Abstract
Enterolignans, products of gut bacterial metabolism of plant lignans, have been associated with reduced risk of chronic diseases, but their association with other plasma metabolites is unknown. We examined plasma metabolite profiles according to urinary enterolignan excretion in a cross-sectional analysis using data from a randomized crossover, controlled feeding study. Eighty healthy adult males and females completed two 28-day feeding periods differing by glycemic load, refined carbohydrate, and fiber content. Lignan intake was calculated from food records using a polyphenol database. Targeted metabolomics was performed by LC-MS on plasma from fasting blood samples collected at the end of each feeding period. Enterolactone (ENL) and enterodiol (END), were measured in 24-h urine samples collected on the penultimate day of each study period using GC-MS. Linear mixed models were used to test the association between enterolignan excretion and metabolite abundances. Pathway analyses were conducted using the Global Test. Benjamini-Hochberg false discovery rate (FDR) was used to control for multiple testing.
Of the metabolites assayed, 121 were detected in all samples. ENL excretion was associated positively with plasma hippuric acid and melatonin, and inversely with epinephrine, creatine, glycochenodeoxycholate, and glyceraldehyde (P <0.05). Hippuric acid only satisfied the FDR of q < 0.1. END excretion was associated with myristic acid and glycine (q <0.5). Two of 57 pathways tested were associated significantly with ENL, ubiquinone and terpenoid-quinone biosynthesis, and inositol phosphate metabolism. These results suggest a potential role for ENL or ENL-metabolizing gut bacteria in regulating plasma metabolites.
Graphical abstract
Introduction
Cancer and other chronic diseases result from aberrant signaling in biological pathways relating to cell proliferation, inflammation and oxidative stress, among others.1 There is evidence that select phytochemicals or bioactive compounds in plant foods, such as lignans, modulate these pathways, thereby reducing cancer risk or progression.2, 3 Lignans are diphenolic compounds found in foods such as fruits, vegetables, seeds, the bran layer of grains, and legumes. Lignans commonly present in plant foods include pinoresinol, lariciresinol, matairesinol, medioresinol, sesamin, syringaresinol, and secoisolariciresinol.4–6 Dietary lignans are metabolized to two major enterolignans: enterodiol (END) and enterolactone (ENL) by gut bacteria involved in various enzymatic steps including deglycosylation, demethylation, dehydroxylation, reduction, and dehydrogenation.7
END and ENL are absorbed, conjugated in the gut epithelium or liver with sulfate or glucuronic acid, and excreted in the urine and bile. ENL accounts for the majority of enterolignans in circulation or excreted in the urine, and has a longer half-life than END.8 Aglycones such as secoisolariciresinol and matairesinol, and others, may be absorbed directly, or converted to intermediates before conversion to END and ENL, and subsequent excretion.9
END and ENL may exert weak estrogenic effects through binding of the estrogen receptor, and there is evidence of anti-inflammatory and antitumorigenic activity, as well as reduced oxidative stress with increased consumption of lignans.2, 10–13 High intake of dietary sources of lignans, as well as plasma END and ENL concentrations, have been associated with reduced risk of colorectal,14, 15 prostate, and breast cancer.16–18
The use of metabolomics, i.e. measurement of small metabolites in biospecimens or biofluids in biomedical research, systems biology and biomarker discovery, is rapidly increasing.19–23 The metabolic state reflects a number of genetic and environmental factors including diet, and metabolites may serve as biomarkers of dietary exposure. Metabolites may also be associated with signaling pathways related to the development of cancer, and therefore can serve as predictors of disease.24 Enterolignans, which eventually undergo enterohepatic circulation, may affect metabolic pathways by regulating sterol transporters25, steroid hormones and their receptors26, 27, and liver enzymes28. Hence, it is possible that enterolignans alter plasma metabolite abundances as a result of their activity on various tissues.
The mechanisms underlying the potentially beneficial health effects of lignans are not clear. We hypothesize that enterolignans affect metabolic signaling in multiple pathways influencing biological responses, and ultimately oncogenesis. There are at least two possible rationales for the association between ENL and metabolites: 1) ENL regulates endogenous metabolism, consequently altering metabolite concentrations, or 2) high ENL excretion reflects the presence of a microbial community that also alters metabolism of dietary compounds or produces signaling molecules that modify endogenous pathways and circulating metabolites. Our aim in this study was to conduct an exploratory analysis of the association of urinary excretion of enterolignans with metabolite profiles in a targeted plasma metabolomics approach in the context of a controlled feeding trial.
Methods
Study Design and Participants
The current study is a cross-sectional analysis based on dietary and biospecimen data obtained from a previous randomized crossover, controlled feeding trial, the Carbohydrate and Related Biomarkers (CARB) Study, which was designed to evaluate effects of specific diet patterns on biomarkers of cancer-risk pathways29. This trial tested two controlled diets for 28 days each. All food and beverages were prepared in the human nutrition laboratory at the Fred Hutchinson Cancer Research Center (Fred Hutch). Height and weight measurements were obtained at baseline, as well as whole-body dual-energy X-ray absorptiometry (DXA) scans to obtain body fat percentage, and weight was monitored throughout the study periods. Participants completed a daily checklist for consumption, and returned any unconsumed food, which was weighed and recorded. Fasting blood samples and 24-h urine samples were collected at the end of each diet period. Blood was collected after a 12 h fast in order to examine more stable biomarkers reflective of the dietary patterns. Weight was monitored throughout the study periods.
For each diet, a 7-day menu rotation was designed (ProNutra version 3.2, Viocare). Overall macronutrient distribution was the same (as % of energy), and fat and protein-containing foods were similar on both diets. While the diets were initially designed to differ by glycemic load, there were other notable differences. The low glycemic index diet was high in whole grains, including foods with more slowly absorbable carbohydrates (WG diet), while the other diet included more refined grains and rapidly absorbed carbohydrates from food sources of higher glycemic indices (RG diet). A total of 80 participants with complete biospecimen data were included in the present study. All study activities were performed in accordance with the Declaration of Helsinki and the study protocol was approved by the Fred Hutch Institutional Review Board (Initial approval received February 2006 with continuation review and approval obtained through May 2018) and Clinical Trials Office. All participants gave informed written consent. This trial was registered at https://www.clinicaltrials.gov as NCT00622661.
Assessment of Lignan Intake
Dietary lignan intakes of lariciresinol, matairesinol, pinoresinol, secoisolariciresinol, syringaresinol, and medioresinol were calculated for the 80 participants from the CARB study using diet records of all foods consumed seven days prior to urine collection at the end of each diet period, and thus representative of the complete cycle of study foods. Calculations were based primarily on the Phenol Explorer database of polyphenol content in foods30, which provides concentrations for common plant lignans, and other nutrient databases were referenced for unrepresented foods.31–36 Total lignan consumption was calculated by summing intakes of the 6 plant lignans. Values were summed and converted into mean daily intake for each participant.
Data Collection and Laboratory Analysis
END and ENL were measured from 24-h urine samples collected at the end of each feeding period. Urine was extracted and enzymatically hydrolyzed and aglycones, along with deuterated internal standards, were analyzed as trimethylsilyl derivatives by gas chromatography–mass spectrometry (GC-MS)37, in a total of 8 batches. The lowest level of quantification (LOQ) for END and ENL in 4 ml of urine was 3.12 ng/ml. Mean intra- and inter-run coefficients of variation (CV) for quality control samples were 4.8% and 9.5% for END and 2.4% and 2.9% for ENL, respectively. Values are represented as µmol excreted in a 24-h urine sample.
