Abstract
Oncolytic virus therapy has recently been recognized as a promising new therapeutic approach for cancer treatment. In this study, we are proposing for the first time to evaluate the in vitro and in vivo oncolytic capacities of the Cowpox virus (CPXV). To improve the tumor selectivity and oncolytic activity, we developed a thymidine kinase (TK)-deleted CPXV expressing the suicide gene FCU1, which converts the non-toxic prodrug 5-fluorocytosine (5-FC) into cytotoxic 5-fluorouracil (5-FU) and 5-fluorouridine-5′-monophosphate (5-FUMP). This TK-deleted virus replicated efficiently in human tumor cell lines; however, it was notably attenuated in normal primary cells, thus displaying a good therapeutic index. Furthermore, this new recombinant poxvirus rendered cells sensitive to 5-FC. In vivo, after systemic injection in mice, the TK-deleted variant caused significantly less mortality than the wild-type strain. A biodistribution study demonstrated high tumor selectivity and low accumulation in normal tissues. In human xenograft models of solid tumors, the recombinant CPXV also displayed high replication, inducing relevant tumor growth inhibition. This anti-tumor effect was improved by 5-FC co-administration. These results demonstrated that CPXV is a promising oncolytic vector capable of expressing functional therapeutic transgenes.
Keywords: cowpox, oncolytic, armed
Introduction
Recent advances in the molecular biology of cancer have led to the development of novel therapeutic agents, including viruses that destroy cancer by lytic viral replication. This approach has been termed viral oncolysis, and several recent studies have demonstrated the successful application of oncolytic viruses (OVs) as effective therapeutics to tumors that have become resistant to conventional chemotherapy.1 The acceptable safety and tolerability of various OVs (herpes simplex virus, vaccinia virus, adenovirus, reovirus, parvovirus, measles virus, and Newcastle disease virus) in patients have also been demonstrated.2
In 2015, the U.S. Food and Drug Administration (FDA) approved the first oncolytic virus Imlygic (talimogene laherparevec, also known as T-VEC) for local treatment of patients with recurrent melanoma.3
The genome organization, lysis capacity, and wide tumor tropism of vaccinia virus, the most commonly used poxvirus vector for cancer therapy,4, 5 make it an ideal oncolytic agent for cancer treatment. The lead product JX-594 (Pexa-Vec) has demonstrated effective results in many clinical trials.6 The vaccinia phylum includes many strains differing by their antitumor potency as well as the frequency of associated viral complications.7 In the perspective of finding attenuated Vacinia virus (VACV) variants, several gene deletions have been tested.8, 9 Other members of the Poxviridae family have been studied for their potential oncolytic properties, such as Myxoma virus,10 Yaba-like disease virus,11 ORF virus,12 and Raccoonpox virus13 and have shown encouraging results in terms of safety and in vitro efficacy in preclinical studies.
In this article, we characterized another member of the Poxviridae family, the Cowpox virus (CPXV), as a putative new oncolytic virus.
CPXV is a European close variant of VACV that belongs to the genus Orthopoxvirus (OPV) of the Poxviridae family. CPXV is described as the source of the first vaccine used by Edward Jenner, who provided the first scientific description of vaccination by detailing the efficacy of CPXV scarification in inducing protective immunity against challenge with variola virus.14, 15 CPXV has a broad host range and is believed to persist in a reservoir comprising various rodents (particularly voles and wood mice) indigenous to parts of Europe and Asia.16 Human CPXV infections are sometimes acquired from cows, sheep, and rodents,17 but the domestic cat is responsible for the majority of these human CPXV cases.18, 19 In humans, the pathogenicity of CPXV is very limited because cowpox is generally a self-limited disease.20 Infection is usually relatively benign in immunocompetent individuals and treatment is largely supportive.21, 22, 23 Human-to-human transmission of Cowpox has never been reported.
In contrast to other double-stranded DNA viruses, poxviruses encode their own DNA replication and transcription machinery and are able to replicate in the cytoplasm, avoiding the risk of integration into the host genome. Among Orthopoxvirus species, CPXV have the largest genome, averaging above 220 kbp, about 30 kbp larger than the VACV genome. CPXV grows in vitro in monkey cells under similar conditions as VACV. Visible plaques are formed in approximately 1 to 2 days on Vero cells. CPXV is able to infect human cells, as described in several clinical cases,24, 25, 26 including epithelial cells, fibroblasts, and macrophages in vivo.27 Several publications mentioned the use of human tumoral cell culture for the amplification of CPXV.28, 29 Until now, the oncolytic potential of this virus had not been described.
Like the other members of the Orthopoxviridae family, CPXV possesses essential characteristics of use in virotherapy: an easily modifiable genome that offers the possibility to insert large therapeutic foreign genes, no risk of genomic integration, ability to replicate in human cells, rapid lytic cycle, and no pre-immunity that might hinder vector replication. Consequently, we hypothesized that CPXV could be an ideal alternative platform for oncolytic virotherapy.
Armed oncolytic vectors are widely used in current clinical trials.30 In a previous study, we described a target tumor cell killing strategy by associating an oncolytic vaccinia virus deleted in thymidine kinase (TK), combined with a suicide gene therapy.31 The TK-deleted VACV expressing the FCU1 fusion suicide gene, combined with the administration of the 5-fluorocytosine (5-FC) prodrug, displayed a highly potent anti-tumor effect both in vitro and in vivo.
In this study, we have explored the feasibility of this approach using CPXV as a viral vector and thus constructed a CPXV armed with the FCU1 gene. The FCU1 gene was inserted into the TK locus of the CPXV genome under the control of the strong p11k7.5 promoter. In vitro, we assessed the ability of the recombinant CPXV virus to replicate and kill various human tumor cell lines. We also evaluated the behavior of the virus on human primary cells.
We further examined the safety profile, biodistribution, and anti-tumor effect of this TK-deleted virus in vivo. We demonstrated here that CPXV possesses the features of a good oncolytic platform, amenable to arming with therapeutic transgenes.
Results
Virus Engineering
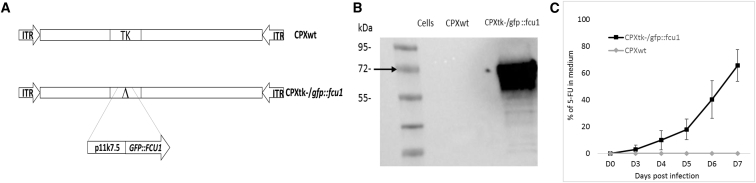
CPXV shares with VACV an essential characteristic of OVs: a large easily modifiable genome, especially with homologous recombination protocol. Due to this high homology between the two viruses (78.5% of genome identity) and particularly in the TK region (97.4% identity), a VACV shuttle plasmid was used to create the CPXtk−/gfp::fcu1 vector. Genomic structures of wild-type and recombinant CPXVs (CPXtk−/gfp::fcu1 expressing the GFP::FCU1 fusion gene) are shown in Figure 1A. The coding sequence of GFP-FCU1 (GFP::FCU1) fusion was introduced into the TK locus under transcriptional control of the synthetic vaccinia promoter p11k7.5, as described in the Materials and Methods. This chimeric GFP::FCU1 gene was generated by directly fusing in frame the coding sequences of eGFP and FCU1, followed by a precise deletion of the translation stop and start codons of eGFP and FCU1, respectively. This GFP::FCU1 fusion protein exhibits cytidine deaminase (CDase) and uracil-phosphoribotransferase (UPRTase) activities similar to those of the FCU1 protein. This chimeric protein displays a fluorescent signal intensity equivalent to the one of native eGFP protein (P.E., unpublished data).
Figure 1.
Generation of CPX Expressing the GFP::FCU1 Fusion Gene and Evaluation of the GFP-FCU1 Protein Expression
(A) Schematic representation of viruses used in this study. CPXtk−/gfp::fcu1 contains a deletion of TK gene replaced by a fusion gene between the eGFP and FCU1 genes. The GFP::FCU1 fusion gene is driven by the synthetic p11k7.5 early late promoter. (B) Western blot detection of the GFP-FCU1 protein by anti-FCU1 monoclonal antibody. Lane 1 (left to the right), mock-infected Lovo cells; lane 2, LoVo cells infected with CPXwt; lane 3, LoVo cells infected with CPXtk−/gfp::fcu1. Molecular weight standards are shown in kDa on the left. The presence of GFP-FCU 1 protein (Mr 72,000) is indicated (arrow). (C) Conversion of 5-FC to 5-FU and release of 5-FU in the cell culture supernatant. LoVo cells were infected with the indicated vector at a MOI of 0.001 and then incubated with 0.1 mM 5-FC from day 2 to day 5 post-infection. The relative concentration of 5-FC and 5-FU in the culture supernatant was measured by HPLC. The results are expressed as the percentage of 5-FU released relative to the total amount of 5-FC+5-FU. Each data point represents the mean of triplicate determinations ± SD.
