ABSTRACT
Porphyromonas gingivalis is a keystone pathogen that contributes to periodontal pathogenesis by disrupting host-microbe homeostasis and promoting dysbiosis. The virulence of P. gingivalis likely reflects an alteration in the lipid A composition of its lipopolysaccharide (LPS) from the penta-acylated (PgLPS1690) to the tetra-acylated (PgLPS1435/1449) form. Mast cells play an important role in periodontitis, but the mechanisms of their activation and regulation remain unknown. The expression of epithelium- and neutrophil-derived host defense peptides (HDPs) (LL-37 and human β-defensin-3), which activate mast cells via Mas-related G protein-coupled receptor X2 (MRGPRX2), is increased in periodontitis. We found that MRGPRX2-expressing mast cells are present in normal gingiva and that their numbers are elevated in patients with chronic periodontitis. Furthermore, HDPs stimulated degranulation in a human mast cell line (LAD2) and in RBL-2H3 cells stably expressing MRGPRX2 (RBL-MRGPRX2). PgLPS1690 caused substantial inhibition of HDP-induced mast cell degranulation, but PgLPS1435/1449 had no effect. A fluorescently labeled HDP (FAM-LL-37) bound to RBL-MRGPRX2 cells, and PgLPS1690 inhibited this binding, but PgLPS1435/1449 had no effect. These findings suggest that low-level inflammation induced by HDP/MRGPRX2-mediated mast cell degranulation contributes to gingival homeostasis but that sustained inflammation due to elevated levels of both HDPs and MRGPRX2-expressing mast cells promotes periodontal disease. Furthermore, differential regulation of HDP-induced mast cell degranulation by PgLPS1690 and PgLPS1435/1449 may contribute to the modulation of disease progression.
KEYWORDS: Porphyromonas gingivalis, antimicrobial peptides, host response, lipopolysaccharide, mast cell, periodontal immunology
INTRODUCTION
Periodontitis is a biofilm-induced chronic inflammatory disease that leads to the destruction of the tooth-supporting structures, including alveolar bone (1). The initial host response to biofilm bacteria is a protective acute inflammatory response (gingivitis) that maintains host-microbe homeostasis in the periodontium. However, the presence of microbial factors, such as keystone pathogens (e.g., Porphyromonas gingivalis), shifts the balance toward a dysbiosis in which former commensals behave as proinflammatory pathobionts (2, 3). Thus, P. gingivalis, which does not directly induce a strong inflammatory response, likely contributes to pathogenesis via the subversion or evasion of the immune system. This promotes the growth and development of a dysbiotic microbial community that contains a number of well-characterized Gram-negative anaerobes, such as Aggregatibacter actinomycetemcomitans, Tannerella forsythia, and Treponema denticola. Through their interactions with cells of the innate and adaptive immune systems, these organisms contribute to the perpetuation and exacerbation of the inflammatory response. Ultimately, the host response elicited by the dysbiotic microbiota induces the degradation of the soft and hard tissues characteristic of periodontitis (2–4).
P. gingivalis has been shown to evade innate immunity via a number of mechanisms, including inhibition of chemokine generation from epithelial cells (5) and immune subversion via microbial exploitation of communication between the C5a receptor, complement receptor 3 (CR3), the chemokine receptor CXCR4, and Toll-like receptor 2 (TLR2) (6, 7). It has been proposed that P. gingivalis uses these evasion maneuvers in concert with tactics to subvert autophagy to exploit macrophages as “Trojan horses” for subsequent infection of other cell types, and potentially for dissemination from the oral cavity to systemic tissues (8).
P. gingivalis lipopolysaccharide (PgLPS) is one of the key virulence factors that contribute to periodontitis (9–11). Lipid A is the most biologically active component of LPS, and its structure differs greatly among Gram-negative bacterial species. The canonical Escherichia coli LPS (EcLPS) possesses a hexa-acylated lipid A structure and promotes a strong inflammatory response via the activation of TLR4. In contrast, PgLPS is heterogeneous, consisting of penta-acylated (LPS1690) and tetra-acylated (LPS1435/1449) lipid structures that generally have opposing effects on innate immune responses, thereby playing a key role in modulating host immune-inflammatory reactions (10, 12, 13). It is noteworthy that P. gingivalis strains expressing penta-acylated lipid A activate TLR4 and are more susceptible to killing by host defense peptides (HDPs) and macrophages than strains that predominantly generate tetra-acylated lipid A (9, 10, 14, 15). Recent studies have shown that lipid A modifications promote evasion of the noncanonical inflammasome by preventing caspase-1 activation (16). Hence, the heterogeneity of these structures is thought to play a role in oral immune homeostasis and contributes to the microbial shift characteristic of periodontitis (2, 17, 18). Thus, alteration of the lipid A composition of P. gingivalis may provide a strategy for it to evade host defense mechanisms in gingival tissue, contributing to dysbiosis and periodontal disease (9, 10).
Mast cells are multifunctional immune cells that are found in all vascularized tissues throughout the body, including the gingiva, and they play a critical role in host defense (19–21). This function of mast cells is mediated via the release of proteases, which degrade fibrinogen and disrupt tight junctions, leading to the transmigration of neutrophils across the vascular endothelium (22). It has been proposed that mast cells contribute to both gingival homeostasis and periodontitis (23–28). Not surprisingly, mast cells are found in healthy gingiva and in gingivitis lesions. Their numbers are increased in chronic periodontitis (CP), and the degree of their degranulation correlates well with disease severity (23, 25–27, 29, 30). Malcolm et al. (31) recently showed that mast-deficient (Wsh/Wsh) mice are protected from P. gingivalis-induced periodontal bone loss. Furthermore, restoration of mast cells in Wsh/Wsh mice resulted in P. gingivalis-induced bone loss that was associated with increased mast cell degranulation. Based on these findings, it has been proposed that mast cell degranulation plays an important role in periodontal bone loss. However, the mechanisms of mast cell activation and regulation in periodontitis remain unknown.
Mas-related G protein-coupled receptor X2 (MRGPRX2; previously named MrgX2) is expressed on human mast cells and contributes to host defense, pseudoallergic drug reactions, and chronic inflammatory diseases, such as chronic urticaria (32–36). Given that mast cells are tissue-resident cells and are found close to the blood vessels, it is likely that they are activated by mediators derived from the epithelium and from invading neutrophils. Indeed, antimicrobial HDPs released from the epithelium (human β-defensins [hBDs]) and from neutrophils (the cathelicidin LL-37) cause both mast cell chemotaxis and degranulation via the activation of MRGPRX2 (37–42). It is noteworthy that expression of HDPs is upregulated in the gingiva and saliva of patients with periodontitis compared to that in the gingiva and saliva of healthy subjects (43–48). These findings suggest that MRGPRX2-mediated activation of mast cells by HDPs contributes to host defense. It is also plausible that the recruitment and activation of mast cells via MRGPRX2 in response to elevated levels of HDPs found in patients with periodontitis are involved in the cascade of events leading to alveolar bone loss. However, the possibility that mast cells residing within periodontal tissues express MRGPRX2 has not previously been determined.
The purpose of this study was 2-fold: (i) to determine if MRGPRX2 is expressed in gingival tissues obtained from healthy subjects as well as from patients with CP and (ii) to characterize the effects of PgLPS1690 and PgLPS1435/1449 on HDP-induced mast cell signaling and degranulation. The data presented herein demonstrate increased levels of MRGPRX2-expressing mast cells in CP patients compared to those in healthy subjects and suggest a previously unrecognized cross talk between HDPs and PgLPS, which may be relevant to gingival homeostasis and periodontal disease.
RESULTS
MRGPRX2-tryptase double-positive mast cells are present in healthy gingiva, and their numbers are increased in CP gingiva.
