Abstract
Absence seizures are generalized, cortico-thalamo-cortical (CTC) high power electroenecephalographic (EEG) or electrocorticographic (ECoG) events that initiate and terminate suddenly. ECoG recordings of absence seizures in animal models of genetic absence epilepsy show a sudden spike-wave-discharge (SWD) onset that rapidly emerges from normal ECoG activity. However, given that absence seizures occur most often during periods of drowsiness or quiet wakefulness, we wondered whether SWD onset correlates with pre-ictal changes in network activity. To address this, we analyzed ECoG recordings of both spontaneous and induced SWDs in rats with genetic absence epilepsy. We discovered that the duration and intensity of spontaneous SWDs positively correlate with pre-ictal 20–40 Hz (β) spectral power and negatively correlate with 4-7 Hz (Ø) power. In addition, the output of thalamocortical neurons decreases within the same preictal window of time. In separate experiments we found that the propensity for SWD induction was correlated with pre-ictal β power. These results argue that CTC networks undergo a pre-seizure state transition, possibly due to a functional reorganization of cortical microcircuits, which leads to the generation of absence seizures.
INTRODUCTION
Genetic absence epilepsy is a pathological disorder that involves the dynamic interplay between cortico-thalamic (CT) and thalamo-cortical (TC) circuits(Crunelli and Leresche, 2002; Danober et al., 1998; Snead, 1995). Arousal-related desynchronized cortico-thalamo-cortical (CTC) activity, which can be visualized as low amplitude, high frequency electroencephalographic (EEG) or electrocorticographic (ECoG) voltage fluctuation(Hirata and Castro-Alamancos, 2010; McCormick et al., 2015), is suddenly interrupted in absence seizures by rhythmic, highly synchronized CT and TC output with characteristic EEG/ECoG spike-wave discharges (SWDs)(Coenen et al., 1992; Danober et al., 1998; Huguenard, 1999; Panayiotopoulos, 2001). These rapidly generalize bilaterally and lead to behavioral arrest and loss of consciousness(Danober et al., 1998; Gotman et al., 2005; Huguenard, 1999; Panayiotopoulos et al., 1989; Sitnikova and van Luijtelaar, 2006; Steriade, 2000). Absence seizures in rodent models of genetic absence epilepsy are state-dependent – mostly occurring during periods of quite wakefulness and drowsiness(Coenen et al., 1991; Danober et al., 1998). This is likely due to increased hyperpolarization of TC, CT, and reticular thalamus (RT) neurons, which increases the probability of phasic burst-firing throughout the CTC network necessary for SWD generation and maintenance(Beenhakker and Huguenard, 2009; Huguenard and McCormick, 2007; Kostopoulos, 2000; Llinás and Steriade, 2006; McCormick et al., 2015; Steriade, 2000; Sorokin et al. 2017). Thus, there is evidence for a state transition within the CTC network that increases its susceptibility to hypersynchronization and seizures.
Despite the known state-dependence of absence seizures, there is little evidence to suggest that EEG or ECoG recordings display pre-ictal changes prior to SWD onset(Danober et al., 1998; Panayiotopoulos, 2001). As such, although many algorithms have been established for SWD detection(Alkan et al., 2005; Ovchinnikov et al., 2010; Paz et al., 2013; Sorokin et al. 2017), few exist for SWD prediction. Recently, some studies have revealed the existence of pre-ictal changes in non-linear metrics including permutation entropy (Li et al., 2007) and granger causality (Lüttjohann and Van Luijtelaar, 2012), as well as in linear metrics such as the power in certain frequency bands obtained via magnetoencephalography(Jacobs-Brichford et al., 2014) and EEG (Van Luijtelaar et al., 2011) and an increase in functional magnetic resonance imaging (fMRI) bold activity(Bai et al., 2010). Moreover, a recent study discovered that EEG power and fMRI amplitude during SWDs in humans were positively correlated with behavioral impairment(Guo et al., 2016). However, to our knowledge the relationship between preictal predictors and the severity and susceptibility of SWDs has not been investigated.
To address this question, here we have analyzed our previous ECoG recordings from Wistar Albino Glaxo Rats from Rijswijk (WAGRij), a well-established animal model of genetic absence epilepsy (Coenen, Drinkenburg, Inoue, & van Luijtelaar, 1992; van Luijtelaar & Coenen, 1986; van Luijtelaar & Sitnikova, 2006, Sorokin et al., 2016). In our previous study, we demonstrated that bilateral SWDs could be induced in freely behaving epileptic rodents by unilaterally driving phasic, clustered spiking in TC neurons in the ventrobasal (VB) thalamus (Sorokin et al., 2016) via single or periodic optical activation of the inhibitory opsin, halorhodopsin (eNpHR3.0)(Gradinaru et al., 2008). Thus we have focused our analysis on pre-ictal periods of both spontaneous and induced seizures, to determine if (1) cortical activity displays pre-ictal changes that can be detected via ECoG recordings in WAGRij rats, (2) if pre-ictal changes predict features of SWDs, and (3) if the ability to induce SWDs depends on the pre-ictal state of the animal. For our analysis, we used a variation of the discrete wavelet transform (DWT) to measure changes in the frequency distribution of the ECoG from pre-ictal to ictal states. We discovered that ECoG activity does display pre-ictal shifts in frequency bands, most notably high β (20–40 Hz), which positively correlates with SWD duration and power, and further that the probability of inducing SWDs positively correlates with β power prior to the optical stimulus. Our results demonstrate that cortical oscillatory behavior undergoes changes prior to generalized absence seizures that correlate with the SWD severity, which may reflect a shift in the resonant activity of microcircuits within the cortex and thalamus that prime the CTC network for hypersynchrony.
RESULTS
Automatic detection of SWDs and artifact rejection
To extract SWDs from our recordings, we developed an offline algorithm to detect absence seizures based on the maximal-overlap discrete wavelet transform (MODWT, Fig. 1a, see methods), which has been used EEG processing (Khalighi et al., 2011; Xie and Krishnan, 2013). While the MODWT, like the related discrete wavelet transform (DWT), efficiently decomposes the frequencies contained in a signal over time by using coarse frequency-resolution bandwidths, it avoids the time-resolution pitfalls of the DWT(Nason and Silverman, 1995), rendering it superior for precise detection of time-localized events such as SWDs.
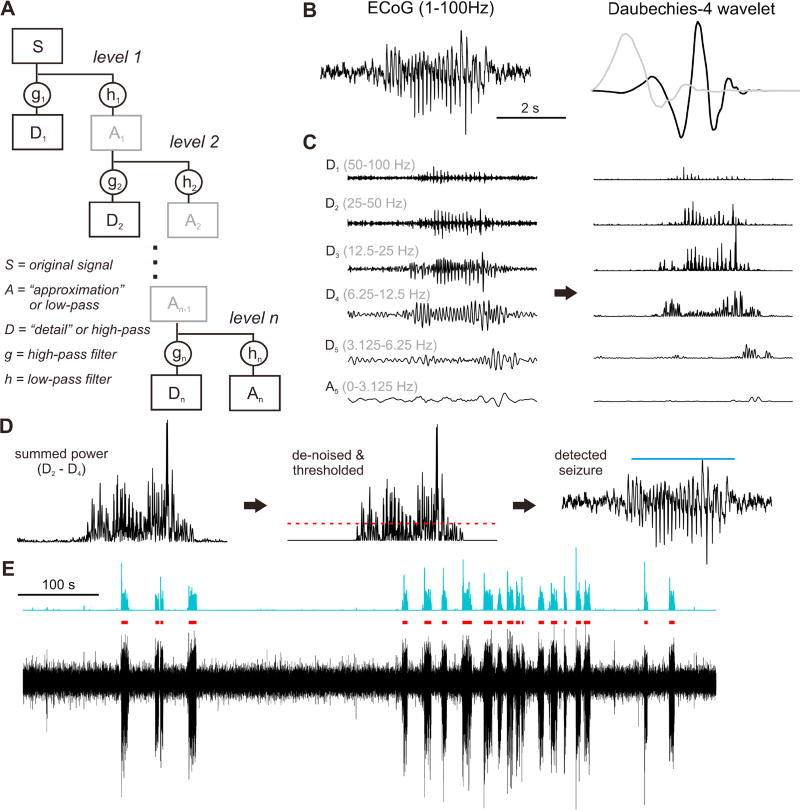
Figure 1. automatic SWD-detection algorithm based on the maximal-overlap discrete wavelet transform.