Blood was drawn after a 12-hour fast from each participant on day 28 of each diet period, processed and stored at −80 °C. Targeted HILIC liquid chromatography (LC)-mass spectrometry (MS) metabolite analysis of human plasma samples was performed at the University of Washington Northwest Metabolomics Research Center as previously described.38, 39 Following a standard protocol, frozen plasma was thawed at 4 °C and a 50 µL aliquot was spiked with a 50 µL mixture containing 22 stable isotope-labeled amino and organic acid internal standards (SILISs) of known concentrations. Methanol (250 µL) containing two SILISs was added and the mixture was vortexed for 10 sec. After 20 min at −20 °C, the samples were centrifuged at 18,000 × g for 15 min. The supernatant (150 µL) was collected and then dried at 30 °C in a Speed-Vac for 105 min. At the end, dried samples were reconstituted in 0.5 mL HILIC LC solvent containing two more SILISs, centrifuged at 18,000 × g for 5 min, and collected supernatants were transferred to LC vials for targeted LC-MS analysis. Samples were prepared in batches of 30 (60 samples per day).
The targeted platform consisted of a dual pump Agilent 1260 LC system running HILIC chromatography coupled to AB-Sciex 5500 QTrap MS operating in multiple-reaction-monitoring (MRM) mode. 103 (81 metabolites plus 22 stable isotope-labeled internal standards) and 123 (119 metabolites and 4 stable isotope-labeled internal standards) compounds belonging to38 major metabolic pathways based on the KEGG database were targeted in positive and negative ionization modes, respectively (total: 200 metabolites and 26 stable isotope-labeled internal standards). In total, 121 metabolites and 24 internal standards were measured across samples. In order to monitor the LC-MS assay performance and to assess the reproducibility of the measurements, a quality control plasma sample was injected for every 10 study samples. The LC-MS assay was run over 10-day period of non-stop data acquisition. The average CV over the 10-day period was 8.5% (based on MRM peak areas without any MS signal normalization) and 80% of measured MRM peaks had CV < 10%. Only six measured metabolic species had CV > %15. The use of 26 stable isotope-labeled internal standards enabled sample prep monitoring and the absolute quantitation of 24 metabolites.
Statistical Analysis
Linear mixed models were used to test the association of 24-h urinary enterolignan (END and ENL) excretion as a continuous variable with plasma metabolite concentrations individually, with fixed effects including age, sex, percentage body fat, batch (metabolomics data), study diet, and diet sequence, and participant as the random effect. A very small value (1 × 10−4) was imputed for zero values for ENL or END (a total of 4 and 21, respectively). All statistical analyses were performed on log-transformed values of enterolignan excretion and metabolite abundances. Statistical analyses were conducted in SAS 9.4.
Pathway analysis using the Global Test in MetaboAnalyst 3.0 was performed on all 160 samples (from both diet periods) without accounting for the repeated measures.40–42 Benjamini-Hochberg false discovery rate (FDR) was used to control for multiple testing with a significance level of q < 0.1.
Results
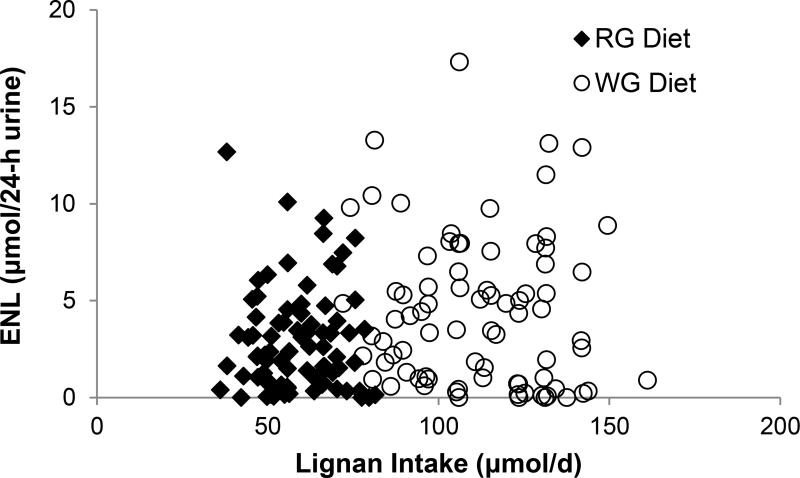
Characteristics of 80 CARB study participants completing both the WG and RG diets, stratified by urinary excretion of ENL and END (above vs. below-median) are shown in Table 1, as we were interested in examining whether there were any differences at baseline between high and low ENL excreters in factors that might also affect plasma metabolite profiles. Overall, the majority of participants were White, followed by Hispanic. A greater proportion of Black participants were in the lower-median excretion categories for END and ENL (82%) compared to the other ethnic groups on the WG diet, and Black participants also excreted low amounts of END on the RG diet. There were no notable differences in dietary intake of lignans between participants in the above- and below-median excretion groups for END or ENL. Consistent with this, there was no linear correlation between dietary lignan intake and ENL excretion for participants on either the WG or RG diets (Figure 1), and regression analysis revealed no significant association (data not shown).
Table 1.
Baseline characteristics of participants in the CARB Study stratified by enterolactone (ENL) and enterodiol (END) excretion on the whole grain (WG) and refined grain (RG) diets.a
| WG Diet | RG Diet | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
||||||
| All | ENL | END | ENL | END | |||||
|
|
|
|
|
||||||
| <4.13 | ≥ 4.13 | <0.45 | ≥ 0.45 | <2.20 | ≥ 2.20 | <0.16 | ≥ 0.16 | ||
| Participants, n | 80 | 41 | 39 | 43 | 37 | 41 | 39 | 40 | 40 |
| Males, n (%) | 40 (50) | 20 (50) | 20 (50) | 23 (57.5) | 17 (42.5) | 21 (52.5) | 19 (47.5) | 21 (52.5) | 19 (47.5) |
| Females, n (%) | 40 (50) | 21 (52.5) | 19 (47.5) | 20 (50) | 20 (50) | 21 (52.5) | 19 (47.5) | 19 (47.5) | 21 (52.5) |
| Ethnicity, n (%) | |||||||||
| White | 35 (44) | 12 (34) | 23 (66) | 16 (46) | 19 (54) | 13 (37) | 22 (63) | 17 (49) | 18 (51) |
| Hispanic | 20 (25) | 10 (50) | 10 (50) | 7 (35) | 13 (65) | 11 (65) | 9 (35) | 9 (45) | 11 (55) |
| Black | 17 (21) | 13 (76) | 4 (24) | 14(82) | 3 (18) | 11 (55) | 6 (45) | 11 (65) | 6 (35) |
| Other | 8 (10) | 6 (75) | 2 (25) | 6 (75) | 2 (25) | 6 (75) | 2 (25) | 3 (37.5) | 5 (62.5) |
| Age, y (SD) | 29.6 (8.1) | 28.7 (8.3) | 30.6 (8.0) | 30.1 (8.2) | 29.0 (8.2) | 28.4 (8.1) | 30.9 (8.1) | 29.5 (8.1) | 29.8 (8.3) |
| Weight, kg (SD) | 81.1 (21.6) | 82.5 (23.3) | 79.7 (20.0) | 80.8 (25.0) | 81.5 (17.5) | 79.4 (23.3) | 82.9 (20.0) | 82.2 (23.6) | 80.1 (19.8) |
| BMI, kg/m2 (SD) | 27.4 (5.9) | 27.6 (6.0) | 27.2 (5.9) | 27.0 (6.2) | 27.9 (5.6) | 26.8 (5.8) | 28.1 (6.0) | 27.6 (5.5) | 27.3 (6.4) |
| Body fat, % (SD) | 32.8 (11.8) | 33.9 (10.8) | 31.8 (12.9) | 31.2 (10.5) | 34.7 (13.1) | 32.8 (10.6) | 32.9 (13.1) | 33.3 (22.4) | 32.4 (12.4) |
| Total lignans, mg/d (SD) | 30.7 (11.2) | 40.4 (7.8) | 40.0 (7.1) | 40.8 (7.9) | 39.6 (7.5) | 21.2 (4.2) | 21.3 (3.8) | 21.0 (4.3) | 21.5 (3.7) |
NOTE: Values are expressed as means or proportions
Urinary enterolignan excretion in µmol/24 h and separated by the median
ENL=enterolactone; END=enterodiol; WG= whole grain diet; RG = refined grain diet
Figure 1.
