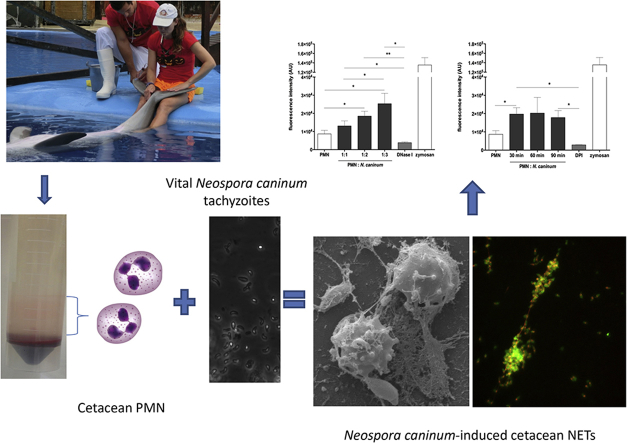
Abstract
Neutrophil extracellular traps (NETs) are web-like structures composed of nuclear DNA decorated with histones and cytoplasmic peptides which antiparasitic properties have not previously been investigated in cetaceans. Polymorphonuclear neutrophils (PMN) were isolated from healthy bottlenose dolphins (Tursiops truncatus), and stimulated with Neospora caninum tachyzoites and the NETs-agonist zymosan. In vitro interactions of PMN with the tachyzoites resulted in rapid extrusion of NETs. For the demonstration and quantification of cetacean NETs, extracellular DNA was stained by using either Sytox Orange® or Pico Green®. Scanning electron microscopy (SEM) and fluorescence analyses demonstrated PMN-derived release of NETs upon exposure to tachyzoites of N. caninum. Co-localization studies of N. caninum induced cetacean NETs proved the presence of DNA adorned with histones (H1, H2A/H2B, H3, H4), neutrophil elastase (NE), myeloperoxidase (MPO) and pentraxin (PTX) confirming the molecular properties of mammalian NETosis. Dolphin-derived N. caninum-NETosis were efficiently suppressed by DNase I and diphenyleneiodonium (DPI) treatments. Our results indicate that cetacean-derived NETs represent an ancient, conserved and relevant defense effector mechanism of the host innate immune system against N. caninum and probably other related neozoan parasites circulating in the marine environment.
Keywords: Tursiops truncatus, cetaceans; Neutrophil extracellular traps; Innate immunity; Neospora caninum.
Graphical abstract
Highlights
-
•
Cetacean derived-NETosis were quickly activated upon contact with N. caninum.
-
•
Fluorescence analyses proved dolphin-NETs molecules upon exposure to N. caninum.
-
•
N. caninum-triggered NETosis in dolphins was a NOX-dependent process.
-
•
DNase I treatment suppressed NET formation signals in cetacean PMN.
1. Introduction
Bottlenose dolphins (Tursiops truncatus) are the most common and well-known members of the family Delphinidae (Cetacea). Female bottlenose dolphins live in groups composed of 10–30 members, but group sizes can vary up to more than hundred specimens. In contrast, adult males live mostly in small groups joining female dolphin pods strictly for mating purposes for short periods of time. Bottlenose dolphins are known to inhabit warm as well as temperate ocean seas worldwide and to be present in all oceans except for the Antarctic and Arctic Circle areas.
Investigations on the dolphin adaptive immune system are quite abundant in literature (Romano et al., 1992, De Guise et al., 2002, Mancia et al., 2007, Beineke et al., 2010, Sitt et al., 2010, Zafra et al., 2015, White et al., 2017). Conversely, investigations on the cetacean innate immune system are less commonly found (Kato and Perrin, 2009, Schwacke et al., 2010, Keogh et al., 2011) despite the fact that PMN are at the forefront of defense against infection (Brinkmann and Zychlinsky, 2012, Silva et al., 2016), resolution of inflammation and wound healing (Rodríguez-Espinosa et al., 2015). Some cetacean PMN data are available on the impact of heavy metals (Cámara Pellissó et al., 2008) and fungi infections (Reif et al., 2009) but any data existing on cetacean PMN effector mechanisms against invasive parasites are still missed.
Human activities and domestic animal industry clearly impact the ocean health system (Dubey, 2003, Conrad et al., 2005) and recently identified neozoan parasite infections in free-ranging marine mammals, such as Neospora caninum, Toxoplasma gondii, Giardia intestinalis, Cryptosporidium parvum, Cryptosporidium hominis, Sarcocystis neurona, Entamoeba sp. and Balantidium coli may all originate from human and animal waste/sewage or their related activities (Buergelt and Bonde, 1983, Olsen et al., 1997, Parveen et al., 1997, Johnson et al., 1998, LaPointe et al., 1999, Dubey, 2003, Conrad et al., 2005, Kleinertz et al., 2014, Hermosilla et al., 2016). Consistently to these observations, antibodies against abortive and neurotropic parasites, such as N. caninum, T. gondii and S. neurona, have been reported to occur around the world, particularly in dolphins (Inskeep et al., 1990; Lapointe et al., 1998; Jardine and Dubey, 2002, Bowater et al., 2003; Cabezón et al, 2004; Santos et al., 2011), whales (Mikaelian et al., 2000, Omata et al., 2006), sea otters (Cole et al., 2000, Conrad et al., 2005) and seals (Dubey, 2003, Fujii et al., 2007), demonstrating the circulation of these typically terrestrial parasitoses in the marine ecosystem.
Commonly in terrestrial susceptible hosts, such as cattle, goats, sheep, horses and dogs, infections of N. caninum underlie complex cellular as well as molecular immunological regulations (see Gazzinelli et al., 1998; Boysen et al., 2006; Taubert et al., 2006, Taubert et al., 2010; Klevar et al., 2007; Wei et al., 2016, Villagra-Blanco et al., 2017a, Villagra-Blanco et al., 2017b). In bottlenose dolphins, the innate immune system comprehends also PMN and monocytes, which comprise between 22-72% and 0–11% of the circulating leukocytes, respectively (Goldstein et al., 2006, Hall et al., 2007, Venn-Watson et al., 2007, Schwacke et al., 2009). Consistently, PMN are known to play a key role in host innate immunity against apicomplexan protozoan infections (Behrendt et al., 2010, Muñoz-Caro et al., 2014, Muñoz-Caro et al., 2015a, Reichel et al., 2015, Silva et al., 2016, Wei et al., 2016, Villagra-Blanco et al., 2017a, Villagra-Blanco et al., 2017b), since they are the most abundant leukocytes and the first ones that reach apicomplexan parasite infection in vivo (Baker et al., 2008, Abi Abdallah et al., 2012, Muñoz-Caro et al., 2016). Mammalian PMN elicit several effector mechanisms to combat protozoan parasites, such as phagocytosis, production of reactive oxygen species (ROS), the excretion of anti-parasitic peptides/proteins and the release of neutrophil extracellular traps (NETs) (for reviews see Brinkmann and Zychlinsky, 2012, Hermosilla et al., 2014, Silva et al., 2016). Accordingly, marine mammalian PMN are also capable of ROS production and to perform phagocytic activities in dolphins (Itou et al., 2001, Noda et al., 2006). Moreover, harbour seal (Phoca vitulina) PMN and monocytes have probed to trigger extracellular traps (ETs) against vital T. gondii-tachyzoites as an efficient host effector mechanism (Reichel et al., 2015). NETs are generally released via a novel PMN cell death process known as NETosis (Fuchs et al., 2007, Brinkmann and Zychlinsky, 2012). Invasive parasites may either be immobilized within NETs (Behrendt et al., 2010, Muñoz-Caro et al., 2014, Muñoz-Caro et al., 2015a, Muñoz-Caro et al., 2015b, Muñoz-Caro et al., 2016, Silva et al., 2016, Wei et al., 2016, Villagra-Blanco et al., 2017a, Villagra-Blanco et al., 2017b) or killed via locally high concentrations of antimicrobial histones, peptides and proteases as postulated elsewhere (Brinkmann et al., 2004, Von Köckritz-Blickwede and Nizet, 2009, Cheng and Palaniyar, 2013).
