ABSTRACT
The human pathogen Burkholderia pseudomallei and the related species Burkholderia thailandensis are facultative intracellular bacteria characterized by the ability to escape into the cytosol of the host cell and to stimulate the formation of multinucleated giant cells (MNGCs). MNGC formation is induced via an unknown mechanism by bacterial type VI secretion system 5 (T6SS-5), which is an essential virulence factor in both species. Despite the vital role of the intracellular life cycle in the pathogenesis of the bacteria, the range of host cell types permissive for initiation and completion of the intracellular cycle is poorly defined. In the present study, we used several different types of human primary cells to evaluate bacterial entry, intracellular survival, and MNGC formation. We report the capacity of B. pseudomallei to enter, efficiently replicate in, and mediate MNGC formation of vein endothelial and bronchial epithelial cells, indicating that the T6SS-5 is important in the host-pathogen interaction in these cells. Furthermore, we show that B. pseudomallei invades fibroblasts and keratinocytes and survives inside these cells as well as in monocyte-derived macrophages and neutrophils for at least 17 h postinfection; however, MNGC formation is not induced in these cells. In contrast, infection of mixed neutrophils and RAW264.7 macrophages with B. thailandensis stimulated the formation of heterotypic MNGCs in a T6SS-5-dependent manner. In summary, the ability of the bacteria to enter and survive as well as induce MNGC formation in certain host cells may contribute to the pathogenesis observed in B. pseudomallei infection.
KEYWORDS: Burkholderia pseudomallei, primary cells, type VI secretion system
INTRODUCTION
Burkholderia pseudomallei is a Gram-negative soil bacterium that causes the potentially fatal disease melioidosis in humans and animals (1, 2). B. pseudomallei is found in the tropics worldwide, with the majority of infections being reported in Southeast Asia and Northern Australia (3). A recent study examining documented human and animal melioidosis cases as well as the environmental presence of B. pseudomallei estimates that the disease is underreported in 45 countries in which it is endemic (4). Underreporting may occur because of the wide variety of manifestations of melioidosis complicating diagnosis of the disease. A common clinical manifestation of an infection with B. pseudomallei is pneumonia (5–7). However, the capability of B. pseudomallei to infect virtually every tissue produces many other manifestations such as encephalitis, osteomyelitis, and abscesses of the skin and internal organs (5).
B. pseudomallei is a facultative intracellular bacterium that enters and survives in nonphagocytic and phagocytic cells, including neutrophils (8–14). Following entry into the host cell, the bacteria escape from the endocytic vacuole into the cytosol, where they exhibit intracellular motility based on actin polymerization and flagella (15–17). A characteristic feature of the intracellular life cycle of B. pseudomallei is the formation of multinucleated giant cells (MNGCs), which result from the fusion of an infected mononuclear cell with one or more neighboring cells (18). Although the physiological role of B. pseudomallei-induced host cell fusion remains to be elucidated, it is conceivable that MNGC formation facilitates localized dissemination of B. pseudomallei, access to nutrients, and escape from extracellular immune defense mechanisms and antibiotics. MNGCs have been observed in vivo in autopsy lung tissue samples of melioidosis patients and in pulmonary lesions of C57BL/6 mice challenged with a low dose of B. pseudomallei (19, 20). Furthermore, a previous analysis of B. pseudomallei-infected cockroaches indicates MNGC formation of hemocytes (21). This finding may suggest that the bacteria are able to induce the fusion of leukocytes during bacteremia in humans.
While the identity of host cellular factors involved in MNGC formation is as yet unknown, recent work has demonstrated that the bacterial type VI secretion system (T6SS) has a central role in this process. The T6SS is a bacteriophage-related secretion apparatus that translocates effector proteins into bacteria or eukaryotic cells by a contraction-based mechanism (22–25). Inactivation of T6SS-5 (also named T6SS-1) of B. pseudomallei abrogates MNGC formation (17, 26, 27). Similarly, a homologue of this T6SS-5 present in the closely related species Burkholderia thailandensis is essential for host cell fusions (28, 29). B. thailandensis shares the same intracellular life cycle and is used as a low-virulence model organism to study B. pseudomallei-host interactions. Disruption of the T6SS-5 causes a virulence defect of both species in animal models of infection (27, 30, 31). A recent study revealed that the expression of the conserved T6SS-5 gene hcp-5 (and likely therefore the whole T6SS-5 gene cluster) is triggered by the glutathione present in the cytoplasm of the host cell (32). Glutathione is synthesized in all cells of the human body, suggesting that production of T6SS-5 proteins is not cell type specific and may occur in all tissues during infection. Importantly, however, the signal(s) for induction of T6SS-5 effector translocation, presumably via an as yet unidentified host-derived molecule(s) or via contact with host cell membranes, remains elusive. Likewise, the host factor(s) that is targeted by the T6SS-5 to mediate host cell fusions is not known.
The range of host cells that are infected by B. pseudomallei and support intracellular survival during in vivo infection has not been fully explored. In vitro, the bacteria actively invade various nonphagocytic cells. However, to the best of our knowledge, this notion is based on findings obtained with immortalized cells except for two studies using primary alveolar epithelial cells (8, 33–36). Furthermore, the ability of B. pseudomallei to infect every tissue and the ubiquitous presence of glutathione in human cells raises the question of cell type specificity of T6SS-5 activity and therefore of MNGC formation. Although MNGC formation in B. pseudomallei-infected cells was first described 20 years ago, surprisingly little is known about the types of host cells stimulated to form cell-cell fusions following infection (9). To the best of our knowledge, B. pseudomallei and B. thailandensis T6SS-5-induced MNGC formation has been analyzed only in assays using epithelial, fibroblast, and monocyte/macrophage-like cell lines or HEK293 cells of unknown type (9, 17, 18, 37–40). In the present study, we investigated the capacity of B. pseudomallei and B. thailandensis to enter and survive in host cells and promote host cell fusions in primary cells that the bacteria are likely to encounter during infection in vivo. To this end, we included human endothelial cells, epithelial cells, fibroblasts, keratinocytes, and mesenchymal stem cells in the study. In addition, we used various epithelial cell lines originating from different organs to test for tissue-specific differences and analyzed infected neutrophils and monocyte-derived macrophages (MDMs) for the formation of MNGCs.
RESULTS
B. pseudomallei is able to invade primary endothelial cells, epithelial cells, fibroblasts, keratinocytes, and mesenchymal stem cells.
