Abstract
Subcutaneous specific immunotherapy (SCIT) has been shown to modify the Dermatophagoides pteronissinus (DP) allergic response, characterized by generation of Treg cells. However, studies have reported no changes in the proportion of Treg cells after immunotherapy, indicating that the effects may be due to modifications in their regulatory activities. We aimed to determine whether Tregs generated by DP-SCIT can switch the allergic response to tolerant and study the involvement of suppressive cytokines on it. Twenty-four DP-allergic rhinitis patients were recruited, 16 treated with DP-SCIT and 8 untreated. Treg and T effector cells were isolated before and after DP-SCIT, and cocultured in different combinations with α-IL-10, α-TGF-β blocking antibodies and nDer p 1. Treg cells after DP-SCIT increased Th1 and decreased Th2 and Th9 proliferation. Similarly, they increased IL-10 and decreased IL-4 and IL-9-producing cells. α-IL-10 affected the activity of Treg cells obtained after DP-SCIT only. Finally, DP-specific IgG4 levels, Treg percentage and IL-10 production were correlated after DP-SCIT. These results demonstrate that DP-SCIT induces Treg cells with different suppressive activities. These changes could be mediated by IL-10 production and appear to play an important role in the induction of the tolerance response leading to a clinical improvement of symptoms.
Introduction
Allergic rhinitis (AR) can affect up to 30% of the population and its prevalence is increasing1. Frequently, AR presents comorbidities such as allergic conjunctivitis and other respiratory disorders like rhinosinusitis and asthma2,3. It is typically caused by common aeroallergens derived from grass, birch, pet or house dust mites4,5. It can greatly affect quality of life and be a burden to public health systems6 due in part to the cost of pharmacological control of symptoms.
Adaptive immune reactions can occur in AR, being T lymphocytes, particularly Th2 (producing IL-4, IL-5 and IL-13, amongst others) and Th9 cells (producing IL-9 and IL-10)7 the main effector T cells (Teff). These cells have an essential role in the induction of other effector cells involved in AR-related inflammation, such as mast cells, basophils and eosinophils8,9.
Specific immunotherapy (sIT) with allergens such as grass pollen or Dermatophagoides pteronyssinus (DP) is an effective treatment for IgE-mediated allergic respiratory diseases10, inducing long-term clinical benefits and immunological tolerance11,12. The underlying immunological mechanisms of sIT include immune deviation from a Th2 to Th1 cell pattern13, blocking antibody production14 and regulatory T cell (Treg) induction15,16. Moreover, as shown for DP subcutaneous immunotherapy (DP-SCIT) in patients with AR, clinically effective sIT is correlated with immunological changes at both the humoral and cellular levels. The former consists of a decreased ratio of specific IgE/IgG4 and an increase in sIgG4. The latter consists of a decreased effector cell response for cells such as Th2, Th9 and Th17 as well as inflammatory plasma cells, and increased levels of Th1 cells17,18. Similar results have been found at the humoral level for DP-SCIT in patients with the AR subphenotype, local AR19.
It is well known that allergen-specific Tregs have an important role in the immunological induction of tolerance observed during sIT. They are able to inhibit the activation, proliferation and effector functions of a wide range of target cells including innate cells, antigen-presenting cells and Teff cells (mainly Th2 and Th9)20. Tregs also release cytokines such as IL-10 and TGF-β, which have key suppressive activities21,22. Different subsets of Tregs have been described depending on their origin: the natural Tregs (nTreg), which originate in the thymus and are the major cell population for the control of immune self-tolerance; and induced Tregs (iTreg), which are peripherally generated from naïve CD4+ T-cells in response to foreign antigens, during sIT and also in vitro 23. These Treg subsets use different suppression mechanisms: while nTregs predominantly use cell-cell contact-dependent mechanisms, iTreg suppression is generally performed via immunomodulatory cytokines such as IL-10 and TGF-β24. Tregs are characterized by the Foxp3 protein, which is a member of the forkhead transcription factor family. This protein plays an important role in the regulation of the expression of genes involved in many Treg cell processes25.
Although several studies have attributed the beneficial clinical effects during sIT to an increase in the Treg population26,27, other studies did not find this change28,29. Moreover, many authors claim that the efficacy is largely related to changes in the number of IL-10 producing T-cells30, suppressing Th2 production of IL-4 and leading to reduced IgE production by plasma cells17. We postulate that various mechanisms of regulation occur during sIT, affecting the function of allergen-specific-Tregs and leading to the suppression of the Th2-type response. Therefore, the effectiveness of sIT could be due not only to the increased percentage of Treg cells but also to their enhanced activity. Whether a reduced capacity of Tregs to suppress the allergen-specific T-cell response in allergic-patients is modified during sIT needs to be established. We hypothesise that DP-SCIT will induce Tregs with a high suppressive activity that acts on effector Th2 cells, shunting the immunological profile towards a Th1/Treg pattern and a tolerant response. To explore this, we have determined the changes in Treg activity produced after 1-year of DP-SCIT in a group of patients with AR to DP compared to a group of untreated patients. More specifically, we examined the proliferation of Th1/Th2/Th9 cells and IL-10, IL-4 and IL-9 cytokine producing-cells in different combinations of Treg and Teff cells, before and after DP-SCIT, as a primary endpoint. We also examined the effects of IL-10 and TGF-β on the proliferation of Th1/Th2/Th9 cells as a secondary endpoint.
Results
Twenty-four patients with AR were included, 16 were to receive DP-SCIT and 8 to remain untreated as controls. The median age of all subjects was 28 (22–30). A total of 8 subjects (33.33%) were female. No significant differences were found in terms of clinical characteristics (sex, age, asthma symptoms, rinoconjunctivitis clinical score symptoms (RCSS), skin prick test (SPT), intradermal test (IDT) and nasal provocation test (NPT)) between groups at baseline. No patients presented any systemic adverse symptoms during DP-SCIT.
After 1 year of DP-SCIT, we observed a significant decrease in IDT area (p = 0.041) and a reduction of RCSS score (p = 0.036). On the contrary, during this period of time, untreated patients showed an increased IDT area and a significant decrease (p = 0.037) in the DP concentration needed to induce a positive NPT, indicating higher reactivity (Table 1).
Table 1.