NETosis is known as a NADPH oxidase (NOX)-dependent mechanism (Behrendt et al., 2010, Von Köckritz-Blickwede et al., 2010, Brinkmann and Zychlinsky, 2012, Muñoz-Caro et al., 2015a, Muñoz-Caro et al., 2015b), which leads to extrusion of DNA-enriched fibers adorned with histones and granular proteins, e. g. neutrophil elastase (NE), myeloperoxidase (MPO), pentraxin, lactoferrin, cathepsin, bacterial permeability-increasing protein (BPI), peptidoglycan recognition proteins (PGRPs) and other PMN granular components (for reviews see Von Köckritz-Blickwede and Nizet, 2009, Brinkmann and Zychlinsky, 2012, Hermosilla et al., 2014, Silva et al., 2016). Currently, different protozoan parasites have been described to produce NETosis in humans as well as in wild and domestic animals, such as Plasmodium falciparum (Baker et al., 2008), Leishmania spp. (Guimarães-Costa et al., 2009, Wang et al., 2011), Eimeria bovis (Behrendt et al., 2010, Muñoz-Caro et al., 2015a), E. arloingi (Silva et al., 2014), E. ninakohlyakimovae (Pérez et al., 2016), T. gondii (Abi Abdallah et al., 2012), Besnoitia besnoiti (Muñoz-Caro et al., 2014), C. parvum (Muñoz-Caro et al., 2015b), Trypanosoma cruzi (Sousa-Rocha et al., 2015), Entamoeba histolytica (Ventura-Juárez et al., 2016), Naegleria fowleri (Contis-Montes de Oca et al., 2016) and more recently N. caninum (Wei et al., 2016, Villagra-Blanco et al., 2017a, Villagra-Blanco et al., 2017b).
To the best our current knowledge there is only one report focusing on NETosis occurring in marine mammals, namely in pinniped-derived PMN and monocytes casting ETs against T. gondii (Reichel et al., 2015). Thus, aim of the herein work was to confirm that cetacean PMN can also cast NETs against neozoan apicomplexan parasites. Therefore isolated PMN from bottlenose dolphins (T. truncatus) were exposed to vital N. caninum-tachyzoites and further analyzed in detail to describe molecules as well as signaling pathways implicated in this ancient host innate immune effector mechanism.
2. Materials and methods
2.1. Ethic statement
All animal procedures were performed according to the dolphinarium Mundomar (Benidorm, Spain) Animal Care Committee guidelines, and approved by the Bioethical Committee of Murcia University (Murcia, Spain) and the local Committees for animal research (REGA ES300305440012), and in accordance to the current European Animal Welfare Legislation: ART13TFEU.
2.2. Parasites
All NET-related experiments were performed with tachyzoites of N. caninum (strain Nc1) which were cultivated in vitro as described elsewhere (Dubey et al., 1988, Taubert et al., 2006, Villagra-Blanco et al., 2017a, Villagra-Blanco et al., 2017b). In brief, N. caninum tachyzoites were maintained by several passages in permanent African green monkey kidney epithelial cells (MARC-145) according to methods described before by Taubert et al. (2006) and Muñoz-Caro et al. (2014). Vital N. caninum-tachyzoites were collected in supernatants of infected host cell monolayers, filtered with 5 ųm sterile syringe filters (Sartorius AG) to removed cell debris, washed thrice with sterile PBS (400 × g, 12 min), counted using a Neubauer haemocytometer chamber (Marienfeld) and re-suspended in sterile RPMI 1640 medium without phenol red (Gibco) until further experimental use as recently reported elsewhere (Villagra-Blanco et al., 2017a, Villagra-Blanco et al., 2017b).
2.3. Host cells
MARC-145 cell monolayers were cultured in DMEM (Sigma-Aldrich) cell culture medium supplemented with 1% penicillin (500 U/ml; Sigma-Aldrich, St. Louis, MO, USA), streptomycin (500 mg/ml; Sigma-Aldrich) and 2% fetal calf serum (FCS; Gibco) and incubated at 37 °C and 5% CO2 until confluency. Then, MARC-145 monolayers were infected with viable N. caninum tachyzoites and cultured at 37 °C and 5% CO2 atmosphere until release of new vital tachyzoites. Cell medium was changed every second day.
2.4. Dolphin blood collection and PMN isolation
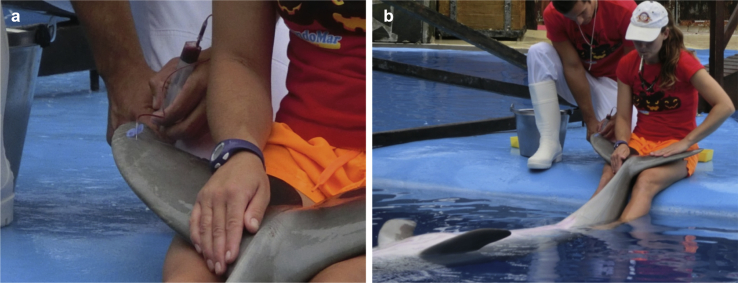
Healthy adult male bottlenose dolphins (T. truncatus; n = 3) kept at the dolphinarium of the Marine Animal Park Mundomar (Benidorm, Spain) were bled by puncture of the ventral superficial fluke plexus from the caudal peduncle bundle (see Fig. 1a). To reduce stress of blood donor animals, professional trainers stayed with the animals to facilitate the physical restraint by using whistles to give them instructions, associated with a positive reinforcement (see Fig. 1b). All dolphin blood extractions were performed during the morning-feeding daily routines of the dolphinarium, when animals were accustomed to periodically medical procedures, such as extraction of urine-, faeces-, semen-, milk- and blood samples.
Fig. 1.
Minimally-invasive blood extraction method for cetaceans. (a) Puncture of the ventral superficial fluke plexus with a fine needle attached to infusion system and one syringe to create a vacuum for blood extraction. (b) Professional trainers performed physical restraint of one dolphin using whistle to give a positive reinforcement during sampling.
The blood was collected in 5 ml sterile plastic tubes containing lithium heparin as anticoagulant (BD Vacutainer®) and thereafter immediately transported using cold ice-packs to the Faculty of Veterinary Medicine, University of Murcia, Murcia, Spain. Heparinized blood of each dolphin (20 ml) was diluted in 30 ml of sterile PBS containing 0.02% EDTA (Sigma-Aldrich), layered on Biocoll Separating Solution® (Biochrom AG) and centrifuged (800 × g, 45 min at 4 °C) as recently described for other marine mammals (Reichel et al., 2015). After the removal of plasma, two different protocols of PMBC extraction were herein tested: i) the pellet containing erythrocytes and PMN was re-suspended in 15 ml RPMI medium 1640 without phenol red, treated with Red Blood Cell Lysis® buffer (1 ml, Sigma-Aldrich) to remove erythrocytes and centrifuged (500 × g, 7 min). Alternatively, ii) the pellet containing the PMN and erythrocytes was suspended in 25 ml distilled water and shaken for 40 s to lyse erythrocytes according to Muñoz-Caro et al. (2014). Osmolarity was immediately re-adjusted by adding the appropriate amount of Hanks salt solution (4 ml, HBSS 10x, Biochrom AG). This procedure was repeated twice to wash the dolphin PMN. In both protocols, the pellets were re-suspended in RPMI medium (Gibco) and PMN were counted in a Neubauer haemocytometer chamber. Finally, cetacean-derived PMN were incubated for 30 min to allow repose at 37 °C and 5% CO2 atmosphere until use. In our hands, the second PMN isolation protocol achieved better results due to the fact that dolphin erythrocytes revealed to be quite resistant to applied Blood Cell Lysis® buffer (Sigma-Aldrich).