(a) Schematic of the maximal-overlap discrete wavelet transform. The original ECoG S is split and convolved with low-pass (h) and high-pass (g) scaled filter coefficients to create a low-pass approximation (A) and high-pass detail (D) signal. At each level j the approximation Aj is further spilt into detail and approximation signals and convolved with new wavelet functions (see methods), until the maximum level n is reached. All D1 – Dn as well as the final approximation An are kept for spectral analysis (gray boxes are ignored). (b) Left: an example ECoG recording with a prominent SWD; ECoG were filtered between 1-100 Hz for all of our analyses. Right: the daubechies-4 wavelet (black) and scaling factor (gray) used for our wavelet decomposition. (c) Left: detail and approximation wavelet coefficients for a level-5 decomposition (the frequencies contained within each coefficient time series are in gray). Right: wavelet power as the square of the wavelet coefficients. (d) Left: summed power for D2 – D4Middle: de-noised power with an automatic threshold (red dashed line). Right: the resulting detected seizure (blue bar). (e) Example ECoG recording (black) with summed and denoised D2 – D4 wavelet power (turquoise), and detected SWDs (red bars).
In our algorithm, first the raw ECoG traces (Fig. 1b left) were decomposed to 5 levels via MODWT using a daubechies-4 wavelet (Fig. 1b right), and the resulting wavelet coefficients (Fig. 1c left) were then squared to obtain power in different frequency bands (Fig. 1c right). Because rodent absence seizures are primarily dominated by frequencies between 6 and 50 Hz(Danober et al., 1998), power derived from wavelet coefficients outside of this bandwidth were set to zero, while the power in the remaining levels were summed (Fig. 1d left). The summed wavelet power was then automatically de-noised and subsequently thresholded using the standard deviation of wavelet power from baseline ECoG segments (Fig. 1d middle, see methods). Finally, the detected events were automatically checked against rigorous criteria to eliminate detection of artifacts and transients (see methods), and the remaining detected events were flagged as putative SWDs (Fig. 1d right, 1e). SWDs extracted from induced-seizure trials were checked against a second set of criteria for semi-automatic artifact rejection (see methods).
Spontaneous SWDs correlate with pre-ictal changes in ECoG spectral power
We first asked whether the state-dependence of spontaneous SWDs in WAGRij rats could be quantified via spectral analysis. Indeed, certain recorded rhythms are highly correlated with behavioral state. For instance, Ø (4–7 Hz) power is associated with active exploration and attention(Buzsáki and Moser, 2013; Hasselmo et al., 2002), and ∂ (1–4 Hz) with drowsiness and slow-wave sleep(Achermann and Borbély, 1998; McCormick and Bal, 1997; Steriade, 1997). To accurately measure spectral power within narrow frequency bands, we decomposed the ECoG recordings from four WAGRij rats using wavelet packet transformation (WPT), which provides higher frequency-resolution compared to MODWT (Fig. 2a, see methods).
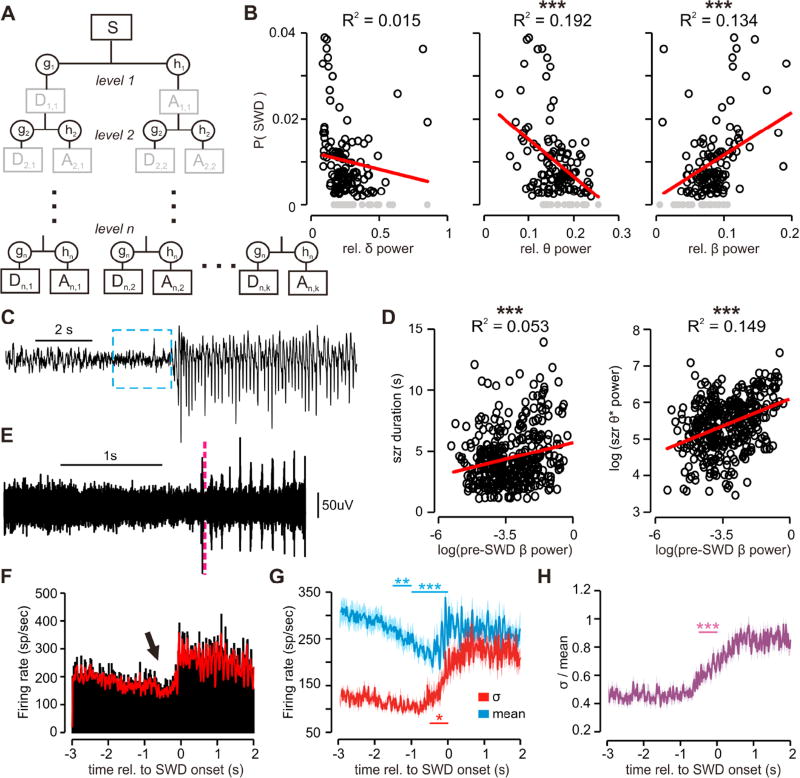
Figure 2. Spontaneous absence seizures are preceded by increases in high β (20-40 Hz) ECoG oscillations and decreased TC firing.
(a) Schematic of the wavelet-packet transform (WPT) used for spectral analysis. Like the discrete wavelet transform (DWT, see Fig. 1a and methods), signals are split and convolved with low-pass (h) and high-pass (g) wavelet filters to give approximation (A) and detail (D) coefficients. At each level, both A and D are then decomposed using scaled versions of coefficients in h and g, resulting in a final set of wavelet coefficients with high frequency-resolution (gray boxes are ignored). (b) Regression of the probability of a spontaneous seizure P(swd) against relative levels of ∂, Ø, and β power. Each scatter point represents a segment of ECoG taken from a recording trial in which the P(swd) and spectral power were computed (gray points represent segments in which no seizures were detected). Red line = best linear fit (∂: p = 0.1662; Ø: p = 2.91e−7; β: p = 1.67e−5) (c) Example ECoG recording showing the transition from normal EEG to pre-ictal high-frequency β oscillations, and a subsequent SWD. The blue box indicates the 2s window used to calculate pre-ictal spectral power. (d) Regression of seizure duration (left) and SWD Ø* (7-9Hz) power (right) against pre-ictal β power. Each scatter point represents a single seizure. Red line = best linear fit. (duration: p = 3.75e−6, t = 4.69; Ø* power: p = 6.66e−16, t = 8.42). (e) MU activity recorded from the right VB (different trial than c); the red dashed line indicates the seizure onset (note the rhythmic clustered firing at the onset of the seizure and the drop in the standard deviation of the signal prior to seizure onset). (f) PSTH (black) and convolved firing rate (red) of averaged over all seizures from the same recording as e. There is a clear pre-ictal drop in population firing rate (arrow). (g) Mean MU firing rate (blue) and S.D. (red) across trials, demonstrating the reduction in firing rate prior to seizure onset. Note that the S.D. remains low prior to seizure onset except for ∼ 0.5s prior to onset. (h) Ratio of S.D. vs. mean firing rate from g, showing a low noise/signal ratio even as MU firing rate decreases. (see Fig. S1; repeated-measures ANOVA, ** p < 0.01, *** p < 0.001; n = 29 trials, 4 animals, DOF: 28).