Changes in clinical characteristics of patients after one year of DP-SCIT.
| 0 months | 12 months | p | |
|---|---|---|---|
| Treated patients (n = 16) | |||
| RCSS | 2.1 (0.46–11.85) | 1.55 (0.82–4.57) | 0.036 |
| Medication score | 0.52 (0–4.67) | 0.25 (0–2.5) | 0.16 |
| IDT DP (mm2) | 168 (64–225) | 100 (48–190) | 0.041 |
| Concentration DP (mg/ml) at positive NPT | 0.04 (0.04–4) | 0.04 (0.04–0.4) | 0.713 |
| Untreated patients (n = 8) | |||
| RCSS | 4.745 (0.5–6.14) | 5.53 (0.92 –7.6) | 0.5 |
| Medication score | 0.07 (0–0.5) | 0.46 (0–2.03) | 0.458 |
| IDT DP (mm2) | 132 (110–324) | 143 (70–225) | 0.716 |
| Concentration DP (mg/ml) at positive NPT | 0.4 (0.04–4) | 0.04 (0.04–0.04) | 0.037 |
RCSS: rhinoconjunctivitis symptom score; IDT: Intradermal test; DP: Dermatophagoides pteronissinus; NPT: Nasal provocation test. Data are represented as medians and range. Statistical analysis for two related samples were carried out by Wilcoxon test; values p ≤ 0.05 are considered statistically significant.
DP-SCIT induces changes in the DP-specific immunoglobulin pattern and stimulates Treg generation
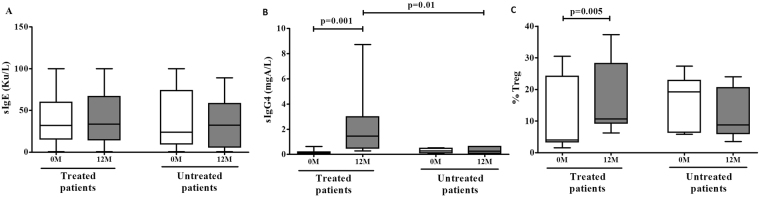
The effects of DP-SCIT on DP-specific immunoglobulin production were evaluated. For this, DP-sIgE and sIgG4 levels were measured in sera samples. No changes were observed for DP-specific IgE (Fig. 1A), however a significant increase in DP-specific IgG4 levels was seen at 12 months (p = 0.001) for treated patients only. Moreover, when we compared treated and untreated patients, although no differences in DP-sIgG4 levels were found between groups for samples taken at 0 months, significantly higher levels of DP-sIgG4 were observed at 12 months (p = 0.01) (Fig. 1B).
Figure 1.
Determination of changes in DP-specific immunoglobulins and Treg populations. (A) DP-sIgE (Ku/L) and (B) DP-sIgG4 (mgA/L) levels for treated and untreated patients before (0 M) and after (12 M) 1-year of DP-SCIT. (C) Comparative analysis of treated (N = 16) and untreated (N = 8) patients before (0 M) and after (12 M) 1-year of treatment. Statistical Wilcoxon (related samples) and Mann-Whitney tests (non-related samples) were performed; p-values ≤ 0.05 are considered statistically significant.
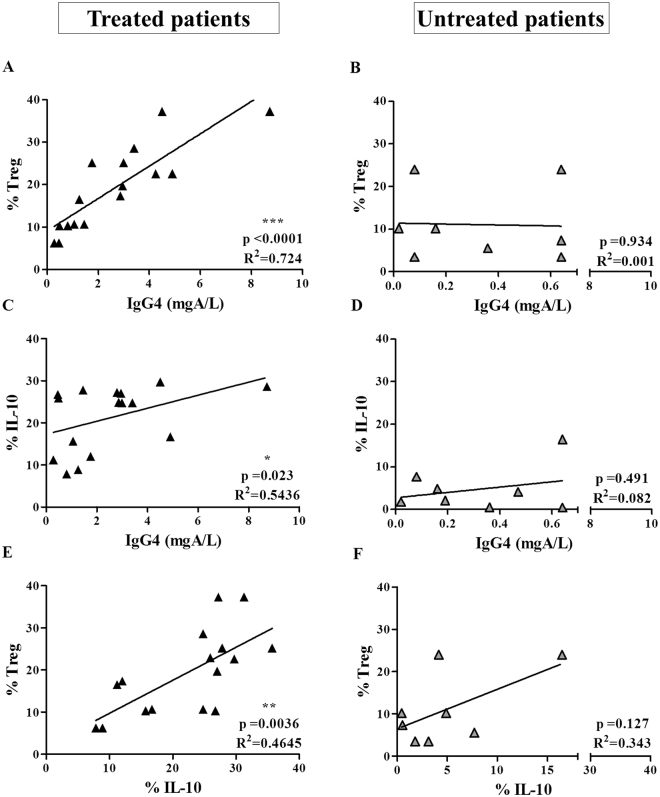
We detected a significant increase in the percentage of Treg cells in peripheral blood mononuclear cells (PBMCs) at 12 months for treated patients (p = 0.005) (Fig. 1C). This can be considered a potential specific marker of successful sIT18. We also found a significant positive correlation of DP-sIgG4 levels with the percentage of Treg cells (Fig. 2A) and IL-10 producing cells (Fig. 2C), and between the percentage of Treg cells and IL-10 producing cells (Fig. 2E) for treated patients after 12 months of DP-SCIT (R2 = 0.724, p < 0.0001; R2 = 0.5436, p = 0.023 and R2 = 0.464, p = 0.0036 respectively). No correlations were found for untreated patients (Fig. 2B,D and F).
Figure 2.
DP-sIgG4 production after 12 months of DP-SCIT correlates with IL-10 production and iTregs. Correlations between percentage of Treg and DP-sIgG4 levels (mgA/L) at 12 months in treated (N = 16) (A) and untreated (N = 8) patients (B). Correlations between percentage of IL-10 secreting cells and DP-sIgG4 levels (mgA/L) at 12 months in treated (N = 16) (C) and untreated (N = 8) patients (D). Correlations between percentage of Treg and percentage of IL-10 secreting cells at 12 months in treated (N = 16) (E) and untreated (N = 8) patients (F). Statistical analysis was carried out using the Pearson correlation coefficient; p-values ≤ 0.05 are considered statistically significant.
The proliferative response to DP by Teff cells is modified by Treg cells induced during DP-SCIT
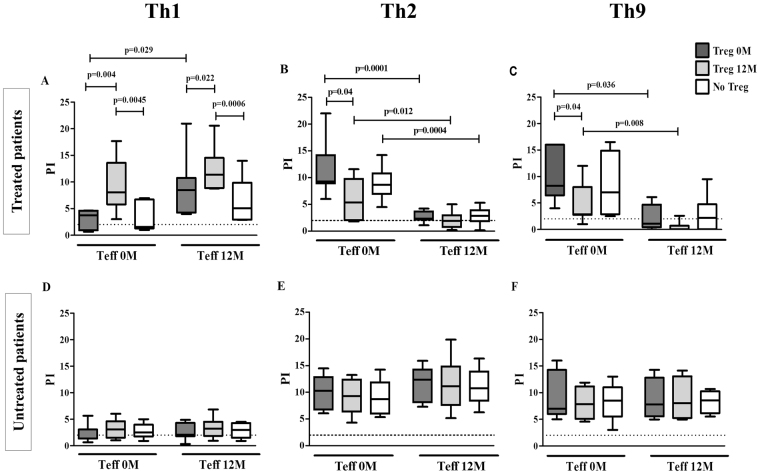
In order to analyze changes in Treg functionality during DP-SCIT, we determined Treg-mediated changes in the proliferative response to DP for different T cell subpopulations. For this purpose, we co-cultured Tregs obtained at 0 and 12 months, with effector T-cells from both time points and determined the proliferative response of these Teff subpopulations. In the treated group, we found that Treg cells obtained at 12 months led to increased Th1 proliferation compared with Treg cells obtained at 0 months and in the absence of Tregs, in cocultures with Teff cells obtained at both 0 months (p = 0.004 and p = 0.0045, respectively) and 12 months (p = 0.022 and p = 0.0006, respectively) (Fig. 3A). Moreover, Treg cells after 12 months led to decreased Th2 and Th9 proliferation compared with Treg cells from 0 months, in cocultures with Teff cells obtained only at 0 months (p = 0.04 in both cases) (Fig. 3B,D, respectively for Th2 and Th9).
