Abstract
Aerobic anoxygenic phototrophic (AAP) bacteria are microorganisms that can harvest light energy using bacteriochlorophyll a to supplement their predominantly organotrophic metabolism. Growth enhancement by light has repeatedly been demonstrated in laboratory experiments with AAP isolates. However, the ecological advantage of light utilization is unclear, as it has never been proven in the natural environment. Here, we conducted manipulation experiments in the NW Mediterranean and found that AAP bacteria display high growth rates which are controlled to a large extent by intense grazing pressure and phosphorous availability. Foremost, we found that, contrarily to the bulk bacterioplakton, AAP bacteria display higher growth rates when incubated under light-dark cycles than in complete darkness. These results represent the first direct evidence that natural populations of marine AAP bacteria can be stimulated by light.
The discovery of marine photoheterotrophs challenged previous simplistic views of the structure of ocean microbial food webs. Marine photoheterotrophs include two main groups, the aerobic anoxygenic phototrophic (AAP) bacteria and the proteorhodopsin (PR)-containing bacteria, which make up large fractions of the microbial communities in the sunlit ocean. Despite having different physiology, both groups are heterotrophs that rely mainly on organic matter and are capable of harvesting light (Koblížek, 2015; Pinhassi et al., 2016).
AAP bacteria are a phylogenetically and metabolically diverse group of organisms utilizing a wide range of carbon sources (Koblížek, 2015). The stimulation of AAP growth by energy captured using bacteriochlorophyll-based reaction centers has been demonstrated in laboratory cultures (Yurkov and van Gemerden, 1993; Biebl and Wagner-Döbler, 2006; Hauruseu and Koblížek, 2012), but not under natural conditions yet. AAP bacteria are relatively common in the euphotic zone of the oceans (Koblížek, 2015) and exhibit faster growth rates than bulk bacterioplankton (Koblížek et al., 2007; Liu et al., 2010; Ferrera et al., 2011). However, field experiments have failed to observe a light effect on AAP bacteria (Schwalbach et al., 2005; Stegman et al., 2014). The reason for these negative results is not clear. It has been hypothesized that AAP bacteria are more vulnerable to grazing than other bacteria due to their large cell sizes (Koblížek et al., 2007; Ferrera et al., 2011) and it is thus possible that the light-induced increase in biomass is removed by intense grazing. Alternatively, AAP growth in field experiments could have been limited by nutrient availability. In such a case, light-derived energy would provide only an irrelevant advantage, as growth would have been dependent on unavailable nutrients.
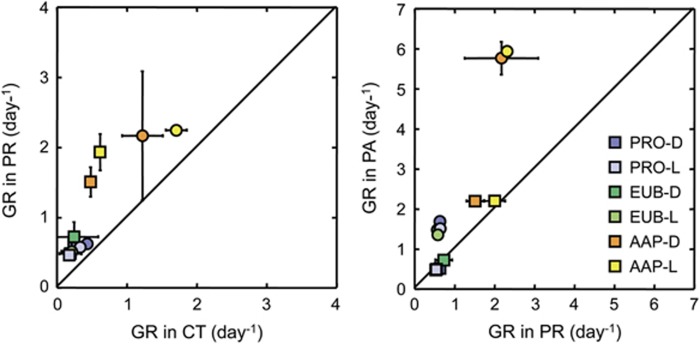
To solve this question, we performed manipulation experiments with natural seawater collected at the Blanes Bay Microbiology Observatory (BBMO). Controls, predator-reduced, as well as treatments with both predation reduction and phosphorus amendment (Supplementary Information) were included to analyze the main constrains on AAP growth. Incubation of seawater promoted growth of all prokaryotes, total bacteria (Eubacterial cells) and AAPs in all treatments, yet the AAPs grew at much faster rates than the bulk community (Supplementary Figure S1, Supplementary Table S1). Removal of predators led to a substantial increase in the GRs of AAPs, as compared with total prokaryotes and Eubacteria (Figure 1). These results confirm that AAP bacteria use high growth rates to cope with more intense grazing than other bacteria.
