Abstract
Background
Ferula assa foetida commonly consumed as a healthy beverage has been demonstrated to have various biological activities, including antioxidation, anti-obesity and anti-cancer.
Objective
Our study aims to investigate the antitumor effect of asafoetida in vivo using mouse mammary carcinoma 4T1 cells.
Materials and methods
In the study, female BALB/c mice were divided into two groups (n = 6), which were control (untreated) and other group of mice with breast cancer treated with 100 mg/kg of asafoetida, respectively, by oral gavage. All mice were injected into the mammary fat pad with 4T1 cells (1 × 105 4T1 cells/0.1 ml of phosphate buffer solution). Asafoetida was administered on day 15 after the tumor had developed for 3 weeks. At end of experiment, tumor weight, tumor volume and tumor burden were measured and lung, liver, kidney and tumor were harvested and sections were prepared for histopathological analysis. Lipoxygenase inhibitory and antioxidant activity of asafoetida was also determined.
Results
Our results showed that treatment with asafoetida was effective in decreasing the tumor weight and tumor volume in treated mice. Body weight significantly increased in female BALB/c mice against control. Apart from the antitumor effect, asafoetida decreased lung, liver and kidney metastasis and also increased areas of necrosis in the tumor tissue respectively.
Conclusions
The present study demonstrated that asafoetida has potent antitumor and antimetastasis effects on breast cancer and is a potential source of natural antitumor products.
Keywords: Asafoetida, Breast cancer, 4T1 cell, Metastasis
1. Introduction
Cancer is a major public health problem in many parts of the world. It is currently the second leading cause of death and is expected to surpass heart diseases as the leading cause of death in the next few years [1]. In industrialized countries, breast cancer is the most common type of cancer among women [2]. Despite the advance of cancer prevention methods early diagnostic detection and used of anti-estrogenic drugs such as tamoxifen and raloxifene, breast cancer is still the most commonly diagnosed cancer that contributes to the second highest cause of cancer associated mortality in women [3]. The conventional therapies for cancer include chemo- and radiotherapies mediated by inducing apoptosis or inhibiting proliferation in neoplastic cells [4]. These therapies cause damage to healthy tissues around the tumors [5] and also develop resistance by numerous tumors [6]. Researchers have been studying alternatives of cancer therapy by applying potential biological molecules to target neoplastic tumors [7]. Herbs have been identified as an important source of novel bioactive compounds for medicine development including cancer chemotherapeutic drugs [8].The efficacy of these herbal chemopreventive agents has been related with their antioxidant potential to reduce or inhibit free radical mediated damage to cellular macromolecules, such as DNA, lipids and proteins [9]. Ferula assa-foetida L. is one of these species that grows wildly in central area of Iran. The part used of this plant and several other species of Ferula is an oleo gum resin [asafoetida] that obtained by incision of stem and root [10]. In Iranian folk medicine, asafoetida is considered as an antispasmodic, anthelminthic, sedative, anticonvulsant and carminative agent [11]. Recent pharmacological and biological studies have verified several pharmacological activities such as antioxidant [12], antileishmanial [13], anticonvulsant [14] anti-diabetic [15], antispasmodic [16], hypotensive [17], and antinociceptive [18] from this oleo gum resin. A meta-analysis study showed that in countries like Japan, Russia, China, Indonesia, the rate of cancer is higher in the comparison of countries that usage of asafoetida is common [19]. Recent studies have revealed its potential antioxidant, antimutagenicity and cancer chemopreventive activities [11]. Although some evidences has been shown that asafoetida could act as anticarcinogen, to our knowledge, no studies are available demonstrating the breast cancer chemopreventive efficacy of F. assa foetida oleo gum resin using an in vivo model of breast cancer. This study provides the scientific evidence for the chemopreventive potential of asafoetida against breast cancer induced by 4T1 cells in BALB/c mice.
2. Materials and methods
2.1. Plant oleo-gum resin
F. assa foetida oleo-gum-resin was collected during the summer from Tabas region (Yazd province, Iran) during the summer and the plant species was botanically identified by Dr. Abbas Zarezadeh in Yazd Agricultural Research Center. The dried powder of asafoetida was soaked in distilled water overnight at room temperature and the yielded suspension was used orally. Concentrations and dosages of the extract were expressed as crude amount of dried oleo-gum-resin used in preparing the stock solution [20].
