Abstract
Background
Oxitard, a polyherbal formulation comprising the extracts of Withania somnifera, Mangifera indica, Glycyrrhiza glabra, Daucus carota, Vitis vinifera, powders of Syzygium aromaticum, Yashada bhasma and Emblica officinalis; and oils of Triticum sativum.
Objective
Current study deals with the assessment of Oxitard (a marketed polyherbal formulation) for its adaptogenic potential in chronic unpredictable stress (CUS) and chronic stress (CS) induced dysfunctional homeostasis in rodents.
Materials & methods
Animals were immobilized for 2 h every day for ten days to induce CS. In order to induce CUS, animals were employed in a battery of stressors of variable value and duration for ten days. Following administration of Oxitard, stress was induced in the animals. Stress-induced efficient changes were evaluated by assessing organ (adrenal gland) weights, ulcer index, hematological parameters and biochemical levels of reduced glutathione (GSH), thiobarbituric acid reactive substances (TBARS) and catalase (CAT).
Results
CS and CUS significantly modified the oxidative stress parameters (increased MDA and decreased GSH). Furthermore, CS and CUS lead to weight reduction, adrenal hypertrophy and gastric ulceration. Pre-treatment with Oxitard (200 and 400 mg/kg, p.o.) significantly modified CS and CUS induced hematological changes, oxidative stress parameters and pathological effects.
Conclusion
In conclusion, Oxitard-intervened antioxidant actions are accountable for its adaptogenic effects in stress-induced dysfunctional homeostasis.
Keywords: Oxitard, Adaptogenic, Oxidative stress, Herbal
1. Introduction
Now-a-days, stress has turned into an essential part of human life. Furthermore creatures are continuously exposed to stressful events which lead to various physiological changes. Stressful stimuli are all around archived to trigger the central monoaminergic systems and hypothalamic–pituitary–adrenal (HPA) axis [1]. Hypothalamic paraventricular nucleus surges the release of corticotrophin-releasing hormone upon activation of the HPA axis, which leads to the secretion of adrenocorticotropin from the anterior pituitary, and ultimately adrenal cortex discharges glucocorticoids [2], [3]. Stress, a non-specific response of the body characterized as physical and psychological alterations that disturb the homeostasis and the balance of the organism resulting in various neuronal, endocrine and visceral dysfunction [4], [5]. Physical stressor like loud noise, big crowds and cluttered surrounding causes stress. According to previous reports, glucocorticoids released by CS disrupt the useful homeostasis of the body and are also associated in the etiopathogenesis of a diversity of disease conditions like coronary heart disease, hypertension, diabetes, gastric ulcers, mental depression, immunosuppression and memory loss [6], [7], [8], [9]. No such medicine is mentioned in current pharmacopoeia which could be utilized as a treatment for stress. Nevertheless, a few plants in folkore medication such as Bacopa monniera, Panax ginseng, Emblica officinalis, Withania somnifera, Evolvulus alsinoides and Ocimum sanctum have been assessed for their adaptogenic effects [10], [11], [12], [13], [14]. Previously, some polyherbal formulations have been found to be effective in stress [15], [16], [17]. Oxitard is one more herbal antioxidant formulation comprising the extracts of W. somnifera, Mangifera indica, Glycyrrhiza glabra, Daucus carota, Vitis vinifera, powders of Syzygium aromaticum, Yashada bhasma and E. officinalis; and oils of Triticum sativum [18]. Previous reports indicate the beneficial role of Oxitard in oral submucous fibrosis [19].
According to general hypothesis, intelligence (I) energy (E), and organization (O), the three fundamental elements control the strength of an individual [20], and adaptogens adjust these battleaxes of healthiness by providing energy, informational and organizational aid to cell arrangements establishing the body [21]. Non-ideal prescriptions perform by improving maybe a couple components, but concurrently destroy the residual one(s), leading to adverse effects [22]. Nevertheless, adaptogens activate bi-directional alteration by triggering and re-establishing the balance of all three factors in an ideal way [22], [23]. Animals are exposed to the similar kind of stressor for diverse phases to induce CS experimentally. Previous reports state that animals have a tendency to adjust to exposure to a similar kind of stressor in CS. To overcome this, the animals are exposed to flexible stressors of diverse amount and time in the form of CUS [24], [25]. Additionally, CUS model shows high level of stress related outcomes as compared to CS. Taking into account these findings, in the present study the two models (i.e., CS and CUS) were utilized to assess the adaptogenic effects of Oxitard (a polyherbal formulation), as there are no reports found about the adaptogenic potential of Oxitard till date. The current study was therefore designed to explore the adaptogenic potential of Oxitard in CS and CUS-induced dysfunctional homeostasis.
2. Materials and methods
2.1. Animals
Male albino Wistar rats (180–220 gm) and male Swiss albino mice (25–30 gm) maintained at standard laboratory diet (Kisan Feeds Ltd., Chandigarh, India) and having free access to water ad libitum, were utilized in the present study. Animals were kept in the departmental animal house and were exposed to a normal light and dark cycle. The experimental protocol was duly approved by the Institutional Animal Ethics Committee and care was given animals according to the guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Ministry of Environment and Forest, Government of India (Reg. No. 1088/PO/Re/S/2007/CPCSEA).
2.2. Oxitard
Composition: Each Oxitard capsule contains extracts of W. somnifera (Solanaceae, 71 mg), G. glabra L. (Papilionaceae, rhizome, 29 mg), Mangifera indica L. (Anacardiaceae, bark, 94 mg), V. vinifera L. (Vitaceae, fruit, 12 mg), D. carota L. Daucus vulgaris (Umbelliferae, root, 47 mg), and powders of S. aromaticum L. Merr. and L.M. Perry (Myrtaceae, flower bud, 29 mg), E. officinalis L. (Euphorbiaceae, fruit, 141 mg), Yashada bhasma (2.5 mg) and oil of T. sativum (Poaceae, 6.5 mg).
