Abstract
Objective
To evaluate predictive factors for retrograde ureteral stent failure in patients with non-urological malignant ureteral obstruction.
Materials and methods
Between 2005 and 2014, medical records of 284 malignant ureteral obstruction patients with 712 retrograde ureteral stent trials including 63 (22.2%) having bilateral malignant ureteral obstruction were retrospectively reviewed. Retrograde ureteral stent failure was defined as the inability to place ureteral stents by cystoscopy, recurrent stent obstruction within one month, or non-relief of azotemia within one week from the prior retrograde ureteral stent. The clinicopathological parameters and first retrograde pyelographic findings were analyzed to investigate the predictive factors for retrograde ureteral stent failure and conversion to percutaneous nephrostomy in multivariate analysis with a statistical significance of p < 0.05.
Results
Retrograde ureteral stent failure was detected in 14.1% of patients. The mean number of retrograde ureteral stent placements and indwelling duration of the ureteral stents were 2.5 ± 2.6 times and 8.6 ± 4.0 months, respectively. Multivariate analyses identified several specific RGP findings as significant predictive factors for retrograde ureteral stent failure (p < 0.05). The significant retrograde pyelographic findings included grade 4 hydronephrosis (hazard ratio 4.10, 95% confidence interval 1.39–12.09), irreversible ureteral kinking (hazard ratio 2.72, confidence interval 1.03–7.18), presence of bladder invasion (hazard ratio 4.78, confidence interval 1.81–12.63), and multiple lesions of ureteral stricture (hazard ratio 3.46, confidence interval 1.35–8.83) (p < 0.05).
Conclusion
Retrograde pyelography might prevent unnecessary and ineffective retrograde ureteral stent trials in patients with advanced non-urological malignant ureteral obstruction.
Introduction
Malignant ureteral obstruction (MUO) due to extrinsic ureteral compression by advanced pelvic or retroperitoneal tumors is an urgent situation resulting in hydroureteronephrosis (HUN) and azotemia in patients with advanced, incurable non-urological cancer with an approximate life expectancy of fewer than seven to twelve months [1–3]. Treatment options include retrograde ureteral stenting (RUS), percutaneous nephrostomy (PCN), and surgical resection of the obstructed segment under general or local anesthesia. Usually, RUS is recommended initially because RUS is simpler and less invasive than PCN, and does not require hospitalization [4–6]. Once RUS fails, a prompt alternative intervention of PCN usually must be performed, and further anterograde stenting via PCN may be attempted [6].
Successful cystoscopic RUS insertion for MUO resolution is a challenging procedure even for the most experienced urologists, with a mean failure rate of 15.0‑34.6% [1, 5, 7–14], and does not always guarantee resolution of the obstruction and amelioration of azotemia [8]. Clinicians must always determine when to convert to PCN after considering the prognosis, quality of life, and complications of each procedure. Therefore, to significantly reduce the number of unnecessary procedures of RUS as well as the associated pain, it is important to accurately predict risk factors of RUS failure. Especially, the clinical significance of the first intraoperative retrograde pyelographic (RGP) findings should be evaluated for prediction of RUS failure and prevention of unnecessary and ineffective RUS trials in the management of patients with non-urological MUO from a single institute.
Materials and methods
Ethical statements
All study protocols were conducted according to the ethical guidelines of the World Medical Association Declaration of Helsinki Ethical Principles for Medical Research Involving Human Subjects. This study was approved by the Institutional Review Board (IRB) of the Research Institute and Hospital National Cancer Center (IRB No. NCC 2016–0102). The need for written informed consent was waived by the IRB.
Patient selection
We retrospectively analyzed the medical charts and radiologic images of 284 patients with MUO with a total of 712 instances of RUS between 2005 and 2014. Indwelling RUS placement was indicated when MUO was strongly suspected from radiographic evidence with azotemia. The exclusion criteria were any patients with a history of urological intervention or surgical treatment, urological malignancy, kidney transplantation, intraoperative iatrogenic ureteral injury and prophylactic stent insertion, outside RUS insertion history, congenital urogenital anomaly, two stents inserted in one ureter, urinary calculi, PCN without RUS trials, bladder fistula, or non-availability of operative records, septic or febrile conditions, imaging, or follow-up records.