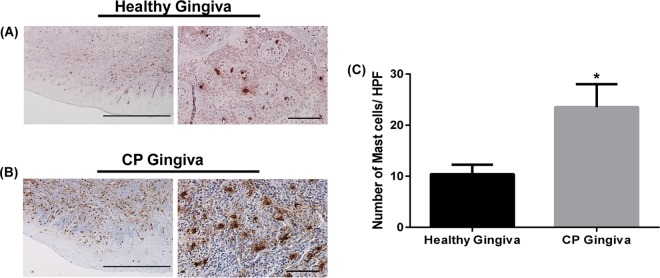
Recent studies demonstrated that mast cell numbers are increased in CP gingiva compared to those in healthy gingiva or gingivitis lesions (23, 25–27, 29, 30), with the degree of mast cell degranulation correlating well with disease severity (25). In contrast, an earlier study showed a decrease in mast cells in CP gingiva compared to the level in normal gingiva (49). Therefore, our first goal was to determine the status of mast cells in normal and CP gingival samples. Immunohistochemical (IHC) analysis with anti-tryptase antibody revealed that mast cells were present in gingival tissues from both healthy subjects and patients with CP (Fig. 1A and B). Quantitative analysis demonstrated a significant increase in mast cell numbers in CP patients compared to the numbers in healthy subjects (Fig. 1C).
FIG 1.
IHC analysis of mast cells in periodontal tissues from healthy subjects and patients with CP. Healthy gingivae (A) and gingivae from patients diagnosed with CP (B) were stained with anti-human mast cell tryptase antibody. Representative photomicrographs are shown. Bars = 2 mm (left) and 200 μm (right). (C) Quantitative analysis of mast cells was done by counting mast cells in a total of 75 high-power fields (HPF) from 5 different tissue sections of both healthy and CP gingivae. Data presented are means and SEM for mast cell counts for 5 donors. Statistical significance was determined by two-way ANOVA with Bonferroni's posttest (*, P < 0.01).
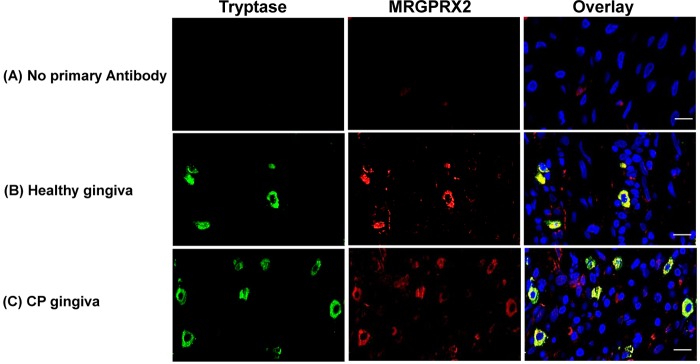
MRGPRX2 is expressed on human skin mast cells and dorsal ganglia but not in other immune or structural cells (36, 50, 51). To determine if MRGPRX2 is expressed in human gingival mast cells, we performed immunofluorescence (IF) staining with anti-tryptase and anti-MRGPRX2 antibodies. Consistent with the immunohistochemistry data (Fig. 1), mast cells were detected in normal gingiva, and their numbers were increased in CP gingiva (Fig. 2B and C, green cells). MRGPRX2 was also expressed in normal gingival tissue, and the number of cells expressing this receptor was increased in CP tissue (Fig. 2B and C, red cells). The overlay images in Fig. 2B and C show that mast cells in both normal and CP samples expressed MRGPRX2 (yellow cells). These findings demonstrate that MRGPRX2-expressing mast cells are present in normal periodontal tissue and that their numbers are increased in CP tissues. We found that several MRGPRX2-expressing cells were not stained by use of anti-tryptase antibody (Fig. 2, overlay images). Fujisawa et al. (50) recently showed that in the skin of patients with chronic urticaria, most MRGPRX2+ cells are mast cells, with nerve cells accounting for ∼10% of MRGPRX2-expressing cells. Similarly, the MRGPRX2+ tryptase− cells in the gingiva are likely to be nerve cells.
FIG 2.
Analysis of tryptase- and MRGPRX2-positive mast cells in periodontal tissue. Representative photomicrographs (n = 5 donors) of double immunofluorescence staining of gingival tissue are shown. Original magnification, ×60. (A) Control. (B and C) A healthy gingiva (B) and the gingiva of a patient with CP (C) were stained with anti-tryptase (green) and anti-MRGPRX2 (red) antibodies. Overlays of double-staining samples are shown. Bars = 50 μm.
PgLPS1690 inhibits HDP-induced Ca2+ mobilization and degranulation in human LAD2 mast cells, but PgLPS1435/1449 does not.
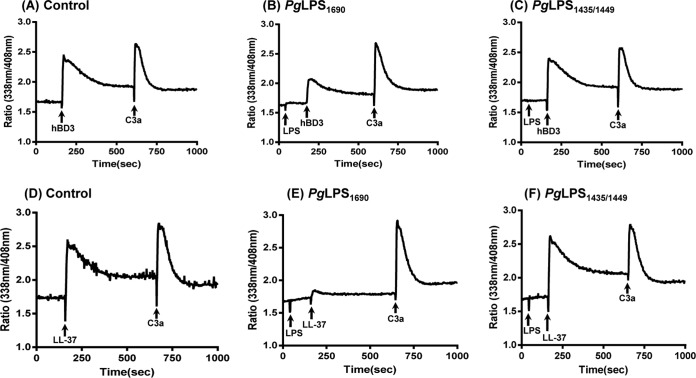
MRGPRX2 expressed on human mast cells is activated by epithelium- and neutrophil-derived HDPs, i.e., hBD3 and LL-37 (37, 38). Mast cells are activated via two major pathways: high-affinity IgE receptors (FcεRI) and G protein-coupled receptors (GPCRs), such as MRGPRX2 (52). While mast cell activation via both pathways results in intracellular Ca2+ mobilization, signaling via FcεRI leads to degranulation and cytokine generation, but MRGPRX2 activation causes strong degranulation and little to no cytokine generation (52). We therefore wanted to determine if PgLPS1690 and PgLPS1435/1449 modulate HDP-induced degranulation in human mast cells. Because of the difficulty in obtaining sufficient numbers of human tissue mast cells, a number of laboratories, including our own, have utilized a human mast cell line, LAD2, for functional studies (32, 38, 50). Although there are limitations associated with the use of cell lines, LAD2 cells are well characterized and express functional MRGPRX2 (33, 34, 37, 38). hBD3 (100 nM) and LL-37 (1 μM) caused intracellular Ca2+ mobilization (Fig. 3A and D). Preincubation of cells with PgLPS1690 did not induce Ca2+ mobilization but resulted in substantial inhibition of the responses to both hBD3 and LL-37 (Fig. 3, panel A versus panel B and panel D versus panel E). The complement component C3a activates human mast cells via a GPCR, the C3a receptor (53, 54). To determine the specificity of PgLPS1690 for HDPs, we tested its effect on Ca2+ mobilization induced by C3a. While PgLPS1690 inhibited Ca2+ responses to HDPs, it had no effect on the response to C3a (Fig. 3, panel A versus panel B and panel D versus panel E). Unlike PgLPS1690, PgLPS1435/1449 did not inhibit the hBD3- or LL-37-induced Ca2+ responses (Fig. 3, panel A versus panel C and panel D versus panel F).
FIG 3.
PgLPS1690 inhibits hBD3- and LL-37-induced Ca2+ mobilization in LAD2 cells, but PgLPS1435/1449 does not. (A and D) Indo-1-loaded cells were sequentially exposed to hBD3 (100 nM) or LL-37 (1 μM) and C3a (10 nM), and intracellular Ca2+ mobilization was determined. (B and E) Cells were pretreated with PgLPS1690 (10 μg/ml) before exposure to hBD3 (100 nM) or LL-37 (1 μM) and C3a (10 nM), and intracellular Ca2+ mobilization was determined. (C and F) Cells were pretreated with PgLPS1435/1449 (10 μg/ml) before exposure to hBD3 (100 nM) or LL-37 (1 μM) and C3a (10 nM), and intracellular Ca2+ mobilization was determined. Traces are representative of three independent experiments.
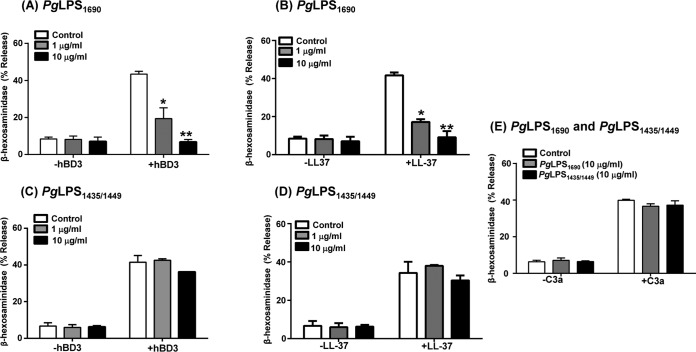
Mast cells express TLR2 and TLR4, key receptors for LPS recognition, yet previous studies have shown that PgLPS does not induce degranulation in rat peritoneal mast cells (55). Similarly, we found that PgLPS1690 and PgLPS1435/1449 did not cause degranulation in human LAD2 cells (Fig. 4A and B). However, preincubation of LAD2 cells with PgLPS1690 resulted in substantial inhibition of degranulation in response to both hBD3 and LL-37 (Fig. 4A and B) but had no effect on the response to C3a (Fig. 4E). In contrast, PgLPS1435/1449 had no effect on degranulation in response to hBD3, LL-37, or C3a (Fig. 4C to E).
