ABSTRACT
Activation of caspase-11 by some Gram-negative bacteria triggers the caspase-1/interleukin 1β (IL-1β) pathway, independent of canonical inflammasomes. Acinetobacter baumannii is a Gram-negative, conditionally pathogenic bacterium that can cause severe pulmonary infection in hospitalized patients. A. baumannii was revealed to activate canonical and noncanonical inflammasome pathways in bone marrow-derived macrophages (BMDMs). Pulmonary infection of caspase-11−/− mice with A. baumannii showed that caspase-11 deficiency impaired A. baumannii clearance, exacerbated pulmonary pathological changes, and enhanced susceptibility to A. baumannii. These data indicate that the caspase-11-mediated innate immune response plays a crucial role in defending against A. baumannii.
KEYWORDS: caspase-11, caspase-1, inflammasome, A. baumannii
INTRODUCTION
Innate immune cells, such as macrophages, recognize invasive pathogens through pattern recognition receptors (PRRs). NOD-like receptors (NLRs), belonging to the PRRs, recruit pro-caspase-1 to assemble inflammasomes (1–3). Pro-caspase-1 is autocleaved into active caspase-1, which further cleaves pro-interleukin 1β (IL-1β) and pro-IL-18 to form mature IL-1β and IL-18. Activation of NLRs/caspase-1 inflammasomes is usually called the canonical inflammasome pathway. The NLRP3 inflammasome is one of the most important canonical inflammasomes and can be activated by a variety of microorganisms, including bacteria, viruses, and fungi (4–6).
In recent years, another member of the inflammatory caspase family, caspase-11, has been demonstrated to activate caspase-1/IL-1β independently of NLRs, which is called the noncanonical inflammasome pathway (7–9). Gram-negative bacteria are the major factors in caspase-11 activation, and lipopolysaccharide (LPS) has been identified as a cytoplasmic ligand for caspase-11 (7–9). Activation of both caspase-1 and caspase-11 can induce cell pyroptosis.
As a Gram-negative, conditionally pathogenic bacterium, Acinetobacter baumannii can cause severe skin, pulmonary, or urinary tract infection in hospitalized patients (10–12). It has been revealed that A. baumannii can activate Toll-like receptor 4 (TLR4), another cellular membrane PRR, as well as NF-κB (13–15). However, it is unclear whether A. baumannii can activate the caspase-11 pathway. In the present study, caspase-11 knockout (caspase-11−/−) mice were infected with A. baumannii to explore the role of caspase-11 in pulmonary A. baumannii infection.
RESULTS
A. baumannii can activate the canonical and noncanonical inflammasome pathways in BMDMs.
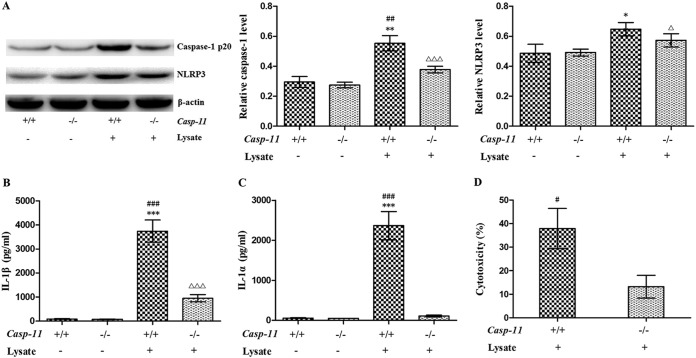
To investigate whether A. baumannii can activate the canonical and noncanonical inflammasome pathways, bone marrow-derived macrophages (BMDMs) from wild-type (caspase-11+/+) and caspase-11−/− mice were treated with A. baumannii lysate for 12 h. Caspase-1 and NLRP3 expression and IL-1β secretion were upregulated in both caspase-11+/+ BMDMs and caspase-11−/− BMDMs, indicating that A. baumannii can activate NLRP3 inflammasomes even though caspase-11 is deficient (Fig. 1A and B). However, induction of caspase-1 expression and IL-1β secretion was partially abrogated, and induction of IL-1α was completely abrogated, in caspase-11+/+ BMDMs when caspase-11 was knocked out (Fig. 1A to C); furthermore, the cytotoxicity of A. baumannii for caspase-11−/− BMDMs was significantly lower than that for caspase-11+/+ BMDMs (Fig. 1D), suggesting that A. baumannii can also activate the noncanonical inflammasome pathway.
