Abstract
M. tuberculosis; contains an unusually high number of serine hydrolases by proteome percentage compared to other common bacteria or humans. This letter describes a method to probe the global substrate specificity of mycobacterial serine hydrolases with ester-protected prodrugs of ethambutol, a first-line antibiotic treatment for TB. These compounds were synthesized directly from ethambutol using a selective o-acylation to yield products in high yield and purity with minimal workup. A library of derivatives was screened against M. smegmatis, a non-infectious model for M. tuberculosis, which displayed significantly lowered biological activity compared to ethambutol. Incubation with a general serine hydrolase reactivated each derivative to near-ethambutol levels, demonstrating that esterification of ethambutol should provide a simple screen for mycobacterial hydrolase activity.
Keywords: Hydrolases, M. tuberculosis, Prodrugs, Antibiotics, Tuberculosis, ethambutol
Graphical abstract
Tuberculosis (TB) is the deadliest infectious disease worldwide.1 The complex bioavailability and cell permeability of Mycobacterium tuberculosis, the causative agent of TB, complicates traditional drug discovery pathways.2 Among the methods used to overcome the impermeability of M. tuberculosis is the utilization of prodrugs, which can be designed with the proper polarity balance for high cell permeability before being activated by internal enzymes.3 TB prodrugs have additional advantages, including trapping the activated drug within the mycobacterial cell4 and introducing additional disease specificity.5 Pyrazinamide and isoniazid, two frontline medications for TB, are both prodrugs that are metabolized by M. tuberculosis enzymes as part of their respective mechanisms of action (Figure 1).6, 7 The threat posed by TB has led to considerable investment into prodrug strategies,5 and several novel TB treatments, such as lansoprazole8 and pretomanid (formerly PA-824)9, are currently in clinical development.
Figure 1.
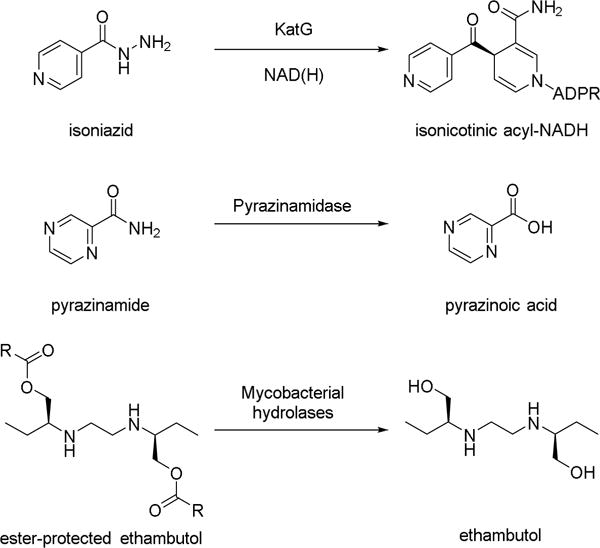
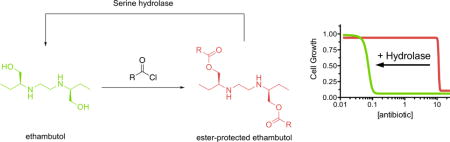
Tuberculosis prodrugs and their activated forms. Isoniazid is converted to the acyl radical by KatG, which reacts with NAD(H) to form the prodrug complex. Pyrazinamide is converted to the active pyrazinoic acid by reaction with pyrazinamidase. We proposed that ester-protected prodrugs of ethambutol would regulate its native antibacterial activity and provide a simple screen for mycobacterial hydrolase activity.