Figure 3.
Proliferative response of Teff after DP stimulation in absence or presence of Treg cells. Box plots represent comparative analysis of proliferation index (PI) of different T cell subsets Th1 (A), Th2 (B) and Th9 (C) from treated (N = 16) and Th1 (D), Th2 (E) and Th9 (F) from untreated (N = 8) patients in different combinations of Teff and Treg at different times: 0 or 12 months (0 M and 12 M, respectively). Statistical analysis was carried out by Wilcoxon test; p-values ≤ 0.05 are considered statistically significant. PI: proliferative index.
Regarding the effects of the immunotherapy on Teff cells, we observed a significant increase of Th1 proliferation at 12 months in the treated group when cocultured with Treg cells from 0 months (p = 0.029) (Fig. 3A). The opposite trend was found for Th2 and Th9 cells (p = 0.0001 and p = 0.036, respectively). In addiction Th2 and Th9 proliferation decreased between 0 and 12 months when cocultured with 12 months Treg cells (p = 0.012 and p = 0.008, respectively) (Fig. 3B,C). Th2 proliferation also decreased between 0 and 12 months in the absence of Tregs (p = 0.0004) (Fig. 3B). No changes were observed for untreated patients (Fig. 3D,E and F). Similar results had been published by our group18.
The pattern of effector T cell cytokine production in response to DP is modified by Tregs induced by DP-SCIT
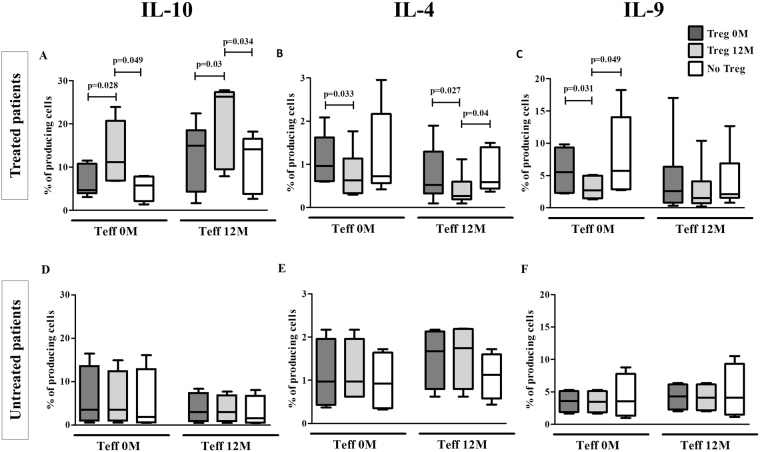
We analyzed the effect of Tregs on cytokine production before and after treatment. For the treated group, we found that Treg cells obtained at 12 months increased the percentage of IL-10-producing cells compared with Tregs obtained at 0 months and in absence of Treg, for cocultures with Teff cells obtained both at 0 months (p = 0.028 and p = 0.049, respectively) and 12 months (p = 0.03 and p = 0.034, respectively) (Fig. 4A).
Figure 4.
Cytokines produced by Teff cells after DP stimulation in absence or presence of Treg cells. Box plots represent comparative analysis of percentages of Teff cells producing IL-10 (CD3+CD4+IL-10+) (A), IL-4 (CD3+CD4+IL-4+) (B) and IL-9 (CD3+CD4+IL-9+) (C) from treated (N = 16) and IL-10 (CD3+CD4+IL-10+) (D), IL-4 (CD3+CD4+IL-4+) (E) and IL-9 (CD3+CD4+IL-9+) (F) from untreated (N = 8) patients in different combinations of Teff and Treg at 0 or 12 months (0 M and 12 M, respectively). Statistical analysis was carried out by Wilcoxon test; p-values ≤ 0.05 are considered statistically significant.
Moreover, Treg cells at 12 months led to a significant decrease in the percentage of IL-4-producing cells in coculture with Teff cells at both 0 and 12 months, compared to the effect induced by Tregs at 0 months (p = 0.033 and p = 0.027, respectively). Similar results were found comparing Teff cells at 12 months in the absence of Treg cells (p = 0.04 for Teff at 12 months) (Fig. 4B). Similarly, Treg cells at 12 months led to a lower percentage of IL-9-producing cells compared to Treg of 0 months and in the absence of Treg, in cocultures with Teff cells obtained at 0 months (p = 0.031 and p = 0.049, respectively) (Fig. 4C). No changes in the percentage of cytokine-producing cells were observed for untreated patients (Fig. 4D–F).
The iTreg suppressive activity is IL-10-dependent
Using blocking antibodies (α-IL-10 and α-TGF-β), we tried to elucidate the potential role of these cytokines in the suppression of the allergic response induced during 1 year of DP-SCIT, and their effects on the proliferation of T-cell subsets.
Results indicated that the presence of these inhibitors only affected the Teff cultures taken at 0 and 12 months, from treated patients and when cocultured with Tregs at 12 months. Data showed that Th1 proliferation was significantly reduced for both 0 and 12 months Teff cells with the presence of α-IL-10 both alone (p = 0.018, p = 0.01 respectively) and together with α-TGF-β (p = 0.006, p = 0.002 respectively) (Fig. 5A,B). The opposite pattern was seen for Th2 proliferation: Th2 levels increased for both 0 and 12 month Teff cells in the presence of α-IL-10, both alone (p = 0.008 for Teff at 0 months and p = 0.002 for Teff at 12 months) and together with α-TGF-β (p = 0.004 for Teff at 0 months and p = 0.008 for Teff at 12 months) (Fig. 5C,D). Moreover, although a tendency to an increased Th9 proliferative response could be observed when Teff 0 M and Treg 12 M were cocultured in the presence of α-IL-10, no significant differences were found. No changes were observed for untreated patients (Fig. S1).
Figure 5.