2.3. Chronic stress (CS)
CS was induced according to the method devised by Kvetnansky and Mikulai [26]. Rats were immobilized in inclined position by fixing each of the four limbs on the immobilization board with sticky tape. Animals were immobilized in this position for 2 h daily for 10 days to induce CS.
2.4. Chronic unpredictable stress (CUS)
CUS was induced in the mice according to a method described by Ortiz et al. [27] which involved acquaintance to numerous stressors in adjustable schedules (Table 1).
Table 1.
The procedure used in CUS.
| Day | Stress type and schedule |
|---|---|
| 1 | 1900 h (earlier night), kept on moist sawdust, overnight; 1000 h restriction, 60 min |
| 2 | 1500 h, cold (4 °C) segregation, 60 min; 1900 h, kept on lights, overnight |
| 3 | 1200 h, kept on dark, 180 min; 1500 h, stress induced by swim, 4 min |
| 4 | 0700 h, kept on moist sawdust, all day; 1900 h, kept deprived of food/water, overnight |
| 5 | 1300 h, stress induced by swim, 3 min; 1900 h, sequestration housing, overnight |
| 6 | 1400 h, isolated at cold (4 °C), 15 min; 1500 h, kept in dark, 120 min |
| 7 | 1900 h, kept on moist sawdust in dark, overnight |
| 8 | 1900 h, isolated with deprived of food/water, overnight |
| 9 | 1600 h, restriction, 60 min; 1900 h, kept in lights, overnight |
| 10 | 0900 h, stress induced by swim, 4 min; 1000 h, restriction, 60 min |
2.5. Treatment
Albino Wistar rats (n = 30) were divided into five groups. CS was induced in all the groups excluding the control group [0.1% carboxy methyl cellulose (CMC)] after treatment with the standard drug and Oxitard. Buspirone (10 mg/kg) and Oxitard (200 and 400 mg/kg) were administered orally. All the drug solutions were prepared in 0.1% CMC. Stress was induced by CS 30 min after administration of each drug. Dose of Oxitard was decided after dose deciding pilot study (data not shown here).
Animals (Swiss albino mice) induced with CUS were randomly assigned to 4 groups [vehicle, buspirone (10 mg/kg) and Oxitard (200 and 400 mg/kg); n = 6 for each group] excluding control group. CUS was induced in all the groups excluding the control group [0.1% carboxy methyl cellulose (CMC)] after treatment with the standard drug and the Oxitard. Buspirone (10 mg/kg) and Oxitard (200 and 400 mg/kg) were administered orally. All the drug solutions were prepared in 0.1% CMC. Stress was induced by CUS 30 min after administration of each drug.
2.6. Hematological parameters
Following anesthesia, blood was collected by retro-orbital plexus to estimate hematological parameters viz. RBC and WBC counts [28].
2.7. Neurochemical analysis
Following behavioral studies, animals were sacrificed; skull cut open and the whole brain was dissected out and stored at −80 °C for further procedure. Homogenization buffer (10 ml/g of brain tissue) with the subsequent composition (12.5 mM sodium phosphate buffer pH 7.0 and 400 mM NaCl) was used to homogenize the brains using glass Teflon homogenizer. After homogenization, the homogenates were centrifuged at 1000× g for 10 min at 4 °C. The supernatant was collected and utilized for the enzyme assay. Reduced glutathione (GSH) assay [29], thiobarbituric acid reactive substances (TBARS) assay [30] and catalase assay [31] were performed for the measurement of GSH and MDA activities respectively.
2.8. Reduced glutathione (GSH)
GSH was analyzed in the brain homogenate. In brief, equal volumes of 20% trichloroacetic acid (TCA) and tissue homogenate (supernatant) were mixed. The precipitated portion was centrifuged. 2.0 ml of 5, 5′-dithiobis-(2-nitrobenzoic acid) (DTNB) reagent (0.6 mM) was added in 0.25 ml of obtained supernatant. Phosphate buffer (0.2 M, pH 8.0) was used to make up the final volume up to 3.0 ml. The colour developed was measured at 412 nm against the reagent blank. Standard reduced glutathione (10–50 μg concentrations) were used for procurement of standard curve. The amount of reduced glutathione was expressed as μg of GSH/mg protein.
2.9. Lipid peroxidation potential (LPO)
LPO in brain was measured by estimating MDA levels. In brief, 2.0 ml of the tissue homogenate (supernatant) was mixed with 2.0 ml of freshly prepared 10% v/v TCA and the resulting mixture was kept in ice bath for 15 min. Later, centrifugation was done to separate the precipitate. 2.0 ml of obtained clear supernatant solution was mixed with 2.0 ml of freshly prepared thiobarbituric acid (TBA) (0.67% v/v). The resulting solution was heated in boiling water bath for 10 min and then instantaneously cooled on ice bath for 5 min. The colour so developed was measured at 532 nm against the reagent blank. Standard MDA (0–23 nM concentrations) were used to obtain the standard graph. Values were expressed as nM of MDA/mg of protein.
2.10. Catalase activity
Catalase activity in the brain was measured. Briefly, 2.0 ml of the homogenate was taken and 1.0 ml of hydrogen peroxide (30 mM) was added in the sample to initiate the reaction. The blank was set by mixing 2.0 ml of the diluted sample (similar dilution) with 1.0 ml of phosphate buffer (50 mM; pH 7.0). The reduction in absorbance was measured at 240 nm. Catalase level was expressed as units/mg of protein.
2.11. Organ weight
The weight of organs (liver, spleen, adrenal gland and testes) after washing with ethanol was recorded per 100 g body weight.