Expression of the GFP::FCU1 fusion protein was confirmed by western blot using the mouse monoclonal antibody directed against FCU1 (Figure 1B). Western blot showed the expected band at 72 kDa for GFP::FCU1 protein.
Analysis of the FCU1 Enzymatic Assays and Bystander Effect
Expression of functional FCU1 by CPXtk−/gfp::fcu1 was confirmed by quantification of the enzymatic activities of FCU1. The CDase and UPRTase activities were determined in lysates of LoVo cells infected at a MOI of 0.01, 48 hr post infection, by analyzing the enzymatic conversions of 5-FC to 5-fluorouracil (5-FU), and 5-FU to 5-fluorouridine-5′-monophosphate (5-FUMP), respectively.
As shown in Table 1, elevated CDase activity was found in cells infected with CPXtk−/gfp::fcu1, whereas no CDase activity was detectable in mock or CPXwt-infected cells. In the same way, UPRTase activity was only found in cells infected by the recombinant CPXV.
Table 1.
Specific CDase and UPRTase Activities in the LoVo Cell Line
| Vector | CDase 5-FC→5-FU | UPRTase 5-FU→5-FUMP |
|---|---|---|
| Mock | ND | ND |
| CPXwt | ND | ND |
| CPXtk−/gfp::fcu1 | 106 ± 17 | 5.65 ± 0.3 |
CDase and UPRTase activities are expressed as the number of nanomol of 5-FC deaminated per min per mg of protein and the number of nmol of 5-FU phosphorylated per min per mg of protein, respectively. The indicated enzymatic activities were measured as described in the Materials and Methods. Each value represents the average of three independent experiments ± SD. Abbreviations: CDase, cytosine deaminase; 5-FC, 5-fluorocytosine; 5-FU, 5-fluorouracil; 5-FUMP, 5-fluorouridine-5’-monophosphate; ND, not detectable; UPRTase, uracil phosphoribosyltransferase.
A major strength of any prodrug activation model is the potential act on neighboring untransfected cells, after diffusion of the cytotoxic drug. In the case of FCU1/5-FC combination, an efficient bystander effect has been reported because 5-FU is highly diffusible around the replication site of the oncolytic virus.32
The analysis of LoVo cell supernatants by high-pressure liquid chromatography (HPLC) showed a progressive release of 5-FU in extracellular medium of LoVo cells transduced with CPXtk−/gfp::fcu1 at MOI of 0.001 and incubated with 0.1 mM 5-FC (Figure 1C). 7 days after infection, approximately 70% of 5-FC was converted to 5-FU in supernatants of CPXtk−/gfp::fcu1 LoVo-transduced cells. Moreover, no 5-FU was detected in supernatants of CPXwt-infected cells, confirming functionality and efficacy of the FCU1 protein expressed by the recombinant CPXtk−/gfp::fcu1 vector. Together, these in vitro enzymatic activity results demonstrate that CPXV can express a functional therapeutic FCU1 gene and is an efficient vector for viral directed enzyme prodrug therapy (VDEPT).
CPXV Infects, Replicates, and Kills Human Tumor Cells In Vitro
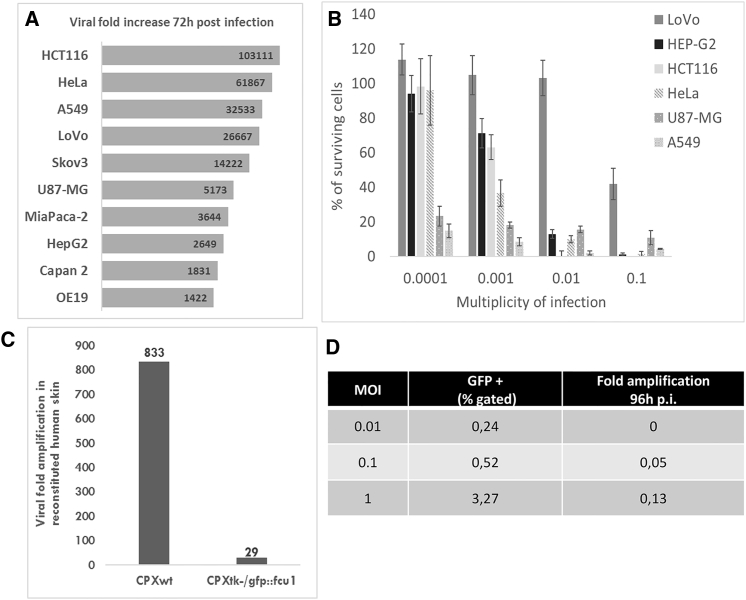
To evaluate the ability of CPXV to enter and replicate in human tumor cell lines, we measured the level of viral particles after 72 hr of infection in various cell lines (Figure 2A). The CPXtk−/gfp::fcu1 virus was able to infect and replicate in all the tumor cell lines tested. However, these results highlighted a cell-line-dependent heterogeneity of replication. High replication was observed in HCT116 cells, where CPXtk−/gfp::fcu1 viral replication reached a 100,000-fold increase (Figure 2A), and lower amplification was shown in OE19, Hep G2, and Capan-2 cells, with 1,400-, 1,800-, and 2,700-fold increases, respectively.
Figure 2.
Replication and Oncolytic Activity of CPXtk−/gfp::fcu1
(A) Amplification factor of CPXtk−/gfp::fcu1 in a panel of human tumoral cells infected with 300 pfu and collected 72 hr post-infection. (B) Tumor cell viability by Trypan blue exclusion: 3 × 105 cells/well were infected with CPXtk−/gfp::fcu1 at a MOI ranging from 0.0001 to 0.1. 5 days after, cell viability was determined by ViCell cell counter automate based on Trypan blue exclusion method. Each data point represents the mean of triplicate determinations ± SD. (C) 3D Phenion FT skin models were infected with 8 × 104 pfu of CPXwt and CPXtk−/gfp::fcu1 by scarification. 7 days post infection, 3D skin and supernatant were collected and sonicated, and viral titers were determined by plaque assay. Results are expressed as viral fold increased (corresponding to output/input ratio). (D) Infection and replication of CPX TK-deleted virus on hPBMCs. Fresh hPBMCs were infected by CPXtk−/gfp::fcu1 at different MOI. 16 hr post infection, eGFP level was measured on flow cytometry. 4 days post infection, cells and supernatants were harvested and sonicated. Viral titers were determined by plaque assay on Vero cells. Results are expressed as viral fold increased (corresponding to output/input ratio).
Nevertheless, antitumoral activity assays confirmed that CPXtk−/gfp::fcu1 was able to infect and kill all tested tumor cell lines with a dose-dependent effect (Figure 2B). Hep G2 cell viability after infection with CPXtk−/gfp::fcu1 varied from 95% at an MOI of 0.0001 to 2% at a MOI of 0.1. Furthermore, CPXtk−/gfp::fcu1 showed an efficient anti-tumor activity on A549 (lung carcinoma) and U-87 MG (glioblastoma) cells with less than 25% viability at a low MOI (0.0001).
Cell Killing by Combination of Prodrug Activation with Viral Oncolysis
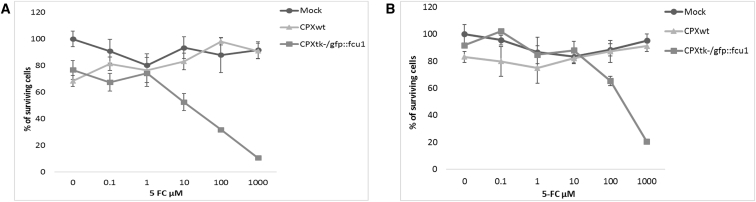
In order to compare the antitumoral activity of CPXtk−/gfp::fcu1 vector alone or combined with 5-FC treatment, LoVo and A549 cancer cells were infected at MOI of 0.01 and 0.000001, respectively, corresponding to a low cytotoxicity (20%–30% mortality). 2 days post-infection, 5-FC was added to the culture supernatants at a range of concentrations (0.1–1,000 μM). Cell viability was determined 3 days later by Trypan blue exclusion (Figure 3). Oncolytic activity of both CPXwt and CPXtk−/gfp::fcu1, in the absence of 5-FC prodrug, was similar and resulted in a low antitumoral effect. The addition of 5-FC had no impact on the viability of mock and CPXwt-infected tumor cells. In contrast, the 5-FC conferred increased toxicity in a prodrug dose-dependent manner to CPXtk−/gfp::fcu1 on human tumor cells. The combination of a low amount of CPXtk−/gfp::fcu1 with 1 mM of 5-FC induced 90% mortality of LoVo cells and 80% mortality of A549 cells.
