Abstract
Zinc is an essential mineral of which its functions have potential implications on exercise performance and beneficial adaptations of physical activity. While the effects of aerobic exercise on zinc metabolism acutely have been well described, the effect of long-term exercise training on zinc status remains unclear. The present review aims to determine the effects of exercise training on markers of zinc status in an apparently healthy adult population. We conducted a systematic literature search on PubMed, Scopus, SPORTDiscus and Cochrane Library from inception to 28 January 2016 to identify interventional or cohort studies that investigated the effects of exercise training on indices of zinc status. Pairwise comparisons of mean differences in within-group change were calculated and summarised visually in forest plots. Six studies satisfied the inclusion criteria for the systematic review, of which 5 studies included data on changes in serum zinc concentrations and 3 studies provided changes in dietary zinc intake. Two comparisons showed significantly higher increase of serum zinc concentrations in the exercise group compared to control, while one comparison reported significantly lower change in serum zinc for the exercising group. The exercise groups consumed significantly higher dietary zinc compared to controls in two comparisons. The present review revealed an incomplete evidence base in evaluating the effect of long-term exercise training on markers of zinc status. Further well-designed investigations are required to elucidate the relationship for establishment of dietary recommendation in populations who are continuing exercise interventions.
Introduction
Zinc is an essential trace element with numerous metabolic functions [1]. While zinc is found ubiquitously in the human body, a significant portion of zinc is located within the musculoskeletal system [2]. In the context of exercise, zinc provides structural integrity and supports catalytic functions for metalloenzymes, such as carbonic anhydrase, superoxide dismutase (SOD) and lactate dehydrogenase [3]. Furthermore, zinc regulates intracellular signalling pathways and corresponding downstream effects on immune function [4,5] and redox homeostasis [6,7], with potential implications for performance [8] and related metabolic benefits of exercise [9].
Exercise and physical activity have been the cornerstone of lifestyle recommendations for the healthy population and those with chronic diseases [10,11]. The public health recommendations for physical activity are based on the benefits of exercise on metabolic, musculoskeletal and neuromotor health [12]. Metabolic adaptations of exercise can be modulated by nutritional status, such as the availability of macronutrients, specifically protein and carbohydrates [13]. The role of adequate micronutrient status in supporting the beneficial adaptations of exercise has gained research attention [14], particularly with respect to the effects of physical activity on the zinc status and subsequent consequences on exercise performance and metabolic effects [15].
A number of cross-sectional studies have investigated the association between exercise/physical activity and zinc status, in particular the zinc status in athletic and control populations. While some studies showed lower serum zinc concentration in athletes [16,17], other papers report no significant differences in zinc status between athletes and controls [18,19]. The lack of conformity in the results may be driven by factors other than physical activity levels, for example differences in dietary habits between the populations. The current evidence is inconclusive in determining the relationship between exercise and zinc status in cross-sectional data, however the examination of longitudinal and cohort studies may be able to elucidate the potential relationship.
We have previously reported significant acute fluctuations in serum zinc concentrations as a result of a single bout of aerobic exercise [20,21]. The changes in zinc metabolism were proposed to be influenced by the events that occur during exercise and recovery, including leakage of zinc ions from damaged myocytes and exercise-induced inflammatory processes that follow aerobic exercise. Preliminary evidence suggests that training status is a modulating factor for the acute effects of exercise on zinc metabolism [20], but it is currently unclear whether exercise training itself imposes adaptations of zinc status and/or metabolism during exercise. Aerobic exercise has been shown to significantly increase systemic zinc concentration immediately following the exercise bout with decline of zinc concentration to below the baseline values in the hours after aerobic exercise; it is uncertain whether the acute exercise-induced changes in zinc metabolism are sustained in the long term. Therefore, the current review aims to determine the long term effects of exercise training on markers of zinc status in an apparently healthy adult population, as identified by interventional or cohort trials.
Methods
Search strategy
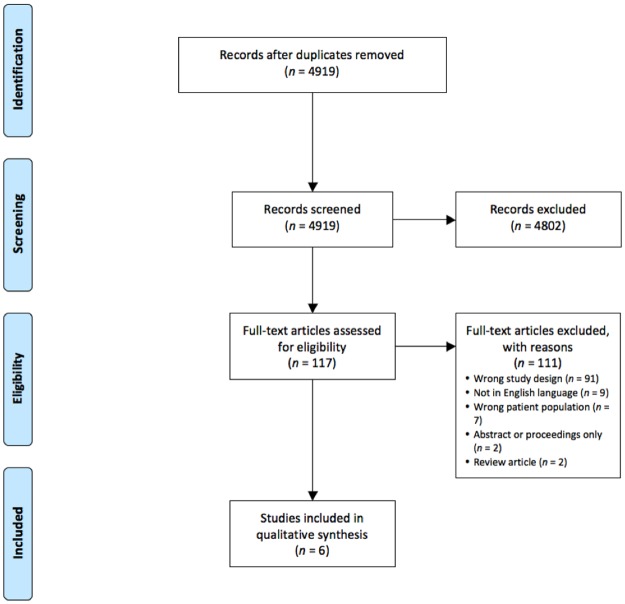
A literature search of PubMed, Scopus, SPORTDiscus and the Cochrane Library electronic databases were conducted from inception to the 28th of January 2016. The search terms included: zinc, exercis*, athlet*, physical activity, train*. Related terms and MeSH terms were used where appropriate. Searches were limited to human subjects and the English language. Fig 1 shows the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) flowchart describing the electronic search outcomes and selection process [22]. Review questions, search strategies and inclusion criteria for this review were prospectively specified and registered with PROSPERO at http://www.crd.york.ac.uk/PROSPERO/ (CRD42015026336).