Figure 1.
Growth rates in the predator-reduced (PR) vs the control (CT) treatments (left panel) and in the phosphorus-amended (PA) vs the PR treatments (right panel). Circles correspond to Experiment 1 and squares to Experiment 2. Dots falling on the 1:1 line indicate that growth rates have the same value for both x-y treatments; if dots are above or below the line, growth rates from y treatments are higher or lower than in x treatments respectively. Error bars represent the standard deviations. AAP, aerobic anoxygenic phototrophic bacteria; D, Dark incubations; EUB, Eubacteria; L, Light-dark incubations; PRO, total prokaryotes.
The Mediterranean is a predominantly P-limited ecosystem (Krom et al., 1991). A previous report from the Mediterranean Sea suggested that AAP growth rates were controlled primarily by P-limitation (Hojerová et al., 2011), whereas experiments conducted in the BBMO showed that grazing had a much stronger effect than nutrient availability (Ferrera et al., 2011). Our results show that the response to P-amendment differed among experiments (Figure 1); whereas a much stronger response to P than to predation was found in Experiment 1, the response was weak in Experiment 2. Nevertheless, the response to P-amendment was stronger for AAP bacteria than for total prokaryotes and Eubacteria in both experiments (Figure 1). In fact, in Experiment 1 AAPs grew at rates of 5.86 day−1 in the P-amended treatment, which is among the highest values reported for AAP bacteria (Koblížek et al., 2007; Ferrera et al., 2011; Hojerová et al., 2011). Our results show that P-limitation can be a stronger growth control factor than grazing, but only under certain circumstances.
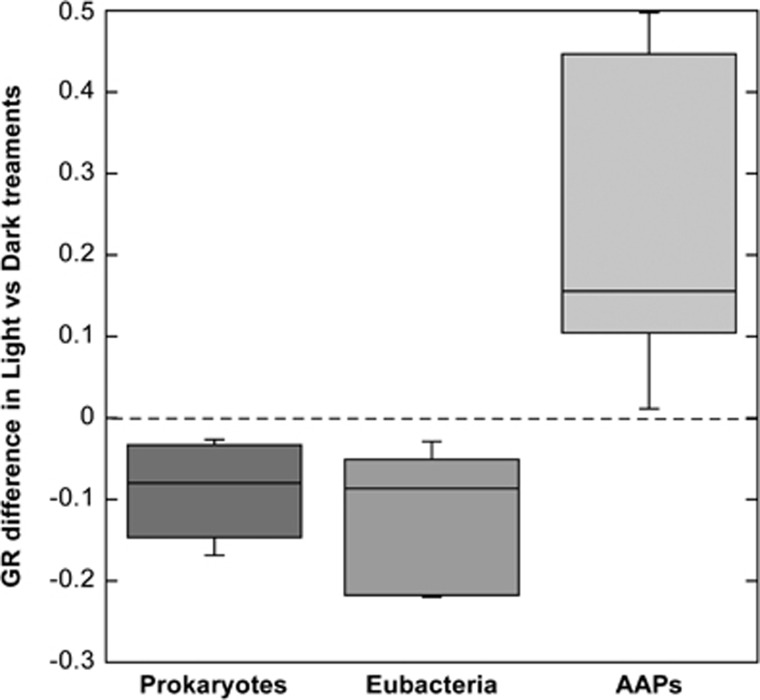
To test whether light stimulates growth, the treatments were subjected to either continuous dark or to natural light-dark cycles, excluding ultraviolet radiation to prevent that its detrimental effect could mask a positive effect of photosynthetically active radiation (Ruiz-González et al., 2013). Overall, either minor inhibition or stimulation by light was observed in the experiments, but interestingly, when comparing the differences in GRs between light regimes, clear differences were found among the groups analyzed (Figure 2). While growth rates were on average 0.11 and 0.09 day−1 higher in the dark for Eubacteria and total prokaryotes respectively, AAP growth rates where 0.23 day−1 higher under light. These differences were larger in the predator-reduced than in the control treatment, and even larger in the P-amended treatment in Experiment 1 (Figure 1) supporting our hypothesis that failure to demonstrate the light stimulation in previous experiments may have been related to nutrient limitation.