Changes induced by α-IL-10 and α-TGF-β blocking antibodies in different subpopulations of DP-specific Teff cells from DP-SCIT treated patients (N = 16). Bars represent different comparisons of IL-10 and TGF-β inhibitions performed in absence or presence of Tregs (from 0 or 12 months) on Th1 0 (A) and 12 months(B), Th2 0 (C) and 12 months (D) and Th9 0 (E) and 12 months (F). Statistical analysis was carried out by Wilcoxon test; p-values ≤ 0.05 are considered statistically significant. PI: proliferative index; 0 M: Treg cells obtained at 0 months; 12 M: Treg cells obtained at 12 months.
Discussion
Historically, the clinical effect of allergen-sIT has been linked to immunological changes such as increased levels of sIgG4 blocking antibodies31, accompanied by the suppression of allergen-specific Th2 cell responses by Tregs30,32.
Although several studies into the effects of Tregs on sIT have been carried out24,33 and the generation of CD4+CD25+IL-10+ Treg cells after sIT has been reported16, the changes in the suppressive activity of these cells brought about during treatment with a specific allergen had not been analyzed until now. The goal of this study was to assess whether sIT can affect Treg suppressive activity in order to redirect the allergic response to a tolerant one. This was evaluated by analyzing Tregs before and after 12 months of DP-SCIT and the immunological changes that these cells induced in terms of a DP-specific proliferative cell response and their cytokine expression patterns. One year of treatment led to a decrease in IDT area. This agrees with previous studies using grass-SCIT34 and DP-SCIT18,19.
Additionally, we found changes in Treg quantity and function, at both the humoral level, evidenced by changes in antibody levels, and the cellular level, evidenced by changes in Th cell subsets and cytokine production.
These responses are closely related, in fact Tregs can interact with B-cells by an IL-10 mediated mechanism, stimulating IgG4 production and inhibiting IgE production35. In previous studies17, we found that increases in the number of IgG4 secreting cells and sIgG4 levels were linked to an improvement of the clinical response to DP-SCIT. Moreover, although no changes in sIgE levels were found here, a significant increase in serum sIgG4 and in the percentage of Treg cells was observed in treated patients, in agreement with other studies36,37. Our findings also revealed a significant correlation between Treg-cell percentage, DP-sIgG4 levels and IL-10 production after DP-SCIT for treated patients, as has been shown previously38,39. These data suggest that during DP-SCIT there is an increase in iTreg cells that produce IL-10, which is involved in modulating the immunoglobulin secretion pattern towards sIgG4, blocking sIgE40.
It is generally accepted that Tregs play a key role in the induction of tolerance during sIT. Previous studies found an increase in the percentage of these cells during treatment18,26,27,41, however, others did not28,29. Moreover, it has been observed that Treg cells from non-sensitized donors have a higher capacity to suppress allergen-specific proliferative responses compared to Treg from sensitized individuals42. These data suggest the involvement of other regulatory mechanisms related to the function of Treg cells during the sIT. In keeping with this, epigenetic changes in Tregs have been shown to influence their suppressive functions and thus could play a role in the generation of immune tolerance32,43. We found that iTregs from treated patients led to changes in the DP-specific proliferative response. Moreover, these iTregs from treated patients were able to increase the percentage of IL-10 secreting T cells, probably due to the IL-10 production by iTreg cells. Similar results have been found for sublingual DP-sIT44,45. Taken together, these results suggest that sIT can induce iTreg, with higher suppressive activity compared to nTreg, and indicates a key role for these cells in switching an allergic response to tolerance46,47.
The iTregs present several differences compared to nTregs48,49. They carry out their suppressive activity through the secretion of cytokines such as IL-10 and TGF- β27,50. IL-10 can inhibit proinflammatory cytokines and IgE production and inducing IgG4 secretion51. On the other hand, TGF-β has been shown to inhibit proinflamatory cytokines production, promote IL-10 production by T-cells52 and induce Treg cells53,54. In order to elucidate whether the suppressive activity of iTregs is mediated by IL-10 or TGF- β, we analyzed changes in the T cell proliferative response in the presence or absence of cytokine-neutralizing antibodies. We found that the previously mentioned effects were only inhibited significantly when α-IL-10 was present. This neutralizing antibody inhibited Th1 proliferation and blocked the suppression of the proliferative responses of Th2 in treated patients. These results consistently match data from other authors for sublingual sIT-treated patients21,55.
Moreover, when both cytokines were blocked simultaneously, no differential effects were observed compared to blocking IL-10 alone, suggesting there is no cooperative effect between the two cytokines.
There are some studies indicating the role of TGF-β in Treg activity56, however in our study the TGF-β mediated mechanism does not seem to have an important effect. These discrepancies may due to the time of performance of each study, since the effect of TGF-β-mediated T-cell suppression may be more important during a relatively early phase of the treatment (for example, after 6 months of sublingual sIT) and not at later time points (12 and 24 months)56. Our results are in line with those published by Bohle and colleagues57, who showed that IL-10 but not TGF-β mediated suppression of allergen-specific T-cell proliferation occurs after birch pollen sublingual sIT.
One limitation of this study is due to the small number of available cells, which limited the number of cytokines we were able to measure. Therefore, we chose cytokines that were representative of each cellular subset (IL-10→Tregs, IL-4→Th2 and IL-9→Th9) in order to achieve as broad a vision of the changes in the immune response as possible. For the same reason, have been unable to study the cell-cell interaction effects using transwell plates to isolate the cells from each other. In any case, we believe the most important factor underlying the suppressive mechanisms of iTregs is cytokine mediated interaction.
In conclusion, our study revealed that DP-SCIT induces Treg cells with a high capacity to suppress the allergic response. The switch in immunologic response towards tolerance during DP-SCIT has been shown to involve the down-regulation of Th2 responses produced by iTreg cells via an IL-10-mediated mechanism. This suppression leads to decreased levels of Th2 cytokines (i.e. IL4 and, IL13) that are involved in the inhibition of Th1 generation58–61. Although more investigation is needed, the iTregs with IL-10-mediated suppressive action represent promising targets for future allergy therapies, and potential efficacy biomarker for novel types of allergen-specific immunotherapy and cell-based therapies.
Methods
Patient work-up
We included patients with a history of persistent AR to DP. Patients were diagnosed by SPT, NPT and having DP-specific IgE levels higher than 0.35 Ku/L (ImmunoCAP-FEIA). Patients were treated with SCIT to DP (Acaroid©, Allergopharma KG, Reinbeck, Germany) for 12 months using a conventional schedule17.
To assess the clinical improvement in response to DP-SCIT, both RCSS and medication score were calculated as described18. A group of patients with AR to DP but not treated with DP-SCIT and with similar clinical characteristics to the treated patients was included as a control.
The study was conducted in accordance with the declaration of Helsinki, all patients participating in the study gave their informed consent and all protocols were approved by the institutional ethical committee (Comité de Ética de la Investigación Biomédica Provincial de Málaga).