Fig 1. PRISMA diagram showing the systematic review process.
Study inclusion criteria
Interventional and cohort studies that were published in peer-reviewed journals and examined the effects of exercise training on zinc status were included. Exercise training was defined as an intervention, over a defined period of time, which involves more than one bout of continuous physical activity [23]. Males and females, aged between 18 and 65 years, who were apparently healthy and not diagnosed with any major health condition or illness were included in the present review. The included studies must include a control group and also report indices of zinc status, such as dietary zinc intake, zinc-related enzymes and/or concentrations of zinc measured in serum/plasma, urine, erythrocytes, hair and nail, before and after the exercise training period. Two investigators independently reviewed each citation, and where appropriate, each full report, to determine whether the study satisfy the inclusion criteria.
Data extraction and quality assessment of included studies
Two investigators extracted published data from all included studies independently, and any differences were resolved by discussion. Data extracted included descriptive information, such as the study authors, year of publication, country where the study was conducted, the number, sex and other characteristics of the control and study populations. The duration, intensity and mode of exercise performed in the study were detailed in the data extraction process. Primary outcomes extracted were plasma/serum zinc concentration and dietary zinc intake, before and following exercise training. Plasma and serum concentrations were grouped to represent systemic zinc concentration; in this report the term “serum” will be used to represent both serum and/or plasma. Other potential markers of zinc status, including zinc concentrations in urine and erythrocyte, and activity or concentration of Cu-Zn superoxide dismutase were extracted as secondary outcomes.
Risk of bias assessment
All included studies were assessed for risk of biases that are applicable for non-randomised interventional studies as recommended by the Grading of Recommendations Assessment, Development and Evaluation (GRADE) guidelines [24]. Two independent reviewers assessed the potential risk of biases including recruitment bias, valid measurement of primary outcomes (serum zinc concentration and dietary zinc intake), incomplete accounting for participants in control or exercising groups, and selective outcome reporting. The ratings of risk included low, unclear and high. Studies with exercise group matched with control group in age, sex and body composition represent a low risk of bias in recruitment. Valid measurement of serum zinc was determined by the use of trace element free collection tubes, appropriate method of analysis i.e. atomic absorption spectroscopy or inductively coupled plasma mass spectroscopy, and timing of blood collection. Similarly, low risk of bias for the valid measurement of dietary zinc was achieved by the use of appropriate method of collection (food record or 24 h recall), reporting of energy or macronutrient intake and consideration of supplemental intake. Data on risk of bias in individual studies were entered into Review Manager 5.3 [25].
Statistical analyses
Changes in the primary outcome measures of serum zinc concentration and dietary zinc intake were calculated by subtracting final from baseline values, with calculated estimation of SD of change using an assumed correlation of 0.7 between the two values [26]. The differences between exercise and control groups were calculated by subtracting pre-post change in the exercise group from pre-post change in the control group. Standard error of between-group differences was estimated from calculation using SD of change and number of participants in the two groups. Pairwise comparisons of mean differences in change between the exercise and control groups were calculated in Review Manager 5.3 [25] and summarised visually in forest plots. Meta-analysis for the effect of exercise training on zinc status was deemed inappropriate due to the variations in the exercise training reported.
Results
The electronic database search identified a total of 4919 citations following removal of duplicates. After initial screening of titles and abstracts, 4802 citations were excluded as they were irrelevant to the current review. Of the remaining 117 full texts assessed for eligibility, six studies met the inclusion criteria. Fig 1 shows the details of study selection and reasons for full text exclusion.
