD) | 10.3 (5.9) | 11.8 (6.4) | 11.7 (6.4) | 0.108 |
|
| ||||
| Median (Min, Max) | 9.0 (3.0, 24.0) | 10.0 (3.0, 53.0) | 10.0 (3.0, 53.0) | |
|
| ||||
| Duration (Days from enrollment) to Intensified Immunosuppressive Therapy* | ||||
|
| ||||
| Mean (SD) | 369.3 (205.1) | |||
|
| ||||
| Median (Min, Max) | 351.0 (64.0, 709.0) | |||
| Local Biopsy Diagnosed Rejection | Total (N=380) | P Value | ||
|---|---|---|---|---|
|
| ||||
| Yes (N=34) | No (N=346) | |||
| MLVI | ||||
| Mean (SD) | 2.6 (3.8) | 1.6 (1.1) | 1.7 (1.6) | 0.0068 |
| Median (Min, Max) | 2.0 (0.0, 23.1) | 1.3 (0.1, 10.0) | 1.3 (0.0, 23.1) | |
| MLVI > 2.5 | 11 (32.4%) | 58 (16.8%) | 69 (18.2%) | 0.0343 |
| Number of Tacrolimus Levels per Participant | ||||
|
| ||||
| Mean (SD) | 11.2 (6.5) | 12.0 (6.5) | 11.9 (6.5) | 0.443 |
|
| ||||
| Median (Min, Max) | 9.0 (3.0, 27.0) | 10.0 (3.0, 53.0) | 10.0 (3.0, 53.0) | |
|
| ||||
| Duration (Days from enrollment) to Rejection | ||||
|
| ||||
| Mean (SD) | 383.9 (196.8) | |||
|
| ||||
| Median (Min, Max) | 388.5 (76.0, 706.0) | |||
One participant in the “Yes” group has immuno-suppressive modification date missing.
TABLE 3.
Relationship between MLVI> 2.5 and Predefined Secondary Liver Outcomes
MLVI = Medication Level Variability Index
| Characteristicss | MLVI > 2.5 | Total | P Value 1 | |
|---|---|---|---|---|
|
| ||||
| Yes | No | |||
| Maximum ALT (U/L) | ||||
| N | 70 | 309 | 379 | <.0001 |
| Mean (SD) | 147.4 (155.9) | 83.0 (112.1) | 94.9 (123.7) | |
| Median (Min, Max) | 81.0 (13.0, 701.0) | 41.0 (13.0, 774.0) | 46.0 (13.0, 774.0) | |
| Mean ALT (U/L) | ||||
| N | 70 | 309 | 379 | <.0001 |
| Mean (SD) | 53.8 (39.0) | 34.8 (24.6) | 38.4 (28.7) | |
| Median (Min, Max) | 41.5 (11.2, 180.3) | 26.1 (10.8, 177.0) | 27.9 (10.8, 180.3) | |
| Maximum GGT (U/L) | ||||
| N | 70 | 309 | 379 | <.0001 |
| Mean (SD) | 222.5 (370.3) | 76.5 (124.4) | 103.5 (202.2) | |
| Median (Min, Max) | 75.0 (12.0, 2394) | 29.0 (8.0, 871.0) | 33.0 (8.0, 2394) | |
| Mean ALT (U/L) | ||||
| N | 70 | 309 | 379 | <.0001 |
| Mean (SD) | 105.2 (158.1) | 39.1 (55.4) | 51.3 (87.9) | |
| Median (Min, Max) | 42.3 (9.1, 659.4) | 18.6 (6.5, 359.8) | 20.7 (6.5, 659.4) | |
Non-parametric Kruskal-Wallis test was performed.
TABLE 4.