Scatter plot of dietary lignan intake and urinary enterolactone (ENL) excretion in 80 participants. Dietary intake (µmol/d) during the final seven days before urine collection was calculated using Phenol Explorer. ENL excretion was measured from 24-h urine samples collected at the end of the RG and WG diet periods. Plot shows the association between lignan intake and ENL during each feeding period (black diamond = refined grain (RG) diet, open circle= whole grain (WG) diet). There was no significant association between ENL excretion and dietary lignan intake when analyzed in a multiple linear regression model.
Table 2 shows average daily intake of six common plant lignans as well as the total amount of lignans and fiber consumed on each study diet. Consumption of all plant lignans was significantly higher on the WG diet compared to the RG diet. Lariciresinol and pinoresinol together accounted for 85–88% of total lignans consumed. Similar to total dietary lignan intake, dietary fiber content of the WG diet was approximately twice that of the RG diet. Mean excretion of END and ENL were also increased on the WG diet relative to the RG diet.
Table 2.
Comparison of dietary lignan intake and enterolignan excretion between whole grain (WG) and refined grain (RG) dietary patterns in 80 participants in the CARB Study
| WG Diet | RG Diet | |||
|---|---|---|---|---|
| Dietary plant lignan intake | ||||
| Mean, SD (range)a | mg/d | µmol/d | mg/d | µmol/d |
| Lariciresinol | 20.8, 3.9 (12.8 – 29.4) | 57.7, 10.8 (35.6 – 81.7) | 12.9, 2.5 (7.0 – 17.8) | 35.9, 7.0 (19.4 – 49.3) |
| Pinoresinol | 13.6, 2.5 (8.6 – 20.0) | 38.0, 7.0 (23.9 – 55.7) | 6.7, 1.3 (4.3 – 10.7) | 18.8, 3.6 (12.0 – 29.8) |
| Secoisolariciresinol | 4.0, 0.8 (1.3 – 6.1) | 11.2, 2.4 (3.5 – 16.8) | 1.4, 0.2 (0.87 – 1.9) | 3.8, 0.68 (2.4 – 5.1) |
| Matairesinol | 0.48, 0.12 (0.10 – 0.70) | 1.3, 0.33 (0.27 – 2.0) | 0.11, 0.03 (0.07 – 0.24) | 0.31, 0.07 (0.20 – 0.68) |
| Syringaresinol | 0.20, 0.04 (0.13 – 0.28) | 0.48, 0.09 (0.31 -0.68) | 0.11, 0.02 (0.07 – 0.15) | 0.26, 0.05 (0.16 – 0.35) |
| Medioresinol | 0.25, 0.05 (0.12 – 0.36) | 0.63, 0.13 (0.30 – 0.92) | 0.006, 0.001 (0.001 – 0.009) | 0.02, 0.003 (0.003 – 0.02) |
| Sesamin | 0.83, 0.22 (0 – 1.2) | 2.3, 0.62 (0 – 3.4) | 0 | 0 |
| Total lignans | 40.2, 7.5 (25.9 – 58.0) | 111.6, 20.7 (72.0 – 161.1) | 21.3, 4.0 (13.0 – 29.3) | 59.1, 11.1 (36.1–81.5) |
| Dietary fiber intake (g/d) | ||||
| Mean, SD (range)b | 55.3, 14 (35.2 – 77.9) | 28.1, 8 (17.7 – 38.0) | ||
| Urinary enterolignan excretion (µmol/24-h urine) | ||||
| Mean, SD (range) | ||||
| Enterolactone (ENL) | 4.5, 3.8 (0 – 17.3) | 2.9, 2.6 (0 – 12.7) | ||
| Enterodiol (END) | 0.8, 1.7 (0 – 13.7) | 0.4, 0.7 (0 – 5.3) | ||
Corresponds to mean consumption over the final 7 days before urine collection for the respective controlled diet
Corresponds to mean consumption during study diet period
We examined the association between enterolignan excretion and plasma abundances of metabolites determined by targeted LC-MS based metabolomics (Table 3). Seven plasma metabolites associated linearly with ENL excretion at P<0.05. Only one metabolite satisfied the FDR of q < 0.1 (hippuric acid). ENL excretion was positively associated with plasma hippuric acid and melatonin, and inversely associated with epinephrine, creatine, glycochenodeoxycholate, and glyceraldehyde. Two plasma metabolites were associated with END excretion; myristic acid was inversely associated (P =0.003), and glycine was positively associated (P=0.02). However, these metabolites did not satisfy the FDR threshold of q < 0.1.
Table 3.
Metabolites associated with enterolactone (ENL) and enterodiol (END) excretion (µmol/24-h)a
| Metabolite | Metabolic Pathway | Estimateb | P-valuec | q-valued |
|---|---|---|---|---|
| ENL | ||||
| Hippuricacid | phenylalanine | 0.13 | 0.0008 | 0.09* |
| Melatonin | tryptophan | 0.05 | 0.009 | 0.48 |
| Epinephrine | tyrosine | −0.02 | 0.014 | 0.48 |
| Creatine | amino acid | −0.03 | 0.019 | 0.48 |
| Glycochenodeoxycholate | bile acid | −0.08 | 0.020 | 0.48 |
| Glyceraldehyde | pentose phosphate | −0.02 | 0.042 | 0.85 |
| END | ||||
| Myristic acid | lipid/fatty acid | −0.03 | 0.003 | 0.35 |
| Glycine | amino acid, nucleotide, microbial | 0.01 | 0.021 | 0.95 |
Multiple linear regression from a mixed model with fixed effects urinary enterolignan excretion (continuous), age, sex, batch, percentage body fat, diet sequence, study diet
Beta coefficient from linear mixed models
Significant at p <0.05
Benjamini-Hochberg (False Discovery Rate; FDR)
Significant at FDR q <0.1
Among the 57 pathways containing metabolites represented in our panel, two were significantly associated with ENL at an FDR level of q < 0.1 using the Global Test. These included the inositol phosphate metabolism, and ubiquinone and other terpenoid-quinone biosynthesis pathways, which were sparsely represented, with 1 and 2 metabolites each, respectively (data not shown).
Discussion
In this randomized crossover controlled feeding trial, we found that urinary excretion of ENL was positively associated with higher plasma concentrations of hippuric acid, and the association was significant with FDR correction. Other metabolites that were significant at p < 0.05 were melatonin, epinephrine, creatine, glycochenodeoxycholate and glyceraldehyde. By analyzing plasma metabolites involved in a range of biological processes, we have taken a step towards uncovering mechanisms associated with lignan-mediated health effects in this exploratory analysis.