FIG 1.
Activation of the NLRP3 inflammasome pathway and the noncanonical inflammasome pathway by A. baumannii in BMDMs. Lysate from 1 × 108 A. baumannii bacteria was used to treat 1 × 106 BMDMs. (A) Caspase-1 and NLRP3 expression in BMDMs was detected by Western blotting. (B and C) IL-1β and IL-1α secretion was measured by ELISA. (D) Cytotoxicity was analyzed with a lactate dehydrogenase kit. *, P < 0.05 versus casp-11+/+ lysate−; **, P < 0.01 versus casp-11+/+ lysate−; ***, P < 0.001 versus casp-11+/+ lysate−; #, P < 0.05 versus casp-11−/− lysate+; ##, P < 0.01 versus casp-11−/− lysate+; ###, P < 0.001 versus casp-11−/− lysate+; △, P < 0.05 versus casp-11−/− lysate−; △△△, P < 0.001 versus casp-11−/− lysate−. casp-11, caspase-11. The data are expressed as means ± SD, and all the experiments were performed three times.
Caspase-11 deficiency impairs A. baumannii clearance.
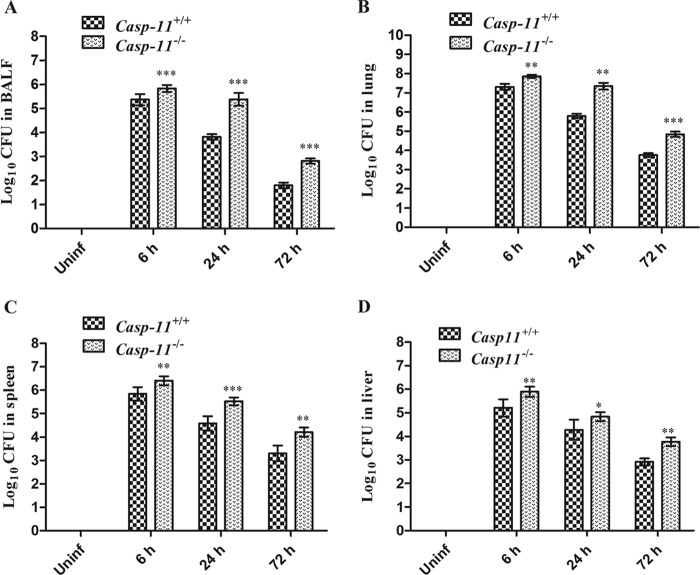
Both caspase-11+/+ and caspase-11−/− mice were infected with A. baumannii (2 × 108 bacteria). The number of CFU in bronchoalveolar lavage fluid (BALF) from caspase-11−/− mice was much higher than in BALF from caspase-11+/+ mice 6, 24, and 72 h after infection (Fig. 2A). Consistently, the number of CFU in lung tissue of caspase-11−/− mice was also higher than in that of caspase-11+/+ mice 6, 24, and 72 h after infection (Fig. 2B). Moreover, bacterial burdens in spleens and livers of caspase-11−/− mice were also higher than those in caspase-11+/+ mice at the above-mentioned time points (Fig. 2C and D).
FIG 2.
Bacterial burdens in caspase-11+/+ and caspase-11−/− mice. (A and B) CFU in BALF and lungs of caspase-11+/+ and caspase-11−/− mice infected with 2 × 108 A. baumannii bacteria. (C and D) CFU in spleens and livers of caspase-11+/+ and caspase-11−/− mice infected with 2 × 108 A. baumannii bacteria. *, P < 0.05 versus casp-11+/+; **, P < 0.01 versus casp-11+/+; ***, P < 0.001 versus casp-11+/+. casp-11, caspase-11; Uninf, uninfected. The data are expressed as means ± SD. Each group contained 5 mice.