2.12. Ulcer index
The stomach was separated out and opened beside the greater curvature by applying cut for evaluating the occurrence of ulcer. The ulcer index was scored according to the technique described by Takagi and Okabe [32]. The technique can be used to assess the ulcer index as well as the severity of gastric lesions:
0 = no lesion
1 = mucosal edema and petechiae
2 = one to five small lesions (1–2 mm)
3 = above five small lesions or one intermediate lesion (3–4 mm)
4 = two to more intermediate lesions or one gross lesion (>4 mm)
5 = perforated ulcers
The ulcer index is given by the following equation:
2.13. Statistical analysis
GraphPad InStat software (version 5.00, San Diego, CA) was used for data analysis. All data are expressed as mean ± SD. The mean significant difference in the experimental groups was analyzed using one way ANOVA followed by Bonferroni test. Values of p < 0.05 were judged statistically significant.
3. Results
3.1. Effect of Oxitard on oxidative stress markers
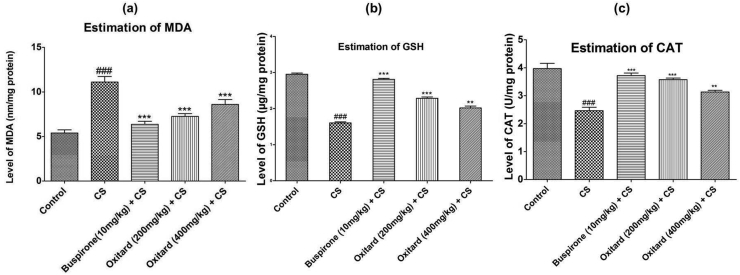
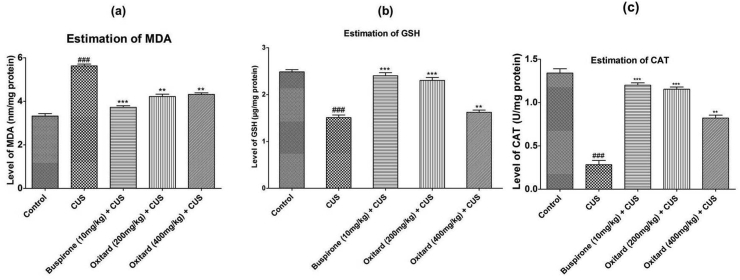
CS and CUS produced a significant decrease in GSH and CAT levels and increase in MDA levels as compared to the control group. Oxitard (200 mg/kg p.o.), significantly improvised the oxidative parameters in the CS and CUS models. To evaluate whether Oxitard exerts antioxidative effect, brain tissues of rats treated with Oxitard were subjected to colorimetric estimation to establish its antioxidative properties in the brain. The results showed that CS and CUS significantly (p < 0.001) increased the brain MDA levels (11.10 ± 1.09 and 5.627 ± 0.142 nM/mg protein) compared to the control groups (5.397 ± 0.602 and 3.320 ± 0.195 nM/mg protein). Treatment with buspirone (10 mg/kg, p.o.) and Oxitard (200 mg/kg, p.o.) significantly decreased brain MDA levels (Table 2; Fig. 1, Fig. 2a) [Buspirone (6.363 ± 0.578 and 3.723 ± 0.127) and Oxitard (7.250 ± 0.547 and 4.217 ± 0.189) nM/mg protein] compared to the corresponding CS and CUS groups (Table 2; Fig. 1, Fig. 2a). Further, CS and CUS significantly (p < 0.001) decreased the brain GSH level (1.607 ± 0.051 and 1.510 ± 0.096 μg/mg protein) compared to the control groups (2.953 ± 0.055 and 2.487 ± 0.081 μg/mg proteins). Treatment with buspirone (10 mg/kg, p.o.) and Oxitard (200 mg/kg, p.o.) significantly increased brain GSH levels (Table 2; Fig. 1, Fig. 2b) [Buspirone (2.813 ± 0.051 and 2.403 ± 0.112), and Oxitard (2.287 ± 0.065 and 2.303 ± 0.11) μg/mg protein] compared to the corresponding CS and CUS groups (Table 2; Fig. 1, Fig. 2b). In addition, CS and CUS significantly (p < 0.001) decreased the brain CAT level (2.467 ± 0.051 and 0.283 ± 0.085 U/mg protein) as compared to the control groups (3.970 ± 0.32 and 1.340 ± 0.087 U/mg proteins). Treatment with buspirone (10 mg/kg, p.o.) and Oxitard (200 mg/kg, p.o.) significantly increased brain CAT levels (Table 2; Fig. 1, Fig. 2c) [Buspirone (3.720 ± 0.154 and 1.20 ± 0.045), and Oxitard (3.573 ± 0.097 and 1.153 ± 0.045) U/mg protein] compared to the corresponding CS and CUS groups (Table 2; Fig. 1, Fig. 2c). Oxitard (400 mg/kg, p.o.) showed less antioxidant activity as compared to Oxitard (200 mg/kg, p.o.). This may be due to saturated dose of Oxitard.
Table 2.
Effect of Oxitard on oxidative stress and hematological parameters in control and CS and CUS-induced stress in rodents.