We assayed the relationship between the occurrence of spontaneous seizures and the distribution of ECoG spectral power by dividing each WPT-decomposed recording trial into three segments of equal length, and calculating the probability of spontaneous SWD occurrence as well as relative ∂, Ø, and high β (20–40Hz) power for each segment (see methods for details). We divided each trial into distinct windows of time to account for changes in arousal level throughout each recording trial. By dividing the trials as such, we improved the temporal resolution and ability to differentiate relative ∂, Ø, and β power, as well seizure probability. We discovered that both ∂ and Ø power were negatively correlated with SWD-probability, which is in agreement with the infrequent occurrences of SWDs during active exploration and sleep(Danober et al., 1998). By contrast, β power was positively correlated (Fig. 2b).
Given the positive correlation between SWD-probability and β power, we wondered whether individual SWDs correlated with increases in pre-ictal β power. To test this, for each detected seizure we summed β power over two seconds prior to SWD onset (Fig. 2c) and regressed the power against two metrics of SWD severity: duration and Ø* (7–9 Hz) power, the fundamental frequency of rodent SWDs(Coenen et al., 1992; Danober et al., 1998). We discovered that pre-ictal β power positively correlated with SWD duration (Fig. 2d left) and even more strongly with SWD Ø* power. Interestingly, a similar pre-ictal increase in high-frequency oscillations has been reported in patients with complex partial epilepsy(Fisher et al., 1992), neocortical epilepsy(Worrell et al., 2004), and mesial temporal lobe epilepsy(Bartolomei et al., 2004), as well as in animal models of acquired epilepsy(Gnatkovsky et al., 2008; Hughes, 2008) and thus may represent a similar pre-seizure shift in circuits responsible for hypersynchronous oscillations. Although in some instances ECoG recordings did not display a visually obvious pre-ictal change in spectral power (compare Fig. 1b to Fig. 2c) due to the variability of pre-ictal states, our results identify a relationship between pre-ictal spectral power and absence seizures.
To further investigate this phenomenon, we analyzed multi-unit (MU) activity simultaneously recorded from the right VB via implanted tungsten wires as described in (Paz et al., 2013, Sorokin et al., 2016). We hypothesized that the correlation between β power and SWDs may be due in part to altered CTC dynamics such thalamocortical (TC) firing patterns, as TC neurons can undergo sub-threshold oscillations in the β range that may facilitate cortico-cortical spread of activity(Contreras and Steriade, 1996; Steriade et al., 1996). Although the low-impedance electrodes used to record MU signals did not allow us to resolve single units, we were nonetheless able to capture and analyze high quality MU activity from a local population of TC neurons (Fig. 2e).
For each seizure, we extracted three seconds of MU activity both preceding and following the seizure onset and calculated the population firing rate over time (see methods). Interestingly, we observed that the TC population firing rate steadily decreased prior to SWD onset (Fig. 2f arrow), and was significantly lower starting1.5s prior to SWD onset compared to 3s prior to onset (Fig. 2f, Fig. S1a). This steady decrease was robust across trials on average (Fig. 2g blue) with low inter-trial variance as indicated by the stable pre-ictal standard deviation of the mean firing rate (Fig. 2g red, Fig. S1b) and ratio of the standard deviation over the mean firing rate (Fig. 2h, Fig. S1c). The increase in the standard deviation of the mean firing rate 0.5s prior to SWD onset (Fig. 2g,f, Fig. S1b,c) is primarily due to the jitter surrounding SWD detection and alignment of MU spiking. Nonetheless, these results suggest that TC, and likely other players in the CTC circuit, undergo a dynamic shift in firing patterns prior to SWD onset, which may contribute to the β oscillations observed in the ECoG. However, the precise mechanisms responsible for this pre-ictal change in brain state remain to be determined.
The probability of inducing a SWD depends on EcoG spectral power
In our previous work, we discovered that SWDs could be induced in WAGRij rats expressing eNpHR in VB neurons with a single pulse of 594nm light delivered unilaterally to the VB(Sorokin et al. 2017) (Fig. 3a). While light pulses could effectively induce SWDs, some fraction of stimuli (<50%) failed to do so. Having observed that spontaneous seizures correlate with pre-ictal changes in β power, we wondered whether the ability to induce SWDs also correlates with the spectral distributions of the ECoG. To address this, we used WPT to decompose ECoG recordings from induced trials in four WAGRij-eNpHR rats and analyzed the average spectral power over two seconds prior to the pulse onset. Interestingly, we discovered that pre-pulse β, but not ∂ and Ø, power negatively correlated with the latency to onset of SWDs (Fig. 3b). This result suggests that even during normal behavior, certain CTC dynamics, such as those associated with increased β power and possibly others, appear to be more susceptible to perturbations that evolve into network hypersynchrony.
Figure 3. SWD induction probability correlates with pre-pulse β power.
(a) Example ECoG recording of an induced seizure. Yellow bar = 594nm pulse delivered unilaterally to the right VB thalamus. Right: zoom of SWD onset indicated by the black dashed box. (b) Regressions of the latency to SWD onset against pre-pulse ∂, Ø, and β power. Red line = best linear fit. Each scatter point represents one induced seizure. (∂: p = 0.380, t = −0.88; Ø: p = 0.691, t = 0.399; β: p = 0.003, t = −3.06). (c) Regression of the probability of inducing an SWD P(swd) against pre-pulse ∂, Ø, and β power. For each regression, we created 20 bins that spanned the range of the pre-pulse power and calculated P(swd) by summing the number of successfully induced seizures divided by the total number of pulses (see methods). Red line = best linear fit. (∂: p = 0.751, t = −0.322; Ø: p = 0.244, t = −1.21; β: p = 0.009, t = 2.94). (d) Example MU recording from an induced SWD trial; note the large cluster of population spikes (arrow) in response to the inhibitory 594nm pulse (yellow bar), and the subsequent organization into rhythmic clustered spiking. (e) PSTH and estimated firing rate (red) calculated from d. The large population spike is apparent (arrow), but there is a noticeable drop in firing rate and ramping into rhythmic bouts of activity following the initial response. (f) Mean firing rate (blue) and S.D. (red) across induced trials. Here the prolonged drop in firing rate is highly apparent (arrow) and significantly lower than the pre-stimulus firing rate (See Fig. S2; paired t-test, *** p < .001, n = 9 trials, 4 animals) and lasts for ∼ 0.2s, following by a gradual ramping of rhythmic firing (see Fig. S2).
To better understand SWD induction probability, we regressed the probability of inducing seizures against pre-pulse β, ∂, and Ø power. The probability of SWD induction did not depend on pre-pulse ∂ or Ø power, but strongly correlated with β power (Fig. 3c), suggesting that the ability to drive the CTC network into an absence seizure may depend on a similar pre-ictal state as occurs during spontaneous SWDs. This led us to wonder whether MU activity recorded from the VB also undergoes similar dynamics following 594nm pulses as seen prior to spontaneous seizures (Fig. 3d; for reference see Fig. 2e–h).