RUS and PCN procedures
RUS under local or general anesthesia was performed with rigid cystoscopy under fluoroscopy by four onco-urologists each with at least 10 years of experience. The type of anesthesia was dependent on the clinicians’ discretion after considering the patients’ general condition for general anesthesia. The stents were typically scheduled to be changed every three months. All ureteral stents had the same HydroPlus coating material. RUS failure was defined as the inability to place RUS by cystoscopy, recurrent hydronephrosis within one month after stenting, or non-relief of azotemia within one week from prior RUS. The patients were then referred for placement of a PCN tube with/without anterograde stenting trial at the affected kidney with MUO. All PCN procedures were performed under local anesthesia by a single uro-radiologist with 15 years of experience.
Prognostic clinical/pathological factors were reviewed including age, sex, body mass index, Eastern Cooperative Oncology Group performance status, type of primary malignancy, current therapeutic modalities before stenting, ureteral level of obstruction, pre‑stenting laboratory parameters including serum creatinine, degree of hydronephrosis from 1 to 4 [15], RGP or anterograde pyelographic or cystoscopy findings, PCN or RUS caliber used (6, 7, or 8 Fr), and median overall survival and PCN-free survival times.
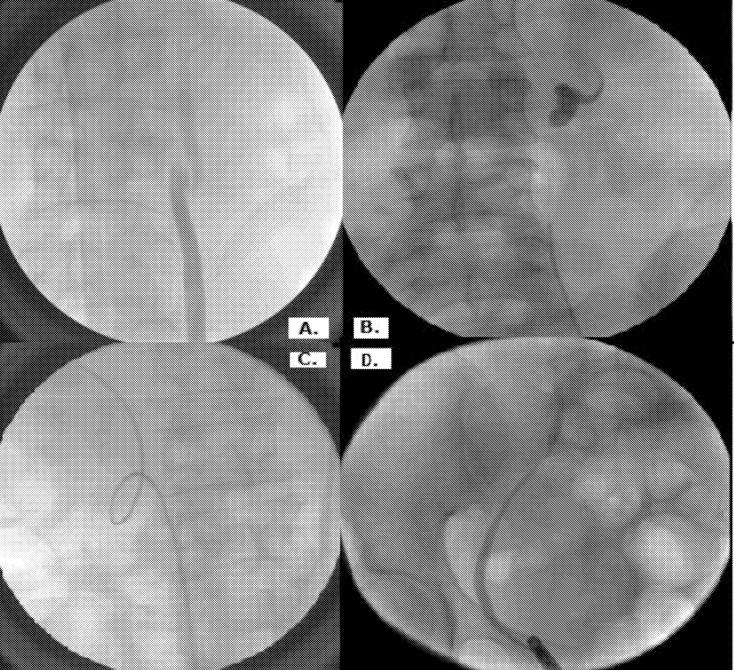
RGP and cystoscopy findings were described in the operative records for each RUS episode as follows: laterality of hydroureteronephrosis, obstruction level of MUO (distal, mid, or proximal ureter), presence of abnormal ureteral direction (either lateralization or normal direction), shape of reversible ureteral kinking with Z-shaped or pigtail-shaped kinking, presence of irreversible ureteral kinking when the ureter failed to straighten even with placement of a ureteral guidewire or stent, and presence of bladder invasion on cystoscopy (Fig 1). In terms of the statistical analysis of predictive risk factors of RUS failure, the first RGP and cystoscopic findings were utilized in the univariate and multivariate models.
Fig 1. Retrograde pyelographic findings.
A) Z-shaped ureteral kinking, B) pigtail shaped kinking, C) irreversible ureteral kinking, and D) ureter lateralization.