2.5. Quantification of dolphin NETs
Dolphin-derived NET formation was quantified by using Pico Green® (Invitrogen), an extracellular DNA-binding fluorescent dye, as reported elsewhere (Muñoz-Caro et al., 2015a, Muñoz-Caro et al., 2015b, Villagra-Blanco et al., 2017a, Villagra-Blanco et al., 2017b). Therefore, cetacean PMN (n = 3) were re-suspended in serum-free cell culture medium RPMI 1640 lacking phenol red and incubated in duplicates with vital N. caninum-tachyzoites (37 °C, 60 min, 3:1 ratio: 7,5 × 105 N. caninum tachyzoites versus 2,5 × 105 dolphin PMN/200 μl). For NET blockage, PMN were pre-treated with the NOX-inhibitor [DPI, 10 μM, Sigma-Aldrich, according to O'Donnell et al. (1993)] for 30 min at 37 °C prior to exposure to N. caninum tachyzoites and DNase I (90 U/well, Roche Diagnostics, addition was performed 15 min before the end of the incubation period). For NET quantification, the samples were treated with micrococcal nuclease (0.1 U/μl, New England Biolabs, 15 min, 37 °C) and centrifuged (300 × g, 5 min). The supernatant was transferred into a 96-well flat-bottom plate (100 μl per well in duplicates). Then, Pico Green® (50 μl/sample, diluted 1:200 in 10 nMTris/1 mM EDTA buffer, in the dark) was added. NET formation was determined by spectrofluorometric analysis at an excitation wavelength of 484 nm and an emission wavelength of 520 nm using an automated plate monochrome reader (FLUOstarOmega, BMG Labtech). NETs were quantified by fluorescence intensity analyses. For negative controls, PMN alone in plain medium were used and for positive controls served PMN stimulated with zymosan (1 mg/ml; Invitrogen) according to Muñoz-Caro et al., 2015a, Muñoz-Caro et al., 2015b.
In order to evaluate parasite dose-dependent effects, different PMN: tachyzoites ratios were applied (1:1, 1:2, 1:3) during 90 min of incubation and processed as described earlier. For NET-kinetic analyses, PMN and parasites were co-cultured with parasites for different time periods (i. e. 30, 60, 90 min).
Visualization of extracellular DNA adorned with histones, neutrophil elastase (NE), myeloperoxidase (MPO), pentraxin (PTX) in Neospora caninum-induced dolphin NETs.
After the co-culture of cetacean PMN with tachyzoites (ratio 1:3, 90 min) on pre-coated poly-L-lysine coverslips, fixation of the samples (4% paraformaldehyde, Merck) and three PBS washings, the samples were carefully transported in a flat-bottom cell culture 6-well plates to the Institute of Parasitology (Justus Liebig University Giessen, Germany) and thereafter blocked with BSA (2%, Sigma-Aldrich, 15 min, RT), incubated in antibody solutions [1 h, RT] and finally mounted in ProLongGold® Antifade (Invitrogen) stained with Sytox Orange® [Invitrogen, 5 mM Sytox Orange®, 10 min, RT, in dark, according to Martinelli et al. (2004)]. For the detection of dolphin histones, MPO, NE and PTX in NETs the following antibodies were used: anti-histone (H1, H2A/H2B, H3, H4) monoclonal (mouse clone H11-4, 1:1000, Merck Millipore), anti-MPO (Alexa Fluor 488, 1:1000, ABIN906866, Antibodies-online.com), anti-NE (AB68672, 1:1000, Abcam) and anti-PTX (SAB2104614-50UG, 1:1000, Sigma-Aldrich) antibodies. NETs-visualization and illustration were achieved by using an inverted Olympus® IX81 fluorescence microscope equipped with a digital camera.
2.6. Scanning electron microscopy (SEM)
Dolphin PMN were co-cultured with vital N. caninum-tachyzoites (ratio: 1:3) for 60 min on poly-L-lysine (Sigma-Aldrich) pre-coated coverslips (10 mm of diameter; Nunc). Cells were fixed in 2.5% glutaraldehyde (Merck), post-fixed in 1% osmium tetroxide (Merck), washed in distilled water, dehydrated, critical point dried by CO2-treatment and sputtered with gold particles. Specimens were examined using a Philips XL30 scanning electron microscope at the Institute of Anatomy and Cell Biology of the Justus Liebig University Giessen, Germany.
2.7. Statistical analysis
Statistical analyses were performed by using Graph Pad Prism® 6 software. One- or two-factorial analyses of variance (ANOVA) with repeated measures were applied to compare co-culture/stimulation conditions using a normal distribution of data. Differences were regarded as significant at a level of p ≤ 0.05 (*); p ≤ 0.01 (**); p ≤ 0.001 (***) and p ≤ 0.0001 (****).
3. Results and discussion
Cetacean PMN, likewise terrestrial mammalian hosts, are well-known to elicit phagocytic and respiratory burst activities resulting in the production of ROS (Keogh et al., 2011). PMN extrusion of NETs is nowadays considered one of the main effector mechanisms of this leukocyte population to combat infectious agents (Brinkmann and Zychlinsky, 2012, Hahn et al., 2013), including protozoan and metazoan parasites (for reviews see Hermosilla et al., 2014, Silva et al., 2016). Since the first description of NETs by Brinkmann et al. (2004), very little has been investigated on NETosis in marine mammals, with the exception of one report on T. gondii-triggered NETs in exposed harbour seal PMN (Reichel et al., 2015). Both, T. gondii and N. caninum, are considered terrestrial neozoan parasites which were recently identified within the marine environment (Fujii et al., 2007, Dubey et al., 2008), and have emerged as important neozoan parasitic pathogens for dolphins (Dubey et al., 2008), pinnipeds (Cabezón et al., 2011), whales (Mazzariol et al., 2012) and sea otters (Conrad et al., 2005, Miller et al., 2008). Alongside, N. caninum has recently been identified as potent NET inducer of PMN in dogs (Wei et al., 2016), goats (Villagra-Blanco et al., 2017a) and cattle (Villagra-Blanco et al., 2017b). To our best knowledge, the present study represents the first description of cetacean-extruded NETs against N. caninum tachyzoites.
3.1. Dolphin PMN-mediated NETosis in presence of N. caninum tachyzoites
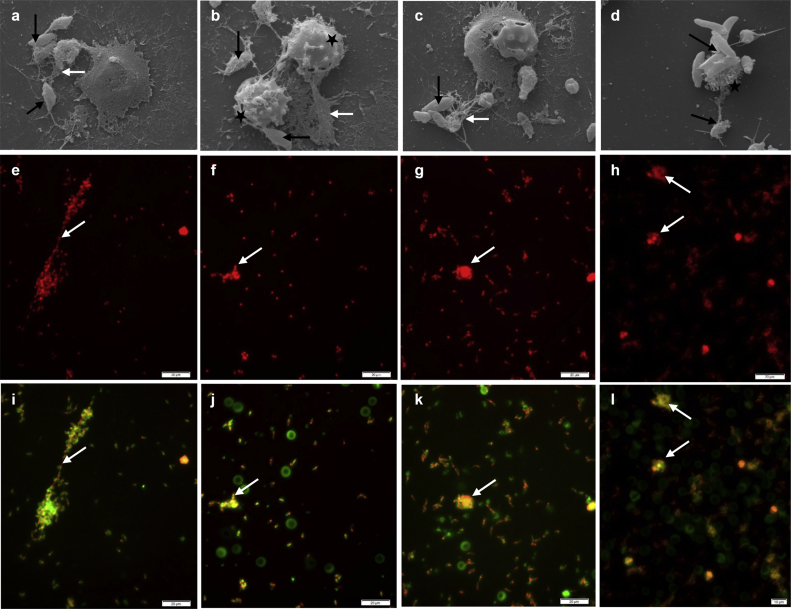
SEM analyses unveiled that exposure of dolphin-derived PMN to N. caninum tachyzoites resulted in the formation of a fine network and slimmer strands of fibers originating from PMN and being attached to the parasites, seemingly entrapping them (Fig. 2a). During NETosis, some dolphin PMN still showed the morphology of intact vital cells (Fig. 2b, 60 min) and others demonstrated the morphological features of PMN activation. Later on, N. caninum tachyzoites were observed entrapped in meshworks of PMN-released filaments (Fig. 2c and, 60 min) or even entangled by a single activated PMN (Fig. 2d, 60 min). Accordingly to these SEM findings, NETosis is most probably a relevant innate effector mechanism by which dolphin PMN confirmly attach and subsequently entrap N. caninum tachyzoites, as reported for other apicomplexan parasites in vitro and in vivo (see Behrendt et al., 2010, Muñoz-Caro et al., 2014, Muñoz-Caro et al., 2015b, Muñoz-Caro et al., 2016, Reichel et al., 2015, Silva et al., 2016).
