Abstract
The importance of the renin angiotensin aldosterone system in cardiovascular physiology and pathophysiology has been well described whereas the detailed molecular mechanisms remain elusive. The angiotensin II type 1 receptor (AT1 receptor) is one of the key players in the renin angiotensin aldosterone system. The AT1 receptor promotes various intracellular signaling pathways resulting in hypertension, endothelial dysfunction, vascular remodeling and end organ damage. Accumulating evidence shows the complex picture of AT1 receptor-mediated signaling; AT1 receptor-mediated heterotrimeric G protein-dependent signaling, transactivation of growth factor receptors, NADPH oxidase and ROS signaling, G protein-independent signaling, including the β-arrestin signals and interaction with several AT1 receptor interacting proteins. In addition, there is functional cross-talk between the AT1 receptor signaling pathway and other signaling pathways. In this review, we will summarize an up to date overview of essential AT1 receptor signaling events and their functional significances in the cardiovascular system.
Keywords: angiotensin II, signal transduction, vascular smooth muscle cell, endothelial cell, ADAM17, EGF receptor
1. Introduction
The Renin-angiotensin-aldosterone system (RAAS) plays an integral role in cardiovascular and renal physiology and pathophysiology, exerting direct autocrine and paracrine as well as endocrine effects. The system influences a large range of homeostatic and modulatory processes including regulation of salt and water balance, vasoconstriction, cell/tissue remodeling and dysfunction in the cardiovascular system. Angiotensin (AngII), the major bioactive peptide of the RAAS, mediates many of its effects by binding to two major G protein-coupled receptors (GPCRs): AngII type 1 receptor (AT1 receptor) and the AngII type 2 receptor (AT2 receptor) (1). Although the AT2 receptor is thought to oppose the effects of the AT1 receptor, many of the effects of AngII are mediated through the activation of the AT1 receptor. The AT1 receptor is predominantly expressed in various tissues throughout the cardiovascular system including vascular smooth muscle, endothelium, heart and kidney. The AT1 receptor promotes intracellular signaling pathways through the activation of various protein kinases, subunits of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, growth factor receptor transactivation (1–5), or direct interactions with AT1 receptor interacting proteins such as Janus kinase 2 (JAK2), phospholipase C (PLC) γ1, AT1 receptor associated protein (ATRAP), type 1 angiotensin II receptor-associated protein (ARAP1) and Guanine nucleotide exchange factor (GEF)-like protein (GLP) (1, 2, 6, 7).
Although the physiological/pathophysiological roles of the AT2 receptor have not been fully elucidated, there is an increasing interest in AT2 receptor functions in the cardiovascular system (8–10). AT2 receptor stimulation causes vasorelaxation through protein kinase A (PKA)-dependent endothelial nitric oxide (NO) synthase (eNOS) activation or paracrine signaling induced by bradykinin/NO/cyclic GMP production (1, 8). Mice overexpressing the AT2 receptor exhibit vasodilation (11), and pharmacological stimulation of AT2 receptor promotes natriuresis, lowering of blood pressure, and inhibits AngII-mediated hypertension (12, 13). In systemic AT2 receptor transgenic mice with AngII infusion, perivascular fibrosis but not cardiac hypertrophy is attenuated compared with wild-type mice (14). By contrast, AT2 receptor stimulation (candesartan plus AngII) promotes vasoconstriction in isolated mesenteric arteries from spontaneously hypertensive rats (15). Mice with ventricular specific AT2 receptor overexpression develop dilated cardiomyopathy and heart failure (16). These confusing results regarding AT2 receptor function is suggested to be largely dependent on expression levels of AT2 receptor in addition to Nox2 regulation and transforming growth factor beta 1 (TGFβ1) signaling pathways (17). The AT2 receptor will not be discussed further in this review, however, there are excellent review articles summarizing recent findings regarding the role of AT2 receptor in hypertension, vascular remodeling and cardiovascular dysfunction (9, 10, 18).
Angiotensin-1-7 (Ang1-7) is converted by angiotensin converting enzyme 2 (ACE2) and is thought to balance the RAAS system by promoting an antagonistic effect on the responses elicited by AngII such as vasodilation (19, 20). Ang1-7 exerts its effects through the Mas receptor. The Ang1-7 activation of Mas receptor contributes to NO production through eNOS Ser1177 phosphorylation (21). However, Ang1-7 attenuates cardiac hypertrophy and fibrosis induced by AngII independent of alterations in blood pressure (22). While the Mas receptor is a GPCR, its G protein coupling is controversial and Mas may also signal G protein independently or constitutively without ligand binding (1). Please note that detailed review articles focused on Ang1-7/Mas functions and mechanisms have been published, whereas more emphasis is needed regarding Mas signaling and how it interacts with AT1 receptor signal transduction (19, 20).
The AT1 receptor is widely expressed in various tissues such as heart, kidney, adrenal gland, brain and adipose tissues (23), with these systems in turn affecting the cardiovascular system directly or indirectly via circulatory as well as local/tissue RAAS (24–26). This review will outline recent findings regarding the AT1 receptor signaling pathways in the cardiovascular system, highlighting its influence on vascular smooth muscle cells (VSMC) and endothelial cells (EC) functions.
2. AT1 receptor signaling in the cardiovascular system
AngII mediates AT1 receptor activation via stacking interactions between Phe8(AngII)/His256(AT1 receptor) and Tyr4(AngII)/Asn111(AT1 receptor) (1), resulting in a conformational change in transmembrane (TM)3-TM6 helices and interaction between TM2 and TM7 (27). Upon AngII binding, the AT1 receptor facilitates a variety of cytoplasmic signaling pathways that mediate VSMC remodeling including hypertrophy and migration. The AT1 receptor interacts with heterotrimeric G-proteins (Gq/11, Gi, G12 and G13) which transduce signals to the cognate effectors and downstream second messengers including PLCβ and Rho GEFs, and inositol triphosphate, diacylglycerol and reactive oxygen species (ROS), respectively. This in turn regulates VSMCs contraction via activation of myosin light chain kinase (MLCK) or inhibition of myosin light chain phosphatase (MLCP) (2, 28, 29). Src family kinases also regulate vascular contraction via MLCP, attenuating myosin light chain phosphorylation and contraction in AngII infused mice (30). AngII-dependent hypertension, but not vascular remodeling, is attenuated in c-Src+/− mice (31). Similarly, Ras-related protein 1 (Rap1b) knockdown or PDZ-RhoGEF/RhoA/Rho kinase signaling cascade promotes vascular contraction induced by AngII through inhibition of MLCP (32, 33). The AT1 receptor has also been shown to regulate vasoconstriction through phosphorylation of with-no-lysine (WNK) and Ste20/SPS1-related proline/alanine-rich kinase (SPAK) and subsequent modulation of Na-K-Cl cotransporter isoform 1 (NKCC1) (34). In addition, calcium activated chloride channel anoctamin-1 (ANO1) is induced by AngII via AT1 receptor-phosphatidylinositol 3-kinase (PI3K)/AKT pathway and regulates vasoconstriction (35), with VSMC specific ANO1 knockdown attenuating AngII-induced contractile responses (36).
AT1 receptor expressed in the cardiovascular system has been shown to activate a variety of intracellular protein kinases including mitogen-activated protein kinase (MAPK) family [extracellular signal regulated kinase (ERK), c-Jun N terminal kinase (JNK), p38MAPK], p70 S6 kinase, AKT/protein kinase B(PKB), various protein kinase C (PKC) isoforms, receptor and non-receptor tyrosine kinases and serine/threonine kinases (2, 28, 37–40). These kinases are believed to stimulate NADPH oxidase, ROS generation and protein synthesis, causing hypertrophy, hyperplasia and migration of VSMCs (2, 41–45), cardiac hypertrophy (46) and renal deterioration (47).
3. Growth factor receptor transactivation
Activation of the AT1 receptor can transactivate receptor tyrosine kinases, thereby enabling AngII to regulate a multitude of signaling pathways downstream of growth factor receptors. Transactivation of the epidermal growth factor (EGF) receptor (EGFR), the major model of AT1 receptor ‘cross-talk,’ drives cellular process distal to the EGFR (5) (Figure 1). AngII stimulation causes rapid activation of the EGFR and subsequent activation of Ras/ERK cascade and various intracellular signaling such as the AKT/p70 S6 kinase cascade and endoplasmic reticulum (ER) stress/unfolded protein response (5). Upon activation of the AT1 receptor, second messengers such as Ca2+ and ROS mediate activation of A Disintegrin And Metalloproteinase 17 (ADAM17) (2, 5). Activation of transmembrane ADAM17 leads to the cleavage of inactive membrane-bound precursors and the production of their complementary active form (Heparin-binding EGF-like growth factor (HB-EGF), neuregulin, EGF, etc). Although some reports show Gq-independent EGFR transactivation (48, 49), ADAM17-mediated HB-EGF shedding by AngII requires Gq activation (45, 50) and subsequent ADAM17 Tyr702 phosphorylation (51–53). The ADAM17-dependent EGFR transactivation causes hypertrophy and migration of VSMCs through the Ras/ERK pathway and the PI3K/Akt/mechanistic target of rapamycin (mTOR)/p70S6K/eukaryotic translation initiation factor 4E (eIF4E) pathway (51–53). In addition, BMX (bone marrow kinase), CHKA (choline kinase alpha) and TRIO [triple functional domain (PTPRF interacting)] have been identified as upstream signaling molecules required for AngII-induced EGFR transactivation by siRNA library screening (54). In ECs, AT1 receptor-mediated EGFR transactivation promotes cell migration via focal adhesion kinase and paxillin phosphorylation (55). It also promotes release of microparticles from ECs, resulting in inflammatory activation (56). Furthermore, systemic inhibition of ADAM17, EGFR or ER stress attenuates aortic wall thickening induced by AngII (57, 58), suggestive of a role for AT1 receptor-EGFR transactivation in cardiovascular pathology (5).
Figure 1. AT1 receptor signal transduction cascade through EGFR transactivation.
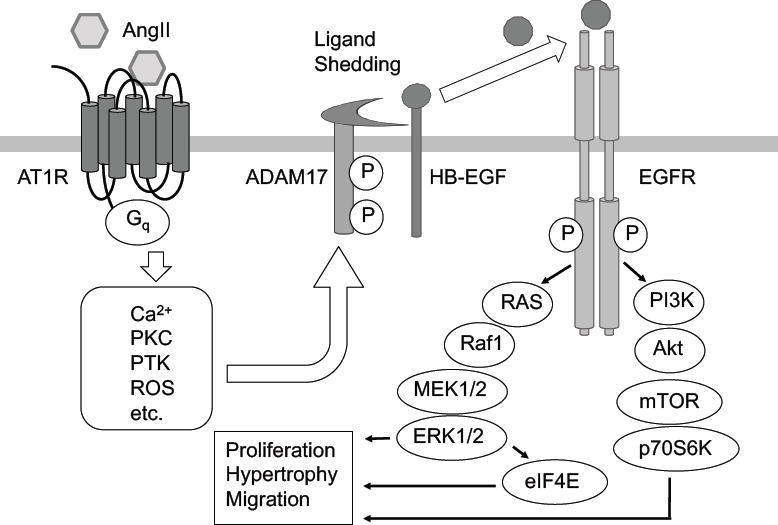
AngII-activated AT1 receptor initiates classical second messenger-mediated signals such as Ca2+, elevation and PKC activation as well as PTK activation and ROS production via heterotrimeric G proteins, which then activate ADAM17 via phosphorylation. Activated ADAM17 causes shedding of EGFR ligands such as pro-HB-EGF, and activates EGFR. EGFR transactivation by AT1 receptor facilitates cellular hypertrophy, proliferation and migration via the Ras/Raf/MEK/ERK pathway and PI3K/Akt-PKB/mTOR pathway. EGFR: epidermal growth factor receptor; ADAM17: A Disintegrin And Metalloproteinase 17; MEK: MAPK/ERK kinase; mTOR: mamalian target of rapamycin; eIF4E: eukaryotic translation initiation factor 4E.
AngII also leads to activation of the platelet-derived growth factor receptor (PDGFR) in cardiovascular tissue (59, 60), mediating ERK activation (61), and regulating vascular hypertrophy and fibrosis (62, 63). A lot less is known in regard to the physiological significance of the Insulin-like growth factor-1 receptor (IGF-1R) transactivation induced by AngII in VSMCs (64). IGF-1R transactivation is Src-dependent, and is required for PI3K and p70 S6 kinase activation by AngII, but not for ERK stimulation (65). IGF-1R transactivation is important for Src kinase mediated cortactin phosphorylation and cytoskeletal reorganization in response to AngII (66). ROS production by AngII is suggested to depend partially on IGF-1R transactivation, leading to p38MAPK and ERK5 activation in VSMCs (67). However, unlike the EGFR, the picture is not as complete, with little information about the role of transactivation of PDGFR and IGF-1R in cardiovascular pathophysiology.