Study characteristics
The characteristics of studies included in the current review are described in Table 1. Three of the included studies were prescribed exercise interventions [27–29] whereby two studies assigned specific exercise interventions to sedentary [28] or athletic populations [29]; another study randomised untrained men into either an exercise group or a control group [27]. The remaining three cohort studies examined athletes before and after a period of time that was relevant for their sport, i.e. competition season [30,31] and a 20 day sailing race [32]. The types of exercise in the included studies were heterogeneous; five of the included studies included exercise that is mostly in aerobic activity [28–32] while one study included a resistance exercise program [27]. The duration of the exercise training was between 20 days and 6 months. The number of total participants in each study ranged from 20 to 75. The majority of the studies examined males [27,29,30,32], with the exception of two studies [28,31]. The relevant zinc outcomes reported in the included studies comprise of zinc concentration in serum [28,30–32], urine [30] and erythrocyte [28], dietary zinc intake [27,30,31], and erythrocyte Cu,Zn- SOD [27,31]. Other zinc-dependent enzymes, such as lactate dehydrogenase or carbonic anhydrase, were not reported in the included studies.
Table 1. Characteristics of included studies.
| Study (author, year) | Study type1 | Study group | Control group | Study n | Study Age (y)2 | Control n | Control Age (y) | Sex (M/F) | Exercise training | Indices of zinc status |
|---|---|---|---|---|---|---|---|---|---|---|
| Azizbeigi et al. 2013 | I, R | Untrained men | Untrained men | 10 | 21.1 ± 2.1 | 10 | 23.3 ± 2.5 | M | Progressive resistance exercise training 3 x/week for 8 weeks | Dietary, SOD3 |
| Cordova & Navas 1998 | C | Spanish League of Volleyball players | Moderately trained university students | 12 | 25.9 ± 2.6 | 12 | 22.3 ± 1.2 | M | Volleyball season training, 5 h/d, 7 x/week, approximately 8 weeks | Dietary, serum, urine |
| Fogelholm et al. 1991 | C | Sailors | Bank clerks | 14 | 28 ± 0.27 | 11 | 33 ± 0.6 | M | Transatlantic sailing for 20 d | Serum |
| Fogelholm 1992 | I, NR | University students | University students | 21 | 24 ± 0.6 | 18 | 26 ± 0.6 | F | Progressive aerobic exercise training for 24 weeks, from 2 x/week to 6 x/week, 30–45 min/d at 60–80% HRR | Serum, erythrocyte |
| Lukaski et al. 1990 | C | Varsity swimmers | Non-training university students | 13 | NS | 15 | NS | M | Competitive swimming season for 24 weeks, not specified | Dietary, plasma, SOD |
| Varsity swimmers | Non-training university students | 16 | NS | 13 | NS | F | Competitive swimming season for 24 weeks, not specified | Dietary, plasma, SOD | ||
| Peake et al. 2003 | I, NR | Well-trained distance runners | Sedentary males | 10 | 28 ± 7 | 7 | 21 ± 0 | M | 16% increase in running training volume over 4 weeks | Plasma |
1 C, cohort; I, interventional; NR, not randomised; R, randomised;
2 presented as mean ± SD; NS, not specified;
3 SOD, superoxide dismutase
Change in serum zinc concentration
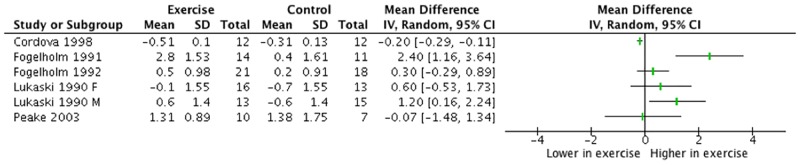
Five studies [28–32] provided data on changes in serum zinc concentrations for six comparisons. Statistical significance of between-group changes in serum zinc concentration were not reported for three studies [29–31], while one study reported non-significant increases in serum zinc concentration in both exercise and control groups [28]. One study found a significantly higher change in serum zinc concentration in the exercising group compared to the control group [32]. Fig 2 shows a forest plot that summaries the mean difference ± 95% CI in serum zinc concentration between the exercise and control groups. The mean differences of change ranged from -0.20 to 2.40 μmol/L; two out of six comparisons elicited significantly higher increase of serum zinc concentrations in the exercise group compared to control [31,32], while one comparison reported significantly lower change in serum zinc for the exercising group [30]. Two studies reported serum zinc concentration with comparison to the reference range. No participants presented with plasma zinc concentration below the reference range in Lukaski et al.’s study. A small percentage of participants displayed low serum zinc concentration in another study [28], with 5% of the exercise group presenting with low serum zinc at all time points and 6% of the control group only at the end of the intervention period.
Fig 2. Pairwise comparisons of the change in serum zinc concentration (μmol/L) between exercise and control groups in interventional trials.
Data are presented as mean difference (95% CI).