| Groups | Oxidative stress parameters |
Hematological parameters |
|||
|---|---|---|---|---|---|
| MDA level (nM/mg protein) | GSH level (μg/mg protein) | CAT level (U/mg protein) | RBC (×106/μl) | WBC (×103/μl) | |
| Control in CS | 5.397 ± 0.602 | 2.953 ± 0.055 | 3.970 ± 0.320 | 5.36 ± 0.29 | 4.20 ± 0.22 |
| Control in CUS | 3.320 ± 0.195 | 2.487 ± 0.081 | 1.340 ± 0.087 | – | – |
| CS | 11.10 ± 1.09### | 1.607 ± 0.051### | 2.467 ± 0.208### | 7.71 ± 0.42### | 6.47 ± 0.42### |
| CUS | 5.627 ± 0.142### | 1.510 ± 0.096### | 0.283 ± 0.085### | – | – |
| Buspirone + CS | 6.363 ± 0.578∗∗∗ | 2.813 ± 0.051∗∗∗ | 3.720 ± 0.154∗∗∗ | 5.51 ± 0.33∗∗∗ | 4.30 ± 0.26∗∗∗ |
| Buspirone + CUS | 3.723 ± 0.127∗∗∗ | 2.403 ± 0.112∗∗∗ | 1.200 ± 0.046∗∗∗ | – | – |
| Oxitard (200 mg/kg) + CS | 7.250 ± 0.547∗∗∗ | 2.287 ± 0.065∗∗∗ | 3.573 ± 0.097∗∗∗ | 5.18 ± 0.21∗∗∗ | 4.28 ± 0.29∗∗∗ |
| Oxitard (200 mg/kg) + CUS | 4.217 ± 0.189∗∗ | 2.303 ± 0.11∗∗ | 1.153 ± 0.045∗∗∗ | – | – |
| Oxitard (400 mg/kg) + CS | 8.60 ± 0.957∗∗∗ | 2.02 ± 0.089∗∗ | 3.133 ± 0.10∗∗ | 6.32 ± 0.25∗∗ | 4.76 ± 0.21∗∗ |
| Oxitard (400 mg/kg) + CUS | 4.32 ± 0.126∗∗∗ | 1.62 ± 0.08∗∗ | 0.820 ± 0.061∗∗ | – | – |
Values are mean ± SD (n = 6). CS= Chronic stress, CUS= Chronic unpredictable stress. Significant values were compared with ***p < 0.001 vs CS/CUS groups, **p < 0.01 vs CS/CUS groups & ###p < 0.001 vs control groups. Hematological parameters were not evaluated in CUS model.
Fig. 1.
Effect of Oxitard on ex vivo antioxidant properties in CS model Values are expressed as mean ± SD. (n = 6) Significant values were compared with ***p < 0.001 vs CS group, **p < 0.01 vs CS group & ###p < 0.001 vs control group.
Fig. 2.
Effect of Oxitard on ex vivo antioxidant properties in CUS model Values are expressed as mean ± SD. (n = 6) Significant values were compared with ***p < 0.001 vs CUS group, **p < 0.01 vs CUS group & ###p < 0.001 vs control group.
3.2. Effect of Oxitard on hematological parameters
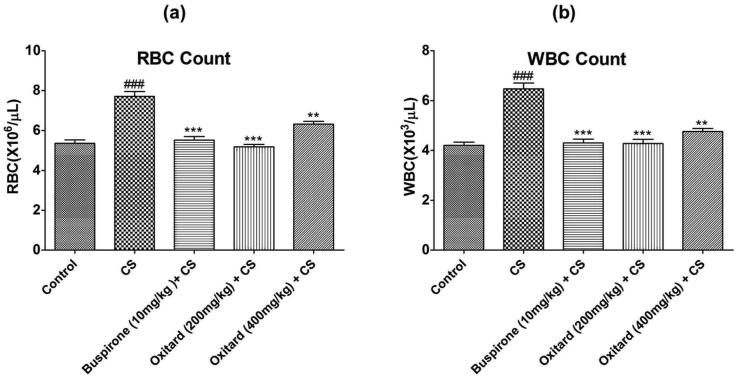
Exposure to CS elevated the RBC and WBC count (Table 2; Fig. 3a and b). Treatment with Oxitard (200 and 400 mg/kg; p.o.) showed significant decline in RBC and WBC count as compared to stressed animals (Table 2; Fig. 3a and b).
Fig. 3.
Effect of Oxitard on hematological parameters in CS induced stress in rats. Values are expressed as mean ± SD. (n = 6) Significant values were compared with ***p < 0.001 vs CS group, **p < 0.01 vs CS group & ###p < 0.001 vs control group.
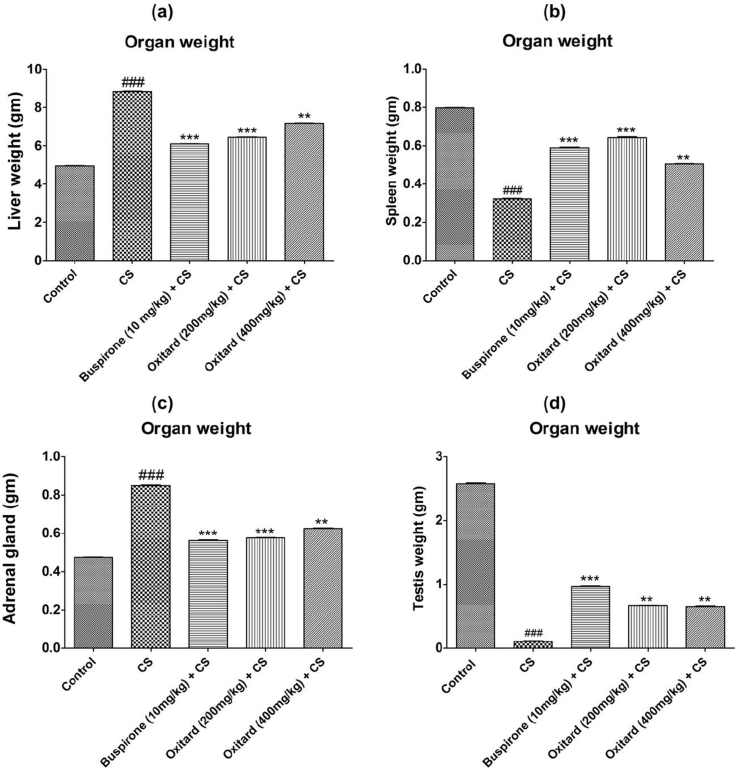
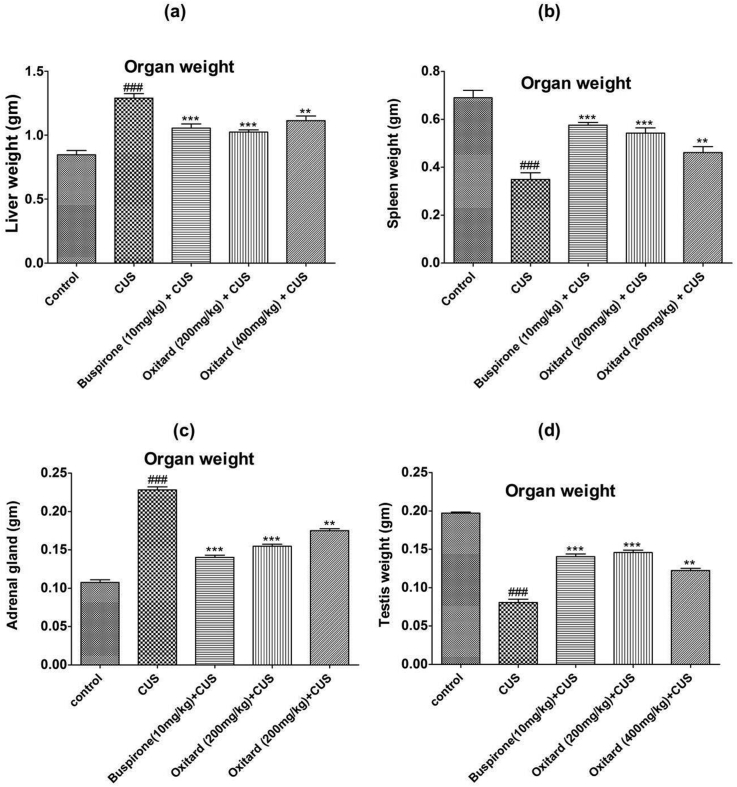
3.3. Effect of Oxitard on organ weight
The weights of the organs like liver and adrenal gland were increased, while the weight of spleen and testis was reduced in stressed animals (Table 3; Fig. 4, Fig. 5a–d). Treatment with Oxitard (200 mg/kg and 400 mg/kg; p.o.) significantly reduced the weight of the liver, and adrenal gland, and significantly increased the weight of the spleen and testis as compared to stressed animals (Fig. 4, Fig. 5a–d).