Comparative analysis was statistically performed between the successful stenting group (Stent group) and the failed stenting group with PCN placement (PCN group) using the Student’s t-test or Wilcoxon rank sum test and chi-square/Fisher’s exact test. To investigate the risk factors affecting stent failure, we examined clinical pathologic factors (age, sex, BMI, anesthesia, pre-stent treatment, and first creatine) and eight factors found on RGP. We explored the association between stent failure and numerous risk factors using the binary logistic regression model where the outcome variable was RUS success versus RUS failure. The clinical pathologic factors with p < 0.05 were adjusted using a multivariate logistic regression model including factors found on RGP. Subsequently, we identified RGP findings that were risk factors for stent failure using a backward variable selection method with a significance level of 0.05. P values less than 0.05 were considered statistically significant, and all statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
The male/female ratio, RUS failure rate or PCN conversion rate, three-year survival rate, and median survival time among the 284 patients including 63 (22.2%) patients with bilateral MUO were 191/93 (67.3%/32.7%), 14.1%, 14.4%, and 8.8 months, respectively (Table 1). Gastric (37.0%), colorectal (24.6%), and gynecologic (22.5%) cancers were the most frequent cancers causing MUO. The mean number of stent changes and indwelling stent duration were 2.5 ± 2.6 times and 8.6 ± 4.0 months, respectively. The remaining baseline demographics and intraoperative RGP findings are described in Table 1.
Table 1. The 284 patients’ baseline clinical characteristics and intraoperative findings of retrograde pyelography.
| Variables | N (%) or mean ± SD |
|---|---|
| Age (years) | 60.5 ± 13.6 |
| Sex, Male/Female | 191/93 (67.3/32.7) |
| Body mass index (kg/cm2) | 21.4 ± 3.6 |
| Pre-stent Treatment | |
| Surgery | 40 (14.1) |
| Radiotherapy | 33 (11.6) |
| Chemotherapy | 182 (64.1) |
| No further therapy | 29 (10.2) |
| Hydronephrosis, Bilateral/Unilateral | 63/221 (22.2/77.8) |
| Primary cancer | |
| Gynecologic cancer | 64 (22.5) |
| Lung cancer | 10 (3.5) |
| Head and neck cancer | 1 (0.4) |
| Osteologic cancer | 2 (0.7) |
| Breast cancer | 15 (5.3) |
| Colorectal cancer | 70 (24.6) |
| Hepatobiliary cancer | 8 (2.8) |
| Stomach cancer | 105 (37.0) |
| Hematologic cancer | 5 (1.8) |
| Others | 4 (1.4) |
| Degree of hydronephrosis1/2/3/4 | 22/91/98/73 (7.8/32.0/34.5/25.7) |
| Patient’s ECOG 0/1/2/3 | 125/121/33/5 (44.0/42.6/11.6/1.8) |
| Serum Creatinine level before stenting | 1.5 ± 1.2 |
| sCr category (mg/dL) < 1.3 | 167 (58.8) |
| ≥ 1.31 | 117 (41.2) |
| Retrograde pyelography findings | |
| Ureteral kinking shape | |
| none/Z-shape/pigtail shape | 167/95/22 (58.8/33.5/7.7) |
| Irreversibility of ureteral kinking | 41 (14.4) |
| Ureteral direction, normal/lateralization | 264/20 (93.0/7.0) |
| Bladder invasion | 37 (13.0) |
| Stent duration (mean, months) | 8.6 ± 4.0 |
| Stenting failure | 40 (14.1) |
| Intra/postoperative stent failure | 10/30 (3.5/10.6) |
| Survival | 41 (14.4) |
| Overall Survival time (median, months) | 8.8 |
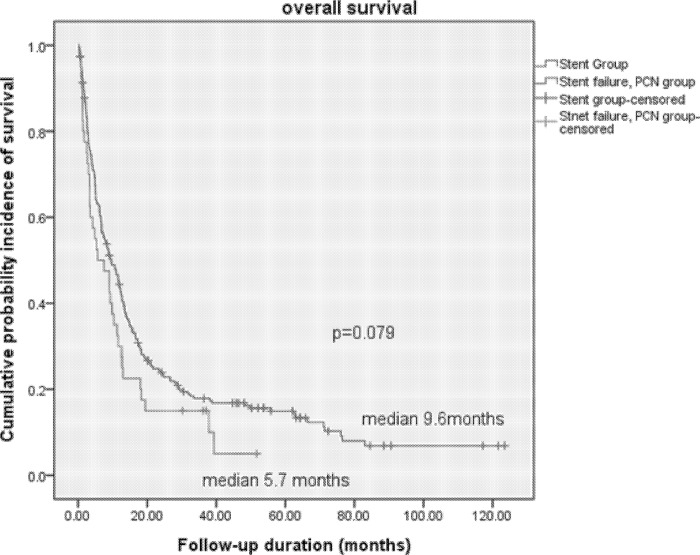
Between the Stent group and PCN groups, the stent duration and median overall survival rates were significantly different in terms of baseline characteristics such as the presence of bilateral hydronephrosis and pre-RUS serum creatinine level (p < 0.05, Table 2). The irreversibility rates of ureteral kinking on first RGP and the presence of bladder invasion on first cystoscopy were also significantly different (p < 0.05). However, the overall survival curve showed statistically insignificant differences between the Stent group (9.6 months) and PCN group (5.7 months) (p = 0.079, Fig 2).
