Main Text
We have known for a long time that cells communicate with their neighbors by secreting a wide array of small molecules and macromolecules. However, in the past three decades, it has become increasingly apparent that cells also release membrane-bounded vesicles not merely to discard selected cellular contents but as an extension of the intercellular communication network. Indeed, there is now vigorous and widespread interest in the roles of these extracellular vesicles (ECVs) as purveyors between cells of growth factors, selected receptors, cytoplasmic signaling proteins, transcription factors, a range of nucleic acid transcripts, DNA, and selected lipids (1). Although generation of ECVs may be a ubiquitous cellular activity, interest in this process has been especially intense with regard to communication within embryonic tissues during development, between cancer cells and their surrounding stroma, between stem cells and their more differentiated neighbors, and as part of modulatory interactions in the immune system (1, 2). Evidently, deciphering where and how ECVs are formed and how cargoes are selected are key goals in ongoing efforts to understand this multi-molecular mode of export. A new article by Jackson et al appearing in this special issue of the Biophysical Journal has not only clarified a key step in the export mechanism but more importantly has reported facile strategies for resolving and analyzing ECVs having distinct origins within the cell (3).
As highlighted in this article, formation of ECVs is known to occur by two pathways. In one, vesicles bud and undergo scission at the plasma membrane, leading to direct ECV formation, whereas in the other, vesicles bud into the interior of late endosomes, which subsequently fuse with the plasma membrane, resulting in indirect release of the ECVs (see Fig. 1). Those arising from the plasma membrane are heterogeneous and generally larger (∼0.15–1.0 μm) than those discharged via endosomes, which are more homogeneous and smaller (0.04–0.15 μm) (1). Proteomic studies on ECVs that have been isolated in bulk and subfractionated based on size, density, and immunopurification strategies have cataloged numerous overlapping components in the subfractions, but also have identified markers for larger and smaller ECVs (4). What Jackson et al. (3) convincingly clarify in their study is that the tetraspanins CD9 and CD63 are selective and useful markers for ECVs of plasma membrane origin (referred to as ectosomes) and ECVs of endosomal origin (referred to as exosomes), respectively. Using these markers as analytical tools, the authors have developed an ECV enrichment protocol involving sequential polyethylene glycol precipitation and adsorption to immobilized lectin concanavalin A. The polyethylene glycol/concanavalin A procedure recovers both ectosomes and exosomes, rids them of serum contaminants, and, strikingly, can be used to efficiently harvest the vesicles from as little as 1 mL of conditioned cell medium. Collected ECVs are also readily resolved into well-separated ectosomal and exosomal subfractions of differing density by sucrose-gradient centrifugation. These new strategies enabled the authors to demonstrate further that ectosomes are released more robustly than exosomes in response to serum stimulation and that exosomal, but not ectosomal export, requires intact microtubules. With these approaches, one can now envision that it will be possible to follow ectosome and exosome production kinetically and differentially and to correlate the release of the varied export cargoes with the specific pathways.
Figure 1.
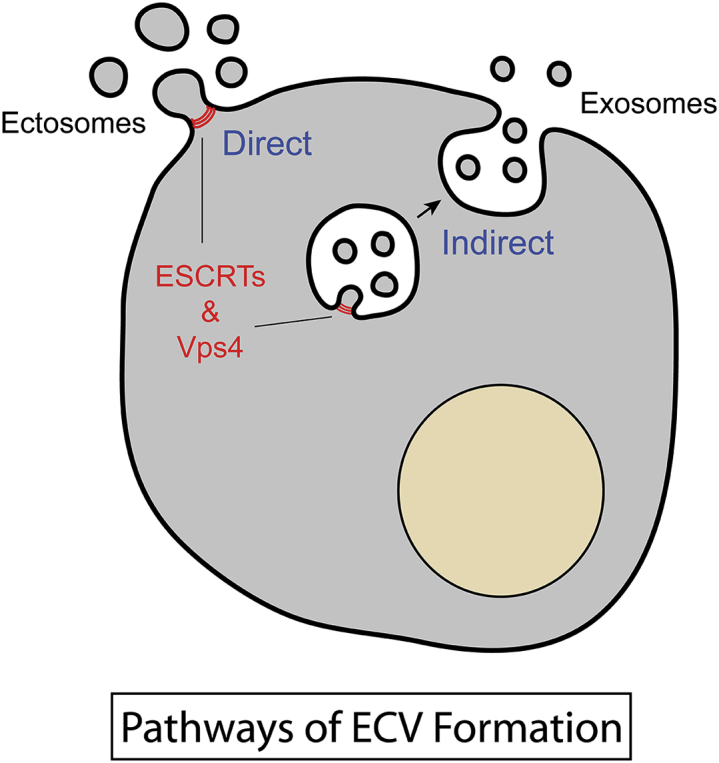
Pathways of ESCRT-mediated export of extracellular vesicles (ECVs). Ectosomes bud directly from the plasma membrane, whereas exosomes are released indirectly via sequential formation in endosomes and exocytosis at the cell surface.