Table 3.
Effect of Oxitard on organ weight and ulcer index in control, CS and CUS-induced-stress in rodents.
| Groups | Organ weights (gm/100 gm body weight) |
Ulcer Index | |||
|---|---|---|---|---|---|
| Liver | Spleen | Adrenal | Testis | ||
| Control in CS | 4.943 ± 0.04 | 0.80 ± 0.002 | 0.48 ± 0.004 | 2.57 ± 0.02 | 0 |
| Control in CUS | 0.847 ± 0.06 | 0.690 ± 0.054 | 0.107 ± 0.07 | 0.197 ± 0.002 | 0 |
| CS | 8.83 ± 0.05### | 0.32 ± 0.004### | 0.85 ± 0.008### | 0.109 ± 0.012### | 14 ± 0.64### |
| CUS | 1.29 ± 0.06### | 0.349 ± 0.05### | 0.228 ± 0.006### | 0.081 ± 0.007### | 13 ± 0.64### |
| Buspirone + CS | 6.09 ± 0.01∗∗∗ | 0.59 ± 0.006∗∗∗ | 0.56 ± 0.006∗∗∗ | 0.973 ± 0.015∗∗∗ | 8 ± 0.62∗∗∗ |
| Buspirone + CUS | 1.06 ± 0.06∗∗∗ | 0.576 ± 0.02∗∗∗ | 0.140 ± 0.005∗∗∗ | 0.140 ± 0.006∗∗∗ | 7 ± 0.65∗∗∗ |
| Oxitard (200 mg/kg) + CS | 6.44 ± 0.03∗∗∗ | 0.64 ± 0.007∗∗∗ | 0.58 ± 0.004∗∗∗ | 0.670 ± 0.007∗∗ | 8 ± 0.64∗∗∗ |
| Oxitard (200 mg/kg) + CUS | 1.02 ± 0.03∗∗∗ | 0.542 ± 0.04∗∗∗ | 0.155 ± 0.005∗∗∗ | 0.146 ± 0.005∗∗∗ | 8 ± 0.55∗∗∗ |
| Oxitard (400 mg/kg) + CS | 7.18 ± 0.04∗∗ | 0.51 ± 0.003∗∗ | 0.63 ± 0.005∗∗ | 0.653 ± 0.021∗∗ | 12 ± 0.77∗∗ |
| Oxitard (400 mg/kg) + CUS | 1.11 ± 0.06∗∗ | 0.461 ± 0.04∗∗ | 0.175 ± 0.004∗∗ | 0.122 ± 0.004∗∗ | 11 ± 1.30∗∗ |
Values are mean ± SD (n = 6). CS= Chronic stress, CUS= Chronic unpredictable stress. Significant values were compared with ***p < 0.001 vs CS/CUS groups, **p < 0.01 vs CS/CUS groups & ###p < 0.001 vs control groups.
Fig. 4.
Effect of Oxitard on organ weight in CS induced-stress rats. Values are expressed as mean ± SD. (n = 6) Significant values were compared with ***p < 0.001 vs CS group, **p < 0.01 vs CS group & ###p < 0.001 vs control group.
Fig. 5.
Effect of Oxitard on organ weight in CUS induced-stress rats Values are expressed as mean ± SD. (n = 6) Significant values were compared with ***p < 0.001 vs CUS group, **p < 0.01 vs CUS group & ###p < 0.001 vs control group.
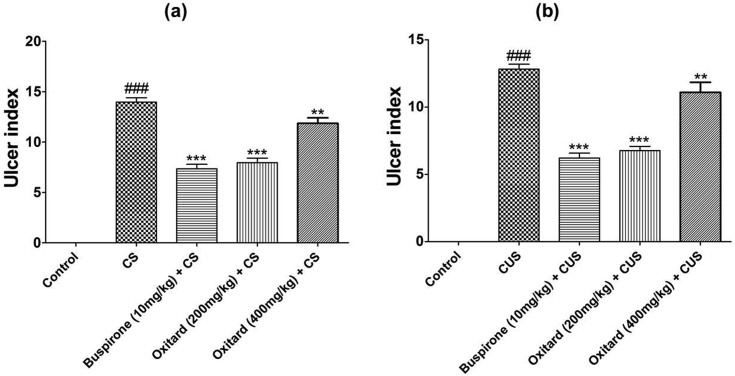
3.4. Effect of Oxitard on pathological changes
CS and CUS produced significant increase in ulcer index as compared to the control groups (Table 3; Fig. 6a and b). Treatment with Oxitard (200 and 400 mg/kg p.o.) significantly attenuated the stress-induced rise in ulcer index (Table 3; Fig. 6a and b).