FIG 4.
PgLPS1690 inhibits hBD3- and LL-37-induced degranulation in LAD2 cells, but PgLPS1435/1449 does not. (A to D) Cells were pretreated with buffer (control), PgLPS1690 (1 or 10 μg/ml, 5 min) (A and B), or PgLPS1435/1449 (1 or 10 μg/ml, 5 min) (C and D) and then exposed to hBD3 (100 nM, 30 min) or LL-37 (1 μM, 30 min), and degranulation (β-hexosaminidase release) was determined. (E) Cells were pretreated with PgLPS1690 (10 μg/ml, 5 min) or PgLPS1435/1449 (10 μg/ml, 5 min) and then exposed to C3a (10 nM, 30 min), and degranulation (β-hexosaminidase release) was determined. Data are means and SEM for three independent experiments. Statistical significance was determined by two-way ANOVA with Bonferroni's posttest (*, P < 0.01; **, P < 0.001).
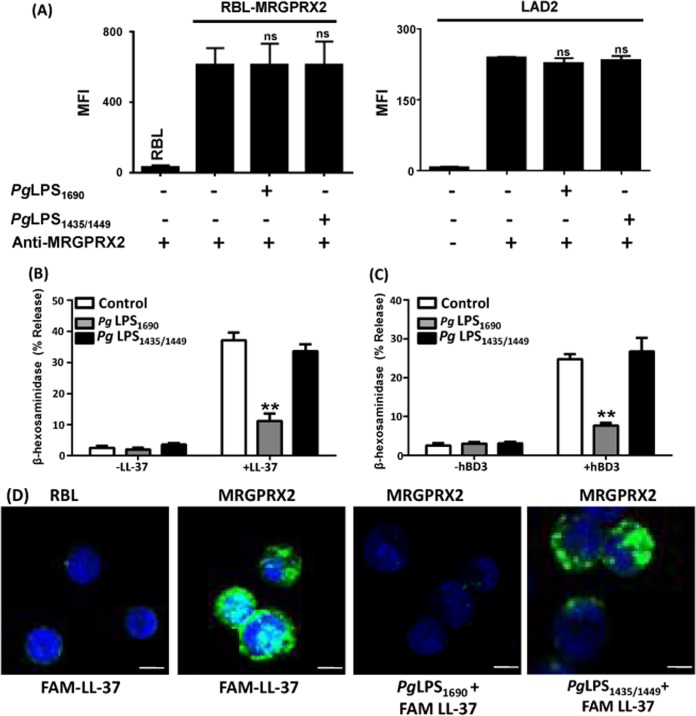
PgLPS1690 has no effect on cell surface MRGPRX2 expression but inhibits degranulation through its association with LL-37.
LPS treatment has been shown to alter the phenotype and function of mast cells (56). It is therefore possible that PgLPS1690 can inhibit HDP-induced mast cell degranulation via downregulation of MRGPRX2. To test this possibility, we first determined the effects of PgLPS1690 and PgLPS1435/1449 on cell surface expression of MRGPRX2 on transfected RBL-2H3 (RBL-MRGPRX2) cells by flow cytometry. In untransfected RBL-2H3 cells, there was minimal binding of anti-MRGPRX2 antibody, which was increased ∼20-fold in RBL-MRGPRX2 cells. There was no significant change in MRGPRX2 expression upon incubation of cells with either PgLPS1690 or PgLPS1435/1449 (Fig. 5A, left panel). Similarly, PgLPS1690 or PgLPS1435/1449 pretreatment had no effect on cell surface MRGPRX2 on LAD2 cells, which natively express the receptor (Fig. 5A, right panel). These findings suggest that the inhibitory effect of PgLPS1690 on HDP-induced mast cell degranulation is not mediated via the downregulation of cell surface MRGPRX2.
FIG 5.
PgLPS1690 has no effect on MRGPRX2 expression but inhibits degranulation through its association with LL-37. (A) RBL-MRGPRX2 (left) or LAD2 (right) cells were incubated at 37°C for 5 min with buffer (control), PgLPS1690 (50 μg/ml), or PgLPS1435/1449 (50 μg/ml). The reaction was stopped, and the cell surface MRGPRX2 level was determined by flow cytometry as described in Materials and Methods. MFI, mean fluorescence intensity. Untransfected RBL cells and LAD2 cells incubated with an isotype-matched antibody were used as controls. ns, not significant. (B and C) RBL-MRGPRX2 cells were preincubated with buffer, PgLPS1690, or PgLPS1435/1449 at 37°C for 5 min and then exposed to LL-37 (3 μM, 30 min) (B) or hBD3 (300 nM, 30 min) (C), and degranulation (β-hexosaminidase release) was determined. Results are the means and SEM for three independent experiments. Statistical significance was determined by two-way ANOVA with Bonferroni's posttest (**, P < 0.001). (D) Untransfected RBL cells (first panel) and RBL-MRGPRX2 cells (second panel) were exposed to FAM-LL-37 (1 μM) and analyzed by confocal microscopy. RBL-MRGPRX2 cells were preincubated with PgLPS1690 (50 μg/ml) (third panel) or PgLPS1435/1449 (50 μg/ml) (last panel), exposed to FAM-LL-37 (1 μM, 30 min), and analyzed by confocal microscopy. Bars = 20 μm.
Similar to that in human LAD2 cells (Fig. 4), hBD3- and LL-37-induced degranulation in RBL-MRGPRX2 cells was inhibited by PgLPS1690 but not by PgLPS1435/1449 (Fig. 5B and C). Because PgLPS1690 did not modulate cell surface MRGPRX2 expression (Fig. 5A), we sought to determine if it inhibits the binding of ligand to the receptor. To test this possibility, we utilized fluorescence-labeled LL-37 (FAM-LL-37) and RBL-MRGPRX2 cells. We found that FAM-LL-37 bound to RBL-MRGPRX2 cells but not to untransfected cells (Fig. 5D). Furthermore, preincubation with PgLPS1690 prevented the binding of FAM-LL-37 to RBL-MRGPRX2 cells, which was associated with inhibition of FAM-LL-37-induced degranulation (Fig. 5B). Conversely, PgLPS1435/1449, which did not block LL-37-induced mast cell degranulation (Fig. 5B), had no inhibitory effect on FAM-LL-37 binding to RBL-MRGPRX2 cells (Fig. 5D).
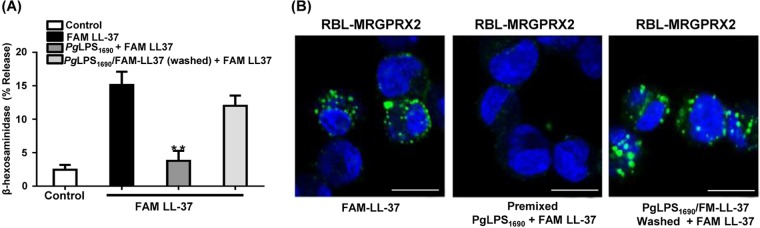
There are two possible mechanisms by which PgLPS1690 inhibits LL-37/MRGPRX2-mediated mast cell degranulation. One involves a direct interaction of anionic PgLPS1690 with the cationic LL-37 molecule, and the other occurs via binding of LPS to MRGPRX2 on mast cells, where it may function as a receptor antagonist. To distinguish between these possibilities, we premixed PgLPS1690 and FAM-LL-37 and tested the ability of LPS to modulate MRGPRX2-mediated degranulation. We found that PgLPS1690 inhibited FAM-LL-37-induced degranulation without requiring preincubation (Fig. 6A). Furthermore, when cells were washed to remove the mixture and restimulated with FAM-LL-37, there was restoration of degranulation. Premixing of FAM-LL-37 with PgLPS1690 prevented the ability of the ligand to bind to MRGPRX2 (Fig. 6B). Furthermore, when the cells were washed to remove the PgLPS1690/FAM-LL-37 and then restimulated with FAM-LL-37, there was restoration of ligand binding to the receptor (Fig. 6B). These findings suggest that PgLPS1690 inhibits LL-37-induced mast cell degranulation via its interaction with ligand rather than by acting as an MRGPRX2 antagonist.