Table 2. Comparison of basic characteristics and intraoperative findings between the stent succeeding group (Stent) and stent failing group (PCN).
| Variables | Stent (N = 244) | PCN (N = 40) | p-value |
|---|---|---|---|
| Age (years) | 54.1 ± 13.1 | 53 ± 13.8 | 0.620 |
| Sex, Male/Female | 166/78 (32.0/68.0) | 25/15 (37.5/62.5) | 0.468 |
| Body mass index (kg/cm2) | 0.901 | ||
| Underweight | 21 (8.6) | 2 (5.0) | |
| Normal | 217 (88.9) | 37 (92.5) | |
| Obese | 6 (2.5) | 1 (2.5) | |
| Patient’s ECOG 0/1/2 | 205/35/4 (84/14.4/1.6) | 33/7/0 (82.5/17.5/0) | 0.801 |
| Pre-stent Treatment | 0.974 | ||
| Surgery | 35 (14.3) | 5 (12.5) | |
| Radiotherapy | 28 (11.5) | 5 (12.5) | |
| Chemotherapy | 155 (64.5) | 27 (67.5) | |
| None or follow-up | 26 (10.7) | 3 (1.1) | |
| Primary cancer | 0.524 | ||
| Gynecologic cancer | 55 (22.5) | 9 (22.5) | |
| Lung cancer | 8 (3.3) | 2 (58.0) | |
| Head and neck cancer | 1 (0.4) | 0 | |
| Osteologic cancer | 11 (4.5) | 4 (10.0) | |
| Breast cancer | 62 (25.4) | 8 (20.0) | |
| Colorectal cancer | 6 (2.5) | 2 (5.0) | |
| Hepatobiliary cancer | 91 (37.3) | 14 (35.0) | |
| Stomach cancer | 5 (2.0) | 0 | |
| Hematologic cancer | 4 (1.6) | 0 | |
| Baseline sCreatinine level (mg/dL) | 1.3 ± 0.8 | 1.5 ± 1.1 | 0.062 |
| sCr category (mg/dL) < 1.3 | 143 (58.6) | 24 (60.0) | 1.000 |
| ≥ 1.31 | 101 (41.4) | 16 (40.0) | |
| Retrograde pyelography findings | |||
| Severity of hydronephrosis 1-3/4 | 196/48 (80.3/19.7) | 15/25 (37.5/72.5) | 0.007 |
| Obstruction level | 0.514 | ||
| None/distal/mid/proximal/multiple | 2/103/67/45/27 (0.8/42.2/27.5/18.4/11.1) |
0/18/8/8/6 (0/45/20/20/15) |
|
| Ureteral kinking | 0.658 | ||
| none/Z-shape/ pigtail shape | 142/84/18 (58.2/34.4/7.4) | 25/11/4 (62.5/27.5/10.0) | |
| Irreversibility of ureteral kinking | 31 (12.7) | 10 (25.0) | 0.022 |
| Ureteral lateralization | 18 (7.4) | 2 (5.0) | 1.000 |
| Bladder invasion | 24 (9.8) | 13 (32.5) | < 0.001 |
| Stent caliber (Fr.) | 6.8 ± 4.0 | 6.5 ± 0.5 | 0.679 |
| Times of Stenting | 2.5 ± 2.6 | 2.7 ± 2.6 | 0.668 |
| Stent duration (mean, months) | 8.6 ± 12.5 | 8.5 ± 11.1 | 0.028 |
| Survival | 37 (15.2) | 4 (10.0) | 0.475 |
| Overall Survival (median, months) | 17.9 ± 23.2 | 11.4 ± 12.9 | 0.013 |
Fig 2. Overall survival curve between patients with retrograde ureteral stenting and percutaneous nephrostomy.