In the present study, higher urinary excretion of the enterolignans ENL and END was observed on the WG diet relative to the RG diet, which is to be expected given the greater proportion of lignan-containing foods on the WG diet. Major lignan-contributing food sources on the WG diet included strawberries, carrots, bell peppers, and grapefruit, and apricots, cauliflower, potatoes, and bell peppers on the RG diet. Interestingly, we found lignan intakes to be much higher than those reported previously. Even on the RG diet, total lignan intakes (ranging from 13 to 29 mg/day) exceeded the previous reports of about 1 mg/day (and ~23 g/d of fiber).43 For Western countries, reported mean intakes range from 0.1 – 2 mg/day.44 These differences could be explained in part by the expanded databases that now include a more complete plant lignan profile; earlier estimates were often limited to two or three compounds.45 Furthermore, results from controlled feeding studies are likely to be different from those obtained with food frequency questionnaires. Food records from controlled feeding studies allow for calculation of intakes of very specific items that may be considerably high in polyphenol content, whereas food frequency questionnaires might only obtain an overall estimate of a food type in a line item combination of several similar foods.
Gut bacteria play a prominent role in production of enterolignans. After consumption of dietary plant lignans, conversion to the bioactive compounds, END and ENL occurs through the activity of gut bacteria. Accordingly, we previously reported that increased excretion of ENL is associated with composition and diversity of the gut microbial community.46 Unlike plant lignan intakes, mean excretion of ENL (4.6 µmol on WG diet and 3.0 µmol on RG diet) and END (0.82 µmol on WG diet and 0.41 µmol on RG diet) of participants in our study was consistent with what has been reported previously,47–50 although not as high as END and ENL levels obtained (9–12 µmol/24h) after ingestion of a single dose of SDG (1.3 µmol/kg body weight).8 We did not observe a statistically significant linear association between ENL and plant lignan intakes. This is consistent with previous observational studies, in which weak correlations were reported (Spearman r = 0.16 – 0.21).51, 52 In general, excretion of END and ENL varied widely among participants in our study, which, beyond differences in lignan intakes, is likely attributable to inter-individual variation in the gut microbiota, intestinal transit time, rate of absorption, or other differences in the intestinal environment affecting metabolite production, as well as genetic variation in transporters and biotransformation enzymes .8, 9, 46, 53, 54 Interindividual variation in ENL has been observed previously under controlled conditions in human participants8. Such interindividual variation may be expected given the various precursors involved in ENL conversion and possible intermediate metabolites. In our study there were no observed differences by sex, and participants had not used antibiotics within the previous 3 months. Hence, the absence of a linear association between ENL excretion and total lignan intakes among participants in our study further supports the significance of the gut microbial composition and activity in ENL production, and ultimately ENL exposure by the host.
The most significant difference observed with increasing ENL excretion was increased plasma hippuric acid, the only metabolite satisfying the FDR threshold of 0.1. Urinary excretion of hippuric acid correlates with consumption of polyphenols, including flavonoid-rich fruits and vegetables, notably, anthocyanin-rich berries, as well as green and black tea55–57. Polyphenols commonly require transformation by gut bacteria for absorption. Consequently, hippuric acid production, like ENL, is highly dependent on activity of the gut bacteria. Hippuric acid is rapidly synthesized from conversion of polyphenolic compounds into the smaller phenolic acids benzoic acid and cinnamic acid (by activity of microbiota in the gut), which may also be derived from phenylalanine58–60 Benzoic acid is then conjugated with glycine to form hippuric acid.61 Other glycine conjugated aromatic compounds derived from phenylalanine catabolites are also generated by gut bacteria, and can be absorbed from the intestine. Hippuric acid was found to be 17-fold higher in plasma from conventional mice compared with their germ-free counterparts.59 Gram negative bacteria in particular play a role in its metabolism, as demonstrated through inhibition of hippuric acid production in the presence of neomycin.62 Additionally, urinary excretion of hippurate is reduced with antibiotic treatment.63,64 Blautia producta (previously Ruminococcus productus), Eubacterium limosum, and strains of Clostridium and Eubacterium genera which have been associated with metabolism of plant polyphenols,65 may also be implicated in ENL metabolism15, 53, 59. In a separate study modeling microbial-host connections in healthy humans, bacteria of the Clostridia class, particularly, were shown to positively correlate with hippuric acid.58 It follows that hippuric acid is increased synonymously with ENL likely as a consequence of increases of select communities of gram negative bacteria, and this is consistent with other reported correlations between gut bacteria and metabolic phenotypes. Nonetheless, there is also the possibility that positive associations between ENL and plasma metabolites, such as hippuric acid, reflect shared metabolic pathways. Further, variation in genes coding for biotransformation enzymes and transporter proteins which results in functional differences, may also explain some of the interindividual variation.66, 67
Melatonin, which was also increased with ENL, is an endogenous metabolite produced by the pineal gland involved in regulation of the circadian rhythm. Its biosynthesis begins with tryptophan, a metabolite of the shikimate pathway, which is converted to serotonin before conversion to melatonin. Interestingly, ENL was shown to regulate expression of circadian clock genes through activity of the estrogen receptor in mice.68 Furthermore, enterolignans regulate signaling via NFκB and Nrf-2, and may interact with CREB or CRB binding protein, which are also associated with increased melatonin synthesis, to regulate transcription of genes associated with inflammatory signaling.69–71 Melatonin is synthesized by plants72, including food-source ones such as banana, tomato, rice, oats, corn, barley, and it is also present in tea, coffee, and wine.73–75 Thus, in addition to potential ENL-regulated endogenous metabolism, increases in melatonin may have been associated with increased consumption of plant foods and particularly grains on the WG diet.
Melatonin is also produced by bacteria, particularly aerobic photosynthetic bacteria and cyanobacteria, with some variations in enzymatic reactions.76 Some (indole-containing) metabolites derived from tryptophan can be produced by Clostridium, which are also involved in metabolism of enterolignans7, and other enteric bacteria expressing tryptophanase,59, 77 potentially altering the availability of tryptophan for melatonin synthesis. While gut bacteria may affect the availability of tryptophan, it is unlikely that they have direct effects on endogenous melatonin production, which is very tightly regulated.
Interestingly, ENL excretion was inversely associated with concentrations of a major bile acid, glycochenodeoxycholate. Chenodeoxycholic acid is synthesized endogenously from cholesterol, and conjugated with glycine to become glycochenodeoxycholate. Subsequently it is secreted into the gut, where it can be deconjugated by the activity of gut bacteria, or reabsorbed from the small intestine. C. Scindens and other 7α-hydroxylating bacteria containing bile acid-inducible genes regulate the levels of chenodeoxycholic acid,9, 78 and consequently, glycochenodeoxycholate in the circulation. It is conceivable that regulation of this bile acid is explained by increases in select communities of gut bacteria associated with higher ENL. ENL may also regulate pregnane X receptor, which is involved in bile acid metabolism27. Importantly, changes in deoxycholic acid and other bile acids can delineate metabolic diseases or conditions.79–81
Epinephrine is a neurotransmitter synthesized endogenously from tyrosine. Gut bacteria of the Clostridia genera, play an important role in fermentation of amino acids in both the small and large intestine82, 83 and consequently, may alter the bioavailability of amino acids, as may other species.84 Therefore, it is possible that the observed differences in epinephrine with ENL are partially explained by inter-individual differences in activity or composition of the gut microbial community. Alternatively, it is possible that lower plasma epinephrine is a result of downstream signaling associated with increased melatonin.85 Besides epinephrine, creatine, and glyceraldehyde, other metabolites related to amino acid metabolism, were all decreased with increasing ENL excretion, although not satisfying the FDR threshold. Decreases in glycine and other amino acids in the hepatic portal vein were recently reported in conventional mice compared to germ-free animals, with the implication that amino acids are likely consumed by bacteria as part of their own metabolism and supporting their growth.86 Therefore decreases in amino acid-related plasma metabolites associated with higher ENL may be explained by higher gut bacterial metabolic activity.
Findings from pathway analysis are limited, as this was an exploratory analysis which did not take into account the crossover design or additional covariates. Only two pathways were associated with ENL excretion: ubiquinone and terpenoid quinone biosynthesis, and inositol phosphate metabolism. However, there was no overlap with plasma metabolites identified in individual analyses.