FIG 6.
PgLPS1690 inhibits FAM-LL-37-induced mast cell degranulation through association with LL-37, not MRGPRX2. (A) RBL-MRGPRX2 cells were exposed to FAM-LL-37 (3 μM) or premixed PgLPS1690 (50 μg/ml) and FAM-LL-37 (3 μM) for 30 min, and degranulation was determined. In addition, the PgLPS1690/FAM-LL-37 mixture was removed by washing, the cells were reexposed to FAM-LL-37 for 30 min, and degranulation was determined. Data are means and SEM for three independent experiments. Statistical significance was determined by two-way ANOVA with Bonferroni's posttest (**, P < 0.001). (B) Samples from each of the three experimental conditions described for panel A were used for confocal microscopy analyses, and representative photomicrographs are shown. Bars = 10 μm.
DISCUSSION
This study provides the first demonstration that MRGPRX2-expressing mast cells are present in normal gingival tissue and that their numbers are increased in patients with CP. The unique features of MRGPRX2 are that it is selectively expressed in mast cells and no other immune cells and that it is activated by multiple cationic ligands, such as HDPs (hBDs, the cathelicidin LL-37, retrocyclin, and protegrin), neuropeptides (substance P and cortistatin), and eosinophil granule proteins (major basic protein [MBP] and eosinophil peroxidase), as well as by a number of FDA-approved cationic drugs (32, 36–38, 50, 57). Activation of MRGPRX2 contributes to host defense, pseudoallergic drug reactions, and chronic inflammatory diseases, such as chronic urticaria (32, 33, 35). The data presented herein suggest that MRGPRX2 contributes to both gingival homeostasis and CP and that differential regulation of HDP-induced mast cell degranulation by PgLPS1690 and PgLPS1435/1449 has a role in the modulation of disease progression.
The epithelium and infiltrating neutrophils play an important role in gingival homeostasis, in part via the secretion of HDPs, such as defensins and the cathelicidin LL-37 (58). These cationic HDPs have direct antimicrobial activities due to their ability to bind to negatively charged residues on microbial membranes, thereby forming pores (59, 60). In addition, HDPs modulate the host response and wound healing via the recruitment and activation of mast cells, monocytes, and T cells (58, 61–64). It now appears that these properties play a greater role in host defense against microbial infection than their direct antimicrobial activity (65, 66). We recently showed that HDPs induce mast cell degranulation via MRGPRX2 (33, 34, 37, 38). Based on these findings, we proposed that MRGPRX2 contributes to the host defense following microbial infection. It is noteworthy that two types of human mast cells have been identified based on the protease content of their secretory granules. Mast cells that are found in the connective tissue of skin contain both tryptase and chymase and are known as MCTC (67, 68). In contrast, the majority of mast cells that are found in the lungs and gut express only tryptase and are known as MCT. Recent studies demonstrated that while skin MCTC express MRGPRX2 at high levels, lung mast cells do not (50). Taken together, these findings imply that the ability of LL-37 and hBDs to modulate host defense and wound healing is restricted to tissues that contain MRGPRX2-expressing MCTC. In the present study, we have shown that mast cells in healthy gingival tissue express MRGPRX2. This suggests that HDPs derived from oral epithelial cells and infiltrating neutrophils found in normal tissue (69) contribute to gingival homeostasis by inducing mast cell degranulation via MRGPRX2. This effect is likely mediated via the action of mast cell-derived proteases that cause increased vascular permeability and disrupt tight junctions, leading to the transmigration of neutrophils across the vascular endothelium (22).
Studies with human subjects and a mouse model of periodontitis suggested that mast cell degranulation contributes to periodontal bone loss (25–27, 29, 31). However, the mechanisms of mast cell activation and regulation in periodontitis have not been determined. The findings of the current study provide the first demonstration that a significant increase in MRGPRX2-expressing mast cells is present in CP patients compared to the level in gingival tissue of healthy subjects. Considered in the context of studies reporting enhanced expression of hBDs and LL-37 in inflamed periodontal tissue relative to that in healthy tissue (43–48), it is feasible that HDP-induced gingival mast cell degranulation via MRGPRX2 contributes to periodontal disease progression. In total, these findings imply that while low-level inflammation induced by HDP/MRGPRX2-mediated mast cell degranulation contributes to gingival homeostasis, sustained inflammation, associated with elevated levels of both HDPs and MRGPRX2-expressing mast cells, promotes periodontal disease.
The observation that PgLPS1690 inhibits HDP-induced degranulation of human mast cells via MRGPRX2 is intriguing, and its significance is currently unknown. It is plausible that during gingival homeostasis PgLPS1690 inhibits HDP-induced mast cell degranulation, thus compromising host defense. However, due to the relative absence of pathogens associated with homeostasis, there might be an insufficient amount of PgLPS1690 to inhibit mast cell degranulation induced by HDPs released from resident epithelial cells and recruited neutrophils. Furthermore, it is conceivable that the virulence of P. gingivalis reflects an alteration in the lipid A composition of its LPS from PgLPS1690 to PgLPS1435/1449, implying that PgLPS1690 contributes to gingival homeostasis. Thus, the possibility that PgLPS1690 disrupts gingival homeostasis by inhibiting the HDP/MRGPRX2-mediated host defense function of mast cells is unlikely.
In periodontal disease, both HDP levels (43–48) and the number of MRGPRX2-expressing mast cells are elevated (Fig. 2). Thus, PgLPS1690 may serve to control disease progression by blocking MRGPRX2-expressing mast cell-mediated inflammation, thereby providing a novel mechanism for obstructing the growth of proinflammatory pathobionts. It has been shown that hemin, a component of gingival crevicular fluid, has an impact on the generation of different types of PgLPS (9). Thus, low concentrations of hemin lead to the generation of mostly penta-acylated lipid A structures, but at high concentrations mostly tetra-acylated lipid A structures are found (9). Consequently, it is likely that alteration of the lipid A composition from the penta- to the tetra-acylated form in the presence of a high hemin concentration removes PgLPS1690's inhibitory signal for blocking mast cell degranulation. Under the influence of high levels of hemin, preferential synthesis of PgLPS1435/1449, which does not inhibit HDP-induced mast cell degranulation, may represent a previously unrecognized strategy for P. gingivalis to evade host defense mechanisms in gingival tissue, thereby contributing to dysbiosis and the pathogenesis of periodontal disease.
In addition to chronic inflammation at the initial site of infection, P. gingivalis also contributes to systemic inflammation, such as atherosclerosis, which is dependent on the presence of mast cells (70–73). The differential effects of PgLPS1690 and PgLPS1435/1449 on HDP/MRGPRX2-mediated mast cell degranulation may therefore modulate chronic inflammatory reactions at distal sites. In this context, P. gingivalis mutants with differences in the lipid A composition of LPS were recently shown to vary in the ability to modulate systemic chronic inflammation (15). Thus, similar P. gingivalis mutants may be required for future studies to delineate the roles of PgLPS, HDP, and MRGPRX2 in mast cell-mediated local periodontal pathogenesis and distal chronic inflammatory diseases, such as atherosclerosis.
The mechanism by which PgLPS1690 modulates HDP-induced signaling and mast cell degranulation has not been determined. PgLPS1690 stimulates autophagy in Saos-2 osteosarcoma cells, and this effect is reversed by PgLPS1435/1449 (74). Furthermore, autophagy is critical for IgE-dependent mast cell degranulation (75). The finding in the present study that PgLPS1690 inhibits MRGPRX2-mediated mast cell degranulation suggests that autophagy is unlikely to be involved. One possibility is that PgLPS1690 binds to MRGPRX2 and acts as a receptor antagonist to inhibit HDP-induced mast cell responses. This is unlikely for several reasons. We found that although preincubation of cells with PgLPS1690 both prevented binding of FAM-LL-37 to MRGPRX2 and blocked degranulation, similar results were obtained when cells were exposed to premixed PgLPS1690 and FAM-LL-37. Furthermore, when cells were washed to remove the PgLPS1690 and FAM-LL-37 mixture and restimulated with FAM-LL-37, there was restoration of degranulation, and this was associated with binding of FAM-LL-37 to MRGPRX2. These findings suggest that the mechanism via which PgLPS1690 inhibits LL-37-induced mast cell degranulation does not involve a direct antagonist effect on MRGPRX2 but may be mediated via its interaction with ligand. We recently showed that E. coli LPS also inhibits hBD3- and LL-37-induced degranulation in mast cells (34). Thus, it is likely that electrostatic interactions between negatively charged E. coli LPS or PgLPS1690 and cationic HDPs provide similar mechanisms for the modulation of MRGPRX2-mediated degranulation in human mast cells.