Indeed, following an initial strong cluster of spikes in response to the 594nm pulse – almost certainly due to T-type calcium channel mediated rebound bursts(Destexhe et al., 1998; Jahnsen and Llinás, 1984) – MU recordings showed first a reduction in firing rate followed by a gradual ramping composed of phasic, rhythmic bouts of MU spikes indicative of oscillatory CTC seizure activity (Fig. 3d–f, Fig. S2). Interestingly, the post-stimulus drop lasted for ∼ 0.2 – 0.3 seconds – longer than is necessary for recovery from a rebound burst(Jahnsen and Llinás, 1984; Steriade et al., 1993; Sorokin et al. 2017), as is evident by the oscillatory spiking observed quickly following the initial light-driven rebound burst when averaging over a single trial (Fig. 3e). We propose that this prolonged reduction of firing rate is not simply due to recovery from the inhibitory 594nm pulse, but may instead reflect a dynamic shift in CTC activity analogously to the pre-ictal reduction in TC firing rate prior to spontaneous SWDs (Fig. 2e–h). In other words, the initiation of both spontaneous and induced SWDs may involve a similar reformation of CTC network activity, which could be used to predict seizure onset.
DISCUSSION
Here we have shown that spontaneous absence seizures in WAGRij rats display precursor changes in both the distribution of ECoG spectral power and the population firing rate of TC neurons (Fig. 2). These changes were robust across our recordings, and may reflect a shift in CTC networks that increases their susceptibility to runaway oscillatory activity. Moreover, we discovered that the same pre-ictal observations in spontaneous seizures positively correlate with the duration and occurrence of artificially induced SWDs (Fig. 3). Thus, our results argue that there are certain CTC dynamics that are more likely to evolve into global absences, whether these occur naturally or through external perturbations.
The correlation we observed between spontaneously occurring SWD severity and high pre-ictal β (20–40 Hz) power has, to our knowledge, not been previously investigated. Previous studies have discovered a similar high-frequency pre-ictal predictor in both humans and animals with various forms of epilepsy(Fisher et al., 1992; Gnatkovsky et al., 2008; Jirsch et al., 2006; Navarro et al., 2002; Staba et al., 2004; Worrell et al., 2004). Further, the appearance of pre-ictal and inter-ictal high-frequency oscillations (HFOs) with frequencies greater than 80 Hz are indicators of epileptogenic zones in patients with temporal lobe epilepsy(Staba et al., 2004; Zijlmans et al., 2012), and removal of tissue generating HFOs correlates with improved post-operative outcome(Jacobs et al., 2010). The mechanisms responsible for fast oscillations may be multifaceted. Some findings suggest that they are linked to extremely rapid inter-spike intervals during population bursts in epileptic tissue(Bragin et al., 2007), while other findings argue they are the result of tightly-coupled, but not perfectly synchronous, firing of pyramidal cells(Jiruska et al., 2010).
Possible mechanisms behind 20–40 Hz oscillations prior to SWDs
Although HFOs and the fast β oscillations that we observed prior to SWDs are likely not directly related, their emergences may be due to an analogous reorganization of epileptic network activity. Given that absence seizures are most common during periods of drowsiness/quiet wakefulness(Danober et al., 1993), and that β oscillations are often associated with attention(Benchenane et al., 2011), it is quite surprising to observe correlations between SWDs and pre-ictal β power. While the relationship between β oscillations and background states of quiet wakefulness require further investigation, one possible mechanism behind the pre-ictal increase in β power could be a recruitment of CT output at the 20–40 Hz range. Indeed 30–40 Hz cortical oscillations throughout the CTC are hypothesized to link cortical areas together via resonant activation of TC neurons(Steriade et al., 1996, 1991). Although evidence suggests that these fast cortical oscillations are heightened during behavioral vigilance due to increases in these oscillations with mesopontine activation(Steriade et al., 1991), their appearance may nonetheless lead to pathological CTC oscillations in epileptic networks via heightened RT-TC synaptic inhibition(Huguenard, 1999; Li et al., 2006).
Since CT oscillations in this range can drive oscillatory RT output, which subsequently inhibits TC neurons(Charpier et al., 1999; Steriade et al., 1996), in epileptic networks TC neurons may be sensitive to such resonant inhibitory inputs and strongly hyperpolarize in response to the repetitive, asynchronous discharges from RT. Following such CT- β -based hyperpolarization, a subsequent strong CT input to the RT – perhaps following the termination of the pre-ictal β oscillations – could induce synchronized RT inhibition and promote synchronized TC post-inhibitory rebound (PIR) bursts(Jahnsen and Llinás, 1984). Additionally, direct CT input to hyperpolarized TC cells could also initiate CTC activity. The result in either case would be launching an absence seizure by reactivating both the RT and CT (McCormick and Bal, 1997). In addition, the 20–40 Hz resonant oscillations in the CTC network may lead to synchronization of cortical regions outside of a possible epileptic cortical focus (Meeren, Hanneke; Lopes da Silva, 2005; Polack et al., 2007; Steriade et al., 1991), which could contribute to the rapid spread of absence seizures. Our observation that TC neurons display a pre-ictal reduction in population firing rate may reflect resonant and fast inhibitory inputs from the RT that initially shunt and hyperpolarize TC neurons, but perhaps following a synchronized CT input at the onset of the SWD (Meeren, Hanneke; Lopes da Silva, 2005; Polack et al., 2007) drive synchronized PIR bursts.
By contrast, the source of fast cortical oscillations may reflect an increase in cortical inhibition, as certain cortical and entorhinal interneurons have been shown to drive oscillations in this range in vitro(Gnatkovsky et al., 2008; Llinás et al., 1991), and computational models of reciprocally connected excitatory-inhibitory networks demonstrate emergent high frequency oscillations due to inhibitory coupling(Sohal and Huguenard, 2005). Increases in cortical inhibition could shunt CT output and effectively remove the tonic depolarizing CT influence from TC neurons, thus leading to dysfacilitation (Contreras et al., 1996) and hyperpolarization with an increased likelihood of PIR bursts in response to RT input(Llinás and Steriade, 2006). A sudden burst of CT action potentials could then elicit a strong and synchronized PIR response in TC neurons via the RT. There may of course be other microcircuits and long-range projections both within and outside of the CTC network that contribute to the pre-ictal changes we have observed, and a more detailed sampling is necessary to uncover the possible mechanisms behind our observations.
Developing new predictive algorithms for absence seizures
We have shown using two distinct methods – spectral decomposition of ECoG and quantification of TC firing rate – that pre-ictal predictors of SWDs are robust and correlate with features of SWD severity such as duration and power. Current algorithms for SWD detection are limited in their ability to predict seizure onset before it occurs, rendering them more useful for offline analysis than for closed-loop intervention. While recent studies have also demonstrated pre-ictal changes in brain dynamics prior to absence seizures(Li et al., 2007; Lüttjohann and Van Luijtelaar, 2012), to our knowledge we are the first to demonstrate a correlation between pre-ictal features and the severity of absence seizures. While further analyses remain to better quantify the relationships between pre-ictal state and absence seizures, we believe our results are a step forward toward designing novel closed-loop therapies that could be adaptively tuned on a perseizure and per-subject basis based on detected pre-ictal features.
EXPERIMENTAL METHODS
Recordings used for our analyses were performed according to protocols approved by the Institutional Animal Care and Use Committee, and every precaution was taken to minimize stress and the number of animals used.