A classic strategy for prodrug development is the conversion of alcohol or carboxylic acid functionalities, which can limit cell permeability, into less-polar ester groups.3 Ester-based prodrugs increase the oral bioavailability of TB therapeutics in preclinical studies, and potentially offer additional benefits over other prodrug strategies. For example, pyrazinoic acid esters have been found to be effective against pyrazinamide-resistant strains of M. tuberculosis,10–14 with some compounds demonstrating dual effects through inhibition by both the masked drug and the masking group itself.15 A drawback to ester-prodrugs is the assumption that nonspecific human hydrolases will remove any ester protecting groups prior to cell internalization, removing the additional therapeutic selectivity of the ester-prodrug. Certain simple ester-protected derivatives of molecular probes are, however, resistant to activation by a broad range of mammalian hydrolases, suggesting the potential for orthogonal ester masking groups.16 M. tuberculosis contains a significantly higher number of serine hydrolases (186 predicted serine hydrolases; ~4% of the proteome)17 by proteome percentage than other common bacteria18 or even humans (>200 predicted serine hydrolases; ~1% of the proteome).19, 20 We hypothesized that this large, diverse family of mycobacterial serine hydrolases might encode orthogonal ester reactivity for endowing mycobacterial specificity into ester-based prodrugs.
As a proof of principle for a TB-specific prodrug, we chose the simple antibiotic ethambutol. Ethambutol is a bacteriostatic, antimycobacterial drug that has been part of front-line TB treatment since the 1960s. While effective, ethambutol usage can present with several serious side effects, which include hepatotoxicity21 and ocular neuritis.22 SAR studies undertaken to ameliorate these effects have found ethambutol to be a poor lead structure for analogues. In particular, modification of its alcohol functionalities, including to a methyl ester, completely abolished antimycobacterial activity.23 Thus, ester-protection of ethambutol should create a simple screen for mycobacterial hydrolase activity where the protected version is nontoxic, but the activated version has TB specific activity (Figure 1). In addition to serving as a useful tool for evaluating hydrolase activity, ester prodrug derivatives of ethambutol could be a novel strategy for decreasing in vivo toxicity. Herein, we report the synthesis and biological evaluation of a series of acylated ethambutol derivatives.
With the goal of probing global serine hydrolase activity in Mycobacteria, compounds 3a–h were chosen for the initial library to test simple ester features (Figure 2). Branching patterns (3f, 3g, and 3h) were chosen to mimic the complex mycolic acids adorning the outside of the mycobacterial cell wall.24, 25 Tertiary ester functionalities (3d and 3e) were also added, as these esters were not hydrolyzed in many mammalian cell types.16, 26 Double-protected derivatives were also targeted over single-protected variants, as they were found to be more stable and compatible with cellular activation. Compounds were prepared from commercially-available (S,S)-ethambutol dihydrochloride 1 and a corresponding acid chloride as outlined in Figure 2, following a procedure developed for selective O-acylation of amino alcohols.27 Acid chlorides were either commercially sourced or prepared from the precursor carboxylic acid using oxalyl chloride in dichloromethane and catalytic DMF. Ethambutol derivatives were prepared by adding the desired acid chloride to a solution of 1 dissolved in trifluoroacetic acid, which was stirred at room temperature for ten minutes. Diethyl ether was added to the mixture to precipitate the products as hydrochloride salts, which were collected using vacuum filtration. These proved to be soluble in CDCl3, which allowed for NMR characterization without conversion to the free base. IR spectroscopy of products showed the disappearance of the ethambutol O-H stretch at 3300 cm−1 while NMR indicated the presence of ammonium protons, demonstrating that ester products were synthesized instead of amides (see Supplemental Information).
Figure 2.