Caspase-11 deficiency aggravates the pulmonary inflammatory response to A. baumannii infection.
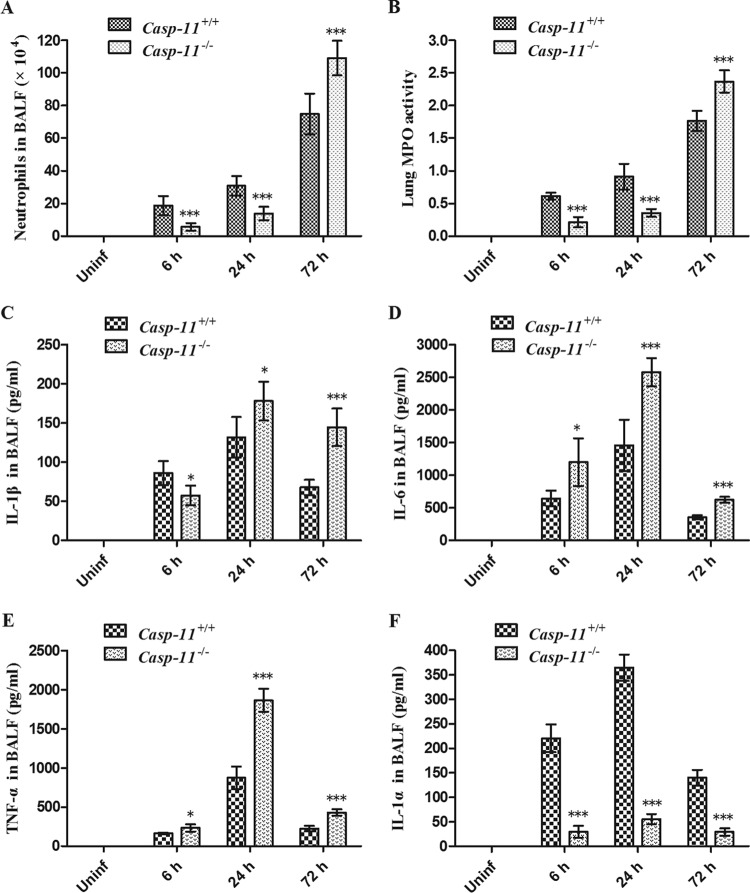
There were fewer neutrophils in BALF from caspase-11−/− mice than in BALF from caspase-11+/+ mice 6 and 24 h after infection but notably more than in BALF from caspase-11+/+ mice 72 h after infection (Fig. 3A). Consistently, myeloperoxidase (MPO) activity in lung tissue of caspase-11−/− mice was lower than that in caspase-11+/+ mice 6 and 24 h after infection but remarkably higher than that in caspase-11+/+ mice 72 h after infection (Fig. 3B). Levels of IL-1β in BALF from caspase-11−/− mice were lower at 6 h but higher at 24 and 72 h after infection than in BALF from caspase-11+/+ mice (Fig. 3C). Moreover, BALF from caspase-11−/− mice contained more IL-6 and tumor necrosis factor alpha (TNF-α) but less IL-1α than BALF from caspase-11+/+ mice at each time point (Fig. 3D to F).
FIG 3.
Pulmonary inflammatory response to A. baumannii in caspase-11+/+ and caspase-11−/− mice. (A) Neutrophils in BALF from caspase-11+/+ and caspase-11−/− mice infected with 2 × 108 A. baumannii bacteria. (B) MPO activity in lung tissue of caspase-11+/+ and caspase-11−/− mice infected with 2 × 108 A. baumannii bacteria. (C to F) Levels of IL-1β, IL-6, TNF-α, and IL-1α in BALF from caspase-11+/+ and caspase-11−/− mice infected with 2 × 108 A. baumannii bacteria. *, P < 0.05 versus casp-11+/+; **, P < 0.01 versus casp-11+/+; ***, P < 0.001 versus casp-11+/+. casp-11, caspase-11; Uninf, uninfected. The data are expressed as means ± SD. Each group contained 5 mice.