2.2. Animals and treatment
8-week old female BALB/c mice bred in animal house of Medical School of Shahid Sadoughi University of Medical Sciences were selected. The mice were housed in 12 h of light and dark cycle and fed with standard pellet and water ad libitum. 10 mice were inoculated with 1 × 105 4T1 cells/mice on left mammary fat pad subcutaneously. 2 weeks after cancer cell implantation, mice were randomly assigned into two groups: control group (normal saline) and asafoetida group (100 mg/kg BW, orally fed every day). Asafoetida treatment was continued until day 21 post-inoculation. At the end of the experiment, mice were sacrificed and tumor, lung, liver and kidney were removed for histopathological studies [21].
2.3. Measurement of body weight and tumor sizes
Body weight was measured every five days. Tumors sizes (tumor weight, tumor volume and tumor burden) was calculated at the end of experiment using the following formula: tumor volume (mm3) = [(width) 2 × length]/2 and tumor burden (%) = tumor volume (mm3)/body weight (g) × 100 [21].
2.4. Histopathological studies
Lungs, liver, kidney and tumor were harvested, fixed in 10% formaldehyde (Sigma–Aldrich, USA) and embedded in paraffin, cut into 5-μm sections, and stained with hematoxylin and eosin (H&E). Sections were evaluated for tumor cell cytology, mitotic rate, growth pattern, necrosis, and metastatic tumor nodules present on these tissues.
2.5. Lipoxygenase inhibition activity of asafoetida
The soybean 15-lipoxygenase was used to test the 15-LOX inhibitory activity of asafoetida. For this purpose, 50 ml of extract solution was added to test solution containing: 3 ml of phosphate buffer (0.1 M, pH = 8), 50 ml enzyme solution (final concentration: 167 U/ml) to achieve the enzyme inhibition between 20 and 80%. After 4 min incubation of test solution, the substrate (Linoleic acid, final concentration: 134 mM) was added and the change in absorbance was measured for 60 s at 234 nm. The IC50 value was calculated graphically using the slopes of absorbance curves. The enzyme solution was kept in ice and tested at intervals to ensure that the enzyme activity was constant. All experiments were performed by UV/Vis Unico Double Beam Spectrophotometer at 25 °C in triplicate [22].
2.6. Antioxidant activity assay of asafoetida
To determine the ability of asafoetida to scavenge DPPH radicals, freshly prepared ethanolic DPPH (0.1 mM; 1 ml) solution was added to different concentrations of asafoetida (20–200 μg/ml). After half an hour, the absorbance was recorded at 517 nm. Results were expressed as percentage inhibition as:
Where A Control is the absorbance of a DPPH solution without asafoetida, A Extract is the absorbance of the tested extract, which is equal to the absorbance of the asafoetida plus the DPPH (20 mg/L) minus the blank extract absorbance. The samples were run in triplicate and the mean value of three of them was recorded. The percentage inhibition was plotted against the sample concentration in order to calculate the IC50 values, which is the concentration (μg/ml) of asafoetida that causes 50% loss of DPPH activity [12].
2.7. Statistical analysis
Differences in the experiments were analyzed for statistical significance by Student's t-test (two-tailed). Results were expressed as mean ± standard error (SEM). Values of p < 0.05 were considered to be statistically significant.
3. Results
3.1. Measurement of body weight and tumor sizes
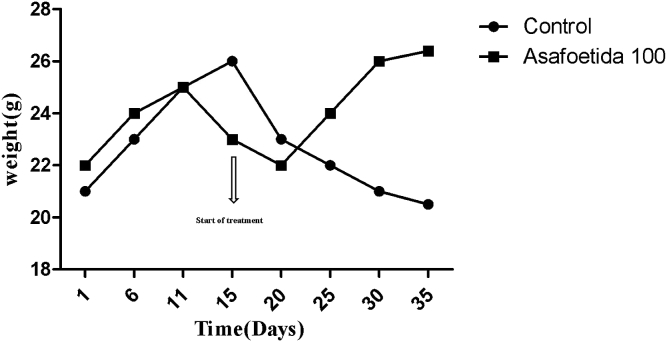
At the end of the experiment, tumors were excised from each animal for examination of tumor weight and assessment of the antitumor effect of asafoetida. Tumor weight, tumor volume and tumor burden decrease significantly in the asafoetida-treated group when compared with control (Table 1). Changes in the body weight of animals were studied during the experimental period of 5 weeks. It was observed that significant body weight loss was found in control group. After asafoetida administration, the body weight started to increase suggesting that asafoetida treatment ameliorated damage to overall body metabolism (Fig. 1).
Table 1.