Fig. 2.
Neospora caninum tachyzoite-triggered dolphin NET structures (SEM) and co-localization of extracellular DNA with histones (H1, H2A/H2B, H3 and H4), NE, MPO and PTX. (a–d) Scanning electron microscopy (SEM) analyses revealed NETs being formed by dolphin PMN after co-culture with N. caninum tachyzoites. (a) Mesh of DNA-structures (white arrow) derived from dolphin PMN attached to N. caninum-tachyzoites (black arrows). (b) Intact cetacean-PMN (black stars) derived a fine filaroid structure (white arrow) being attached to tachyzoites (black arrows). (c) Conglomerates of several tachyzoites (black arrow) being entrapped in a rather chunky meshwork of cetacean-PMN-released thicker extracellular filaments (white arrow) (d) Dolphin PMN activated (black star) entrapping diverse N. caninum-tachyzoites (black arrows). (e–l) Co-cultures of dolphin PMN and N. caninum tachyzoites were fixed, permeabilized, stained for analysis of co-localization (i-l; merge, white arrows) of extracellular DNA (e-h; red; Sytox Orange®) and classical NETs components (all green, white arrows) such as histones (i), NE (j), MPO (k) and pentraxin (l). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.2. Immunofluorescence analyses of N. caninum-induced dolphin NETs
Sytox Orange® staining-based fluorescence investigations further proved the presence of dolphin-derived NETs containing DNA (Fig. 2e–l). N. caninum tachyzoites were located in close proximity to extruded cetacean-triggered NETs and often being trapped within these extracellular structures. Extruded dolphin NETs demonstrated classical NETs components, i. e. histones (H1, H2B/H2B, H3, H4) (Fig. 2e and i), NE (Fig. 2f and j), MPO (Fig. 2g and k) and PTX (Fig. 2h, l), as proofed by co-localization of extracellular DNA adorned with these molecules in parasite-entrapping structures (Fig. 2i, j, 2k, 2l). Co-localization assays of dolphin-derived NETs demonstrated the concomitant existence of histones (H1, H2A/H2B, H3, H4), NE, MPO and PTX, confirming typical molecular characteristics of NETs (Brinkmann and Zychlinsky, 2012), and agreed with previous reports on other apicomplexan-triggered NETosis (Baker et al., 2008, Behrendt et al., 2010, Abi Abdallah et al., 2012, Silva et al., 2014, Muñoz-Caro et al., 2014, Muñoz-Caro et al., 2015a, Muñoz-Caro et al., 2015b, Muñoz-Caro et al., 2016, Reichel et al., 2015, Maksimov et al., 2016). In this context, the pivotal role of MPO and NE in N. caninum-triggered NETs has been recently proven by functional inhibition assays, leading to the reduction of tachyzoite-mediated NETosis in the canine (Wei et al., 2016), the goat (Villagra-Blanco et al., 2017a) and the bovine systems (Villagra-Blanco et al., 2017b). The presence of PTX in dolphin-derived NETs is in accordance to recent PTX findings obtained from extruded NETs against the same parasite in goats (Villagra-Blanco et al., 2017a) and cattle (Villagra-Blanco et al., 2017b). PTX is known to be stored in PMN granules and relevant in early host innate immune reactions. In common with proteinase 3 and MPO, NE is expressed on the apoptotic PMN surface as reported elsewhere (Bottazzi et al., 2009). In dolphin-derived NET formation, it can be speculated that PTX might therefore participate in N. caninum recognition thereby facilitating the entrapment of tachyzoites as previously demonstrated for bacterial pathogens (Bottazzi et al., 2009). More importantly, whole PMN proteome analysis unveiled that PTX forms a complex with other NETs-related molecules in human PMN and appears to have a crucial role in boosting the actions of different NETs components (Daigo and Hamakudo, 2012). Similar synergistic properties have been described for other PMN-excreted pro-inflammatory cytokines/chemokines in response to N. caninum-, T. gondii- (Taubert et al., 2006) and C. parvum-exposed bovine and human PMN (Muñoz-Caro et al., 2016).
3.3. Neospora caninum-induced dolphin NETosis at different tachyzoite ratios
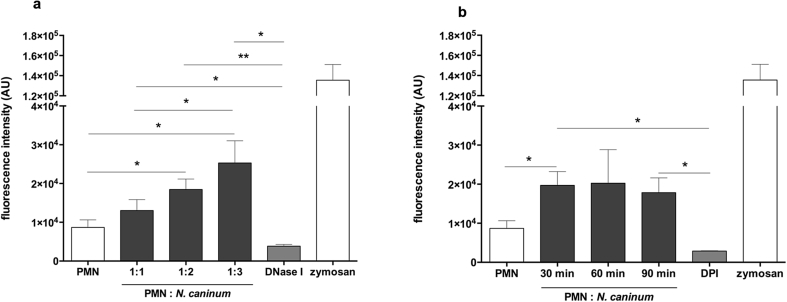
Quantification of dolphin NET induction through tachyzoites of N. caninum confirmed a strong dose-dependent NET formation, as increasing amounts of N. caninum tachyzoites led to significantly enhanced Pico Green®-derived fluorescence intensities (p ≤ 0.05) when compared to negative controls, coinciding to published data on T. gondii-mediated NETosis in pinniped PMN (Reichel et al., 2015). However, time dependency was not observed. As expected, DNase I treatments leading to NET disintegration reduced NETosis (p ≤ 0.05) under the basal levels of the negative controls (Fig. 3a), which confirmed the DNA backbone nature of N. caninum-triggered cetacean NETosis.
Fig. 3.
Dose, kinetic and functional inhibition assays of N. caninum tachyzoites-triggered NET formation in dolphins. PMN were incubated with tachyzoites, zymosan (1 mg/ml, positive control) or plain medium (negative control) at different ratios (a; PMN: tachyzoites = 1:1, 1:2, 1:3) and time periods (b; 30, 60 and 90 min). To prove the DNA nature of NETs, the samples were treated with DNase I (a; 15 min). Moreover, cetacean PMN cells were pre-treated with NOX-inhibitor (b; DPI, 10 μM) for 30 min prior to N. caninum stimulation (1:3 ratio; 90 min). After incubation, all samples were analyzed for extracellular DNA by quantifying Pico Green®-derived fluorescence intensities. Each condition was performed in duplicates. Geometric means of three PMN donors. Differences were regarded as significant at a level of p < 0.05 (*) and p < 0.01 (**).
3.4. Neospora caninum-triggered dolphin NETosis is a NOX-dependent effector mechanism
Kinetic-related studies revealed a fast parasite-triggered NET induction in exposed dolphin PMN. As such, N. caninum tachyzoites induced NETosis was detected as fast as 30 min of exposure, i. e. at the earliest time point measured in this assay (until 90 min of exposure, as the latest time point). In contrast, tachyzoite-free negative controls of dolphin PMN contributed very low to extracellular DNA extrusion (p ≤ 0.05, Fig. 3b) when compared to parasite-exposed PMN (ratio 1:3). Besides, functional inhibition experiment performed with DPI, a potent inhibitor of NOX, resulted in a significant reduction of tachyzoite-triggered dolphin NET formation (p ≤ 0.05, Fig. 3b). Regarding signal pathways, the NOX pathway clearly participates in N. caninum-induced dolphin NETosis, since DPI treatment assays resulted in significant reduction of NET formation. NOX-dependent NETosis findings, were recently reported for other closely related apicomplexan-related NETosis investigations, i. e. E. bovis (Muñoz-Caro et al., 2015a), T. gondii (Abi Abdallah et al., 2012, Reichel et al., 2015), B. besnoiti (Muñoz-Caro et al., 2014), N. caninum (Wei et al., 2016) and C. parvum (Muñoz-Caro et al., 2015b), highlighting the relevance of NOX in protozoan-mediated NETosis (Silva et al., 2016). Nonetheless, also NOX-independent parasite-induced NETosis has recently been reported for N. caninum in the caprine system (Villagra-Blanco et al., 2017a), showing differences depending on the parasite species as well as the origin of the PMN donor host species.