Fig. 6.
Effect of Oxitard on ulcer index CS and CUS induced stress in rats. Values are expressed as mean ± SD (n = 6). Significant values were compared with ***p < 0.001 vs CS, CUS group, **p < 0.01 vs CS, CUS group & ###p < 0.001 vs control group.
4. Discussion
In the present study, CS and CUS brought about noteworthy rise of MDA (a marker of lipid peroxidation) and decrease in the levels of GSH (an endogenous anti-oxidant). According to previous reports, free radicals have been produced by stress [33], which further increase corticosterone secretion through hyper-actuation of the HPA axis [34]. On the other hand, HPA over-stimulation brought about by free radicals elevates corticosterone secretion by disrupting hippocampal neurons, which sustain the homeostasis of the HPA axis by negative feedback system [35]. Previous study signifies the key role of free radicals in stress-induced related pathological effects and biochemical imbalance [36]. Additionally, stress reduces the GSH level and prompts to elevated ROS levels in rodent tissues [37]. Strong evidence suggests that the GSH system facilitates an enzymatic antioxidant defense system against hydrogen peroxide (H2O2) [4], [38]. Furthermore, an immobilization stress leads to lipid peroxidation by overproduction of free radicals, mainly in cell membranes [39]. Previous studies also showed a significant reduction of CAT level in stressed animals [40]. In the present study, pre-treatment with Oxitard improvised CS and CUS-associated oxidative stress parameters in terms of elevation of GSH, CAT and decrease in MDA levels, signifying that adaptogenic potential of Oxitard might be contributed by its free radical scavenging property.
Increased secretion of stress hormones, which are identified to increase the mRNA levels and metabolic activities in the hepatic cells, could be the reason behind increased weight of liver during stress. Furthermore, adrenal hyperplasia and hypertrophy is known to be caused by powerful stimulation of the adrenal glands throughout delayed stress conditions [41], [42]. Stress induced adrenomedullary response leads to hyperactivity of adrenals in stressed animals which increases the production of corticotropic hormone and ultimately results in increased weight of adrenals [43], [44]. The result indicates that the decreased weight of liver and adrenal glands following pre-treatment with Oxitard might be due to the reversal of stress induced adrenomedullary response and consequently decreased production of corticotropic hormone. Decreased weight of spleen during stress is due to its contraction which releases more amount of blood (RBC) into circulation [45]. Increased weight of spleen following pre-treatment with Oxitard might be due to the inhibition of recruitment of lymphocytes to blood from spleen. The weight of testis decreases because there is destruction of spermatogenesis and reduction of testosterone levels during stress [46]. Pre-treatment with Oxitard significantly increased weight of testis indicating its anti-stress property. Stress also causes alteration in hematological parameters like increase in total and differential WBC counts [43], [47]. Significant reduction of RBC and WBC counts as compared to stressed rats following pre-treatment of Oxitard authenticated the adaptogenic potential of Oxitard. Since gastric mucosal blood flow plays an essential role in defensive mechanism of gastric mucosa, its disruption results in beginning of ulcers [48]. In our study, Oxitard treatment significantly diminished the occurrences and severities of ulcers caused by CS and CUS. This further validated the adaptogenic potential of Oxitard. Thus Oxitard could be a better candidate in the treatment of stress.
5. Conclusion
Oxitard showed antioxidant property and prevented the stress-induced alterations in body weight, organ weight, ulcer index and hematological parameters, indicating its protective effect against stress. Oxitard-facilitated antioxidant actions might be the reason behind its defensive effects in stress-induced dysfunctional homeostasis. However, further experiments are required to discover the mechanism behind the adaptogenic potential of Oxitard utilizing different animal models of stress.
Conflict of interest
The authors declare that there is no conflict of interest.
Acknowledgement
The authors acknowledge the financial support provided by the management of Hygia Institute of Pharmaceutical Education & Research, Lucknow.
Footnotes
Peer review under responsibility of Transdisciplinary University, Bangalore.