Electrode Implantation and ECoG Recordings
Animals used for our analyses were previously implanted as described in(Sorokin et al. 2017). Briefly, implants were custom made by securing electrodes and optical fibers to male-female dual-row mill-max connectors (Mill-Max Co. Oyster Bay, NY). Cortical ECoG electrodes were manufactured by hand-soldering small .047” × 1/8” self-tapping screws onto 1” strips of insulated silver wire, and securing the non-soldered end of the wire to the mill-max sockets. MU electrodes were manufactured by encapsulating a 200um-core optical fiber and four tungsten wires with a small piece of 0.015” polyimide tubing, securing the bundle with cyanoacrylate glue, and inserting the free-ends of the tungsten wires through the remaining Mill-Max sockets. Each implant contained a total of four tungsten wires and four ECoG screws, plus one optical fiber.
For the surgical implantation, animals were anesthetized with isoflurane and injected with analgesic, and then secured in a stereotaxic frame. The skulls of rats were exposed and burr holes were drilled in the skull bilaterally over anterior and posterior somtatosensory cortex, ipsilaterally over the right VB, and over the cerebellum for reference. Screws were secured first (∼ 3mm lateral and 2mm or 3mm posterior relative to bregma), and the tungsten/optical fiber bundle was then lowered into the thalamus so that the tip of the optical fiber rested just above the VB (2.8mm posterior, 2.7mm lateral, 5.5mm ventral relative to bregma), while the tungsten wires spanned the VB (2.8mm posterior, 2.7mm lateral, 5.6-6mm ventral relative to bregma). Implants were then secured with dental cement, and lidocaine and antibiotic were applied to the surrounding skin. Animals were monitored for one week following implantation and given further analgesic as necessary. Following recovery, ECoG and MU activity were recorded for two weeks between the hours of 10:00 AM − 4:00 PM to control for circadian rhythms. Animals were recorded in a quiet, isolated room, primarily displayed quiet wakefulness with occasional bouts of exploratory behavior. Note that although four ECoG and four MU electrodes were used previously, for this study we only analyzed ECoG from the anterior somatosensory cortex, contralateral to the implanted VB, and only one MU electrode. For further details, see(Sorokin et al. 2017).
The Discrete Wavelet Transform (DWT) and its variants
We used wavelet transformations to decompose ECoG recordings into both time and frequency. While our previous work used the continuous wavelet transform (CWT)(Sorokin et al. 2017), which provides a highly detailed time-frequency map(Torrence and Compo, 1998), we instead used a much more efficient wavelet decomposition based on the discrete wavelet transform (DWT) for our analysis. Given an input signal S with [1,2,3,… n] samples, the DWT decomposes the input signal by first performing the following convolutions:
over every sample in S and for decomposition level j = 1, where ψ, and Ф are internally and mutually orthogonal low-pass and high-pass filters (known as scaling and wavelet bases), respectively, and Hk and Gk are the low-pass and high-pass outputs of the convolution at each point k = [1,2, 3,… n]. Then, the low- and high-pass outputs H and G are decimated such that the final approximation A and detail D wavelet coefficients are:
Aj and Dj are vectors of length . Following the first level decomposition, Aj is re-convolved by shifted and scaled versions of ψ and Ф:
where j is the level of decomposition, k is the time-shift, and g and h are the high-pass and low-pass coefficients of the wavelet and scaling bases, defined by the relationship:
Thus, at each level j, the non-zero elements of the wavelet and scaling functions defined are expanded in time and reduced in amplitude, leading to successive low-pass filtering of the signal. Note that ψ and Ф are sparse signals, containing mostly 0’s except for points defined by the coefficients g and h.
Unlike the DWT, the maximal-overlap DWT (MODWT) avoids the pitfalls of down-sampling, which results in a non-shift invariant decomposition(Nason and Silverman, 1995). This makes the MODWT superior to the traditional DWT for localizing time-delimited events such as seizures. Additionally, the MODWT makes organization of decomposition coefficients convenient as they retain equal lengths. Detail coefficient vectors [D1,D2,… DJmax], and the final approximation vector AJmax are stored into a matrix W = [D1,D2,…,DJmax, AJmax], where each column contains lower frequencies than the previous column, with higher frequency and lower time-resolution by a scale of 2j.
In addition to the MODWT variant of the DWT, we also implemented the wavelet-packet transformation (WPT) for our spectral analysis. While WPT retains the same drawback as the DWT in terms of time-localization since approximation and detail coefficients are down-sampled at each level j, it has much finer frequency-resolution than either the DWT or the MODWT (Gu et al., 2011). This is accomplished by decomposing both Aj and Dj at each subsequent level, which provides better separation of frequencies -particularly important when analyzing changes in neighboring frequency bands.
The choice of particular wavelet bases depends on the application. For our analyses, we used the daubechies-4 (Db4) wavelet, a well-established wavelet basis used heavily in EEG research(Adeli et al, 2003; Al-Qazzaz et al, 2015). In particular, the Db4 wavelet shares similar features with individual spike-wave discharges, and its near symmetry improves the accuracy of time and frequency localization.
Automatic Seizure Detection
We implemented the MODWT outlined to 5 levels using a Db4 wavelet to decompose ECoG traces for seizure detection. First, ECoG were filtered between 1–100 Hz, resampled to 200 Hz, and z-score normalized (Fig. 1b left). Wavelet coefficients for D1–5 and A5 were then computed using MODWT (Fig. 1c left) and power was computed as:
where w represents one of the 6 wavelet coefficient vectors extracted via MODWT (Fig. 1b right). We then summed coefficients P2–4 (representing frequencies from 50 – 6 Hz), since rodent SWDs have little power outside of this range(Danober et al, 1998), and automatically denoised the summed power:
where α > 0. Following denoising, power was automatically thresholded for putative seizure detection using power from non-ictal, baseline segments. Baseline power was extracted by first splitting the non-denoised summed power P into N segments, and sorting the standard deviation of those segments:
then extracting the lowest n < N subset, and using their indices to extract power for baseline segments:
We finally calculated the upper and lower 95% confidence bounds from Pn, and defined our seizure threshold on a per-trial basis as:
where Cu is the upper 95% confidence bound, and λ sets the threshold level. Because SWDs are such high-energy events compared to background ECoG activity, we set λ to a high value (5–8), which robustly localized SWDs while avoiding artifacts (see Fig. 1d,e). Following detection, all putative seizures were automatically tested against a set of conservative criteria to further eliminate artifacts. SWDs that were shorter than 1.5 seconds were eliminated, and consecutive events separated by less than 1 second were merged. The efficiency of our algorithm makes it suitable for on-line applications.
Spontaneous Seizure Analysis
For spectral analysis, ECoG signals were downsampled to 128 Hz and decomposed using WPT up to 6 levels. Power from frequencies in the ∂ (1–4 Hz), Ø (4–7 Hz), Ø* (7–9 Hz), and high β (20–40 Hz) were summed for our analysis. We analyzed 2 seconds of recordings prior to each seizure, and regressed ∂, Ø, and β power in this window against features of the SWDs including duration and Ø* power (the fundamental frequency of SWDs). Because of the large ranges of values for the different power bands, we took the log of the power. Linear regressions were run using robust linear-regression. For MU analysis, we calculated the population firing rate from 300–6000 Hz band-passed MU TC signals from −3:3 seconds surrounding each seizure by first detecting MU spikes(see Sorokin et al. 2017 for details), computing a peri-stimulus time histogram (PSTH) using 10ms bins, and finally convolving the PSTH with a 40ms gaussian kernel to estimate a continuously-varying firing rate (Fig. 2e,f). We then stored the average computed firing rate as well as the standard deviations across seizures, and averaged these values across trials (Fig. 2g,h).