Final Multivariate Logistic Regression Model Assessing Effect of MLVI > 2.5 on Central Pathologist Confirmed Rejection1.
| Factor | Reference Group | Comparison Group | Odds Ratio | 95% CI | Pairwise P Value | Overall P Value |
|---|---|---|---|---|---|---|
| MLVI > 2.5 | No | Yes | 2.54 | (1.17, 5.53) | 0.02 | 0.02 |
| Age at baseline | 1–12 years | 13–17 years | 2.66 | (1.30, 5.46) | 0.007 | 0.007 |
| Center | Center A | Center B | 3.91 | (1.35, 11.28) | 0.012 | 0.02 |
| Center C | 2.41 | (0.73, 7.96) | 0.15 | |||
| Center D | 1.03 | (0.29, 3.66) | 0.96 | |||
| Center E | 0.94 | (0.27, 3.35) | 0.93 |
The baseline factors evaluated included primary diagnosis, age at baseline (enrollment in MALT), race, time from transplant to MALT enrollment, primary insurance (state/federal funded vs traditional private insurance), and center. The backward elimination method was used to develop the final multivariate model.
Sensitivity analyses
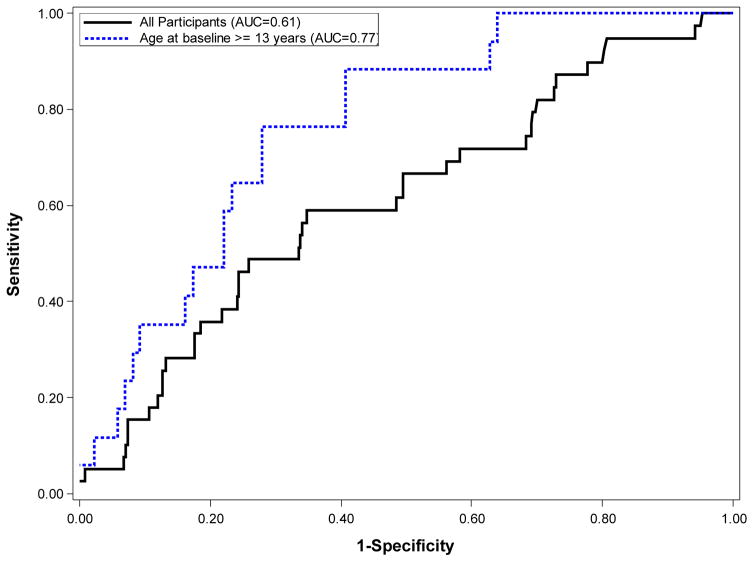
We found an AUC of 0.61 (95% C.I. 0.51–0.70) for MLVI’s overall ability to predict LAR. The AUC for the adolescent (age>12 years old) group was higher (0.77; 95% C.I. 0.67 – 0.88), as predicted (Figure 2). Thresholds of 2.5, 2.0. or 1.5 resulted in sensitivity of 0.6, 0.7, and 0.8 and specificity of 0.77, 0.73, and 0.59, respectively suggesting that the optimal threshold for adolescents was 2 (not 2.5 as pre-defined for the primary analysis). When MLVI’s were calculated on a rolling basis, MLVIs were lower as well as more stable in younger children as compared with adolescents (table s5).
FIGURE 2.
Receiver-Operator Characteristic Curve for prediction of centrally read rejection in participants.
Effect of number of levels and dosing frequency on the MLVI
There was no significant difference between the number of levels used to calculate MLVI and the value of MLVI (Table 5). There was no significant difference in the number of blood levels used in the rejection vs. no-rejection groups (table s6). The number of levels used for analyses varied:for the predictor analyses, only the levels obtained before the event were used (table 2). When we repeated the analyses using all available levels, the MLVI remained significantly associated with LAR (table s7). Target tacrolimus levels, reported for each patient by the sites, were not associated with MLVIs. 29 patients in MALT had once-a-day dosing. 10.3% of those had an above-threshold MLVI, as compared with 19.1% of those with more than once a day dosing; p=0.33, Fisher-Exact test.
Table 5.
Number of Tacrolimus Levels per Participant Used in the Primary Analysis, and MLVI Scores
| MLVI Scores | Total | P Value 1 | ||
|---|---|---|---|---|
|
| ||||
| Less than or equal to 2.5 | Greater than 2.5 | |||
| Numer of Blood Levels Records per Participant | ||||
| N | 309 | 70 | 379 | 0.1032 |
| Mean (SD) | 11.5 (6.4) | 13.1 (7.3) | 11.8 (6.6) | |
| Median (Min, Max) | 10.0 (3.0, 53.0) | 12.0 (3.0, 38.0) | 10.0 (3.0, 53.0) | |
Two sample t test performed to determine relationship between total number of TLV records per participant and MLVI scores (>2.5 and <=2.5).