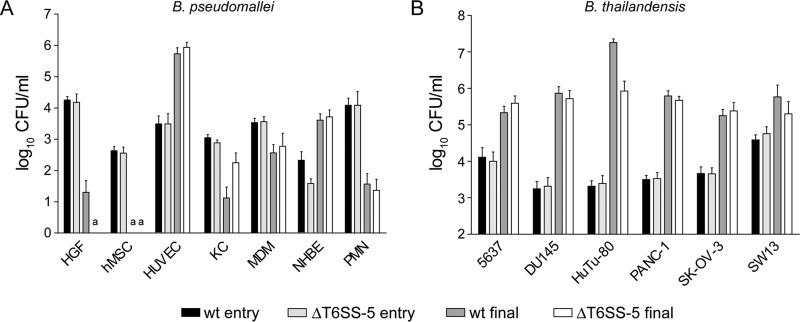
We included a selection of normal primary human phagocytic and nonphagocytic cells as well as epithelial cell lines derived from diverse organs in this study (Table 1). The multiplicity of infection (MOI) and postinfection time were adjusted for each cell and cell line to optimize bacterial entry, MNGC formation, and host cell viability. Entry into host cells was determined after infection with B. pseudomallei and antibiotic treatment for 2 h to kill extracellular bacteria. The cells were infected with wild-type B. pseudomallei E8 or B. thailandensis E264 and mutants thereof that harbor a disrupted T6SS-5 (ΔBPSS1504 and ΔBTH_II0857 mutants, respectively). These unmarked mutants were generated and subjected to complementation analysis in previous studies (31, 41). For the sake of simplicity throughout the remainder of this report, we will refer to B. pseudomallei ΔBPSS1504 and B. thailandensis ΔBTH_II0857 as ΔT6SS-5 mutants. In addition, it should be noted that the purpose of this study was to examine the capability of B. pseudomallei to complete the intracellular life cycle in a particular cell type rather than comparing infection efficiencies between different cells. Using antibiotic protection assays, we found that the B. pseudomallei wild-type and ΔT6SS-5 mutant strains are capable of invading vein endothelial cells (HUVEC), bronchial epithelial cells (NHBE), gingival fibroblasts (HGFs), undifferentiated keratinocytes (KCs), and mesenchymal stem cells (hMSC) and were also detected inside MDMs and neutrophils (PMNs) (Fig. 1A). Likewise, B. thailandensis invaded all epithelial cell lines derived from diverse human tissues: adrenal glands (SW 13), bladder (5637), duodenum (HuTu-80), ovary (SK-OV-3), pancreas (PANC-1), and prostate (DU 145) (Fig. 1B).
TABLE 1.
Primary cells, cell lines, and culture conditions used in this studya
| Cell/cell line | Type/origin (source/reference) | Culture medium (source) | Bacterium/MOI/time p.i. (h) | No. of experiments/ no. of replicates |
Range of MOIs tested for MNGC formation |
|---|---|---|---|---|---|
| HGF | Fibroblast/gingiva (CLS) | FGM-MG (CLS) | B. pseudomallei/200/24 | 2/2 | 50–500 |
| hMSC | Stem cell/bone marrow (ATCC) | Mesenchymal stem cell medium (ATCC) | B. pseudomallei/100/23 | 1/2 | 10–400 |
| HUVEC | Endothelial/vascular (Lonza) | EGM-2 (Lonza) | B. pseudomallei/1/21 | 2/1 or 2 | NA |
| KC | Keratinocyte/epidermal foreskin (healthy volunteers) | KGM Gold, KGM-2 BulletKit (Lonza) | B. pseudomallei/50/21 | 2/3 | 50–200 |
| MDM | Peripheral blood mononuclear cells/blood (healthy volunteers) | RPMI 1640 (Biochrom), 10% FBS (Sigma) | B. pseudomallei/10/20 | 2/2 | 1–50 |
| NHBE | Epithelial/airways above bifurcation of the lungs (Lonza) | BEGM (Lonza) | B. pseudomallei/1/19 | 3/3 | NA |
| PMN | Granulocyte/blood (healthy volunteers) | RPMI 1640 (Biochrom), 10% FBS (Sigma) | B. pseudomallei/10/17 | 2/3 | 1–50 |
| 5637 | Carcinoma epithelial/bladder (CLS/51) | RPMI 1640 (Biochrom), 10% FBS (Sigma) | B. thailandensis/10/40 | 3/3 | 10–100b |
| DU-145 | Epithelial/brain derived metastatic prostate cancer (CLS/52) | DMEM (Gibco), 10% FBS (Sigma) | B. thailandensis/100/26 | 2/3 | 10–100b |
| HuTu-80 | Adenocarcinoma epithelial/duodenum (CLS/53) | DMEM (Gibco), 10% FBS (Sigma), 1% NEA (Biochrom) | B. thailandensis/50/18 | 2/3 | 10–100b |
| PANC-1 | Carcinoma epithelial/pancreas (CLS/54) | DMEM (Gibco), 10% FBS (Sigma) | B. thailandensis/100/14 | 3/3 | 10–100b |
| SK-OV-3 | Adenocarcinoma epithelial/ovary (CLS/51) | McCoy 5A (Biochrom), 2 mM l-glutamine (Gibco), 1× NEA (Gibco), 5% FBS (Sigma) | B. thailandensis/100/25 | 2/3 | 10–100b |
| SW13 | Carcinoma epithelial/adrenal gland (CLS/55) | 1:1 DMEM/Ham's F-12 (Gibco), 2 mM l-glutamine (Gibco), 5% FBS (Sigma) | B. thailandensis/50/20 | 3/3 | 10–100b |
Abbreviations: ATCC, American Type Culture Collection (www.atcc.org); CLS, Cell lines services (http://www.clsgmbh.de); DMEM, Dulbecco's modified Eagle medium; DSMZ, German Collection of Microorganisms and Cell Cultures (https://www.dsmz.de/); MDM, monocyte-derived macrophages; NEA, nonessential amino acids; NA, not applicable; p.i., postinfection.
MNGCs were observed at all MOIs used.
FIG 1.
(A) Entry and intracellular survival of B. pseudomallei wild type (wt) and T6SS-5 mutant (ΔT6SS-5) in human primary cells (HUVEC, human vein endothelial cells; NHBE, bronchial epithelial cells; HGF, gingival fibroblasts; KC, undifferentiated keratinocytes; hMSC, mesenchymal stem cells; MDM, monocyte-derived macrophages; PMN, neutrophils). Entry and intracellular survival were determined at 3 h and 17 to 24 h postinfection, respectively, using antibiotic protection assays. The MOI and time of infection were optimized for each cell and are listed in Table 1. (B) Entry and intracellular survival of B. thailandensis wild type (wt) and T6SS-5 mutant (ΔT6SS-5) in human epithelial cell lines derived from different tissues (SW 13, adrenal gland cell line; 5637, bladder cell line; HuTu-80, duodenal cell line; SK-OV-3, ovary cell line; PANC-1, pancreatic cell line; DU 146, prostate cell line). Entry and intracellular survival were measured at 3 h and 14 to 40 h postinfection, respectively, using antibiotic protection assays. The MOI and time of infection were optimized for each cell line and are listed in Table 1. Shown are mean values + standard deviation. a, not detected.
B. pseudomallei exhibits prolonged intracellular survival in all cell types analyzed except for mesenchymal stem cells.
Compared with the entry (invasion) time point, intracellular CFU of both B. pseudomallei wild-type and ΔT6SS-5 strains increased substantially in HUVEC and NHBE after approximately 24 h postinfection (Fig. 1A). These data show that the bacteria are able to efficiently proliferate inside these types of host cells, cells that the bacteria might encounter upon infection via percutaneous inoculation and inhalation, respectively. Compared with the initial CFU count, at subsequent time points intracellular CFU were lower in all other cell types infected with B. pseudomallei wild-type or ΔT6SS-5 strains, although to different degrees. Viable wild-type and ΔT6SS-5 bacteria were isolated from undifferentiated KCs at 21 h postinfection; however, only wild-type B. pseudomallei was harvested from HGFs at 24 h postinfection. The bacteria were not detected in hMSC at MOIs of 100 (Fig. 1A) and 400 under the conditions used (data not shown), indicating that these cells do not support intracellular survival of B. pseudomallei (Fig. 1A). Both MDMs and PMNs contained viable wild-type and ΔT6SS-5 bacteria at approximately 20 h and 17 h postinfection, respectively. B. thailandensis was able to efficiently multiply in all epithelial cell lines tested, displaying a >14-fold increase in intracellular CFU for both wild-type and ΔT6SS-5 bacteria at 14 to 40 h postinfection compared to 3 h postinfection (Fig. 1B).