Only two plasma metabolites were associated with END excretion, with none satisfying the FDR. END is an intermediate in microbial metabolism, and consequently, a less useful marker of gut microbial activity than ENL, the predominant enterolignan in urine and serum. Furthermore, differences in metabolite abundances associated with END and ENL excretion may also be explained by distinct communities of enterolignan-producing bacteria.87, 88 As a saturated fatty acid, myristic acid is obtained from the diet, and has been associated with increased serum cholesterol and inflammation89, 90. Hence, an inverse relationship between myristic acid and END might be expected. There was, on the other hand, a marginal increase in glycine with increasing END. This increase correlates with the increase in hippuric acid noted with increased ENL. Additionally, glycine has been associated previously with decreases in free fatty acids in plasma91. Thus, the decrease in myristic acid is perhaps partly attributable to increased glycine.
To our knowledge this is the first study investigating the relationship between urinary ENL and END excretion and plasma metabolites. A major strength of this study is the controlled feeding design. With such a design, the effect of inter-individual variation in diet on enterolignans is diminished since individuals consumed all the same foods, though in varying amounts, while on each controlled diet. Therefore, it is unlikely that differences in ENL profiles in this study may be attributed to unknown confounding by consumption of select lignans or overall lignan consumption. The use of targeted metabolite profiling currently allows for detection of ~200 metabolites in many important metabolic pathways with good reproducibility. However, while our targeted platform contained metabolites in 38 different metabolic pathways, some pathways were underrepresented. Moreover, our findings are limited without associated data on gut microbial community structure. Also, there was still a range in lignan dose as a result of differences in energy and food needs among participants. Differing or missing values for lignan content for some study foods consumed may have also have resulted in underestimates of plant lignan intake. Additionally, it should be pointed out that diet, and other related factors or behaviors unaccounted for in the current study, may affect both metabolite abundances and ENL excretion independently. Thus, in such a cross-sectional analysis, a direct regulatory mechanism cannot be ascertained.
Conclusions
In conclusion, increased urinary excretion of ENL was significantly associated with increased hippuric acid in plasma, and to a lesser degree, decreases in plasma metabolites mediating nucleic acid, amino acid, and bile acid metabolism. These findings suggest a role for ENL excretion as a surrogate for a gut microbial profile associated with metabolic processes, as well as a possible role of ENL in regulation of endogenous metabolism, although the mechanisms are unknown.
Acknowledgments
Research for this study was supported by the NIH (R25 CA092408, R01 CA192222, R01 CA192222-01A1S1, R01 GM114029, P30 CA015704and U54 CA116847), and the Nutrition and Obesity Research Center (P30 DK35816).
Footnotes
Conflicts of Interest
The authors have no conflicts of interest to declare.
References
- 1.Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB. Oxidative stress, inflammation, and cancer: how are they linked? Free Radic Biol Med. 2010;49(11):1603–16. doi: 10.1016/j.freeradbiomed.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adolphe JL, Whiting SJ, Juurlink BH, Thorpe LU, Alcorn J. Health effects with consumption of the flax lignan secoisolariciresinol diglucoside. Br J Nutr. 2010;103(7):929–38. doi: 10.1017/S0007114509992753. [DOI] [PubMed] [Google Scholar]
- 3.Webb AL, McCullough ML. Dietary lignans: potential role in cancer prevention. Nutr Cancer. 2005;51(2):117–31. doi: 10.1207/s15327914nc5102_1. [DOI] [PubMed] [Google Scholar]
- 4.Axelson M, Sjovall J, Gustafsson BE, Setchell KD. Origin of lignans in mammals and identification of a precursor from plants. Nature. 1982;298(5875):659–60. doi: 10.1038/298659a0. [DOI] [PubMed] [Google Scholar]
- 5.Heinonen S, Nurmi T, Liukkonen K, Poutanen K, Wahala K, Deyama T, et al. In vitro metabolism of plant lignans: new precursors of mammalian lignans enterolactone and enterodiol. J Agric Food Chem. 2001;49(7):3178–86. doi: 10.1021/jf010038a. [DOI] [PubMed] [Google Scholar]
- 6.Penalvo JL, Nurmi T, Haajanen K, Al-Maharik N, Botting N, Adlercreutz H. Determination of lignans in human plasma by liquid chromatography with coulometric electrode array detection. Anal Biochem. 2004;332(2):384–93. doi: 10.1016/j.ab.2004.05.046. [DOI] [PubMed] [Google Scholar]
- 7.Clavel T, Henderson G, Engst W, Dore J, Blaut M. Phylogeny of human intestinal bacteria that activate the dietary lignan secoisolariciresinol diglucoside. FEMS Microbiol Ecol. 2006;55(3):471–8. doi: 10.1111/j.1574-6941.2005.00057.x. [DOI] [PubMed] [Google Scholar]
- 8.Kuijsten A, Arts IC, Vree TB, Hollman PC. Pharmacokinetics of enterolignans in healthy men and women consuming a single dose of secoisolariciresinol diglucoside. J Nutr. 2005;135(4):795–801. doi: 10.1093/jn/135.4.795. [DOI] [PubMed] [Google Scholar]
- 9.Clavel T, Dore J, Blaut M. Bioavailability of lignans in human subjects. Nutr Res Rev. 2006;19(2):187–96. doi: 10.1017/S0954422407249704. [DOI] [PubMed] [Google Scholar]
- 10.Pietrofesa RA, Velalopoulou A, Arguiri E, Menges CW, Testa JR, Hwang WT, et al. Flaxseed lignans enriched in secoisolariciresinol diglucoside prevent acute asbestos-induced peritoneal inflammation in mice. Carcinogenesis. 2016;37(2):177–87. doi: 10.1093/carcin/bgv174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shimizu S, Ishigamori R, Fujii G, Takahashi M, Onuma W, Terasaki M, et al. Involvement of NADPH oxidases in suppression of cyclooxygenase-2 promoter-dependent transcriptional activities by sesamol. J Clin Biochem Nutr. 2015;56(2):118–22. doi: 10.3164/jcbn.14-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qiang L, Yuan J, Shouyin J, Yulin L, Libing J, Jian-An W. Sesamin Attenuates Lipopolysaccharide-Induced Acute Lung Injury by Inhibition of TLR4 Signaling Pathways. Inflammation. 2016;39(1):467–72. doi: 10.1007/s10753-015-0270-6. [DOI] [PubMed] [Google Scholar]
- 13.Cassani RS, Fassini PG, Silvah JH, Lima CM, Marchini JS. Impact of weight loss diet associated with flaxseed on inflammatory markers in men with cardiovascular risk factors: a clinical study. Nutr J. 2015;14:5. doi: 10.1186/1475-2891-14-5. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 14.Jenab M, Thompson LU. The influence of flaxseed and lignans on colon carcinogenesis and beta-glucuronidase activity. Carcinogenesis. 1996;17(6):1343–8. doi: 10.1093/carcin/17.6.1343. [DOI] [PubMed] [Google Scholar]