Change in dietary zinc intake
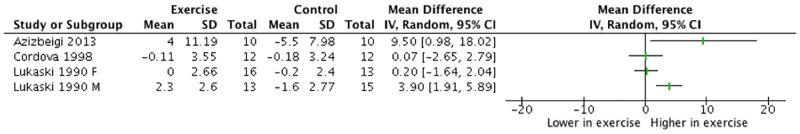
Three studies [27,30,31] provided data on change in dietary zinc intake for four comparisons. The results from the included studies did not include statistical differences of the change in dietary zinc intake between the exercise and control group. Forest plot summarising the calculated between group mean difference ± 95% CI of dietary zinc intake is shown in Fig 3. The mean differences of change ranged from 0.07 to 9.5 mg/d; the exercising groups consumed significantly higher dietary zinc compared to controls in two out of four comparisons. Two studies reported dietary zinc intake of participants with comparisons to the recommended intakes. Lukaski et al. [31] reported dietary zinc intake of control females only to be lower than 67% of RDI at both preseason and postseason. Fogelholm [28] reported that 21% of those in the exercising group were consuming dietary zinc intakes below the Nordic recommendations during the intervention; similarly, 27% of the control group presented with low dietary zinc intake. In the only study that reported zinc density [31], no significant changes were noted in zinc density within the diets before and after the competitive swimming season in both male and female participants.
Fig 3. Pairwise comparisons of the change in dietary zinc intake (mg/d) between exercise and control groups in interventional trials.
Data are presented as mean difference (95% CI).
Other outcomes (RBC and urinary zinc concentrations and, erythrocyte Cu, Zn-SOD)
Results for other zinc outcomes are presented in Table 2. One study reported significant increases in erythrocyte zinc concentration of the exercise group compared to the control group [28]. Another study reported urinary zinc losses, however no statistical significance was reported [30]. Two studies [27,31] reported data on changes in erythrocyte Cu, Zn-SOD activity for three comparisons (Table 2). Significant increase in SOD activity following exercise was reported in the exercise group compared to the control group in one study [27].
Table 2. Change in erythrocyte zinc concentration, urinary zinc loss and Cu,Zn SOD activity in the included studies.
| Study (author, year) | Outcomes | Change in control group | Change in exercise group | Units | Statistical significance of between group difference |
|---|---|---|---|---|---|
| Cordova & Navas 1998 | Urinary zinc excretion | -15 ± 208.68 | 145 ± 173.36 | μg/day | NR |
| Fogelholm 1992 | Erythrocyte zinc concentration | -0.01 ± 0.003 | 0.05 ± 0.004 | μmol/g Hb | < 0.001 |
| Azizbeigi et al. 2013 | Erythrocyte Cu, Zn-SOD | -48.73 ± 232.65 | 127.53 ± 154.66 | U/g Hb | 0.014 |
| Lukaski et al. 1990 M | -145 ± 379.66 | 795 ± 387.31 | U/g Hb | NR | |
| Lukaski et al. 1990 F | -197 ± 347.33 | 1566 ± 413.22 | U/g Hb | NR |
Hb, haemoglobin; NR, not reported; SOD, superoxide dismutase
Risk of bias assessment
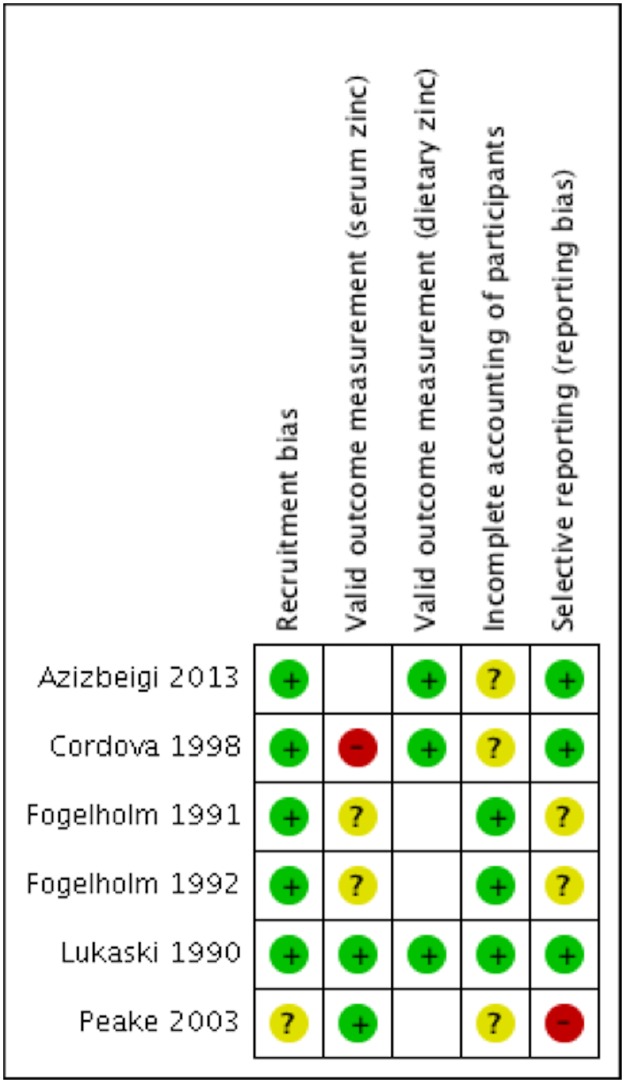
Fig 4 shows the risk of biases summary for all studies. The support for judgements is presented in S1 Table. The majority of the studies demonstrated low risks of bias for recruitment with control group matched in age, sex and BMI with the exercise group [27,28,30–32]. All included studies scored low or unclear risks of bias for valid measurement of serum zinc, with the exception of one study scoring high risk of bias, where non-fasting blood samples were collected for the analysis of serum zinc [30]. Similarly, all of the studies presented with low risk of bias for valid measurement of dietary zinc. Most of the studies reported follow up data for all participants thereby scoring low risk of bias for incomplete accounting of participants. The majority of the studies reported all relevant outcomes with the exception of one study that failed to report measured dietary intake data [29] and another study which did not present longitudinal changes of dietary zinc intake over the intervention period [28].
