Abstract
Objective
Aggressive laryngeal, tracheal and pulmonary papilloma is an extremely challenging clinical problem without proven treatment options. A recent German report documented promising results with systemic bevacizumab. The objective of this study is to report the initial experience of this novel treatment in the United States for Recurrent Respiratory Papillomatosis (RRP).
Study Design
Cases series
Methods
Electronic survey of the RRP Task Force of the American Society of Pediatric Otolaryngology, American Broncho-Esophagological Association, and physicians known to the authors to have used systemic bevacizumab for RRP.
Results
Eleven completed surveys were obtained. In three cases, systemic bevacizumab was considered clinically but not administered. Eight patients were treated with systemic bevacizumab, all for aggressive papillomatosis uncontrolled by surgical and adjuvant therapy, including 7 of 8 with pulmonary disease. Treatment dosing ranged from 5–10 mg/kg every 2–4 weeks, with all patients responding (7/8 partial response, 1/8 complete response). In four patients who had post-bevacizumab chest imaging, three demonstrated improvement of disease and one stabilization. Treatment interval could be lengthened in seven patients and clinical response maintained. One patient with long-standing pulmonary disease (>10 years) was diagnosed with malignant transformation while on treatment and bevacizumab was discontinued in lieu of other chemotherapeutic agents. All other patients continue on systemic bevacizumab with minimal complications (hemoptysis n=1; proteinuria n=1).
Conclusions
Systemic bevacizumab appears to have significant promise in the most treatment-resistant and aggressive forms of papillomatosis with a low complication profile. These results suggest bevacizumab should be studied in a formal clinical trial for RRP.
Keywords: Recurrent Respiratory Papillomatosis, RRP, laryngeal papilloma, tracheal papilloma, pulmonary papilloma, Human Papillomavirus, HPV, bevacizumab, Avastin
Introduction
The management of complex laryngotracheal and pulmonary papillomatosis has proven to be an elusive and unsatisfying endeavor. There is no uniformity in the responses to surgical and adjuvant therapies, and to date, no systemic therapy has shown to be consistently effective in the management of this disease1.
In 2014, Mohr and his team in Germany published their initial experience with management of recurrent respiratory papillomatosis (RRP) in four adults and one pediatric patient using systemic bevacizumab2. The responses were dramatic with a significant reduction in interval to surgical debridements and good long-term effect, a result also seen in the first case report of systemic bevacizumab for pulmonary RRP3. A similar result was reported recently in the American literature, describing the impact of intravenous bevacizumab on a child with severe laryngotracheal and pulmonary papillomatosis4. Since adjuvant therapy with systemic bevacizumab became recognized as a potential therapy, several centers across the country have begun using it for management in their most difficult cases.
As there are no clinical trials utilizing bevacizumab for RRP or standardized protocols for its dosing, there is little information available to clinicians who would consider this treatment modality for their patients. Therefore, the objective of this report is to describe the use of intravenous bevacizumab for the treatment of severe RRP in the United States to better understand the dosing, disease response, and complications associated with the use of this novel treatment.
Methods
An electronic survey was designed to query physicians who regularly treat patients with RRP on their experience with systemic bevacizumab. The survey was distributed to 1) RRP Task Force of the American Society of Pediatric Otolaryngology, 2) Members of the American Broncho-Esophagological Association and 3) physicians who had directly contacted any of the authors for assistance in dosing bevacizumab due to the authors’ experience with this treatment modality. The study was vetted through the Johns Hopkins IRB, who determined it was exempt from IRB approval since 1) the research involved the collection or study of existing data, documents, and records and 2) all data was de-identified before obtained by study members with no access to patient identifiers. Physician consent to collection of data was implied by completing the survey, and this was stated explicitly in survey instructions. Treating physicians were asked to provide details of: clinical situation that led to consideration of bevacizumab treatment, whether bevacizumab was used, patient’s demographics and prior treatment course, bevacizumab dosing, treatment response, and complications. Treatment response was surveyed as a forced choice – Disease Progression, Disease Stabilization, Partial Response, or Complete Response. Statistical analysis was limited to descriptive statistics due to the small numbers of cases.
Results
Eleven completed surveys were obtained from nine medical centers, and in three cases systemic bevacizumab was considered but not administered. Reasons provided to not administer systemic bevacizumab were lack of hospital approval (1), lack of data in young children (1), and initial treatment with intralesional bevacizumab instead of systemic treatment (1). Eight completed surveys representing seven medical centers where systemic bevacizumab was used were, therefore, available for analysis.