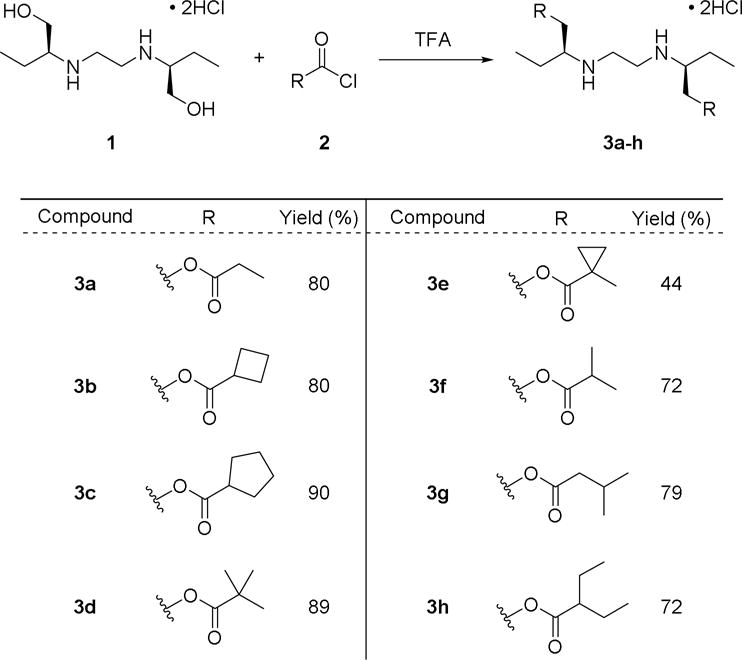
Esterified derivatives of ethambutol. All reactions were run at RT for 15 minutes with stirring, before cooling to 0°C and adding Et2O to precipitate products as hydrochloride salts.
Compounds 3a–h were screened for their ability to inhibit the growth of M. smegmatis, a non-infectious model mycobacterium for TB infection. M. smegmatis has a high conservation of serine hydrolases with M. tuberculosis, including 22/24 Lip family hydrolases, all cutinases, and other hydrolases identified as therapeutic targets.28–30 Initial screening was performed using disc diffusion measurements (Figure 3). By comparing the relative sizes of the inhibition zones across each compounds, disc diffusion provided a qualitative assessment of the relative MIC values of ester prodrugs in comparison to ethambutol. As expected, ester protection significantly decreased the toxicity of ethambutol, with significantly higher concentrations of compounds 3b (25 μg/mL), 3e (6.25 μg/mL), and 3h (3.125 μg/mL) required for similar zones of inhibition as 1 (0.39 μg/mL). Levels of inhibition, however, varied across ester-protected derivatives, with 3h showing significantly greater inhibition zones across all concentrations. Matching with previous observations about the high stability of methyl cyclopropyl esters,16 compound 3e showed the lowest relative toxicity.
Figure 3.
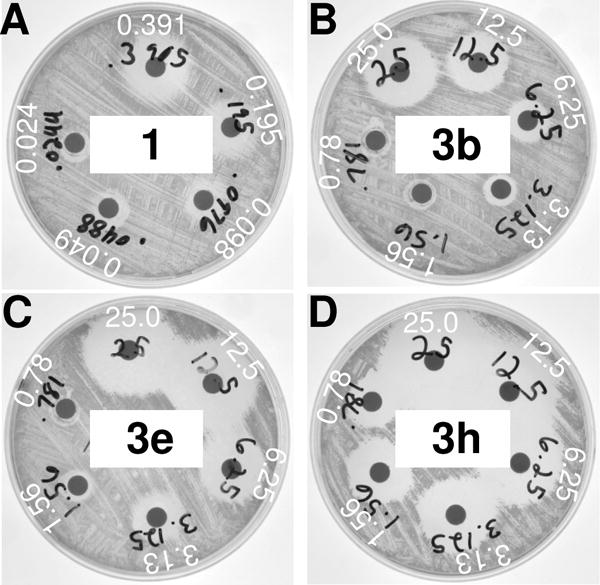
Disc diffusion measurements for ethambutol and selected derivatives in Middlebrook agar media against M. smegmatis. Derivatives were serially diluted from 25 μg/mL to 0.781 μg/mL, while ethambutol was diluted from 0.391 μg/mL to 0.0244 μg/mL. Inhibition was measured after two days of growth at 37°C.