4. Small G proteins activated by AT1 receptor
AngII activates various small G proteins including Ras, Rho and Rac, potentially regulating vascular remodeling (68). GTP-bound RhoA and RhoA in the particulate fraction is upregulated by AngII in VSMCs (69, 70). G12/13 mediates RhoA activation induced by AngII, and this regulation is independent of Gq/11 signaling stimulated by AngII (71, 72). Cardiac specific G13 deficient mouse is protected from AngII-induced cardiac hypertrophy and fibrosis (71). Moreover, the Rho/Rho-associated protein kinase (ROCK) pathway is also important in vascular remodeling (68) and cardiovascular diseases (73–75). We have previously shown that a tyrosine kinase, PYK2, and its upstream activation by PKCδ is essential for AngII-induced Rho/ROCK pathway activation in VSMCs, and this is in parallel with EGFR transactivation pathways in VSMCs (76). Activation of Rho/ROCK is also required for JNK activation and subsequent VSMCs migration (76). Alternatively, AngII promotes Jak2-dependent Arhgef1 phosphorylation, resulting in RhoA activation and subsequent blood pressure elevation (77, 78). In addition, RhoA activation mediates nuclear factor kB (NF-kB) activation and subsequent IL-6 expression in VSMCs (79). ROCK inhibitor suppresses the expression of monocyte chemoattractant protein-1 (MCP-1) or plasminogen activator inhibitor-1 (PAI-1) induced by AngII in VSMCs (80, 81). Taken together, RhoA/ROCK appears essential in the vascular contraction, remodeling as well as inflammation induced by AngII (Figure 2). Rac activates p21-activated kinase 1 (PAK1) in VSMCs, resulting in JNK activation and VSMC hypertrophy (82–84) as well as promoting ROS production in VSMCs (85).
Figure 2. AT1 receptor signal transduction cascade through the Rho/ROCK pathway.
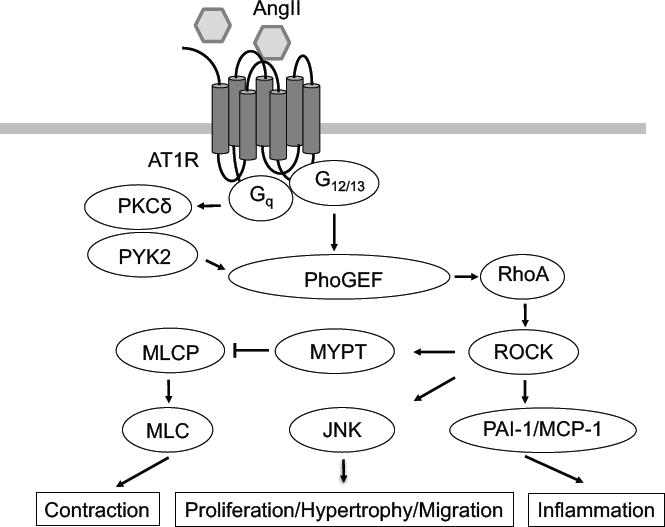
The AT1 receptor interacts with heterotrimeric G proteins and activates Rho/ROCK pathway via RhoGEF. Through this pathway, AT1 receptor stimulates cellular contraction via MLC by inhibition of MLCP, cellular proliferation/hypertrophy via JNK, and inflammation via PAI-1/MCP-1. MLC: myosin light chain; MLCP: MLC phosphatase.
5. NADPH oxidase subunits and ROS
Various NADPH oxidase subunits including Nox1, p22 phox, p47 phox and p67 phox, are stimulated via AT1 receptor activation. This produces H2O2 and superoxide (3, 4) and results in the stimulation of p38MAPK/AKT pathway and protein synthesis (28). In ECs, AT1 receptor signaling mediates endothelial dysfunction via inhibition of NO production and induction of vascular insulin resistance (86, 87). The role of AngII-induced oxidative stress in the pathogenesis of endothelial dysfunction has also been validated with AT1 receptor antagonists (88, 89). Acute AngII stimulation of AT1 receptor increases NO production via eNOS phosphorylation (63, 90–92) and eNOS gene transfer inhibits VSMC hypertrophy induced by AngII (63). However, AngII infusion or endothelial NADPH oxidase-derived H2O2 induced by AngII also causes eNOS uncoupling and superoxide production (90, 93, 94). AngII also activates poly (ADP-ribose) polymerase (PARP), resulting in a decrease in intracellular NAD+, ATP levels and EC dysfunction (95). Deficiency of p47 phox in mice ablates enhanced medial thickness of the aorta induced by AngII infusion (96). AngII-induced hypertension and vascular remodeling are exaggerated in superoxide dismutase 1 (SOD1) knockout mice, whereas these responses are reduced in SOD1 transgenic mice (97). In contrast, AngII-induced hypertension is unaltered in NOX2 deficient mice, while vascular remodeling is attenuated in cerebral arterioles (98). These results suggest that AT1 receptor mediates endothelial dysfunction and vascular remodeling by vascular ROS production which likely includes peroxynitrite generation.
6. AT1 receptor interacting proteins
β-arrestin, initially discovered to mediate desensitization and subsequent uncoupling of activated AT1 receptor with associated G proteins, also serves as a signaling system. GPCR kinases (GRK) phosphorylate activated GPCRs, enabling β-arrestin to bind to the receptor, terminate further G protein-mediated signaling and target the receptor for internalization (99). Binding of β-arrestin 2 to the AT1 receptor is essential for ERK1/2 and Akt activation stimulating protein synthesis through Akt-mTOR-p70/85S6K and ERK1/2-p90RSK pathways in VSMCs (100, 101). In addition, mechanical stretch facilitates β-arrestin 2-biased pro-survival signaling through AT1 receptor mediated EGFR transactivation in AngII- or G protein-independent manner in cardiac myocytes (102). Thus, β-arrestin-biased AT1 receptor agonists have been created in attempts to treat heart failure (103–105). There are conflicting studies suggesting both benefit and harm of β-arrestin signals in cardiovascular hypertrophy or heart failure. It appears that β-arrestin 2 inhibition and/or β-arrestin 1 stimulation might be desirable for the treatment of VSMC hypertrophy, hyperplasia and atherosclerosis, contrary to cardiac hypertrophy and heart failure, for which β-arrestin 2 stimulation appears to be a potential therapeutic strategy. It is also important to note the role of β-arrestin 1 in mediating ERK1/2-dependent aldosterone production and secretion induced by adrenal AT1 receptor stimulation (106). Inhibition of adrenal β-arrestin 1 may be beneficial in heart failure. This mechanism may also explain aldosterone escape seen in certain patients treated with AT1 receptor blockers (107). Currently utilized AT1 receptor blockers have been classified as dual G protein/β-arrestin 1 inhibitors or G protein selective inhibitors (108).
AT1 receptor forms heterodimer with other GPCRs (α1D adrenergic receptor, β1 adrenergic receptor, β2 adrenergic receptor, bradykinin receptor B2, dopamin receptor D1, prostaglandin F receptor, and P2Y purinergic receptor 6) (109–114) in addition to other receptors including the lectin-like oxidized low density lipoprotein receptor oxLDL receptor (115) and EGFR (116). An altered interaction between AT1 receptor and these receptors is suggested to affect physiological/pathophysiological conditions such as vasoconstriction, hypertension, atherosclerosis or impaired sodium excretion.
The AT1 receptor also interacts directly with various other proteins (2, 6). The C-terminal cytoplasmic domain of AT1 receptor is an important feature of AT1 receptor structure and regulation, known to interact with JAK2 and PLCγ1 (117, 118). AT1 interaction is necessary for AngII-induced JAK2 activation. Both JAK2 and PLCγ1 share the YIPP motif binding site at the C terminus of the AT1 receptor. As mentioned, this JAK2 activation contributes to AngII-induced vasoconstriction (77, 78). Except for contribution to inositol 1,4,5-trisphosphate production, functional significance of PLCγ1 activation by AngII remains obscure (117, 118). ATRAP, a three-transmembrane protein, binds to the C-terminal cytoplasmic domain, regulating AT1 receptor internalization in VSMCs (119) and cardiac myocytes (120) in addition to negatively modulating AT1 receptor-induced signal transduction (121, 122). ATRAP attenuates AT1 receptor-mediated vascular senescence via calcineurin/Nuclear factor of activated T-cells (NFAT) pathway (123). ATRAP transgenic mice did not reveal a significant phenotype but neo-intimal formation induced by vascular injury was inhibited and ERK, STAT1 and STAT3 activity was attenuated (124). Similarly, in cardiac specific ATRAP transgenic mice, cardiac hypertrophy induced by AngII infusion is attenuated (125). ARAP1 also binds to AT1 receptor and regulates AT1 receptor recycling to the plasma membrane (126). Proximal tubules-specific ARAP1 transgenic mice show hypertension and kidney hypertrophy through enhancement of AT1 signaling (127). Overexpression of GLP, a cytosolic protein, causes hypertrophy in VSMCs and renal proximal tubular cells via, at least in part, activation of Akt and inhibition of p28kip1 protein expression (128).
Gamma-aminobutyric acid (GABA) receptor-associated protein (GABARAP), a protein involved in the trafficking of intracellular GABA(A) receptor through microtubule networks, interacts with C-terminal domain of AT1 receptor and enhances the trafficking of AT1 receptor to the plasma membrane (129). Tubulin directly binds to the AT1 receptor, regulating AT1 receptor trafficking from the ER to the cell surface (130). AT1 receptor also directly binds to filamin A, an actin cross-linking protein, with agonist activation of the AT1 receptor promoting filamin phosphorylation, suggestive of a direct role of AT1 receptor in actin remodeling mediated by filamin (131). β-COP (Coatomer subunit β), a component of Coat Protein I (COPI) transport vesicles involved in the transport between different Golgi stacks and transport from the Golgi to the ER, interacts with AT1 receptor on Lys308 and regulates AT1 receptor export trafficking to the cell surface (132). Taken together, AT1 receptor binds to various interacting proteins through C-terminal domain and facilitates diverse signaling including AT1 receptor trafficking and cell surface expression (Table 1).
Table 1.
Interactions between AT1 receptor and other proteins.
| General function | ATI related function | Ref | ||
|---|---|---|---|---|
| GPCRs | α1D adrenergic receptor | growth/proliferation | promotes preeclampsia | (112) |
| β1 adrenergic receptor | increase cardiac output | enhance AT1 signaling | (110) | |
| β2 adrenergic receptor | vasodilation | enhance AT1 signaling | (110) | |
| Bradykinin receptor B2 | vasodilation | enhance activation of Gq and Gi | (109) | |
| Dopamine receptor D1 | natriuresis, vasorelaxation | renal vascular resistance/sodium transport | (111) | |
| Prostaglandin F receptor | vasoconstriction | enhance vasoconstriction | (114) | |
| P2Y purinergic receptor 6 | vasocontraction | mediate vascular remodeling | (113) | |
| Other receptors | EGFR | growth/proliferation | mediate vascular remodeling | (116) |
| OxLDL receptor | atherosclerosis formation | AT1 activation | (115) | |
| Other proteins | ARAP1 | unknown | AT1 trafficking to cell surface | (126) |
| ATRAP | unknown | AT1 internalization and inhibition | (119) | |
| β-arrestin | receptor desensitization | biased agonism | (101) | |
| β-COP | intraGolgi transport | AT1 trafficking to cell surface | (132) | |
| Filamin A | anchoring membrane proteins | AT1 cytoskeleton coupling | (131) | |
| GABARAP | GABA(A) receptor trafficking | AT1 trafficking to cell surface | (129) | |
| GLP | unknown | stimulate hypertrophy | (128) | |
| Tublin | microtubule dynamics | AT1 trafficking to cell surface | (130) |
AT1 receptor binds to various intracellular proteins through its C-terminal domain and facilitates diverse signaling.
7. Cascades of Wnt, Notch, Hippo and mitochondria
In addition, there is functional crosstalk between AT1 signaling pathway and other signaling pathways. AngII upregulates receptor activator of nuclear factor-κB (RANKL) system in VSMCs, with AT1 receptor blockade attenuating RANKL expression and vascular calcification (133). Wnt/β-catenin pathway has an important role in embryonic development, tissue regeneration, cell proliferation and migration. AngII-induced β-catenin signaling pathway activation was suppressed by a nuclear orphan receptor, Nur77. Nur77 negatively regulates AngII-induced VSMC proliferation and migration by promoting β-catenin degradation and inhibition of its transcriptional activity (134). Animal models suggest β-catenin is required for adaptive cardiac remodeling by AngII infusion (135). A pro-growth factor, Wnt1 inducible signaling pathway protein 1 (WISP1), is a target of TCF/LEF (T-cell factor/lymphoid enhancer factor) and promotes cardiac hypertrophy. AT1 receptor physical association with Nox2 is further enhanced following AngII stimulation, mediating WISP1 induction and cardiomyocyte hypertrophy (136). Similarly, Wnt/β-catenin pathway is suggested to be involved in AngII-induced renal injury and renal fibrosis (137, 138). AngII enhances Wnt1 expression, β-catenin nuclear translocation in mouse podocytes, with inhibition of Wnt/β-catenin pathway attenuating podocyte injury (139). β-catenin destabilization reagent also inhibits AngII-induced β-catenin, collagen I, fibronectin and osteopontin in mouse collecting duct cell or kidney of renovascular hypertensive rat (140, 141). Thus, there is accumulating evidence indicating a close relationship between the Wnt/β-catenin pathway and AT1 receptor in regard to cardiovascular remodeling and chronic kidney diseases.