B. pseudomallei stimulates MNGC formation in primary endothelial and epithelial cells.
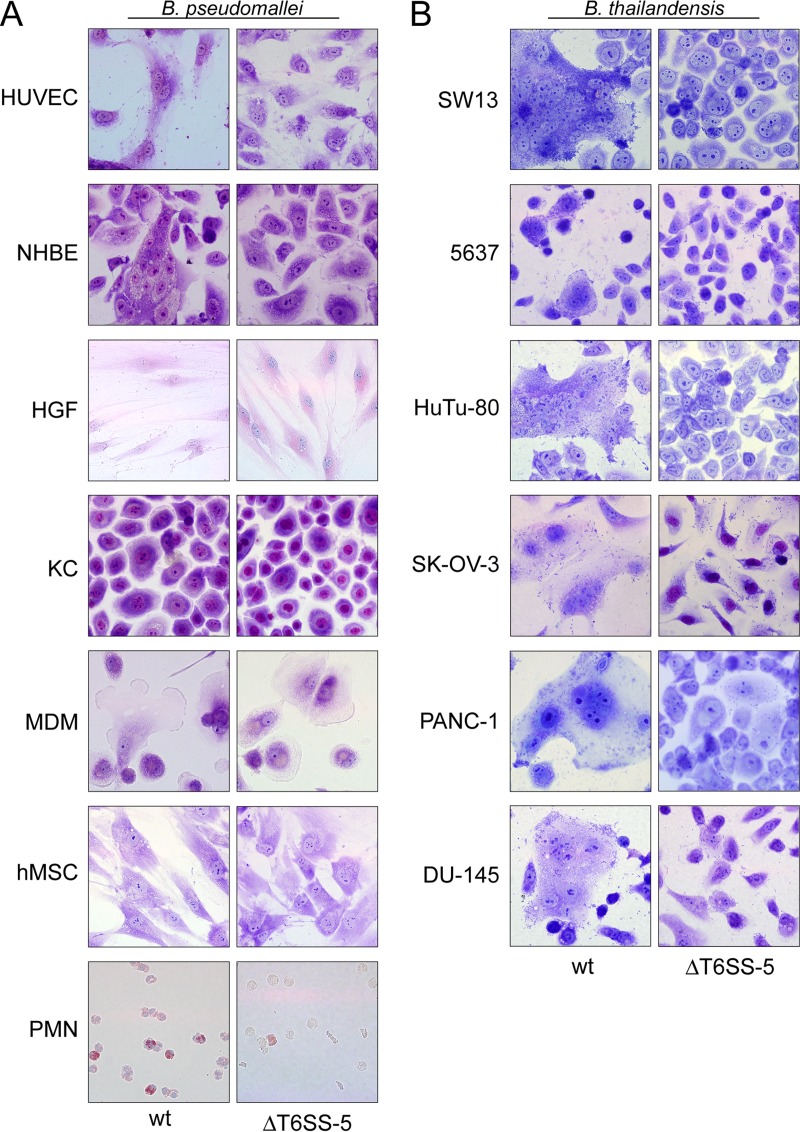
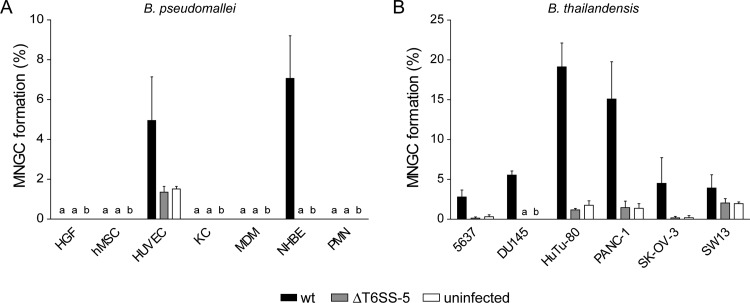
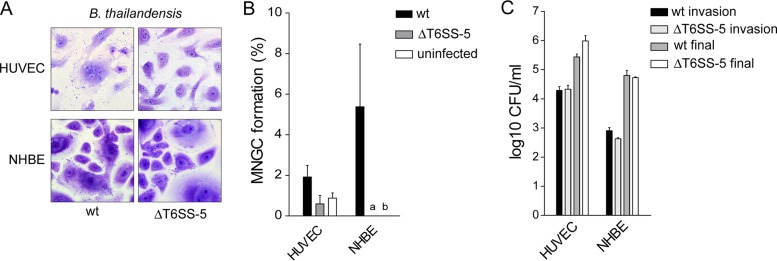
Next, we analyzed the capacity of B. pseudomallei to induce MNGC formation in the different types of primary cells. For this, the same time point postinfection as for the quantification of intracellular survival was used. To allow for primary cells and cell lines displaying more than three nuclei per cell in the absence of bacteria (which could be the result of mitosis occurring without cytokinesis), MNGC formation was also quantified in the uninfected state for some cells. Using Giemsa stain, MNGC formation was detected in HUVEC and NHBE upon infection with B. pseudomallei (Fig. 2A; see also Fig. 4A). Likewise, B. thailandensis was able to invade, survive in, and induce MNGC formation in HUVEC and NHBE (Fig. 3). Despite the presence of intracellular B. pseudomallei, no host cell fusions were observed in fibroblasts and keratinocytes at MOIs of 200 and 50, respectively (Fig. 2A and 4A). The infection of HGFs and KCs at increased MOIs of 500 and 200, respectively, also failed to result in MNGC formation (data not shown). Similarly, infected MDMs and PMNs were monitored for MNGC production until 20 and 17 h postinfection, respectively. However, no MNGCs were detected (Fig. 2A and 4A).
FIG 2.
(A) Human primary cells infected with B. pseudomallei wild type (wt) and T6SS-5 mutant (ΔT6SS-5) for 17 to 24 h using antibiotic protection assays and stained with Giemsa. The same MOI and time of infection were used as for the determination of intracellular survival of B. pseudomallei in these cells and are listed in Table 1. MNGC formation was observed in NHBE and HUVEC infected with wild-type B. pseudomallei. (B) Human epithelial cell lines infected with B. thailandensis wild type (wt) and T6SS-5 mutant (ΔT6SS-5) for 14 to 40 h using antibiotic protection assays and stained with Giemsa (see legend to Fig. 1 for abbreviations of cell lines). The same MOI and time of infection were used as for the determination of intracellular survival of B. thailandensis in these cell lines and are listed in Table 1. Wild-type B. thailandensis stimulated MNGC formation in all cell lines tested.
FIG 4.
(A) Quantification of MNGC formation in primary cells (see legend to Fig. 1 for abbreviations) infected with B. pseudomallei wild type (wt) and T6SS-5 mutant (ΔT6SS-5) or left uninfected. The same MOI and time of infection as for the determination of intracellular survival of B. pseudomallei in these cells were used and are listed in Table 1. (B) MNGC formation in epithelial cell lines infected with B. thailandensis wild type (wt) or ΔT6SS-5 or left uninfected (see legend to Fig. 1 for abbreviations of cell lines). The same MOI and time of infection were used as for the determination of intracellular survival of B. thailandensis in these cells and are listed in Table 1. Shown are mean values + standard deviation. a, not detected; b, not determined.