To quantitate the relative activation of these prodrugs and the dependence of this activation on serine hydrolase activity, values for MIC99 were determined for compounds 3a–h under a variety of conditions (Table 1 and Figure 4). For each compound, the MIC99 for M. smegmatis was compared with the MIC99 after pre-incubation of the compounds with pig liver esterase (PLE), a general serine hydrolase, to confirm that the prodrugs could be activated by serine hydrolases. If compounds were fully activated, MIC99 values +PLE should be comparable to the MIC99 value for the parent compound 1. Additionally, pre-incubation of each compound with PLE in tandem with the general serine hydrolase inhibitor, phenylmethylsulfonyl fluoride (PMSF), was performed to demonstrate that ester-prodrug activation was dependent on the serine hydrolase activity of PLE rather than nonspecific hydrolysis. If ester activation was dependent on the serine hydrolase activity of PLE, the MIC99 values for +PLE +PMSF should be comparable to the ester-protected compound.
Table 1.
MIC99 for ester-protected ethambutol derivatives.
| Compound | MIC99 (μg/mL)* | MIC99 + PLE (μg/mL) | MIC99 + PLE + PMSF (μg/mL) |
|---|---|---|---|
| 1 | 0.098 | 0.098 | 0.098 |
| 3a | 0.78 | 0.78 | 0.78 |
| 3b | 3.1 | 0.39 | 6.2 |
| 3c | 6.3 | 0.098 | 1.6 |
| 3d | 6.3 | 0.78 | 6.3 |
| 3e | 13 | 0.78 | 25 |
| 3f | 6.3 | 0.39 | 6.3 |
| 3g | 6.3 | 0.20 | 0.39 |
| 3h | 1.6 | 0.20 | 0.78 |
MIC99 was measured against M. smegmatis. The highest concentration sample in the MIC99 dilutions was preincubated with PMSF (1mM), PLE (0.1 mg/mL), or the combination of PLE and PMSF for 1 hour at RT before dilution. The A600 of the growth plates were measured after 72 h of culture growth at 37 °C and 225 rpm.
Figure 4.
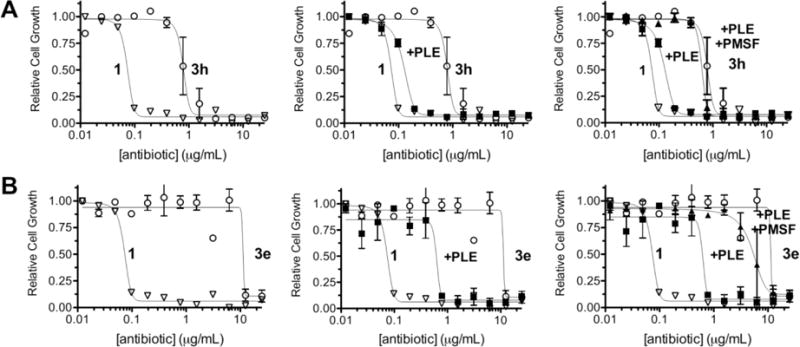
Dose-dependency curves for branched (3h, A) and tertiary (3e, B) ethambutol derivatives, along with PLE treatment (middle) and PLE + PMSF (right). Derivatives were serially diluted in Middlebrook media and further diluted 1:1 with M. smegmatis in Middlebrook (final concentrations 25 μg/mL to 0.012 μg/mL). Pig liver esterase treatment was performed at the highest dilution (50 μg/mL) by incubating with PLE (0.1 mg/mL) or PLE + PMSF (1 mM) for 1h at RT. Plates were incubated at 37°C for 3 days, and cell growth was measured at A600.
Similar to the measurements with the disc diffusion, ester protection of ethambutol increased the MIC99 values from compound 1 between 8- and 130-fold (Table 1). The methyl cyclopropyl ester (3e) again showed the greatest stability and least toxicity with an MIC99 value at or close to the solubility limit for this compound (Figure 4), whereas the propyl ester (3a) showed the greatest activation. This profile matches the relative stability of other carboxylic ester libraries where small, unbranched esters like 3a were activated in a variety of cell types and conditions, but tertiary esters like 3d and 3e required either specialized enzymes16 or enzyme engineering31, 32 to accomplish these reactions. Relative activation varied 16-fold across all ester-protected derivatives, suggesting differential activation by mycobacterial serine hydrolases.