Logistic regression analysis revealed that only general anesthesia (hazard ratio [HR] 9.80, 95% confidence interval [CI] 1.59–60.64, p = 0.014) was significant in univariate analysis among clinicopathological parameters; however, the type of anesthesia became insignificant when analyzed with RGP parameters (p = 0.494, Table 3). The final multivariate analysis revealed that grade 4 hydronephrosis (HR 4.1, CI 1.39–12.09), multiple ureteral stricture lesions (HR 3.46, CI 1.35–8.83), irreversible ureteral kinking (HR 2.72, CI 1.18–6.31), and bladder invasion (HR 4.78, CI 1.81–12.63) were significant independent factors for RUS failure and PCN conversion (p < 0.05, Table 3).
Table 3. Logistic regression analysis of predictive risk factors for stenting failure.
| Univariate | Multivariate | |||
|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | |
| Age | 0.99 (0.97–1.02) | 0.621 | ||
| Sex, female | 0.76 (0.38–1.53) | 0.446 | ||
| BMI, Low | 1 (ref) | (0.746) | ||
| Normal | 1.79 (0.40–7.96) | 0.444 | ||
| Obese | 1.75 (0.13–22.78) | 0.669 | ||
| Anesthesia, local | 1 (ref) | 1 (ref) | ||
| general | 9.80 (1.59–60.64) | 0.014 | 4.12 (0.07–239.34) | 0.494 |
| Presenting therapy | ||||
| Surgery | 1 (ref) | (0.955) | ||
| Radiotherapy | 1.25 (0.33–4.75) | 0.743 | ||
| Chemotherapy | 1.22 (0.44–3.39) | 0.704 | ||
| None | 0.91 (0.20–4.20) | 0.907 | ||
| sCr < 1.3 | 1 (ref) | |||
| > 1.3 | 0.92 (0.47–1.83) | 0.821 | ||
| RGP findings | ||||
| HUN degree 1+2+3 | 1 (ref) | 1 (ref) | ||
| 4 | 3.46 (1.36–8.82) | 0.009 | 4.10 (1.39–12.09) | 0.010 |
| Laterality, unilateral | 1 (ref) | |||
| bilateral | 2.27 (1.06–4.84) | 0.035 | ||
| Stricture site, single | 1 (ref) | 1 (ref) | ||
| multiple | 2.51 (1.16–5.44) | 0.019 | 3.46 (1.35–8.83) | 0.010 |
| Ureter kinking, none | 1 (ref) | (0.710) | ||
| Z-shaped | 0.88 (0.39–1.97) | 0.753 | ||
| Pig-tailed | 1.49 (0.45–4.93) | 0.514 | ||
| Irrev. Ureteral kinking, | 2.73 (1.18–6.31) | 0.019 | 2.72 (1.03–7.18) | 0.043 |
| Ureteral lateralization | 1.34 (0.30–6.05) | 0.707 | ||
| Bladder invasion s | 5.99 (2.61–13.73) | < 0.001 | 4.78 (1.81–12.63) | 0.002 |
| Stent caliber, 6Fr. | 1 (ref) | |||
| 7Fr. ≤ | 0.77 (0.36–1.67) | 0.513 | ||
BMI, body mass index; sCr, serum creatinine level; RGP, retrograde pyelography; HUN, hydronephrosis; Irrev., irreversible
Discussion
The relationship between MUO and RUS failure
MUO is an emergent condition characterized by uremia or azotemia, and delayed intervention can adversely affect the planning of further treatment and even result in death. The therapeutic options and timing of any intervention should be determined cautiously after consideration of risk of complications, quality of life, renal function preservation, and balancing kidney laterality. Once inadequate MUO decompression after RUS is detected, the decision to convert to PCN with/without anterograde stenting should be made promptly. However, a general consensus has not been reached regarding which treatment modalities are the safest and most effective or the proper timing of treatment [10, 16, 17].
Some risk factors for RUS failure were identified, but other factors were not significant predictive factors for RUS failure relating to prognostic survival because adequate MUO management by successful RUS improves survival in patients with advanced or metastatic tumors [1, 3–5, 7–14, 16, 18–20]. This study also evaluated the significant predictive factors for RUS failure to identify several specific RGP imaging parameters (p < 0.05, Table 3).