The patient demographics and prior treatment history are summarized in Table 1. These were for the most part patients with a long clinical history of juvenile-onset RRP, with aggressive clinical courses requiring very frequent surgical interventions, in many cases at least once a month. Numerous adjuvant treatments had been previously administered, including intralesional cidofovir (7), systemic interferon (4), and celecoxib (3). Spread of disease beyond the larynx was common, with tracheal (6) and pulmonary (7) disease in the majority of patients. The two adult-onset patients in the study had primary pulmonary papilloma without an antecedent history of laryngeal papilloma, a patient population with very limited surgical options.
Table 1.
Patient Demographics
| Case | Age | Gender | Onset | Sites affected | Surgical Interval |
Prior Therapy | ||
|---|---|---|---|---|---|---|---|---|
| Larynx | Trachea | Lung | ||||||
| 1 | 20 | F | Juvenile | Y | Y | Y | 3 weeks | Local: cidofovir, bevacizumab, photodynamic therapy |
| Systemic: interferon | ||||||||
|
| ||||||||
| 2 | 12 | F | Juvenile | Y | Y | Y | 1–4 weeks | Local: cidofovir |
| Systemic: interferon, propranolol, celecoxib, Gardasil | ||||||||
|
| ||||||||
| 3 | 16 | F | Juvenile | Y | N | N | 4–6 weeks | Local: cidofovir |
| Systemic: celecoxib | ||||||||
|
| ||||||||
| 4 | 10 | M | Juvenile | Y | Y | Y | 4 weeks | Local: cidofovir |
| Systemic: interferon, indole 3 carbinol, celecoxib | ||||||||
|
| ||||||||
| 5 | 18 | M | Juvenile | Y | Y | Y | 6 weeks | Local: cidofovir, bevacizumab |
| Systemic: interferon, leflunomide | ||||||||
|
| ||||||||
| 6 | 21 | M | Juvenile | Y | Y | Y | 6 weeks | Local: cidofovir |
|
| ||||||||
| 7 | 86 | M | Adult | N | N | Y | 3 months | Local: cidofovir |
|
| ||||||||
| 8 | 62 | M | Adult | N | Y | Y | 12 months | None |
Consistent with the only reports describing the use of bevacizumab for RRP2–4, dosing was initiated in all patients in the range of 5 to 10 mg/kg per dose, most frequently at three week intervals (Table 2). Seven of the eight patients were characterized by their physicians as having a Partial Response (PR) to treatment, with one complete response. Three example partial responses are shown in Figure 1 and Figure 2 that all demonstrate significant regression of laryngeal and tracheal papilloma. Reduction of the exophytic papilloma alleviated airway obstruction and allowed for lengthening of the inter-surgical interval in all of these cases, demonstrating how even a partial response can have a dramatic effect on patient symptomatology.
Table 2.
Bevacizumab Dosing and Response
| Case | Dose (mg/kg) |
Initial Dosing Interval (q week) |
Duration of Treatment (months) |
Stable Dosing Interval (q week) |
Response | Pulmonary Response |
Post- bevacizumab Surgical Interval |
Complications |
|---|---|---|---|---|---|---|---|---|
| 1 | 10 | 3 | 12 | 8 | PR | Not known | q3months | Treatment changed secondary to diagnosis of malignancy |
|
| ||||||||
| 2 | 10 | 4 | 21 | 8 | PR | CR | q4months | Proteinuria |
|
| ||||||||
| 3 | 10 | 2 | 10 | 12 | CR | -- | No surgery required | None |
|
| ||||||||
| 4 | 5 | 3 | 18 | 3 | PR | Stable | q6months | None |
|
| ||||||||
| 5 | 10 | 3 | 7 | 6 | PR | Not known | q6week with no excision needed | Hemoptysis |
|
| ||||||||
| 6 | 10 | 3 | 5 | 12 | PR | Not known | q3months | None |
|
| ||||||||
| 7 | 5 | 3 | 10 | 8 | PR | PR | No surgery required | None |
|
| ||||||||
| 8 | 10 | 3 | 8 | 6 | PR | PR | No surgery required | None |
Abbreviations: PR – Partial Response, CR – Complete Response
Figure 1.