Pre-incubation with PLE to confirm serine hydrolase-dependent activation of the ester prodrugs found that each compound was activated by PLE either to nearly equivalent MIC99 values with 1 (Figure 4A) or within 8-fold of 1 for the stable tertiary esters (Figure 4B). Propyl ester 3a showed no further activation, suggesting that this compound was already fully activated either by mycobacterial hydrolases or was inherently hydrolytically unstable. Pre-incubation of PLE along with PMSF (+PLE +PMSF) decreased biological activity compared to that of PLE alone, confirming that ester prodrug activation was dependent on serine hydrolase activity (Table 1 and Figure 4). To determine any potential contributions from nonspecific hydrolysis, each compound was dissolved in water and Middlebrook media and incubated at 37°C for 10 days, using comparative TLC to track degradation. After 72h, no ethambutol was observed in either medium (Figure S1), although 3a, 3b, 3c and 3g showed some breakdown in Middlebrook based on the disappearance of the parent compound. Degradation products only began appearing in compounds 3a, 3b, 3c and 3f in water after a full 10 days of exposure (Figure S2). Additionally, the observed TLC changes suggest that only one of the two ester functionalities is being hydrolyzed rather than reverting completely to ethambutol, which may also explain the observed incomplete PLE activation of all derivatives. This high hydrolytic stability strongly implicates differences in mycobacterial hydrolase activity in the shifted MIC values of the ester prodrugs.
Overall, the esterified derivatives of ethambutol displayed significantly lowered biological activity towards M. smegmatis compared to the parent compound, confirming that the alcohol is necessary for the biological activity of ethambutol. The MIC99 values were also dependent on the structure of the ester functionality, with 3a and 3h displaying the greatest activity. Incubation of each compound with PLE also resulted in the restoration of biological activity, demonstrating that the ester functionality can be cleaved by a broad-spectrum serine hydrolase. Thus, esterification of ethambutol successfully attenuates its biological activity while remaining susceptible to activation by serine hydrolase, creating a simple screen for determining the substrate specificity of mycobacterial hydrolases. Ester-protected ethambutol derivatives were easily synthesized and their susceptibility to hydrolases such as PLE makes them excellent biological probes for mycobacteria. Building on this proof of principle, efforts to expand this library and explore the specificity of mycobacterial hydrolases are ongoing.
Supplementary Material
Highlights.
-
-
A library of ester-prodrugs of ethambutol was synthesized
-
-
Selective o-acylation was used to generate derivatives in one step
-
-
Esterified derivatives were deactivated relative to ethambutol
-
-
Incubation with a general serine hydrolase restored ethambutol-like activity
Acknowledgments
We would like to thank Brent Kochert for help with the initial biological assays. This project was funded by NIH 1 R15 GM110641-01A1 to RJJ.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.CDC | TB | Data and Statistics. https://www.cdc.gov/tb/statistics/default.htm. Accessed May 18 2017.