FIG 3.
MNGC formation in primary endothelial (HUVEC) and bronchial epithelial cells (NHBE) induced by B. thailandensis. (A) Giemsa stain of NHBE and HUVEC infected with B. thailandensis wild type and ΔT6SS-5 mutant at MOIs of 200 and 50, respectively, for approximately 20 h. (B) Quantification of MNGC formation efficiency under the same conditions. (C) Invasion and intracellular survival of B. thailandensis in HUVEC and NHBE at 3 h and 20 h postinfection, respectively. Shown are mean values + standard deviation. a, not detected; b, not determined.
B. thailandensis stimulated the production of MNGCs in all six epithelial cell lines included in the study irrespective of the organ from which they were derived (Fig. 2B). The degree of MNGC formation differed considerably among the cell lines, ranging between 19.1% for duodenum cells (HuTu-80) and 2.8% for bladder cells (5637) (Fig. 4B). Importantly, disruption of the T6SS-5 in B. pseudomallei or in B. thailandensis abolished MNGC formation in all cell lines (infected with B. thailandensis) and in NHBE and HUVEC (infected with B. pseudomallei). Taken together, the results verify the essentiality of the secretion system for host cell fusions in a wide range of nonphagocytic cell types, including primary epithelial and endothelial cells.
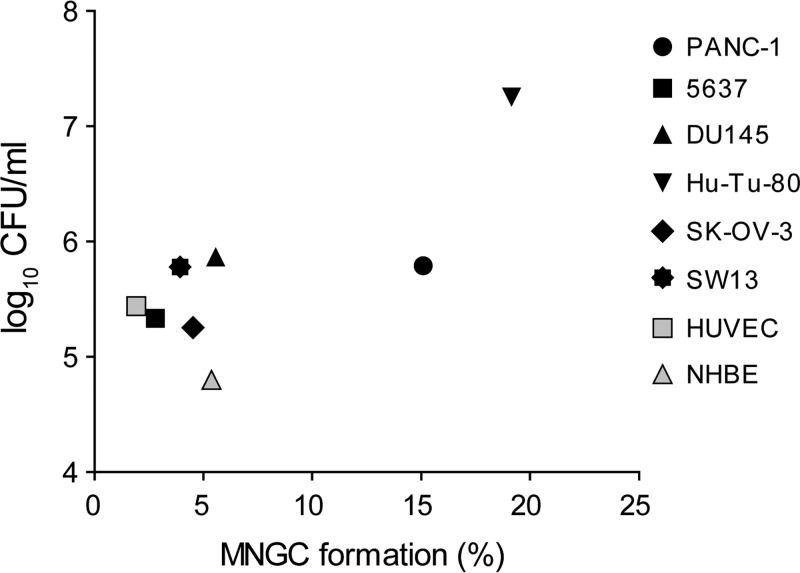
Association of MNGC formation with the number of bacteria residing within host cells.
MNGC formation was observed in NHBE and HUVEC primary cells, which supported efficient intracellular growth of B. pseudomallei and B. thailandensis and was most pronounced in the HuTu-80 cell line, which had the highest bacterial load of all the cell lines studied. These findings led us to examine whether there is an association between the number of intracellular bacteria and cell-cell fusion efficiency. For this, we plotted the average CFU of wild-type B. thailandensis used to determine intracellular survival against the average MNGC formation among HUVEC and NHBE and each of six cell lines (Fig. 5). The correlation between intracellular CFU and MNGC formation was significant (Pearson's correlation coefficient [r] = 0.77; P = 0.024) when all data points were included. However, when the data point of the HuTu-80 cell line was excluded—as it might represent an outlier based on the CFU value, which exceeds that of all the other cell lines more than 1,000-fold—no correlation between the intracellular bacterial burden and MNGC formation was observed (r = 0.34; P = 0.45). In addition, the CFU of wild-type B. thailandensis isolated, for example, from HUVEC were 4.3-fold higher than those of NHBE, yet MNGC formation of HUVEC was 2.8-fold lower. These observations may indicate that host cell-specific factors contribute to cell-cell fusion or that the activity of the T6SS-5 differs between cell types.
FIG 5.
Intracellular loads of wild-type B. thailandensis in relation to the extent of MNGC formation. The diagram shows average values of final intracellular CFU of wild-type B. thailandensis plotted against average values of MNGC formation (%) for each cell line (black symbols), primary endothelial cells (HUVEC), and epithelial cells (NHBE) (gray symbols).
PMNs engage in heterotypic MNGC formation.
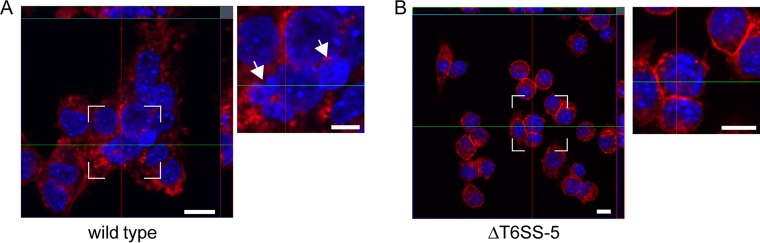
Although viable bacteria were isolated from PMNs after 17 h postinfection, B. pseudomallei did not stimulate MNGC formation in these cells. To test if this was caused by PMN-driven impairment of the intracellular bacteria to induce MNGCs or if this was attributable to an inherent inability of the PMNs themselves to fuse (e.g., absence of a surface protein functioning as a receptor of the T6SS-5), we performed mixed MNGC formation assays. For this, adherent RAW264.7 cells, which facilitate efficient intracellular proliferation of B. thailandensis and form pronounced MNGCs, were mixed with PMNs at a ratio of 1:2 and infected with wild-type B. thailandensis and the ΔT6SS-5 mutant for 22 h. Plasma membrane and DNA of infected cells were stained with wheat germ agglutinin Alexa Fluor 594 and DAPI (4′,6-diamidino-2-phenylindole), respectively, to identify MNGCs. Nuclei from PMNs and macrophages can be morphologically distinguished by their distinct nuclear shapes. Indeed, after wild-type infection, several MNGCs containing multilobed and round nuclei were reproducibly observed (Fig. 6A). No MNGCs were detected in ΔT6SS-5-infected cells (Fig. 6B). These data suggest that PMNs were not inherently unable to fuse and that a mechanism for the absence of MNGC formation observed in PMNs alone may be due to impairment of bacterial functions.
FIG 6.
Confocal fluorescence microscopy z-stack imaging of mixed PMN and RAW264.7 macrophages infected with wild-type B. thailandensis (A) and B. thailandensis ΔT6SS-5 (B) for 22 h at an MOI of 50. z-stack images were collected at 0.6-μm sections, and the red and green lines indicate the orthogonal planes of the y-z and x-z projections, respectively. Heterotypic MNGC containing macrophage (single-lobed) and neutrophil (multilobed) nuclei were detected upon infection with wild-type B. thailandensis. Shown are representative images of two experiments performed in triplicate. Plasma membrane was stained with wheat germ agglutinin Alexa Fluor 594 conjugate (red), and DNA was stained with DAPI (blue). Arrows mark multilobed nuclei. Bars, 10 μm.