References
- 1.Kvetnansky R., Pacak K., Fukuhara K., Viskupic E., Hiremagalur B., Nankova B. Sympathoadrenal system in stress. Interaction with the hypothalamic–pituitary–adrenocortical system. Ann NY Acad Sci. 1995;771:131–158. doi: 10.1111/j.1749-6632.1995.tb44676.x. [DOI] [PubMed] [Google Scholar]
- 2.Bhatia N., Jaggi A.S., Singh N., Anand P., Dhawan R. Adaptogenic potential of curcumin in experimental chronic stress and chronic unpredictable stress-induced memory deficits and alterations in functional homeostasis. J Nat Med. 2011;65:532–543. doi: 10.1007/s11418-011-0535-9. [DOI] [PubMed] [Google Scholar]
- 3.Venihaki M., Gravanis A., Margioris A.N. Comparative study between normal rat chromaffin and PC12 rat pheochromocytoma cells: production and effects of corticotropin releasing hormone (CRH) Endocrinology. 1997;138:698–704. doi: 10.1210/endo.138.2.4916. [DOI] [PubMed] [Google Scholar]
- 4.Sahin E., Gumuslu S. Immoblization stress in rat tissue: alterations in protein oxidation, lipid peroxidation and antioxidant defense system. Comperative Biochem Physiol Part C Toxicol Pharmacol. 2007;144:342–347. doi: 10.1016/j.cbpc.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 5.Shivakumar H., Javed T., Prakash T., Rao R.N., Swamy B.J., Goud A.V. Adaptogenic activity of ethenolic extract of Tribulus terrestris L. J Nat Remedies. 2006;6:87–95. [Google Scholar]
- 6.Yadin E., Thomas E. Stimulation of the lateral septum attenuates immobilization-induced stress ulcers. Physiol Behav. 1993;59:883–886. doi: 10.1016/0031-9384(95)02184-1. [DOI] [PubMed] [Google Scholar]
- 7.Roy M.P., Kirschbaum C., Steptoe A. Psychological cardiovascular and metabolic correlates of individual differences in cortisol stress recovery in young men. Psychoneuroendocrinology. 2001;26:375–391. doi: 10.1016/s0306-4530(00)00061-5. [DOI] [PubMed] [Google Scholar]
- 8.Purret S.B. Quantitative aspects of stress-induced immunomodulation. Int Immunol Pharmacol. 2001;1:507–520. doi: 10.1016/s1567-5769(00)00030-8. [DOI] [PubMed] [Google Scholar]
- 9.Fitzpatrick F., Christedd N., Durant S., Dardenne M., Nunez E.A., HomoDelarche F. Glucocorticoids in the nonobese diabetic (NOD) mouse: basal serum levels, effect of endocrine manipulation and immobilization stress. Life Sci. 1992;50:1063–1069. doi: 10.1016/0024-3205(92)90102-u. [DOI] [PubMed] [Google Scholar]
- 10.Rai D., Bhatia G., Palit G., Pal R., Singh S., Singh H.K. Adaptogenic effects of Bacopa monniera (Brahmi) Pharmacol Biochem Behav. 2003;75:823–830. doi: 10.1016/s0091-3057(03)00156-4. [DOI] [PubMed] [Google Scholar]
- 11.Rai D., Bhatia G., Sen T., Palit G. Anti-stress effects of Ginkgo biloba and Panax ginseng: a comparative study. J Pharamcol Sci. 2001;93:458–464. doi: 10.1254/jphs.93.458. [DOI] [PubMed] [Google Scholar]
- 12.Ravindran R., Rathinaswamy S.D., Samson J., Senthilvelan M. Noise-stress-induced brain neurotransmitter changes and the effect of Ocimum sanctum (Linn) treatment in albino rats. J Pharm Sci. 2005;98:354–360. doi: 10.1254/jphs.fp0050127. [DOI] [PubMed] [Google Scholar]
- 13.Muruganandam A.V., Bhattacharya S.K. Adaptogenic activity of Withania somnifera: an experimental study using a rat model of chronic stress. Pharmacol Biochem Behav. 2003;75:547–555. doi: 10.1016/s0091-3057(03)00110-2. [DOI] [PubMed] [Google Scholar]
- 14.Siripurapu K.B., Gupta P., Bhatia G., Maurya R., Nath C., Palit G. Adaptogenic and anti-amnesic properties of Evolvulus alsinoides in rodents. Pharmacol Biochem Behav. 2005;81:424–432. doi: 10.1016/j.pbb.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 15.Muruganandam A.V., Kumar V., Bhattacharya S.K. Effect of poly herbal formulation, EuMil, on chronic stress-induced homeostatic perturbations in rats. Indian J Exp Biol. 2002;40:1151–1160. [PubMed] [Google Scholar]
- 16.Anssari M.Z., Fasiuddin M., Salman S., Nazer S., Imran M., Toufeeq M. Pharmacological screening of polyherbal formulation for anti-stress activity on albino rats. Int J Pharmacol Res. 2015;5:125–128. [Google Scholar]
- 17.Bhattacharya S.K., Bhattacharya A., Chakrabarti A. Adaptogenic activity of Siotone, a polyherbal formulation of Ayurvedic rasayanas. Indian J Exp Biol. 2000;28:119–128. [PubMed] [Google Scholar]
- 18.Patil S., Halgatti V., Maheshwari S., Santosh B.S. Comparative study of the efficacy of herbal antioxidants oxitard and aloe vera in the treatment of oral submucous fibrosis. J Clin Exp Dent. 2014;6:265–270. doi: 10.4317/jced.51424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singh B.P., Mittal N., Sharma V., Palani Evaluation of role of oxitard capsules in the treatment of oral submucous fibrosis. Antiseptic. 2009;106:103–107. [Google Scholar]
- 20.Olalde Rangel J.A. The systemic theory of living systems and relevance to CAM. Part I: the theory. Evid Based Complement Alternat Med. 2005;2:13–18. doi: 10.1093/ecam/neh068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Olalde Rangel J.A., Magarici M., Amendola F., del Castillo O. The systemic theory of living systems. Part IV: systemic medicine the Praxis. Evid Based Complement Alternat Med. 2005;2:429–439. doi: 10.1093/ecam/neh139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Olalde Rangel J.A. The systemic theory of living systems and relevance to CAM: the theory (Part II) Evid Based Complement Alternat Med. 2005;2:129–137. doi: 10.1093/ecam/neh093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Olalde Rangel J.A. The systemic theory of living systems and relevance to CAM: the theory (Part III) Evid Based Complement Alternat Med. 2005;2:267–275. doi: 10.1093/ecam/neh119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schneiderman N., Ironson G., Siegel S.D. Stress and health: psychological, behavioral, and biological determinants. Annu Rev Clin Psychol. 2005;1:607–628. doi: 10.1146/annurev.clinpsy.1.102803.144141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zheng J., Dobner A., Babygirija R., Ludwig K., Takahashi T. Effects of repeated restraint stress on gastric motility in rats. Am J Physiol Regul Integr Comp Physiol. 2009;296:R1358–R1365. doi: 10.1152/ajpregu.90928.2008. [DOI] [PubMed] [Google Scholar]
