ABSTRACT
Innate-immunity-related genes in humans are activated during urinary tract infections (UTIs) caused by pathogenic strains of Escherichia coli but are suppressed by commensals. Epigenetic mechanisms play a pivotal role in the regulation of gene expression in response to environmental stimuli. To determine whether epigenetic mechanisms can explain the different behaviors of pathogenic and commensal bacteria, we infected larvae of the greater wax moth, Galleria mellonella, a widely used model insect host, with a uropathogenic E. coli (UPEC) strain that causes symptomatic UTIs in humans or a commensal-like strain that causes asymptomatic bacteriuria (ABU). Infection with the UPEC strain (CFT073) was more lethal to larvae than infection with the attenuated ABU strain (83972) due to the recognition of each strain by different Toll-like receptors, ultimately leading to differential DNA/RNA methylation and histone acetylation. We used next-generation sequencing and reverse transcription (RT)-PCR to correlate epigenetic changes with the induction of innate-immunity-related genes. Transcriptomic analysis of G. mellonella larvae infected with E. coli strains CFT073 and 83972 revealed strain-specific variations in the class and expression levels of genes encoding antimicrobial peptides, cytokines, and enzymes controlling DNA methylation and histone acetylation. Our results provide evidence for the differential epigenetic regulation of transcriptional reprogramming by UPEC and ABU strains of E. coli in G. mellonella larvae, which may be relevant to understanding the different behaviors of these bacterial strains in the human urinary tract.
KEYWORDS: DNA methylation, Galleria mellonella, antimicrobial peptides, asymptomatic bacteriuria, epigenetics, histone acetylation, innate immunity, Toll-like receptors, urology, uropathogenic E. coli
INTRODUCTION
Commensal bacteria can persist for long periods in a host without triggering a destructive immune response. In contrast, pathogens can gain a short-term advantage by expressing virulence factors that maintain their fitness in a predominantly hostile environment. The contrasting effects of commensals and pathogens are clearly displayed in the human urinary tract, which serves as a model for studying host-parasite coevolution (1). The primary etiological agents responsible for more than 85% of uncomplicated urinary tract infections (UTIs) in humans are uropathogenic Escherichia coli (UPEC) strains, which are often resistant to antibiotics (2). UPEC strains can cause symptomatic UTIs by expressing virulence factors, such as toxins, superantigens, adhesins, and invasins, often encoded by pathogenicity islands (PAIs) in the bacterial genome (3). UPEC strains that fail to express these virulence factors can colonize the urinary tract for extended periods, causing asymptomatic bacteriuria (ABU). The genomic analysis of ABU E. coli isolates has shown that UPEC strains have adapted to long-term growth in the urinary bladder by reductive evolution and loss of the ability to express functional virulence factors (1, 4–6).
ABU resembles commensalism in the human intestinal tract but involves a bacterial monoculture rather than a complex microbiota (5). Asymptomatic colonization of the human bladder by ABU E. coli strain 83972 involves the blocking of disease-associated signaling pathways (7, 8). This remarkable evolutionary adaptation of UPEC strains to commensalism is possible only when the bactericidal innate immune response of the host is attenuated to allow the survival and persistence of asymptomatic strains (9). For example, colonization of the urinary tract by ABU E. coli strain 83972 requires the inhibition of Toll-like receptor 4 (TLR4) signaling to suppress the mucosal immune response, which remains active during symptomatic UTIs caused by UPEC strains (10–13). Innate immunity is a major line of defense against UTIs, and ABU strains must therefore ensure that innate immune responses are prevented by reducing the expression of host immunity-related genes. It is not clear whether the host can self-regulate its innate-immunity-related genes and proteins to selectively block colonization by UPEC but not ABU strains. An understanding of the underlying molecular mechanisms will provide insight into the adaptation of UPEC strains to commensalism and will eventually yield new disease markers and drug targets for UTIs.