DISCUSSION
Understanding the pathogenesis of melioidosis requires the identification of the cell types susceptible to infection with B. pseudomallei in vivo. In the present study, we performed in vitro infection assays utilizing primary phagocytes and nonphagocytic cells to gain insight into the cell type specificity of the intracellular life cycle of B. pseudomallei. We show that B. pseudomallei has the capacity to invade normal bronchial epithelial cells (NHBE), vein endothelial cells (HUVEC), gingival fibroblasts (HGFs), and keratinocytes (KCs) and to survive in these cells for at least 19 h. The discovery of invasion and intracellular survival of B. pseudomallei in endothelial cells and keratinocytes is a novel finding, which has also not been reported for endothelial or keratinocyte cell lines. Infection of bronchial epithelial NHBE and HGFs is in agreement with previous studies reporting the isolation of viable bacteria from primary alveolar epithelial cells and immortalized fibroblast cells, respectively (9, 34, 36, 42). HGFs and KCs appear to restrict efficient intracellular replication of B. pseudomallei as indicated by decreasing levels of intracellular CFU counts over the course of the infection. However, the cells may constitute a niche for intracellular survival of B. pseudomallei during in vivo infection. Unlike fibroblasts and keratinocytes, NHBE and HUVEC facilitated extensive multiplication of B. pseudomallei (177- and 19-fold increases in CFU, respectively) and B. thailandensis (78- and 14-fold increases in CFU, respectively), which was similar to that observed for some of the cell lines. These results suggest that bronchial epithelial and vein endothelial cells provide a major replication niche for B. pseudomallei in vivo and as such likely play an important role in the pathogenesis of airway and bloodstream infection. Previous work showed that infections of olfactory neurons or phagocytes able to cross the blood-brain barrier (BBB) are routes of access for B. pseudomallei to the brain (43, 44). The ability, therefore, of B. pseudomallei to invade HUVEC could suggest transcellular crossing of the endothelium potentially by cell lysis-mediated disruption of BBB integrity as another mechanism to access the brain. Moreover, invasion of vascular endothelial cells would provide a means of spread of B. pseudomallei from airway tissue into the bloodstream and from the bloodstream into organs as observed for other intracellular pathogens (45, 46).
MNGC formation was detected following B. thailandensis infection in all six epithelial cell lines derived from different organs. Though these are cancer cell lines, the finding might indicate that the T6SS-5 does not exhibit tissue-specific activity. These data are in line with previous studies reporting MNGC formation in, for example, cervical and lung cancer cell lines (47, 48). Using physiologically more-relevant primary NHBE cells, we confirmed the capability of B. pseudomallei to mediate cell-cell fusions in lung epithelial cells. We speculate that epithelial cells of other organs produce MNGCs upon infection with the bacteria as well. Furthermore, we present the novel finding that B. pseudomallei stimulates the fusion of endothelial cells. Importantly, MNGC formation by cells and cell lines of NHBE and HUVEC was strictly dependent on a functional T6SS-5. To date, cell lines but not primary cells have been used to investigate the role of the T6SS-5 in cell-cell fusion (17, 27–29, 41). Thus, the lack of MNGCs of NHBE and HUVEC infected with B. pseudomallei ΔT6SS-5 provides further evidence for the fundamental importance of the secretion system for mediating the fusion of host cells. These data strongly suggest that MNGCs observed in the tissue of melioidosis patients are induced by the T6SS-5 (20). Previous studies detected MNGCs in B. pseudomallei- or B. thailandensis-infected monocyte/macrophage, epithelial, and fibroblast cell lines, pointing toward the lack of strict cell type specificity of T6SS-5 function (18, 37, 39). Our finding that B. pseudomallei stimulates cell-cell fusions of primary epithelial and endothelial cells verifies that T6SS-5 activity is not restricted to a single cell type. Whether the T6SS-5 employs a host structure(s) found in both cell types for inducing cell-cell fusions or has the ability to target multiple and cell-specific host factors remains to be determined. A secreted protein of the T6SS-5 with effector activity essential for MNGC formation and virulence has been identified; however, the effector has not been experimentally characterized and lacks significant sequence similarity to proteins of known function, precluding speculation as to the host cellular target of the T6SS-5 (27–29).
Despite the presence of viable intracellular bacteria in PMNs, HGFs, KCs, and MDMs, no MNGCs were observed under the conditions studied. In contrast to HGF cells, fibroblast cell lines were previously shown to be permissive for MNGC formation mediated by B. pseudomallei (8, 9, 18). Thus, our data might point toward distinct differences between primary cells and cell lines or host/tissue-specific differences between the cells influencing the susceptibility for B. pseudomallei-induced host cell fusions. A potential explanation for the basis of this is the lack of expression of host factor(s) targeted by the T6SS-5 to induce fusions or functioning as the signal for activating the T6SS-5. Another possibility for the absence of MNGCs in infected MDMs (but not keratinocytes) is the lower cell density and smaller number of cell-cell contacts than in MNGC-positive cells and cell lines. In addition, this study involved a single B. thailandensis isolate and a single B. pseudomallei isolate, and interstrain variation in, for example, MNGC formation was not determined. However, given that the T6SS-5 is highly conserved in B. pseudomallei, it appears unlikely that the lack of MNGC formation in some of the primary cells is due to strain-specific differences.
Consistent with previous work, we recovered viable bacteria from PMNs after extended incubation times (8, 49, 50). PMNs filled with B. pseudomallei were found adjacent to other PMNs, and no cell-cell fusions were detected. To determine whether the properties of the bacteria inside PMNs or the properties of the PMNs themselves, e.g., the absence of surface structures, account for this, we performed mixed-infection assays with RAW264.7 macrophages, which facilitate efficient intracellular replication of B. thailandensis. Although further studies are required to clarify the mechanism of heterotypic MNGC formation, the detection of MNGCs consisting of both macrophages and PMNs indicates that B. thailandensis located inside macrophages is able to mediate the fusion with adjacent PMNs and suggests that this assay can be used to assess the susceptibility of other host cells to T6SS-5-induced cell-cell fusion.
MATERIALS AND METHODS
Bacteria.
B. thailandensis wild-type strain E264, B. thailandensis ΔtssK-5 (ΔBTH_II0857), B. pseudomallei wild-type strain E8, and B. pseudomallei ΔBPSS1504 were grown in LB medium or on blood agar plates. The unmarked in-frame deletion ΔtssK-5 and ΔBPSS1504 mutants produce a nonfunctional T6SS-5 (31, 41). Work with B. pseudomallei was performed under biosafety level 3 (BSL-3) containment. The ability of B. thailandensis E264 (wild type) and B. pseudomallei E8 (wild type) to stimulate MNGC formation was confirmed using the RAW264.7 macrophage cell line.
Cells and cell lines.
The human primary cells and cell lines used in this study are listed in Table 1. All cells were cultured at 37°C with 5% CO2. The experiments with nonphagocytic primary cells were performed at passages 3 to 9.
Granulocyte, monocyte, and keratinocyte isolation.