Nowadays, there is growing evidence on the crucial role of NETosis as efficient defense mechanism in diverse terrestrial vertebrate host species against several protozoan (Silva et al., 2016) and metazoan parasites (Chuah et al., 2013, Bonne-Annèe et al., 2014, Muñoz-Caro et al., 2015a, Muñoz-Caro et al., 2015b, Lange et al., 2017). Nonetheless, with respect to NETosis research still scarce data are available for marine mammals and whether this efficient defense mechanism might actively participate in vivo against neozoan parasites, needs to be addressed in the near future, but in vitro evidence here presented strongly suggest that this is most probably occurring. Presented results clearly evidence that N. caninum is a competent parasite species capable to trigger NETs in the cetacean immune system, and consistent to previous N. caninum observations of NETs in terrestrial host species (Wei et al., 2016, Villagra-Blanco et al., 2017a, Villagra-Blanco et al., 2017b). Considering the biology of N. caninum, which include obligate intracellular parasite stages, the entrapment/immobilization of extracellular tachyzoites through NETosis might have a significant impact on the outcome of the disease as already demonstrated for other related apicomplexan protozoa in vitro and in vivo (Baker et al., 2008, Behrendt et al., 2010, Abi Abdallah et al., 2012, Hermosilla et al., 2014, Muñoz-Caro et al., 2014, Muñoz-Caro et al., 2016, Silva et al., 2014, Silva et al., 2016).
4. Conclusion
The current study describes for the first time the ability of bottlenose-dolphin PMN to cast NETs against the abortive protozoan parasite N. caninum providing evidence of the importance of this ancient and well conserved effector mechanism of the host innate immune system of marine cetacean species.
5. Declaration
5.1. Ethics approval and consent to participate
Not applicable.
5.2. Consent for publication
Not applicable.
5.3. Availability of data and material
All data generated or analyzed during this study are included in this published article.
Competing interests
The authors declare that they have no competing interests in the present study.
Funding
This project received any funding by third parties and was exclusively covered by the Institute of Parasitology, Justus Liebig University Giessen (Germany) and the Department of Animal Health, University of Murcia, Spain (Convenio Específico de Colaboración entre la Universidad de Murcia y Aqualandia España, S.A).
Acknowledgements
The authors would like to acknowledge the veterinary surgeons and animal training staff at the Marine Animal Park Mundomar (Benidorm, Spain) for their kind collaboration in blood collection. We also deeply thank the Cell Culture Unity of SAI (University of Murcia, Spain), as well as Brigitte Hofmann, Dr. Christin Ritter (JLU-Giessen, Germany) and Dr. Zhengtao Yang (Jilin University, China) for their technical assistance in the in vitro culture of N. caninum.
References
- Abi Abdallah D.S., Lin C., Ball C.J., King M.R., Duhamel G.E., Denkers E.Y. Toxoplasma gondii triggers release of human and mouse neutrophil extracellular traps. Infect. Immun. 2012;80:768–777. doi: 10.1128/IAI.05730-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker V.S., Imade G.E., Molta N.B., Tawde P., Pam S.D., Obadofin M.O., Sagay S.A., Egah D.Z., Iya D., Afolabi B.B., Baker M., Ford K., Ford R., Roux K.H., Keller T.C., 3rd Cytokine-associated neutrophil extracellular traps and antinuclear antibodies in Plasmodium falciparum infected children under six years of age. Malar. J. 2008;7:41. doi: 10.1186/1475-2875-7-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrendt J.H., Ruiz A., Zahner H., Taubert A., Hermosilla C. Neutrophil extracellular trap formation as innate immune reactions against the apicomplexan parasite Eimeria bovis. Vet. Immunol. Immunopathol. 2010;133:1–8. doi: 10.1016/j.vetimm.2009.06.012. [DOI] [PubMed] [Google Scholar]
- Beineke A., Siebert U., Wohlsein P., Baumgärtner W. Immunology of whales and dolphins. Vet. Immunol. Immunopathol. 2010;133:81–94. doi: 10.1016/j.vetimm.2009.06.019. [DOI] [PubMed] [Google Scholar]
- Bonne-Annèe S., Kerepesi L.A., Hess J.A., Wesolowski J., Paumet F., Lok J.B., Nolan T.J., Abraham D. Extracellular traps are associated with human and mouse neutrophil and macrophage mediated killing of larval Strongyloides stercoralis. Microbes. Infect. 2014;16(6):502–511. doi: 10.1016/j.micinf.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottazzi B., Garlanda C., Cotena A., Moalli F., Jaillon S., Deban L., Montavani A. The long pentraxin PTX3 as a phototypic humoral pattern recognition receptor: interplay with cellular innate immunity. Immunol. Rev. 2009;227:9–18. doi: 10.1111/j.1600-065X.2008.00719.x. [DOI] [PubMed] [Google Scholar]
- Bowater R.O., Norton J., Johnson S., Hill B., O'Donoghue P., Prior H. Toxoplasmosis in indo-pacific humpbacked dolphins (Sousa chinensis), from queensland. Aust. Vet. J. 2003;81:627–632. doi: 10.1111/j.1751-0813.2003.tb12509.x. [DOI] [PubMed] [Google Scholar]
- Boysen P., Klevar S., Olsen I., Storset A.K. The protozoan Neospora caninum directly triggers bovine NK cells to produce gamma interferon and to kill infected fibroblasts. Infect. Immun. 2006;74:953–960. doi: 10.1128/IAI.74.2.953-960.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkmann V., Reichard U., Goosmann C., Fauler B., Uhlemann Y., Weiss D.S., Weinrauch Y., Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- Brinkmann V., Zychlinsky A. Neutrophil extracellular traps: is immunity the second function of chromatin? J. Cell. Biol. 2012;198:773–783. doi: 10.1083/jcb.201203170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buergelt C.D., Bonde R.K. Toxoplasmic meningoencephalitis in a west indian manatee. J. Am. Vet. Med. Assoc. 1983;183(11):1294–1296. [PubMed] [Google Scholar]
- Cabezón O., Hall A.J., Vincent C., Pabon M., García-Bocanegra I., Dubey J.P., Almería S. Seroprevalence of Toxoplasma gondii in North-eastern Atlantic harbor seal (Phoca vitulina) and grey seal (Halichoerus grypus) Vet. Parasitol. 2011;179(1–3):253–256. doi: 10.1016/j.vetpar.2011.01.046. [DOI] [PubMed] [Google Scholar]
- Cabezon O., Resendes A.R., Domingo M., Raga J.A., Agustí C., Alegre F., Mons J.L., Dubey J.P., Almería S. Seroprevalence of Toxoplasma gondii antibodies in wild dolphins from the Spanish Mediterranean coast. J. Parasitol. 2004;90(3):643–644. doi: 10.1645/GE-257R. [DOI] [PubMed] [Google Scholar]
- Cámara Pellissó S., Muñoz M.J., Carballo M., Sánchez-Vizcaíno J.M. Determination of the immunotoxic potential of heavy metals on the functional activity of bottlenose dolphin leukocytes in vitro. Vet. Immunol. Immunopathol. 2008;121:189–198. doi: 10.1016/j.vetimm.2007.09.009. [DOI] [PubMed] [Google Scholar]