Notch signaling pathway, a regulator of cell fate in the developing heart, is also implicated in cardiovascular pathophysiology (142). AT1 receptor stimulates Notch signaling pathway through an increase of γ-secretase enzymatic activity, mediating VSMCs proliferation and migration (143). Notch inhibition or γ-secretase complex silencing in mice attenuates hypertension induced by AngII (144, 145). Notch3 −/− mice show attenuated renal vascular constriction, vessel wall thickening and hypertension induced by AngII. In contrast, Notch3 −/− mice show enhanced cardiac hypertrophy, tubular dilation or fibrosis in kidney, and greater mortality due to heart failure induced by AngII. This is suggestive of a role of Notch3 in end organ adaptation in hypertension (146). In addition, activation of Notch1 signaling is observed in AngII-induced abdominal aortic aneurysm (AAA). AAA formation induced by AngII is attenuated by Notch1 haploinsufficiency via modulation of macrophage infiltration or inflammatory activation (147). AngII-induced AAA formation and vascular inflammation is also attenuated by pharmacological inhibition of Notch signaling (148).
Hippo signaling pathway is a complex signaling network, regulating cell proliferation and apoptosis to control organ size, with recent studies revealing GPCRs serve as upstream regulators of the Hippo pathway (149). AngII binding to AT1 receptor inhibits Hippo signaling by decreasing the activity of the large tumor suppressor kinase (LATS), leading to nuclear translocation of Yes-associated protein (YAP) in HEK293T cells. In contrast, AngII does not affect Hippo pathway activity in podocytes (150). Altogether, recent studies reveal various crosstalk between the AT1 receptor signaling pathways and other signaling pathways (Figure 3). Although not all of functional significance has been elucidated, they show new roles for AngII-mediated signaling mechanisms and cardiovascular pathophysiology.
Figure 3. The new and complex mechanisms of AT1 receptor-mediated signaling in cardiovascular pathophysiology.
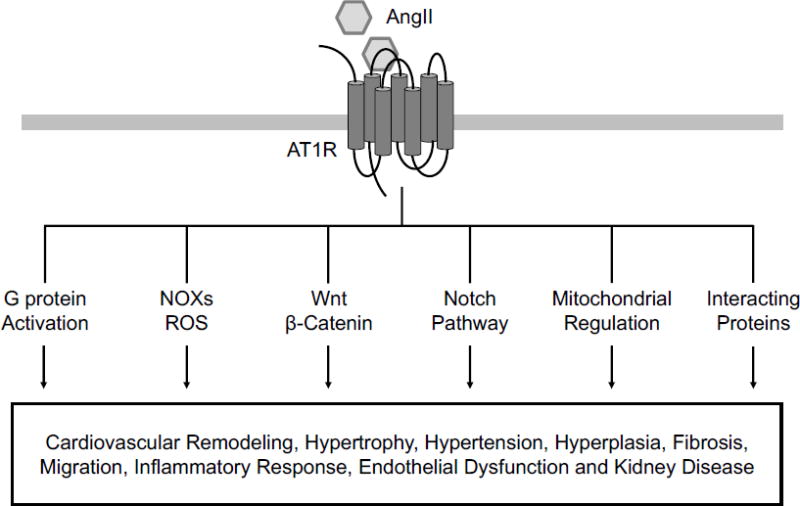
Traditionally AT1 receptor is known to cause cardiovascular remodeling, hypertension and end organ damage via a few cascades including Gq/Ca2+-PKC, ERK/MAPK and NOX/ROS. Although not all the functional significances have been identified, recent studies explored new signaling mechanisms by which the AT1 receptor may contribute to cardiovascular disorders, including Wnt, Notch, mitochondrial regulation and AT1 receptor interacting proteins. Elucidating the complexity of AT1 receptor signaling seems to be on the forefront of RAAS research to conquer cardiovascular disorders.
AngII has been shown to induce mitochondrial dysfunction leading to mitochondrial ROS generation which modulates various AngII responses including experimental hypertension (151). Mitochondrial ROS production induced by AngII appears to require NADH/NADPH oxidase such as NOX2 in ECs (152). Pharmacological and genetic inhibition of mitochondrial ROS (152, 153) are effective in reducing AngII-induced hypertension and vascular dysfunction in rodents. AngII-induced hypertension and vascular dysfunction also involve mitochondrial ROS-dependent activation of the L-type Ca2+ channel in VSMCs (154). In addition, AngII induces mitochondrial fragmentation via dynamin-related protein 1 (Drp1) phosphorylation in VSMCs and neuroblastoma cells (155, 156). siRNA silencing of Drp1 attenuates AngII-induced ERK activation and matrix metalloproteinase 2/9 induction in VSMCs (155). Mitofusion 2 (MFN2), another GTPase, controls mitochondrial fusion. MFN2 over-expression attenuates AngII-induced cardiac myocyte hypertrophy in vitro and in vivo (157). These data suggest a presence of AngII-mediated mitochondrial signal transduction in regulating cardiovascular pathophysiology.
8. Tissue-specific roles of AT1 receptor and transcriptional factors
Recently, tissue specific knockouts of the AT1 receptor has enabled researchers to disassociate the effects of AT1 receptor signaling in a variety of tissues. AT1 receptor in VSMCs is essential for AngII-mediated regulation of renal blood flow, and mice with VSMC specific AT1 receptor depletion show increased urinary sodium excretion and attenuated AngII-induced high blood pressure (158). Mice with principal cell specific AT1 receptor depletion show enhanced natriuresis and a modest decrease in blood pressure in the initial phase of AngII-dependent hypertension (159). AT1 receptor depletion in VSMCs or ECs did not affect AngII-induced medial thickening, AAA formation and atherosclerosis (160, 161), however depletion of AT1 receptor in ECs attenuates thoracic aortic aneurysm (TAA) formation (162). Depletion in fibroblasts attenuated AngII-induced medial hyperplasia in the ascending aorta (161). These recent studies suggest the importance of tissue specific AT1 receptor signaling on hypertension and vascular remodeling.
Hypoxia-inducible factor 1 alpha (HIF-1α) and peroxisome proliferator-activated receptor gamma (PPARγ) are also considered to regulate AngII functions in tissue/cell type specifically. We have shown that AngII upregulates HIF-1α expression and induces ADAM17 expression in VSMCs via transcriptional up-regulation (163). In addition, reduced medial wall thickening and hypertension induced by AngII is observed in VSMC specific HIF-1α knockout mice (164). However, VSMCs specific HIF-1α knockout mice are also reported to show increased AT1 receptor expression in vasculature and elevated blood pressure with downregulated PPARγ (165). It has been demonstrated that vascular smooth muscle-specific overexpression of a dominant negative human PPARγ mutation in mice (P467L) leads to increased angiotensin-II-dependent vasoconstriction (166) and enhanced VSMC ERK activation (167). Thus, PPARγ agonist inhibits AngII-induced PKCζ activation, ERK1/2 activation, Krueppel-like factor 5 expression and VSMC proliferation (168). In addition, enhanced AngII-induced vascular remodeling, contractility, inflammation and endothelial dysfunction are observed in inducible vascular smooth muscle-specific PPARγ deficient mice, which seems to involve oxidative stress due to decreased expression of SOD3 (169). Interestingly, it was also shown that endothelial-specific expression of dominant negative PPARγ (V290M) exhibited endothelial dysfunction and an augmented pressor response to AngII (170). The enhancement of AngII-induced endothelial dysfunction in this mouse is associated with enhanced oxidative stress and decline in antioxidant genes (catalase and SOD3) in carotid arteries (171). The above findings illustrate the complex relations among the AT1 receptor, HIF-1α and PPARγ in mediating hypertension, vascular remodeling and endothelial dysfunction.
9. Conclusions and future directions
An overview of AT1 receptor signaling and recent findings in cardiovascular physiology are summarized in this review. AT1 receptor signal transduction pathways are a central cascade in RAAS, and emerging evidence reveals the complexity of AT1 receptor signaling, including crosstalk with other signaling cascades in addition to direct interaction with other receptors and proteins. AT1 receptor facilitates various intracellular signaling pathways thus contributing to vascular remodeling, endothelial dysfunction, cardiovascular diseases, atherosclerosis and end organ damage. Therefore, elucidating the complete picture of AT1 signaling is beneficial to control hypertension and cardiovascular diseases. Genomic and proteomic approaches in coordinance with system biology will help further understand the mechanism by which AT1 receptor mediates cardiovascular dysfunctions. In addition, since most of the findings reviewed here are based on cell/animal models, the need to expand research in human samples and translational research will be important to control cardiovascular diseases in humans.
Acknowledgments
Supported by National Institute of Health grants HL128324 (SE and V.R.), HL133248 (SE), and F31HL127971 (S.J.F.) and American Heart Association grants 13GRNT17060036 (SE), 16GRNT30410007 (SE), 16GRNT30130013 (V.R.) and 16POST3051004 (T.K.).
Abbreviations
- AAA
abdominal aortic aneurysm
- ADAM17
a disintegrin and metalloproteinase 17
- ANO1
anoctamin-1
- ARAP1
type 1 angiotensin II receptor-associated protein
- ATRAP
AT1 receptor associated protein
- AngII
angiotensin II
- AT1 receptor
angiotensin II type 1 receptor
- AT2 receptor
angiotensin II type 2 receptor
- β-COP
Coatomer subunit β
- BMX
bone marrow kinase
- CHKA
choline kinase alpha
- COPI
Coat Protein I
- Drp1
dynamin-related protein 1
- EC
endothelial cells
- EGF
epidermal growth factor
- EGFR
epidermal growth factor receptor
- eIF4E
eukaryotic translation initiation factor 4E
- eNOS
endothelial nitric oxide synthase
- ER
endoplasmic reticulum
- ERK
extracellular signal regulated kinase
- GABA
Gamma-aminobutyric acid
- GABARAP
Gamma-aminobutyric acid receptor-associated protein
- GEF
Guanine nucleotide exchange factor
- GLP
GEF-like protein
- GPCR
G protein-coupled receptor
- GRK
G protein-coupled receptor kinase
- HB-EGF
Heparin-binding EGF-like growth factor
- HIF-1α
hypoxia-inducible factor 1 alpha
- IGF-1R
insulin-like growth factor-1 receptor
- JAK2
Janus kinase 2
- JNK
c-Jun N terminal kinase
- LATS
large tumor suppressor kinase
- MAPK
mitogen-activated protein kinase
- MEK
MAPK/ERK kinase
- MCP-1
monocyte chemoattractant protein-1
- MFN2
Mitofusion 2
- MLCK
myosin light chain kinase
- MLCP
myosin light chain phosphatase
- mTOR
mechanistic target of rapamycin
- NADPH
nicotinamide adenine dinucleotide phosphate
- NF-kB
nuclear factor kappa B
- NFAT
Nuclear factor of activated T-cells
- NKCC1
Na-K-Cl cotransporter isoform 1
- NLRP3
NLR family pyrin domain containing 3
- NO
nitric oxide
- oxLDL
oxidized low density lipoprotein
- PAI-1
plasminogen activator inhibitor-1
- PAK1
p21-activated kinase 1
- PARP
poly (ADP-ribose) polymerase
- PDGFR
platelet-derived growth factor receptor
- PI3K
phosphatidylinositol 3-kinase
- PKA
protein kinase A
- PKB
protein kinase B
- PKC
protein kinase C
- PLC
phospholipase C
- PPARγ
peroxisome proliferator-activated receptor gamma
- RAAS
renin-angiotensin-aldosterone system
- RANKL
receptor activator of nuclear factor-kappa B
- RAP1b
Ras-related protein
- ROCK
Rho-associated protein kinase
- ROS
reactive oxygen species
- SOD
superoxide dismutase
- SPAK
Ste20/SPS1-related proline/alanine-rich kinase
- TAA
thoracic aortic aneurysm
- TCF/LEF
(T-cell factor/lymphoid enhancer factor)
- TGFβ1
transforming growth factor beta 1
- TRIO
triple functional domain (PTPRF interacting)
- VSMC
vascular smooth muscle cells
- WISP1
Wnt1 inducible signaling pathway protein 1
- WNK
with-no-lysine
- YAP
Yes-associated protein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors are unaware of any affiliations, memberships, or financial holdings that might be perceived as affecting the objectivity of this review.