Time to rejection
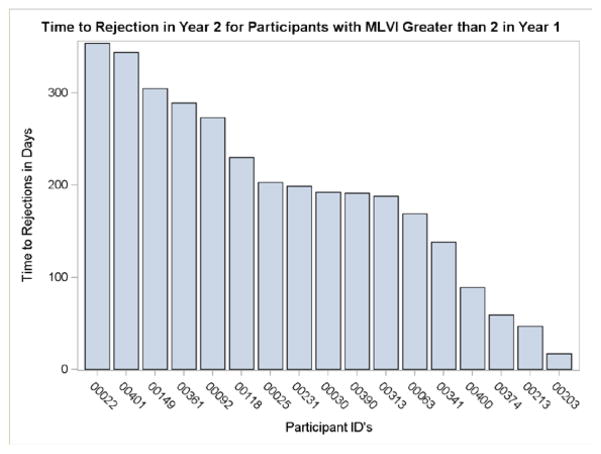
An exploratory analysis, using a model which included year-1 MLVI data, found that adolescents who had an above-threshold MLVI in year 1 (>2, n=25) had a rejection rate of 53% by the end of year 2, as compared with a rate of only 6% for adolescents who did not meet that threshold (n=71). 17 patients (all ages) who met the MLVI threshold in year 1 developed LAR in year 2. For those patients, the average time from meeting the MLVI threshold to rejection was 193.4 days (SD = 101.0), and the median was 192.0 days (range, 17–354 days). Our “time to rejection” analyses do not include pre-MALT data (which we do not have), and so it is quite possible that the MLVI has been high for a much longer period. Figure 3 presents the time to rejection for each of those patients. A rolling quarterly analysis, in which MLVI was recalculated each quarter in year 2, showed that only 6% of patients who reached the MLVI threshold in one quarter only had a rejection during the study period, as compared with 42% of those who reached that threshold in 4 quarters, showing that persistently elevated MLVI’s confer a higher risk
FIGURE 3.
time to rejection.
Discussion
This prospective multisite study found that the MLVI predicts LAR and is associated with liver injury in a representative cohort of 379 pediatric liver transplant recipients. MLVI predicts poor outcomes in any age group (primary analysis). Secondary analyses show that MLVI is a particularly robust predictor of rejection in adolescents. This may be because adherence is less stable in adolescents as compared with younger children whose medication taking is governed by their parents to a larger extent. Our results suggest that nonadherence is a more important risk in adolescents and that adherence monitoring in the transplant setting should concentrate on the adolescent age group.
MLVI is an inexpensive extension of clinical practice (it can be calculated from existing information in medical charts). Relying on clinical readings meant that we did not standardize the number and timing of blood levels, and left it to the centers’ discretion. The number of blood levels, however, was not associated with the value of MLVI. This suggests that even patients who are nonadherent to medications do perform clinically-mandated blood tests as recommended. Our reliance on clinically-obtained information suggests that our results are likely to be generalizable to clinical settings. Since MLVI monitoring imposes no additional burden on the patient, it mitigates some aspects of selection bias that typically happens in adherence research,18,20,21 as demonstrated by the fact that the MALT sample was nationally representative inasmuch as LAR rates were considered. MLVI monitoring typically detects nonadherence months before LAR occurs. As a marker of behavior, it carries with it an implication to practice, as it pinpoints a specific risk that can be addressed by behavioral interventions27,28.
Most known objective measures of adherence, including electronic monitoring devices, are indirect measures, the use of which requires participants who are motivated to use the device. This motivation is likely to be lacking in nonadherent patients who, by definition, do not follow treatment recommendations, making it difficult to monitor those patients who need the monitoring the most.18,20 Combined approaches to measure adherence have been proposed, but not fully validated17,33,34 – they may increase burden on participants or clinicians.34 Whether combining MLVI with another measure confers an advantage could be examined in clinical settings.