- 15.Yoder S, Lancaster S, Hullar M, Lampe J. Gut Microbial Metabolism of Plant Lignans:Influence on Human Health. In: Rio KTDD, editor. Diet-Microbe Interactions In The Gut Effects on Human Health and Disease. Elsevier, Inc.; 2015. pp. 103–17. [Google Scholar]
- 16.Anderson LN, Cotterchio M, Boucher BA, Kreiger N. Phytoestrogen intake from foods, during adolescence and adulthood, and risk of breast cancer by estrogen and progesterone receptor tumor subgroup among Ontario women. Int J Cancer. 2013;132(7):1683–92. doi: 10.1002/ijc.27788. [DOI] [PubMed] [Google Scholar]
- 17.Jackson MD, McFarlane-Anderson ND, Simon GA, Bennett FI, Walker SP. Urinary phytoestrogens and risk of prostate cancer in Jamaican men. Cancer Causes Control. 2010;21(12):2249–57. doi: 10.1007/s10552-010-9648-9. [DOI] [PubMed] [Google Scholar]
- 18.McCann MJ, Rowland IR, Roy NC. Anti-proliferative effects of physiological concentrations of enterolactone in models of prostate tumourigenesis. Mol Nutr Food Res. 2013;57(2):212–24. doi: 10.1002/mnfr.201200362. [DOI] [PubMed] [Google Scholar]
- 19.Gu H, Carroll PA, Du J, Zhu J, Neto FC, Eisenman RN, et al. Quantitative Method to Investigate the Balance between Metabolism and Proteome Biomass: Starting from Glycine. Angew Chem Int Ed Engl. 2016;55(50):15646–50. doi: 10.1002/anie.201609236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sperber H, Mathieu J, Wang Y, Ferreccio A, Hesson J, Xu Z, et al. The metabolome regulates the epigenetic landscape during naive-to-primed human embryonic stem cell transition. Nat Cell Biol. 2015;17(12):1523–35. doi: 10.1038/ncb3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu J, Djukovic D, Deng L, Gu H, Himmati F, Chiorean EG, et al. Colorectal cancer detection using targeted serum metabolic profiling. J Proteome Res. 2014;13(9):4120–30. doi: 10.1021/pr500494u. [DOI] [PubMed] [Google Scholar]
- 22.Johnson CH, Ivanisevic J, Siuzdak G. Metabolomics: beyond biomarkers and towards mechanisms. Nat Rev Mol Cell Biol. 2016;17(7):451–9. doi: 10.1038/nrm.2016.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nagana Gowda GA, Raftery D. Recent Advances in NMR-Based Metabolomics. Anal Chem. 2017;89(1):490–510. doi: 10.1021/acs.analchem.6b04420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Armitage EG, Barbas C. Metabolomics in cancer biomarker discovery: current trends and future perspectives. J Pharm Biomed Anal. 2014;87:1–11. doi: 10.1016/j.jpba.2013.08.041. [DOI] [PubMed] [Google Scholar]
- 25.Liang YT, Chen J, Jiao R, Peng C, Zuo Y, Lei L, et al. Cholesterol-lowering activity of sesamin is associated with down-regulation on genes of sterol transporters involved in cholesterol absorption. J Agric Food Chem. 2015;63(11):2963–9. doi: 10.1021/jf5063606. [DOI] [PubMed] [Google Scholar]
- 26.Dikshit A, Gomes Filho MA, Eilati E, McGee S, Small C, Gao C, et al. Flaxseed reduces the pro-carcinogenic micro-environment in the ovaries of normal hens by altering the PG and oestrogen pathways in a dose-dependent manner. Br J Nutr. 2015;113(9):1384–95. doi: 10.1017/S000711451500029X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jacobs MN, Nolan GT, Hood SR. Lignans, bacteriocides and organochlorine compounds activate the human pregnane X receptor (PXR) Toxicol Appl Pharmacol. 2005;209(2):123–33. doi: 10.1016/j.taap.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 28.Xu C, Liu Q, Zhang Q, Jiang ZY, Gu A. Urinary enterolactone associated with liver enzyme levels in US adults: National Health and Nutrition Examination Survey (NHANES) Br J Nutr. 2015;114(1):91–7. doi: 10.1017/S000711451500149X. [DOI] [PubMed] [Google Scholar]
- 29.Neuhouser ML, Schwarz Y, Wang C, Breymeyer K, Coronado G, Wang CY, et al. A low-glycemic load diet reduces serum C-reactive protein and modestly increases adiponectin in overweight and obese adults. J Nutr. 2012;142(2):369–74. doi: 10.3945/jn.111.149807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Neveu V, Perez-Jimenez J, Vos F, Crespy V, du Chaffaut L, Mennen L, et al. Phenol-Explorer: an online comprehensive database on polyphenol contents in foods. Database (Oxford) 2010;2010 doi: 10.1093/database/bap024. bap024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Durazzo A, Zaccaria M, Polito A, Maiani G, Carcea M. Lignan Content in Cereals, Buckwheat and Derived Foods. Foods. 2013;2(1):53–63. doi: 10.3390/foods2010053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kuhnle GG, Dell'aquila C, Aspinall SM, Runswick SA, Mulligan AA, Bingham SA. Phytoestrogen content of cereals and cereal-based foods consumed in the UK. Nutr Cancer. 2009;61(3):302–9. doi: 10.1080/01635580802567141. [DOI] [PubMed] [Google Scholar]
- 33.Milder IE, Arts IC, van de Putte B, Venema DP, Hollman PC. Lignan contents of Dutch plant foods: a database including lariciresinol, pinoresinol, secoisolariciresinol and matairesinol. Br J Nutr. 2005;93(3):393–402. doi: 10.1079/bjn20051371. [DOI] [PubMed] [Google Scholar]
- 34.Moreno-Franco B, Garcia-Gonzalez A, Montero-Bravo AM, Iglesias-Gutierrez E, Ubeda N, Maroto-Nunez L, et al. Dietary alkylresorcinols and lignans in the Spanish diet: development of the alignia database. J Agric Food Chem. 2011;59(18):9827–34. doi: 10.1021/jf2015446. [DOI] [PubMed] [Google Scholar]
- 35.Penalvo JL, Adlercreutz H, Uehara M, Ristimaki A, Watanabe S. Lignan content of selected foods from Japan. J Agric Food Chem. 2008;56(2):401–9. doi: 10.1021/jf072695u. [DOI] [PubMed] [Google Scholar]
- 36.Thompson LU, Boucher BA, Liu Z, Cotterchio M, Kreiger N. Phytoestrogen content of foods consumed in Canada, including isoflavones, lignans, and coumestan. Nutr Cancer. 2006;54(2):184–201. doi: 10.1207/s15327914nc5402_5. [DOI] [PubMed] [Google Scholar]
- 37.Atkinson C, Lampe JW, Scholes D, Chen C, Wahala K, Schwartz SM. Lignan and isoflavone excretion in relation to uterine fibroids: a case-control study of young to middle-aged women in the United States. Am J Clin Nutr. 2006;84(3):587–93. doi: 10.1093/ajcn/84.3.587. [DOI] [PubMed] [Google Scholar]
- 38.Chiao YA, Kolwicz SC, Basisty N, Gagnidze A, Zhang J, Gu H, et al. Rapamycin transiently induces mitochondrial remodeling to reprogram energy metabolism in old hearts. Aging (Albany NY) 2016;8(2):314–27. doi: 10.18632/aging.100881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parent BA, Seaton M, Sood RF, Gu H, Djukovic D, Raftery D, et al. Use of Metabolomics to Trend Recovery and Therapy After Injury in Critically Ill Trauma Patients. JAMA Surg. 2016;151(7):e160853. doi: 10.1001/jamasurg.2016.0853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xia J, Wishart DS. MetPA: a web-based metabolomics tool for pathway analysis and visualization. Bioinformatics. 2010;26(18):2342–4. doi: 10.1093/bioinformatics/btq418. [DOI] [PubMed] [Google Scholar]