References
- 1.Karnik SS, Unal H, Kemp JR, Tirupula KC, Eguchi S, Vanderheyden PML, Thomas WG. International Union of Basic and Clinical Pharmacology. XCIX. Angiotensin Receptors: Interpreters of Pathophysiological Angiotensinergic Stimuli. Pharmacological Reviews. 2015;67(4):754–819. doi: 10.1124/pr.114.010454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Higuchi S, Ohtsu H, Suzuki H, Shirai H, Frank GD, Eguchi S. Angiotensin II signal transduction through the AT1 receptor: novel insights into mechanisms and pathophysiology. Clin Sci (Lond) 2007;112(8):417–428. doi: 10.1042/CS20060342. [DOI] [PubMed] [Google Scholar]
- 3.Garrido AM, Griendling KK. NADPH oxidases and angiotensin II receptor signaling. Mol Cell Endocrinol. 2009;302(2):148–158. doi: 10.1016/j.mce.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nguyen Dinh Cat A, Montezano AC, Burger D, Touyz RM. Angiotensin II, NADPH oxidase, and redox signaling in the vasculature. Antioxid Redox Signal. 2013;19(10):1110–1120. doi: 10.1089/ars.2012.4641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Forrester SJ, Kawai T, O’Brien S, Thomas W, Harris RC, Eguchi S. Epidermal Growth Factor Receptor Transactivation: Mechanisms, Pathophysiology, and Potential Therapies in the Cardiovascular System. Annu Rev Pharmacol Toxicol. 2016;56:627–653. doi: 10.1146/annurev-pharmtox-070115-095427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nouet S, Amzallag N, Li J-M, Louis S, Seitz I, Cui T-X, Alleaume A-M, Di Benedetto M, Boden C, Masson M, Strosberg AD, Horiuchi M, Couraud P-O, Nahmias C. Trans-inactivation of Receptor Tyrosine Kinases by Novel Angiotensin II AT2 Receptor-interacting Protein, ATIP. Journal of Biological Chemistry. 2004;279(28):28989–28997. doi: 10.1074/jbc.M403880200. [DOI] [PubMed] [Google Scholar]
- 7.Mogi M, Iwai M, Horiuchi M. Emerging concepts of regulation of angiotensin II receptors: new players and targets for traditional receptors. Arterioscler Thromb Vasc Biol. 2007;27(12):2532–2539. doi: 10.1161/ATVBAHA.107.144154. [DOI] [PubMed] [Google Scholar]
- 8.Padia SH, Carey RM. AT2 receptors: beneficial counter-regulatory role in cardiovascular and renal function. Pflugers Arch. 2013;465(1):99–110. doi: 10.1007/s00424-012-1146-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matavelli LC, Siragy HM. AT2 receptor activities and pathophysiological implications. J Cardiovasc Pharmacol. 2015;65(3):226–232. doi: 10.1097/FJC.0000000000000208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sumners C, de Kloet AD, Krause EG, Unger T, Steckelings UM. Angiotensin type 2 receptors: blood pressure regulation and end organ damage. Curr Opin Pharmacol. 2015;21:115–121. doi: 10.1016/j.coph.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsutsumi Y, Matsubara H, Masaki H, Kurihara H, Murasawa S, Takai S, Miyazaki M, Nozawa Y, Ozono R, Nakagawa K, Miwa T, Kawada N, Mori Y, Shibasaki Y, Tanaka Y, Fujiyama S, Koyama Y, Fujiyama A, Takahashi H, Iwasaka T. Angiotensin II type 2 receptor overexpression activates the vascular kinin system and causes vasodilation. Journal of Clinical Investigation. 1999;104(7):925–935. doi: 10.1172/JCI7886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kemp BA, Howell NL, Gildea JJ, Keller SR, Padia SH, Carey RM. AT(2) receptor activation induces natriuresis and lowers blood pressure. Circ Res. 2014;115(3):388–399. doi: 10.1161/CIRCRESAHA.115.304110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kemp BA, Howell NL, Keller SR, Gildea JJ, Padia SH, Carey RM. AT2 Receptor Activation Prevents Sodium Retention and Reduces Blood Pressure in Angiotensin II-Dependent Hypertension. Circ Res. 2016;119(4):532–543. doi: 10.1161/CIRCRESAHA.116.308384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kurisu S, Ozono R, Oshima T, Kambe M, Ishida T, Sugino H, Matsuura H, Chayama K, Teranishi Y, Iba O, Amano K, Matsubara H. Cardiac Angiotensin II Type 2 Receptor Activates the Kinin/NO System and Inhibits Fibrosis. Hypertension. 2003;41(1):99–107. doi: 10.1161/01.hyp.0000050101.90932.14. [DOI] [PubMed] [Google Scholar]
- 15.You D, Loufrani L, Baron C, Levy BI, Widdop RE, Henrion D. High blood pressure reduction reverses angiotensin II type 2 receptor-mediated vasoconstriction into vasodilation in spontaneously hypertensive rats. Circulation. 2005;111(8):1006–1011. doi: 10.1161/01.CIR.0000156503.62815.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yan X, Price RL, Nakayama M, Ito K, Schuldt AJT, Manning WJ, Sanbe A, Borg TK, Robbins J, Lorell BH. Ventricular-specific expression of angiotensin II type 2 receptors causes dilated cardiomyopathy and heart failure in transgenic mice. American Journal of Physiology - Heart and Circulatory Physiology. 2003;285(5):H2179–H2187. doi: 10.1152/ajpheart.00361.2003. [DOI] [PubMed] [Google Scholar]
- 17.Xu J, Sun Y, Carretero OA, Zhu L, Harding P, Shesely EG, Dai X, Rhaleb NE, Peterson E, Yang XP. Effects of cardiac overexpression of the angiotensin II type 2 receptor on remodeling and dysfunction in mice post-myocardial infarction. Hypertension. 2014;63(6):1251–1259. doi: 10.1161/HYPERTENSIONAHA.114.03247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chow BS, Allen TJ. Angiotensin II type 2 receptor (AT2R) in renal and cardiovascular disease. Clin Sci (Lond) 2016;130(15):1307–1326. doi: 10.1042/CS20160243. [DOI] [PubMed] [Google Scholar]
- 19.Ferrario CM. New physiological concepts of the renin-angiotensin system from the investigation of precursors and products of angiotensin I metabolism. Hypertension. 2010;55(2):445–452. doi: 10.1161/HYPERTENSIONAHA.109.145839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferreira AJ, Santos RA, Bradford CN, Mecca AP, Sumners C, Katovich MJ, Raizada MK. Therapeutic implications of the vasoprotective axis of the renin-angiotensin system in cardiovascular diseases. Hypertension. 2010;55(2):207–213. doi: 10.1161/HYPERTENSIONAHA.109.140145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chappell MC. Emerging Evidence for a Functional Angiotensin-Converting Enzyme 2-Angiotensin-(1–7)-Mas Receptor Axis: More Than Regulation of Blood Pressure? Hypertension. 2007;50(4):596–599. doi: 10.1161/HYPERTENSIONAHA.106.076216. [DOI] [PubMed] [Google Scholar]
- 22.Grobe JL, Mecca AP, Lingis M, Shenoy V, Bolton TA, Machado JM, Speth RC, Raizada MK, Katovich MJ. Prevention of angiotensin II-induced cardiac remodeling by angiotensin-(1–7) Am J Physiol Heart Circ Physiol. 2007;292(2):H736–742. doi: 10.1152/ajpheart.00937.2006. [DOI] [PubMed] [Google Scholar]
- 23.de Gasparo M, Catt KJ, Inagami T, Wright JW, Unger T. International union of pharmacology. XXIII. The angiotensin II receptors. Pharmacol Rev. 2000;52(3):415–472. [PubMed] [Google Scholar]
- 24.Coble JP, Grobe JL, Johnson AK, Sigmund CD. Mechanisms of brain renin angiotensin system-induced drinking and blood pressure: importance of the subfornical organ. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology. 2015;308(4):R238–R249. doi: 10.1152/ajpregu.00486.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Satou R, Shao W, Navar LG. Role of stimulated intrarenal angiotensinogen in hypertension. Ther Adv Cardiovasc Dis. 2015;9(4):181–190. doi: 10.1177/1753944715585512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sparks MA, Crowley SD, Gurley SB, Mirotsou M, Coffman TM. Classical Renin-Angiotensin system in kidney physiology. Compr Physiol. 2014;4(3):1201–1228. doi: 10.1002/cphy.c130040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miura S, Zhang J, Boros J, Karnik SS. TM2-TM7 interaction in coupling movement of transmembrane helices to activation of the angiotensin II type-1 receptor. J Biol Chem. 2003;278(6):3720–3725. doi: 10.1074/jbc.M211338200. [DOI] [PubMed] [Google Scholar]
- 28.Mehta PK, Griendling KK. Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system. American Journal of Physiology - Cell Physiology. 2007;292(1):C82–C97. doi: 10.1152/ajpcell.00287.2006. [DOI] [PubMed] [Google Scholar]
- 29.Nguyen Dinh Cat A, Touyz RM. Cell signaling of angiotensin II on vascular tone: novel mechanisms. Curr Hypertens Rep. 2011;13(2):122–128. doi: 10.1007/s11906-011-0187-x. [DOI] [PubMed] [Google Scholar]
- 30.Qin B, Zhou J. Src Family Kinases (SFK) Mediate Angiotensin II-Induced Myosin Light Chain Phosphorylation and Hypertension. PLoS One. 2015;10(5):e0127891. doi: 10.1371/journal.pone.0127891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Callera GE, Antunes TT, He Y, Montezano AC, Yogi A, Savoia C, Touyz RM. c-Src Inhibition Improves Cardiovascular Function but not Remodeling or Fibrosis in Angiotensin II-Induced Hypertension. Hypertension. 2016 doi: 10.1161/HYPERTENSIONAHA.116.07699. [DOI] [PubMed] [Google Scholar]
- 32.Hilgers RH, Todd J, Jr, Webb RC. Increased PDZ-RhoGEF/RhoA/Rho kinase signaling in small mesenteric arteries of angiotensin II-induced hypertensive rats. J Hypertens. 2007;25(8):1687–1697. doi: 10.1097/HJH.0b013e32816f778d. [DOI] [PubMed] [Google Scholar]
- 33.Lakshmikanthan S, Zieba BJ, Ge ZD, Momotani K, Zheng X, Lund H, Artamonov MV, Maas JE, Szabo A, Zhang DX, Auchampach JA, Mattson DL, Somlyo AV, Chrzanowska-Wodnicka M. Rap1b in smooth muscle and endothelium is required for maintenance of vascular tone and normal blood pressure. Arterioscler Thromb Vasc Biol. 2014;34(7):1486–1494. doi: 10.1161/ATVBAHA.114.303678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zeniya M, Sohara E, Kita S, Iwamoto T, Susa K, Mori T, Oi K, Chiga M, Takahashi D, Yang S-S, Lin S-H, Rai T, Sasaki S, Uchida S. Dietary Salt Intake Regulates WNK3– SPAK–NKCC1 Phosphorylation Cascade in Mouse Aorta Through Angiotensin II. Hypertension. 2013;62(5):872–878. doi: 10.1161/HYPERTENSIONAHA.113.01543. [DOI] [PubMed] [Google Scholar]
- 35.Wang B, Li C, Huai R, Qu Z. Overexpression of ANO1/TMEM16A, an arterial Ca2+-activated Cl- channel, contributes to spontaneous hypertension. J Mol Cell Cardiol. 2015;82:22–32. doi: 10.1016/j.yjmcc.2015.02.020. [DOI] [PubMed] [Google Scholar]
- 36.Heinze C, Seniuk A, Sokolov MV, Huebner AK, Klementowicz AE, Szijarto IA, Schleifenbaum J, Vitzthum H, Gollasch M, Ehmke H, Schroeder BC, Hubner CA. Disruption of vascular Ca2+-activated chloride currents lowers blood pressure. J Clin Invest. 2014;124(2):675–686. doi: 10.1172/JCI70025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Griendling KK, Ushio-Fukai M, Lassegue B, Alexander RW. Angiotensin II signaling in vascular smooth muscle. New concepts. Hypertension. 1997;29(1 Pt 2):366–373. doi: 10.1161/01.hyp.29.1.366. [DOI] [PubMed] [Google Scholar]