Figure 3.
In Vitro Sensitivities of Infected Human Tumor Cells to 5-FC
Combination of oncolytic and prodrug activation cytotoxicity. (A and B) LoVo (A) and A549 (B) human tumor cells were infected with the CPXwt and CPXtk−/gfp::fcu1 at a MOI of 0.01 on LoVo cells and at a MOI of 0.000001 on A549 cells. After 48 hr, cells were grown in the presence of increasing concentrations of 5-FC. Cell survival was determined 3 days later, as described in the Materials and Methods section. Cell viability results are expressed as the percentage of viable cells relative to untreated/non-infected cells. Values are represented as mean ± SD of three individual determinations.
These results indicate that recombinant CPXtk−/gfp::fcu1 acquired an enhanced in vitro anti-tumor activity as compared to CPXwt virus in the presence of 5-FC prodrug.
Pathogenicity Evaluation In Vitro and In Vivo
Phenion full thickness is a 3D multilayered skin equivalent that simulates histological and physiological properties of human skin. The model represents a multilayered epidermis and a dermal compartment.33, 34 The pathogenesis of several different virus infections, including papillomaviruses, adenoviruses, parvoviruses, poxviruses, and herpesviruses was tested with 3D skin substitutes.35, 36 In the case of poxviruses, especially CPXV, it has already been demonstrated that epithelial raft culture can be used as a predictive model for pox lesions.37 In our experiment (Figure 2C), we evaluated the replication of both CPXwt and the TK-deleted virus. After 7 days of infection, CPXtk−/gfp::fcu1 reached lower titers than the CPXwt (<30-fold and 833-fold amplifications, respectively). These results confirmed the benefit of deleting TK gene for safety improvement.
We also demonstrated that human peripheral blood mononuclear cells (hPBMCs) were weakly infected by our TK-deleted CPXV and that the virus was not able to replicate in these primary cells. As shown in Figure 2D, after 16 hr of infection, CPXtk−/gfp::fcu1 penetrated poorly into PBMCs, with less than 5% of cells infected at MOI 1 and no viral amplification observed 4 days post infection. The replication of the recombinant CPXV was totally abortive in these blood cells: CPXtk−/gfp::fcu1 had a negligible impact on these cells.
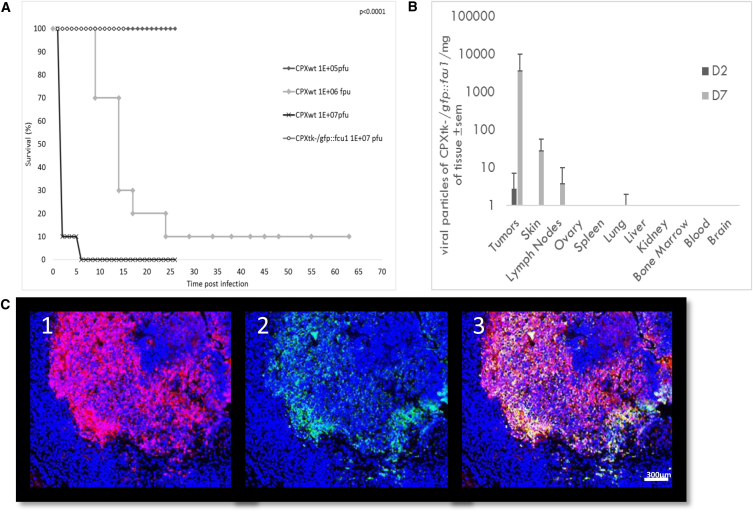
In vivo, the virulence of CPXV was assessed in immunocompetent BALB/c mice. Animals were injected intravenously (i.v.) with CPXwt or CPXtk−/gfp::fcu1 at a dose between 1 × 104 and 1 × 107 particle forming units (pfu)/mouse (Figure 4A). Weight loss, mortality, and side effects were monitored for more than 60 days. As expected, considering mice as the natural reservoir of the virus, the CPXwt demonstrated a high and rapid toxicity after injection at the highest dose (1 × 107 pfu), with 90% mortality 1-day post injection. At 106 pfu, we still observed a high mortality, but with a delay in the time to death (90% mortality 24 days post infection). At lower doses (105 and 104 pfu), no mortality was observed but mice infected with CPXwt exhibited pock lesions around the genital area, footpad, and mouth. These side effects appeared 9 days post-infection and persisted until the end of the experiment.
Figure 4.
Virulence Studies and Biodistribution of CPXV in Mice
(A)Virulence studies in CPXV-infected mice. Immunocompetent BalB/c mice were i.v. injected with CPXwt or CPXtk−/gfp::fcu1 at concentration ranging from 1 × 105 to 1 × 107 pfu/mouse (n = 10 per group). The highest dose of deleted virus is represented. Animals were individually weighted and monitored for signs of disease for more than 60 days. Mice were euthanized when their initial weight loss reached 20% of their initial weight. Survival data are presented as Kaplan-Meier plots. p values are obtained by statistical analysis (log-rank). (B) Organ distribution of CPXVtk-/gfp::fcu1 in mice. A biodistribution experiment was performed in Swiss Nude mice bearing subcutaneous glioblastoma tumor cells. Mice were injected i.v. with CPXtk−/gfp::fcu1 at 1 × 106 pfu . At days 2 and 7, 3 mice were euthanized, and tumors and organs were collected and homogenized. Viral titers were determined by plaque assay on Vero cells. Results are expressed in pfu/mg of tissue ± SEM. (C) Viral immunostaining was performed after fixation in formaldehyde (FA) as described in Materials and Methods. Cellular DNA was stained in blue (C1, C2, and C3) with DAPI, gfp::fcu1 protein was stained in red (C1 and C3), and virus was stained in green (C2 and C3). The merged picture is presented in C3.
In comparison, the recombinant CPXtk−/gfp::fcu1 virus was significantly attenuated. All treated mice remained healthy, with no weight loss (data not shown). At the doses of 106 and 107 pfu, pock lesions were detected on the tail and face of the mice. In contrast to pock lesions induced by CPXwt, these mild and self-limiting lesions healed in approximately 20 days. No clinical signs of illness were observed at the lowest doses.
Biodistribution of CPXtk−/gfp::fcu1 and Detection of FCU1 Protein in Tumors
As an in vivo demonstration of the tumor selectivity of CPXtk−/gfp::fcu1 replication, Swiss nude mice bearing subcutaneous (s.c.) U-87 MG human glioblastoma xenograft tumors were treated with CPXtk−/gfp::fcu1 via the i.v. route. Viral titers were determined by a standard plaque assay on Vero cells.
A significant difference between infectivity of tumor and normal tissue was observed 2 days after inoculation of the virus (Figure 4B). CPXtk−/gfp::fcu1 was only detected in tumors. 7 days after injection, CPXtk−/gfp::fcu1 viral particles had titers 100-fold or higher in tumor tissues compared to any other organ tissue tested, including the brain, bone marrow, kidneys, liver, ovaries, blood, and spleen. Low amounts of virus were detected in the skin (with the highest level at 30 pfu/mg of tissue), lymph nodes, and, to a smaller extent, in the lung (with less than 1 pfu/mg of tissue). The titration of CPXtk−/gfp::fcu1 in the entire tumor at day 7 after a single injection of 1 × 106 pfu revealed a viral load of up to 7 × 106 pfu, demonstrating that CPXV was able to reach and fully replicate in the tumor after systemic injection.
The presence of CPXV and the expression of GFP::FCU1 gene into the tumor was analyzed by immunostaining in LoVo human colorectal tumor-bearing mice treated intratumorally (IT) by the recombinant CPXV vector. FCU1 and viral immunostaining were performed 5 days after virus administration. Fluorescence microscopy of tumor sections confirmed the expression of FCU1 (Figure 4C1) and the presence of viral proteins (Figure 4C2). As expected, the distribution of the FCU1 fusion protein correlated with the area of virus detection (Figure 4C3). Virus was detected in almost all the tumoral mass, showing that CPXV was able to replicate and rapidly invade the tumor. The merge picture clearly showed the spread of the virus and a strong expression of the therapeutic FCU1 protein (Figure 4C3).