Caspase-11 deficiency exacerbates pulmonary pathological changes caused by A. baumannii infection.
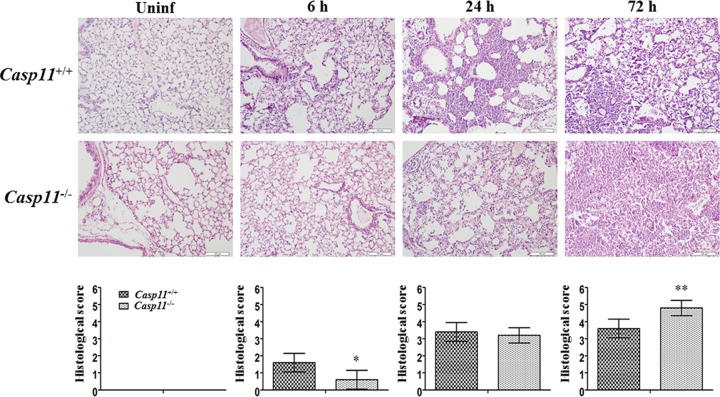
Pulmonary pathological changes were evaluated by hematoxylin-eosin (HE) staining. No obvious pathological change was observed in the lungs of either caspase-11+/+ or caspase-11−/− mice without A. baumannii infection (Fig. 4, Uninf). Six hours after infection, fewer neutrophils had infiltrated into lungs of caspase-11−/− mice than into those of caspase-11+/+ mice (Fig. 4, 6 h). However, 24 h after infection, lungs from both types of mice exhibited comparable pathological changes, with infiltration of large quantities of neutrophils and local consolidation (Fig. 4, 24 h). Seventy-two hours after infection, pulmonary pathological changes in caspase-11−/− were mice significantly exacerbated and were characterized by extensive neutrophil infiltration and consolidation (Fig. 4, 72 h).
FIG 4.
Pulmonary pathological changes of caspase-11+/+ and caspase-11−/− mice. Lung tissues were stained with HE, and lung pathology was scored blindly in caspase-11+/+ and caspase-11−/− mice after infection with 2 × 108 A. baumannii bacteria. *, P < 0.05 versus casp-11+/+; **, P < 0.01 versus casp-11+/+. casp-11, caspase-11; Uninf, uninfected. The data are expressed as means ± SD. Each group contained 5 mice.
Caspase-11 deficiency enhances susceptibility to A. baumannii.
To investigate the tolerance of mice for A. baumannii, both caspase-11+/+ and caspase-11−/− mice were infected with 1 × 108 or 5 × 108 A. baumannii bacteria. It was found that both types of mice survived infection with 1 × 108 A. baumannii bacteria; however, after infection with 5 × 108 A. baumannii bacteria, the survival rate of caspase-11+/+ mice (7/8; 87.5%) was significantly higher than that of caspase-11−/− mice (2/8; 25%) (Fig. 5A). HE staining showed that 24 h after infection with 5 × 108 A. baumannii bacteria, caspase-11−/− mice displayed very serious pathological changes characterized by diffuse inflammatory cell infiltration and consolidation (Fig. 5B and C).
FIG 5.