- 38.Eguchi S, Frank GD, Mifune M, Inagami T. Metalloprotease-dependent ErbB ligand shedding in mediating EGFR transactivation and vascular remodelling. Biochem Soc Trans. 2003;31(Pt 6):1198–1202. doi: 10.1042/bst0311198. [DOI] [PubMed] [Google Scholar]
- 39.Yin G, Yan C, Berk BC. Angiotensin II signaling pathways mediated by tyrosine kinases. Int J Biochem Cell Biol. 2003;35(6):780–783. doi: 10.1016/s1357-2725(02)00300-x. [DOI] [PubMed] [Google Scholar]
- 40.Suzuki H, Motley ED, Frank GD, Utsunomiya H, Eguchi S. Recent progress in signal transduction research of the angiotensin II type-1 receptor: protein kinases, vascular dysfunction and structural requirement. Curr Med Chem Cardiovasc Hematol Agents. 2005;3(4):305–322. doi: 10.2174/156801605774322355. [DOI] [PubMed] [Google Scholar]
- 41.Eguchi S, Numaguchi K, Iwasaki H, Matsumoto T, Yamakawa T, Utsunomiya H, Motley ED, Kawakatsu H, Owada KM, Hirata Y, Marumo F, Inagami T. Calcium-dependent epidermal growth factor receptor transactivation mediates the angiotensin II-induced mitogen-activated protein kinase activation in vascular smooth muscle cells. J Biol Chem. 1998;273(15):8890–8896. doi: 10.1074/jbc.273.15.8890. [DOI] [PubMed] [Google Scholar]
- 42.Eguchi S, Dempsey PJ, Frank GD, Motley ED, Inagami T. Activation of MAPKs by angiotensin II in vascular smooth muscle cells. Metalloprotease-dependent EGF receptor activation is required for activation of ERK and p38 MAPK but not for JNK. J Biol Chem. 2001;276(11):7957–7962. doi: 10.1074/jbc.M008570200. [DOI] [PubMed] [Google Scholar]
- 43.Seshiah PN, Weber DS, Rocic P, Valppu L, Taniyama Y, Griendling KK. Angiotensin II stimulation of NAD(P)H oxidase activity: upstream mediators. Circ Res. 2002;91(5):406–413. doi: 10.1161/01.res.0000033523.08033.16. [DOI] [PubMed] [Google Scholar]
- 44.Touyz RM, Wu XH, He G, Salomon S, Schiffrin EL. Increased angiotensin II-mediated Src signaling via epidermal growth factor receptor transactivation is associated with decreased C-terminal Src kinase activity in vascular smooth muscle cells from spontaneously hypertensive rats. Hypertension. 2002;39(2 Pt 2):479–485. doi: 10.1161/hy02t2.102909. [DOI] [PubMed] [Google Scholar]
- 45.Ohtsu H, Higuchi S, Shirai H, Eguchi K, Suzuki H, Hinoki A, Brailoiu E, Eckhart AD, Frank GD, Eguchi S. Central role of Gq in the hypertrophic signal transduction of angiotensin II in vascular smooth muscle cells. Endocrinology. 2008;149(7):3569–3575. doi: 10.1210/en.2007-1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhai P, Galeotti J, Liu J, Holle E, Yu X, Wagner T, Sadoshima J. An angiotensin II type 1 receptor mutant lacking epidermal growth factor receptor transactivation does not induce angiotensin II-mediated cardiac hypertrophy. Circ Res. 2006;99(5):528–536. doi: 10.1161/01.RES.0000240147.49390.61. [DOI] [PubMed] [Google Scholar]
- 47.Lautrette A, Li S, Alili R, Sunnarborg SW, Burtin M, Lee DC, Friedlander G, Terzi F. Angiotensin II and EGF receptor cross-talk in chronic kidney diseases: a new therapeutic approach. Nat Med. 2005;11(8):867–874. doi: 10.1038/nm1275. [DOI] [PubMed] [Google Scholar]
- 48.Miura S, Zhang J, Matsuo Y, Saku K, Karnik SS. Activation of extracellular signal-activated kinase by angiotensin II-induced Gq-independent epidermal growth factor receptor transactivation. Hypertens Res. 2004;27(10):765–770. doi: 10.1291/hypres.27.765. [DOI] [PubMed] [Google Scholar]
- 49.Feng YH, Ding Y, Ren S, Zhou L, Xu C, Karnik SS. Unconventional homologous internalization of the angiotensin II type-1 receptor induced by G-protein-independent signals. Hypertension. 2005;46(2):419–425. doi: 10.1161/01.HYP.0000172621.68061.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mifune M, Ohtsu H, Suzuki H, Nakashima H, Brailoiu E, Dun NJ, Frank GD, Inagami T, Higashiyama S, Thomas WG, Eckhart AD, Dempsey PJ, Eguchi S. G protein coupling and second messenger generation are indispensable for metalloprotease-dependent, heparin-binding epidermal growth factor shedding through angiotensin II type-1 receptor. J Biol Chem. 2005;280(28):26592–26599. doi: 10.1074/jbc.M502906200. [DOI] [PubMed] [Google Scholar]
- 51.Ohtsu H, Dempsey PJ, Frank GD, Brailoiu E, Higuchi S, Suzuki H, Nakashima H, Eguchi K, Eguchi S. ADAM17 mediates epidermal growth factor receptor transactivation and vascular smooth muscle cell hypertrophy induced by angiotensin II. Arterioscler Thromb Vasc Biol. 2006;26(9):e133–137. doi: 10.1161/01.ATV.0000236203.90331.d0. [DOI] [PubMed] [Google Scholar]
- 52.Elliott KJ, Bourne AM, Takayanagi T, Takaguri A, Kobayashi T, Eguchi K, Eguchi S. ADAM17 silencing by adenovirus encoding miRNA-embedded siRNA revealed essential signal transduction by angiotensin II in vascular smooth muscle cells. J Mol Cell Cardiol. 2013;62:1–7. doi: 10.1016/j.yjmcc.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.George AJ, Hannan RD, Thomas WG. Unravelling the molecular complexity of GPCR-mediated EGFR transactivation using functional genomics approaches. FEBS J. 2013;280(21):5258–5268. doi: 10.1111/febs.12509. [DOI] [PubMed] [Google Scholar]
- 54.George AJ, Purdue BW, Gould CM, Thomas DW, Handoko Y, Qian H, Quaife-Ryan GA, Morgan KA, Simpson KJ, Thomas WG, Hannan RD. A functional siRNA screen identifies genes modulating angiotensin II-mediated EGFR transactivation. Journal of Cell Science. 2013;126(23):5377–5390. doi: 10.1242/jcs.128280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Montiel M, de la Blanca EP, Jimenez E. Angiotensin II induces focal adhesion kinase/paxillin phosphorylation and cell migration in human umbilical vein endothelial cells. Biochem Biophys Res Commun. 2005;327(4):971–978. doi: 10.1016/j.bbrc.2004.12.110. [DOI] [PubMed] [Google Scholar]
- 56.Burger D, Montezano AC, Nishigaki N, He Y, Carter A, Touyz RM. Endothelial microparticle formation by angiotensin II is mediated via Ang II receptor type I/NADPH oxidase/Rho kinase pathways targeted to lipid rafts. Arterioscler Thromb Vasc Biol. 2011;31(8):1898–1907. doi: 10.1161/ATVBAHA.110.222703. [DOI] [PubMed] [Google Scholar]
- 57.Takayanagi T, Kawai T, Forrester SJ, Obama T, Tsuji T, Fukuda Y, Elliott KJ, Tilley DG, Davisson RL, Park JY, Eguchi S. Role of epidermal growth factor receptor and endoplasmic reticulum stress in vascular remodeling induced by angiotensin II. Hypertension. 2015;65(6):1349–1355. doi: 10.1161/HYPERTENSIONAHA.115.05344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Takayanagi T, Forrester SJ, Kawai T, Obama T, Tsuji T, Elliott KJ, Nuti E, Rossello A, Kwok HF, Scalia R, Rizzo V, Eguchi S. Vascular ADAM17 as a Novel Therapeutic Target in Mediating Cardiovascular Hypertrophy and Perivascular Fibrosis Induced by Angiotensin II. Hypertension. 2016;68(4):949–955. doi: 10.1161/HYPERTENSIONAHA.116.07620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Heeneman S, Haendeler J, Saito Y, Ishida M, Berk BC. Angiotensin II induces transactivation of two different populations of the platelet-derived growth factor beta receptor. Key role for the p66 adaptor protein Shc. J Biol Chem. 2000;275(21):15926–15932. doi: 10.1074/jbc.M909616199. [DOI] [PubMed] [Google Scholar]
- 60.Kim S, Zhan Y, Izumi Y, Yasumoto H, Yano M, Iwao H. In vivo activation of rat aortic platelet-derived growth factor and epidermal growth factor receptors by angiotensin II and hypertension. Arterioscler Thromb Vasc Biol. 2000;20(12):2539–2545. doi: 10.1161/01.atv.20.12.2539. [DOI] [PubMed] [Google Scholar]
- 61.Mondorf UF, Geiger H, Herrero M, Zeuzem S, Piiper A. Involvement of the platelet-derived growth factor receptor in angiotensin II-induced activation of extracellular regulated kinases 1 and 2 in human mesangial cells. FEBS Lett. 2000;472(1):129–132. doi: 10.1016/s0014-5793(00)01433-2. [DOI] [PubMed] [Google Scholar]
- 62.Schellings MW, Baumann M, van Leeuwen RE, Duisters RF, Janssen SH, Schroen B, Peutz-Kootstra CJ, Heymans S, Pinto YM. Imatinib attenuates end-organ damage in hypertensive homozygous TGR(mRen2)27 rats. Hypertension. 2006;47(3):467–474. doi: 10.1161/01.HYP.0000202487.68969.f7. [DOI] [PubMed] [Google Scholar]
- 63.Suzuki H, Eguchi K, Ohtsu H, Higuchi S, Dhobale S, Frank GD, Motley ED, Eguchi S. Activation of endothelial nitric oxide synthase by the angiotensin II type 1 receptor. Endocrinology. 2006;147(12):5914–5920. doi: 10.1210/en.2006-0834. [DOI] [PubMed] [Google Scholar]
- 64.Du J, Sperling LS, Marrero MB, Phillips L, Delafontaine P. G-protein and tyrosine kinase receptor cross-talk in rat aortic smooth muscle cells: thrombin- and angiotensin II-induced tyrosine phosphorylation of insulin receptor substrate-1 and insulin-like growth factor 1 receptor. Biochem Biophys Res Commun. 1996;218(3):934–939. doi: 10.1006/bbrc.1996.0165. [DOI] [PubMed] [Google Scholar]
- 65.Zahradka P, Litchie B, Storie B, Helwer G. Transactivation of the insulin-like growth factor-I receptor by angiotensin II mediates downstream signaling from the angiotensin II type 1 receptor to phosphatidylinositol 3-kinase. Endocrinology. 2004;145(6):2978–2987. doi: 10.1210/en.2004-0029. [DOI] [PubMed] [Google Scholar]
- 66.Zahradka P, Storie B, Wright B. IGF-1 receptor transactivation mediates Src-dependent cortactin phosphorylation in response to angiotensin II. Can J Physiol Pharmacol. 2009;87(10):805–812. doi: 10.1139/Y09-052. [DOI] [PubMed] [Google Scholar]
- 67.Touyz RM, Cruzado M, Tabet F, Yao G, Salomon S, Schiffrin EL. Redox-dependent MAP kinase signaling by Ang II in vascular smooth muscle cells: role of receptor tyrosine kinase transactivation. Can J Physiol Pharmacol. 2003;81(2):159–167. doi: 10.1139/y02-164. [DOI] [PubMed] [Google Scholar]
- 68.Ohtsu H, Suzuki H, Nakashima H, Dhobale S, Frank GD, Motley ED, Eguchi S. Angiotensin II signal transduction through small GTP-binding proteins: mechanism and significance in vascular smooth muscle cells. Hypertension. 2006;48(4):534–540. doi: 10.1161/01.HYP.0000237975.90870.eb. [DOI] [PubMed] [Google Scholar]
- 69.Yamakawa T, Tanaka S, Numaguchi K, Yamakawa Y, Motley ED, Ichihara S, Inagami T. Involvement of Rho-kinase in angiotensin II-induced hypertrophy of rat vascular smooth muscle cells. Hypertension. 2000;35(1 Pt 2):313–318. doi: 10.1161/01.hyp.35.1.313. [DOI] [PubMed] [Google Scholar]
- 70.Seko T, Ito M, Kureishi Y, Okamoto R, Moriki N, Onishi K, Isaka N, Hartshorne DJ, Nakano T. Activation of RhoA and inhibition of myosin phosphatase as important components in hypertension in vascular smooth muscle. Circ Res. 2003;92(4):411–418. doi: 10.1161/01.RES.0000059987.90200.44. [DOI] [PubMed] [Google Scholar]
- 71.Takefuji M, Wirth A, Lukasova M, Takefuji S, Boettger T, Braun T, Althoff T, Offermanns S, Wettschureck N. G(13)-mediated signaling pathway is required for pressure overload-induced cardiac remodeling and heart failure. Circulation. 2012;126(16):1972–1982. doi: 10.1161/CIRCULATIONAHA.112.109256. [DOI] [PubMed] [Google Scholar]