- Cheng O.Z., Palaniyar N. NET balancing: a problem in inflammatory lung diseases. Front. Immunol. 2013;4:1. doi: 10.3389/fimmu.2013.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuah C., Jones M.K., Burke M.L., Owen H.C., Anthony B.J., McManus D.P., Ramm G.A., Gobert G.N. Spatial and temporal transcriptomics of Schistosoma japonicum-induced hepatic granuloma formation reveals novel roles for neutrophils. J. Leukoc. Biol. 2013;94(2):353–365. doi: 10.1189/jlb.1212653. [DOI] [PubMed] [Google Scholar]
- Cole R.A., Lindsay D.S., Howe D.K., Roderick C.L., Dubey J.P., Thomas N.J., Baeten L.A. Biological and molecular characterizations of Toxoplasma gondii strains obtained from southern sea otters (Enhydra lutris nereis) J. Parasitol. 2000;86:526–530. doi: 10.1645/0022-3395(2000)086[0526:BAMCOT]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Conrad P.A., Miller M.A., Kreuder C., James E.R., Mazet J., Dabritz H., Jessup D.A., Gulland F., Grigg M.E. Transmission of Toxoplasma: clues from the study of sea otters as sentinels of Toxoplasma gondii flow into the marine environment. Int. J. Parasitol. 2005;35:1155–1168. doi: 10.1016/j.ijpara.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Contis-Montes de Oca A., Carrasco-Yépez M., Campos-Rodríguez R., Pacheco-Yérez J., Bonilla-Lemus P., Pérez-López J., Rojas-Hernández S. Neutrophil extracellular traps damage Naegleria fowleri trophozoites opsonized with human IgG. Parasite Immunol. 2016;38(8):481–495. doi: 10.1111/pim.12337. [DOI] [PubMed] [Google Scholar]
- Daigo K., Hamakudo T. Host-protective effect of circulating pentraxin 3 (PTX3) and complex formation with neutrophil extracellular traps. Front. Immun. 2012;3:378. doi: 10.3389/fimmu.2012.00378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Guise S., Erickson K., Blanchard M., DiMolfetto L., Lepper H., Wang J., Stott J., Ferrick D. Monoclonal antibodies to lymphocyte surface antigens for cetacean homologues to CD2, CD19 and CD21. Vet. Immunol. Immunopathol. 2002;84:209–221. doi: 10.1016/s0165-2427(01)00409-3. [DOI] [PubMed] [Google Scholar]
- Dubey J.P., Hattel A.L., Lindsay D.S., Topper M.J. Neonatal Neospora caninum infection in dogs: isolation of the causative agent and experimental transmission. J. Amer. Vet. Med. Assn. 1988;193:1259–1263. [PubMed] [Google Scholar]
- Dubey J.P. Review of Neospora caninum and neosporosis in animals. Korean J. Parasitol. 2003;41:1–16. doi: 10.3347/kjp.2003.41.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey J.P., Fair P.A., Sundar N., Velmurugan G., Kwok O.C., McFee W.E., Majumdar D., Su C. Isolation of Toxoplasma gondii from bottlenose dolphins (Tursiops truncatus) J. Parasitol. 2008;94(4):821–823. doi: 10.1645/GE-1444.1. [DOI] [PubMed] [Google Scholar]
- Fuchs T.A., Abed U., Goosmann C., Hurwitz R., Schulze I., Wahn V., Weinrauch Y., Brinkmann V., Zychlinsky A. Novel cell death program leads to neutrophil extracellular traps. J. Cell. Biol. 2007;176:231–241. doi: 10.1083/jcb.200606027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii K., Kakumoto C., Kobayashi M., Saito S., Kariya T., Watanabe Y., Xuan X., Igarashi I., Suzuki M. Seroepidemiology of Toxoplasma gondii and Neospora caninum in seals around Hokkaido, Japan. J. Vet. Med. Sci. 2007;69(4):393–398. doi: 10.1292/jvms.69.393. [DOI] [PubMed] [Google Scholar]
- Gazzinelli R.T., Talvani A., Camargo M.M., Santiago H.C., Oliveira M.A., Vieira L.Q., Martins G.A., Aliberti J.C., Silva J.S. Induction of cell-mediated immunity during early stages of infection with intracellular protozoa. Braz. J. Med. Biol. Res. 1998;31:89–104. doi: 10.1590/s0100-879x1998000100012. [DOI] [PubMed] [Google Scholar]
- Goldstein J.D., Reese E., Reif J.S., Varela R.A., Mcculloch S.D., Dfran R.H., Fair P.A., Bossart G.D. Hematologic, biochemical, and cytologic findings from apparently healthy Atlantic bottlenose dolphins (Tursiops truncatus) inhabiting the India River Lagoon, Florida, USA. J. Wildl. Dis. 2006;42:447–454. doi: 10.7589/0090-3558-42.2.447. [DOI] [PubMed] [Google Scholar]
- Guimarães-Costa A.B., Nascimento M.T., Froment G.S., Soares R.P., Morgado F.N., Conceição-Silva F., Saraiva E.M. Leishmania amazonensis promastigotes induce and are killed by neutrophil extracellular traps. Proc. Natl. Acad. Sci. U. S. A. 2009;106:6748–6753. doi: 10.1073/pnas.0900226106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn S., Giaglis S., Chowdhury C.S., Hösli I., Hasler P. Modulation of neutrophil NETosis: interplay between infectious agents and underlying host physiology. Semin. Immunopathol. 2013;35:439–453. doi: 10.1007/s00281-013-0380-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall A.J., Wells R.S., Sweeney J.C., Townsend F.I., Balmer B.C., Hohn A.A., Rhinehart H.L. Annual, seasonal and individual variation in hematology and clinical blood chemistry profiles in bottlenose dolphins (Tursiops truncatus) from Sarasota Bay, Florida. Comp. Biochem. Physiol. A. 2007;148:266–277. doi: 10.1016/j.cbpa.2007.04.017. [DOI] [PubMed] [Google Scholar]
- Hermosilla C., Muñoz-Caro T., Silva L.M.R., Ruiz A., Taubert A. The intriguing host innate immune response: novel anti-parasitic defence by neutrophil extracellular traps. Parasitology. 2014;141:1489–1498. doi: 10.1017/S0031182014000316. [DOI] [PubMed] [Google Scholar]
- Hermosilla C., Silva L.M.R., Navarro M., Taubert A. Anthropozoonotic endoparasites in free-ranging “urban” South American sea lions (Otaria flavescens) J. Vet. Med. 2016;2016:7507145. doi: 10.1155/2016/7507145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inskeep W., II, Gardiner C.H., Harris R.K., Dubey J.P., Goldston R.T. Toxoplasmosis in atlantic bottle-nosed dolphins (Tursiops truncatus) J. Wildl. Dis. 1990;26:377–382. doi: 10.7589/0090-3558-26.3.377. [DOI] [PubMed] [Google Scholar]
- Itou T., Sugisawa H., Inoue Y., Jinbo T., Sakai T. Oxygen radical generation and expression of NADPH oxidase genes in bottlenose dolphin (Tursiops truncatus) neutrophils. Dev. Comp. Immunol. 2001;25:47–53. doi: 10.1016/s0145-305x(00)00037-9. [DOI] [PubMed] [Google Scholar]
- Jardine J.E., Dubey J.P. Congenital toxoplasmosis in a indo-pacific bottlenose dolphin (Tursiops aduncus) J. Parasitol. 2002;88:197–199. doi: 10.1645/0022-3395(2002)088[0197:CTIAIP]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Johnson S.P., Nolan S., Gulland F.M.D. Antimicrobial susceptibility of bacteria isolated from pinnipeds stranded in central and northern California. J. Zoo. Wildl. Med. 1998;29:288–294. [PubMed] [Google Scholar]
- Kato H., Perrin W.F. Bryde's whale: balaenoptera edeni/brydei. In: Perrin W.F., Würsig B., Thewissen J.G.M., editors. Encyclopedia of Marine Mammals. second ed. Academic Press; San Diego: 2009. pp. 158–163. [Google Scholar]