Fig 4. Risk of bias summary judgements on each risk of bias item for each included study.
Green (+) symbols represent low risk of bias for the specific criteria for the included study. Yellow (?) symbols represent unclear risk of bias and red (-) symbols denote high risk of bias. Support for judgements is presented in S1 Table.
Discussion
The current review of interventional and cohort studies has identified incomplete evidence for the effects of exercise training on zinc status in an apparently healthy adult population. In total, six studies satisfied the inclusion criteria for the present analysis. The limited evidence suggests that exercise is associated with higher dietary zinc intake and erythrocyte SOD activity; while minimal differences were observed in serum zinc concentrations. We have revealed distinct gaps in the current literature regarding the effects of exercise training on zinc status with implications on establishing dietary zinc requirements in populations that exercise regularly.
The studies included in the current review consisted of exercise interventions that are dissimilar in mode, duration and intensity. Three out of six studies included exercise that is predominately aerobic in nature, for example running and swimming [28,29,31]. A mix of aerobic and anaerobic activities were used in two studies [30,32], while another study utilised resistance exercise training only [27]. The heterogeneity in exercise interventions in the included studies contributed to the variance of the outcomes measured. Further, while changes in early markers of exercise training are evident within hours following the first exercise session [33], repeated and progressive exercise bouts are required to develop and maintain beneficial adaptations of exercise. The duration of exercise interventions described in the current analysis ranged from 3 to 24 weeks; therefore, there may be substantial differences in the extent of exercise adaptations presented by the included studies. Combined with the small number of studies in the current review, a quantitative summation of the changes in zinc status, beyond pairwise analyses, was deemed inappropriate.
Based on six comparisons for exercise-induced changes in serum zinc concentration, the estimated mean difference of change for two comparisons indicated that exercise induces significant increase in serum zinc; while one comparison suggested that serum zinc changes are lower in the exercise group compared to control. The current evidence suggests equivocal changes in serum zinc concentration as a result of exercise interventions. The limitations of serum zinc concentration as a marker of zinc status in humans are well described [34]; changes in a multitude of factors, such as inflammation, hormones and age can affect the relationship between zinc status and serum zinc concentration. As such, the majority of studies reported other measures of zinc status, such as erythrocyte and urinary zinc concentration, to describe changes in zinc metabolism under exercise training.
The most commonly measured zinc outcome, other than serum zinc concentration, was erythrocyte Cu, Zn-SOD activity. SOD plays a key role in antioxidative activity by providing catalytic function for the disassociation of the radical oxygen species, superoxide, into oxygen or hydrogen peroxide. In erythrocytes where oxygen exchange occurs with haemoglobin, significant intracellular oxidative stress is induced by exercise acutely through increases in oxygen consumption [35]. As a result, exercise training per se can induce significant increases in SOD activity, with erythrocyte SOD correlating positively to training status [36]. It is unclear from the current evidence whether dietary zinc intake and/or baseline zinc status may influence the exercise-induced increases in erythrocyte SOD activity. Further, while copper status may influence the enzymatic activity of SOD, only two of the included studies reported copper intake of the study populations. Future studies will benefit from the inclusion of copper status to further elucidate the relationship between zinc status and SOD activity. Moreover, the examination of other zinc-dependent enzymes, such as lactate dehydrogenase or carbonic anhydrase, may provide insights into the effects of regular exercise and zinc intake on the enzymatic functions of zinc.
Exercise also impacts on immunity, both acutely and chronically. Following a bout of prolonged high intensity exercise, acute decreases in multiple components of immune function are evident including the reductions in B lymphocyte production of immunoglobulins and antigen-presenting capacity [37]. Whilst the greatest effects of exercise on immune functions are observed following high intensity exercise, prolonged moderate intensity exercise may also exert clinically significant changes in immunity [38]. The acute fluctuations in immune functions contribute to the increased susceptibility to potential pathogens in the hours following exercise. As one of the functions of zinc is to support innate and adaptive immune function, the provision of zinc deficient diets has been associated with lowered immunity in humans [39]. However, the evidence for zinc status in modulating exercise-induced immune changes is less clear. The included study by Peake et al. failed to find significant relationships between plasma zinc levels and immune outcomes following a period of increased training in runners [29]. Further investigations into the relationships between zinc status, immune function and exercise are required.