- 72.Suzuki H, Kimura K, Shirai H, Eguchi K, Higuchi S, Hinoki A, Ishimaru K, Brailoiu E, Dhanasekaran DN, Stemmle LN, Fields TA, Frank GD, Autieri MV, Eguchi S. Endothelial nitric oxide synthase inhibits G12/13 and rho-kinase activated by the angiotensin II type-1 receptor: implication in vascular migration. Arterioscler Thromb Vasc Biol. 2009;29(2):217–224. doi: 10.1161/ATVBAHA.108.181024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Seasholtz TM, Brown JH. RHO SIGNALING in vascular diseases. Mol Interv. 2004;4(6):348–357. doi: 10.1124/mi.4.6.8. [DOI] [PubMed] [Google Scholar]
- 74.Noma K, Oyama N, Liao JK. Physiological role of ROCKs in the cardiovascular system. Am J Physiol Cell Physiol. 2006;290(3):C661–668. doi: 10.1152/ajpcell.00459.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kolluru GK, Majumder S, Chatterjee S. Rho-kinase as a therapeutic target in vascular diseases: striking nitric oxide signaling. Nitric Oxide. 2014;43:45–54. doi: 10.1016/j.niox.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 76.Ohtsu H, Mifune M, Frank GD, Saito S, Inagami T, Kim-Mitsuyama S, Takuwa Y, Sasaki T, Rothstein JD, Suzuki H, Nakashima H, Woolfolk EA, Motley ED, Eguchi S. Signal-crosstalk between Rho/ROCK and c-Jun NH2-terminal kinase mediates migration of vascular smooth muscle cells stimulated by angiotensin II. Arterioscler Thromb Vasc Biol. 2005;25(9):1831–1836. doi: 10.1161/01.ATV.0000175749.41799.9b. [DOI] [PubMed] [Google Scholar]
- 77.Guilluy C, Bregeon J, Toumaniantz G, Rolli-Derkinderen M, Retailleau K, Loufrani L, Henrion D, Scalbert E, Bril A, Torres RM, Offermanns S, Pacaud P, Loirand G. The Rho exchange factor Arhgef1 mediates the effects of angiotensin II on vascular tone and blood pressure. Nat Med. 2010;16(2):183–190. doi: 10.1038/nm.2079. [DOI] [PubMed] [Google Scholar]
- 78.Kirabo A, Kearns PN, Jarajapu YP, Sasser JM, Oh SP, Grant MB, Kasahara H, Cardounel AJ, Baylis C, Wagner K-U, Sayeski PP. Vascular smooth muscle Jak2 mediates angiotensin II-induced hypertension via increased levels of reactive oxygen species. Cardiovascular Research. 2011;91(1):171–179. doi: 10.1093/cvr/cvr059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cui R, Tieu B, Recinos A, Tilton RG, Brasier AR. RhoA mediates angiotensin II-induced phospho-Ser536 nuclear factor kappaB/RelA subunit exchange on the interleukin-6 promoter in VSMCs. Circ Res. 2006;99(7):723–730. doi: 10.1161/01.RES.0000244015.10655.3f. [DOI] [PubMed] [Google Scholar]
- 80.Funakoshi Y, Ichiki T, Shimokawa H, Egashira K, Takeda K, Kaibuchi K, Takeya M, Yoshimura T, Takeshita A. Rho-kinase mediates angiotensin II-induced monocyte chemoattractant protein-1 expression in rat vascular smooth muscle cells. Hypertension. 2001;38(1):100–104. doi: 10.1161/01.hyp.38.1.100. [DOI] [PubMed] [Google Scholar]
- 81.Takeda K, Ichiki T, Tokunou T, Iino N, Fujii S, Kitabatake A, Shimokawa H, Takeshita A. Critical role of Rho-kinase and MEK/ERK pathways for angiotensin II-induced plasminogen activator inhibitor type-1 gene expression. Arterioscler Thromb Vasc Biol. 2001;21(5):868–873. doi: 10.1161/01.atv.21.5.868. [DOI] [PubMed] [Google Scholar]
- 82.Woolfolk EA, Eguchi S, Ohtsu H, Nakashima H, Ueno H, Gerthoffer WT, Motley ED. Angiotensin II-induced activation of p21-activated kinase 1 requires Ca2+ and protein kinase C{delta} in vascular smooth muscle cells. Am J Physiol Cell Physiol. 2005;289(5):C1286–1294. doi: 10.1152/ajpcell.00448.2004. [DOI] [PubMed] [Google Scholar]
- 83.Ohtsu H, Dempsey PJ, Eguchi S. ADAMs as mediators of EGF receptor transactivation by G protein-coupled receptors. Am J Physiol Cell Physiol. 2006;291(1):C1–10. doi: 10.1152/ajpcell.00620.2005. [DOI] [PubMed] [Google Scholar]
- 84.Hinoki A, Kimura K, Higuchi S, Eguchi K, Takaguri A, Ishimaru K, Frank GD, Gerthoffer WT, Sommerville LJ, Autieri MV, Eguchi S. p21-activated kinase 1 participates in vascular remodeling in vitro and in vivo. Hypertension. 2010;55(1):161–165. doi: 10.1161/HYPERTENSIONAHA.109.143057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gregg D, Rauscher FM, Goldschmidt-Clermont PJ. Rac regulates cardiovascular superoxide through diverse molecular interactions: more than a binary GTP switch. Am J Physiol Cell Physiol. 2003;285(4):C723–734. doi: 10.1152/ajpcell.00230.2003. [DOI] [PubMed] [Google Scholar]
- 86.Andreozzi F, Laratta E, Sciacqua A, Perticone F, Sesti G. Angiotensin II impairs the insulin signaling pathway promoting production of nitric oxide by inducing phosphorylation of insulin receptor substrate-1 on Ser312 and Ser616 in human umbilical vein endothelial cells. Circ Res. 2004;94(9):1211–1218. doi: 10.1161/01.RES.0000126501.34994.96. [DOI] [PubMed] [Google Scholar]
- 87.Nakashima H, Suzuki H, Ohtsu H, Chao JY, Utsunomiya H, Frank GD, Eguchi S. Angiotensin II regulates vascular and endothelial dysfunction: recent topics of Angiotensin II type-1 receptor signaling in the vasculature. Curr Vasc Pharmacol. 2006;4(1):67–78. doi: 10.2174/157016106775203126. [DOI] [PubMed] [Google Scholar]
- 88.Luscher TF. Endothelial dysfunction: the role and impact of the renin-angiotensin system. Heart. 2000;84(Suppl 1):i20–22. doi: 10.1136/heart.84.suppl_1.i20. discussion i50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Prasad A, Tupas-Habib T, Schenke WH, Mincemoyer R, Panza JA, Waclawin MA, Ellahham S, Quyyumi AA. Acute and chronic angiotensin-1 receptor antagonism reverses endothelial dysfunction in atherosclerosis. Circulation. 2000;101(20):2349–2354. doi: 10.1161/01.cir.101.20.2349. [DOI] [PubMed] [Google Scholar]
- 90.Yan C, Kim D, Aizawa T, Berk BC. Functional interplay between angiotensin II and nitric oxide: cyclic GMP as a key mediator. Arterioscler Thromb Vasc Biol. 2003;23(1):26–36. doi: 10.1161/01.atv.0000046231.17365.9d. [DOI] [PubMed] [Google Scholar]
- 91.Imanishi T, Kobayashi K, Kuroi A, Mochizuki S, Goto M, Yoshida K, Akasaka T. Effects of angiotensin II on NO bioavailability evaluated using a catheter-type NO sensor. Hypertension. 2006;48(6):1058–1065. doi: 10.1161/01.HYP.0000248920.16956.d8. [DOI] [PubMed] [Google Scholar]
- 92.Ramchandran R, Takezako T, Saad Y, Stull L, Fink B, Yamada H, Dikalov S, Harrison DG, Moravec C, Karnik SS. Angiotensinergic stimulation of vascular endothelium in mice causes hypotension, bradycardia, and attenuated angiotensin response. Proc Natl Acad Sci U S A. 2006;103(50):19087–19092. doi: 10.1073/pnas.0602715103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mollnau H, Wendt M, Szocs K, Lassegue B, Schulz E, Oelze M, Li H, Bodenschatz M, August M, Kleschyov AL, Tsilimingas N, Walter U, Forstermann U, Meinertz T, Griendling K, Munzel T. Effects of angiotensin II infusion on the expression and function of NAD(P)H oxidase and components of nitric oxide/cGMP signaling. Circ Res. 2002;90(4):E58–65. doi: 10.1161/01.res.0000012569.55432.02. [DOI] [PubMed] [Google Scholar]
- 94.Chalupsky K, Cai H. Endothelial dihydrofolate reductase: critical for nitric oxide bioavailability and role in angiotensin II uncoupling of endothelial nitric oxide synthase. Proc Natl Acad Sci U S A. 2005;102(25):9056–9061. doi: 10.1073/pnas.0409594102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Szabo C, Pacher P, Zsengeller Z, Vaslin A, Komjati K, Benko R, Chen M, Mabley JG, Kollai M. Angiotensin II-mediated endothelial dysfunction: role of poly(ADP-ribose) polymerase activation. Mol Med. 2004;10(1–6):28–35. doi: 10.2119/2004-00001.szabo. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Owens AP, 3rd, Subramanian V, Moorleghen JJ, Guo Z, McNamara CA, Cassis LA, Daugherty A. Angiotensin II induces a region-specific hyperplasia of the ascending aorta through regulation of inhibitor of differentiation 3. Circ Res. 2010;106(3):611–619. doi: 10.1161/CIRCRESAHA.109.212837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Carlstrom M, Lai EY, Ma Z, Steege A, Patzak A, Eriksson UJ, Lundberg JO, Wilcox CS, Persson AE. Superoxide dismutase 1 limits renal microvascular remodeling and attenuates arteriole and blood pressure responses to angiotensin II via modulation of nitric oxide bioavailability. Hypertension. 2010;56(5):907–913. doi: 10.1161/HYPERTENSIONAHA.110.159301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chan SL, Baumbach GL. Deficiency of Nox2 prevents angiotensin II-induced inward remodeling in cerebral arterioles. Front Physiol. 2013;4:133. doi: 10.3389/fphys.2013.00133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Shukla AK, Xiao K, Lefkowitz RJ. Emerging paradigms of beta-arrestin-dependent seven transmembrane receptor signaling. Trends Biochem Sci. 2011;36(9):457–469. doi: 10.1016/j.tibs.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tilley DG. G protein-dependent and G protein-independent signaling pathways and their impact on cardiac function. Circ Res. 2011;109(2):217–230. doi: 10.1161/CIRCRESAHA.110.231225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kendall RT, Lee MH, Pleasant DL, Robinson K, Kuppuswamy D, McDermott PJ, Luttrell LM. Arrestin-dependent angiotensin AT1 receptor signaling regulates Akt and mTor-mediated protein synthesis. J Biol Chem. 2014;289(38):26155–26166. doi: 10.1074/jbc.M114.595728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rakesh K, Yoo B, Kim IM, Salazar N, Kim KS, Rockman HA. beta-Arrestin-Biased Agonism of the Angiotensin Receptor Induced by Mechanical Stress. Science Signaling. 2010;3(125) doi: 10.1126/scisignal.2000769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Boerrigter G, Lark MW, Whalen EJ, Soergel DG, Violin JD, Burnett JC., Jr Cardiorenal actions of TRV120027, a novel ss-arrestin-biased ligand at the angiotensin II type I receptor, in healthy and heart failure canines: a novel therapeutic strategy for acute heart failure. Circ Heart Fail. 2011;4(6):770–778. doi: 10.1161/CIRCHEARTFAILURE.111.962571. [DOI] [PubMed] [Google Scholar]
- 104.Boerrigter G, Soergel DG, Violin JD, Lark MW, Burnett JC., Jr TRV120027, a novel beta-arrestin biased ligand at the angiotensin II type I receptor, unloads the heart and maintains renal function when added to furosemide in experimental heart failure. Circ Heart Fail. 2012;5(5):627–634. doi: 10.1161/CIRCHEARTFAILURE.112.969220. [DOI] [PubMed] [Google Scholar]