- 2.Koul A, Arnoult E, Lounis N, Guillemont J, Andries K. The challenge of new drug discovery for tuberculosis. Nature. 2011;469(7331):483–490. doi: 10.1038/nature09657. [DOI] [PubMed] [Google Scholar]
- 3.Rautio J, Kumpulainen H, Heimbach T, et al. Prodrugs: design and clinical applications. Nature Reviews Drug Discovery. 2008;7(3):255–270. doi: 10.1038/nrd2468. [DOI] [PubMed] [Google Scholar]
- 4.Hanoulle X, Wieruszeski J-M, Rousselot-Pailley P, et al. Selective intracellular accumulation of the major metabolite issued from the activation of the prodrug ethionamide in mycobacteria. J Antimicrob Chemother. 2006;58(4):768–772. doi: 10.1093/jac/dkl332. [DOI] [PubMed] [Google Scholar]
- 5.Mori G, Chiarelli LR, Riccardi G, Pasca MR. New prodrugs against tuberculosis. Drug Discovery Today. 2016 doi: 10.1016/j.drudis.2016.09.006. [DOI] [PubMed] [Google Scholar]
- 6.Shi W, Zhang X, Jiang X, et al. Pyrazinamide inhibits trans-translation in Mycobacterium tuberculosis. Science. 2011;333(6049):1630–1632. doi: 10.1126/science.1208813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suarez J, Ranguelova K, Jarzecki AA, et al. An oxyferrous heme/protein-based radical intermediate is catalytically competent in the catalase reaction of Mycobacterium tuberculosis catalase-peroxidase (KatG) J Biol Chem. 2009;284(11):7017–7029. doi: 10.1074/jbc.M808106200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rybniker J, Vocat A, Sala C, et al. Lansoprazole is an antituberculous prodrug targeting cytochrome bc1. Nature Communications. 2015;6:7659. doi: 10.1038/ncomms8659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ginsberg AM, Laurenzi MW, Rouse DJ, Whitney KD, Spigelman MK. Safety, tolerability, and pharmacokinetics of PA-824 in healthy subjects. Antimicrob Agents Chemother. 2009;53(9):3720–3725. doi: 10.1128/AAC.00106-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cynamon MH, Klemens SP, Chou TS, Gimi RH, Welch JT. Antimycobacterial activity of a series of pyrazinoic acid esters. J Med Chem. 1992;35(7):1212–1215. doi: 10.1021/jm00085a007. [DOI] [PubMed] [Google Scholar]
- 11.Cynamon MH, Gimi R, Gyenes F, et al. Pyrazinoic acid esters with broad spectrum in vitro antimycobacterial activity. J Med Chem. 1995;38(20):3902–3907. doi: 10.1021/jm00020a003. [DOI] [PubMed] [Google Scholar]
- 12.Bergmann KE, Cynamon MH, Welch JT. Quantitative structure–activity relationships for the in vitro antimycobacterial activity of pyrazinoic acid esters. J Med Chem. 1996;39(17):3394–3400. doi: 10.1021/jm950538t. [DOI] [PubMed] [Google Scholar]
- 13.Simões MF, Valente E, Gómez MJR, Anes E, Constantino L. Lipophilic pyrazinoic acid amide and ester prodrugs: stability, activation and activity against M. tuberculosis. European Journal of Pharmaceutical Sciences. 2009;37(3):257–263. doi: 10.1016/j.ejps.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 14.Young E, Perkowski E, Malik S, et al. Inhaled pyrazinoic acid esters for the treatment of tuberculosis. Pharm Res. 2016;33(10):2495–2505. doi: 10.1007/s11095-016-1974-5. [DOI] [PubMed] [Google Scholar]
- 15.Pires D, Valente E, Simões MF, et al. Esters of pyrazinoic acid are active against pyrazinamide-resistant strains of mycobacterium tuberculosis and other naturally resistant mycobacteria in vitro and ex vivo within macrophages. Antimicrob Agents Chemother. 2015;59(12):7693–7699. doi: 10.1128/AAC.00936-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tian L, Yang Y, Wysocki LM, et al. Selective esterase–ester pair for targeting small molecules with cellular specificity. Proceedings of the National Academy of Sciences. 2012;109(13):4756–4761. doi: 10.1073/pnas.1111943109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tallman KR, Levine SR, Beatty KE. Small-molecule probes reveal esterases with persistent activity in dormant and reactivating Mycobacterium tuberculosis. ACS Infect Dis. 2016;2(12):936–944. doi: 10.1021/acsinfecdis.6b00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cole ST, Brosch R, Parkhill J, et al. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature. 1998;393(6685):537–544. doi: 10.1038/31159. [DOI] [PubMed] [Google Scholar]