On the basis of four comparisons, dietary zinc intake appears to be higher in exercise groups compared to control in two comparisons; therefore, we deemed the effects of exercise training on dietary zinc intake to be equivocal. In a study by Lukaski et al., while dietary zinc intake increased in male swimmers over the competition period, zinc density (mg/MJ) remained unchanged [31]. This suggests that higher total zinc intake derived from increased amount of food consumed. In the maintenance of homeostasis, exercise and associated energy expenditure can impact on food and energy intake [40]. It is difficult to elucidate the influence of changing dietary patterns, as a result of exercise, on zinc status and related outcomes. The majority of the included studies did not provide details on the sources of zinc intake or its potential impact on gastrointestinal zinc absorption and zinc status. Further, a number of studies collected dietary data but failed to report quantitative values for dietary zinc intake before and following exercise intervention. The selective reporting of outcomes limited the number of comparisons and evidence available in the present review.
All included studies within this review were low in number of participants and therefore contributed to the limited power of the comparisons in determining the effect of exercise training on markers of zinc status. Further, four out of six included studies recruited men only; similar level of sex bias were noted previously [21], highlighting the sex bias that exists in health research [41]. Moreover, the current review included mostly participants who were highly trained at baseline, for example volleyball players and swimmers. Therefore, the results of this analysis may not be applicable for untrained populations who are initiating exercise training protocols. The implications of the effects on zinc metabolism from initiating exercise training as part of a lifestyle program in patients with chronic diseases, such as diabetes, may be an important consideration particularly in populations at risk of marginal zinc status.
This review is the first to determine the effects of exercise training on markers of zinc status in an adult population. The current report extends our knowledge of the acute effects of exercise on markers of zinc metabolism [20,21]. In the previous reports we described acute fluctuations in serum zinc concentrations in the hours following a bout of aerobic activity, thereby providing the basis to determine the potential long term effects of repeated bouts of exercise on markers of zinc status. One of the strengths of the present review is the determination of risk of biases as per the recommendations by the GRADE working party [24]. In our evaluation, a number of risk of biases were identified, including factors that have implications in the valid measurement of serum zinc concentration, and the risk of selective reporting, particularly in regards to dietary zinc intake. As serum zinc concentrations are affected by numerous factors, including diurnal fluctuations, fasting status and inflammation [1], it is important for investigators to control for potential confounding variables in the determination of the relationship between exercise and serum zinc concentrations. Further, the omission of dietary zinc intake at baseline and following exercise interventions in some studies does not allow for the determination of the effect of exercise on dietary intake and the potential modulation of diet on exercise-induced changes in zinc metabolism. Future studies should consider including additional information regarding zinc density, i.e. amount of dietary zinc compared to energy intake, thereby furthering the understanding of the relationship between energy and zinc intake.
The present review revealed an incomplete evidence base in evaluating the effect of long term exercise training on markers of zinc status. Further, the strength of the presented evidence is limited by the majority of study designs reported being non-randomised or cohort studies. The limited evidence suggests that dietary zinc intake may increase in those who are physically active, by homeostatic adjustments for increases in energy expenditure. Additional studies, with different study populations such as those participating in moderate levels of physical activity, are required to extend the evidence to the general population. Future evaluation on the effects of zinc intake on physical performance will present implications for clinical sports nutrition practice. Exercise training was found to be associated with increases in SOD activity as part of the adaptations of exercise, consistent with the current literature [35]. As the turnover of erythrocyte SOD requires sufficient zinc status to support the adaptations of exercise, the current dietary recommendations for populations who are initiating or continuing exercise intervention should be to consume dietary zinc levels, to at least the Recommended Daily Intake [42].