- 105.Monasky MM, Taglieri DM, Henze M, Warren CM, Utter MS, Soergel DG, Violin JD, Solaro RJ. The beta-arrestin-biased ligand TRV120023 inhibits angiotensin II-induced cardiac hypertrophy while preserving enhanced myofilament response to calcium. Am J Physiol Heart Circ Physiol. 2013;305(6):H856–866. doi: 10.1152/ajpheart.00327.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lymperopoulos A, Bathgate A. Arrestins in the cardiovascular system. Prog Mol Biol Transl Sci. 2013;118:297–334. doi: 10.1016/B978-0-12-394440-5.00012-7. [DOI] [PubMed] [Google Scholar]
- 107.Lymperopoulos A, Sturchler E, Bathgate-Siryk A, Dabul S, Garcia D, Walklett K, Rengo G, McDonald P, Koch WJ. Different potencies of angiotensin receptor blockers at suppressing adrenal beta-Arrestin1-dependent post-myocardial infarction hyperaldosteronism. J Am Coll Cardiol. 2014;64(25):2805–2806. doi: 10.1016/j.jacc.2014.09.070. [DOI] [PubMed] [Google Scholar]
- 108.Dabul S, Bathgate-Siryk A, Valero TR, Jafferjee M, Sturchler E, McDonald P, Koch WJ, Lymperopoulos A. Suppression of adrenal betaarrestin1-dependent aldosterone production by ARBs: head-to-head comparison. Sci Rep. 2015;5:8116. doi: 10.1038/srep08116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.AbdAlla S, Lother H, Quitterer U. AT1-receptor heterodimers show enhanced G-protein activation and altered receptor sequestration. Nature. 2000;407(6800):94–98. doi: 10.1038/35024095. [DOI] [PubMed] [Google Scholar]
- 110.Barki-Harrington L, Luttrell LM, Rockman HA. Dual inhibition of beta-adrenergic and angiotensin II receptors by a single antagonist: a functional role for receptor-receptor interaction in vivo. Circulation. 2003;108(13):1611–1618. doi: 10.1161/01.CIR.0000092166.30360.78. [DOI] [PubMed] [Google Scholar]
- 111.Zeng C, Luo Y, Asico LD, Hopfer U, Eisner GM, Felder RA, Jose PA. Perturbation of D1 dopamine and AT1 receptor interaction in spontaneously hypertensive rats. Hypertension. 2003;42(4):787–792. doi: 10.1161/01.HYP.0000085334.34963.4E. [DOI] [PubMed] [Google Scholar]
- 112.Gonzalez-Hernandez Mde L, Godinez-Hernandez D, Bobadilla-Lugo RA, Lopez-Sanchez P. Angiotensin-II type 1 receptor (AT1R) and alpha-1D adrenoceptor form a heterodimer during pregnancy-induced hypertension. Auton Autacoid Pharmacol. 2010;30(3):167–172. doi: 10.1111/j.1474-8673.2009.00446.x. [DOI] [PubMed] [Google Scholar]
- 113.Nishimura A, Sunggip C, Tozaki-Saitoh H, Shimauchi T, Numaga-Tomita T, Hirano K, Ide T, Boeynaems JM, Kurose H, Tsuda M, Robaye B, Inoue K, Nishida M. Purinergic 2Y6 receptors heterodimerize with angiotensin AT1 receptors to promote angiotensin II-induced hypertension. Sci Signal. 2016;9(411):ra7. doi: 10.1126/scisignal.aac9187. [DOI] [PubMed] [Google Scholar]
- 114.Goupil E, Fillion D, Clement S, Luo X, Devost D, Sleno R, Petrin D, Saragovi HU, Thorin E, Laporte SA, Hebert TE. Angiotensin II type I and prostaglandin F2alpha receptors cooperatively modulate signaling in vascular smooth muscle cells. J Biol Chem. 2015;290(5):3137–3148. doi: 10.1074/jbc.M114.631119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yamamoto K, Kakino A, Takeshita H, Hayashi N, Li L, Nakano A, Hanasaki-Yamamoto H, Fujita Y, Imaizumi Y, Toyama-Yokoyama S, Nakama C, Kawai T, Takeda M, Hongyo K, Oguro R, Maekawa Y, Itoh N, Takami Y, Onishi M, Takeya Y, Sugimoto K, Kamide K, Nakagami H, Ohishi M, Kurtz TW, Sawamura T, Rakugi H. Oxidized LDL (oxLDL) activates the angiotensin II type 1 receptor by binding to the lectin-like oxLDL receptor. FASEB J. 2015;29(8):3342–3356. doi: 10.1096/fj.15-271627. [DOI] [PubMed] [Google Scholar]
- 116.Olivares-Reyes JA, Shah BH, Hernandez-Aranda J, Garcia-Caballero A, Farshori MP, Garcia-Sainz JA, Catt KJ. Agonist-induced interactions between angiotensin AT1 and epidermal growth factor receptors. Mol Pharmacol. 2005;68(2):356–364. doi: 10.1124/mol.104.010637. [DOI] [PubMed] [Google Scholar]
- 117.Ali MS, Sayeski PP, Dirksen LB, Hayzer DJ, Marrero MB, Bernstein KE. Dependence on the motif YIPP for the physical association of Jak2 kinase with the intracellular carboxyl tail of the angiotensin II AT1 receptor. J Biol Chem. 1997;272(37):23382–23388. doi: 10.1074/jbc.272.37.23382. [DOI] [PubMed] [Google Scholar]
- 118.Venema RC, Ju H, Venema VJ, Schieffer B, Harp JB, Ling BN, Eaton DC, Marrero MB. Angiotensin II-induced association of phospholipase Cgamma1 with the G-protein-coupled AT1 receptor. J Biol Chem. 1998;273(13):7703–7708. doi: 10.1074/jbc.273.13.7703. [DOI] [PubMed] [Google Scholar]
- 119.Cui T, Nakagami H, Iwai M, Takeda Y, Shiuchi T, Tamura K, Daviet L, Horiuchi M. ATRAP, novel AT1 receptor associated protein, enhances internalization of AT1 receptor and inhibits vascular smooth muscle cell growth. Biochem Biophys Res Commun. 2000;279(3):938–941. doi: 10.1006/bbrc.2000.4055. [DOI] [PubMed] [Google Scholar]
- 120.Tanaka Y, Tamura K, Koide Y, Sakai M, Tsurumi Y, Noda Y, Umemura M, Ishigami T, Uchino K, Kimura K, Horiuchi M, Umemura S. The novel angiotensin II type 1 receptor (AT1R)-associated protein ATRAP downregulates AT1R and ameliorates cardiomyocyte hypertrophy. FEBS Lett. 2005;579(7):1579–1586. doi: 10.1016/j.febslet.2005.01.068. [DOI] [PubMed] [Google Scholar]
- 121.Lopez-Ilasaca M, Liu X, Tamura K, Dzau VJ. The angiotensin II type I receptor-associated protein, ATRAP, is a transmembrane protein and a modulator of angiotensin II signaling. Mol Biol Cell. 2003;14(12):5038–5050. doi: 10.1091/mbc.E03-06-0383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tsurumi Y, Tamura K, Tanaka Y, Koide Y, Sakai M, Yabana M, Noda Y, Hashimoto T, Kihara M, Hirawa N, Toya Y, Kiuchi Y, Iwai M, Horiuchi M, Umemura S. Interacting molecule of AT1 receptor, ATRAP, is colocalized with AT1 receptor in the mouse renal tubules. Kidney Int. 2006;69(3):488–494. doi: 10.1038/sj.ki.5000130. [DOI] [PubMed] [Google Scholar]
- 123.Min L-J, Mogi M, Tamura K, Iwanami J, Sakata A, Fujita T, Tsukuda K, Jing F, Iwai M, Horiuchi M. Angiotensin II type 1 receptor-associated protein prevents vascular smooth muscle cell senescence via inactivation of calcineurin/nuclear factor of activated T cells pathway. Journal of Molecular and Cellular Cardiology. 2009;47(6):798–809. doi: 10.1016/j.yjmcc.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 124.Oshita A, Iwai M, Chen R, Ide A, Okumura M, Fukunaga S, Yoshii T, Mogi M, Higaki J, Horiuchi M. Attenuation of inflammatory vascular remodeling by angiotensin II type 1 receptor-associated protein. Hypertension. 2006;48(4):671–676. doi: 10.1161/01.HYP.0000238141.99816.47. [DOI] [PubMed] [Google Scholar]
- 125.Wakui H, Tamura K, Tanaka Y, Matsuda M, Bai Y, Dejima T, Masuda S, Shigenaga A, Maeda A, Mogi M, Ichihara N, Kobayashi Y, Hirawa N, Ishigami T, Toya Y, Yabana M, Horiuchi M, Minamisawa S, Umemura S. Cardiac-specific activation of angiotensin II type 1 receptor-associated protein completely suppresses cardiac hypertrophy in chronic angiotensin II-infused mice. Hypertension. 2010;55(5):1157–1164. doi: 10.1161/HYPERTENSIONAHA.109.147207. [DOI] [PubMed] [Google Scholar]
- 126.Guo DF, Chenier I, Tardif V, Orlov SN, Inagami T. Type 1 angiotensin II receptor-associated protein ARAP1 binds and recycles the receptor to the plasma membrane. Biochem Biophys Res Commun. 2003;310(4):1254–1265. doi: 10.1016/j.bbrc.2003.09.154. [DOI] [PubMed] [Google Scholar]
- 127.Guo DF, Chenier I, Lavoie JL, Chan JS, Hamet P, Tremblay J, Chen XM, Wang DH, Inagami T. Development of hypertension and kidney hypertrophy in transgenic mice overexpressing ARAP1 gene in the kidney. Hypertension. 2006;48(3):453–459. doi: 10.1161/01.HYP.0000230664.32874.52. [DOI] [PubMed] [Google Scholar]
- 128.Guo DF, Tardif V, Ghelima K, Chan JS, Ingelfinger JR, Chen X, Chenier I. A novel angiotensin II type 1 receptor-associated protein induces cellular hypertrophy in rat vascular smooth muscle and renal proximal tubular cells. J Biol Chem. 2004;279(20):21109–21120. doi: 10.1074/jbc.M401544200. [DOI] [PubMed] [Google Scholar]
- 129.Cook JL, Re RN, deHaro DL, Abadie JM, Peters M, Alam J. The trafficking protein, GABARAP, binds to and enhances plasma membrane expression and function of the angiotensin AT(1) receptor. Circulation research. 2008;102(12):1539–1547. doi: 10.1161/CIRCRESAHA.108.176594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhang X, Wang H, Duvernay MT, Zhu S, Wu G. The Angiotensin II Type 1 Receptor C-Terminal Lys Residues Interact with Tubulin and Modulate Receptor Export Trafficking. PLoS ONE. 2013;8(2):e57805. doi: 10.1371/journal.pone.0057805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Tirupula KC, Ithychanda SS, Mohan ML, Naga Prasad SV, Qin J, Karnik SS. G protein-coupled receptors directly bind filamin A with high affinity and promote filamin phosphorylation. Biochemistry. 2015;54(44):6673–6683. doi: 10.1021/acs.biochem.5b00975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhu S, Zhang M, Davis JE, Wu WH, Surrao K, Wang H, Wu G. A single mutation in helix 8 enhances the angiotensin II type 1a receptor transport and signaling. Cellular signalling. 2015;27(12):2371–2379. doi: 10.1016/j.cellsig.2015.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Osako MK, Nakagami H, Shimamura M, Koriyama H, Nakagami F, Shimizu H, Miyake T, Yoshizumi M, Rakugi H, Morishita R. Cross-Talk of Receptor Activator of Nuclear Factor-κB Ligand Signaling With Renin–Angiotensin System in Vascular Calcification. Arteriosclerosis, Thrombosis, and Vascular Biology. 2013;33(6):1287–1296. doi: 10.1161/ATVBAHA.112.301099. [DOI] [PubMed] [Google Scholar]
- 134.Cui M, Cai Z, Chu S, Sun Z, Wang X, Hu L, Yi J, Shen L, He B. Orphan Nuclear Receptor Nur77 Inhibits Angiotensin II-Induced Vascular Remodeling via Downregulation of beta-Catenin. Hypertension. 2016;67(1):153–162. doi: 10.1161/HYPERTENSIONAHA.115.06114. [DOI] [PubMed] [Google Scholar]
- 135.Baurand A, Zelarayan L, Betney R, Gehrke C, Dunger S, Noack C, Busjahn A, Huelsken J, Taketo MM, Birchmeier W, Dietz R, Bergmann MW. Beta-catenin downregulation is required for adaptive cardiac remodeling. Circ Res. 2007;100(9):1353–1362. doi: 10.1161/01.RES.0000266605.63681.5a. [DOI] [PubMed] [Google Scholar]