Induced Seizure Analysis
We performed similar analyses for induced-trials as we did for spontaneous trials. To analyze the relationship between pre-pulse ∂, Ø, and β power and the ability to induce SWDs, we regressed power from these frequency bands against the latency to SWD onset following the pulse, as well as the probability of inducing SWDs. For the latter analysis, each pulse was marked as:
and the probability of inducing SWDs was calculated by segmenting ∂, Ø, and β power into 20 bins, and computing:
where M is the number of pulses with pre-pulse ∂, Ø, or β power lying within a certain range defined by bin b. Thus, we obtained a more continuous representation of P(swd) based on pre-pulse spectral power, and regressed P(swd) against binned ∂, Ø, and β power separately (Fig. 3c).
Statistics and Design of Analyses
Unless otherwise, stated, means are presented as ± S.E., and statistics are based on parametric tests. We used MATLAB 2013a for all analysis and statistical tests (Math Works Inc., Natick, MA). We wrote custom software for our seizure detection and analyses, and used built-in MATLAB functions for wavelet decomposition. Linear regressions were performed using robust-linear regression (which is more robust against outliers than standard ordinary least squares), and p-values for linear regressions tests the hypothesis that true correlation exists against a null-correlation. For regressions, we used n = # divided segments in trials (2b), total seizures recorded (2d, 3b), or binned spectral power from all seizures (20 bins, 3d).
To assess changes in MU firing prior to spontaneous seizures, we binned the average firing rates for each trial into 0.5s bins, and ran a repeated-measures ANOVA over the binned firing rates from −3s to 0s relative to SWD onset. We repeated this analysis for the standard deviation of firing rates over trials, as well as the ratio of the standard deviation over the mean (see Fig. S1). To measure changes in MU firing rates following 594nm pulses, we binned the average firing rates into 0.2s bins and compared the binned rates centered at −0.4s and 0.2s relative to stimulus onset using a paired t-test (Fig. S2a). We also ran a linear regression on the trial-averaged firing rate from 0.1s to 0.5s following the 594nm pulse to statistically evaluate the observed ramping of firing rate (Fig. S2b). We corrected for multiple comparisons using the Bonferroni correction and for repeated-measured ANOVA, the Tukey-Kramer correction. We also corrected for heteroskedasticity of the linear regression on the average MU firing rate (Fig. S2b) using the revised variance estimator of the fit:
where , the diagonal matrix of the squared residuals (White 1980), which we then used to correct the estimated variances of the fit coefficients for statistical testing.
Supplementary Material
Highlights.
Spontaneous SWDs in WAGRij rats correlate with pre-ictal 20–40 Hz (β) power.
Thalamocortical (TC) firing rate decreases prior to spontaneous SWD onset
Induced SWDs are more likely when preceded by higher β power
TC firing rate displays similar dynamics between induced and spontaneous SWDs
Acknowledgments
J.T.P. is supported by NIH-NINDS R01NS096369 and R00NS078118-01 and Gladstone Institutes. J.R.H. is supported by NIH-NINDS 5R01NS034774. J.S. is supported by the Stanford Neuroscience Graduate program and the Center for Mind, Brain, and Computation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CODE AVAILABILITY
Please contact Jordan Sorokin (jorsor@stanford.edu) or John Huguenard (john.huguenard@stanford.edu) for custom software written for analysis.
References
- Achermann P, Borbély AA. Coherence analysis of the human sleep electroencephalogram. Neuroscience. 1998;85:1195–1208. doi: 10.1016/S0306-4522(97)00692-1. [DOI] [PubMed] [Google Scholar]
- Adeli H, Zhoub Z, Dadmehr N. Analysis of EEG records in an epileptic patient using wavelet transform. J. Neurosci. Methods. 2003;123:69–87. doi: 10.1016/S0165-0270(02)00340-0. [DOI] [PubMed] [Google Scholar]
- Al-Qazzaz N, Hamid Bin Mohd Ali S, Ahmad S, Islam M, Escudero J. Selection of Mother Wavelet Functions for Multi-Channel EEG Signal Analysis during a Working Memory Task. Sensors. 2015;15:29015–29035. doi: 10.3390/s151129015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkan A, Koklukaya E, Subasi A. Automatic seizure detection in EEG using logistic regression and artificial neural network. J. Neurosci. Methods. 2005;148:167–176. doi: 10.1016/j.jneumeth.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Bai X, Vestal M, Berman R, Negishi M, Spann M, Vega C, Desalvo M, Novotny EJ, Constable RT, Blumenfeld H. Dynamic time course of typical childhood absence seizures: EEG, behavior, and functional magnetic resonance imaging. J. Neurosci. 2010;30:5884–93. doi: 10.1523/JNEUROSCI.5101-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartolomei F, Wendling F, Régis J, Gavaret M, Guye M, Chauvel P. Pre-ictal synchronicity in limbic networks of mesial temporal lobe epilepsy. Epilepsy Res. 2004;61:89–104. doi: 10.1016/j.eplepsyres.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Beenhakker MP, Huguenard JR. Neurons that Fire Together Also Conspire Together: Is Normal Sleep Circuitry Hijacked to Generate Epilepsy? Neuron. 2009 doi: 10.1016/j.neuron.2009.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benchenane K, Tiesinga PH, Battaglia FP. Oscillations in the prefrontal cortex: A gateway to memory and attention. Curr. Opin. Neurobiol. 2011 doi: 10.1016/j.conb.2011.01.004. [DOI] [PubMed] [Google Scholar]
- Bragin A, Wilson CL, Engel J. Voltage depth profiles of high-frequency oscillations after kainic acid-induced status epilepticus. Epilepsia; 2007. pp. 35–40. [DOI] [PubMed] [Google Scholar]
- Buzsáki G, Moser EI. Memory, navigation and theta rhythm in the hippocampal-entorhinal system. Nat. Neurosci. 2013;16:130–8. doi: 10.1038/nn.3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charpier S, Leresche N, Deniau JM, Mahon S, Hughes SW, Crunelli V. On the putative contribution of GABA(B) receptors to the electrical events occuring during spontaneous spike and wave discharges. Neuropharmacology. 1999;38:1699–1706. doi: 10.1016/S0028-3908(99)00139-2. [DOI] [PubMed] [Google Scholar]
- Coenen AML, Drinkenburg WHIM, Peeters BWMM, Vossen JMH, van Luijtelaar ELJM. Absence epilepsy and the level of vigilance in rats of the WAG/Rij strain. Neurosci. Biobehav. Rev. 1991;15:259–263. doi: 10.1016/S0149-7634(05)80005-3. [DOI] [PubMed] [Google Scholar]
- Coenen aML, Drinkenburg WHIM, Inoue M, van Luijtelaar ELJM. Genetic models of absence epilepsy, with emphasis on the WAG/Rij strain of rats. Epilepsy Res. 1992;12:75–86. doi: 10.1016/0920-1211(92)90029-S. [DOI] [PubMed] [Google Scholar]