- 26.Kvetnansky R., Mikulai L. Adrenal and urinary catecholamines in rats during adaptation to repeated immobilization stress. Endocrinology. 1970;81:738–743. doi: 10.1210/endo-87-4-738. [DOI] [PubMed] [Google Scholar]
- 27.Ortiz J., Fitizgerald L.W., Lane S., Terwillinger R., Nestler E.J. Biochemical adaptations in the mesolimbic dopamine system in response to repeated stress. Neuropsychopharmacology. 1996;14:443–452. doi: 10.1016/0893-133X(95)00152-4. [DOI] [PubMed] [Google Scholar]
- 28.Kozinets G.I., Makarov V.A. Triada-X; Moscow: 1997. Investigations of blood systems in clinical practice; pp. 189–193. [in Russian] [Google Scholar]
- 29.Moron M.S., Depierre J.W. Levels of glutathione, glutathione reductase and glutathione S- transferase activities in rat lung and liver. Biochem Biophys Acta. 1979;582:67–78. doi: 10.1016/0304-4165(79)90289-7. [DOI] [PubMed] [Google Scholar]
- 30.Mihara M., Uchiyama M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal Biochem. 1978;86:271–278. doi: 10.1016/0003-2697(78)90342-1. [DOI] [PubMed] [Google Scholar]
- 31.Sinha A.K. Colorimetric assay of catalase. Anal Biochem. 1972;47:389–394. doi: 10.1016/0003-2697(72)90132-7. [DOI] [PubMed] [Google Scholar]
- 32.Takagi K., Okabe S. The effects of drugs on the production and recovery processes of the stress ulcer. Jpn J Pharmacol. 1968;18:9–11. doi: 10.1254/jjp.18.9. [DOI] [PubMed] [Google Scholar]
- 33.Liu J., Wang X., Mori A. Immobilization stress-induced antioxidant defense changes in rat plasma: effect of treatment with reduced glutathione. Int J Biochem. 1994;26:511–517. doi: 10.1016/0020-711x(94)90008-6. [DOI] [PubMed] [Google Scholar]
- 34.Liu J., Wang X., Shigenaga M.K., Yeo H.C., Mori A., Ames B.N. Immobilization stress causes oxidative damage to lipid, protein, and DNA in the brain of rats. FASEB J. 1996;10:1532–1538. [PubMed] [Google Scholar]
- 35.Sapolsky R.M., Krey L.C., McEwen B.S. The neuroendocrinology of stress and aging: the glucocorticoid cascade hypothesis. Endocr Rev. 1986;7:284–301. doi: 10.1210/edrv-7-3-284. [DOI] [PubMed] [Google Scholar]
- 36.Olivenza R., Moro M.A., Lizasoain I., Lorenzo P., Fernandez A.P., Rodrigo J. Chronic stress induces the expression of inducible nitric oxide synthase in rat brain cortex. J Neurochem. 2000;74:785–791. doi: 10.1046/j.1471-4159.2000.740785.x. [DOI] [PubMed] [Google Scholar]
- 37.Tang Y., Zhong Z. Obtusifolin treatment improves hyperlipidemia and hyperglycemia: possible mechanism involving oxidative stress. Cell Biochem Biophy. 2014;70:1751–1757. doi: 10.1007/s12013-014-0124-0. [DOI] [PubMed] [Google Scholar]
- 38.Gupta V., Lahiri S.S., Sultana S., Tulsawani R.K., Kumar R. Anti-oxidative effect of Rhodiola imbricata root extract in rats during cold, hypoxia and restraint (C–H–R) exposure and poststress recovery. Food Chem Toxicol. 2010;48:1019–1025. doi: 10.1016/j.fct.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 39.Dringen R. Metabolism and functions of glutathione in brain. Prog Neurobiol. 2000;62:649–671. doi: 10.1016/s0301-0082(99)00060-x. [DOI] [PubMed] [Google Scholar]
- 40.Samarghandian S., Farkhondeh T., Samini F., Borji A. Protective effects of carvacrol against oxidative stress induced by chronic stress in rat's brain, liver, and kidney. Biochem Res Int. 2016;2016:1–7. doi: 10.1155/2016/2645237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tuli J.S., Smith J.A., Morton D.B. Effects of acute and chronic restraint on the adrenal gland weight and serum corticosterone concentration of mice and their fecal output of oocyst after infection with eimueria apionodes. Res Vet Sci. 1995;59:82–86. doi: 10.1016/0034-5288(95)90036-5. [DOI] [PubMed] [Google Scholar]
- 42.Marti O., Gavalda A., Jolin T., Armario A. Effect of regulatory exposure to chronic immobilization stress on the circadian pattern of pituitary adrenal hormones, growth hormone and thyroid stimulating hormone in the adult male rat. Psychoneuroendocrinol. 1993;18:67–77. doi: 10.1016/0306-4530(93)90056-q. [DOI] [PubMed] [Google Scholar]
- 43.Jiban D., Tigari P., Roopa K., Dupadahalli K., Praveen S. An experimental evaluation of anti-stress effects of Terminalia chebula. J Physiol Biomed Sci. 2011;24:13–19. [Google Scholar]
- 44.Lakshmi B.V.S., Sudhakar M. Adaptogenic activity of Lagenaria siceraria: an experimental study using acute stress models on rats. J Pharmacol Toxicol. 2009;4:300–306. [Google Scholar]
- 45.Sharma B., Gouda T.S., Rao N.V., Shalam M., Shantakumar S.M., Narasu M.A. Study on adaptogenic activity of stem extracts of Tinospora malabarica (lamk) Pharmacol Online. 2007;1:349–358. [Google Scholar]
- 46.Jyoti R., Pandey S.N., Srivastava R.K. Effect of immobilization stress on spermatogenesis of albino rats. J Anat Soc India. 2003;52:55–57. [Google Scholar]
- 47.Takano T. Additive effect of nitrogen oxides and cold stress on circulating leukocyte counts in rats. Toxicol Lett. 1983;17:289–291. doi: 10.1016/0378-4274(83)90240-0. [DOI] [PubMed] [Google Scholar]
- 48.Singh G.K., Rai G., Chatterjee S.S., Kumar V. Beneficial effects of fumaria indica on chronic stress-induced neurobehavioral and biochemical perturbations in rats. Chin Med. 2012;3:49–60. [Google Scholar]