- 136.Shanmugam P, Valente AJ, Prabhu SD, Venkatesan B, Yoshida T, Delafontaine P, Chandrasekar B. Angiotensin-II type 1 receptor and NOX2 mediate TCF/LEF and CREB dependent WISP1 induction and cardiomyocyte hypertrophy. J Mol Cell Cardiol. 2011;50(6):928–938. doi: 10.1016/j.yjmcc.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zhou D, Tan RJ, Fu H, Liu Y. Wnt/beta-catenin signaling in kidney injury and repair: a double-edged sword. Lab Invest. 2016;96(2):156–167. doi: 10.1038/labinvest.2015.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zhou L, Liu Y. Wnt/beta-catenin signaling and renin-angiotensin system in chronic kidney disease. Curr Opin Nephrol Hypertens. 2016;25(2):100–106. doi: 10.1097/MNH.0000000000000205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Jiang L, Xu L, Song Y, Li J, Mao J, Zhao AZ, He W, Yang J, Dai C. Calmodulin-dependent protein kinase II/cAMP response element-binding protein/Wnt/beta-catenin signaling cascade regulates angiotensin II-induced podocyte injury and albuminuria. J Biol Chem. 2013;288(32):23368–23379. doi: 10.1074/jbc.M113.460394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Cuevas CA, Gonzalez AA, Inestrosa NC, Vio CP, Prieto MC. Angiotensin II increases fibronectin and collagen I through the beta-catenin-dependent signaling in mouse collecting duct cells. Am J Physiol Renal Physiol. 2015;308(4):F358–365. doi: 10.1152/ajprenal.00429.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Cuevas CA, Tapia-Rojas C, Cespedes C, Inestrosa NC, Vio CP. beta-Catenin-Dependent Signaling Pathway Contributes to Renal Fibrosis in Hypertensive Rats. Biomed Res Int. 2015;2015:726012. doi: 10.1155/2015/726012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.de la Pompa JL, Epstein JA. Coordinating tissue interactions: Notch signaling in cardiac development and disease. Dev Cell. 2012;22(2):244–254. doi: 10.1016/j.devcel.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ozasa Y, Akazawa H, Qin Y, Tateno K, Ito K, Kudo-Sakamoto Y, Yano M, Yabumoto C, Naito AT, Oka T, Lee JK, Minamino T, Nagai T, Kobayashi Y, Komuro I. Notch activation mediates angiotensin II-induced vascular remodeling by promoting the proliferation and migration of vascular smooth muscle cells. Hypertens Res. 2013;36(10):859–865. doi: 10.1038/hr.2013.52. [DOI] [PubMed] [Google Scholar]
- 144.Basu S, Srinivasan DK, Yang K, Raina H, Banerjee S, Zhang R, Fisher SA, Proweller A. Notch transcriptional control of vascular smooth muscle regulatory gene expression and function. J Biol Chem. 2013;288(16):11191–11202. doi: 10.1074/jbc.M112.442996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Rivera-Torres J, Guzman-Martinez G, Villa-Bellosta R, Orbe J, Gonzalez-Gomez C, Serrano M, Diez J, Andres V, Maraver A. Targeting gamma-secretases protect against angiotensin II-induced cardiac hypertrophy. J Hypertens. 2015;33(4):843–850. doi: 10.1097/HJH.0000000000000463. discussion 850. [DOI] [PubMed] [Google Scholar]
- 146.Boulos N, Helle F, Dussaule JC, Placier S, Milliez P, Djudjaj S, Guerrot D, Joutel A, Ronco P, Boffa JJ, Chatziantoniou C. Notch3 is essential for regulation of the renal vascular tone. Hypertension. 2011;57(6):1176–1182. doi: 10.1161/HYPERTENSIONAHA.111.170746. [DOI] [PubMed] [Google Scholar]
- 147.Hans CP, Koenig SN, Huang N, Cheng J, Beceiro S, Guggilam A, Kuivaniemi H, Partida-Sanchez S, Garg V. Inhibition of Notch1 signaling reduces abdominal aortic aneurysm in mice by attenuating macrophage-mediated inflammation. Arterioscler Thromb Vasc Biol. 2012;32(12):3012–3023. doi: 10.1161/ATVBAHA.112.254219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Cheng J, Koenig SN, Kuivaniemi HS, Garg V, Hans CP. Pharmacological inhibitor of notch signaling stabilizes the progression of small abdominal aortic aneurysm in a mouse model. J Am Heart Assoc. 2014;3(6):e001064. doi: 10.1161/JAHA.114.001064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Meng Z, Moroishi T, Guan KL. Mechanisms of Hippo pathway regulation. Genes Dev. 2016;30(1):1–17. doi: 10.1101/gad.274027.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wennmann DO, Vollenbroker B, Eckart AK, Bonse J, Erdmann F, Wolters DA, Schenk LK, Schulze U, Kremerskothen J, Weide T, Pavenstadt H. The Hippo pathway is controlled by Angiotensin II signaling and its reactivation induces apoptosis in podocytes. Cell Death Dis. 2014;5:e1519. doi: 10.1038/cddis.2014.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Dikalov SI, Nazarewicz RR. Angiotensin II-induced production of mitochondrial reactive oxygen species: potential mechanisms and relevance for cardiovascular disease. Antioxid Redox Signal. 2013;19(10):1085–1094. doi: 10.1089/ars.2012.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Dikalov SI, Nazarewicz RR, Bikineyeva A, Hilenski L, Lassegue B, Griendling KK, Harrison DG, Dikalova AE. Nox2-induced production of mitochondrial superoxide in angiotensin II-mediated endothelial oxidative stress and hypertension. Antioxid Redox Signal. 2014;20(2):281–294. doi: 10.1089/ars.2012.4918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Widder JD, Fraccarollo D, Galuppo P, Hansen JM, Jones DP, Ertl G, Bauersachs J. Attenuation of angiotensin II-induced vascular dysfunction and hypertension by overexpression of Thioredoxin 2. Hypertension. 2009;54(2):338–344. doi: 10.1161/HYPERTENSIONAHA.108.127928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Chaplin NL, Nieves-Cintron M, Fresquez AM, Navedo MF, Amberg GC. Arterial Smooth Muscle Mitochondria Amplify Hydrogen Peroxide Microdomains Functionally Coupled to L-Type Calcium Channels. Circ Res. 2015;117(12):1013–1023. doi: 10.1161/CIRCRESAHA.115.306996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Lim S, Lee SY, Seo HH, Ham O, Lee C, Park JH, Lee J, Seung M, Yun I, Han SM, Lee S, Choi E, Hwang KC. Regulation of Mitochondrial Morphology by Positive Feedback Interaction Between PKC delta and Drp1 in Vascular Smooth Muscle Cell. Journal of Cellular Biochemistry. 2015;116(4):648–660. doi: 10.1002/jcb.25016. [DOI] [PubMed] [Google Scholar]
- 156.Qi X, Disatnik MH, Shen N, Sobel RA, Mochly-Rosen D. Aberrant mitochondrial fission in neurons induced by protein kinase C{delta} under oxidative stress conditions in vivo. Mol Biol Cell. 2011;22(2):256–265. doi: 10.1091/mbc.E10-06-0551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Yu H, Guo Y, Mi L, Wang X, Li L, Gao W. Mitofusin 2 inhibits angiotensin II-induced myocardial hypertrophy. J Cardiovasc Pharmacol Ther. 2011;16(2):205–211. doi: 10.1177/1074248410385683. [DOI] [PubMed] [Google Scholar]
- 158.Sparks MA, Stegbauer J, Chen D, Gomez JA, Griffiths RC, Azad HA, Herrera M, Gurley SB, Coffman TM. Vascular Type 1A Angiotensin II Receptors Control BP by Regulating Renal Blood Flow and Urinary Sodium Excretion. J Am Soc Nephrol. 2015;26(12):2953–2962. doi: 10.1681/ASN.2014080816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Chen D, Stegbauer J, Sparks MA, Kohan D, Griffiths R, Herrera M, Gurley SB, Coffman TM. Impact of Angiotensin Type 1A Receptors in Principal Cells of the Collecting Duct on Blood Pressure and Hypertension. Hypertension. 2016;67(6):1291–1297. doi: 10.1161/HYPERTENSIONAHA.115.06987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Rateri DL, Moorleghen JJ, Knight V, Balakrishnan A, Howatt DA, Cassis LA, Daugherty A. Depletion of Endothelial or Smooth Muscle Cell-Specific Angiotensin II Type 1a Receptors Does Not Influence Aortic Aneurysms or Atherosclerosis in LDL Receptor Deficient Mice. PLoS ONE. 2012;7(12):e51483. doi: 10.1371/journal.pone.0051483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Poduri A, Rateri DL, Howatt DA, Balakrishnan A, Moorleghen JJ, Cassis LA, Daugherty A. Fibroblast Angiotensin II Type 1a Receptors Contribute to Angiotensin II-Induced Medial Hyperplasia in the Ascending Aorta. Arterioscler Thromb Vasc Biol. 2015;35(9):1995–2002. doi: 10.1161/ATVBAHA.115.305995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Rateri DL, Moorleghen JJ, Balakrishnan A, Owens AP, Howatt DA, Subramanian V, Poduri A, Charnigo R, Cassis LA, Daugherty A. Endothelial Cell-specific Deficiency of AngII Type 1a Receptors Attenuates AngII-induced Ascending Aortic Aneurysms in LDL Receptor −/− Mice. Circulation research. 2011;108(5):574–581. doi: 10.1161/CIRCRESAHA.110.222844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Obama T, Tsuji T, Kobayashi T, Fukuda Y, Takayanagi T, Taro Y, Kawai T, Forrester Steven J, Elliott Katherine J, Choi E, Daugherty A, Rizzo V, Eguchi S. Epidermal growth factor receptor inhibitor protects against abdominal aortic aneurysm in a mouse model. Clinical Science. 2015;128(9):559–565. doi: 10.1042/CS20140696. [DOI] [PubMed] [Google Scholar]
- 164.Imanishi M, Tomita S, Ishizawa K, Kihira Y, Ueno M, Izawa-Ishizawa Y, Ikeda Y, Yamano N, Tsuchiya K, Tamaki T. Smooth muscle cell-specific Hif-1α deficiency suppresses angiotensin II-induced vascular remodelling in mice. Cardiovascular Research. 2014;102(3):460–468. doi: 10.1093/cvr/cvu061. [DOI] [PubMed] [Google Scholar]
- 165.Huang Y, Di Lorenzo A, Jiang W, Cantalupo A, Sessa WC, Giordano FJ. Hypoxia-inducible factor-1alpha in vascular smooth muscle regulates blood pressure homeostasis through a peroxisome proliferator-activated receptor-gamma-angiotensin II receptor type 1 axis. Hypertension. 2013;62(3):634–640. doi: 10.1161/HYPERTENSIONAHA.111.00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ketsawatsomkron P, Lorca RA, Keen HL, Weatherford ET, Liu X, Pelham CJ, Grobe JL, Faraci FM, England SK, Sigmund CD. PPARgamma regulates resistance vessel tone through a mechanism involving RGS5-mediated control of protein kinase C and BKCa channel activity. Circ Res. 2012;111(11):1446–1458. doi: 10.1161/CIRCRESAHA.112.271577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Carrillo-Sepulveda MA, Keen HL, Davis DR, Grobe JL, Sigmund CD. Role of vascular smooth muscle PPARgamma in regulating AT1 receptor signaling and angiotensin II-dependent hypertension. PLoS One. 2014;9(8):e103786. doi: 10.1371/journal.pone.0103786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Gao D, Hao G, Meng Z, Ning N, Yang G, Liu Z, Dong X, Niu X. Rosiglitzone Suppresses Angiotensin II-Induced Production of KLF5 and Cell Proliferation in Rat Vascular Smooth Muscle Cells. PLOS ONE. 2015;10(4):e0123724. doi: 10.1371/journal.pone.0123724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Marchesi C, Rehman A, Rautureau Y, Kasal DA, Briet M, Leibowitz A, Simeone SMC, Ebrahimian T, Neves MF, Offermanns S, Gonzalez FJ, Paradis P, Schiffrin EL. Protective role of vascular smooth muscle cell PPARγ in angiotensin II-induced vascular disease. Cardiovascular Research. 2013;97(3):562–570. doi: 10.1093/cvr/cvs362. [DOI] [PubMed] [Google Scholar]
- 170.Beyer AM, de Lange WJ, Halabi CM, Modrick ML, Keen HL, Faraci FM, Sigmund CD. Endothelium-specific interference with peroxisome proliferator activated receptor gamma causes cerebral vascular dysfunction in response to a high-fat diet. Circ Res. 2008;103(6):654–661. doi: 10.1161/CIRCRESAHA.108.176339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Hu C, Lu KT, Mukohda M, Davis DR, Faraci FM, Sigmund CD. Interference with PPARgamma in endothelium accelerates angiotensin II-induced endothelial dysfunction. Physiol Genomics. 2016;48(2):124–134. doi: 10.1152/physiolgenomics.00087.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]