Effect of asafoetida on tumor sizes in animals treated and compared to control group.
| Groups | Tumor volume (mm3) | Tumor weight (g) | Tumor burden (%) |
|---|---|---|---|
| Control | 1512 ± 125 | 2.3 ± 0.6 | 74.2 ± 9.2 |
| Asafoetida100 | 498 ± 44** | 1.2 ± 0.2* | 18.8 ± 4.5** |
Values represent the mean ± SEM, n = 5. *p < 0.05 and **p < 0.01.
Fig. 1.
Change in body weight of mice in control group and after treatment with asafoetida.
3.2. Microscopic study of breast cancer cells
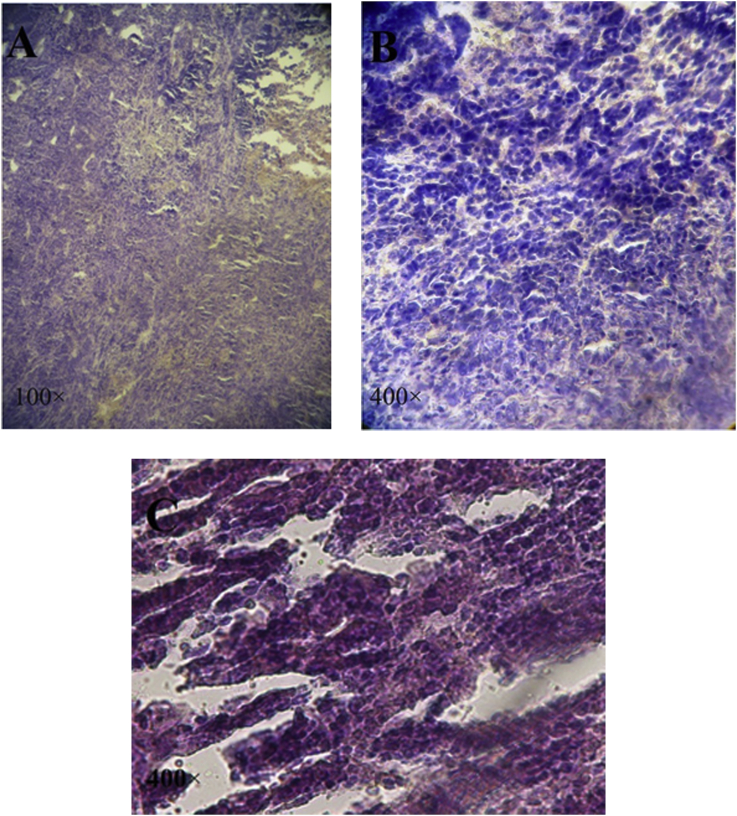
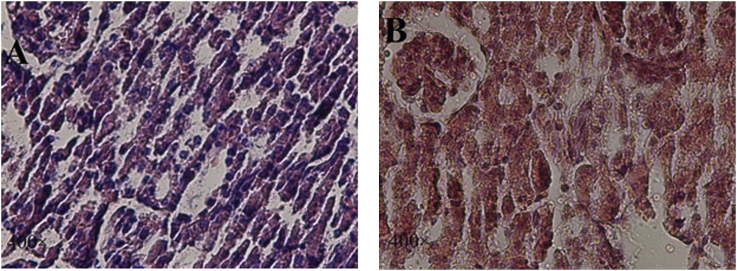
The histology sections of breast showed infiltration of tumor cells. In neoplastic cells large hyperchromatic nuclei and relatively small amount of cytoplasm were noted. These neoplastic cells showed special characteristics, including pleomorphic cells and nuclei, and giant cells. The results indicate cells under mitotic division in some areas. The neoplastic cells in primary tumor appeared in clusters or clumps (Fig. 2A and B). In most areas of samples treated with asafoetida, the tumor cells were necrotic and the results showed larger areas of necrosis. Tumor clumps in some areas of tissue were still seen (Fig. 2C).
Fig. 2.
Histology evaluation of primary tumor induced by 4T1, sections from mice untreated and treated with asafoetida. H&E staining as observed under a light microscope (100×, 400×). In primary tumor, the neoplastic cells were characterized by small amount of cytoplasm. Some cells were under mitotic division (Fig. 2A and B). In sections of treated asafoetida breast, numbers of tumor clumps were decreased (Fig. 2C).