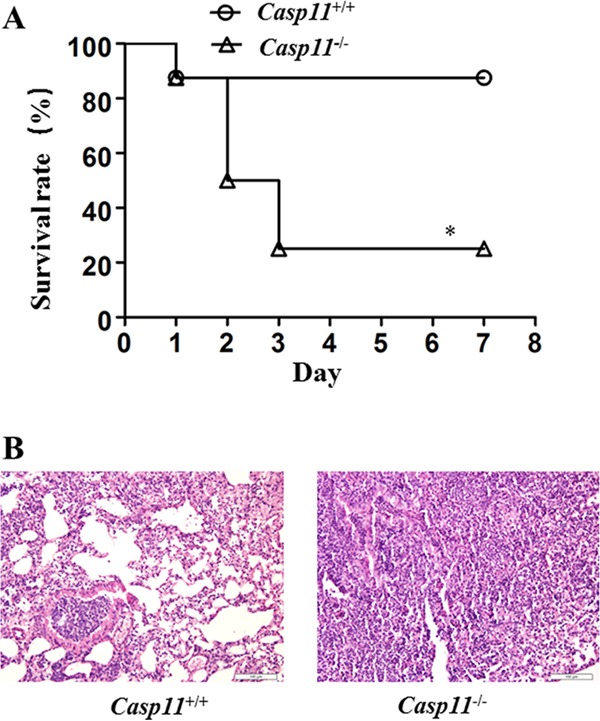
Survival rate of caspase-11+/+ and caspase-11−/− mice. (A) Kaplan-Meier analysis of the survival rate of caspase-11+/+ and caspase-11−/− mice after infection with 5 × 108 A. baumannii bacteria. (B) Pulmonary pathology of caspase-11+/+ and caspase-11−/− mice 24 h after infection with 5 × 108 A. baumannii bacteria. *, P < 0.05 versus casp-11+/+. Each group contained 8 mice.
DISCUSSION
Some Gram-negative bacteria, such as Escherichia coli, Salmonella enterica serovar Typhimurium, and Citrobacter freundii, have been shown to activate both the NLRP3 inflammasome and noncanonical inflammasome pathways (8, 16–18). Our in vitro study showed that A. baumannii upregulated caspase-1 and NLRP3 expression and IL-1β secretion in both caspase-11+/+ and caspase-11−/− BMDMs, indicating that A. baumannii can activate the NLRP3 inflammasome. Consistently, Kang et al. found that A. baumannii-induced IL-1β secretion was dependent on NLRP3, the adaptor ASC (apoptosis-associated speck-like protein containing a CARD), and caspase-1, involving the P2X7 receptor, K+ efflux, reactive oxygen species (ROS) production, and cathepsin release (19).
In addition to the NLRP3 inflammasome, our in vitro study has revealed that A. baumannii can activate the caspase-11-mediated noncanonical inflammasome, based on the following observations. (i) Caspase-11 knockout partially abrogated A. baumannii-induced caspase-1 expression and IL-1β secretion. (ii) Caspase-11 knockout completely abrogated A. baumannii-induced IL-1α secretion. (iii) Caspase-11 knockout reduced the cytotoxicity of A. baumannii on BMDMs. Therefore, these data suggest that caspase-11 deficiency may impair the innate immune response to A. baumannii infection.
Actually, our in vivo study showed that the numbers of CFU in BALF and lung tissue from caspase-11−/− mice were much higher than those in caspase-11+/+ mice 24 h or 72 h after infection with the same quantity of A. baumannii bacteria, indicating that caspase-11 knockout impaired the ability of macrophages to clear A. baumannii. Similarly, caspase-11−/− mice exhibited decreased capability to clear Salmonella Typhimurium in the intestine compared with caspase-11+/+ mice (20). In addition, caspase-11−/− mice also displayed deficiency in Legionella pneumophila clearance (21).
As a consequence of deficiency in bacterial clearance, caspase-11−/− mice demonstrated exacerbated pulmonary pathological changes characterized by extensive neutrophil infiltration and consolidation for the duration of infection with A. baumannii. Consistently, the survival rate of caspase-11−/− mice was much lower than that of caspase-11+/+ mice, indicating that caspase-11 knockout increases susceptibility to A. baumannii infection. The increased bacterial burden may activate NLRs or TLRs to increase cytokine release and inflammatory response.
In summary, our studies have shown that A. baumannii can activate the noncanonical caspase-11 inflammasome and that caspase-11 deficiency impairs pulmonary A. baumannii clearance, resulting in exaggerated pulmonary inflammation and consolidation. These data indicate that the caspase-11-mediated innate immune response plays a crucial role in defending against A. baumannii invasion.
MATERIALS AND METHODS
Animals.