Skin tests
SPT were performed with a battery of 17 common inhalant allergens, including pollens, DP, molds and animal danders (ALK, Madrid, Spain). IDT was carried out with freshly reconstituted freeze dried DP (0.4 mg/ml) (ALK, Madrid, Spain) as previously described62 and results expressed as mm2. IDT to DP was performed at 0 and 12 months in order to assess clinical improvement.
Nasal provocation test
NPT was carried out at 0 and 12 months in both treated and untreated groups as described previously63. Briefly, symptom-free patients [total visual analogue scale (VAS), <60 mm] were intranasally challenged with two puffs (100 mL) of saline in each nostril to exclude nasal hyperreactivity. If this result was negative, 15 min later we began administering reconstituted freeze-dried allergen solutions of DP (0.0, 0.4 and 4 mg/mL) at 15-min intervals. Two puffs (100 mL) of the solution at room temperature were applied in each nostril. Responses were monitored by means of acoustic rhinometry (SRE 2000 rhinometer; Rhinometrics, Lynge, Denmark) and symptom score. Acoustic rhinometry was performed following the guidelines of the Standardization Committee on Acoustic Rhinometry (E4), measuring the volume of the nasal cavity that corresponds to the lower turbinate (VOL 2–6 cm) in each nostril. Nasal-ocular symptoms, including obstruction, rhinorrhea, itching and sneezing were monitored at each time-point by placing a vertical mark on a horizontal VAS of 100 mm. The total range of the VAS during NPT was 0–500 mm. The response to nasal challenge was evaluated based on subjective (VAS of nasal-ocular symptoms) and objective (VOL 2–6 cm) parameters. A NPT was considered positive when an increase greater than 30% in the VAS score and a decrease greater than 30% in nasal cavity volume was observed, compared to the baseline value.
Sample collection and storing
Peripheral blood samples were collected from treated and untreated patients, at 0 and 12 months and processed immediately. PBMCs were isolated by Ficoll-Paque density gradient (Pharmacia Biotech, Barcelona, Spain) and frozen in RPMI 1640 medium supplemented with 2 mM of L-glutamine (BioWhittaker, Pittsburgh, PA, USA), gentamicin (5 mg/ml) (Normon, Madrid, Spain), streptomycin (50 ng/ml), penicillin (100 IU/ml), 40% of fetal bovine serum (BioWhittaker) and 10% of DMSO (Sigma, St. Louis, MO, USA) and stored in liquid nitrogen.
Serum was collected for specific IgE (sIgE) and IgG4 (sIgG4) determination, and stored at −20 °C.
Samples were managed by the Málaga Hospital-IBIMA Biobank, which belongs to the Andalusian Public Health System Biobank.
Specific IgE and IgG4 determination
Levels of sIgE and sIgG4 to DP (d1) were determined by ImmunoCAP-FEIA in serum samples, according to the manufacturer’s instructions (Thermo Fisher Scientific, Massachusetts, USA).
Treg cells determination
The percentage of Treg cells (CD3+CD4+CD25+CD127low) (Fig. S2) during DP-SCIT was determined in PBMCs at 0 and 12 month using specific MoAbs: CD3-PerCP, CD4-APC (BD™ Biosciences), CD25-PECy7 and CD127-PE (Biolegend®, San Diego, CA, USA). Cells were phenotyped using a BDTM FACS Canto II flow cytometer (BDTM Biosciences). Results were analyzed with FlowJo® software (Tree Star, Inc, Ashland, OR, USA) and dead cells were excluded using Near-IR fluorescent LIVE/DEAD® Fixable Dead Cell dyes (Thermo Fisher Scientific).
Cell isolation
Treg and Teff cells were isolated from PBMCs at 0 and 12 month using the immunomagnetic EasySepTM Human CD4+CD127lowCD25+ Regulatory T Cell Isolation Kit (STEMCELLTM Technologies, Vancouver, Canada).
Analysis of the suppressive capacity of Treg cells during DP-SCIT
Teff cells were obtained at 0 and 12 months and cocultured at a 5:1 ratio with Tregs from 0 and 12 months, respectively. The 5:1 ratio was chosen based on previous studies56. Teff cells were also cultured alone (without Treg) as a control. These cultures were then stimulated with nDer p 1 (10 µg/ml) (Indoor Biotechnologies INC, Charlottesville, VA, USA) for 6 days at 5% CO2 and 37 °C conditions. In order to determine the Teff proliferative response, they were pre-stained with CFSE (5, 6-carboxifluoresceindiacetate N-succinimidyl ester) (Life Technologies, Eugene, OR, USA). During the last two hours of culture, GolgiSTOP (BDTM Biosciences, San Jose, CA, USA) was added. Surface and intracellular characterization was carried out with specific MoAbs: CD3-PerCP, CD4-APC, CD4-APCH7 (BD™ Biosciences), CD27-PerCP and CD294-AF (Biolegend®). For intracellular cytokine staining, cells were fixed using the BD Cytofix/CytoPermFixation/Permeabilization solution kit (BDTM Biosciences) and stained with IL-10-PE, IFN-γ-PECy7, IL-9-PE (BDTM Bioscience) and IL-4-PECy7 (Biolegend®). T-cell subpopulations were defined as follows: CD3+CD4+IFN-γ+ (Th1); CD3+CD4+CD27-CD294+ (Th2); CD3+CD4+CD294-IL9+ (Th9). Production of IL-10, IL-4 and IL-9 cytokines was determined for all CD3+CD4+ cells. Cells were phenotyped using a BDTM FACS Canto II flow cytometer (BDTM Biosciences) and analyzed with FlowJo® software (Tree Star). Dead cells were excluded using Near-IR fluorescent LIVE/DEAD® Fixable Dead Cell dyes (Thermo Fisher Scientific).
Results are expressed as percentage of cells or as proliferation index (PI). This was calculated by dividing the percentage of CFSEdim cells after Der p1 stimulation with the percentage of CFSEdim cells without stimuli. A PI higher than 2 was considered positive64–68.
Analysis of the cytokine mediated mechanism of Treg suppression
The effect on the proliferative response was determined using α-IL-10 (monoclonal mouse IgG2B) and α-TGF-β1 (monoclonal mouse IgG1) blocking antibodies (10 µg/ml) (R&D Systems, Minneapolis, MN, USA). These were added separately or together in all cell culture combinations described above and incubated for 6 days (5% CO2 and 37 °C). Surface and intracellular characterization was carried out as previously described and results expressed as PI.
Statistical analysis
Data are presented as individual values and mean with s.d. or median and ranges as indicated. Qualitative variables were analyzed by chi-squared and Fisher tests. Quantitative variables were analyzed using the Mann-Whitney U-test. Comparisons of related samples were carried out by Wilcoxon test. Correlation analysis was performed by calculating the Pearson correlation coefficient. All reported p-values represented two-tailed tests, and p-values ≤ 0.05 are considered statistically significant.
The authors declare that all data supporting of this study are available within the paper and its supplementary files.