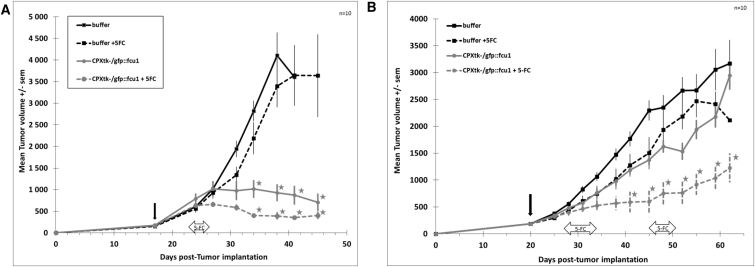
CPXtk−/gfp::fcu1 Combined with 5-FC Therapy Reduces Tumor in Human Glioblastoma and Colorectal Xenograft Models
The clinical potential of the virus was monitored in two human cancer cells lines: U-87 MG as a human glioblastoma model (Figure 5A) and LoVo as a human colorectal cancer model (Figure 5B).
Figure 5.
In Vivo CPXV Anti-tumor Activity in Glioblastoma and Colorectal Xenograft Models
(A and B) Immunodeficient Swiss nude mice were implanted subcutaneously with U-87 MG glioblastoma cells (A) or LoVo colorectal carcinoma cells (B). Mice (n = 10/group) were injected IT with CPXtk−/gfp::fcu1 (1 × 106 pfu) or CPXwt 3 weeks after tumor cell transplantation. 5 days after viral injection, 5-FC was administered to mice by gavage at a dose of 200 mg/kg/day. Mice were monitored until sacrifice based on high tumor volume. The vertical arrow indicates the time of virus injection and the horizontal arrow indicates the duration of 5-FC treatment. Stars represent p value < 0.05 compared to control groups (buffer and buffer + 5-FC). Results are expressed in mean tumor volume ± SEM.
Nude mice bearing established s.c. tumors (100–300 mm3) were treated IT with 106 pfu CPXwt or CPXtk−/gfp::fcu1 . In order to obtain the full efficacy of the combined therapy, the 5-FC treatment started 5 days after virus injection with daily oral gavage at the dose of 200 mg/kg/day. This time period should enable virus spread in the whole tumor.
In the U-87 MG glioblastoma model, as shown in Figure 5A, CPXV treatment resulted in the stabilization of the tumor growth, with a reduction of more than 70% of tumoral mass as compared to the control group (p < 0.0005). This strong tumor growth inhibition was also improved by the addition of 5-FC that led to an additional but not significant tumor size reduction of 10% as compared to CPXV alone. In this U-87 MG glioblastoma model, mice showed classic signs of illness (mainly diarrhea and weight loss) after 5 days of 5-FC treatment, presumably due to 5-FU toxicity. These side effects were reversible upon 5-FC treatment discontinuation.
A single IT injection of CPXV in the LoVo colorectal model tumor (Figure 5B) resulted in a weak antitumoral effect in comparison to the control group (p > 0.05). The administration of 5-FC alone had no effect on tumor growth. The lack of antitumoral activity of the virus alone was compensated by 5-FC treatment, which demonstrated improved efficacy, resulting in approximately 60% tumor regression after 60 days (p < 0.05). In this LoVo model, in contrast to the U-87 MG model, no 5-FU-related toxicity was observed. Consequently, a second cycle of 5-FC treatment was administered to the mice.
Taken together, these results confirmed the strong efficacy of the combined action of CPXtk−/gfp::fcu1 virus and 5-FC treatment on U-87 MG tumor cells and to a lesser extend in LoVo tumor cells.
Discussion
The data presented in this article underscore the interest of using CPXV as a new platform for vaccine delivery. In this study, the virus ability to control cellular proliferation was notable in all the tested human tumor cell lines. The most remarkable results were obtained using HeLa and Hep G2 cells, in which cell proliferation was reduced by 85% following CPXV treatment at a MOI of 0.01. Equally impressive, HCT116 colon carcinoma cell proliferation was totally inhibited at the same multiplicity of infection, confirming the strong oncolytic potential of CPXV. Our data demonstrated that CPXV is able to infect and replicate in a number of human tumor cell lines and it is also able to kill them.
Nevertheless, complete infection and lysis of the whole tumor is difficult. Therefore, oncolytic viruses are often armed. One example of arming is enzyme-prodrug systems, which are capable of exerting a strong bystander effect and which may enhance the oncolytic efficacy of the virus therapy by eliminating surrounding uninfected tumor cells. The FCU1/5-FC enzyme-prodrug system has been extensively investigated in vitro and in preclinical models of xenografts in using a variety of delivery systems, including replication-defective viruses32, 38 and replication-selective oncolytic viruses.31, 39, 40, 41, 42, 43 The proof of this suicide gene concept has also been demonstrated in humans using TG4023, a non-propagative Vaccinia virus (MVA) hosting the FCU1 gene.44 In this phase I study, after a single percutaneous IT injection of TG4023 in primary or metastatic liver tumors in combination with systemic administration of 5-FC, therapeutic 5-FU concentrations in tumors were detected without significant systemic exposure to the cytotoxic anticancer drug.
The expression of the fusion gene GFP::FCU1 in the CPXV backbone allows infection and replication monitoring of the virus and of the enzymatic activation of the 5-FC prodrug by the bifunctional suicide protein FCU1. The FCU1 gene, through its expression by CPXtk−/gfp::fcu1, was able to convert non-toxic prodrug 5-FC into cytotoxic 5-FU and 5-FUMP. The produced 5-FU diffuses in and out of cells and does not require cell-to-cell contact for cell toxicity. This bystander effect enhances the antitumor efficacy of the VDEPT approach by eliminating surrounding uninfected tumor cells via in situ production of cytotoxic 5-FU.45 In a human colorectal cancer in vitro model, the recombinant CPXtk−/gfp::fcu1 vector exhibited less than 10% of cytotoxicity, but reached more than 90% cytotoxicity upon addition of 5-FC and production of 5-FU in the culture media. In our recombinant CPXV, the expression of the transgene is under control of the early/late p11k7.5 VACV synthetic promoter. The use of a vaccinia promoter in a Cowpox vector was already demonstrated by the expression of marker genes (eGFP and mRFP) under early and late vaccinia promoter.46 Moreover, we previously demonstrated the superiority of p11k7.5 compared to natural p7.5 and pH5R VACV promoters for gene expression in the MVA backbone.38 In our study, the efficacy of the p11k7.5 synthetic promoter in the cowpox context was largely confirmed by western blot analysis, showing a strong expression of the FCU1 therapeutic gene in a human cancer cell line.
These results were confirmed in vivo by imaging: the IT administered recombinant CPXtk−/gfp::fcu1 vector invaded the tumor tissues and the FCU1 protein was correctly and largely expressed in the tumor. Moreover, the IT injection resulted in a significant reduction of the tumor progression, with or without 5-FC administration. Indeed, we showed here that CPXV replicated in the glioblastoma xenograft model and rapidly killed tumoral cells, demonstrating a potent antitumor efficacy due to viral replication in the tumor. In a model more resistant to viral replication, like Lovo colorectal cancer, the expression of the therapeutic transgene is particularly beneficial. In this model, the recombinant virus alone had weak antitumoral activity, whereas the concomitant 5-FC treatment led to significant inhibition of tumoral growth. However, this setting can still be optimized. It might also be possible, in order to increase the efficacy of the combination and reduce side effects to extend the viral injection/5-FC treatment interval and dose. Numerous schedules of administration could also be evaluated, starting with the route of injection. In our study, the single injection of the recombinant CPXV at 1 × 106 pfu by i.v. failed (data not shown). Our hypothesis is that a too low dose was injected. Indeed, we know, thanks to a biodistribution experiment, that virus injected by a systemic route is able to reach the tumor. Another possibility could be to maintain the same dose, but with two or three injections. All these regimens require further investigations.
We then demonstrated in a series of experiments that inactivation of the TK gene leads to very low levels of virus multiplication in an in vitro skin model as well as attenuation in mice, presumably due to restricted multiplication in non-dividing cells. In an immunocompetent mouse model, a natural CPXV host,16 TK-deleted CPXV was significantly attenuated compared to wild-type CPXV. Unlike CPXwt, no mortality was observed in mice infected by CPXtk−/gfp::fcu1 during the study and only few reversible skin side effects were identified. Moreover, we demonstrated the ability of the recombinant CPXV to specifically replicate in implanted tumors after i.v. injection while leaving, apart from skin and nodes, most organs unaffected.