Significance of bladder invasion
Among the factors identified, the presence of bladder invasion (HR 4.78) on cystoscopy was found to be significant (p = 0.002, Table 3). Urinary drainage was hindered when the bladder was invaded by pelvic cancer, and it was difficult to identify the intravesical ureteral orifices with the retroscopic cystoscopic approach for stenting. The stent patency was not maintained because of the continued extrinsic compression from the perivesical tumors.
Significance of the degree of hydronephrosis
An increased degree of hydronephrosis (grade 4; HR 4.10) before stenting was associated with a greater likelihood of RUS failure in patients with MUO, similar to previous studies [3, 8]. A severe degree of hydronephrosis indicates a progressively azotemic state referred to as a chronic malfunctioning drainage system from the renal pelvis via the ureteropelvic junction. A contralateral side RUS or PCN would be also considered if RUS on the ipsilateral side with hydronephrosis failed to prevent further aggravation of azotemia.
Significance of RGP findings
The significance of specific RGP findings was shown in predicting RUS failure and thus preventing unnecessary RUS trials. This study focused on the deformed renal pelvis and ureter itself in terms of direction, location, laterality, shape, kinking, and reversibility using RGP. Irreversible fixed ureteral kinking (HR 2.72) and multiple stricture lesions (HR 3.46) were significant predictive factors for RUS failure, whereas laterality of MUO, ureteral lateralization, and ureteral kinking shape were not significant predictive factors for RUS failure (p > 0.05, Table 3). RUS failure might be associated with bending, deformation, and reversible kinking status resulting from extrinsic compression or tumor invasion of the ureter, thus increasing resistance during RUS insertion and recurrence of irreversible ureter kinking in most advanced or metastatic cancers [21, 22]. Irreversible ureteral kinking with delayed or unsuccessful RUS could cause not only ureteritis, periureteral fibrosis, and periureteral lymph node progression, but also a poor general condition with azotemia or uremia resulting in interruption or delay of further chemotherapeutic treatment with poor survival outcomes.
Other significant baseline demographic parameters
In terms of baseline patient’s clinicopathological demographics, those previously reported as significant for RUS failure (male sex, age, BMI, anesthetic type, and presenting therapy) were not significantly related to RUS failure in this study (p > 0.05, Table 3). Only anesthetic type was significant in univariate analysis (p = 0.014), but it became insignificant when other RGP findings were considered that were potentially powerful indicators of RUS failure in this study.
Differential risk factors between intraoperative and postoperative RUS failure and between first RUS failure and sequential RUS failure
Among the 40 cases of RUS failure, there was both intraoperative and postoperative RUS failure. Further subanalysis showed that, among other clinicopathological and RGP parameters, the presence of multiple lesions causing ureteral stricture was the only significant difference remaining between intraoperative and postoperative RUS failure, which would immediately lead to PCN insertion (S1 Table). Another sub-analysis for the differential risk factors was performed between first attempted RUS failure (N = 17, 42.5%) and RUS failure after sequential successful stent changes (N = 23, 57.5%). Among the clinicopathological and RGP parameters, age was the only significant difference remaining between first RUS failure (59.1 ± 14.4 year-old) and RUS failure after sequential successful stenting (48.5 ± 11.7 year-old) groups (p = 0.014, S2 Table). Further studies with a large cohort would be needed to identify differential risk factors of postoperative RUS failure after sequential successful stenting and to compare the success rate or the effectiveness of restoring renal function between RUS and PCN in first attempt of decompressing procedure.
Limitations
This retrospective study had some inherent limitations including heterotrophic cancer etiologies with different baseline cancer stages, different references of radiologic interpretation in operative records, and different guidelines for choosing either RUS or PCN without any formal guidelines. Additionally, an increasing number of stent changes and their related RGP findings that could influence RUS failure were not considered. Despite these limitations, our findings using RGP imaging could predict urinary drainage malfunction and RUS ineffectiveness so that early PCN placement or contralateral RUS could be performed without attempting unnecessary RUS trials. This could reduce pain, improve the quality of life, and increase chemotherapy response with better survival outcomes and lower medical costs.