Electronic supplementary material
Acknowledgements
The authors would like to thank Ana Molina for her excellent technical assistance. The study was funded by grants from the Institute of Health “Carlos III” of the Ministry of Economy and Competitiveness: PI12/02481, PI15/02256 and PI15/00559, RETICS ARADyAL (RD16/0006/0001; RD16/0006/0015), Juan Rodes Program (JR15/00036) and Sara Borrell Program (CD14/00242). Salud 2000 project, SEAIC Foundation, PI-0542-2010, Andalusian Regional Ministry of Economy and Knowledge (CTS-7433), Andalusian Regional Ministry of Health, Nicolas Monardes Program (C-0044-2012 SAS2013). Grants were co-funded by the European Regional Development Fund (ERDF).
Author Contributions
M.J.T. and C.M. made substantial contributions to conception and design of experiments; M.G., I.D., F.P., P.C., M.J.R., C.R., F.G. and L.G. made substantial contributions to acquisition of data or analysis and interpretation of data; M.G., I.D., J.R.P., M.M.E., M.J.T. and C.M. contributed to drafting the article or revising it critically for important intellectual content; M.J.T. and C.M. made substantial contributions to the final version of the manuscript. All authors have read and approved the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
M. Gonzalez, I. Doña, M. J. Torres and C. Mayorga contributed equally to this work.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-017-12261-2.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.EFA. EFA Book on Respiratory Allergies in Europe. (European Federation of Allergy and Airways Diseases Patients Associations 2011).
- 2.Williams, D. C., Edney, G., Maiden, B. & Smith, P. K. Recognition of allergic conjunctivitis in patients with allergic rhinitis. World Allergy Organ J6, 4, doi:1939-4551-6-4 (2013). [DOI] [PMC free article] [PubMed]
- 3.Passalacqua G. Specific immunotherapy: beyond the clinical scores. Ann Allergy Asthma Immunol. 2011;107:401–406. doi: 10.1016/j.anai.2011.04.017. [DOI] [PubMed] [Google Scholar]
- 4.Bauchau, V. & Durham, S. R. Prevalence and rate of diagnosis of allergic rhinitis in Europe. The European respiratory journal24, 758–764, doi:24/5/758 (2004). [DOI] [PubMed]
- 5.Bousquet J, Demoly P, Michel FB. Specific immunotherapy in rhinitis and asthma. Annals of allergy, asthma & immunology: official publication of the American College of Allergy, Asthma, & Immunology. 2001;87:38–42. doi: 10.1016/S1081-1206(10)62193-X. [DOI] [PubMed] [Google Scholar]
- 6.Meltzer, E. O. & Bukstein, D. A. The economic impact of allergic rhinitis and current guidelines for treatment. Annals of allergy, asthma & immunology: official publication of the American College of Allergy, Asthma, & Immunology106, S12–16, doi:S1081-1206(10)00958-0 (2011). [DOI] [PubMed]
- 7.Veldhoen, M. et al. Transforming growth factor-beta ‘reprograms’ the differentiation of T helper 2 cells and promotes an interleukin 9-producing subset. Nature immunology9, 1341–1346, doi:ni.1659 (2008). [DOI] [PubMed]
- 8.Novak N, Bieber T, Allam JP. Immunological mechanisms of sublingual allergen-specific immunotherapy. Allergy. 2011;66:733–739. doi: 10.1111/j.1398-9995.2010.02535.x. [DOI] [PubMed] [Google Scholar]
- 9.Shamji MH, Durham SR. Mechanisms of immunotherapy to aeroallergens. Clinical and experimental allergy: journal of the British Society for Allergy and Clinical Immunology. 2011;41:1235–1246. doi: 10.1111/j.1365-2222.2011.03804.x. [DOI] [PubMed] [Google Scholar]
- 10.Bousquet, J., Lockey, R. & Malling, H. J. Allergen immunotherapy: therapeutic vaccines for allergic diseases. A WHO position paper. The Journal of allergy and clinical immunology102, 558–562, doi:S0091674998003479 (1998). [DOI] [PubMed]
- 11.Des Roches, A. et al. Immunotherapy with a standardized Dermatophagoides pteronyssinus extract. VI. Specific immunotherapy prevents the onset of new sensitizations in children. The Journal of allergy and clinical immunology99, 450–453, doi:S0091-6749(97)70069-1 (1997). [DOI] [PubMed]
- 12.Durham, S. R. et al. Grass pollen immunotherapy decreases the number of mast cells in the skin. Clinical and experimental allergy: journal of the British Society for Allergy and Clinical Immunology29, 1490–1496, doi:cea678 (1999). [DOI] [PubMed]
- 13.Bellinghausen I, et al. Insect venom immunotherapy induces interleukin-10 production and a Th2-to-Th1 shift, and changes surface marker expression in venom-allergic subjects. European journal of immunology. 1997;27:1131–1139. doi: 10.1002/eji.1830270513. [DOI] [PubMed] [Google Scholar]
- 14.Wachholz, P. A. & Durham, S. R. Induction of ‘blocking’ IgG antibodies during immunotherapy. Clinical and experimental allergy: journal of the British Society for Allergy and Clinical Immunology33, 1171–1174, doi:1765 (2003). [DOI] [PubMed]
- 15.Jutel, M., Akdis, M., Blaser, K. & Akdis, C. A. Mechanisms of allergen specific immunotherapy–T-cell tolerance and more. Allergy61, 796–807, doi:ALL1175 (2006). [DOI] [PubMed]
- 16.Gardner LM, Thien FC, Douglass JA, Rolland JM, O’Hehir RE. Induction of T ‘regulatory’ cells by standardized house dust mite immunotherapy: an increase in CD4+ CD25+ interleukin-10+ T cells expressing peripheral tissue trafficking markers. Clinical and experimental allergy: journal of the British Society for Allergy and Clinical Immunology. 2004;34:1209–1219. doi: 10.1111/j.1365-2222.2004.02009.x. [DOI] [PubMed] [Google Scholar]
- 17.Fernandez, T. D. et al. Differential Plasma-cell evolution is linked with Dermatophagoides pteronyssinus immunotherapy response. Sci Rep5, 14482, doi:srep14482 [pii]10.1038/srep14482 (2015). [DOI] [PMC free article] [PubMed]
- 18.Gomez E, et al. Initial immunological changes as predictors for house dust mite immunotherapy response. Clin Exp Allergy. 2015;45:1542–1553. doi: 10.1111/cea.12578. [DOI] [PubMed] [Google Scholar]
- 19.Rondon C, et al. Efficacy and safety of D. pteronyssinus immunotherapy in local allergic rhinitis: a double-blind placebo-controlled clinical trial. Allergy. 2016;71:1057–1061. doi: 10.1111/all.12889. [DOI] [PubMed] [Google Scholar]