MLVI is not the same as looking at just one level of a medication: not all factors that might affect a single level would also affect MLVI. For example, a stable absorption defect might affect a single level but not MLVI, “white coat” nonadherence is likely to be missed when looking at one level but it would be expected to be detected by MLVI, which would “flag” instances in which patients take too little or too much of the medication (e.g., right before a doctor’s visit). “White coat adherence” may be the reason that, in our cohort, the existence of low medication blood levels in and of itself did not predict LAR. Since MLVI did predict LAR, our data suggest that “white coat adherence” is not an important factor when MLVI is considered.
MALT data show that the MLVI is not sensitive to metabolic or absorption anomalies. We also observed that MLVI is lower and more stable in younger children who would be expected to have more variability in absorption or metabolism of tacrolimus, and that MLVI is not affected by common drug-drug interactions. When used as a threshold, MLVI is not affected by common prescription practices7, and it was found to be correlated with electronic monitoring of adherence35 and with a multidisciplinary panel assessment of adherence2. MLVI values have been responsive to behavioral interventions.27,28 In the aggregate, therefore, those data establish that the index primarily measures a behavioral construct (adherence) and not a genetic or physiologic variability in drug metabolism or absorption. It is possible that some extent of medication level fluctuation would be due to factors other than nonadherence, such as dietary preferences. Because of that, we believe that MLVI should be used as a threshold construct and not as a continuous number.
Limitations of the study include that both rejections and blood tests were obtained clinically and not by a predetermined protocol, and the fact that, in the absence of an intervention component in the MALT study, it is impossible to determine with certainty whether MLVI monitoring can result in clinical benefits. However, the finding that MLVI provides ample warning before the rejection would be expected to occur suggests that it can have substantial clinical utility. The MLVI concept is generalizable to adult recipients29 as well as, potentially, to other clinical contexts in which medication levels are reflective of exposure to a medication.
A recent study36 found that a reminders-based intervention resulted in a substantial improvement in electronic monitor (VITALITY GLOWCAP ©) readings, but did not result in improvements in medication levels (or variability). The impact on clinical outcomes is not reported in this study. In contrast, pilot intervention efforts and MALT results suggest that MLVI scores, as well as changes in MLVI, are closely related to clinical transplant outcomes. Electronic monitors can provide “real time” information, which may be useful in select cases such as with elderly patients who have a genuine memory loss and need daily reminders. Since MLVI utilizes existing chart information, is cheap, and can predict outcomes, it seems to be particularly suitable for a broad clinical monitoring program. Electronic monitors, or other relatively elaborate methods, might help in selective settings or as a way to enhance certain intervention strategies.
This prospective, hypothesis-driven multi-site study has shown that MLVI, an inexpensive, simple extension of clinical practice, predicts LAR in a nationally representative sample of pediatric liver transplant recipients. MLVI did not predict all rejections in the cohort, and certainly should not be used in lieu of sound clinical judgment or other modes of transplant risk assessment. Yet, the MLVI emerges as one of the very few proven biomarkers of outcome in liver transplant recipients. A robust intervention trial that uses MLVI to target patients at-risk is needed before a firm recommendation can be made about the optimal way to use this biomarker. Until such information is available, our results and pilot intervention data27,28 suggest that MLVI monitoring, which is consistent with widely applied measurement theory,37 could inform the delivery of tailored behavioral interventions in a personalized-care paradigm.
Supplementary Material
Figure S1: Distribution of MLVI scores by Site
Table S1: Patient characteristics by enrollment status.
Table S2: Absorption/metabolic issues.
Table S3: Lack of Association between Undetectable Levels and Central Pathologist Determined Rejection
Table S4: Patient characteristics by MLVI status.
Table S5: Distribution of MLVI Calculated for One Year Period on Three Months Rolling Basis by Age
Table S6: Number of Tacrolimus Levels per Participant Used in the Primary Analysis, and Central Pathology
Table S7: Central Pathology Rejection by MLVI Scores
Acknowledgments
Supported by NIH/NIDDK grant # R01DK080740 (ES).