Six- to 8-week-old female specific-pathogen-free (SPF) caspase-11−/− mice (C57BL/6 background) and wild-type C57BL/6 (caspase-11+/+) mice were studied in accordance with the Guide to the Care and Use of Experimental Animals (China). The experimental procedures were approved by the China Medical University Animal Care and Use Committee. Caspase-11−/− mice were purchased from Jackson Laboratory, and wild-type C57BL/6 mice were provided by the Animal Division, China Medical University.
Bone marrow-derived macrophage generation.
Bone marrow was harvested from mouse femurs and tibias. BMDMs were generated by plating bone marrow cells in Dulbecco's modified Eagle's medium (DMEM) with 10% fetal bovine serum (FBS) and 10% macrophage colony-stimulating factor (M-CSF) (L929 cell supernatant).
Bacterial culture, inoculation, and CFU measurement.
A. baumannii (ATCC BAA-1605) bacteria were grown overnight in tryptic soy broth (TSB) at 37°C. A. baumannii bacteria (2 × 108) in 20 μl saline were administered to anesthetized mice intranasally. Twenty-four or 72 h after infection, mice were sacrificed for examination. To quantify CFU, suspension fluid from BALF or homogenized lung tissue was inoculated into sterile tryptic soy agar (TSA) at 37°C for 24 h.
Bacterial lysate preparation.
Bacteria were collected by centrifugation at 4°C and 7,000 × g for 10 min and were suspended in 1 ml phosphate-buffered saline (PBS). Subsequently, the bacteria were sonicated with a JY96-IIN sonifier (Xinzhi, Ningbo, China) on ice. After centrifugation at 4°C and 13,000 × g for 10 min, the supernatant was filtered by using a 0.45-μm membrane (Millipore). Lysate from 1 × 108 bacteria was used to treat 1 × 106 BMDMs.
Western blotting.
Equal amounts of proteins were separated on 10% SDS-polyacrylamide gels and transferred to polyvinylidene difluoride (PVDF) membranes (Millipore) in a wet electron transfer device. The membranes were blocked in 5% bovine serum albumin (BSA) in Tris-buffered saline (TBS) containing 0.05% Tween 20 for 1 h at room temperature. The membranes were incubated with rabbit polyclonal antibody for caspase-1 (1:1,000; Abcam) or rabbit polyclonal antibody for NLRP3 (1:500; Abcam) overnight at 4°C. After being washed in TBS-Tween 20 three times, the membranes were incubated in horseradish peroxidase (HRP)-conjugated goat anti-rabbit (1:5,000) for 2 h at room temperature. An enhanced chemiluminescence (ECL) kit was used to visualize target proteins. The optical densities (ODs) of the bands were determined with Image-J software.
Bronchoalveolar lavage fluid collection and staining.
BALF was collected by injecting into the lungs and withdrawing 1 ml saline. The BALF was centrifuged at 2,000 rpm for 5 min, and the supernatant was collected for enzyme-linked immunosorbent assay (ELISA) detection. Lysis buffer was added to the precipitant to eliminate red blood cells. After another centrifugation at 2,000 rpm for 5 min, the precipitant was resuspended. The suspension fluid was then smeared, and the neutrophils were counted after Wright staining.
ELISA.
Levels of TNF-α, IL-1β, IL-6, and IL-1α in the supernatants of BMDMs and BALF were measured using mouse ELISA kits (eBioscience) for the cytokines according to the manufacturer's instructions. The sensitivity was 8 pg/ml for TNF-α and IL-1β and 4 pg/ml for IL-6 and IL-1α.
Cytotoxicity assay.
The cytotoxicity of A. baumannii for BMDMs was analyzed using a lactate dehydrogenase (LDH) assay kit (Beyotime Biotechnology, China). After 12 h of infection, cell supernatants were harvested for analysis of LDH release by dying cells. The absorbance at 490 nm was measured with a plate reader spectrophotometer, and cytotoxicity values were determined based on the following equation: (ODsample − ODlow control)/(ODhigh control − ODlow control) × 100, where low control indicates BMDMs without bacterial infection and high control indicates BMDMs (without bacterial infection) treated with LDH-releasing agent.