In order to further decrease CPXV-associated side effects, one or multiple gene deletions could be considered. Xu et al.46 demonstrated that 10 genes are involved in CPXV virulence by the induction of hemorrhagic lesions on chorioallantoic membranes. The deletion of the CrmA gene from cowpox had also demonstrated some attenuation in the mouse pulmonary disease model.47 Moreover, CPXV like VACV encodes a large number of immunomodulatory proteins involved in the inhibition or evasion to the host inflammatory and immune responses29, 48 in antiapoptotic proteins (F1L, N1L, and E3L) or proteins acting as growth-stimulating factors like VGF.49, 50 Deletion of these genes could improve the tumoral selectivity and safety profile of CPXV, as demonstrated with VACV.51, 52
Interestingly, CPXV harbors the most complete set of open reading frames expected to encode immune evasion proteins that collectively target a wide range of antiviral host responses.53, 54 Unlike VACV, CPXV prevents stimulation of CD8+ T cells, and this correlated with retention of MHC-I in the endoplasmic reticulum by the CPXV203 protein.55 This particular skill allows CPXV to hide from the immune system. Studies highlighted that CPXV and VACV are clearly distinguishable56 and not immunologically identical.57 Yet sporadic human CPXV infections have been more frequent in recent years than in the past, probably due to the cessation of VACV-based, smallpox vaccination.25, 58, 59, 60 Several studies highlight that VACV vaccination protects against CPXV challenge, although to a different degree, depending on the severity of the challenge.61 These results on animals seem to be confirmed in humans because recent vaccination with VACV, in fact, does not fully protect against Cowpox;60 no or limited neutralizing antibodies are produced. In this context, the possible development of CPXV as a new or second wave in the setting of OV-based treatment requires further investigations. Another hypothesis, related to the emergence of human CPXV infection, is the diversity of CPXV strains.62, 63 Screening different strains as oncolytic viruses would be of definite interest.
In summary, this study represents, to our knowledge, the first characterization of an oncolytic CPXV and its use as a vector for VDEPT. Considering these results and the CPXV characteristics (cytoplasmic replication, high titer production, easily modifiable genome with high capacity of insertion, and ability to escape innate and acquired immunity), CPXV could become an ideal vector candidate for oncolytic viral therapies.
Materials and Methods
Cell Lines
Human colon cancer cell lines LoVo (ATCC CCL-229) and HCT 116 (ATCC CCL-247), human lung cancer cell line A549 (ATCC CCL-185), hepatocarcinoma human cell line HepG2 (ATCC HB 8065), glioblastoma human cancer cell line U-87 MG (ATCC HTB-14), cervix human cancer cell line HeLa (ATCC CCL-2), pancreatic human cancer cell line MIA-Paca-2 (ATCC CRL-1420), and Vero cell line (ATCC CCL-81) were obtained from the American Type Culture Collection (ATCC, Rockville, MD). Human esophagus cancer cell line OE-19 (ECACC n°96071721) was obtained from the European Collection of Cell Culture (ECACC). All cell lines were grown in recommended media supplemented with 10% fetal calf serum (FCS). hPBMCs were obtained after buffy coat extraction from a blood bag (EFS, Strasbourg, France).
Viruses
CPXV strain Brighton (ATCC VR-302) (CPXwt) was obtained from ATCC (Rockville, MD). A recombinant CPXV was created by insertion of the GFP::FCU1 fusion gene into the CPXwt TK locus (CPXV CDS 105). Briefly, Vero cells were infected with CPXwt at a MOI of 0.01 and incubated at 37°C for 3 hr, then transfected with a shuttle plasmid containing the GFP::FCU1 fusion gene under the control of the synthetic p11k7.5 promoter and surrounded by the flanking sequence of the TK gene.
The cells were then incubated for 48 hr at 37°C. Double recombination occurred between TK homologous regions in the shuttle plasmid and the wild-type virus, resulting in the insertion of the GFP::FCU1 fusion gene into the TK locus of the CPX (CPXtk−/gfp::fcu1). Recombinant virus was isolated from eGFP-fluorescent plaques and submitted to additional plaque purification cycles on Vero cells. Virus sequence was confirmed by multiple PCRs and DNA sequencing.
CPXwt and CPXtk−/gfp::fcu1 vector were grown on HeLa cells and purified on a sucrose gradient. Virus stocks were titrated on Vero cells by plaque assay. Viruses were adsorbed on cell monolayers for 30 min at 37°C. Infected cells were incubated in fresh medium supplemented with 5% FCS and 1% agar at 37°C in a 5% CO2 atmosphere. After 72 hr, the infected cells were stained with a second agar overlay supplemented with 0.008% neutral red.
Western Blotting
LoVo tumor cells were infected by CPXwt and CPXtk−/gfp::fcu1 at a MOI of 0.1 and incubated for 24 hr. Cell lysate proteins (30 μg as determined by using a Bio-Rad protein assay) were run on a 10% SDS-PAGE gel under reducing conditions and transferred onto a nitrocellulose membrane. The membrane was incubated with anti-FCU1 mouse monoclonal antibody 3H131 and washed and incubated with anti-mouse secondary antibody coupled to horseradish peroxidase (Amersham, Les Ulis, France). Detection was done using enhanced chemiluminescence (Amersham).
Enzymatic Assays
CDase and UPRTase activities in LoVo cells were determined using 5-FC (Toronto Research Chemicals, North York, Canada) and 5-FU (Sigma, St. Louis, MO) as substrates, respectively. Lovo human tumor cells (3 × 106 cells) were infected by CPXwt and CPXtk−/gfp::fcu1 vector at a MOI of 0.01. 48 hr later, CDase and UPRTase activity detection was performed by enzymatic assays, as previously described.38 Briefly, 5-FC, 5-FU, and 5-FUMP were isocratically separated using HPLC (supelcosil LC-18-S column and UV detection at 260 nm and 280 nm). For detection of CDase activity, the mobile phase was 50 mM phosphoric acid adjust to pH 2.1 with ammonium hydroxide. For detection of UPRTase activity, the mobile phase was 20 mM KH2PO4, 5 mM tetrabutylammoniumsulfate, and 5% methanol adjusted to pH 5 with potassium hydroxide.
CDase activity was also quantified by measuring the amount of 5-FU released in the culture media. LoVo cells were infected with the different vectors at a MOI of 0.0001 and plated in a 6-well culture dish (1 × 106 cells/well). After 48 hr, 1 mM 5-FC was added to the culture medium. Every day for 1 week, 5-FC and 5-FU concentrations in the media were measured by HPLC. 50 μL of media were quenched with 1 mL of ethyl acetate/2-propanol/0.5 M acetic acid solution (84:15:1). The samples were vortexed and centrifuged. The organic supernatant was evaporated to dryness and reconstituted in 50 μL of water and analyzed by HPLC using a mobile phase of 50 mM phosphoric acid adjusted to pH 2.1. Results are expressed as the percentage of 5-FU relative to the total amount of 5FC + 5FU after various incubation times with 5-FC.
In Vitro Viability Assay
Human tumor cells were infected in suspension by CPXwt and CPXtk−/gfp::fcu1 at a different MOI of 0.1, 0.01, 0.001, and 0.0001. A total of 3 × 105 cells/well were plated in 6-well culture dishes in 2 mL of medium supplemented with 10% FCS. Cells were then cultured at 37°C for 5 days, and the viable cells were counted by Trypan blue exclusion using a Vi-Cell cell counter (Beckmann Coulter, CA). Results are expressed as the percentage of viable cells, 100% corresponding to mock treated cells.
In Vitro Cell Sensitivity to 5-FC
Human LoVo and A549 tumor cells in suspension were transduced by CPXtk−/gfp::fcu1 virus at MOI of 0.01 and 0.000001, respectively. A total of 5 × 105 cells/well were plated in 6-well culture dishes in 2 mL of medium supplemented with 10% FCS. After 48 hr of infection, cells were exposed to various concentrations of 5-FC, ranging from 0.1 to 1,000 μmol. 3 days later, cell viability was determined by Trypan blue exclusion using a Vi-Cell cell counter. Results are expressed as percentage of viable cells, 100% corresponding to mock treated cells without 5-FC.
In Vitro Virus Replication
Growing human tumor cells were seeded onto 6-well plates at 5 × 105 cells/well. 24 hr later, cells were infected with CPXtk−/gfp::fcu1 at a MOI of 0.001 and incubated in fresh growth medium supplemented with 10% FCS. Before harvesting, pictures were taken under fluorescent microscopy (by UV excitation) for eGFP expression visualization. Supernatants and cells were collected 72 hr post-infection and submitted to a quick freeze-thaw cycle and sonication to release intracellular viral particles. Viral titer in cell lysates was quantified on Vero cells by plaque assay.
3D Skin Model Infection
The Phenion full-thickness (FT) skin model is a 3D tissue construct that simulates histological and physiological properties of human skin (∅ 1.32 cm; surface 1.3 cm2). It was purchased from Henkel AG & Company KGaA (Düsseldorf, Germany). According to the supplier’s instructions, tissues were kept at 37°C under 5% CO2 overnight and fresh, pre-warmed medium was added. Each Phenion FT skin model was infected with 8 × 104 pfu of CPXwt or CPXtk−/gfp::fcu1 by scarification of the tissue. Cultures were incubated for 7 days at 37°C and medium was changed every other day. Viral replication both in medium and skin was quantified on Vero cells by plaque assay after 2 cycles of sonication in PBS.