Figure 2.
Box plots showing the distribution of the difference between growth rates (GR) in the light and dark treatments (positive values indicate higher growth rates in the light and vice versa) for the microbial groups studied. Values from all treatments and experiments are pooled. From top to bottom, the horizontal lines of the box represent the upper-quartile, median and lower-quartile. Whiskers extending from top and bottom of the box represent maximum and minimum values. AAP, aerobic anoxygenic phototrophic bacteria; EUB, Eubacteria; PRO, total prokaryotes.
The advantage for photoheterotrophs over other bacteria of using light has been intensively debated since their discovery. Its stimulatory effect has been shown in AAP cultures (Yurkov and van Gemerden, 1993; Hauruseu and Koblížek, 2012), but its effect under natural conditions remained doubtful since only indirect evidences existed. In addition, Kirchman and Hanson (2013) used theoretical calculations of the contribution of phototrophy to the organisms’ energy requirements to conclude that even though AAP bacteria harvest more light energy than PR-bacteria, the net yield of phototrophy is only modest. Nevertheless, a positive correlation between AAP abundance and day length was found in seasonal studies in Mediterranean coastal waters and a lagoon (Lamy et al., 2011; Ferrera et al., 2014), and, recently, Cepáková et al. (2016) observed that the GRs of AAP bacteria in lakes were higher than their mortality rates during the day, an indication that light may directly stimulate AAP growth. Contrarily, no clear effect was found in the activity of AAPs in short-term experiments neither in marine nor in freshwater environments (Stegman et al., 2014; Garcia-Chaves et al., 2016). Here, we provide the first evidence that marine AAP bacteria can be directly stimulated by light under natural conditions. Although an indirect enhancement of growth by phytoplankton-driven primary production could occur, the observed increase likely reflects a direct light stimulation since the larger increase occurred in treatments where the majority of phytoplankton had been removed by filtration. Nevertheless, the magnitude of the response was variable between experiments suggesting that heterogeneity in the response of various AAP communities may exist. Further experiments investigating the interplay between light and different environmental parameters on growth rates are required to better evaluate the light-enhanced growth of AAP bacteria. In conclusion, our results indicate that AAP bacteria can grow much faster than bulk bacterioplankton and that growth in the NW Mediterranean is largely controlled both by grazing and P-limitation. These results show for the first time that natural populations of marine AAP bacteria can be directly stimulated by light.
Acknowledgments
This work was supported by grant REMEI (CTM2015-70340-R) from the Spanish Ministry of Economy, Industry and Competitivity. MK was supported by the GA ČR project 13-11281S and the MŠMT project Algatech Plus.
Footnotes
Supplementary Information accompanies this paper on The ISME Journal website (http://www.nature.com/ismej)
The authors declare no conflict of interest.