Conclusion
This study identified the significant importance of first RGP findings, such as multiple ureteral strictures, shape and irreversibility of ureteral kinking, presence of bladder invasion, and degree of hydronephrosis, for preventing RUS failure and unnecessary and ineffective RUS trials. This may help identify which patients should undergo RUS and PCN for the management of MUO.
Supporting information
(DOCX)
(DOCX)
Acknowledgments
Mr. June Nyun Kim and Sung Hwie Kang from the Department of Urology and Center for Prostate Cancer Research Institute and Hospital of National Cancer Center, Goyang, Korea, contributed to database management.
Data Availability
Data are available upon request from the National Cancer Center Institutional Data Access / Ethics Committee for researchers who meet the criteria for access to confidential data. Public access to data is restricted due to containing potentially identifying information, such as patients’ name, date of birth, insurance security number, and ID. To access the data, please contact Dr. Kang Hyun Lee, and he will send the request to the IRB of the National Cancer Center to make the data available (uroonoc@ncc.re.kr). Alternatively, please contact the IRB of the National Cancer Center.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.Wang JY, Zhang HL, Zhu Y, Qin XJ, Dai BO, Ye DW. Predicting the failure of retrograde ureteral stent insertion for managing malignant ureteral obstruction in outpatients. Oncol Lett. 2016;11: 879–883. doi: 10.3892/ol.2015.3961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosenberg BH, Bianco FJ Jr., Wood DP Jr., Triest JA. Stent-change therapy in advanced malignancies with ureteral obstruction. J Endourol. 2005;19: 63–67. doi: 10.1089/end.2005.19.63 [DOI] [PubMed] [Google Scholar]
- 3.Yossepowitch O, Lifshitz DA, Dekel Y, Gross M, Keidar DM, Neuman M, et al. Predicting the success of retrograde stenting for managing ureteral obstruction. Journal Urol. 2001;166: 1746–1749. [PubMed] [Google Scholar]
- 4.Chung SY, Stein RJ, Landsittel D, Davies BJ, Cuellar DC, Hrebinko RL, et al. 15-year experience with the management of extrinsic ureteral obstruction with indwelling ureteral stents. J Urol. 2004;172: 592–595. doi: 10.1097/01.ju.0000130510.28768.f5 [DOI] [PubMed] [Google Scholar]
- 5.Ganatra AM, Loughlin KR. The management of malignant ureteral obstruction treated with ureteral stents. J Urol. 2005;174: 2125–2128. doi: 10.1097/01.ju.0000181807.56114.b7 [DOI] [PubMed] [Google Scholar]
- 6.Harrington KJ, Pandha HS, Kelly SA, Lambert HE, Jackson JE, Waxman J. Palliation of obstructive nephropathy due to malignancy. Br J Urol. 1995;76: 101–107. [DOI] [PubMed] [Google Scholar]
- 7.Izumi K, Mizokami A, Maeda Y, Koh E, Namiki M. Current outcome of patients with ureteral stents for the management of malignant ureteral obstruction. J Urol. 2011;185: 556–561. doi: 10.1016/j.juro.2010.09.102 [DOI] [PubMed] [Google Scholar]
- 8.Kamiyama Y, Matsuura S, Kato M, Abe Y, Takyu S, Yoshikawa K, et al. Stent failure in the management of malignant extrinsic ureteral obstruction: risk factors. Int J Urology. 2011;18: 379–382. [DOI] [PubMed] [Google Scholar]
- 9.Kanou T, Fujiyama C, Nishimura K, Tokuda Y, Uozumi J, Masaki Z. Management of extrinsic malignant ureteral obstruction with urinary diversion. Int J Urol. 2007;14: 689–692. doi: 10.1111/j.1442-2042.2007.01747.x [DOI] [PubMed] [Google Scholar]
- 10.Kouba E, Wallen EM, Pruthi RS. Management of ureteral obstruction due to advanced malignancy: optimizing therapeutic and palliative outcomes. J Urol. 2008;180:444–450. doi: 10.1016/j.juro.2008.04.008 [DOI] [PubMed] [Google Scholar]