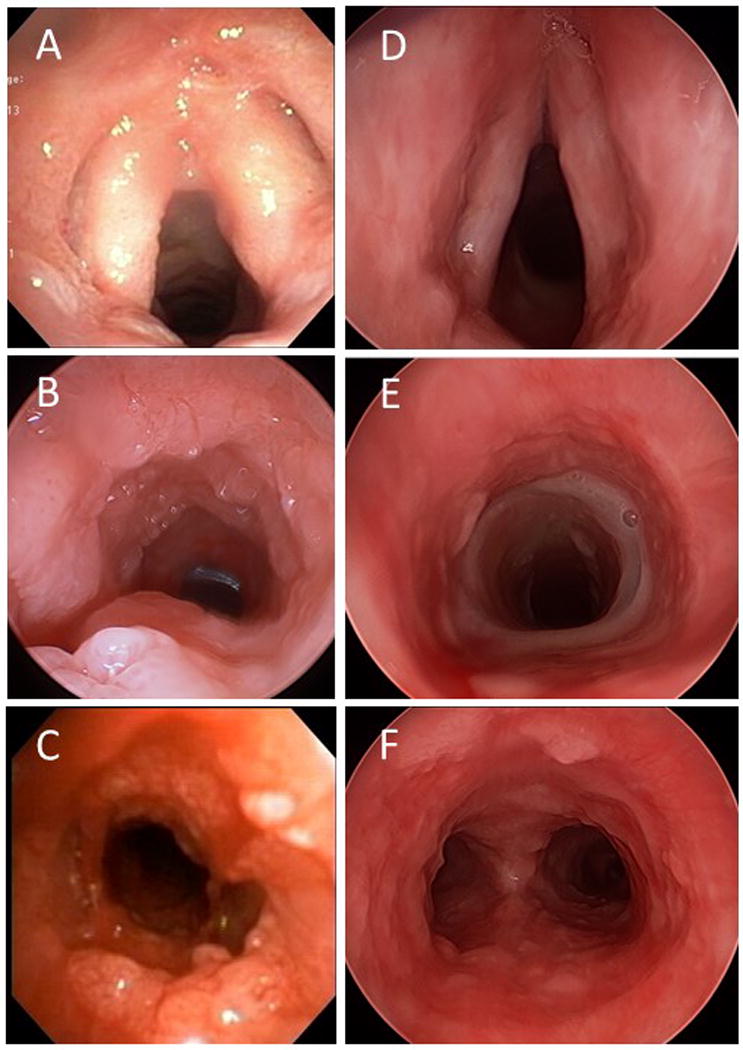
Response to four cycles of bevacizumab (10 mg/kg every three weeks). Pre-bevacizumab (A-C), and post-bevacizumab (D-F). Disease regresses and ceases to obstruct airway but is not completely eliminated. Note indwelling tracheal stent, required for severe tracheal stenosis as a consequence of previous photodynamic therapy. Photos courtesy of Simon R. Best, MD.
Figure 2.
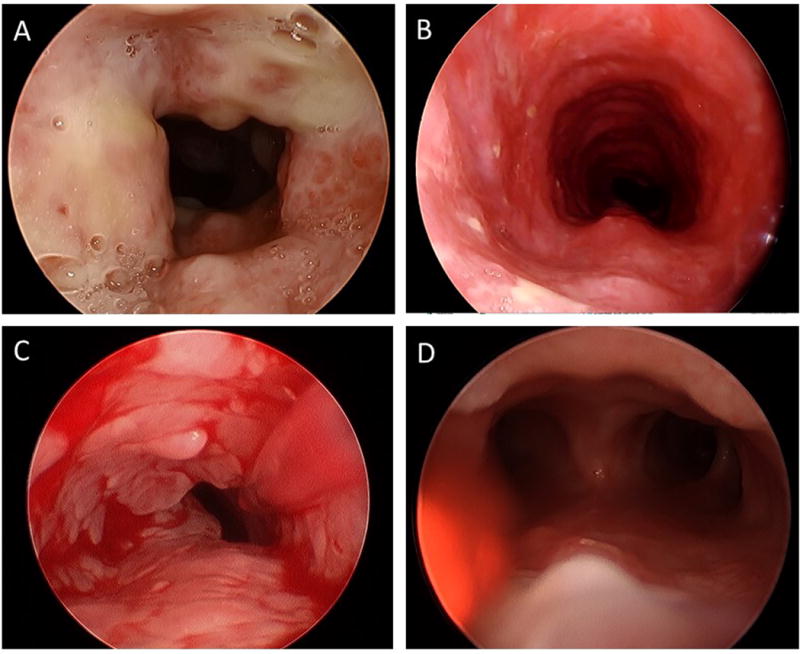
Tracheal papilloma responses to bevacizumab: 10 mg/kg every three weeks (A and B) and 5 mg/kg every three weeks (C and D). Photos courtesy of Douglas Sidell, MD and Diego Preciado, MD.