- Contreras D, Destexhe A, Sejnowski T, Steriade M. Control of spatiotemporal coherence of a thalamic oscillation by corticothalamic feedback. Science. 1996;274:771–774. doi: 10.1126/science.274.5288.771. [DOI] [PubMed] [Google Scholar]
- Contreras D, Steriade M. Spindle oscillation in cats: the role of corticothalamic feedback in a thalamically generated rhythm. J. Physiol. 1996;490(Pt. 1):159–179. doi: 10.1113/jphysiol.1996.sp021133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crunelli V, Leresche N. Childhood absence epilepsy: genes, channels, neurons and networks. Nat. Rev. Neurosci. 2002;3:371–82. doi: 10.1038/nrn811. [DOI] [PubMed] [Google Scholar]
- Danober L, Depaulis a, Marescaux C, Vergnes M. Effects of cholinergic drugs on genetic absence seizures in rats. Eur. J. Pharmacol. 1993;234:263–268. doi: 10.1016/0014-2999(93)90962-H. [DOI] [PubMed] [Google Scholar]
- Danober L, Deransart C, Depaulis A, Vergnes M, Marescaux C. Pathophysiological mechanisms of genetic absence epilepsy in the rat. Prog. Neurobiol. 1998 doi: 10.1016/S0301-0082(97)00091-9. [DOI] [PubMed] [Google Scholar]
- Destexhe A, Neubig M, Ulrich D, Huguenard J. Dendritic low-threshold calcium currents in thalamic relay cells. J. Neurosci. 1998;18:3574–3588. doi: 10.1523/JNEUROSCI.18-10-03574.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher RS, Webber WR, Lesser RP, Arroyo S, Uematsu S. High-frequency EEG activity at the start of seizures. J. Clin. Neurophysiol. 1992 doi: 10.1097/00004691-199207010-00012. [DOI] [PubMed] [Google Scholar]
- Gnatkovsky V, Librizzi L, Trombin F, De Curtis M. Fast activity at seizure onset is mediated by inhibitory circuits in the entorhinal cortex in vitro. Ann. Neurol. 2008;64:674–686. doi: 10.1002/ana.21519. [DOI] [PubMed] [Google Scholar]
- Gotman J, Grova C, Bagshaw a, Kobayashi E, Aghakhani Y, Dubeau F. Generalized epileptic discharges show thalamocortical activation and suspension of the default state of the brain. Proc. Natl. Acad. Sci. U. S. A. 2005;102:15236–15240. doi: 10.1073/pnas.0504935102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradinaru V, Thompson KR, Deisseroth K. eNpHR: A Natronomonas halorhodopsin enhanced for optogenetic applications. Brain Cell Biol. 2008;36:129–139. doi: 10.1007/s11068-008-9027-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu X, Li G, Zhou M, Lo KL. Wavelet transform based approach to harmonic analysis; 11th Int. Conf. Electr. Power Qual. Util; 2011. pp. 1–6. [DOI] [Google Scholar]
- Guo JN, Kim R, Chen Y, Negishi M, Jhun S, Weiss S, Ryu JH, Bai X, Xiao W, Feeney E, Rodriguez-Fernandez J, Mistry H, Crunelli V, Crowley MJ, Mayes LC, Constable RT, Blumenfeld H. Impaired consciousness in patients with absence seizures investigated by functional MRI, EEG, and behavioural measures: a cross-sectional study. Lancet Neurol. 2016;15:1336–1345. doi: 10.1016/S1474-4422(16)30295-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselmo ME, Bodelón C, Wyble BP. A proposed function for hippocampal theta rhythm: separate phases of encoding and retrieval enhance reversal of prior learning. Neural Comput. 2002;14:793–817. doi: 10.1162/089976602317318965. [DOI] [PubMed] [Google Scholar]
- Hirata A, Castro-Alamancos MA. Neocortex network activation and deactivation states controlled by the thalamus. J. Neurophysiol. 2010;103:1147–1157. doi: 10.1152/jn.00955.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JR. Gamma, fast, and ultrafast waves of the brain: Their relationships with epilepsy and behavior. Epilepsy Behav. 2008;13:25–31. doi: 10.1016/j.yebeh.2008.01.011. [DOI] [PubMed] [Google Scholar]
- Huguenard JR. Neuronal circuitry of thalamocortical epilepsy and mechanisms of antiabsence drug action. Adv. Neurol. 1999;79:991–999. [PubMed] [Google Scholar]
- Huguenard JR, McCormick DA. Thalamic synchrony and dynamic regulation of global forebrain oscillations. Trends Neurosci. 2007 doi: 10.1016/j.tins.2007.05.007. [DOI] [PubMed] [Google Scholar]
- Jacobs-Brichford E, Horn PS, Tenney JR. Mapping Preictal Networks Preceding Childhood Absence Seizures Using Magnetoencephalography. J. Child Neurol. 2014 doi: 10.1177/0883073813518107. 0883073813518107- [DOI] [PubMed] [Google Scholar]
- Jacobs J, Zijlmans M, Zelmann R, Chatillon CÉ, Hall J, Olivier A, Dubeau F, Gotman J. High-frequency electroencephalographic oscillations correlate with outcome of epilepsy surgery. Ann. Neurol. 2010;67:209–220. doi: 10.1002/ana.21847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahnsen H, Llinás R. Electrophysiological properties of guinea-pig thalamic neurones: an in vitro study. J. Physiol. 1984;349:205–26. doi: 10.1113/jphysiol.1984.sp015154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jirsch JD, Urrestarazu E, LeVan P, Olivier A, Dubeau F, Gotman J. High-frequency oscillations during human focal seizures. Brain. 2006;129:1593–1608. doi: 10.1093/brain/awl085. [DOI] [PubMed] [Google Scholar]
- Jiruska P, Csicsvari J, Powell AD, Fox JE, Chang W-C, Vreugdenhil M, Li X, Palus M, Bujan AF, Dearden RW, Jefferys JGR. High-frequency network activity, global increase in neuronal activity, and synchrony expansion precede epileptic seizures in vitro. J. Neurosci. 2010;30:5690–5701. doi: 10.1523/JNEUROSCI.0535-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalighi S, Sousa T, Oliveira D, Pires G, Nunes U. Efficient feature selection for sleep staging based on maximal overlap discrete wavelet transform and SVM; Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBS; 2011. pp. 3306–3309. [DOI] [PubMed] [Google Scholar]
- Kostopoulos GK. Spike-and-wave discharges of absence seizures as a transformation of sleep spindles: The continuing development of a hypothesis. Clin. Neurophysiol. 2000;111 doi: 10.1016/S1388-2457(00)00399-0. [DOI] [PubMed] [Google Scholar]
- Li H, Kraus A, Wu J, Huguenard JR, Fisher RS. Selective changes in thalamic and cortical GABAA receptor subunits in a model of acquired absence epilepsy in the rat. Neuropharmacology. 2006;51:121–8. doi: 10.1016/j.neuropharm.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Li X, Ouyang G, Richards DA. Predictability analysis of absence seizures with permutation entropy. Epilepsy Res. 2007;77:70–74. doi: 10.1016/j.eplepsyres.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Llinás RR, Grace AA, Yarom Y. In vitro neurons in mammalian cortical layer 4 exhibit intrinsic oscillatory activity in the 10- to 50-Hz frequency range. Proc. Natl. Acad. Sci. U. S. A. 1991;88:897–901. doi: 10.1073/pnas.88.3.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás RR, Steriade M. Bursting of thalamic neurons and states of vigilance. J. Neurophysiol. 2006;95:3297–308. doi: 10.1152/jn.00166.2006. [DOI] [PubMed] [Google Scholar]