In summary, the data presented herein suggest that mast cells play important roles in both gingival homeostasis and periodontal disease by orchestrating an interaction between gingival epithelial cells and neutrophils via MRGPRX2-mediated degranulation. In vivo studies with wild-type and mast cell-deficient mice have demonstrated an important role for mast cells in P. gingivalis-induced periodontal inflammation and bone loss (31). A mouse ortholog (MrgprB2) of human MRGPRX2 was recently identified, and similar to the situation in humans, this receptor appears to be expressed only in mast cells (32). Thus, future in vivo studies with MrgprB2 knockout mice should provide new information on the role of this receptor in periodontal health and disease.
MATERIALS AND METHODS
Materials.
All cell culture reagents were purchased from Invitrogen (Gaithersburg, MD). Native complement C3a was obtained from Complement Technology (Tyler, TX). hBD3, LL-37, and FAM-LL-37 were purchased from Anaspec (Freemont, CA). PgLPS1690 and PgLPS1435/1449 were obtained from Astarte Biologics (Redmond, WA). Anti-human tryptase antibody was purchased from Santa Cruz (Dallas, TX), and anti-human MRGPRX2 antibody was obtained from Novus Biologicals (Littleton, CO). 4′,6-Diamidino-2-phenylindole (DAPI), ProLong Gold antifade reagent, Alexa Fluor 488-conjugated donkey anti-mouse IgG, and Alexa Fluor 594-conjugated donkey anti-rabbit IgG were purchased from Molecular Probes Inc. (Eugene, OR). Vectastain ABC kits and NovaRED peroxide substrate were obtained from Vector Laboratories, Inc. (Burlingame, CA). Phycoerythrin (PE)-conjugated anti-human MRGPRX2 antibody was obtained from BioLegend (San Diego, CA).
Human gingival tissue.
Human gingival tissue samples were obtained during routine periodontal surgery performed at the University of Pennsylvania, School of Dental Medicine. In all cases, the donated tissue represented secondary gingival flaps that were typically discarded upon removal from the patient's mouth. Healthy tissue came from individuals free of periodontal disease presenting for clinical crown-lengthening surgery. Diseased tissue was procured from individuals diagnosed with CP who presented for pocket reduction surgery. Upon removal, tissue was immediately placed in cold F-12 medium supplemented with 1% antibiotic-antimycotic solution and transported to the laboratory on ice for further processing. The protocol for tissue procurement was approved by the University of Pennsylvania Institutional Review Board, and all donors provided informed consent.
IHC.
Immunohistochemical (IHC) staining was performed on 5-μm-thick, paraffin-embedded healthy and CP gingiva sections by using a mouse anti-human MC tryptase monoclonal antibody as described previously (25), with some modifications. Briefly, deparaffinized, hydrated, and antigen-retrieved sections were incubated with anti-human tryptase (1:500 dilution) at 4°C overnight. An isotype control antibody or incubations without primary antibody served as experimental controls. Sections were washed three times with phosphate-buffered saline containing 0.1% Tween 20 (PBST) and then incubated with biotinylated horse anti-mouse IgG for 30 min. Detection was done using a Vectastain ABC kit. NovaRED peroxidase substrate solution was employed as the chromogen. The sections were then counterstained with Mayer's hematoxylin and mounted using Permount medium. Images were captured using a Nikon E600 microscope with a digital camera attached.
IF assay of human gingival tissue.
Immunofluorescence (IF) staining was performed on 5-μm-thick, paraffin-embedded sections prepared from healthy and CP gingiva. Briefly, deparaffinized, hydrated, and antigen-retrieved sections were incubated with anti-human tryptase (1:750 dilution) overnight at 4°C. An isotype control antibody or incubations without primary antibody served as experimental controls. Sections were then washed thrice with PBST and incubated with secondary antibody, i.e., Alexa Fluor 488-conjugated donkey anti-mouse (1:400 dilution), in conjunction with DAPI (1:4,000) for 1 h at room temperature in the dark. Subsequently, sections were washed three times with PBST and incubated overnight at 4°C with anti-human MRGPRX2 (1:500). The following day, sections were washed and incubated with Alexa Fluor 594-conjugated donkey anti-rabbit IgG (1:400 dilution) at room temperature in the dark for 1 h. The tissue sections were then washed and mounted with ProLong Gold antifade reagent. Images were captured using a Nikon A1R laser scanning confocal microscope with a 60× water objective (numerical aperture [NA] = 1.2) and analyzed using Nikon Elements and Adobe Photoshop software.
Human mast cell culture.
The human mast cell line LAD2 was maintained in complete StemPro-34 medium supplemented with l-glutamine (2 mM), penicillin (100 IU/ml), streptomycin (100 μg/ml), and recombinant human stem cell factor (rhSCF) (100 ng/ml). Hemidepletions were performed weekly with medium containing rhSCF (100 ng/ml) (76).
Transfection of RBL-2H3 cells.
Cells were transfected with plasmids encoding hemagglutinin (HA)-tagged MRGPRX2 by use of an Amaxa Nucleofector device and an Amaxa kit V according to the manufacturer's protocol. Following transfection, cells were cultured in the presence of G418 (1 mg/ml), and cells expressing equivalent levels of the receptor were sorted using an anti-HA specific antibody and fluorescein isothiocyanate (FITC)-conjugated anti-mouse IgG (35, 37, 77).
The LAD2 and RBL-2H3 cells used in this study were authenticated by determining the expression of FcεRI and c-Kit by flow cytometry.
Calcium mobilization.
Ca2+ mobilization was determined as described previously (37). Briefly, LAD2 mast cells (0.2 × 106) were loaded with 1 μM indo-1 AM for 30 min at room temperature. Cells were washed and resuspended in 1.5 ml of HEPES-buffered saline. Ca2+ mobilization was measured using a Hitachi F-2500 spectrophotometer, with an excitation wavelength of 355 nm and an emission wavelength of 410 nm.
Degranulation.
LAD2 (5 × 103) and RBL-MRGPRX2 (5 × 104) cells were seeded into 96-well plates in a total well volume of 50 μl HEPES buffer containing 0.1% bovine serum albumin (BSA) and pretreated with PgLPS1690 or PgLPS1435/1449 for 5 min before stimulation with the indicated peptides for 30 min. Cells without LPS treatment were designated controls. For total β-hexosaminidase release, unstimulated cells were lysed in 50 μl of 0.1% Triton X-100. Aliquots (20 μl) of supernatants or cell lysates were incubated with 20 μl of 1 mM p-nitrophenyl-N-acetyl-β-d-glucosamine for 1.5 h at 37°C. The reaction was stopped by adding 250 μl of a 0.1 M Na2CO3/0.1 M NaHCO3 buffer, and absorbance was measured at 405 nm (37).
Flow cytometry.
Untransfected RBL-2H3 or RBL-MRGPRX2 cells and LAD2 cells (0.5 × 105/50 μl) were preincubated with either PgLPS1690 or PgLPS1435/1449 (50 μg/ml) for 5 min at 37°C. Cells were incubated with PE-conjugated anti-human MRGPRX2 antibody (0.2 μg) for 30 min on ice. Cells were washed in fluorescence-activated cell sorter (FACS) buffer (PBS containing 2% fetal calf serum [FCS] and 0.02% sodium azide) and fixed in 300 μl 1.5% paraformaldehyde. Cells were acquired using a BD LSR II flow cytometer (San Jose, CA), and the resulting data were analyzed using WinList software, version 8.
Confocal microscopy.