- Keogh M.J., Spoon T., Ridgway S.H., Jensen E., Van Bonn W., Romano T.A. Simultaneous measurement of phagocytosis and respiratory burst of leukocytes in whole blood from bottlenose dolphins (Tursiops truncatus) utilizing flow cytometry. Vet. Immunol. Immunopathol. 2011;144:468–475. doi: 10.1016/j.vetimm.2011.08.017. [DOI] [PubMed] [Google Scholar]
- Klevar S., Kulberg S., Boysen P., Storset A.K., Modal T., Björkmann C., Olsen I. Natural killer cells act as early responders in an experimental infection with Neospora caninum in calves. Int. J. Parasitol. 2007;37(3–4):329–339. doi: 10.1016/j.ijpara.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Kleinertz S., Hermosilla C., Ziltener A., Kreicker S., Hirzmann J., Abdel-Ghaffar F., Taubert A. Gastrointestinal parasites of free-living indo-pacific bottlenose dolphins (Tursiops aduncus) in the northern red sea, Egypt. Parasitol. Res. 2014;113:1405–1415. doi: 10.1007/s00436-014-3781-4. [DOI] [PubMed] [Google Scholar]
- Lange M., Penagos-Tabares F., Muñoz-Caro T., Gärtner U., Mejer H., Schaper R., Hermosilla C., Taubert A. Gastropod-derived haemocyte extracellular traps entrap metastrongyloid larval stages of Angiostrongylus vasorum, Aelurostrongylus Abstrusus and Troglostrongylus Brevior. Parasit. Vectors. 2017;10:50. doi: 10.1186/s13071-016-1961-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaPointe J.M., Duignan P.J., Marsh A.E., Gulland F.M., Barr B.C., Naydan D.K., King D.P., Farman C.A., Huntingdon K.A.B., Lowenstine L.J. Meningoencephalitis due to a Sarcocystis neurona-like protozoan in Pacific harbor seals (Phoca vitulina richardsi) J. Parasitol. 1998;84:1184–1189. [PubMed] [Google Scholar]
- Maksimov P., Hermosilla C., Kleinertz S., Hirzmann J., Taubert A. Besnoitia besnoiti infections activate primary bovine endothelial cells and promote PMN adhesion and NET formation under physiological flow condition. Parasitol. Res. 2016;115:1991–2001. doi: 10.1007/s00436-016-4941-5. [DOI] [PubMed] [Google Scholar]
- Mancia A., Lundqvist M.L., Romano T.A., Peden-Adams M.M., Fair P.A., Kindy M.S., Ellis B.C., Gattoni-Celli S., McKillen D.J., Trent H.F., Chen Y.A., Almeida J.S., Gross P.S., Chapman R.W., Warr G.W. A dolphin peripheral blood leukocyte cDNA microarray for studies of immune function and stress reaction. Dev. Comp. Immunol. 2007;31(5):520–529. doi: 10.1016/j.dci.2006.07.011. [DOI] [PubMed] [Google Scholar]
- Martinelli S., Urosevic M., Daryadel A., Oberholzer P.A., Baumann C., Fey M.F., Dummer R., Simon H.U., Yousefi S. Induction of genes mediating interferon dependent extracellular trap formation during neutrophil differentiation. J. Biol. Chem. 2004;279:44123–44132. doi: 10.1074/jbc.M405883200. [DOI] [PubMed] [Google Scholar]
- Mazzariol S., Marker F., Mignone W., Serracca L., Goria M., Marsili L., DiGuardo G., Casalone C. Dolphin Morbillivirus and Toxoplasma gondii co-infection in a Mediterranean fin whale (Balaenoptera physalus) BMC Vet. Res. 2012;8:20. doi: 10.1186/1746-6148-8-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikaelian I., Boisclair J., Dubey J.P., Kennedy S., Martineau D. Toxoplasmosis in beluga whales from the St. Lawrence estuary: two case reports and a serological survey. J. Comp. Pathol. 2000;122:73–76. doi: 10.1053/jcpa.1999.0341. [DOI] [PubMed] [Google Scholar]
- Miller M.A., Miller W.A., Conrad P.A., James E.R., Melli A.C., Leutenegger C.M., Dabritz H.A., Packham A.E., Paradies D., Harris M., Ames J., Jessup D.A., Worcester K., Grigg M.E. Type X Toxoplasma gondii in wild mussel and terrestrial carnivores from coastal California: new linkages between terrestrial mammals, runoff and toxoplasmosis of sea otters. Int. J. Parasitol. 2008;38(11):1319–1328. doi: 10.1016/j.ijpara.2008.02.005. [DOI] [PubMed] [Google Scholar]
- Muñoz-Caro T., Hermosilla C., Silva L.M.R., Cortes H., Taubert A. Neutrophil extracellular traps as innate immune reaction against the emerging apicomplexan parasite Besnoitia besnoiti. PLoS One. 2014;9(3):e91415. doi: 10.1371/journal.pone.0091415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz-Caro T., Mena Huertas J.S., Conejeros I., Alarcón P., Hidalgo M.A., Burgos R.A., Taubert A., Hermosilla C. CD11b-, ERK/MAP kinase- and SOCE-dependent Eimeria bovis-triggered neutrophil extracellular trap formation. Vet. Res. 2015;46:23. doi: 10.1186/s13567-015-0155-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz-Caro T., Lendner M., Daugschies A., Hermosilla C., Taubert A. NADPH oxidase, MPO, NE, ERK1/2, p38 MAPK and Ca2+ influx are essential for Cryptosporidium parvum-induced NET formation. Dev. Comp. Immunol. 2015;52:245–254. doi: 10.1016/j.dci.2015.05.007. [DOI] [PubMed] [Google Scholar]
- Muñoz-Caro T., Silva M.R.L., Rentería-Solís Z., Taubert A., Hermosilla C. Neutrophil extracellular traps in the intestinal mucosa of Eimeria-infected animals. Asian Pac. J. Trop. Biomed. 2016;6:301–307. [Google Scholar]
- Noda K., Akiyoshi H., Aoki M., Shimada T., Ohashi F. Relationship between transportation stress and polymorphonuclear cell functions of bottlenose dolphins, Tursiops truncatus. J. Vet. Med. Sci. 2006;69:379–383. doi: 10.1292/jvms.69.379. [DOI] [PubMed] [Google Scholar]
- O'Donnell B.V., Tew D.G., Jones O.T., England P.J. Studies on the inhibitory mechanism of iodonium compounds with special reference to neutrophil NADPH oxidase. Biochem. J. 1993;290:41–49. doi: 10.1042/bj2900041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen M.E., Roch P.D., Stabler M., Chan W. Giardiasis in ringed seals from the western Arctic. J. Wildl. Dis. 1997;33:646–648. doi: 10.7589/0090-3558-33.3.646. [DOI] [PubMed] [Google Scholar]
- Omata Y., Umeshita Y., Watarai M., Tachibana M., Sasaki M., Murata K., Yamada T.K. Investigation for presence of Neospora caninum, Toxoplasma gondii and Brucella-species infection in killer whales (Orcinus orca) mass-stranded on the coast of Shiretoko, Hokkaido. Jpn. J. Vet. Med. Sci. 2006;68:523–526. doi: 10.1292/jvms.68.523. [DOI] [PubMed] [Google Scholar]
- Parveen S.R., Murphree L., Edmiston L., Kaspar C.W., Portier K.M., Tamplin M.L. Association of multiple-antibiotic-resistance profiles with point and non-point sources of Escherichia coli in Apalachicola Bay. Appl. Environ. Microbiol. 1997;63:2607–2612. doi: 10.1128/aem.63.7.2607-2612.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez D., Muñoz M.C., Molina J.M., Muñoz-Caro T., Silva L.M.R., Taubert A., Hermosilla C., Ruiz A. Eimeria ninakohlyakimovae induces NADPH oxidase-dependent monocyte extracellular trap formation and upregulates IL-12 and TGF-α, IL-6 and CCL2 gene transcription. Vet. Parasitol. 2016;227:143–150. doi: 10.1016/j.vetpar.2016.07.028. [DOI] [PubMed] [Google Scholar]