Granulocytes and monocytes were isolated from heparinized human whole blood from healthy donors, using 5% dextran sedimentation and Biocoll (1.077 g/ml; Biochrom) density gradient centrifugation, after informed consent from the donors. Cells present in the monocyte/lymphocyte layer were washed, and monocytes were enriched by adherence to a petri dish for 1 h in RPMI 1640 medium. Adherent monocytes were washed and stimulated with macrophage colony-stimulating factor (M-CSF; 50 ng/ml) for 7 days. The cells were washed, and fresh medium supplemented with M-CSF (50 ng/ml) was added every other day. Granulocytes found in the pellet fraction after Biocoll centrifugation were >95% pure after hypotonic water lysis of erythrocytes. Primary foreskin keratinocytes were obtained from routine circumcisions performed at the Loretto Clinic in Tuebingen by A. Frunder. After removal of surplus fatty and vascular tissue, the skin sample was cut into 0.5- to 1-cm2 pieces and incubated overnight at 4°C in epidermal keratinocyte medium with supplements (CELLnTEC) with 10 μg/ml gentamicin and 0.25 μg/ml amphotericin B (CELLnTEC) containing 10 mg/ml Dispase II (Roche) to digest the basal lamina. The following day, the epidermis was carefully separated from the dermis, and small slices of the epidermis were incubated in 0.05% trypsin–EDTA (Merck Millipore) for 30 min. Trypsin digestion was stopped using RPMI 1640 medium (Thermo Fisher Scientific) containing 10% fetal calf serum (FCS), and single cells were obtained using a 100-μm-pore-size cell strainer (Corning Incorporated). After centrifugation, keratinocytes were resuspended in epidermal keratinocyte medium with supplements (CELLnTEC) and cultured in a collagen-coated tissue flask (Corning Incorporated). The cells were allowed to adhere for 4 h, and then the medium was replaced with fresh medium. Cells were used for experimental assays until passage 5.
Antibiotic protection assay and intracellular CFU counts.
Infection of host cells was initiated by spinning B. thailandensis or B. pseudomallei at the indicated MOI onto the cell monolayer at 800 × g for 5 min using the media described in Table 1. Following infection for 1 h, except for PMNs and keratinocytes, which were infected for 30 min and 1.5 h, respectively, the cells were washed with phosphate-buffered saline (PBS) and incubated with medium supplemented with 100 μg/ml imipenem to kill extracellular bacteria. The infected cells were incubated in the presence of the antibiotic for 2 h to determine bacterial entry or for longer incubation times as indicated in Table 1 to determine intracellular survival. At the indicated time points, the cells were washed with PBS and lysed with 1% Triton X-100. Serial dilutions were plated on LB plates to quantify the CFU per milliliter.
Giemsa stain and MNGC formation.
Cells were grown on coverslips, infected as described above, and fixed in methanol at the indicated time points postinfection (Table 1). Following staining of the cells with Giemsa stain (Sigma) for 20 to 50 min, the coverslips were rinsed in distilled water and mounted in 100% glycerol. Images were acquired on an Olympus BX51 microscope using a 40× (63× for neutrophils) oil lens objective. MNGCs were defined as cells containing at least three nuclei. MNGC formation efficiency (as a percentage) was determined with a 10× or 20× objective using the following formula: (N within multinucleated giant cells/total N) × 100, where N is the number of nuclei (18). A minimum of 2,300 nuclei were counted for each condition and time point.
Mixed granulocyte-RAW264.7 macrophage coinfection and confocal microscopy.
Granulocytes were isolated as described above, immediately mixed at a 1:2 ratio with RAW264.7 cells in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS), and seeded onto poly-d-lysine-coated coverslips. The cell mixture was infected with B. thailandensis wild-type and ΔtssK-5 strains at an MOI of 10 for approximately 22 h, fixed with 4% formaldehyde, and stained with wheat germ agglutinin Alexa Fluor 594 conjugate and DAPI (Invitrogen). The samples were mounted using ProLong Diamond (Invitrogen), and images were collected using the confocal mode of an inverted Zeiss LSM 710 NLO microscope equipped with a spectral detector, a Zeiss Plan-Apochromat 63×/1.40 oil DIC M27 objective and the Zeiss Zen 2011 software. Images were adjusted for brightness and contrast in Photoshop CS5. Two independent experiments were performed in triplicate.
Ethics statement.
Isolation and use of human skin tissue and human peripheral blood leukocytes were approved by the Medical Ethics Committee of the University of Tuebingen (331/2010BO2 and 406/2016BO2, respectively) and performed according to the principles of the Declaration of Helsinki. Use of the human skin tissue involved written consent to the physician in charge and anonymization of the samples.
ACKNOWLEDGMENTS
We thank Julia Frick's laboratory for sharing equipment and Michael Buhl, Florian Hölzl, Ariane Dinkelacker, and Matthias Willmann for excellent technical assistance. We thank Salvatore Chiantia for critical reading of the manuscript and helpful comments.
This work was funded within the framework of Germany's Excellence Initiative by the Deutsche Forschungsgemeinschaft (ZUK63) to S.S. B.S. was supported by the Transregio TRR 156 of the Deutsche Forschungsgemeinschaft.
REFERENCES
- 1.Thomas AD, Forbes-Faulkner J, Parker M. 1979. Isolation of Pseudomonas pseudomallei from clay layers at defined depths. Am J Epidemiol 110:515–521. doi: 10.1093/oxfordjournals.aje.a112832. [DOI] [PubMed] [Google Scholar]
- 2.Wiersinga WJ, van der Poll T, White NJ, Day NP, Peacock SJ. 2006. Melioidosis: insights into the pathogenicity of Burkholderia pseudomallei. Nat Rev Microbiol 4:272–282. doi: 10.1038/nrmicro1385. [DOI] [PubMed] [Google Scholar]
- 3.Limmathurotsakul D, Wongratanacheewin S, Teerawattanasook N, Wongsuvan G, Chaisuksant S, Chetchotisakd P, Chaowagul W, Day NP, Peacock SJ. 2010. Increasing incidence of human melioidosis in Northeast Thailand. Am J Trop Med Hyg 82:1113–1117. doi: 10.4269/ajtmh.2010.10-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Limmathurotsakul D, Golding N, Dance DA, Messina JP, Pigott DM, Moyes CL, Rolim DB, Bertherat E, Day NP, Peacock SJ, Hay SI. 2016. Predicted global distribution of and burden of melioidosis. Nat Microbiol 1(1):15008. [DOI] [PubMed] [Google Scholar]
- 5.Currie BJ, Ward L, Cheng AC. 2010. The epidemiology and clinical spectrum of melioidosis: 540 cases from the 20 year Darwin prospective study. PLoS Negl Trop Dis 4:e900. doi: 10.1371/journal.pntd.0000900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Easton A, Haque A, Chu K, Lukaszewski R, Bancroft GJ. 2007. A critical role for neutrophils in resistance to experimental infection with Burkholderia pseudomallei. J Infect Dis 195:99–107. doi: 10.1086/509810. [DOI] [PubMed] [Google Scholar]