In all patients reported here, the surgical interval between excisions for papilloma was significantly lengthened after systemic bevacizumab, and in some cases surgical debridement was no longer necessary. Due to the response seen, the treatment interval was able to be lengthened in seven patients with maintenance of clinical response. Pulmonary imaging was not available in all patients to monitor response to treatment, but in three of the four patients followed with chest imaging, there was improvement or resolution of the pulmonary papilloma in three, and stabilization of disease in the fourth.
The treatment appears to have been very well tolerated in most patients, with only two reported minor complications (hemoptysis and proteinuria). One adult patient with long-standing tracheal, bronchial, and pulmonary disease had a dramatic response to bevacizumab (Figure 1) and went from requiring debridement of tracheal disease every three weeks to a surveillance bronchoscopy every three months where no debridement was needed. After over a year on therapy, and 11 cycles of bevacizumab, a friable tissue mass was identified in a secondary bronchus and a biopsy was consistent with squamous cell carcinoma. Bevacizumab was then discontinued in lieu of other chemotherapeutic agents.
Discussion
We believe that systemic bevacizumab shows significant promise as a treatment modality for severe RRP, as demonstrated by results at multiple centers across the United States. As is appropriate for a novel treatment such as systemic bevacizumab, the patient population in whom this has been used thus far clearly represents the most aggressive and treatment-refractory cases of RRP. Despite this selection bias for the most aggressive cases, all eight patients reported here responded to systemic bevacizumab, with seven partial but dramatic responses and one complete response.
All primary laryngeal RRP patients in this report are juvenile-onset RRP (JORRP), with 5 of 6 patients having tracheal and pulmonary spread of disease. All cases had been exposed to prior adjuvant therapy, in many cases multiple systemic agents, but all still suffered from significant disease progression. The pre-bevacizumab surgical interval for this population ranged from 1 to 6 weeks, a tremendous burden of disease for patients and their caregivers. After systemic bevacizumab, all treated patients had significant lengthening of their inter-surgical interval, with surgical intervals now on the order of months, or not required at all. While the presence of flat airway papilloma (Figure 1 and Figure 2) classifies most of these responses as partial rather than complete, these are still significant improvements in quality of life and dramatic reductions in the need for anesthesia. This reduction in the exophytic papilloma which alleviates the airway obstruction but doesn’t completely eliminate the papilloma appears to be the typical response to systemic bevacizumab, likely reflecting the direct anti-angiogenic action of bevacizumab. For the four patients with known pulmonary disease and available post-bevacizumab chest imaging, including the two adults with primary pulmonary papilloma, 1 of the 4 had complete resolution of the lung papillomatosis, 2 had partial response and 1 had stabilization of the lung lesions.
There was near uniformity in dosing, as the majority of the centers utilized a 10 mg/kg dosing schedule. The one JORRP patient with lower dosing (5mg/kg) required more frequent infusions for maintenance compared to the group with 10 mg/kg dosing, a consideration for future studies. Side effects were minor, and included one case with proteinuria and one with temporary hemoptysis after the first dose of bevacizumab which subsequently resolved. However, the long term effects of the medication, which appears to require continued dosing to keep the papilloma under control, is not yet known. Some insights into continued dosing for RRP may come from the use of systemic bevacizumab as a maintenance therapy for Neurofibromatosis 2 (NF2), where long-term dosing of bevacizumab is used as a non-surgical modality for skull base schwannomas5. In this cohort, which received systemic bevacizumab for an average of 33 months, hypertension (24%), proteinuria (18%), and fatigue were common side effects.
The report of systemic bevacizumab from Mohr et al. includes one patient with a significant clinical response and regression of papilloma, who later presented with a diagnosis of laryngeal squamous cell carcinoma after two cycles of bevacizumab2. Our current report also includes a patient with long-standing tracheal and pulmonary disease who developed an invasive carcinoma while receiving bevacizumab. The natural history of pulmonary papilloma is well-described, and patients with pulmonary disease are at risk of developing malignant transformation6,7. Risk factors include pulmonary disease of long-standing duration, and HPV-11 viral infection, both of which were present in the patient reported here. As an anti-angiogenesis agent, bevacizumab does not induce DNA damage or apoptosis in papillomas2 and, therefore, wouldn’t appear to be directly implicated in malignant transformation. Nevertheless, this end-point should be closely evaluated in further studies of systemic bevacizumab for RRP.