- 19.Simon GM, Cravatt BF. Activity-based proteomics of enzyme superfamilies: serine hydrolases as a case study. J Biol Chem. 2010;285(15):11051–11055. doi: 10.1074/jbc.R109.097600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Long JZ, Cravatt BF. The metabolic serine hydrolases and their functions in mammalian physiology and disease. Chem Rev. 2011;111(10):6022–6063. doi: 10.1021/cr200075y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lees A, Allan G, Smith J, Tyrrell W, Fallon R. Toxicity from rifampicin plus isoniazid and rifampicin plus ethambutol therapy. Tubercle. 1971;52(3):182–190. doi: 10.1016/0041-3879(71)90041-9. [DOI] [PubMed] [Google Scholar]
- 22.Kumar A, Sandramouli S, Verma L, Tewari H, Khosla P. Ocular ethambutol toxicity: is it reversible? Journal of Neuro-Ophthalmology. 1993;13(1):15–17. [PubMed] [Google Scholar]
- 23.Häusler H, Kawakami RP, Mlaker E, Severn WB, Stütz AE. Ethambutol analogues as potential antimycobacterial agents. Bioorg Med Chem Lett. 2001;11(13):1679–1681. doi: 10.1016/s0960-894x(01)00258-x. [DOI] [PubMed] [Google Scholar]
- 24.Layre E, Sweet L, Hong S, et al. A comparative lipidomics platform for chemotaxonomic analysis of Mycobacterium tuberculosis. Chemistry & Biology. 2011;18(12):1537–1549. doi: 10.1016/j.chembiol.2011.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sartain MJ, Dick DL, Rithner CD, Crick DC, Belisle JT. Lipidomic analyses of Mycobacterium tuberculosis based on accurate mass measurements and the novel “Mtb LipidDB”. J Lipid Res. 2011;52(5):861–872. doi: 10.1194/jlr.M010363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bender DM, Peterson JA, McCarthy JR, Gunaydin H, Takano Y, Houk K. Cyclopropanecarboxylic acid esters as potential prodrugs with enhanced hydrolytic stability. Org Lett. 2008;10(3):509–511. doi: 10.1021/ol702892e. [DOI] [PubMed] [Google Scholar]
- 27.Kristensen TE, Hansen FK, Hansen T. The selective O-acylation of hydroxyproline as a convenient method for the large-scale preparation of novel proline polymers and amphiphiles. Eur J Org Chem. 2009;2009(3):387–395. [Google Scholar]
- 28.Singh G, Singh G, Jadeja D, Kaur J. Lipid hydrolizing enzymes in virulence: Mycobacterium tuberculosis as a model system. Crit Rev Microbiol. 2010;36(3):259–269. doi: 10.3109/1040841X.2010.482923. [DOI] [PubMed] [Google Scholar]
- 29.Shen G, Singh K, Chandra D, et al. LipC (Rv0220) is an immunogenic cell surface esterase of Mycobacterium tuberculosis. Infect Immun. 2012;80(1):243–253. doi: 10.1128/IAI.05541-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.West NP, Chow FM, Randall EJ, et al. Cutinase-like proteins of Mycobacterium tuberculosis: characterization of their variable enzymatic functions and active site identification. FASEB J. 2009;23(6):1694–1704. doi: 10.1096/fj.08-114421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Herter S, Nguyen G-S, Thompson ML, et al. Comparative analysis of tertiary alcohol esterase activity in bacterial strains isolated from enrichment cultures and from screening strain libraries. Appl Microbiol Biotechnol. 2011;90(3):929–939. doi: 10.1007/s00253-011-3124-7. [DOI] [PubMed] [Google Scholar]
- 32.Bartsch S, Kourist R, Bornscheuer UT. Complete inversion of enantioselectivity towards acetylated tertiary alcohols by a double mutant of a Bacillus subtilis esterase. Angew Chem Int Ed. 2008;47(8):1508–1511. doi: 10.1002/anie.200704606. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