Untransfected RBL-2H3 or RBL-MRGPRX2 cells (1 × 105) were stimulated with FAM-LL-37 at 37°C for 30 min in the presence or absence of PgLPS1690 or PgLPS1435/1449. Cells were then washed and stimulated with FAM-LL-37 at 37°C for 30 min. After washing three times with PBS, samples were fixed with 1.5% formaldehyde and cytospun onto glass slides. Images were captured using a Nikon A1R laser scanning confocal microscope with a 60× water objective (NA = 1.2) and analyzed using Nikon NIS Elements software.
Statistical analysis.
Data were expressed as means ± standard errors of the means (SEM) for 3 to 5 independent experiments, and statistical significance was determined by two-way analysis of variance (ANOVA) with Bonferroni's posttest. The significance of differences is indicated in the figures and was analyzed by use of GraphPad Prism, version 5.01.
ACKNOWLEDGMENTS
This work was supported by NIH grants R21-AI108585 and R01-AI124182 to H.A. It was also supported by grant K99-HL121073 to H.S. and grant R0-1DE022465 to K.B.-B.
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
We thank Arnold Kirshenbaum and Dean Metcalfe (NIAID/NIH) for providing LAD2 mast cells.
We have no conflicts of interest to declare.
REFERENCES
- 1.Darveau RP. 2010. Periodontitis: a polymicrobial disruption of host homeostasis. Nat Rev Microbiol 8:481–490. doi: 10.1038/nrmicro2337. [DOI] [PubMed] [Google Scholar]
- 2.Darveau RP, Hajishengallis G, Curtis MA. 2012. Porphyromonas gingivalis as a potential community activist for disease. J Dent Res 91:816–820. doi: 10.1177/0022034512453589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hajishengallis G. 2014. Immunomicrobial pathogenesis of periodontitis: keystones, pathobionts, and host response. Trends Immunol 35:3–11. doi: 10.1016/j.it.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hajishengallis G, Lamont RJ. 2014. Breaking bad: manipulation of the host response by Porphyromonas gingivalis. Eur J Immunol 44:328–338. doi: 10.1002/eji.201344202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Darveau RP, Belton CM, Reife RA, Lamont RJ. 1998. Local chemokine paralysis, a novel pathogenic mechanism for Porphyromonas gingivalis. Infect Immun 66:1660–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang M, Krauss JL, Domon H, Hosur KB, Liang S, Magotti P, Triantafilou M, Triantafilou K, Lambris JD, Hajishengallis G. 2010. Microbial hijacking of complement-Toll-like receptor crosstalk. Sci Signal 3:ra11. doi: 10.1126/scisignal.2000697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zenobia C, Hajishengallis G. 2015. Porphyromonas gingivalis virulence factors involved in subversion of leukocytes and microbial dysbiosis. Virulence 6:236–243. doi: 10.1080/21505594.2014.999567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hajishengallis G. 2009. Porphyromonas gingivalis-host interactions: open war or intelligent guerilla tactics? Microbes Infect 11:637–645. doi: 10.1016/j.micinf.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Al-Qutub MN, Braham PH, Karimi-Naser LM, Liu X, Genco CA, Darveau RP. 2006. Hemin-dependent modulation of the lipid A structure of Porphyromonas gingivalis lipopolysaccharide. Infect Immun 74:4474–4485. doi: 10.1128/IAI.01924-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Curtis MA, Percival RS, Devine D, Darveau RP, Coats SR, Rangarajan M, Tarelli E, Marsh PD. 2011. Temperature-dependent modulation of Porphyromonas gingivalis lipid A structure and interaction with the innate host defenses. Infect Immun 79:1187–1193. doi: 10.1128/IAI.00900-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herath TD, Darveau RP, Seneviratne CJ, Wang CY, Wang Y, Jin L. 2013. Tetra- and penta-acylated lipid A structures of Porphyromonas gingivalis LPS differentially activate TLR4-mediated NF-kappaB signal transduction cascade and immuno-inflammatory response in human gingival fibroblasts. PLoS One 8:e58496. doi: 10.1371/journal.pone.0058496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Darveau RP, Pham TT, Lemley K, Reife RA, Bainbridge BW, Coats SR, Howald WN, Way SS, Hajjar AM. 2004. Porphyromonas gingivalis lipopolysaccharide contains multiple lipid A species that functionally interact with both Toll-like receptors 2 and 4. Infect Immun 72:5041–5051. doi: 10.1128/IAI.72.9.5041-5051.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herath TD, Darveau RP, Seneviratne CJ, Wang CY, Wang Y, Jin L. 2016. Heterogeneous Porphyromonas gingivalis LPS modulates immuno-inflammatory response, antioxidant defense and cytoskeletal dynamics in human gingival fibroblasts. Sci Rep 6:29829. doi: 10.1038/srep29829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coats SR, Do CT, Karimi-Naser LM, Braham PH, Darveau RP. 2007. Antagonistic lipopolysaccharides block E. coli lipopolysaccharide function at human TLR4 via interaction with the human MD-2 lipopolysaccharide binding site. Cell Microbiol 9:1191–1202. doi: 10.1111/j.1462-5822.2006.00859.x. [DOI] [PubMed] [Google Scholar]
- 15.Slocum C, Coats SR, Hua N, Kramer C, Papadopoulos G, Weinberg EO, Gudino CV, Hamilton JA, Darveau RP, Genco CA. 2014. Distinct lipid A moieties contribute to pathogen-induced site-specific vascular inflammation. PLoS Pathog 10:e1004215. doi: 10.1371/journal.ppat.1004215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kayagaki N, Wong MT, Stowe IB, Ramani SR, Gonzalez LC, Akashi-Takamura S, Miyake K, Zhang J, Lee WP, Muszynski A, Forsberg LS, Carlson RW, Dixit VM. 2013. Noncanonical inflammasome activation by intracellular LPS independent of TLR4. Science 341:1246–1249. doi: 10.1126/science.1240248. [DOI] [PubMed] [Google Scholar]
- 17.Jain S, Darveau RP. 2010. Contribution of Porphyromonas gingivalis lipopolysaccharide to periodontitis. Periodontol 2000 54:53–70. doi: 10.1111/j.1600-0757.2009.00333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berezow AB, Darveau RP. 2011. Microbial shift and periodontitis. Periodontol 2000 55:36–47. doi: 10.1111/j.1600-0757.2010.00350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Galli SJ, Maurer M, Lantz CS. 1999. Mast cells as sentinels of innate immunity. Curr Opin Immunol 11:53–59. doi: 10.1016/S0952-7915(99)80010-7. [DOI] [PubMed] [Google Scholar]
- 20.Gekara NO, Weiss S. 2008. Mast cells initiate early anti-Listeria host defences. Cell Microbiol 10:225–236. [DOI] [PubMed] [Google Scholar]
- 21.Marshall JS, Jawdat DM. 2004. Mast cells in innate immunity. J Allergy Clin Immunol 114:21–27. doi: 10.1016/j.jaci.2004.04.045. [DOI] [PubMed] [Google Scholar]
- 22.Wernersson S, Pejler G. 2014. Mast cell secretory granules: armed for battle. Nat Rev Immunol 14:478–494. doi: 10.1038/nri3690. [DOI] [PubMed] [Google Scholar]
- 23.Batista AC, Rodini CO, Lara VS. 2005. Quantification of mast cells in different stages of human periodontal disease. Oral Dis 11:249–254. doi: 10.1111/j.1601-0825.2005.01113.x. [DOI] [PubMed] [Google Scholar]
- 24.Gunhan M, Bostanci H, Gunhan O, Demiriz M. 1991. Mast cells in periodontal disease. Ann Dent 50:25–29. [PubMed] [Google Scholar]
- 25.Huang S, Lu F, Chen Y, Huang B, Liu M. 2013. Mast cell degranulation in human periodontitis. J Periodontol 84:248–255. doi: 10.1902/jop.2012.120066. [DOI] [PubMed] [Google Scholar]