- 20.Vignali, D. A., Collison, L. W. & Workman, C. J. How regulatory T cells work. Nature reviews. Immunology8, 523–532, doi:nri2343 [pii]10.1038/nri2343 (2008). [DOI] [PMC free article] [PubMed]
- 21.Jutel M, et al. IL-10 and TGF-beta cooperate in the regulatory T cell response to mucosal allergens in normal immunity and specific immunotherapy. European journal of immunology. 2003;33:1205–1214. doi: 10.1002/eji.200322919. [DOI] [PubMed] [Google Scholar]
- 22.Akdis, M. et al. Interleukins, from 1 to 37, and interferon-gamma: receptors, functions, and roles in diseases. The Journal of allergy and clinical immunology127, 701–721 e701–770, doi:S0091-6749(10)01949-4 (2011). [DOI] [PubMed]
- 23.Curotto de Lafaille, M. A. & Lafaille, J. J. Natural and adaptive foxp3+ regulatory T cells: more of the same or a division of labor? Immunity30, 626–635, doi:S1074-7613(09)00199-X (2009). [DOI] [PubMed]
- 24.Rolland JM, Gardner LM, O’Hehir RE. Functional regulatory T cells and allergen immunotherapy. Current opinion in allergy and clinical immunology. 2010;10:559–566. doi: 10.1097/ACI.0b013e32833ff2b2. [DOI] [PubMed] [Google Scholar]
- 25.Chatila TA, et al. JM2, encoding a fork head-related protein, is mutated in X-linked autoimmunity-allergic disregulation syndrome. J Clin Invest. 2000;106:R75–81. doi: 10.1172/JCI11679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sorensen, A. E. et al. Human leukocyte antigen-G and regulatory T cells during specific immunotherapy for pollen allergy. International archives of allergy and immunology162, 237–252, doi:000353281 (2013). [DOI] [PubMed]
- 27.Suarez-Fueyo A, et al. Grass tablet sublingual immunotherapy downregulates the TH2 cytokine response followed by regulatory T-cell generation. The Journal of allergy and clinical immunology. 2014;133(130-138):e131–132. doi: 10.1016/j.jaci.2013.09.043. [DOI] [PubMed] [Google Scholar]
- 28.Moed H, Gerth van Wijk R, Hendriks RW, van der Wouden JC. Evaluation of clinical and immunological responses: a 2-year follow-up study in children with allergic rhinitis due to house dust mite. Mediators of inflammation. 2013;2013:345217. doi: 10.1155/2013/345217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim, E. H. et al. Sublingual immunotherapy for peanut allergy: clinical and immunologic evidence of desensitization. J Allergy Clin Immunol127, 640–646 e641, doi:S0091-6749(10)03043-5 (2011). [DOI] [PMC free article] [PubMed]
- 30.Fujimura, T. et al. The induced regulatory T cell level, defined as the proportion of IL-10(+)Foxp3(+) cells among CD25(+)CD4(+) leukocytes, is a potential therapeutic biomarker for sublingual immunotherapy: a preliminary report. International archives of allergy and immunology153, 378–387, doi:000316349 (2010). [DOI] [PubMed]
- 31.Kappen, J. H., Durham, S. R., Veen, H. I. & Shamji, M. H. Applications and mechanisms of immunotherapy in allergic rhinitis and asthma. Therapeutic advances in respiratory disease, doi:10.1177/1753465816669662 (2016). [DOI] [PMC free article] [PubMed]
- 32.Syed A, et al. Peanut oral immunotherapy results in increased antigen-induced regulatory T-cell function and hypomethylation of forkhead box protein 3 (FOXP3) The Journal of allergy and clinical immunology. 2014;133:500–510. doi: 10.1016/j.jaci.2013.12.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stelmaszczyk-Emmel A, Zawadzka-Krajewska A, Glodkowska-Mrowka E, Demkow U. FoxP3 Tregs Response to Sublingual Allergen Specific Immunotherapy in Children Depends on the Manifestation of Allergy. Journal of immunology research. 2015;2015:731381. doi: 10.1155/2015/731381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Francis, J. N. et al. Grass pollen immunotherapy: IL-10 induction and suppression of late responses precedes IgG4 inhibitory antibody activity. The Journal of allergy and clinical immunology121, 1120–1125 e1122, doi:S0091-6749(08)00357-6 (2008). [DOI] [PubMed]
- 35.Meiler F, Klunker S, Zimmermann M, Akdis CA, Akdis M. Distinct regulation of IgE, IgG4 and IgA by T regulatory cells and toll-like receptors. Allergy. 2008;63:1455–1463. doi: 10.1111/j.1398-9995.2008.01774.x. [DOI] [PubMed] [Google Scholar]
- 36.Eifan, A. O. et al. Clinical efficacy and immunological mechanisms of sublingual and subcutaneous immunotherapy in asthmatic/rhinitis children sensitized to house dust mite: an open randomized controlled trial. Clinical and experimental allergy: journal of the British Society for Allergy and Clinical Immunology40, 922–932, doi:CEA3448 (2010). [DOI] [PubMed]
- 37.Park, K. H. et al. Different Responses in Induction of Allergen Specific Immunoglobulin G4 and IgE-Blocking Factors for Three Mite Subcutaneous Immunotherapy Products. Yonsei Med J57, 1427–1434, doi:57.1427 (2016). [DOI] [PMC free article] [PubMed]
- 38.Jeannin P, Lecoanet S, Delneste Y, Gauchat JF, Bonnefoy JY. IgE versus IgG4 production can be differentially regulated by IL-10. Journal of immunology. 1998;160:3555–3561. [PubMed] [Google Scholar]
- 39.Satoguina, J. S., Weyand, E., Larbi, J. & Hoerauf, A. T regulatory-1 cells induce IgG4 production by B cells: role of IL-10. Journal of immunology174, 4718–4726, doi:174/8/4718 (2005). [DOI] [PubMed]
- 40.Zhao, D. et al. The Functional IgE-Blocking Factor Induced by Allergen-Specific Immunotherapy Correlates with IgG4 Antibodies and a Decrease of Symptoms in House Dust Mite-Allergic Children. Int Arch Allergy Immunol169, 113–120, doi:000444391 (2016). [DOI] [PubMed]
- 41.Lou W, Wang C, Wang Y, Han D, Zhang L. Responses of CD4(+) CD25(+) Foxp3(+) and IL-10-secreting type I T regulatory cells to cluster-specific immunotherapy for allergic rhinitis in children. Pediatric allergy and immunology: official publication of the European Society of Pediatric Allergy and Immunology. 2012;23:140–149. doi: 10.1111/j.1399-3038.2011.01249.x. [DOI] [PubMed] [Google Scholar]
- 42.Thunberg S, et al. Immune regulation by CD4+ CD25+ T cells and interleukin-10 in birch pollen-allergic patients and non-allergic controls. Clinical and experimental allergy: journal of the British Society for Allergy and Clinical Immunology. 2007;37:1127–1136. doi: 10.1111/j.1365-2222.2007.02739.x. [DOI] [PubMed] [Google Scholar]