- 7.Wiersinga WJ, Veer C, Wieland CW, Gibot S, Hooibrink B, Day NP, Peacock SJ, van der Poll T. 2007. Expression profile and function of triggering receptor expressed on myeloid cells-1 during melioidosis. J Infect Dis 196:1707–1716. doi: 10.1086/522141. [DOI] [PubMed] [Google Scholar]
- 8.Jones AL, Beveridge TJ, Woods DE. 1996. Intracellular survival of Burkholderia pseudomallei. Infect Immun 64:782–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harley VS, Dance DA, Drasar BS, Tovey G. 1998. Effects of Burkholderia pseudomallei and other Burkholderia species on eukaryotic cells in tissue culture. Microbios 96:71–93. [PubMed] [Google Scholar]
- 10.Stevens MP, Friebel A, Taylor LA, Wood MW, Brown PJ, Hardt WD, Galyov EE. 2003. A Burkholderia pseudomallei type III secreted protein, BopE, facilitates bacterial invasion of epithelial cells and exhibits guanine nucleotide exchange factor activity. J Bacteriol 185:4992–4996. doi: 10.1128/JB.185.16.4992-4996.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Utaisincharoen P, Arjcharoen S, Lengwehasatit I, Limposuwan K, Sirisinha S. 2005. Burkholderia pseudomallei invasion and activation of epithelial cells requires activation of p38 mitogen-activated protein kinase. Microb Pathog 38:107–112. doi: 10.1016/j.micpath.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 12.Vanaporn M, Wand M, Michell SL, Sarkar-Tyson M, Ireland P, Goldman S, Kewcharoenwong C, Rinchai D, Lertmemongkolchai G, Titball RW. 2011. Superoxide dismutase C is required for intracellular survival and virulence of Burkholderia pseudomallei. Microbiology 157:2392–2400. doi: 10.1099/mic.0.050823-0. [DOI] [PubMed] [Google Scholar]
- 13.Horton RE, Morrison NA, Beacham IR, Peak IR. 2012. Interaction of Burkholderia pseudomallei and Burkholderia thailandensis with human monocyte-derived dendritic cells. J Med Microbiol 61:607–614. doi: 10.1099/jmm.0.038588-0. [DOI] [PubMed] [Google Scholar]
- 14.Williams NL, Morris JL, Rush CM, Ketheesan N. 2014. Migration of dendritic cells facilitates systemic dissemination of Burkholderia pseudomallei. Infect Immun 82:4233–4240. doi: 10.1128/IAI.01880-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stevens MP, Stevens JM, Jeng RL, Taylor LA, Wood MW, Hawes P, Monaghan P, Welch MD, Galyov EE. 2005. Identification of a bacterial factor required for actin-based motility of Burkholderia pseudomallei. Mol Microbiol 56:40–53. doi: 10.1111/j.1365-2958.2004.04528.x. [DOI] [PubMed] [Google Scholar]
- 16.Benanti EL, Nguyen CM, Welch MD. 2015. Virulent Burkholderia species mimic host actin polymerases to drive actin-based motility. Cell 161:348–360. doi: 10.1016/j.cell.2015.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.French CT, Toesca IJ, Wu TH, Teslaa T, Beaty SM, Wong W, Liu M, Schroder I, Chiou PY, Teitell MA, Miller JF. 2011. Dissection of the Burkholderia intracellular life cycle using a photothermal nanoblade. Proc Natl Acad Sci U S A 108:12095–12100. doi: 10.1073/pnas.1107183108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kespichayawattana W, Rattanachetkul S, Wanun T, Utaisincharoen P, Sirisinha S. 2000. Burkholderia pseudomallei induces cell fusion and actin-associated membrane protrusion: a possible mechanism for cell-to-cell spreading. Infect Immun 68:5377–5384. doi: 10.1128/IAI.68.9.5377-5384.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Conejero L, Patel N, de Reynal M, Oberdorf S, Prior J, Felgner PL, Titball RW, Salguero FJ, Bancroft GJ. 2011. Low-dose exposure of C57BL/6 mice to Burkholderia pseudomallei mimics chronic human melioidosis. Am J Pathol 179:270–280. doi: 10.1016/j.ajpath.2011.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wong KT, Puthucheary SD, Vadivelu J. 1995. The histopathology of human melioidosis. Histopathology 26:51–55. doi: 10.1111/j.1365-2559.1995.tb00620.x. [DOI] [PubMed] [Google Scholar]
- 21.Fisher NA, Ribot WJ, Applefeld W, DeShazer D. 2012. The Madagascar hissing cockroach as a novel surrogate host for Burkholderia pseudomallei, B. mallei, and B. thailandensis. BMC Microbiol 12:117. doi: 10.1186/1471-2180-12-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pukatzki S, Ma AT, Revel AT, Sturtevant D, Mekalanos JJ. 2007. Type VI secretion system translocates a phage tail spike-like protein into target cells where it cross-links actin. Proc Natl Acad Sci U S A 104:15508–15513. doi: 10.1073/pnas.0706532104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Basler M, Pilhofer M, Henderson GP, Jensen GJ, Mekalanos JJ. 2012. Type VI secretion requires a dynamic contractile phage tail-like structure. Nature 483:182–186. doi: 10.1038/nature10846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cascales E, Cambillau C. 2012. Structural biology of type VI secretion systems. Philos Trans R Soc Lond B Biol Sci 367:1102–1111. doi: 10.1098/rstb.2011.0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hood RD, Singh P, Hsu F, Guvener T, Carl MA, Trinidad RR, Silverman JM, Ohlson BB, Hicks KG, Plemel RL, Li M, Schwarz S, Wang WY, Merz AJ, Goodlett DR, Mougous JD. 2010. A type VI secretion system of Pseudomonas aeruginosa targets a toxin to bacteria. Cell Host Microbe 7:25–37. doi: 10.1016/j.chom.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schell MA, Ulrich RL, Ribot WJ, Brueggemann EE, Hines HB, Chen D, Lipscomb L, Kim HS, Mrazek J, Nierman WC, Deshazer D. 2007. Type VI secretion is a major virulence determinant in Burkholderia mallei. Mol Microbiol 64:1466–1485. doi: 10.1111/j.1365-2958.2007.05734.x. [DOI] [PubMed] [Google Scholar]
- 27.Burtnick MN, Brett PJ, Harding SV, Ngugi SA, Ribot WJ, Chantratita N, Scorpio A, Milne TS, Dean RE, Fritz DL, Peacock SJ, Prior JL, Atkins TP, Deshazer D. 2011. The cluster 1 type VI secretion system is a major virulence determinant in Burkholderia pseudomallei. Infect Immun 79:1512–1525. doi: 10.1128/IAI.01218-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schwarz S, Singh P, Robertson JD, LeRoux M, Skerrett SJ, Goodlett DR, West TE, Mougous JD. 2014. VgrG-5 is a Burkholderia type VI secretion system-exported protein required for multinucleated giant cell formation and virulence. Infect Immun 82:1445–1452. doi: 10.1128/IAI.01368-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Toesca IJ, French CT, Miller JF. 2014. The type VI secretion system spike protein VgrG5 mediates membrane fusion during intercellular spread by pseudomallei group Burkholderia species. Infect Immun 82:1436–1444. doi: 10.1128/IAI.01367-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pilatz S, Breitbach K, Hein N, Fehlhaber B, Schulze J, Brenneke B, Eberl L, Steinmetz I. 2006. Identification of Burkholderia pseudomallei genes required for the intracellular life cycle and in vivo virulence. Infect Immun 74:3576–3586. doi: 10.1128/IAI.01262-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schwarz S, West TE, Boyer F, Chiang WC, Carl MA, Hood RD, Rohmer L, Tolker-Nielsen T, Skerrett SJ, Mougous JD. 2010. Burkholderia type VI secretion systems have distinct roles in eukaryotic and bacterial cell interactions. PLoS Pathog 6:e1001068. doi: 10.1371/journal.ppat.1001068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wong J, Chen Y, Gan YH. 2015. Host cytosolic glutathione sensing by a membrane histidine kinase activates the type VI secretion system in an intracellular bacterium. Cell Host Microbe 18:38–48. doi: 10.1016/j.chom.