- 41.Goeman JJ, Buhlmann P. Analyzing gene expression data in terms of gene sets: methodological issues. Bioinformatics. 2007;23(8):980–7. doi: 10.1093/bioinformatics/btm051. [DOI] [PubMed] [Google Scholar]
- 42.Xia J, Wishart DS. Using MetaboAnalyst 3.0 for Comprehensive Metabolomics Data Analysis. Curr Protoc Bioinformatics. 2016;55:14 0 1–0 91. doi: 10.1002/cpbi.11. [DOI] [PubMed] [Google Scholar]
- 43.Milder IE, Kuijsten A, Arts IC, Feskens EJ, Kampman E, Hollman PC, et al. Relation between plasma enterodiol and enterolactone and dietary intake of lignans in a Dutch endoscopy-based population. J Nutr. 2007;137(5):1266–71. doi: 10.1093/jn/137.5.1266. [DOI] [PubMed] [Google Scholar]
- 44.Peterson J, Dwyer J, Adlercreutz H, Scalbert A, Jacques P, McCullough ML. Dietary lignans: physiology and potential for cardiovascular disease risk reduction. Nutr Rev. 2010;68(10):571–603. doi: 10.1111/j.1753-4887.2010.00319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Norskov NP, Olsen A, Tjonneland A, Bolvig AK, Laerke HN, Knudsen KE. Targeted LC-MS/MS Method for the Quantitation of Plant Lignans and Enterolignans in Biofluids from Humans and Pigs. J Agric Food Chem. 2015;63(27):6283–92. doi: 10.1021/acs.jafc.5b01275. [DOI] [PubMed] [Google Scholar]
- 46.Hullar MA, Lancaster SM, Li F, Tseng E, Beer K, Atkinson C, et al. Enterolignan-producing phenotypes are associated with increased gut microbial diversity and altered composition in premenopausal women in the United States. Cancer Epidemiol Biomarkers Prev. 2015;24(3):546–54. doi: 10.1158/1055-9965.EPI-14-0262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Adlercreutz H, van der Wildt J, Kinzel J, Attalla H, Wahala K, Makela T, et al. Lignan and isoflavonoid conjugates in human urine. J Steroid Biochem Mol Biol. 1995;52(1):97–103. doi: 10.1016/0960-0760(94)00146-d. [DOI] [PubMed] [Google Scholar]
- 48.Lampe JW, Gustafson DR, Hutchins AM, Martini MC, Li S, Wahala K, et al. Urinary isoflavonoid and lignan excretion on a Western diet: relation to soy, vegetable, and fruit intake. Cancer Epidemiol Biomarkers Prev. 1999;8(8):699–707. [PubMed] [Google Scholar]
- 49.Nurmi T, Mursu J, Penalvo JL, Poulsen HE, Voutilainen S. Dietary intake and urinary excretion of lignans in Finnish men. Br J Nutr. 2010;103(5):677–85. doi: 10.1017/S0007114509992261. [DOI] [PubMed] [Google Scholar]
- 50.Stumpf K, Adlercreutz H. Short-term variations in enterolactone in serum, 24-hour urine, and spot urine and relationship with enterolactone concentrations. Clin Chem. 2003;49(1):178–81. doi: 10.1373/49.1.178. [DOI] [PubMed] [Google Scholar]
- 51.Horn-Ross PL, Barnes S, Lee VS, Collins CN, Reynolds P, Lee MM, et al. Reliability and validity of an assessment of usual phytoestrogen consumption (United States) Cancer Causes Control. 2006;17(1):85–93. doi: 10.1007/s10552-005-0391-6. [DOI] [PubMed] [Google Scholar]
- 52.Kilkkinen A, Valsta LM, Virtamo J, Stumpf K, Adlercreutz H, Pietinen P. Intake of lignans is associated with serum enterolactone concentration in Finnish men and women. J Nutr. 2003;133(6):1830–3. doi: 10.1093/jn/133.6.1830. [DOI] [PubMed] [Google Scholar]
- 53.Clavel T, Borrmann D, Braune A, Dore J, Blaut M. Occurrence and activity of human intestinal bacteria involved in the conversion of dietary lignans. Anaerobe. 2006;12(3):140–7. doi: 10.1016/j.anaerobe.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 54.Kilkkinen A, Stumpf K, Pietinen P, Valsta LM, Tapanainen H, Adlercreutz H. Determinants of serum enterolactone concentration. Am J Clin Nutr. 2001;73(6):1094–100. doi: 10.1093/ajcn/73.6.1094. [DOI] [PubMed] [Google Scholar]
- 55.Df de Mello V, M AL, Lindstrom J, Puupponen-Pimia R, D EL, Pihlajamaki J, et al. Fasting serum hippuric acid is elevated after bilberry (Vaccinium myrtillus) consumption and associates with improvement of fasting glucose levels and insulin secretion in persons at high risk of developing type 2 diabetes. Mol Nutr Food Res. 2017 doi: 10.1002/mnfr.201700019. [DOI] [PubMed] [Google Scholar]
- 56.Krupp D, Doberstein N, Shi L, Remer T. Hippuric acid in 24-hour urine collections is a potential biomarker for fruit and vegetable consumption in healthy children and adolescents. J Nutr. 2012;142(7):1314–20. doi: 10.3945/jn.112.159319. [DOI] [PubMed] [Google Scholar]
- 57.Mulder TP, Rietveld AG, van Amelsvoort JM. Consumption of both black tea and green tea results in an increase in the excretion of hippuric acid into urine. Am J Clin Nutr. 2005;81(1 Suppl):256S–60S. doi: 10.1093/ajcn/81.1.256S. [DOI] [PubMed] [Google Scholar]
- 58.Li M, Wang B, Zhang M, Rantalainen M, Wang S, Zhou H, et al. Symbiotic gut microbes modulate human metabolic phenotypes. Proc Natl Acad Sci U S A. 2008;105(6):2117–22. doi: 10.1073/pnas.0712038105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wikoff WR, Anfora AT, Liu J, Schultz PG, Lesley SA, Peters EC, et al. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc Natl Acad Sci U S A. 2009;106(10):3698–703. doi: 10.1073/pnas.0812874106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Armstrong MD, Chao FC, Parker VJ, Wall PE. Endogenous formation of hippuric acid. Proc Soc Exp Biol Med. 1955;90(3):675–9. doi: 10.3181/00379727-90-22134. [DOI] [PubMed] [Google Scholar]
- 61.Chiba M, Poon K, Hollands J, Pang KS. Glycine conjugation activity of benzoic acid and its acinar localization in the perfused rat liver. J Pharmacol Exp Ther. 1994;268(1):409–16. [PubMed] [Google Scholar]
- 62.Asatoor AM. Aromatisation of Quinic Acid and Shikimic Acid by Bacteria and the Production of Urinary Hippurate. Biochim Biophys Acta. 1965;100:290–2. doi: 10.1016/0304-4165(65)90455-1. [DOI] [PubMed] [Google Scholar]
- 63.Swann JR, Tuohy KM, Lindfors P, Brown DT, Gibson GR, Wilson ID, et al. Variation in antibiotic-induced microbial recolonization impacts on the host metabolic phenotypes of rats. J Proteome Res. 2011;10(8):3590–603. doi: 10.1021/pr200243t. [DOI] [PubMed] [Google Scholar]
- 64.Williams HR, Cox IJ, Walker DG, Cobbold JF, Taylor-Robinson SD, Marshall SE, et al. Differences in gut microbial metabolism are responsible for reduced hippurate synthesis in Crohn's disease. BMC Gastroenterol. 2010;10:108. doi: 10.1186/1471-230X-10-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Selma MV, Espin JC, Tomas-Barberan FA. Interaction between phenolics and gut microbiota: role in human health. J Agric Food Chem. 2009;57(15):6485–501. doi: 10.1021/jf902107d. [DOI] [PubMed] [Google Scholar]