- 26.Huang S, Lu F, Li J, Lan T, Huang B, Yin X, Jin H. 2014. Quantification of tryptase-TIM-3 double-positive mast cells in human chronic periodontitis. Arch Oral Biol 59:654–661. doi: 10.1016/j.archoralbio.2014.03.016. [DOI] [PubMed] [Google Scholar]
- 27.Lagdive SS, Lagdive SB, Mani A, Anarthe R, Pendyala G, Pawar B, Marawar PP. 2013. Correlation of mast cells in periodontal diseases. J Indian Soc Periodontol 17:63–67. doi: 10.4103/0972-124X.107500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Steinsvoll S, Helgeland K, Schenck K. 2004. Mast cells—a role in periodontal diseases? J Clin Periodontol 31:413–419. doi: 10.1111/j.1600-051X.2004.00516.x. [DOI] [PubMed] [Google Scholar]
- 29.Agrawal R, Gupta J, Gupta KK, Kumar V. 2016. Correlation of mast cells in different stages of human periodontal diseases: pilot study. J Oral Maxillofac Pathol 20:91–95. doi: 10.4103/0973-029X.180950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marjanovic D, Andjelkovic Z, Brkic Z, Videnovic G, Sehalic M, Matvjenko V, Lestarevic S, Djordjevic N. 2016. Quantification of mast cells in different stages of periodontal disease. Vojnosanit Pregl 73:458–462. doi: 10.2298/VSP141222030M. [DOI] [PubMed] [Google Scholar]
- 31.Malcolm J, Millington O, Millhouse E, Campbell L, Adrados Planell A, Butcher JP, Lawrence C, Ross K, Ramage G, McInnes IB, Culshaw S. 2016. Mast cells contribute to Porphyromonas gingivalis-induced bone loss. J Dent Res 95:704–710. doi: 10.1177/0022034516634630. [DOI] [PubMed] [Google Scholar]
- 32.McNeil BD, Pundir P, Meeker S, Han L, Undem BJ, Kulka M, Dong X. 2015. Identification of a mast-cell-specific receptor crucial for pseudo-allergic drug reactions. Nature 519:237–241. doi: 10.1038/nature14022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gupta K, Kotian A, Subramanian H, Daniell H, Ali H. 2015. Activation of human mast cells by retrocyclin and protegrin highlight their immunomodulatory and antimicrobial properties. Oncotarget 6:28573–28587. doi: 10.18632/oncotarget.3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gupta K, Subramanian H, Ali H. 2016. Modulation of host defense peptide-mediated human mast cell activation by LPS. Innate Immun 22:21–30. doi: 10.1177/1753425915610643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Subramanian H, Gupta K, Ali H. 2016. Roles of Mas-related G protein-coupled receptor X2 on mast cell-mediated host defense, pseudoallergic drug reactions, and chronic inflammatory diseases. J Allergy Clin Immunol 138:700–710. doi: 10.1016/j.jaci.2016.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tatemoto K, Nozaki Y, Tsuda R, Konno S, Tomura K, Furuno M, Ogasawara H, Edamura K, Takagi H, Iwamura H, Noguchi M, Naito T. 2006. Immunoglobulin E-independent activation of mast cell is mediated by Mrg receptors. Biochem Biophys Res Commun 349:1322–1328. doi: 10.1016/j.bbrc.2006.08.177. [DOI] [PubMed] [Google Scholar]
- 37.Subramanian H, Gupta K, Guo Q, Price R, Ali H. 2011. Mas-related gene X2 (MrgX2) is a novel G protein-coupled receptor for the antimicrobial peptide LL-37 in human mast cells: resistance to receptor phosphorylation, desensitization, and internalization. J Biol Chem 286:44739–44749. doi: 10.1074/jbc.M111.277152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Subramanian H, Gupta K, Lee D, Bayir AK, Ahn H, Ali H. 2013. Beta-defensins activate human mast cells via Mas-related gene X2. J Immunol 191:345–352. doi: 10.4049/jimmunol.1300023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Niyonsaba F, Iwabuchi K, Matsuda H, Ogawa H, Nagaoka I. 2002. Epithelial cell-derived human beta-defensin-2 acts as a chemotaxin for mast cells through a pertussis toxin-sensitive and phospholipase C-dependent pathway. Int Immunol 14:421–426. doi: 10.1093/intimm/14.4.421. [DOI] [PubMed] [Google Scholar]
- 40.Niyonsaba F, Iwabuchi K, Someya A, Hirata M, Matsuda H, Ogawa H, Nagaoka I. 2002. A cathelicidin family of human antibacterial peptide LL-37 induces mast cell chemotaxis. Immunology 106:20–26. doi: 10.1046/j.1365-2567.2002.01398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen X, Niyonsaba F, Ushio H, Hara M, Yokoi H, Matsumoto K, Saito H, Nagaoka I, Ikeda S, Okumura K, Ogawa H. 2007. Antimicrobial peptides human beta-defensin (hBD)-3 and hBD-4 activate mast cells and increase skin vascular permeability. Eur J Immunol 37:434–444. doi: 10.1002/eji.200636379. [DOI] [PubMed] [Google Scholar]
- 42.Soruri A, Grigat J, Forssmann U, Riggert J, Zwirner J. 2007. Beta-defensins chemoattract macrophages and mast cells but not lymphocytes and dendritic cells: CCR6 is not involved. Eur J Immunol 37:2474–2486. doi: 10.1002/eji.200737292. [DOI] [PubMed] [Google Scholar]
- 43.Pereira AL, Franco GC, Cortelli SC, Aquino DR, Costa FO, Raslan SA, Cortelli JR. 2013. Influence of periodontal status and periodontopathogens on levels of oral human beta-defensin-2 in saliva. J Periodontol 84:1445–1453. doi: 10.1902/jop.2012.120321. [DOI] [PubMed] [Google Scholar]
- 44.Takeuchi Y, Nagasawa T, Katagiri S, Kitagawara S, Kobayashi H, Koyanagi T, Izumi Y. 2012. Salivary levels of antibacterial peptide (LL-37/hCAP-18) and cotinine in patients with chronic periodontitis. J Periodontol 83:766–772. doi: 10.1902/jop.2011.100767. [DOI] [PubMed] [Google Scholar]
- 45.Salazar MG, Jehmlich N, Murr A, Dhople VM, Holtfreter B, Hammer E, Volker U, Kocher T. 2013. Identification of periodontitis associated changes in the proteome of whole human saliva by mass spectrometric analysis. J Clin Periodontol 40:825–832. doi: 10.1111/jcpe.12130. [DOI] [PubMed] [Google Scholar]
- 46.Yilmaz D, Guncu GN, Kononen E, Baris E, Caglayan F, Gursoy UK. 2015. Overexpressions of hBD-2, hBD-3, and hCAP18/LL-37 in gingiva of diabetics with periodontitis. Immunobiology 220:1219–1226. doi: 10.1016/j.imbio.2015.06.013. [DOI] [PubMed] [Google Scholar]
- 47.Jin L. 2011. An update on innate defense molecules of human gingiva. Periodontol 2000 56:125–142. doi: 10.1111/j.1600-0757.2010.00364.x. [DOI] [PubMed] [Google Scholar]
- 48.Turkoglu O, Emingil G, Kutukculer N, Atilla G. 2009. Gingival crevicular fluid levels of cathelicidin LL-37 and interleukin-18 in patients with chronic periodontitis. J Periodontol 80:969–976. doi: 10.1902/jop.2009.080532. [DOI] [PubMed] [Google Scholar]
- 49.Gemmell E, Carter CL, Seymour GJ. 2004. Mast cells in human periodontal disease. J Dent Res 83:384–387. doi: 10.1177/154405910408300506. [DOI] [PubMed] [Google Scholar]
- 50.Fujisawa D, Kashiwakura J, Kita H, Kikukawa Y, Fujitani Y, Sasaki-Sakamoto T, Kuroda K, Nunomura S, Hayama K, Terui T, Ra C, Okayama Y. 2014. Expression of Mas-related gene X2 on mast cells is upregulated in the skin of patients with severe chronic urticaria. J Allergy Clin Immunol 134:622.e9–633.e9. doi: 10.1016/j.jaci.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 51.Robas N, Mead E, Fidock M. 2003. MrgX2 is a high potency cortistatin receptor expressed in dorsal root ganglion. J Biol Chem 278:44400–44404. doi: 10.1074/jbc.M302456200. [DOI] [PubMed] [Google Scholar]