- 11.Liatsikos EN, Karnabatidis D, Katsanos K, Kallidonis P, Katsakiori P, Kagadis GC, et al. Ureteral metal stents: 10-year experience with malignant ureteral obstruction treatment. J Urol. 2009;182: 2613–2617. doi: 10.1016/j.juro.2009.08.040 [DOI] [PubMed] [Google Scholar]
- 12.Sountoulides P, Pardalidis N, Sofikitis N. Endourologic management of malignant ureteral obstruction: indications, results, and quality-of-life issues. J Endourol. 2010;24: 129–142. doi: 10.1089/end.2009.0157 [DOI] [PubMed] [Google Scholar]
- 13.Wenzler DL, Kim SP, Rosevear HM, Faerber GJ, Roberts WW, Wolf JS Jr. Success of ureteral stents for intrinsic ureteral obstruction. J Endourol. 2008;22: 295–299. doi: 10.1089/end.2007.0201 [DOI] [PubMed] [Google Scholar]
- 14.Wong LM, Cleeve LK, Milner AD, Pitman AG. Malignant ureteral obstruction: outcomes after intervention. Have things changed? J Urol. 2007;178: 178–183; discussion 83. doi: 10.1016/j.juro.2007.03.026 [DOI] [PubMed] [Google Scholar]
- 15.Fernbach SK, Maizels M, Conway JJ. Ultrasound grading of hydronephrosis: introduction to the system used by the Society for Fetal Urology. Pediatr Radiol. 1993;23: 478–480. [DOI] [PubMed] [Google Scholar]
- 16.Jeong IG, Han KS, Joung JY, Seo HK, Chung J. The outcome with ureteric stents for managing non-urological malignant ureteric obstruction. BJU Int. 2007;100: 1288–1291. doi: 10.1111/j.1464-410X.2007.07172.x [DOI] [PubMed] [Google Scholar]
- 17.Modi AP, Ritch CR, Arend D, Walsh RM, Ordonez M, Landman J, et al. Multicenter experience with metallic ureteral stents for malignant and chronic benign ureteral obstruction. J Endourol. 2010;24: 1189–1193. doi: 10.1089/end.2010.0121 [DOI] [PubMed] [Google Scholar]
- 18.Varda B, Sood A, Krishna N, Gandaglia G, Sammon JD, Zade J, et al. National rates and risk factors for stent failure after successful insertion in patients with obstructed, infected upper tract stones. Can Urol Assoc J. 2015;9: E164–171. doi: 10.5489/cuaj.2456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wijayarathna S, Suvendran S, Ishak M, Weligamage A, Epa A, Munasinghe S, et al. Outcome of retrograde ureteric stenting as a urinary drainage procedure in ureteric obstruction related to malignant lesions. Ceylon Med J. 2014;59: 124–127. doi: 10.4038/cmj.v59i4.7864 [DOI] [PubMed] [Google Scholar]
- 20.Yu SH, Ryu JG, Jeong SH, Hwang EC, Jang WS, Hwang IS, et al. Predicting factors for stent failure-free survival in patients with a malignant ureteral obstruction managed with ureteral stents. Korean J Urol. 2013;54: 316–321. doi: 10.4111/kju.2013.54.5.316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sammon JD, Ghani KR, Karakiewicz PI, Bhojani N, Ravi P, Sun M, et al. Temporal trends, practice patterns, and treatment outcomes for infected upper urinary tract stones in the United States. Eur Urol. 2013;64: 85–92. doi: 10.1016/j.eururo.2012.09.035 [DOI] [PubMed] [Google Scholar]
- 22.Goldsmith ZG, Wang AJ, Banez LL, Lipkin ME, Ferrandino MN, Preminger GM, et al. Outcomes of metallic stents for malignant ureteral obstruction. J Urol. 2012;188: 851–855. doi: 10.1016/j.juro.2012.04.113 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
Data Availability Statement
Data are available upon request from the National Cancer Center Institutional Data Access / Ethics Committee for researchers who meet the criteria for access to confidential data. Public access to data is restricted due to containing potentially identifying information, such as patients’ name, date of birth, insurance security number, and ID. To access the data, please contact Dr. Kang Hyun Lee, and he will send the request to the IRB of the National Cancer Center to make the data available (uroonoc@ncc.re.kr). Alternatively, please contact the IRB of the National Cancer Center.