ABBREVIATIONS
- AUC
Area Under the ROC Curve
- ALT
Alanine Aminotransferase
- BAR
Balance of Risk Scale
- CD
Chronic Diarrhea
- CONSORT
Consolidated Standards of Reporting Trials
- D
Duodenitis
- D-MELD
MELD plus added donor characteristics Scale
- DSM-IV
Diagnostic and Statistical Manual of mental Disorders, 4th Edition
- GGT
Gamma-Glutamyl Transferase
- IRB
Institutional Review Board
- LAR
Late Acute Rejection
- MALT
Medication Adherence in children who had a Liver Transplant
- MELD
Model for End-Stage Lived Disease Scale
- MLVI
Medication Level Variability Index
- ROC
Receiver-Operator Characteristic (curve analysis)
- RV
Rotavirus
- SD
Standard Deviation
- SG
Short Gut
- SPLIT
Studies in Pediatric Liver Transplantation (consortium)
- SOFT
Survival Outcomes Following Liver Transplantation
- TRI
Transplant Risk Index Scale
- UC
Ulcerative Colitis
Footnotes
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Additional Supporting Information may be found in the online version of this article.
References
- 1.Shemesh E, Shneider BL, Emre S. Adherence to medical recommendations in pediatric transplant recipients: time for action. Pediatr Transplant. 2008 May;12(3):281–3. doi: 10.1111/j.1399-3046.2008.00920.x. [DOI] [PubMed] [Google Scholar]
- 2.Shemesh E, Shneider BL, Savitzky JL, Arnott L, Gondolesi G, Krieger N, Kerkar N, Magid M, Stuber ML, Schmeidler J, Yehuda R, Emre S. Medication adherence in pediatric and adolescent liver transplant Recipients. Pediatrics. 2004 Apr;113(4):825–32. doi: 10.1542/peds.113.4.825. [DOI] [PubMed] [Google Scholar]
- 3.Shemesh E. Nonadherence to medications following pediatric liver transplantation. Pediatr Transplant. 2004 Dec;8(6):600–5. doi: 10.1111/j.1399-3046.2004.00238.x. [DOI] [PubMed] [Google Scholar]
- 4.Shemesh E, Fine RN. Is calculating the standard deviation of tacrolimus blood levels the new gold standard for evaluating non-adherence to medications in transplant recipients? Pediatr Transplant. 2010 Dec;14(8):940–3. doi: 10.1111/j.1399-3046.2010.01396.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Molmenti E, Mazariegos G, Bueno J, Cacciarelli T, Alasio T, Khanna A, Kosmach B, Jain A, Fung J, Reyes J. Noncompliance after pediatric liver transplantation. Transplant Proc. 1999 Feb-Mar;31(1–2):408. doi: 10.1016/s0041-1345(98)01682-0. [DOI] [PubMed] [Google Scholar]
- 6.Shemesh E, Lurie S, Stuber ML, Emre S, Patel Y, Vohra P, Aromando M, Shneider BL. A pilot study of posttraumatic stress and nonadherence in pediatric liver transplant recipients. Pediatrics. 2000 Feb;105(2):E29. doi: 10.1542/peds.105.2.e29. [DOI] [PubMed] [Google Scholar]
- 7.Venkat VL, Nick TG, Bucuvalas JC. An Objective Measure To Identify Pediatric Liver Transplant Recipients At Risk For Late Allograft Rejection Related To Non-Adherence. Transplantation. 2006 Jul 15;82(1 Suppl 2):335. doi: 10.1111/j.1399-3046.2007.00794.x. [DOI] [PubMed] [Google Scholar]
- 8.Venkat VL, Nick TG, Wang Y, Bucuvalas JB. An objective measure to identify pediatric liver transplant recipients at risk for late allograft rejection related to non-adherence. Pediatr Transplant. 2008 Feb;12(1):67–72. doi: 10.1111/j.1399-3046.2007.00794.x. [DOI] [PubMed] [Google Scholar]
- 9.Stuber ML, Shemesh E, Seacord D, Washington J, 3rd, Hellemann G, McDiarmid S. Evaluating non-adherence to immunosuppressant medications in pediatric liver transplant recipients. Pediatr Transplant. 2008 May;12(3):284–8. doi: 10.1111/j.1399-3046.2008.00923.x. [DOI] [PubMed] [Google Scholar]
- 10.Annunziato RA, Emre S, Shneider BL, Barton C, Dugan CA, Shemesh E. Adherence and medical outcomes in pediatric liver transplant recipients who transition to adult services. Pediatric Transplantation. 2007;11:608–614. doi: 10.1111/j.1399-3046.2007.00689.x. [DOI] [PubMed] [Google Scholar]
- 11.Pollock-Barziv SM, Finkelstein Y, Manlhiot C, Dipchand AI, Hebert D, Ng VL, Solomon M, McCrindle BW, Grant D. Variability in tacrolimus blood levels increases the risk of late rejection and graft loss after solid organ transplantation in older children. Pediatr Transplant. 2010 Dec;14(8):968–75. doi: 10.1111/j.1399-3046.2010.01409.x. [DOI] [PubMed] [Google Scholar]
- 12.National Institutes of Health. [Accessed 4/6/16];Liver Action Plan. :6. http://liverplan.niddk.nih.gov. Executive summary.