Animals
All animal protocols were carried out according to standard operating procedures of Felasa and have been approved by the French Research and Education Ministry (APAFIS#7049-2016060816539934 v6).
In Vivo Viral Pathogenicity
Viral pathogenicity of CPXtk−/gfp::fcu1 was assessed upon infection in immunocompetent BALB/c mice (female, 6 weeks old from Charles Rivers Laboratories [Saint Germain Nuelles, France]). Increasing doses of CPXwt and CPXtk−/gfp::fcu1, ranging from 1 × 104 pfu to 1 × 107 pfu, were injected intravenously in the tail vein. The animals were examined daily throughout the course of the experiment for signs of illness, weight loss, general appearance, and pock lesion formation.
Subcutaneous Tumor Models
Female Swiss nude mice were obtained from Charles River Laboratories. Animals used in the studies were uniform in age (6 weeks) and body weight (20–23 g).
Mice were injected s.c. into the right flank with 5 × 106 human tumor cells (LoVo or U-87 MG). When tumors reached a diameter of 100–300 mm3, mice were assigned in a random, blinded manner to receive the recombinant or wild-type virus.
Biodistribution of the Recombinant CPX Virus
The presence of CPXtk−/gfp::fcu1 virus in tumors and organ samples was evaluated by virus titration. The virus was injected i.v. at the dose of 1 × 106 pfu through tail vein into female nude mice bearing s.c. U-87 MG tumor. Mice were sacrificed 2 and 7 days post infection. Mice were perfused intracardially with an exsanguinating solution (0.9% NaCl with heparin 50 IU/mL) until blood was removed. Tumors and organs were then collected, weighed, homogenized in PBS, and sonicated. Viral titers were quantified by plaque assay on Vero cells and standardized per milligram of tissue.
In Vivo Antitumor Activity of the Recombinant CPX Virus in Subcutaneous Tumor Model
Nude mice bearing established s.c. implanted U-87 MG or LoVo tumors were treated by IT injection of CPXtk−/gfp::fcu1 vector at the dose of 1 × 106 pfu (in 100 μL of PBS) or vehicle (control group). 5 days post-virus or vehicle injection, 5-FC was administered by oral gavage at 100 mg/kg (0.5 mL of 5-FC 0.5% in water) twice a day for 3 weeks. Tumor size was measured twice a week using calipers. Tumor volume was calculated in mm3 using the formula (Π/6)(length × width2).
Immunohistochemistry
Detection of FCU1 and the viral proteins was performed by immunohistochemical labeling. For each treatment, 3 slides were analyzed. 5 days after IT injection of CPXtk−/gfp::fcu1 at the dose of 1 × 106 pfu, resected LoVo tumors were fixed with 4% formaldehyde in 0.1 M phosphate buffer. Tumors where then desiccated and embedded in paraffin wax. Sections (5 μM) were mounted on adhesive glass slides and used for histological analysis.
Detection of FCU1 protein was performed by immunostaining of the slides fixed with tumor with anti-FCU1 mouse monoclonal antibody 3H1, followed by Goat anti Mouse-immunoglobulin-G (IgG)-Polymer Dextran HRP (DAKO, K4001) (red staining). CPXV-infected cells were detected upon incubation of the slide with rabbit IgG anti vaccinia-virus (B 65101R, Biodesign, dilution 1/1,000), followed by goat anti-rabbit IgG-Polymer Dextran HRP (DAKO, K4003, dilution 1/1,000) (green staining). To block non-specific antibody binding, slides were incubated 30 min with Linblock solution between the two staining steps. Coverslips were counterstained with DAPI (B-2883 SIGMA) (blue staining) and mounted on glass slides. Negative control tumors also underwent the same immunostaining treatment for comparison purposes. Slides were analyzed using Nikon microscopy.
Statistical Analysis
Statistical analyses of tumor volumes were performed using the nonparametric Mann-Whitney U test and the log-rank test was applied for statistical survival analysis (Statistica 7.1 software, StatSoft). A p value < 0.05 was considered to be statistically significant and is represented by stars on the figures.
Author Contributions
Conceived the design of the study: P.E., J.F., M.R., S.B., and C.C.-B; Performed the experiments in vitro and in vivo: M.R.; Conducted HPLC experiments: P.C.; Histological study: S.C.; Animal studies: C.P, D.A., and N.S.; Conducted hPBMC studies: C.T.; Analyzed the data: M.R., J.F., and P.E.; Review: E.Q. and Mariette Ducatez. We thank Caroline Schenkels and Alison Munro for English support.
Acknowledgments
M.R. is the primary author of this manuscript and recipient of an Industrial Training Convention for Research (CIFRE) doctoral fellowship. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Lichty B.D., Breitbach C.J., Stojdl D.F., Bell J.C. Going viral with cancer immunotherapy. Nat. Rev. Cancer. 2014;14:559–567. doi: 10.1038/nrc3770. [DOI] [PubMed] [Google Scholar]
- 2.Pol J., Buqué A., Aranda F., Bloy N., Cremer I., Eggermont A., Erbs P., Fucikova J., Galon J., Limacher J.M. Trial watch-oncolytic viruses and cancer therapy. OncoImmunology. 2015;5:e1117740. doi: 10.1080/2162402X.2015.1117740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andtbacka R.H.I., Kaufman H.L., Collichio F., Amatruda T., Senzer N., Chesney J., Delman K.A., Spitler L.E., Puzanov I., Agarwala S.S. Talimogene laherparepvec improves durable response rate in patients with advanced melanoma. J. Clin. Oncol. 2015;33:2780–2788. doi: 10.1200/JCO.2014.58.3377. [DOI] [PubMed] [Google Scholar]
- 4.Mell L.K., Yu Y.A., Brumund K.T., Advani S.J., Onyeama S., Daniels G.A., Weisman R.A., Martin P., Szalay A.A. Phase I trial of attenuated vaccinia virus (GL-ONC1) delivered intravenously with concurrent cisplatin and radiation therapy in patients with locoregionally advanced head-and-neck squamous cell carcinoma. Int. J. Radiat. Oncol. 2014;88:477–478. [Google Scholar]
- 5.Mastrangelo M.J., Maguire H.C., Jr., Eisenlohr L.C., Laughlin C.E., Monken C.E., McCue P.A., Kovatich A.J., Lattime E.C. Intratumoral recombinant GM-CSF-encoding virus as gene therapy in patients with cutaneous melanoma. Cancer Gene Ther. 1999;6:409–422. doi: 10.1038/sj.cgt.7700066. [DOI] [PubMed] [Google Scholar]
- 6.Cripe T.P., Ngo M.C., Geller J.I., Louis C.U., Currier M.A., Racadio J.M., Towbin A.J., Rooney C.M., Pelusio A., Moon A. Phase 1 study of intratumoral Pexa-Vec (JX-594), an oncolytic and immunotherapeutic vaccinia virus, in pediatric cancer patients. Mol. Ther. 2015;23:602–608. doi: 10.1038/mt.2014.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kirn D.H., Thorne S.H. Targeted and armed oncolytic poxviruses: a novel multi-mechanistic therapeutic class for cancer. Nat. Rev. Cancer. 2009;9:64–71. doi: 10.1038/nrc2545. [DOI] [PubMed] [Google Scholar]
- 8.Puhlmann M., Gnant M., Brown C.K., Alexander H.R., Bartlett D.L. Thymidine kinase-deleted vaccinia virus expressing purine nucleoside phosphorylase as a vector for tumor-directed gene therapy. Hum Gene Ther. 1999;10:649–657. doi: 10.1089/10430349950018724. [DOI] [PubMed] [Google Scholar]
- 9.McCart J.A., Ward J.M., Lee J., Hu Y., Alexander H.R., Libutti S.K., Moss B., Bartlett D.L. Systemic cancer therapy with a tumor-selective vaccinia virus mutant lacking thymidine kinase and vaccinia growth factor genes. Cancer Res. 2001;61:8751–8757. [PubMed] [Google Scholar]
- 10.Stanford M.M., McFadden G. Myxoma virus and oncolytic virotherapy: a new biologic weapon in the war against cancer. Expert Opin Biol Ther. 2007;7:1415–1425. doi: 10.1517/14712598.7.9.1415. [DOI] [PubMed] [Google Scholar]