Supporting information
(DOCX)
(DOC)
(XLSX)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Samman S. Zinc. Nutr Diet. 2007;64: S131–S134. doi: 10.1111/j.1747-0080.2007.00200.x [Google Scholar]
- 2.Jackson M. Physiology of zinc: general aspects In: Mills C, editor. Zinc in Human Biology. New York, NY: Springer-Verlag; 1989. pp. 1–14. [Google Scholar]
- 3.Vallee BL, Falchuk KH. The biochemical basis of zinc physiology. Physiol Rev. 1993;73: 79–118. [DOI] [PubMed] [Google Scholar]
- 4.Haase H, Rink L. Functional significance of zinc-related signaling pathways in immune cells. Annu Rev Nutr. 2009;29: 133–52. doi: 10.1146/annurev-nutr-080508-141119 [DOI] [PubMed] [Google Scholar]
- 5.Foster M, Samman S. Zinc and regulation of inflammatory cytokines: implications for cardiometabolic disease. Nutrients. 2012;4: 676–94. doi: 10.3390/nu4070676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oteiza PI. Zinc and the modulation of redox homeostasis. Free Radic Biol Med. 2012;53: 1748–1759. doi: 10.1016/j.freeradbiomed.2012.08.568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Foster M, Samman S. Zinc and redox signaling: perturbations associated with cardiovascular disease and diabetes mellitus. Antioxid Redox Signal. 2010;13: 1549–73. doi: 10.1089/ars.2010.3111 [DOI] [PubMed] [Google Scholar]
- 8.Lukaski HC. Low dietary zinc decreases erythrocyte carbonic anhydrase activities and impairs cardiorespiratory function in men during exercise. Am J Clin Nutr. 2005;81: 1045–1051. [DOI] [PubMed] [Google Scholar]
- 9.Chu A, Foster M, Samman S. Determinants of zinc transport in humans: zinc status, exercise, inflammation and chronic diseases In: Ostojic S, editor. Human health and nutrition: new research. New York: Nova Science; 2015. pp. 17–48. [Google Scholar]
- 10.Eckel RH, Jakicic JM, Ard JD, De Jesus JM, Houston Miller N, Hubbard VS, et al. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: A report of the American College of cardiology/American Heart Association task force on practice guidelines. Circulation. 2014;129: 76–99. doi: 10.1161/01.cir.0000437740.48606.d1 [Google Scholar]
- 11.Colberg SR, Sigal RJ, Fernhall B, Regensteiner JG, Blissmer BJ, Rubin RR, et al. Exercise and type 2 diabetes: the American College of Sports Medicine and the American Diabetes Association: joint position statement. Diabetes Care. 2010;33: e147–67. doi: 10.2337/dc10-9990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garber CE, Blissmer B, Deschenes MR, Franklin B a., Lamonte MJ, Lee IM, et al. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: Guidance for prescribing exercise. Med Sci Sports Exerc. 2011;43: 1334–1359. doi: 10.1249/MSS.0b013e318213fefb [DOI] [PubMed] [Google Scholar]
- 13.Smiles WJ, Hawley JA, Camera DM. Effects of skeletal muscle energy availability on protein turnover responses to exercise. J Exp Biol. 2016;219: 214–225. doi: 10.1242/jeb.125104 [DOI] [PubMed] [Google Scholar]
- 14.Margaritis I, Rousseau A. Does physical exercise modify antioxidant requirements? Nutr Res Rev. 2008;21: 3–12. doi: 10.1017/S0954422408018076 [DOI] [PubMed] [Google Scholar]
- 15.Ganapathy S, Volpe S. Zinc, exercise, and thyroid hormone function. Crit Rev food Sci …. 1999; 37–41. [DOI] [PubMed] [Google Scholar]
- 16.Arikan S, Akkus H, Halifeoglu I, Baltaci AK, Arikan S., Akkus H., et al. Comparison of plasma leptin and zinc levels in elite athletes and sedentary people. Cell Biochem Funct. 2008;26: 655–658. doi: 10.1002/cbf.1480 [DOI] [PubMed] [Google Scholar]
- 17.Haralambie G. Serum zinc in athletes in training. Int J Sport Med. 1981; [DOI] [PubMed] [Google Scholar]
- 18.Nuviala RJ, Lapieza MG, Bernal E. Magnesium, zinc, and copper status in women involved in different sports. Int J Sport Nutr. 1999;9: 295–309. [DOI] [PubMed] [Google Scholar]
- 19.Crespo R, Relea P, Lozano D, Macarro-Sanchez M, Usabuaga J, Villa L, et al. Biochemical markers of nutrition in elite-marathon runners. J Sport Med Phys Fit. 1995;35: 268–72. [PubMed] [Google Scholar]
- 20.Chu A, Petocz P, Samman S. Immediate Effects of Aerobic Exercise on Plasma/Serum Zinc Levels: A Meta-analysis. Med Sci Sports Exerc. 2016;48: 726–733. doi: 10.1249/MSS.0000000000000805 [DOI] [PubMed] [Google Scholar]
- 21.Chu A, Petocz P, Samman S. Plasma/Serum Zinc Status During Aerobic Exercise Recovery: A Systematic Review and Meta-Analysis. Sports Med. 2017;47: 127–134. doi: 10.1007/s40279-016-0567-0 [DOI] [PubMed] [Google Scholar]
- 22.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP a, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6: e1000100 doi: 10.1371/journal.pmed.1000100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boulé NG, Kenny GP, Haddad E, Wells GA, Sigal RJ. Meta-analysis of the effect of structured exercise training on cardiorespiratory fitness in Type 2 diabetes mellitus. Diabetologia. 2003;46: 1071–1081. doi: 10.1007/s00125-003-1160-2 [DOI] [PubMed] [Google Scholar]
- 24.Guyatt GH, Oxman AD, Vist G, Kunz R, Brozek J, Alonso-Coello P, et al. GRADE guidelines: 4. Rating the quality of evidence—study limitations (risk of bias). J Clin Epidemiol. 2011;64: 407–415. doi: 10.1016/j.jclinepi.2010.07.017 [DOI] [PubMed] [Google Scholar]
- 25.The Cochrane Collaboration. Review Manager (RevMan). Copenhagen: The Nordic Cochrane Centre; 2014. [Google Scholar]
- 26.The Cochrane Collaboration. Cochrane handbook for systematic reviews of interventions. 5.1.0. Higgins J, Green S, editors. 2011.