- 13.Bucuvalas JC, Alonso E, Magee JC, Talwalkar J, Hanto D, Doo E. Improving long-term outcomes after liver transplantation in children. Am J Transplant. 2008 Dec;8(12):2506–13. doi: 10.1111/j.1600-6143.2008.02432.x. [DOI] [PubMed] [Google Scholar]
- 14.Kelly DA, Bucuvalas JC, Alonso EM, Karpen SJ, Allen U, Green M, Farmer D, Shemesh E, McDonald RA. Long-term medical management of the pediatric patient after liver transplantation: 2013 practice guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation. Liver Transpl. 2013 Aug;19(8):798–825. doi: 10.1002/lt.23697. [DOI] [PubMed] [Google Scholar]
- 15.Kikkert MJ1, Barbui C, Koeter MW, et al. Assessment of medication adherence in patients with schizophrenia: the Achilles heel of adherence research. J Nerv Ment Dis. 2008;196(4):274–81. doi: 10.1097/NMD.0b013e31816a4346. [DOI] [PubMed] [Google Scholar]
- 16.Shemesh E. Adherence to medical regimens. In: Walker WA, Goulet O, Kleinman RE, Sherman PM, Shneider BL, Sanderson IR, editors. Pediatric Gastrointestinal Disease. 4. BC Decker Inc; Ontario, Canada: 2004. pp. 2102–2110. [Google Scholar]
- 17.Kreys E. Measurements of medication adherence: in search of a gold standard. journal of clinical pathways. 2016;2(8):43–47. [Google Scholar]
- 18.Jeffery RA, Navarro T, Wilczynski NL, Iserman EC, Keepanasseril A, Sivaramalingam B, Agoritsas T, Haynes RB. Adherence measurement and patient recruitment methods are poor in intervention trials to improve patient adherence. J Clin Epidemiol. 2014 Oct;67(10):1076–82. doi: 10.1016/j.jclinepi.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 19.Shellmer DA1, Zelikovsky N. The challenges of using medication event monitoring technology with pediatric transplant patients. Pediatr Transplant. 2007;11(4):422–8. doi: 10.1111/j.1399-3046.2007.00681.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lieber SR, Helcer J, Shemesh E. Monitoring drug adherence. Transplant Rev. 2015;29(2):73–7. doi: 10.1016/j.trre.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Molloy GJ, O’Carroll RE, Witham MD, McMurdo ME. Interventions to enhance adherence to medications in patients with heart failure: a systematic review. Circ Heart Fail. 2012;5(1):126–133. doi: 10.1161/CIRCHEARTFAILURE.111.964569. [DOI] [PubMed] [Google Scholar]
- 22.Jayaraman S1, Rieder MJ, Matsui DM. Compliance assessment in drug trials: has there been improvement in two decades? Can J Clin Pharmacol. 2005;12(3):e251–3. Epub 2005 Oct 24. [PubMed] [Google Scholar]
- 23.Danziger-Isakov L, Frazier TW, Worley S, Williams N, Shellmer D, Dharnidharka VR, Gupta NA, Ikle D, Sweet SC CTOTC-05 Consortium. Perceived barriers to medication adherence in pediatric and adolescent solid organ transplantation. Pediatr Transplant. 2016;20(2):307–15. doi: 10.1111/petr.12648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shemesh E. Barriers to adherence - To screen or not to screen, that is the question. Pediatr Transplant. 2016;20(2):188–90. doi: 10.1111/petr.12671. [DOI] [PubMed] [Google Scholar]
- 25.Shemesh E. Measuring adherence in TORDIA. J Am Acad Child Adolesc Psychiatry. 2011;50(10):1075–6. doi: 10.1016/j.jaac.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 26.Lieber SR, Volk ML. Non-adherence and graft failure in adult liver transplant recipients. Dig Dis Sci. 2013;58(3):824–34. doi: 10.1007/s10620-012-2412-0. [DOI] [PubMed] [Google Scholar]