3.3. Microscopic study of the lung
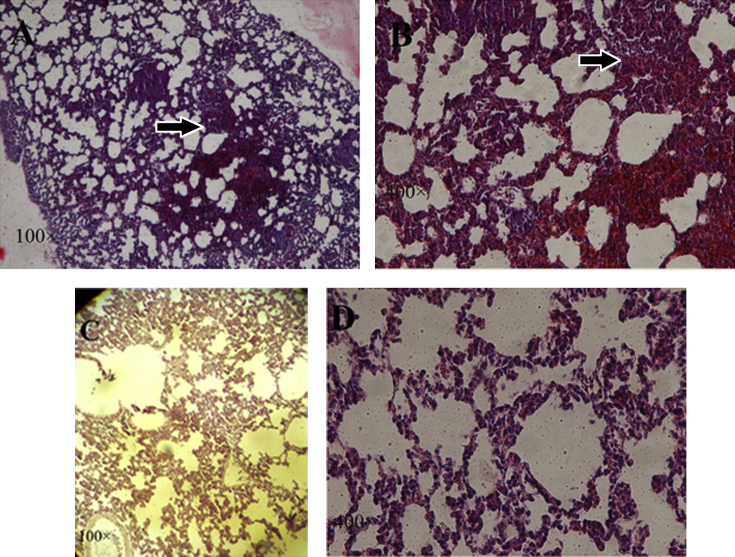
Examination of histological sections of the untreated lungs confirmed the presence of microscopic metastases. Untreated mice exhibited infiltration of neoplastic cells in the lungs and structural destruction of the pulmonary alveoli. The neoplastic cells were characterized by the presence of large hyperchromatic nuclei and small amount of cytoplasm. The metastatic cells in all areas of lungs appeared in clusters or clumps. These cells lining the alveoli and the alveolar septa had several layers (Fig. 3A and B). We investigated the ability of asafoetida to modulate breast cancer metastasis to the lung. In microscopic slides of treated lungs, the broad clumps of tumor cells were removed. Tumor cells were seen in some areas of the lung. The alveolar septum had fewer metastatic cells and therefore is less thick (Fig. 3C and D), suggesting that asafoetida inhibited the growth of breast cancer metastasis to the lung.
Fig. 3.
Histology evaluation of lungs sections from mice untreated and treated with asafoetida. H&E staining as observed under a light microscope (100×, 400×). Untreated mice exhibited infiltration of neoplastic cells in the lungs and in the form of clusters or clumps. These cells line the alveoli and the alveolar septa (Fig. 3A and B). In treated lungs, the clumps of tumor cells were removed. The alveolar septum is less thick (Fig. 3C and D). Metastatic cells were marked by arrows.
3.4. Microscopic study of the liver
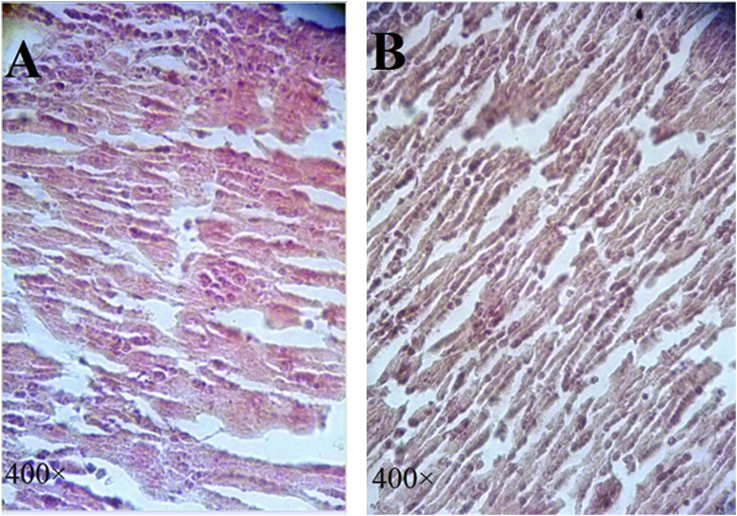
Examination of histological sections of the liver confirmed the presence of infiltration of neoplastic cells in the liver. The neoplastic cells were characterized by the presence of large hyperchromatic nuclei and small amount of cytoplasm. The metastatic cells in liver appeared in clusters or clumps. These metastatic cells were wider than the ropes of normal liver hepatocyte plates. The liver lobules were destroyed and central vein and the portal triad were not seen. A lot of irregular vascular architecture was seen in these samples (Fig. 4A). In treated animals with asafoetida, the number of tumor clusters is less than untreated liver. The tumor clusters have lost their integrity and there were necrotic cells, but in some areas, a few neoplastic cells were seen (Fig. 4B).
Fig. 4.