- 52.Gaudenzio N, Sibilano R, Marichal T, Starkl P, Reber LL, Cenac N, McNeil BD, Dong X, Hernandez JD, Sagi-Eisenberg R, Hammel I, Roers A, Valitutti S, Tsai M, Espinosa E, Galli SJ. 2016. Different activation signals induce distinct mast cell degranulation strategies. J Clin Invest 126:3981–3998. doi: 10.1172/JCI85538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Venkatesha RT, Berla Thangam E, Zaidi AK, Ali H. 2005. Distinct regulation of C3a-induced MCP-1/CCL2 and RANTES/CCL5 production in human mast cells by extracellular signal regulated kinase and PI3 kinase. Mol Immunol 42:581–587. doi: 10.1016/j.molimm.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 54.Kashem SW, Subramanian H, Collington SJ, Magotti P, Lambris JD, Ali H. 2011. G protein coupled receptor specificity for C3a and compound 48/80-induced degranulation in human mast cells: roles of Mas-related genes MrgX1 and MrgX2. Eur J Pharmacol 668:299–304. doi: 10.1016/j.ejphar.2011.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Konopka L, Wierzbicki M, Brzezinska-Blaszczyk E. 2010. Lipopolysaccharide from Porphyromonas gingivalis stimulates rat mast cells to cysteinyl leukotriene generation and upregulates Toll-like receptor -2 and -4 expression. Int J Immunopathol Pharmacol 23:803–810. doi: 10.1177/039463201002300315. [DOI] [PubMed] [Google Scholar]
- 56.Sandig H, Bulfone-Paus S. 2012. TLR signaling in mast cells: common and unique features. Front Immunol 3:185. doi: 10.3389/fimmu.2012.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Motakis E, Guhl S, Ishizu Y, Itoh M, Kawaji H, de Hoon M, Lassmann T, Carninci P, Hayashizaki Y, Zuberbier T, Forrest AR, Babina M, FANTOM Consortium . 2014. Redefinition of the human mast cell transcriptome by deep-CAGE sequencing. Blood 123:e58–e67. doi: 10.1182/blood-2013-02-483792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Greer A, Zenobia C, Darveau RP. 2013. Defensins and LL-37: a review of function in the gingival epithelium. Periodontol 2000 63:67–79. doi: 10.1111/prd.12028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Putsep K, Carlsson G, Boman HG, Andersson M. 2002. Deficiency of antibacterial peptides in patients with morbus Kostmann: an observation study. Lancet 360:1144–1149. doi: 10.1016/S0140-6736(02)11201-3. [DOI] [PubMed] [Google Scholar]
- 60.Burton MF, Steel PG. 2009. The chemistry and biology of LL-37. Nat Prod Rep 26:1572–1584. doi: 10.1039/b912533g. [DOI] [PubMed] [Google Scholar]
- 61.Yang D, Liu ZH, Tewary P, Chen Q, de la Rosa G, Oppenheim JJ. 2007. Defensin participation in innate and adaptive immunity. Curr Pharm Des 13:3131–3139. doi: 10.2174/138161207782110453. [DOI] [PubMed] [Google Scholar]
- 62.Yang D, Chen Q, Schmidt AP, Anderson GM, Wang JM, Wooters J, Oppenheim JJ, Chertov O. 2000. LL-37, the neutrophil granule- and epithelial cell-derived cathelicidin, utilizes formyl peptide receptor-like 1 (FPRL1) as a receptor to chemoattract human peripheral blood neutrophils, monocytes, and T cells. J Exp Med 192:1069–1074. doi: 10.1084/jem.192.7.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gorr SU. 2012. Antimicrobial peptides in periodontal innate defense. Front Oral Biol 15:84–98. doi: 10.1159/000329673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rohrl J, Yang D, Oppenheim JJ, Hehlgans T. 2010. Human beta-defensin 2 and 3 and their mouse orthologs induce chemotaxis through interaction with CCR2. J Immunol 184:6688–6694. doi: 10.4049/jimmunol.0903984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hilchie AL, Wuerth K, Hancock RE. 2013. Immune modulation by multifaceted cationic host defense (antimicrobial) peptides. Nat Chem Biol 9:761–768. doi: 10.1038/nchembio.1393. [DOI] [PubMed] [Google Scholar]
- 66.Mansour SC, Pena OM, Hancock RE. 2014. Host defense peptides: front-line immunomodulators. Trends Immunol 35:443–450. doi: 10.1016/j.it.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 67.Irani AM, Craig SS, DeBlois G, Elson CO, Schechter NM, Schwartz LB. 1987. Deficiency of the tryptase-positive, chymase-negative mast cell type in gastrointestinal mucosa of patients with defective T lymphocyte function. J Immunol 138:4381–4386. [PubMed] [Google Scholar]
- 68.Irani AM, Bradford TR, Kepley CL, Schechter NM, Schwartz LB. 1989. Detection of MCT and MCTC types of human mast cells by immunohistochemistry using new monoclonal anti-tryptase and anti-chymase antibodies. J Histochem Cytochem 37:1509–1515. doi: 10.1177/37.10.2674273. [DOI] [PubMed] [Google Scholar]
- 69.Puklo M, Guentsch A, Hiemstra PS, Eick S, Potempa J. 2008. Analysis of neutrophil-derived antimicrobial peptides in gingival crevicular fluid suggests importance of cathelicidin LL-37 in the innate immune response against periodontogenic bacteria. Oral Microbiol Immunol 23:328–335. doi: 10.1111/j.1399-302X.2008.00433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gibson FC III, Yumoto H, Takahashi Y, Chou HH, Genco CA. 2006. Innate immune signaling and Porphyromonas gingivalis-accelerated atherosclerosis. J Dent Res 85:106–121. doi: 10.1177/154405910608500202. [DOI] [PubMed] [Google Scholar]
- 71.Bot I, de Jager SC, Zernecke A, Lindstedt KA, van Berkel TJ, Weber C, Biessen EA. 2007. Perivascular mast cells promote atherogenesis and induce plaque destabilization in apolipoprotein E-deficient mice. Circulation 115:2516–2525. doi: 10.1161/CIRCULATIONAHA.106.660472. [DOI] [PubMed] [Google Scholar]
- 72.Kupreishvili K, Fuijkschot WW, Vonk AB, Smulders YM, Stooker W, Van Hinsbergh VW, Niessen HW, Krijnen PA. 2017. Mast cells are increased in the media of coronary lesions in patients with myocardial infarction and may favor atherosclerotic plaque instability. J Cardiol 69:548–554. doi: 10.1016/j.jjcc.2016.04.018. [DOI] [PubMed] [Google Scholar]
- 73.Shi GP, Bot I, Kovanen PT. 2015. Mast cells in human and experimental cardiometabolic diseases. Nat Rev Cardiol 12:643–658. doi: 10.1038/nrcardio.2015.117. [DOI] [PubMed] [Google Scholar]
- 74.Blasi I, Korostoff J, Dhingra A, Reyes-Reveles J, Shenker BJ, Shahabuddin N, Alexander D, Lally ET, Bragin A, Boesze-Battaglia K. 2016. Variants of Porphyromonas gingivalis lipopolysaccharide alter lipidation of autophagic protein, microtubule-associated protein 1 light chain 3, LC3. Mol Oral Microbiol 31:486–500. doi: 10.1111/omi.12141. [DOI] [PubMed] [Google Scholar]
- 75.Ushio H, Ueno T, Kojima Y, Komatsu M, Tanaka S, Yamamoto A, Ichimura Y, Ezaki J, Nishida K, Komazawa-Sakon S, Niyonsaba F, Ishii T, Yanagawa T, Kominami E, Ogawa H, Okumura K, Nakano H. 2011. Crucial role for autophagy in degranulation of mast cells. J Allergy Clin Immunol 127:1267.e6–1276.e6. doi: 10.1016/j.jaci.2010.12.1078. [DOI] [PubMed] [Google Scholar]
- 76.Kirshenbaum AS, Akin C, Wu Y, Rottem M, Goff JP, Beaven MA, Rao VK, Metcalfe DD. 2003. Characterization of novel stem cell factor responsive human mast cell lines LAD 1 and 2 established from a patient with mast cell sarcoma/leukemia; activation following aggregation of FcepsilonRI or FcgammaRI. Leuk Res 27:677–682. doi: 10.1016/S0145-2126(02)00343-0. [DOI] [PubMed] [Google Scholar]
- 77.Subramanian H, Kashem SW, Collington SJ, Qu H, Lambris JD, Ali H. 2011. PMX-53 as a dual CD88 antagonist and an agonist for Mas-related gene 2 (MrgX2) in human mast cells. Mol Pharmacol 79:1005–1013. doi: 10.1124/mol.111.071472. [DOI] [PMC free article] [PubMed] [Google Scholar]