Supplementary Material
References
- Biebl H, Wagner-Döbler I. (2006). Growth and bacteriochlorophyll a formation in taxonomically diverse aerobic anoxygenic phototrophic bacteria in chemostat culture: influence of light regimen and starvation. Process Biochem 41: 2153–2159. [Google Scholar]
- Cepáková Z, Hrouzek P, Žišková E, Nuyanzina-Boldareva E, Šorf M, Kozlíková-Zapomělová E et al. (2016). High turnover rates of aerobic anoxygenic phototrophs in European freshwater lakes. Environ Microbiol 18: 5063–5071. [DOI] [PubMed] [Google Scholar]
- Ferrera I, Borrego CM, Salazar G, Gasol JM. (2014). Marked seasonality of aerobic anoxygenic phototrophic bacteria in the coastal NW Mediterranean Sea as revealed by cell abundance, pigment concentration and pyrosequencing of pufM gene. Environ Microbiol 16: 2953–2965. [DOI] [PubMed] [Google Scholar]
- Ferrera I, Gasol JM, Sebastián M, Hojerová E, Kobížek M. (2011). Comparison of growth rates of aerobic anoxygenic phototrophic bacteria and other bacterioplankton groups in coastal mediterranean waters. Appl Environ Microbiol 77: 7451–7458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Chaves MC, Cottrell MT, Kirchman DL, Ruiz-González C, del Giorgio PA. (2016). Single-cell activity of freshwater aerobic anoxygenic phototrophic bacteria and their contribution to biomass production. ISME J 10: 1579–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauruseu D, Koblížek M. (2012). Influence of light on carbon utilization in aerobic anoxygenic phototrophs. Appl Environ Microbiol 78: 7414–7419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hojerová E, Mašín M, Brunet C, Ferrera I, Gasol JM, Koblížek M. (2011). Distribution and growth of aerobic anoxygenic phototrophs in the Mediterranean Sea. Environ Microbiol 13: 2717–2725. [DOI] [PubMed] [Google Scholar]
- Kirchman DL, Hanson TE. (2013). Bioenergetics of photoheterotrophic bacteria in the oceans. Environ Microbiol Rep 5: 188–199. [DOI] [PubMed] [Google Scholar]
- Koblížek M. (2015). Ecology of aerobic anoxygenic phototrophs in aquatic environments. FEMS Microbiol Rev 39: 854–870. [DOI] [PubMed] [Google Scholar]
- Koblížek M, Mašín M, Ras J, Poulton AJ, Prášil O. (2007). Rapid growth rates of aerobic anoxygenic phototrophs in the ocean. Environ Microbiol 9: 2401–2406. [DOI] [PubMed] [Google Scholar]
- Krom MD, Kress N, Brenner S, Gordon LI. (1991). Phosphorus limitation of primary productivity in the Eastern Mediterranean Sea. Limnol Oceanogr 36: 424–432. [Google Scholar]
- Lamy D, De Carvalho-Maalouf P, Cottrell MT, Lami R, Catala P, Oriol L et al. (2011). Seasonal dynamics of aerobic anoxygenic phototrophs in a Mediterranean coastal lagoon. Aquat Microb Ecol 62: 153–163. [Google Scholar]
- Liu R, Zhang Y, Jiao N. (2010). Diel variations in frequency of dividing cells and abundance of aerobic anoxygenic phototrophic bacteria in a coral reef system of the South China Sea. Aquat Microb Ecol 58: 303–310. [Google Scholar]
- Pinhassi J, Delong EF, Béjà O, González JM, Pedrós-alió C. (2016). Marine bacterial and archaeal ion-pumping rhodopsins: genetic diversity. Physiol Ecol 80: 929–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-González C, Simó R, Sommaruga R, Gasol JM. (2013). Away from darkness: a review on the effects of solar radiation on heterotrophic bacterioplankton activity. Front Microbiol 4: 1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwalbach MS, Brown M, Fuhrman JA. (2005). Impact of light on marine bacterioplankton community structure. Aquat Microb Ecol 39: 235–245. [Google Scholar]
- Stegman MR, Cottrell MT, Kirchman DL. (2014). Leucine incorporation by aerobic anoxygenic phototrophic bacteria in the Delaware estuary. ISME J 8: 2339–2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurkov VV, van Gemerden H. (1993). Impact of light/dark regimen on growth rate, biomass formation and bacteriochlorophyll synthesis in Erythromicrobium hydrolyticum. Arch Microbiol 159: 84–89. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.