Histology evaluation of liver sections from mice untreated and treated with asafoetida. H&E staining as observed under a light microscope (400×). In untreated liver group, a large number of the tumors cells appeared in clusters or clumps. Central vein and portal triad were not seen and vascular architecture were irregular (Fig. 4A). In sections of the liver treated with asafoetida, there is mild disruption in tumor clusters or clumps (Fig. 4B).
3.5. Microscopic study of breast cancer metastasis to the kidney
Examination of histological sections of the kidney confirmed the presence of infiltration of neoplastic cells in the kidney. The neoplastic cells were characterized by the presence of large hyperchromatic nuclei and small amount of cytoplasm. The metastatic cells in kidney appeared in clusters or small clumps. A large number of tumor cells were similar to renal tubule epithelial cells. They had regular round nuclei and granular eosinophilic cytoplasm. A few number of tumor cells showed clear cytoplasm (clear cells) (Fig. 5A). Most tumor cells in samples treated with asafoetida were necrotic and a few number of tumor cells remained in the kidney (Fig. 5B).
Fig. 5.
Histology evaluation of kidney sections from mice untreated and treated with asafoetida. H&E staining as observed under a light microscope (400×). In untreated kidney group, a large number of the tumors cells appeared in clusters or clumps (Fig. 5A). In sections of the kidney treated with asafoetida, a few number of tumor cells remained (Fig. 5B).
3.6. Lipoxygenase inhibitory and radical scavenging activity
The LOX activity was measured as an increase in the absorbance at 234 nm, which reflects the formation of hydroperoxylinoleic acid. The IC50 asafoetida highest inhibitory effect was obtained at 32 μg/ml in this work; our results also showed that the IC50 of antioxidant activity of asafoetida was 109 μg/ml (Table 2).
Table 2.
Antioxidant and lipoxygenase inhibitory activities of asafoetida.
| DPPH (IC50) | Lipoxygenase inhibition (IC50) |
|---|---|
| 109 μg/ml | 32 μg/ml |
4. Discussion
In the present study, we aimed to evaluate the antitumor and antimetastasis effects of F. assa foetida oleo gum resin in a mouse mammary tumor model using the metastasis-specific mouse mammary carcinoma 4T1 cells. The 4T1 breast cancer cells are derived spontaneously from BALB/c mammary carcinoma [23]. These cells closely resemble metastatic breast cancer in human patients and could be used as a suitable model for evaluation of the efficacy of anti-cancer drugs [24]. In this study, our results showed that the weight of the asafoetida-treated group decreased for the first 2 weeks after breast cancer induction and started to increase after treatment with asafoetida until the end of the experiment. This effect could be due to the ability of asafoetida to counteract carcinogenicity/tumor burden, thereby preventing cachexia. Cancer cachexia is defined by loss of muscle mass and fat directly caused by tumor factors or indirectly caused by an aberrant host response to tumor presence [25]. According to the UK Home Office Regulation, weight loss is expected in mice induced with breast cancer and the mice can lose up to 25% of their body weight after 4 weeks of breast cancer induction [26]. In addition, the treatment groups showed a reduction in tumor volume and also the weight of tumors of the asafoetida-treated group was lower as compared to the control (Table 1). In a previous study, Bagheri et al. [10] demonstrated the cytotoxic effect of some Ferula species on Artemia salina as a model for evaluating general cytotoxicity [10]. In different studies, beneficial effects of some Ferula species as cancer chemopreventive has been investigated and demonstrated that these species could be as a good source of cancer chemopreventive agents [27]. Asafoetida is an oleo-gum-resin obtained from the Iranian endemic medicinal plant, F. assa foetida and traditionally it is being used for treatment of various disorders and also have several biological activities such as antioxidant and cancer chemopreventive [28]. In spite of some old evidence about genotoxicity and mutagenicity of asafoetida, recent studies have revealed its potential antioxidant, antimutagenicity and cancer chemopreventive activities [11]. In an in vivo study, Saleem et