Myeloperoxidase activity assay.
MPO activity in lung tissue was measured with an MPO assay kit (Nanjing Jiancheng Corp., China) according to the manufacturer's instructions. The absorbance at 460 nm was measured with a plate reader spectrophotometer. The MPO value (units per gram of lung tissue) was determined based on the following equation: (ODsample − ODcontrol)/11.3 × amount of sample (in grams).
Hematoxylin-eosin staining.
Lung tissue was fixed with 10% formalin and embedded in paraffin; 4-μm-thick sections were stained with HE and observed using a Leica DMRB microscope. The degree of pathology was judged blindly and scored according to the following criteria (22): 0, no reaction in alveolar walls; 1, diffuse reaction in alveolar walls, primarily neutrophilic, with no thickening of alveolar walls; 2, diffuse presence of inflammatory cells in alveolar walls with slight thickening; 3, distinct thickening of alveolar walls due to the presence of inflammatory cells; 4, alveolar wall thickening with up to 25% of the lung consolidated; 5, alveolar wall thickening with more than 50% of the lung consolidated.
Statistical analysis.
Data are expressed as means ± standard deviations (SD). Differences were analyzed by Student's t test or one-way analysis of variance (ANOVA). The survival rate was determined by the Kaplan-Meier method and compared by log-rank test. Statistical analysis was performed using SPSS 13.0. Differences were considered significant when the P value was less than 0.05.
REFERENCES
- 1.Sharma D, Kanneganti TD. 2016. The cell biology of inflammasomes: mechanisms of inflammasome activation and regulation. J Cell Biol 213:617–629. doi: 10.1083/jcb.201602089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Man SM, Kanneganti TD. 2015. Regulation of inflammasome activation. Immunol Rev 265:6–21. doi: 10.1111/imr.12296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Latz E, Xiao TS, Stutz A. 2013. Activation and regulation of the inflammasomes. Nat Rev Immunol 13:397–411. doi: 10.1038/nri3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marim FM, Franco MM, Gomes MT, Miraglia MC, Giambartolomei GH, Oliveira SC. 2017. The role of NLRP3 and AIM2 in inflammasome activation during Brucella abortus infection. Semin Immunopathol 39:215–223. doi: 10.1007/s00281-016-0581-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jo EK, Kim JK, Shin DM, Sasakawa C. 2016. Molecular mechanisms regulating NLRP3 inflammasome activation. Cell Mol Immunol 13:148–159. doi: 10.1038/cmi.2015.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Franchi L, Munoz-Planillo R, Nunez G. 2012. Sensing and reacting to microbes through the inflammasomes. Nat Immunol 13:325–332. doi: 10.1038/ni.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shi J, Zhao Y, Wang Y, Gao W, Ding J, Li P, Hu L, Shao F. 2014. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature 514:187–192. doi: 10.1038/nature13820. [DOI] [PubMed] [Google Scholar]
- 8.Kayagaki N, Wong MT, Stowe IB, Ramani SR, Gonzalez LC, Akashi-Takamura S, Miyake K, Zhang J, Lee WP, Muszynski A, Forsberg LS, Carlson RW, Dixit VM. 2013. Noncanonical inflammasome activation by intracellular LPS independent of TLR4. Science 341:1246–1249. doi: 10.1126/science.1240248. [DOI] [PubMed] [Google Scholar]
- 9.Hagar JA, Powell DA, Aachoui Y, Ernst RK, Miao EA. 2013. Cytoplasmic LPS activates caspase-11: implications in TLR4-independent endotoxic shock. Science 341:1250–1253. doi: 10.1126/science.1240988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garcia-Patino MG, Garcia-Contreras R, Licona-Limon P. 2017. The immune response against Acinetobacter baumannii, an emerging pathogen in nosocomial infections. Front Immunol 8:441. doi: 10.3389/fimmu.2017.00441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dexter C, Murray GL, Paulsen IT, Peleg AY. 2015. Community-acquired Acinetobacter baumannii: clinical characteristics, epidemiology and pathogenesis. Expert Rev Anti Infect Ther 13:567–573. doi: 10.1586/14787210.2015.1025055. [DOI] [PubMed] [Google Scholar]