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 33.Ye Z, Lee CM, Sun GW, Gan YH. 2008. Burkholderia pseudomallei infection of T cells leads to T-cell costimulation partially provided by flagellin. Infect Immun 76:2541–2550. doi: 10.1128/IAI.01310-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sim SH, Liu Y, Wang D, Novem V, Sivalingam SP, Thong TW, Ooi EE, Tan G. 2009. Innate immune responses of pulmonary epithelial cells to Burkholderia pseudomallei infection. PLoS One 4:e7308. doi: 10.1371/journal.pone.0007308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bast A, Schmidt IH, Brauner P, Brix B, Breitbach K, Steinmetz I. 2011. Defense mechanisms of hepatocytes against Burkholderia pseudomallei. Front Microbiol 2:277. doi: 10.3389/fmicb.2011.00277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lu R, Popov V, Patel J, Eaves-Pyles T. 2012. Burkholderia mallei and Burkholderia pseudomallei stimulate differential inflammatory responses from human alveolar type II cells (ATII) and macrophages. Front Cell Infect Microbiol 2:165. doi: 10.3389/fcimb.2012.00165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boddey JA, Day CJ, Flegg CP, Ulrich RL, Stephens SR, Beacham IR, Morrison NA, Peak IR. 2007. The bacterial gene lfpA influences the potent induction of calcitonin receptor and osteoclast-related genes in Burkholderia pseudomallei-induced TRAP-positive multinucleated giant cells. Cell Microbiol 9:514–531. doi: 10.1111/j.1462-5822.2006.00807.x. [DOI] [PubMed] [Google Scholar]
- 38.Pegoraro G, Eaton BP, Ulrich RL, Lane DJ, Ojeda JF, Bavari S, DeShazer D, Panchal RG. 2014. A high-content imaging assay for the quantification of the Burkholderia pseudomallei induced multinucleated giant cell (MNGC) phenotype in murine macrophages. BMC Microbiol 14:98. doi: 10.1186/1471-2180-14-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Suparak S, Muangsombut V, Riyapa D, Stevens JM, Stevens MP, Lertmemongkolchai G, Korbsrisate S. 2011. Burkholderia pseudomallei-induced cell fusion in U937 macrophages can be inhibited by monoclonal antibodies against host cell surface molecules. Microbes Infect 13:1006–1011. doi: 10.1016/j.micinf.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 40.Welkos SL, Klimko CP, Kern SJ, Bearss JJ, Bozue JA, Bernhards RC, Trevino SR, Waag DM, Amemiya K, Worsham PL, Cote CK. 2015. Characterization of Burkholderia pseudomallei strains using a murine intraperitoneal infection model and in vitro macrophage assays. PLoS One 10:e0124667. doi: 10.1371/journal.pone.0124667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hopf V, Gohler A, Eske-Pogodda K, Bast A, Steinmetz I, Breitbach K. 2014. BPSS1504, a cluster 1 type VI secretion gene, is involved in intracellular survival and virulence of Burkholderia pseudomallei. Infect Immun 82:2006–2015. doi: 10.1128/IAI.01544-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Breitbach K, Rottner K, Klocke S, Rohde M, Jenzora A, Wehland J, Steinmetz I. 2003. Actin-based motility of Burkholderia pseudomallei involves the Arp 2/3 complex, but not N-WASP and Ena/VASP proteins. Cell Microbiol 5:385–393. doi: 10.1046/j.1462-5822.2003.00277.x. [DOI] [PubMed] [Google Scholar]
- 43.Liu PJ, Chen YS, Lin HH, Ni WF, Hsieh TH, Chen HT, Chen YL. 2013. Induction of mouse melioidosis with meningitis by CD11b+ phagocytic cells harboring intracellular B. pseudomallei as a Trojan horse. PLoS Negl Trop Dis 7:e2363. doi: 10.1371/journal.pntd.0002363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.St John JA, Ekberg JA, Dando SJ, Meedeniya AC, Horton RE, Batzloff M, Owen SJ, Holt S, Peak IR, Ulett GC, Mackay-Sim A, Beacham IR. 2014. Burkholderia pseudomallei penetrates the brain via destruction of the olfactory and trigeminal nerves: implications for the pathogenesis of neurological melioidosis. mBio 5:e00025-14. doi: 10.1128/mBio.00025-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stins MF, Badger J, Sik Kim K. 2001. Bacterial invasion and transcytosis in transfected human brain microvascular endothelial cells. Microb Pathog 30:19–28. doi: 10.1006/mpat.2000.0406. [DOI] [PubMed] [Google Scholar]
- 46.Sheen TR, Ebrahimi CM, Hiemstra IH, Barlow SB, Peschel A, Doran KS. 2010. Penetration of the blood-brain barrier by Staphylococcus aureus: contribution of membrane-anchored lipoteichoic acid. J Mol Med (Berl) 88:633–639. doi: 10.1007/s00109-010-0630-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Suparak S, Kespichayawattana W, Haque A, Easton A, Damnin S, Lertmemongkolchai G, Bancroft GJ, Korbsrisate S. 2005. Multinucleated giant cell formation and apoptosis in infected host cells is mediated by Burkholderia pseudomallei type III secretion protein BipB. J Bacteriol 187:6556–6560. doi: 10.1128/JB.187.18.6556-6560.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Amornrit W, Muangsombut V, Wangteeraprasert T, Korbsrisate S. 2012. Elevated intracellular levels of iron in host cells promotes Burkholderia pseudomallei infection. Asian Biomed 6:465–471. [Google Scholar]
- 49.Chanchamroen S, Kewcharoenwong C, Susaengrat W, Ato M, Lertmemongkolchai G. 2009. Human polymorphonuclear neutrophil responses to Burkholderia pseudomallei in healthy and diabetic subjects. Infect Immun 77:456–463. doi: 10.1128/IAI.00503-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pruksachartvuthi S, Aswapokee N, Thankerngpol K. 1990. Survival of Pseudomonas pseudomallei in human phagocytes. J Med Microbiol 31:109–114. doi: 10.1099/00222615-31-2-109. [DOI] [PubMed] [Google Scholar]
- 51.Fogh J, Fogh JM, Orfeo T. 1977. One hundred and twenty-seven cultured human tumor cell lines producing tumors in nude mice. J Natl Cancer Inst 59:221–226. doi: 10.1093/jnci/59.1.221. [DOI] [PubMed] [Google Scholar]
- 52.Mickey DD, Stone KR, Wunderli H, Mickey GH, Vollmer RT, Paulson DF. 1977. Heterotransplantation of a human prostatic adenocarcinoma cell line in nude mice. Cancer Res 37:4049–4058. [PubMed] [Google Scholar]
- 53.Schmidt M, Deschner EE, Thaler HT, Clements L, Good RA. 1977. Gastrointestinal cancer studies in the human to nude mouse heterotransplant system. Gastroenterology 72:829–837. [PubMed] [Google Scholar]
- 54.Lieber M, Mazzetta J, Nelson-Rees W, Kaplan M, Todaro G. 1975. Establishment of a continuous tumor-cell line (panc-1) from a human carcinoma of the exocrine pancreas. Int J Cancer 15:741–747. doi: 10.1002/ijc.2910150505. [DOI] [PubMed] [Google Scholar]
- 55.Lasfargues EY, Ozzello L. 1958. Cultivation of human breast carcinomas. J Natl Cancer Inst 21:1131–1147. [PubMed] [Google Scholar]