al. showed that pre-treatment of animals with acetone extract of asafoetida could cause the reversal of early events of carcinogenesis [29]. Another study showed that asafoetida reduced the multiplicity and size of palpable mammary tumors in Sprague–Dawley rats [11]. Mallikarjuna et al. has reported reduction in the multiplicity and size in mammary tumors in rats following asafoetida supplementation [30]. Phytochemical analysis showed that asafoetida contains about 40–64% resin, 25% endogenous gum, 10–17% volatile oil and 1.5–10% ash. The resin portion is known to contain farnesiferol, asareninotannols, ferulic acid, umbelliferone, the gum includes glucose, galactose, l-arabinose, rhamnose, glucuronic acid, polysaccharides and glycoproteins, and the volatile fraction contains sulfur-containing compounds, monoterpenes and other volatile terpenoids [11]. Umbelliprenin is one of these components that has been shown to have remarkable cancer chemoprevention in vitro and in vivo [31]. Moreover, it was reported recently that farnesiferol C from F. assa-foetida may be a potential candidate for the treatment of cancer [11]. Phenolic compounds like ferulic acid present in asafoetida have been reported to have strong antioxidants and antitumor properties [32]. Ferulic acid has also been reported to inhibit growth of human breast and colon cancer cells [33]. The efficacy of the natural products against cancer has been related with their antioxidant potential to reduce or inhibit free radical mediated damage to cellular macromolecules, such as DNA, lipids and proteins. Another possible mechanism of action for the active principles could be related to lipoxygenase activity [34]. Interestingly in this study, asafoetida showed a remarkable inhibitory activity against lipoxygenase and also antioxidant activity. According to these results, the IC50 of LOX activity of asafoetida was calculated as 32 μg/ml (Table 2). In this study we also investigated the treatment effects of asafoetida on histopathological and metastasis in lung, liver and kidney in animals treated with asafoetida and compared to histological changes in control group. This is the first time that asafoetida was demonstrated to be effective not only in decreasing the tumor weight but also in inhibiting lung, liver and kidney metastasis. In most areas of samples treated with asafoetida, the tumor cells were necrotic and the results showed larger areas of necrosis. Tumor clumps were still present in some areas of tissue. We also investigated the ability of asafoetida to modulate breast cancer metastasis to the lung. In treated lungs, the broad clumps of tumor cells were removed. The alveolar septum had fewer metastatic cells and therefore is less thick. In liver the tumor clusters have lost their integrity and there were necrotic cells, but in some areas, a few neoplastic cells were seen. Histopathological analysis on kidney of treated animals showed that most tumor cells were necrotic. These observations suggest that asafoetida under experimental conditions can affect breast carcinogenesis by altering histological changes indicating its anti-carcinogenic potential. The observed modulation in histoarchitecture by asafoetida could be ascribed to its ability to maintain cell structure and integrity either by some direct mechanism or indirectly by scavenging the free radicals. However, studies are required for further exploration with regard to other definitive bioassays including protein expression and documentation of specific molecular markers to establish the exact mechanism for asafoetida mediated cancer chemoprevention.
5. Conclusion
The results present the first evidence on the antitumor and antimetastasis effects of asafoetida as demonstrated by in vivo tumor weight and metastasis reduction in BALB/c mice bearing breast cancer tumors. More detailed molecular mechanisms, for instance, genomic and proteomic responses underlying asafoetida-induced breast cancer cell apoptotic cell death, and antimetastasis remain to be elucidated. Besides, further investigation is needed to determine the clinical efficacy and safety of asafoetida in human subjects with breast cancer metastasis.
Sources of funding
The financial of this research was supported by the research deputy of Yazd Shahid Sadoughi Medical University.
Conflict of interest