- 66.Lampe JW, Chang JL. Interindividual differences in phytochemical metabolism and disposition. Semin Cancer Biol. 2007;17(5):347–53. doi: 10.1016/j.semcancer.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hodges RE, Minich DM. Modulation of Metabolic Detoxification Pathways Using Foods and Food-Derived Components: A Scientific Review with Clinical Application. J Nutr Metab. 2015;2015:760689. doi: 10.1155/2015/760689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Damdimopoulou P, Nurmi T, Salminen A, Damdimopoulos AE, Kotka M, van der Saag P, et al. A single dose of enterolactone activates estrogen signaling and regulates expression of circadian clock genes in mice. J Nutr. 2011;141(9):1583–9. doi: 10.3945/jn.111.140277. [DOI] [PubMed] [Google Scholar]
- 69.Maronde E, Pfeffer M, Olcese J, Molina CA, Schlotter F, Dehghani F, et al. Transcription factors in neuroendocrine regulation: rhythmic changes in pCREB and ICER levels frame melatonin synthesis. J Neurosci. 1999;19(9):3326–36. doi: 10.1523/JNEUROSCI.19-09-03326.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ziady AG, Sokolow A, Shank S, Corey D, Myers R, Plafker S, et al. Interaction with CREB binding protein modulates the activities of Nrf2 and NF-kappaB in cystic fibrosis airway epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2012;302(11):L1221–31. doi: 10.1152/ajplung.00156.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mylroie H, Dumont O, Bauer A, Thornton CC, Mackey J, Calay D, et al. PKCepsilon-CREB-Nrf2 signalling induces HO-1 in the vascular endothelium and enhances resistance to inflammation and apoptosis. Cardiovasc Res. 2015;106(3):509–19. doi: 10.1093/cvr/cvv131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hardeland R. Melatonin in plants and other phototrophs: advances and gaps concerning the diversity of functions. J Exp Bot. 2015;66(3):627–46. doi: 10.1093/jxb/eru386. [DOI] [PubMed] [Google Scholar]
- 73.Hattori A, Migitaka H, Iigo M, Itoh M, Yamamoto K, Ohtani-Kaneko R, et al. Identification of melatonin in plants and its effects on plasma melatonin levels and binding to melatonin receptors in vertebrates. Biochem Mol Biol Int. 1995;35(3):627–34. [PubMed] [Google Scholar]
- 74.Kolar J, Machackova I. Melatonin in higher plants: occurrence and possible functions. J Pineal Res. 2005;39(4):333–41. doi: 10.1111/j.1600-079X.2005.00276.x. [DOI] [PubMed] [Google Scholar]
- 75.Tan DX, Hardeland R, Manchester LC, Korkmaz A, Ma S, Rosales-Corral S, et al. Functional roles of melatonin in plants, and perspectives in nutritional and agricultural science. J Exp Bot. 2012;63(2):577–97. doi: 10.1093/jxb/err256. [DOI] [PubMed] [Google Scholar]
- 76.Tilden AR, Becker MA, Amma LL, Arciniega J, McGaw AK. Melatonin production in an aerobic photosynthetic bacterium: an evolutionarily early association with darkness. J Pineal Res. 1997;22(2):102–6. doi: 10.1111/j.1600-079x.1997.tb00310.x. [DOI] [PubMed] [Google Scholar]
- 77.Botsford JL, Demoss RD. Escherichia coli tryptophanase in the enteric environment. J Bacteriol. 1972;109(1):74–80. doi: 10.1128/jb.109.1.74-80.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wells JE, Hylemon PB. Identification and characterization of a bile acid 7alpha-dehydroxylation operon in Clostridium sp. strain TO-931, a highly active 7alpha-dehydroxylating strain isolated from human feces. Appl Environ Microbiol. 2000;66(3):1107–13. doi: 10.1128/aem.66.3.1107-1113.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vitek L, Haluzik M. The role of bile acids in metabolic regulation. J Endocrinol. 2016;228(3):R85–96. doi: 10.1530/JOE-15-0469. [DOI] [PubMed] [Google Scholar]
- 80.Ridlon JM, Harris SC, Bhowmik S, Kang DJ, Hylemon PB. Consequences of bile salt biotransformations by intestinal bacteria. Gut Microbes. 2016;7(1):22–39. doi: 10.1080/19490976.2015.1127483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yoshimoto S, Loo TM, Atarashi K, Kanda H, Sato S, Oyadomari S, et al. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature. 2013;499(7456):97–101. doi: 10.1038/nature12347. [DOI] [PubMed] [Google Scholar]
- 82.Allison C, Macfarlane GT. Influence of pH, nutrient availability, and growth rate on amine production by Bacteroides fragilis and Clostridium perfringens. Appl Environ Microbiol. 1989;55(11):2894–8. doi: 10.1128/aem.55.11.2894-2898.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dai ZL, Wu G, Zhu WY. Amino acid metabolism in intestinal bacteria: links between gut ecology and host health. Front Biosci (Landmark Ed) 2011;16:1768–86. doi: 10.2741/3820. [DOI] [PubMed] [Google Scholar]
- 84.Zhao Y, Wu J, Li JV, Zhou NY, Tang H, Wang Y. Gut microbiota composition modifies fecal metabolic profiles in mice. J Proteome Res. 2013;12(6):2987–99. doi: 10.1021/pr400263n. [DOI] [PubMed] [Google Scholar]
- 85.Fecteau KA, Eiler H, Oliver JW. Effect of combined lignan phytoestrogen and melatonin treatment on secretion of steroid hormones by adrenal carcinoma cells. Am J Vet Res. 2011;72(5):675–80. doi: 10.2460/ajvr.72.5.675. [DOI] [PubMed] [Google Scholar]
- 86.Mardinoglu A, Shoaie S, Bergentall M, Ghaffari P, Zhang C, Larsson E, et al. The gut microbiota modulates host amino acid and glutathione metabolism in mice. Mol Syst Biol. 2015;11(10):834. doi: 10.15252/msb.20156487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Clavel T, Henderson G, Alpert CA, Philippe C, Rigottier-Gois L, Dore J, et al. Intestinal bacterial communities that produce active estrogen-like compounds enterodiol and enterolactone in humans. Appl Environ Microbiol. 2005;71(10):6077–85. doi: 10.1128/AEM.71.10.6077-6085.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Clavel T, Lippman R, Gavini F, Dore J, Blaut M. Clostridium saccharogumia sp. nov. and Lactonifactor longoviformis gen. nov., sp. nov., two novel human faecal bacteria involved in the conversion of the dietary phytoestrogen secoisolariciresinol diglucoside. Syst Appl Microbiol. 2007;30(1):16–26. doi: 10.1016/j.syapm.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 89.Zock PL, de Vries JH, Katan MB. Impact of myristic acid versus palmitic acid on serum lipid and lipoprotein levels in healthy women and men. Arterioscler Thromb. 1994;14(4):567–75. doi: 10.1161/01.atv.14.4.567. [DOI] [PubMed] [Google Scholar]
- 90.Perreault M, Roke K, Badawi A, Nielsen DE, Abdelmagid SA, El-Sohemy A, et al. Plasma levels of 14:0, 16:0, 16:1n-7, and 20:3n-6 are positively associated, but 18:0 and 18:2n-6 are inversely associated with markers of inflammation in young healthy adults. Lipids. 2014;49(3):255–63. doi: 10.1007/s11745-013-3874-3. [DOI] [PubMed] [Google Scholar]
- 91.El Hafidi M, Perez I, Zamora J, Soto V, Carvajal-Sandoval G, Banos G. Glycine intake decreases plasma free fatty acids, adipose cell size, and blood pressure in sucrose-fed rats. Am J Physiol Regul Integr Comp Physiol. 2004;287(6):R1387–93. doi: 10.1152/ajpregu.00159.2004. [DOI] [PubMed] [Google Scholar]