- 11.Hu Y., Lee J., McCart J.A., Xu H., Moss B., Alexander H.R., Bartlett D.L. Yaba-like disease virus: an alternative replicating poxvirus vector for cancer therapy. J Virol. 2001;75:10300–10308. doi: 10.1128/JVI.75.21.10300-10308.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rintoul J.L., Lemay C.G., Tai L.H., Stanford M.M., Falls T.J., de Souza C.T., Bridle B.W., Daneshmand M., Ohashi P.S., Wan Y. ORFV: a novel oncolytic and immune stimulating parapoxvirus therapeutic. Mol. Ther. 2012;20:1148–1157. doi: 10.1038/mt.2011.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Evgin L., Vähä-Koskela M., Rintoul J., Falls T., Le Boeuf F., Barrett J.W., Bell J.C., Stanford M.M. Potent oncolytic activity of raccoonpox virus in the absence of natural pathogenicity. Mol Ther. 2010;18:896–902. doi: 10.1038/mt.2010.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Edward J. P. F. Collier & Son; 1909. The Three Original Publications on Vaccination Against Smallpox, Vol. XXXVIII. [Google Scholar]
- 15.Caroll D.S., Emerson G.L., Sammons S., Olson V., Frace M., Nakazawa Y., Czerny C.P., Tryland M., Kolodziejek J., Nowotny N. Chasing Jenner’s vaccine: revisiting cowpox virus classification. PLoS One. 2011;6:e23086. doi: 10.1371/journal.pone.0023086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chantrey J., Meyer H., Baxby D., Begon M., Bown K.J., Hazel S.M., Jones T., Montgomery W.I., Bennett M. Cowpox: reservoir hosts and geographic range. Epidemiol. Infect. 1999;122:455–460. doi: 10.1017/s0950268899002423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Campe H., Zimmermann P., Glos K., Bayer M., Bergemann H., Dreweck C., Graf P., Weber B.K., Meyer H., Büttner M. Cowpox virus transmission from pet rats to humans, Germany. Emerg. Infect. Dis. 2005;15:777–780. doi: 10.3201/eid1505.090159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carletti F., Bordi L., Castilletti C., Di Caro A., Falasca L., Gioia C., Ippolito G., Zaniratti S., Beltrame A., Viale P. Cat-to-human orthopoxvirus transmission, northeastern Italy. Emerg. Infect. Dis. 2009;15:499–500. doi: 10.3201/eid1503.080813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Świtaj K., Kajfasz P., Kurth A., Nitsche A. Cowpox after a cat scratch - case report from Poland. Ann. Agric. Environ. Med. 2015;22:456–458. doi: 10.5604/12321966.1167713. [DOI] [PubMed] [Google Scholar]
- 20.Ninove L., Domart Y., Vervel C., Voinot C., Salez N., Raoult D., Meyer H., Capek I., Zandotti C., Charrel R.N. Cowpox virus transmission from pet rats to humans, France. Emerg. Infect. Dis. 2009;15:781–784. doi: 10.3201/eid1505.090235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quenelle D.C., Collins D.J., Kern E.R. Cutaneous infections of mice with vaccinia or cowpox viruses and efficacy of cidofovir. Antiviral Res. 2004;63:33–40. doi: 10.1016/j.antiviral.2004.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duraffour S., Mertens B., Meyer H., van den Oord J.J., Mitera T., Matthys P., Snoeck R., Andrei G. Emergence of cowpox: study of the virulence of clinical strains and evaluation of antivirals. PLoS ONE. 2013;8:e55808. doi: 10.1371/journal.pone.0055808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smee D.F., Bailey K.W., Sidwell R.W. Comparative effects of cidofovir and cyclic HPMPC on lethal cowpox and vaccinia virus respiratory infections in mice. Chemotherapy. 2003;49:126–131. doi: 10.1159/000070618. [DOI] [PubMed] [Google Scholar]
- 24.Becker C., Kurth A., Hessler F., Kramp H., Gokel M., Hoffmann R., Kuczka A., Nitsche A. Cowpox virus infection in pet rat owners: not always immediately recognized. Dtsch. Arztebl. Int. 2009;106:329–334. doi: 10.3238/arztebl.2009.0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ducournau C., Ferrier-Rembert A., Ferraris O., Joffre A., Favier A.L., Flusin O., Van Cauteren D., Kecir K., Auburtin B., Védy S. Concomitant human infections with 2 cowpox virus strains in related cases, France, 2011. Emerg. Infect. Dis. 2013;19:1996–1999. doi: 10.3201/eid1912.130256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McCollum A.M., Austin C., Nawrocki J., Howland J., Pryde J., Vaid A., Holmes D., Weil M.R., Li Y., Wilkins K. Investigation of the first laboratory-acquired human cowpox virus infection in the United States. J. Infect. Dis. 2012;206:63–68. doi: 10.1093/infdis/jis302. [DOI] [PubMed] [Google Scholar]
- 27.Martinez M.J., Bray M.P., Huggins J.W. A mouse model of aerosol-transmitted orthopoxviral disease: morphology of experimental aerosol-transmitted orthopoxviral disease in a cowpox virus-BALB/c mouse system. Arch. Pathol. Lab. Med. 2000;124:362–377. doi: 10.5858/2000-124-0362-AMMOAT. [DOI] [PubMed] [Google Scholar]
- 28.Bourquain D., Nitsche A. Cowpox virus but not vaccinia virus induces secretion of CXCL1, IL-8 and IL-6 and chemotaxis of monocytes in vitro. Virus Res. 2013;171:161–167. doi: 10.1016/j.virusres.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alcami A., Smith G.L. Vaccinia, cowpox, and camelpox viruses encode soluble gamma interferon receptors with novel broad species specificity. J Virol. 1995;69:4633–4639. doi: 10.1128/jvi.69.8.4633-4639.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sampath P., Thorne S.H. Novel therapeutic strategies in human malignancy: combining immunotherapy and oncolytic virotherapy. Oncolytic Virother. 2015;4:75–82. doi: 10.2147/OV.S54738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Foloppe J., Kintz J., Futin N., Findeli A., Cordier P., Schlesinger Y., Hoffmann C., Tosch C., Balloul J.M., Erbs P. Targeted delivery of a suicide gene to human colorectal tumors by a conditionally replicating vaccinia virus. Gene Ther. 2008;15:1361–1371. doi: 10.1038/gt.2008.82. [DOI] [PubMed] [Google Scholar]
- 32.Erbs P., Regulier E., Kintz J., Leroy P., Poitevin Y., Exinger F., Jund R., Mehtali M. In vivo cancer gene therapy by adenovirus-mediated transfer of a bifunctional yeast cytosine deaminase/uracil phosphoribotransferase fusion gene. Cancer Res. 2000;60:3813–3822. [PubMed] [Google Scholar]
- 33.Groeber F., Holeiter M., Hampel M., Hinderer S., Schenke-Layland K. Skin tissue engineering--in vivo and in vitro applications. Adv. Drug Deliv. Rev. 2011;63:352–366. doi: 10.1016/j.addr.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 34.De Wever B., Kurdykowski S., Descargues P. Human skin models for research applications in pharmacology and toxicology: introducing NativeSkin, the “missing link” bridging cell culture and/or reconstructed skin models and human clinical testing. Appl In Vitro Toxicol. 2015;1:26–32. [Google Scholar]
- 35.Duraffour S., Snoeck R., Krecmerová M., van Den Oord J., De Vos R., Holy A., Crance J.M., Garin D., De Clercq E., Andrei G. Activities of several classes of acyclic nucleoside phosphonates against camelpox virus replication in different cell culture models. Antimicrob. Agents Chemother. 2007;51:4410–4419. doi: 10.1128/AAC.00838-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Andrei G., Duraffour S., Van den Oord J., Snoeck R. Epithelial raft cultures for investigations of virus growth, pathogenesis and efficacy of antiviral agents. Antiviral Res. 2010;85:431–449. doi: 10.1016/j.antiviral.2009.10.019. [DOI] [PubMed] [Google Scholar]
- 37.Tamošiūnaitė A., Hoffmann D., Franke A., Schluckebier J., Tauscher K., Tischer B.K., Beer M., Klopfleisch R., Osterrieder N. Histopathological and immunohistochemical studies of cowpox virus replication in a three-dimensional skin model. J. Comp. Pathol. 2016;155:55–61. doi: 10.1016/j.jcpa.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 38.Erbs P., Findeli A., Kintz J., Cordier P., Hoffmann C., Geist M., Balloul J.M. Modified vaccinia virus Ankara as a vector for suicide gene therapy. Cancer Gene Ther. 2008;15:18–28. doi: 10.1038/sj.cgt.7701098. [DOI] [PubMed] [Google Scholar]