- 27.Azizbeigi K, Azarbayjni MA, Peeri M, Agha-Alinejad H, Stannard S. The Effect of Progressive Resistance Training on Oxidative Stress and Antioxidant Enzyme Activity in Erythrocytes in Untrained Men. Int J Sport Nutr Exerc Metab. 2013;23: 230–238. [DOI] [PubMed] [Google Scholar]
- 28.Fogelholm M. Micronutrient status in females during a 24-week fitness-type exercise program. Ann Nutr Metab. 1992;36: 209–218. [DOI] [PubMed] [Google Scholar]
- 29.Peake JM, Gerrard DF, Griffin JFT. Plasma zinc and immune markers in runners in response to a moderate increase in training volume. Int J Sports Med. 2003;24: 212–216. doi: 10.1055/s-2003-39094 [DOI] [PubMed] [Google Scholar]
- 30.Córdova A, Navas FJ. Effect of training on zinc metabolism: changes in serum and sweat zinc concentrations in sportsmen. Ann Nutr Metab. 1998;42: 274–282. [DOI] [PubMed] [Google Scholar]
- 31.Lukaski HC, Hoverson BS, Gallagher SK, Bolonchuk WW. Physical training and copper, iron, and zinc status of swimmers. Am J Clin Nutr. 1990;51: 1093–9. [DOI] [PubMed] [Google Scholar]
- 32.Fogelholm GM, Lahtinen PK. Nutritional evaluation of a sailing crew during a transatlantic race. Scand J Med Sci Sports. 1991;1: 99–103. doi: 10.1111/j.1600-0838.1991.tb00278.x [Google Scholar]
- 33.Hawley JA, Hargreaves M, Joyner MJ, Zierath JR. Integrative Biology of Exercise. Cell. 2014;159: 738–749. doi: 10.1016/j.cell.2014.10.029 [DOI] [PubMed] [Google Scholar]
- 34.Lowe NM, Dykes FC, Skinner A-L, Patel S, Warthon-Medina M, Decsi T, et al. EURRECA-Estimating zinc requirements for deriving dietary reference values. Crit Rev Food Sci Nutr. 2013;53: 1110–23. doi: 10.1080/10408398.2012.742863 [DOI] [PubMed] [Google Scholar]
- 35.Nikolaidis MG, Jamurtas AZ. Blood as a reactive species generator and redox status regulator during exercise. Arch Biochem Biophys. 2009;490: 77–84. doi: 10.1016/j.abb.2009.08.015 [DOI] [PubMed] [Google Scholar]
- 36.Speich M, Pineau a, Ballereau F. Minerals, trace elements and related biological variables in athletes and during physical activity. Clin Chim Acta. 2001;312: 1–11. [DOI] [PubMed] [Google Scholar]
- 37.Gleeson M. Immune function in sport and exercise. J Appl Physiol. 2007;103: 693–9. doi: 10.1152/japplphysiol.00008.2007 [DOI] [PubMed] [Google Scholar]
- 38.Diment BC, Fortes MB, Edwards JP, Hanstock HG, Ward MD, Dunstall HM, et al. Exercise intensity and duration effects on in vivo immunity. Med Sci Sports Exerc. 2015;47: 1390–1398. doi: 10.1249/MSS.0000000000000562 [DOI] [PubMed] [Google Scholar]
- 39.Beck FW, Prasad AS, Kaplan J, Fitzgerald JT, Brewer GJ. Changes in cytokine production and T cell subpopulations in experimentally induced zinc-deficient humans. Am J Physiol. 1997;272: E1002–E1007. [DOI] [PubMed] [Google Scholar]
- 40.Manore MM. Weight Management for Athletes and Active Individuals: A Brief Review. Sport Med. Springer International Publishing; 2015;45: 83–92. doi: 10.1007/s40279-015-0401-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim AM, Tingen CM, Woodruff TK. Sex bias in trials and treatment must end. Nature. 2010;465: 688–9. doi: 10.1038/465688a [DOI] [PubMed] [Google Scholar]
- 42.National Health and Medical Research Council. Nutrient Reference Values for Australia and New Zealand Including Recommended Dietary Intakes. 2006.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOC)
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.