None declared.
Acknowledgments
The authors thank all people who have assisted the experimental procedure.
Footnotes
Peer review under responsibility of Transdisciplinary University, Bangalore.
References
- 1.Jemal A., Murray T., Ward E., Samuels A., Tiwari R.C., Ghafoor A. Cancer statistics, 2005. CA Cancer J Clin. 2005;55(1):10–30. doi: 10.3322/canjclin.55.1.10. [DOI] [PubMed] [Google Scholar]
- 2.Kamangar F., Dores G.M., Anderson W.F. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol. 2006;24(14):2137–2150. doi: 10.1200/JCO.2005.05.2308. [DOI] [PubMed] [Google Scholar]
- 3.Yeap S.K., Abu N., Mohamad N.E., Beh B.K., Ho W.Y., Ebrahimi S. Chemopreventive and immunomodulatory effects of Murraya koenigii aqueous extract on 4T1 breast cancer cell-challenged mice. BMC Complement Altern Med. 2015;15(1):1. doi: 10.1186/s12906-015-0832-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ghobrial I.M., Witzig T.E., Adjei A.A. Targeting apoptosis pathways in cancer therapy. CA Cancer J Clin. 2005;55(3):178–194. doi: 10.3322/canjclin.55.3.178. [DOI] [PubMed] [Google Scholar]
- 5.Baskar R., Lee K.A., Yeo R., Yeoh K.-W. Cancer and radiation therapy: current advances and future directions. Int J Med Sci. 2012;9(3):193. doi: 10.7150/ijms.3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morrison R., Schleicher S.M., Sun Y., Niermann K.J., Kim S., Spratt D.E. Targeting the mechanisms of resistance to chemotherapy and radiotherapy with the cancer stem cell hypothesis. J Oncol. 2010;2011 doi: 10.1155/2011/941876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Altieri D.C. The molecular basis and potential role of survivin in cancer diagnosis and therapy. Trends Mol Med. 2001;7(12):542–547. doi: 10.1016/s1471-4914(01)02243-2. [DOI] [PubMed] [Google Scholar]
- 8.Balunas M.J., Kinghorn A.D. Drug discovery from medicinal plants. Life Sci. 2005;78(5):431–441. doi: 10.1016/j.lfs.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 9.Galati G., O'Brien P.J. Potential toxicity of flavonoids and other dietary phenolics: significance for their chemopreventive and anticancer properties. Free Radic Biol Med. 2004;37(3):287–303. doi: 10.1016/j.freeradbiomed.2004.04.034. [DOI] [PubMed] [Google Scholar]
- 10.Bagheri S.M., Sahebkar A., Gohari A.R., Saeidnia S., Malmir M., Iranshahi M. Evaluation of cytotoxicity and anticonvulsant activity of some Iranian medicinal Ferula species. Pharm Biol. 2010;48(3):242–246. doi: 10.3109/13880200903081796. [DOI] [PubMed] [Google Scholar]
- 11.Iranshahy M., Iranshahi M. Traditional uses, phytochemistry and pharmacology of asafoetida (Ferula assa-foetida oleo-gum-resin)—a review. J Ethnopharmacol. 2011;134(1):1–10. doi: 10.1016/j.jep.2010.11.067. [DOI] [PubMed] [Google Scholar]
- 12.Bagheri S.M., Hedesh S.T., Mirjalili A., Dashti-R M.H. Evaluation of anti-inflammatory and some possible mechanisms of antinociceptive effect of Ferula assa foetida oleo gum resin. J Evid Based Complement Altern Med. 2016;21(4):271–276. doi: 10.1177/2156587215605903. 2156587215605903. [DOI] [PubMed] [Google Scholar]
- 13.Bafghi A.F., Bagheri S.M., Hejazian S.H. Antileishmanial activity of Ferula assa-foetida oleo gum resin against Leishmania major: an in vitro study. J Ayurveda Integr Med. 2014;5(4):223. doi: 10.4103/0975-9476.146567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bagheri S.M., Rezvani M.E., Vahidi A.R., Esmaili M. Anticonvulsant effect of Ferula assa-foetida oleo gum resin on chemical and amygdala-kindled rats. N Am J Med Sci. 2014;6(8):408. doi: 10.4103/1947-2714.139296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Azizian H., Ebrahim Rezvani M., Esmaeilidehaj M., Bagheri S.M. Anti-obesity, fat lowering and liver steatosis protective effects of Ferula asafoetida gum in type 2 diabetic rats: possible involvement of leptin. Iran J Diabetes Obes. 2012;4(3):120–126. [Google Scholar]
- 16.Bagheri S., Hejazian S., Dashti-R M. The relaxant effect of seed fs essential oil and oleo-gum-resin of Ferula assa-foetida on isolated rat's ileum. Ann Med Health Sci Res. 2015;4(2):238–241. doi: 10.4103/2141-9248.129050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fatehi M., Farifteh F., Fatehi-Hassanabad Z. Antispasmodic and hypotensive effects of Ferula asafoetida gum extract. J Ethnopharmacol. 2004;91(2):321–324. doi: 10.1016/j.jep.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 18.Bagheri S., Dashti-R M., Morshedi A. Antinociceptive effect of Ferula assa-foetida oleo-gum-resin in mice. Res Pharm Sci. 2014;9(3):207. [PMC free article] [PubMed] [Google Scholar]