As emphasized and addressed further in the article by Jackson et al. (3), the topology of ECV formation in both the direct and indirect pathways mimics processes (e.g., endocytic sorting of membrane proteins for degradation, cytokinesis, and viral budding) that require the endosomal sorting complex required for transport (ESCRT) complex. Although ESCRT components have been identified in proteomic studies of ECVs (4, 5), no consistent picture has emerged indicating that ESCRTs and related machinery are required for ECV formation. Jackson et al. (3) have now provided clarity to this issue in elegant studies that focused on inhibiting the AAA+ ATPase Vps4, which is well known to be essential for ESCRT-mediated budding events (6). In particular, the authors employed cell lines in which they could inducibly and uniformly express ATPase-deficient Vps4 subunits. These Vps4B(EQ) subunits exert a dominant inhibitory effect on ESCRT disassembly and thereby block completion of vesicle budding. Jackson et al. (3) demonstrate that Vps4B(EQ) causes a dramatic reduction in release of both CD9 and CD63, as well as syntenin, a common ectosomal/exosomal component, and two microRNAs, miR-92a and miR-150, that have been detected extracellularly in earlier studies. Importantly, the authors could attribute inhibition to a decrease in the number of released ECVs, as would be expected for a block of ESCRT-mediated vesicle budding.
Taken together, these new achievements by Jackson et al. (3) in distinguishing and efficiently recovering ECVs formed by the direct and indirect pathways, and their illustration that most, if not all, ECVs are “ESCRTed” from cells, nicely complement the recent proteomic studies of ECV subpopulations and set the stage for future efforts to advance our understanding of the mechanisms and cargo-sorting processes involved in ECV formation.
Editor: Lukas Tamm.
References
- 1.Desrochers L.M., Antonyak M.A., Cerione R.A. Extracellular vesicles: satellites of information transfer in cancer and stem cell biology. Dev. Cell. 2016;37:301–309. doi: 10.1016/j.devcel.2016.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tkach M., Théry C. Communication by extracellular vesicles: where we are and where we need to go. Cell. 2016;164:1226–1232. doi: 10.1016/j.cell.2016.01.043. [DOI] [PubMed] [Google Scholar]
- 3.Jackson C.E., Scruggs B.S., Hanson P.I. Effects of inhibiting Vps4 support a general role for ESCRTs in extracellular vesicle biogenesis. Biophys. J. 2017 doi: 10.1016/j.bpj.2017.05.032. Published online June 16, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kowal J., Arras G., Théry C. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc. Natl. Acad. Sci. USA. 2016;113:E968–E977. doi: 10.1073/pnas.1521230113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keerthikumar S., Chisanga D., Mathivanan S. ExoCarta: a web-based compendium of exosomal cargo. J. Mol. Biol. 2016;428:688–692. doi: 10.1016/j.jmb.2015.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang B., Stjepanovic G., Hurley J.H. Vps4 disassembles an ESCRT-III filament by global unfolding and processive translocation. Nat. Struct. Mol. Biol. 2015;22:492–498. doi: 10.1038/nsmb.3015. [DOI] [PMC free article] [PubMed] [Google Scholar]