There are limitations to a retrospective survey and case series, including selection bias, poor recall of patient or medication details, and incomplete data. We sought to minimize selection bias by surveying a wide group of practitioners who would have considered systemic bevacizumab and included the responses for those who ultimately decided against its use. We determined the risk of poor recall and inaccurate data to be low due to the intensity of the work required to get bevacizumab approved by regulatory bodies and the fact that these patients are likely well-known to their treating physicians. All patients included in this study are still being actively treated by their physicians, and we were therefore able to obtain complete information on all treated patients.
Despite the limitations to this study, it is important to recognize that each physician who has incorporated bevacizumab into a treatment regimen has noted a significant response compared to past therapies attempted (Table 1). The response of the pulmonary disease in four patients, a disease for which there exists no known treatment options, is particularly notable. That these responses occurred in the most severe and treatment-refractory group of RRP patients is highly significant, and strongly suggests that systemic bevacizumab should be formally studied in the setting of a clinical trial for severe RRP.
Conclusions
Systemic bevacizumab appears to have promise in the most treatment-resistant and aggressive forms of papillomatosis with a low complication profile. Future studies should be performed to better define the dosing details and response rate to systemic bevacizumab in RRP, and the patient population most likely to benefit from this new treatment modality.
Acknowledgments
Financial Support: Financial Support for this study was provided by the NIDCD Mentored Patient-Oriented Research Career Development Award 1K23DC014758 (S. Best)
The authors thank the following for their participation in this research: Douglas Sidell, MD, Lee Smith, MD, Diego Preciado, MD, Scott Shofer, M.D., Ph.D., Jeffrey Clarke, MD, and Jonathan Bock, MD.
Footnotes
Conflict of Interest: The authors have no financial relationships, or conflicts of interest to disclose.
References
- 1.Healy GB, Gelber RD, Trowbridge AL, Grundfast KM, Ruben RJ, Price KN. Treatment of recurrent respiratory papillomatosis with human leukocyte interferon. Results of a multicenter randomized clinical trial. N Engl J Med. 1988;319(7):401–407. doi: 10.1056/NEJM198808183190704. [DOI] [PubMed] [Google Scholar]
- 2.Mohr M, Schliemann C, Biermann C, et al. Rapid response to systemic bevacizumab therapy in recurrent respiratory papillomatosis. Oncol Lett. 2014;8(5):1912–1918. doi: 10.3892/ol.2014.2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nagel S, Busch C, Blankenburg T, Schutte W. Treatment of respiratory papillomatosis--a case report on systemic treatment with bevacizumab. Pneumologie. 2009;63(7):387–389. doi: 10.1055/s-0029-1214714. [DOI] [PubMed] [Google Scholar]
- 4.Zur KB, Fox E. Bevacizumab chemotherapy for management of pulmonary and laryngotracheal papillomatosis in a child. Laryngoscope. 2016 doi: 10.1002/lary.26450. [DOI] [PubMed] [Google Scholar]
- 5.Morris KA, Golding JF, Blesing C, et al. Toxicity profile of bevacizumab in the UK Neurofibromatosis type 2 cohort. J Neurooncol. 2017;131(1):117–124. doi: 10.1007/s11060-016-2276-9. [DOI] [PubMed] [Google Scholar]
- 6.Gerein V, Rastorguev E, Gerein J, Draf W, Schirren J. Incidence, age at onset, and potential reasons of malignant transformation in recurrent respiratory papillomatosis patients: 20 years experience. Otolaryngol Head Neck Surg. 2005;132(3):392–394. doi: 10.1016/j.otohns.2004.09.035. [DOI] [PubMed] [Google Scholar]
- 7.Gelinas JF, Manoukian J, Cote A. Lung involvement in juvenile onset recurrent respiratory papillomatosis: a systematic review of the literature. Int J Pediatr Otorhinolaryngol. 2008;72(4):433–452. doi: 10.1016/j.ijporl.2007.12.003. [DOI] [PubMed] [Google Scholar]