- 39.Simpson G.R., Horvath A., Annels N.E., Pencavel T., Metcalf S., Seth R., Peschard P., Price T., Coffin R.S., Mostafid H. Combination of a fusogenic glycoprotein, pro-drug activation and oncolytic HSV as an intravesical therapy for superficial bladder cancer. Br. J. Cancer. 2012;106:496–507. doi: 10.1038/bjc.2011.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dias J.D., Liikanen I., Guse K., Foloppe J., Sloniecka M., Diaconu I., Rantanen V., Eriksson M., Hakkarainen T., Lusky M. Targeted chemotherapy for head and neck cancer with a chimeric oncolytic adenovirus coding for bifunctional suicide protein FCU1. Clin. Cancer Res. 2010;16:2540–2549. doi: 10.1158/1078-0432.CCR-09-2974. [DOI] [PubMed] [Google Scholar]
- 41.Quirin C., Rohmer S., Fernández-Ulibarri I., Behr M., Hesse A., Engelhardt S., Erbs P., Enk A.H., Nettelbeck D.M. Selectivity and efficiency of late transgene expression by transcriptionally targeted oncolytic adenoviruses are dependent on the transgene insertion strategy. Hum. Gene Ther. 2011;22:389–404. doi: 10.1089/hum.2010.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kaufmann J.K., Bossow S., Grossardt C., Sawall S., Kupsch J., Erbs P., Hassel J.C., von Kalle C., Enk A.H., Nettelbeck D.M. Chemovirotherapy of malignant melanoma with a targeted and armed oncolytic measles virus. J. Invest. Dermatol. 2013;133:1034–1042. doi: 10.1038/jid.2012.459. [DOI] [PubMed] [Google Scholar]
- 43.Hammer K., Kazcorowski A., Liu L., Behr M., Schemmer P., Herr I., Nettelbeck D.M. Engineered adenoviruses combine enhanced oncolysis with improved virus production by mesenchymal stromal carrier cells. Int. J. Cancer. 2015;137:978–990. doi: 10.1002/ijc.29442. [DOI] [PubMed] [Google Scholar]
- 44.Husseini F., Delord J.P., Fournel-Federico C., Guitton J., Erbs P., Homerin M., Halluard C., Jemming C., Orange C., Limacher J.M. Vectorized gene therapy of liver tumors: proof-of-concept of TG4023 (MVA-FCU1) in combination with flucytosine. Ann. Oncol. 2017;28:169–174. doi: 10.1093/annonc/mdw440. [DOI] [PubMed] [Google Scholar]
- 45.Denning C., Pitts J.D. Bystander effects of different enzyme-prodrug systems for cancer gene therapy depend on different pathways for intercellular transfer of toxic metabolites, a factor that will govern clinical choice of appropriate regimes. Hum. Gene Ther. 1997;8:1825–1835. doi: 10.1089/hum.1997.8.15-1825. [DOI] [PubMed] [Google Scholar]
- 46.Xu Z., Zikos D., Tamošiūnaitė A., Klopfleisch R., Osterrieder N., Tischer B.K. Identification of 10 cowpox virus proteins that are necessary for induction of hemorrhagic lesions (red pocks) on chorioallantoic membranes. J. Virol. 2014;88:8615–8628. doi: 10.1128/JVI.00901-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.MacNeil A.L., Moldawer L.L., Moyer R.W. The role of the cowpox virus crmA gene during intratracheal and intradermal infection of C57BL/6 mice. Virology. 2009;384:151–160. doi: 10.1016/j.virol.2008.10.041. [DOI] [PubMed] [Google Scholar]
- 48.Jackson S.S., Ilyinskii P., Philippon V., Gritz L., Yafal A.G., Zinnack K., Beaudry K.R., Manson K.H., Lifton M.A., Kuroda M.J. Role of genes that modulate host immune responses in the immunogenicity and pathogenicity of vaccinia virus. J Virol. 2005;79:6554–6559. doi: 10.1128/JVI.79.10.6554-6559.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Buller R.M., Chakrabarti S., Cooper J.A., Twardzik D.R., Moss B. Deletion of the vaccinia virus growth factor gene reduces virus virulence. J Virol. 1988;62:866–874. doi: 10.1128/jvi.62.3.866-874.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Andrade A.A., Silva P.N., Pereira A.C., De Sousa L.P., Ferreira P.C., Gazzinelli R.T., Kroon E.G., Ropert C., Bonjardim C.A. The vaccinia virus-stimulated mitogen-activated protein kinase (MAPK) pathway is required for virus multiplication. Biochem J. 2004;381:437–446. doi: 10.1042/BJ20031375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kirn D.H., Wang Y., Le Boeuf F., Bell J., Thorne S.H. Targeting of interferon-beta to produce a specific, multi-mechanistic oncolytic vaccinia virus. PLoS Med. 2007;4:e353. doi: 10.1371/journal.pmed.0040353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Postigo A., Martin M.C., Dodding M.P., Way M. Vaccinia-induced epidermal growth factor receptor-MEK signalling and the anti-apoptotic protein F1L synergize to suppress cell death during infection. Cell Microbiol. 2009;11:1208–1218. doi: 10.1111/j.1462-5822.2009.01327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shchelkunov S.N., Safronov P.F., Totmenin A.V., Petrov N.A., Ryazankina O.I., Gutorov V.V., Kotwal G.J. The genomic sequence analysis of the left and right species-specific terminal region of a cowpox virus strain reveals unique sequences and a cluster of intact ORFs for immunomodulatory and host range proteins. Virology. 1998;243:432–460. doi: 10.1006/viro.1998.9039. [DOI] [PubMed] [Google Scholar]
- 54.Seet B.T., Johnston J.B., Brunetti C.R., Barrett J.W., Everett H., Cameron C., Sypula J., Nazarian S.H., Lucas A., McFadden G. Poxviruses and immune evasion. Annu. Rev. Immunol. 2003;21:377–423. doi: 10.1146/annurev.immunol.21.120601.141049. [DOI] [PubMed] [Google Scholar]
- 55.Byun M., Wang X., Pak M., Hansen T.H., Yokoyama W.M. Cowpox virus exploits the endoplasmic reticulum retention pathway to inhibit MHC class I transport to the cell surface. Cell Host Microbe. 2007;2:306–315. doi: 10.1016/j.chom.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 56.McNeill T.A. The neutralization of pox viruses. II. Relationships between vaccinia, rabbitpox, cowpox and ectromelia. J. Hyg. (Lond.) 1968;66:549–555. doi: 10.1017/s0022172400028291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Baxby D. A comparison of the antigens present on the surface of virus released artificially from chick cells infected with vaccinia virus, and cowpox virus and its white pock mutant. J. Hyg. (Lond.) 1972;70:353–365. doi: 10.1017/s0022172400022403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Żaba R., Jałowska M., Kowalczyk M.J., Bowszyc-Dmochowska M., Adamski Z., Szkaradkiewicz A. Cowpox virus infection in a child after contact with a domestic cat: a case report. New Microbiol. 2017;40:148–150. [PubMed] [Google Scholar]
- 59.Gazzani P., Gach J.E., Colmenero I., Martin J., Morton H., Brown K., Milford D.V. Fatal disseminated cowpox virus infection in an adolescent renal transplant recipient. Pediatr. Nephrol. 2017;32:533–536. doi: 10.1007/s00467-016-3534-y. [DOI] [PubMed] [Google Scholar]
- 60.Baxby D., Bennett M., Getty B. Human cowpox 1969-93: a review based on 54 cases. Br. J. Dermatol. 1994;131:598–607. doi: 10.1111/j.1365-2133.1994.tb04969.x. [DOI] [PubMed] [Google Scholar]
- 61.Dimier J., Ferrier-Rembert A., Pradeau-Aubreton K., Hebben M., Spehner D., Favier A.L., Gratier D., Garin D., Crance J.M., Drillien R. Deletion of major nonessential genomic regions in the vaccinia virus Lister strain enhances attenuation without altering vaccine efficacy in mice. J. Virol. 2011;85:5016–5026. doi: 10.1128/JVI.02359-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Franke A., Pfaff F., Jenckel M., Hoffmann B., Höper D., Antwerpen M., Meyer H., Beer M., Hoffmann D. Classification of cowpox viruses into several distinct clades and identification of a novel lineage. Viruses. 2017;9:142. doi: 10.3390/v9060142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mauldin M.R., Antwerpen M., Emerson G.L., Li Y., Zoeller G., Carroll D.S., Meyer H. Cowpox virus: what’s in a name? Viruses. 2017;9:101. doi: 10.3390/v9050101. [DOI] [PMC free article] [PubMed] [Google Scholar]