- 19.Nigam U., Sachan S. Evaluation of Ferula asafoetida for its anticancerous activity in different countries. J Pharmacogn Phytochem. 2013;2(4) [Google Scholar]
- 20.Bagheri S.M., Yadegari M., Porentezari M., Mirjalili A., Hasanpor A., Dashti R.M.H. Effect of Ferula assa-foetida oleo gum resin on spermatic parameters and testicular histopathology in male wistar rats. J Ayurveda Integr Med. 2015;6(3):175. doi: 10.4103/0975-9476.146552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yazan L.S., Ong Y.S., Zaaba N.E., Ali R.M., Foo J.B., Tor Y.S. Anti-breast cancer properties and toxicity of Dillenia suffruticosa root aqueous extract in BALB/c mice. Asian Pac J Trop Biomed. 2015;5(12):1018–1026. [Google Scholar]
- 22.Mahdavi M., Shirazi M.S., Taherkhani R., Saeedi M., Alipour E., Moghadam F.H. Synthesis, biological evaluation and docking study of 3-aroyl-1-(4-sulfamoylphenyl) thiourea derivatives as 15-lipoxygenase inhibitors. Eur J Med Chem. 2014;82:308–313. doi: 10.1016/j.ejmech.2014.05.054. [DOI] [PubMed] [Google Scholar]
- 23.Tao K., Fang M., Alroy J., Sahagian G.G. Imagable 4T1 model for the study of late stage breast cancer. BMC Cancer. 2008;8(1):1. doi: 10.1186/1471-2407-8-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taheri A., Dinarvand R., Ahadi F., Khorramizadeh M.R., Atyabi F. The in vivo antitumor activity of LHRH targeted methotrexate–human serum albumin nanoparticles in 4T1 tumor-bearing Balb/c mice. Int J Pharm. 2012;431(1):183–189. doi: 10.1016/j.ijpharm.2012.04.033. [DOI] [PubMed] [Google Scholar]
- 25.Argilés J.M., Busquets S., Stemmler B., López-Soriano F.J. Cancer cachexia: understanding the molecular basis. Nat Rev Cancer. 2014;14(11):754–762. doi: 10.1038/nrc3829. [DOI] [PubMed] [Google Scholar]
- 26.Martin T.A., Parr C., Davies G., Watkins G., Lane J., Matsumoto K. Growth and angiogenesis of human breast cancer in a nude mouse tumour model is reduced by NK4, a HGF/SF antagonist. Carcinogenesis. 2003;24(8):1317–1323. doi: 10.1093/carcin/bgg072. [DOI] [PubMed] [Google Scholar]
- 27.Valiahdi S.M., Iranshahi M., Sahebkar A. Cytotoxic activities of phytochemicals from Ferula species. DARU J Pharm Sci. 2013;21(1):1. doi: 10.1186/2008-2231-21-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Panwar R., Satyavati Rana P., Dhawan D.K., Prasad K.K. Chemopreventive efficacy of different doses of Ferula asafoetida oleo-gum-resin against 1, 2-dimethylhydrazine (DMH) induced rat colon carcinogenesis. J Phytopharm. 2015;4(6):282–286. [Google Scholar]
- 29.Saleem M., Alam A., Sultana S. Asafoetida inhibits early events of carcinogenesis: a chemopreventive study. Life Sci. 2001;68(16):1913–1921. doi: 10.1016/s0024-3205(01)00977-8. [DOI] [PubMed] [Google Scholar]
- 30.Mallikarjuna G., Dhanalakshmi S., Raisuddin S., Rao A.R. Chemomodulatory influence of Ferula asafoetida on mammary epithelial differentiation, hepatic drug metabolizing enzymes, antioxidant profiles and N-methyl-N-nitrosourea-induced mammary carcinogenesis in rats. Breast Cancer Res Treat. 2003;81(1):1–10. doi: 10.1023/a:1025448620558. [DOI] [PubMed] [Google Scholar]
- 31.Iranshahi M., Sahebkar A., Takasaki M., Konoshima T., Tokuda H. Cancer chemopreventive activity of the prenylated coumarin, umbelliprenin, in vivo. Eur J Cancer Prev. 2009;18(5):412–415. doi: 10.1097/CEJ.0b013e32832c389e. [DOI] [PubMed] [Google Scholar]
- 32.Yan X., Murphy B.T., Hammond G.B., Vinson J.A., Neto C.C. Antioxidant activities and antitumor screening of extracts from cranberry fruit (Vaccinium macrocarpon) J Agric Food Chem. 2002;50(21):5844–5849. doi: 10.1021/jf0202234. [DOI] [PubMed] [Google Scholar]
- 33.Hudson E.A., Dinh P.A., Kokubun T., Simmonds M.S., Gescher A. Characterization of potentially chemopreventive phenols in extracts of brown rice that inhibit the growth of human breast and colon cancer cells. Cancer Epidemiol Biomarkers Prev. 2000;9(11):1163–1170. [PubMed] [Google Scholar]
- 34.Ikemoto S., Sugimura K., Kuratukuri K., Nakatani T. Antitumor effects of lipoxygenase inhibitors on murine bladder cancer cell line (MBT-2) Anticancer Res. 2004;24(2B):733–736. [PubMed] [Google Scholar]