- 43.Sakaguchi S, Vignali DA, Rudensky AY, Niec RE, Waldmann H. The plasticity and stability of regulatory T cells. Nature reviews. Immunology. 2013;13:461–467. doi: 10.1038/nri3464. [DOI] [PubMed] [Google Scholar]
- 44.Cosmi, L. et al. Sublingual immunotherapy with Dermatophagoides monomeric allergoid down-regulates allergen-specific immunoglobulin E and increases both interferon-gamma- and interleukin-10-production. Clinical and experimental allergy: journal of the British Society for Allergy and Clinical Immunology36, 261–272, doi:CEA2429 (2006). [DOI] [PubMed]
- 45.Ciprandi, G. et al. Induction of interleukin 10 by sublingual immunotherapy for house dust mites: a preliminary report. Annals of allergy, asthma & immunology: official publication of the American College of Allergy, Asthma, & Immunology95, 38–44, doi:S1081-1206(10)61186-6 (2005). [DOI] [PubMed]
- 46.Radulovic, S., Jacobson, M. R., Durham, S. R. & Nouri-Aria, K. T. Grass pollen immunotherapy induces Foxp3-expressing CD4+ CD25+ cells in the nasal mucosa. The Journal of allergy and clinical immunology121, 1467–1472, 1472 e1461, doi:S0091-6749(08)00597-6 (2008). [DOI] [PubMed]
- 47.Ling EM, et al. Relation of CD4+ CD25+ regulatory T-cell suppression of allergen-driven T-cell activation to atopic status and expression of allergic disease. Lancet. 2004;363:608–615. doi: 10.1016/S0140-6736(04)15592-X. [DOI] [PubMed] [Google Scholar]
- 48.Adeegbe DO, Nishikawa H. Natural and induced T regulatory cells in cancer. Frontiers in immunology. 2013;4:190. doi: 10.3389/fimmu.2013.00190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schmitt EG, Williams CB. Generation and function of induced regulatory T cells. Frontiers in immunology. 2013;4:152. doi: 10.3389/fimmu.2013.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Burastero, S. E. et al. Effect of sublingual immunotherapy with grass monomeric allergoid on allergen-specific T-cell proliferation and interleukin 10 production. Annals of allergy, asthma & immunology: official publication of the American College of Allergy, Asthma, & Immunology100, 343–350, doi:S1081-1206(10)60597-2 (2008). [DOI] [PubMed]
- 51.Chung F. Anti-inflammatory cytokines in asthma and allergy: interleukin-10, interleukin-12, interferon-gamma. Mediators of inflammation. 2001;10:51–59. doi: 10.1080/09629350120054518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wan YY, Flavell RA. TGF-beta and regulatory T cell in immunity and autoimmunity. Journal of clinical immunology. 2008;28:647–659. doi: 10.1007/s10875-008-9251-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen, W. & Konkel, J. E. TGF-beta and ‘adaptive’ Foxp3(+) regulatory T cells. J Mol Cell Biol2, 30–36, doi:mjp004 (2010). [DOI] [PMC free article] [PubMed]
- 54.Fu S, et al. TGF-beta induces Foxp3+ T-regulatory cells from CD4+ CD25 - precursors. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2004;4:1614–1627. doi: 10.1111/j.1600-6143.2004.00566.x. [DOI] [PubMed] [Google Scholar]
- 55.Yamanaka, K. et al. Induction of IL-10-producing regulatory T cells with TCR diversity by epitope-specific immunotherapy in pollinosis. The Journal of allergy and clinical immunology124, 842–845 e847, doi:S0091-6749(09)00953-1 (2009). [DOI] [PubMed]
- 56.O’Hehir, R. E. et al. House dust mite sublingual immunotherapy: the role for transforming growth factor-beta and functional regulatory T cells. American journal of respiratory and critical care medicine180, 936–947, doi:200905-0686OC (2009). [DOI] [PubMed]
- 57.Bohle B, et al. Sublingual immunotherapy induces IL-10-producing T regulatory cells, allergen-specific T-cell tolerance, and immune deviation. The Journal of allergy and clinical immunology. 2007;120:707–713. doi: 10.1016/j.jaci.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 58.Rengarajan, J., Szabo, S. J. & Glimcher, L. H. Transcriptional regulation of Th1/Th2 polarization. Immunol Today21, 479–483, doi:S0167-5699(00)01712-6 (2000). [DOI] [PubMed]
- 59.Mullen AC, et al. Role of T-bet in commitment of TH1 cells before IL-12-dependent selection. Science. 2001;292:1907–1910. doi: 10.1126/science.1059835. [DOI] [PubMed] [Google Scholar]
- 60.Li-Weber M, et al. Th2-specific protein/DNA interactions at the proximal nuclear factor-AT site contribute to the functional activity of the human IL-4 promoter. J Immunol. 1998;161:1380–1389. [PubMed] [Google Scholar]
- 61.Zhang DH, Yang L, Ray A. Differential responsiveness of the IL-5 and IL-4 genes to transcription factor GATA-3. J Immunol. 1998;161:3817–3821. [PubMed] [Google Scholar]
- 62.Bousquet J, et al. Practical guide to skin prick tests in allergy to aeroallergens. Allergy. 2012;67:18–24. doi: 10.1111/j.1398-9995.2011.02728.x. [DOI] [PubMed] [Google Scholar]
- 63.Rondon C, et al. Seasonal idiopathic rhinitis with local inflammatory response and specific IgE in absence of systemic response. Allergy. 2008;63:1352–1358. doi: 10.1111/j.1398-9995.2008.01695.x. [DOI] [PubMed] [Google Scholar]
- 64.Rodriguez, M. J. et al. LPS promotes Th2 dependent sensitisation leading to anaphylaxis in a Pru p 3 mouse model. Sci Rep7, 40449, doi:srep40449 (2017). [DOI] [PMC free article] [PubMed]
- 65.Fernandez, T. D. et al. Role of effector cells (CCR7(−)CD27(−)) and effector-memory cells (CCR7(−)CD27(+)) in drug-induced maculopapular exanthema. Int J Immunopathol Pharmacol23, 437–447, doi:6 (2010). [DOI] [PubMed]
- 66.Gomez, E. et al. Effect of Pru p 3 on dendritic cell maturation and T-lymphocyte proliferation in peach allergic patients. Ann Allergy Asthma Immunol109, 52–58, doi:S1081-1206(12)00391-2 (2012). [DOI] [PubMed]
- 67.Filby, A., Begum, J., Jalal, M. & Day, W. Appraising the suitability of succinimidyl and lipophilic fluorescent dyes to track proliferation in non-quiescent cells by dye dilution. Methods82, 29–37, doi:S1046-2023(15)00089-4 (2015). [DOI] [PubMed]
- 68.Tario, J. D. Jr. et al. Optimized staining and proliferation modeling methods for cell division monitoring using cell tracking dyes. J Vis Exp, e4287, doi:4287 (2012). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.