- Lüttjohann A, Van Luijtelaar G. The dynamics of cortico-thalamo-cortical interactions at the transition from pre-ictal to ictal LFPs in absence epilepsy. Neurobiol. Dis. 2012;47:49–60. doi: 10.1016/j.nbd.2012.03.023. [DOI] [PubMed] [Google Scholar]
- McCormick Da, Bal T. Sleep and arousal: thalamocortical mechanisms. Annu. Rev. Neurosci. 1997;20:185–215. doi: 10.1146/annurev.neuro.20.1.185. [DOI] [PubMed] [Google Scholar]
- McCormick Da, McGinley MJ, Salkoff DB. Brain state dependent activity in the cortex and thalamus. Curr. Opin. Neurobiol. 2015;31:133–140. doi: 10.1016/j.conb.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeren Hanneke, Lopes da Silva F. Evolving Concepts on the Pathophysiology of Absence Seizures. Arch. Neurol. 2005;62:371–376. doi: 10.1001/archneur.62.3.371. [DOI] [PubMed] [Google Scholar]
- Nason G, Silverman B. The stationary wavelet transform and some statistical applications. Wavelets Stat. 1995:281–299. doi: 10.1007/978-1-4612-2544-7_17. [DOI] [Google Scholar]
- Navarro V, Martinerie J, Le Van Quyen M, Clemenceau S, Adam C, Baulac M, Varela F. Seizure anticipation in human neocortical partial epilepsy. Brain. 2002;125:640–655. doi: 10.1016/j.cmpb.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Ovchinnikov A, Lüttjohann A, Hramov A, Van Luijtelaar G. An algorithm for real-time detection of spike-wave discharges in rodents. J. Neurosci. Methods. 2010;194:172–178. doi: 10.1016/j.jneumeth.2010.09.017. [DOI] [PubMed] [Google Scholar]
- Panayiotopoulos CP. Treatment of typical absence seizures and related epileptic syndromes. Paediatr. Drugs. 2001;3:379–403. doi: 10.1007/978-1-4471-4042-9. [DOI] [PubMed] [Google Scholar]
- Panayiotopoulos CP, Obeid T, Waheed G. Differentiation of typical absence seizures in epileptic syndromes. A video EEG study of 224 seizures in 20 patients. Brain. 1989;112(Pt 4):1039–1056. doi: 10.1093/brain/112.4.1039. [DOI] [PubMed] [Google Scholar]
- Paz JT, Davidson T, Freschette E, Delord B, Prada I, Peng K, Deisseroth K, Huguenard JR. Closed-loop optogenetic control of thalamus as a new tool to interrupt seizures after cortical injury. Nat. Neurosci. 2013;16:64–70. doi: 10.1038/nn.3269.Closed-loop. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polack P-O, Guillemain I, Hu E, Deransart C, Depaulis A, Charpier S. Deep layer somatosensory cortical neurons initiate spike-and-wave discharges in a genetic model of absence seizures. J. Neurosci. 2007;27:6590–6599. doi: 10.1523/JNEUROSCI.0753-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitnikova E, van Luijtelaar G. Cortical and thalamic coherence during spike-wave seizures in WAG/Rij rats. Epilepsy Res. 2006;71:159–180. doi: 10.1016/j.eplepsyres.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Snead OC. Basic mechanisms of generalized absence seizures. Ann. Neurol. 1995 doi: 10.1002/ana.410370204. [DOI] [PubMed] [Google Scholar]
- Sohal VS, Huguenard JR. Inhibitory coupling specifically generates emergent gamma oscillations in diverse cell types. Proc. Natl. Acad. Sci. U. S. A. 2005;102:18638–43. doi: 10.1073/pnas.0509291102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorokin JM, Davidson TJ, Frechette E, Abramian AM, Deisseroth K, Huguenard JR, Paz JT. Bidirectional control of generalized epilepsy networks via rapid real-time switching of firing mode. Neuron. 2017;93:194–210. doi: 10.1016/j.neuron.2016.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staba RJ, Wilson CL, Bragin A, Jhung D, Fried I, Engel J. High-frequency oscillations recorded in human medial temporal lobe during sleep. Ann. Neurol. 2004;56:108–115. doi: 10.1002/ana.20164. [DOI] [PubMed] [Google Scholar]
- Steriade M. Corticothalamic resonance, states of vigilance and mentation. NeuroScience. 2000;101:243–276. doi: 10.1016/S0306-4522(00)00353-5. [DOI] [PubMed] [Google Scholar]
- Steriade M. Synchronized activities of coupled oscillators in the cerebral cortex and thalamus at different levels of vigilance. Cereb. Cortex. 1997;7:583–588. doi: 10.1093/cercor/7.6.583. [DOI] [PubMed] [Google Scholar]
- Steriade M, Contreras D, Amzica F, Timofeev I. Synchronization of fast (30-40 Hz) spontaneous oscillations in intrathalamic and thalamocortical networks. J. Neurosci. 1996;16:2788–2808. doi: 10.1523/JNEUROSCI.16-08-02788.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, Dossi RC, Pare D, Oakson G. Fast oscillations (2040 Hz) in thalamocortical systems and their potentiation by mesopontine cholinergic nuclei in the cat. Pnas. 1991;88:4396–4400. doi: 10.1073/pnas.88.10.4396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, McCormick DA, Sejnowski TJ. Thalamocortical oscillations in the sleeping and aroused brain. Science. 1993;262:679–685. doi: 10.1126/science.8235588. [DOI] [PubMed] [Google Scholar]
- Torrence C, Compo GP. A Practical Guide to Wavelet Analysis. Bull. Am. Meteorol. Soc. 1998;79:61–78. doi: 10.1175/1520-0477(1998)079<0061:APGTWA>2.0.CO;2. [DOI] [Google Scholar]
- van Luijtelaar ELJM, Coenen AML. Two types of electrocortical paroxysms in an inbred strain of rats. Neurosci. Lett. 1986;70:393–397. doi: 10.1016/0304-3940(86)90586-0. [DOI] [PubMed] [Google Scholar]
- Van Luijtelaar G, Hramov A, Sitnikova E, Koronovskii A. Spike-wave discharges in WAG/Rij rats are preceded by delta and theta precursor activity in cortex and thalamus. Clin. Neurophysiol. 2011;122:687–695. doi: 10.1016/j.clinph.2010.10.038. [DOI] [PubMed] [Google Scholar]
- van Luijtelaar G, Sitnikova E. Global and focal aspects of absence epilepsy: The contribution of genetic models. Neurosci. Biobehav. Rev. 2006 doi: 10.1016/j.neubiorev.2006.03.002. [DOI] [PubMed] [Google Scholar]
- White H. A heteroskedasticity-consistent covariance matrix estimator and a direct test for heteroskedasticity. Econometrica. 1980;48:817–838. [Google Scholar]
- Worrell GA, Parish L, Cranstoun SD, Jonas R, Baltuch G, Litt B. High-frequency oscillations and seizure generation in neocortical epilepsy. Brain. 2004;127:1496–1506. doi: 10.1093/brain/awh149. [DOI] [PubMed] [Google Scholar]
- Xie S, Krishnan S. Wavelet-based sparse functional linear model with applications to EEGs seizure detection and epilepsy diagnosis. Med. Biol. Eng. Comput. 2013;51:49–60. doi: 10.1007/s11517-012-0967-8. [DOI] [PubMed] [Google Scholar]
- Zijlmans M, Jiruska P, Zelmann R, Leijten FSS, Jefferys JGR, Gotman J. High-frequency oscillations as a new biomarker in epilepsy. Ann. Neurol. 2012;71:169–178. doi: 10.1002/ana.22548. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.