- 27.Annunziato RA, Baisley MC, Arrato N, Barton C, Henderling F, Arnon R, Kerkar N. Strangers headed to a strange land? A pilot study of using a transition coordinator to improve transfer from pediatric to adult services. J Pediatr. 2013;163(6):1628–33. doi: 10.1016/j.jpeds.2013.07.031. [DOI] [PubMed] [Google Scholar]
- 28.Shemesh E, Annunziato RA, Shneider BL, Dugan CA, Warshaw J, Kerkar N, Emre S. Improving adherence to medications in pediatric liver transplant recipients. Pediatric Transplantation. 2008;12(3):316–23. doi: 10.1111/j.1399-3046.2007.00791.x. [DOI] [PubMed] [Google Scholar]
- 29.Christina S, Annunziato RA, Schiano TD, Anand R, Vaidya S, Chuang K, Zack Y, Florman S, Shneider BL, Shemesh E. Medication level variability index predicts rejection, possibly due to nonadherence, in adult liver transplant recipients. Liver Transpl. 2014;20(10):1168–77. doi: 10.1002/lt.23930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Porter ME. What Is Value in Health Care? N Engl J Med. 2010;363:2477–2481. doi: 10.1056/NEJMp1011024. [DOI] [PubMed] [Google Scholar]
- 31. [Accessed 12/16/2015];Studies in Pediatric Liver Transplantation. https://secure.emmes.com/splitweb/
- 32.Banff Working Group. Liver biopsy interpretation for causes of late liver allograft dysfunction. Hepatology. 2006;44(2):489–501. doi: 10.1002/hep.21280. [DOI] [PubMed] [Google Scholar]
- 33.Pai AL, Rausch J, Tackett A, Marsolo K, Drotar D, Goebel J. System for integrated adherence monitoring: real-time non-adherence risk assessment in pediatric kidney transplantation. Pediatr Transplant. 2012;16(4):329–34. doi: 10.1111/j.1399-3046.2012.01657.x. [DOI] [PubMed] [Google Scholar]
- 34.Shemesh E. Measuring adherence to medications: are complex methods superior to simple ones? Pediatr Transplant. 2012;16(4):315–7. doi: 10.1111/j.1399-3046.2012.01676.x. [DOI] [PubMed] [Google Scholar]
- 35.Kerkar N, Annunziato RA, Foley L, Schmeidler J, Rumbo C, Emre S, Shneider B, Shemesh E. Prospective analysis of nonadherence in autoimmune hepatitis: a common problem. J Pediatr Gastroenterol Nutr. 2006;43(5):629–34. doi: 10.1097/01.mpg.0000239735.87111.ba. [DOI] [PubMed] [Google Scholar]
- 36.Reese PP, Bloom RD, Trofe-Clark J, Mussell A, Leidy D, Levsky S, Zhu J, Yang L, Wang W, Troxel A, Feldman HI, Volpp K. Automated Reminders and Physician Notification to Promote Immunosuppression Adherence Among Kidney Transplant Recipients: A Randomized Trial. Am J Kidney Dis. 2017 Mar;69(3):400–409. doi: 10.1053/j.ajkd.2016.10.017. [DOI] [PubMed] [Google Scholar]
- 37.Feingenbaum AV. Total Quality Control. 4. McGraw-Hill; USA: 2004. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Distribution of MLVI scores by Site
Table S1: Patient characteristics by enrollment status.
Table S2: Absorption/metabolic issues.
Table S3: Lack of Association between Undetectable Levels and Central Pathologist Determined Rejection
Table S4: Patient characteristics by MLVI status.
Table S5: Distribution of MLVI Calculated for One Year Period on Three Months Rolling Basis by Age
Table S6: Number of Tacrolimus Levels per Participant Used in the Primary Analysis, and Central Pathology
Table S7: Central Pathology Rejection by MLVI Scores