- 12.Peleg AY, Seifert H, Paterson DL. 2008. Acinetobacter baumannii: emergence of a successful pathogen. Clin Microbiol Rev 21:538–582. doi: 10.1128/CMR.00058-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Korneev KV, Arbatsky NP, Molinaro A, Palmigiano A, Shaikhutdinova RZ, Shneider MM, Pier GB, Kondakova AN, Sviriaeva EN, Sturiale L, Garozzo D, Kruglov AA, Nedospasov SA, Drutskaya MS, Knirel YA, Kuprash DV. 2015. Structural relationship of the lipid A acyl groups to activation of murine Toll-like receptor 4 by lipopolysaccharides from pathogenic strains of Burkholderia mallei, Acinetobacter baumannii, and Pseudomonas aeruginosa. Front Immunol 6:595. doi: 10.3389/fimmu.2015.00595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim CH, Jeong YJ, Lee J, Jeon SJ, Park SR, Kang MJ, Park JH, Park JH. 2013. Essential role of Toll-like receptor 4 in Acinetobacter baumannii-induced immune responses in immune cells. Microb Pathog 54:20–25. doi: 10.1016/j.micpath.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 15.March C, Regueiro V, Llobet E, Moranta D, Morey P, Garmendia J, Bengoechea JA. 2010. Dissection of host cell signal transduction during Acinetobacter baumannii-triggered inflammatory response. PLoS One 5:e10033. doi: 10.1371/journal.pone.0010033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kailasan Vanaja S, Rathinam VA, Atianand MK, Kalantari P, Skehan B, Fitzgerald KA, Leong JM. 2014. Bacterial RNA:DNA hybrids are activators of the NLRP3 inflammasome. Proc Natl Acad Sci U S A 111:7765–7770. doi: 10.1073/pnas.1400075111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Broz P, Newton K, Lamkanfi M, Mariathasan S, Dixit VM, Monack DM. 2010. Redundant roles for inflammasome receptors NLRP3 and NLRC4 in host defense against Salmonella. J Exp Med 207:1745–1755. doi: 10.1084/jem.20100257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lupfer CR, Anand PK, Liu Z, Stokes KL, Vogel P, Lamkanfi M, Kanneganti TD. 2014. Reactive oxygen species regulate caspase-11 expression and activation of the non-canonical NLRP3 inflammasome during enteric pathogen infection. PLoS Pathog 10:e1004410. doi: 10.1371/journal.ppat.1004410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kang MJ, Jo SG, Kim DJ, Park JH. 2017. NLRP3 inflammasome mediates interleukin-1beta production in immune cells in response to Acinetobacter baumannii and contributes to pulmonary inflammation in mice. Immunology 150:495–505. doi: 10.1111/imm.12704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Knodler LA, Crowley SM, Sham HP, Yang H, Wrande M, Ma C, Ernst RK, Steele-Mortimer O, Celli J, Vallance BA. 2014. Noncanonical inflammasome activation of caspase-4/caspase-11 mediates epithelial defenses against enteric bacterial pathogens. Cell Host Microbe 16:249–256. doi: 10.1016/j.chom.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Akhter A, Caution K, Abu Khweek A, Tazi M, Abdulrahman BA, Abdelaziz DH, Voss OH, Doseff AI, Hassan H, Azad AK, Schlesinger LS, Wewers MD, Gavrilin MA, Amer AO. 2012. Caspase-11 promotes the fusion of phagosomes harboring pathogenic bacteria with lysosomes by modulating actin polymerization. Immunity 37:35–47. doi: 10.1016/j.immuni.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Szarka RJ, Wang N, Gordon L, Nation PN, Smith RH. 1997. A murine model of pulmonary damage induced by lipopolysaccharide via intranasal instillation. J Immunol Methods 202:49–57. doi: 10.1016/S0022-1759(96)00236-0. [DOI] [PubMed] [Google Scholar]