- Reichel M., Muñoz-Caro T., Sánchez-Contreras G., Rubio-García A., Magdowski G., Gärtner U., Hermosilla C., Taubert A. Harbour seal (Phoca vitulina) PMN and monocytes release extracellular traps to capture the apicomplexan parasite Toxoplasma gondii. Dev. Comp. Immunol. 2015;50:106–115. doi: 10.1016/j.dci.2015.02.002. [DOI] [PubMed] [Google Scholar]
- Reif J.S., Peden-Adams M.M., Romano T.A., Rice C.D., Fair P.A., Bossart G.D. Immune dysfunction in Atlantic bottlenose dolphins (Tursiops truncatus) with lobomycosis. Med. Mycol. 2009;47(2):125–135. doi: 10.1080/13693780802178493. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Espinosa O., Rojas-Espinosa O., Moreno-Altamirano M.M., López-Villegas E.O., Sánchez-García F.J. Metabolic requirements for neutrophil traps formation. Immunol. 2015;145(2):213–224. doi: 10.1111/imm.12437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano T.A., Ridgway S.H., Quaranta V.J. MHC class II molecules and immunoglobulins on peripheric blood lymphocytes of the bottlenosed dolphin, Tursiops truncatus. J. Exp. Zool. 1992;263:96–104. doi: 10.1002/jez.1402630110. [DOI] [PubMed] [Google Scholar]
- Santos P.S., Albuquerque G.R., da Silva V.M., Martin A.R., Marvulo M.F., Souza S.L., Ragozo A.M., Nascimento C.C., Gennari S.M., Dubey J.P., Silva J.C. Seroprevalence of Toxoplasma gondii in free-living amazon river dolphins (Inia geoffrensis) from central amazon, Brazil. Vet. Parasitol. 2011;183:171–173. doi: 10.1016/j.vetpar.2011.06.007. [DOI] [PubMed] [Google Scholar]
- Schwacke L.H., Hall A.J., Townsend F.I., Wells R.S., Hansen L.J., Hohn A.A., Bossart G.D., Fair P.A., Rowles T.K. Hematologic and serum biochemical reference intervals for free-ranging common bottlenose dolphins (Tursiops truncatus) and variation in the distributions of clinicopathologic values related to geographic sampling site. Am. J. Vet. Res. 2009;70:973–985. doi: 10.2460/ajvr.70.8.973. [DOI] [PubMed] [Google Scholar]
- Schwacke L.H., Twiner M.J., De Guise S., Balmer B.C., Wells R.S., Townsend F.I., Rotstein D.C., Varela R.A., Hansen L.J., Zolman E.S., Spradlin T.R., Levin M., Leibrecht H., Wang Z., Rowles T.K. Eosinophilia and bioxin exposure in bottlenose dolphins (Tursiops truncatus) from a coastal area impacted by repeated mortality events. Environ. Res. 2010;110(6):548–555. doi: 10.1016/j.envres.2010.05.003. [DOI] [PubMed] [Google Scholar]
- Silva L.M.R., Muñoz-Caro T., Rüdiger G., Vila-Viçosa M.J.M., Cortes H.C.E., Hermosilla C., Taubert A. The apicomplexan parasite Eimeria arloingi induces caprine neutrophil extracellular traps. Parasitol. Res. 2014;113:2797–2807. doi: 10.1007/s00436-014-3939-0. [DOI] [PubMed] [Google Scholar]
- Silva L.M.R., Muñoz-Caro T., Burgos R.A., Hidalgo M.A., Taubert A., Hermosilla C. Far beyond phagocytosis: phagocyte-derived extracellular traps act efficiently against protozoan parasites in vitro and in vivo. Mediat. Inflamm. 2016;2016:5898074. doi: 10.1155/2016/5898074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitt T., Bowen L., Blanchard M.T., Gershwin L.J., Byrne Ba, Dold C., McBain J., Stott J.L. Cellular immune responses in cetaceans immunized with a porcine erysipelas vaccine. Vet. Immunol. Immunopathol. 2010;137:181–189. doi: 10.1016/j.vetimm.2010.05.003. [DOI] [PubMed] [Google Scholar]
- Sousa-Rocha D., Thomaz-Tobias M., Diniz L.F.A., Souza P.S.S., Pinge-Filho P., Toledo K.A. Trypanosoma cruzi and its soluble antigens induce NET release by stimulating toll-like receptors. PLoS One. 2015;10:e0139569. doi: 10.1371/journal.pone.0139569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taubert A., Krüll M., Zahner H., Hermosilla C. Toxoplasma gondii and Neospora caninum infection of bovine endothelial cells induce endothelial adhesion molecule gene transcription and subsequent PMN adhesion. Vet. Immunol. Immunopathol. 2006;112:214–222. doi: 10.1016/j.vetimm.2006.03.017. [DOI] [PubMed] [Google Scholar]
- Taubert A., Wimmers K., Ponksuksili S., Jimenez C.A., Zahner H., Hermosilla C. Microarray-based transcriptional profiling of Eimeria bovis-infected bovine endothelial host cells. Vet. Res. 2010;41:70. doi: 10.1051/vetres/2010041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venn-Watson S., Jensen E.D., Ridgway S.H. Effects of age and sex on clinic-pathologic reference ranges in a healthy managed Atlantic bottlenose dolphin population. J. Am. Vet. Med. Assoc. 2007;231:596–601. doi: 10.2460/javma.231.4.596. [DOI] [PubMed] [Google Scholar]
- Ventura-Juárez J., Campos-Esparza M.R., Pacheco-Yepez J., López-Blanco J.A., Adabache-Ortíz A., Silva-Briano M., Campos-Rodríguez R. Entamoeba histolytica induces human neutrophils to form NETs. Parasite Immunol. 2016;38:503–509. doi: 10.1111/pim.12332. [DOI] [PubMed] [Google Scholar]
- Villagra-Blanco R., Silva L.M.R., Gärtner U., Wagner H., Failing K., Wehrend A., Taubert A., Hermosilla C. Molecular analyses on Neospora caninum-triggered NETosis in the caprine system. Dev. Comp. Immunol. 2017;72:119–127. doi: 10.1016/j.dci.2017.02.020. [DOI] [PubMed] [Google Scholar]
- Villagra-Blanco R., Silva L.M.R., Muñoz-Caro T., Yang Z., Li J., Gärtner U., Taubert A., Zhang X., Hermosilla C. Bovine PMN cast neutrophil extracellular traps against the abortive parasite Neospora caninum. Front. Immunol. 2017;8:606. doi: 10.3389/fimmu.2017.00606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Köckritz-Blickwede M., Nizet V. Innate immunity turned inside-out: antimicrobial defense by phagocyte extracellular traps. J. Mol. Med. 2009;87:775–783. doi: 10.1007/s00109-009-0481-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Köckritz-Blickwede M., Chow O., Ghochani M., Nizet V. Visualization and functional evaluation of phagocyte extracellular traps. Methods Microbiol. 2010;37:139–160. [Google Scholar]
- Wang Y., Chen Y., Xin L., Beverley S.M., Carlsen E.D., Popov V., Chang K.P., Wang M., Soong L. Differential microbicidal effects of human histone proteins H2A and H2B on Leishmania promastigotes and amastigotes. Infect. Immun. 2011;79:1124–1133. doi: 10.1128/IAI.00658-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Z., Hermosilla C., Taubert A., He X., Wang X., Gong P., Li J., Yang Z., Zhang X. Canine neutrophil extracellular traps release induced by the apicomplexan parasite Neospora caninum in vitro. Front. Immunol. 2016;7:436. doi: 10.3389/fimmu.2016.00436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White N.D., Godard-Codding C., Webb S.J., Bossart G.D., Fair P.A. Immunotoxic effects of in vitro exposure of dolphin lymphocytes to Louisiana sweet crude oil and Corexit™. J. Appl. Toxicol. 2017;37:676–682. doi: 10.1002/jat.3414. [DOI] [PubMed] [Google Scholar]
- Zafra R., Jaber J.R., Pérez J., de la Fuente R., Arbelo M., Andrada M., Fernández A. Immunohistochemical characterisation of parasitic pneumonias of dolphins stranded in the Canary Islands. Res. Vet. Sci. 2015;100:207–212. doi: 10.1016/j.rvsc.2015.03.021. [DOI] [PubMed] [Google Scholar]