Eukaryotic gene expression is regulated by epigenetic mechanisms, such as DNA methylation and histone acetylation, which can result in heritable phenotypic alterations independent of DNA sequence mutations (14). Epigenetic mechanisms can be targeted by effector molecules and toxins released by pathogens, presumably to hijack host innate immune responses (15). Pore-forming toxins released by pathogenic bacteria modulate a variety of host signaling pathways and regulate the phosphorylation and acetylation of histones (16). For example, the wild-type UPEC strain UTI89 can infect human uroepithelial cells by downregulating the tumor suppressor gene CDKN2A via the methylation of exonic CpG islands, whereas the fimH mutant of the same strain lacks this ability (17). Comparable studies concerning the epigenetic basis of infection by ABU E. coli strains have yet to be reported. We hypothesize that selective epigenetic regulation underpins the differential transcriptional reprogramming of innate-immunity-related genes in hosts infected by UPEC or ABU strains.
UPEC pathogenesis has been well characterized in rodent models, but the role of epigenetic mechanisms has not been addressed in detail. Such mechanisms must be investigated to fully understand the regulation of systemic-immunity-related gene expression because it affects the whole body following localized infections of the urinary tract, kidney, and bloodstream (18). The mouse model is not ideal for epigenetic experiments, because large numbers of animals are required, and this raises both economic and ethical issues. Furthermore, innate immunity in the UPEC-infected urinary tract mucosa of mammalian models is difficult to study in isolation due to unavoidable cross talk with the adaptive immune system (19). Insects have emerged as alternative model hosts for human pathogens and are particularly suitable in this context because they are small and can be reared inexpensively in large numbers without ethical concerns, and they lack adaptive immunity, allowing the innate immune system and associated epigenetic mechanisms to be studied in isolation (20–23). The larvae of the greater wax moth, Galleria mellonella, are widely used to study host-parasite coevolution and the virulence of human pathogens, including extraintestinal pathogenic E. coli (ExPEC), which encompasses all known UPEC strains (20, 22, 24–30). The differential virulence of individual UPEC clones isolated from patients suffering from community-acquired or nosocomial UTIs has already been demonstrated in the G. mellonella infection model (25). The larvae can adapt to live at 37°C (the temperature at which humans interact with pathogens and commensals), and transcriptome sequencing has revealed the expression of genes responsible for innate immunity and epigenetic regulation (31).
Here, we established the G. mellonella larval model as a host for the analysis of conserved epigenetic mechanisms and innate immune responses following infection with the UPEC strain CFT073 and the ABU strain 83972. We found that the infection of G. mellonella with UPEC and ABU isolates induced the strain-specific epigenetic regulation and transcriptional reprogramming of the host innate immune system, supporting our hypothesis that differential epigenetic regulation causes the selective transcriptional reprogramming of genes related to innate immunity in hosts infected by these distinct E. coli strains.
RESULTS
Strain-specific pathogenesis and survival of E. coli in G. mellonella larvae.
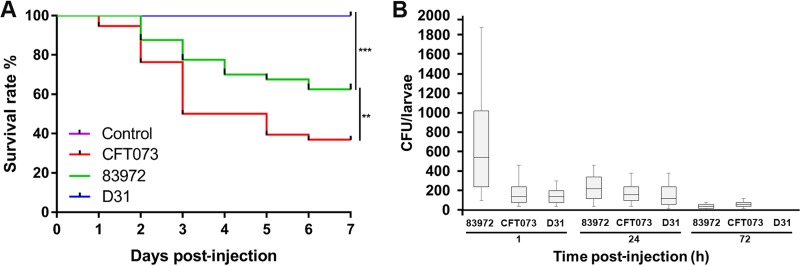
We investigated the susceptibility of G. mellonella larvae by infecting them with UPEC strain CFT073 or ABU strain 83972 and then recording mortality for up to 7 days postinjection. We observed significant differences in mortality rates between the different treatments (P < 0.0001), such as following the injection of CFT073 (survival rate, <35%) and 83972 (survival rate, >60%) at a dose of 1.0 × 103 CFU per larva (Fig. 1A). No deaths were recorded when larvae were injected with the same dose of a nonpathogenic E. coli K-12 strain (D31) or mock injected by pricking them with an empty needle. We therefore injected a dose of 1 × 103 CFU/larva in all subsequent experiments to investigate the effects of virulent and attenuated E. coli strains.
FIG 1.
Strain-specific pathogenesis and survival of E. coli in G. mellonella larvae. Bacteria were grown to log phase in LB medium at 37°C. The survival time course represents larvae inoculated with 103 CFU/larva of UPEC strain CFT073, ABU E. coli strain 83972, or the nonpathogenic E. coli K-12 strain D31. (A) Kaplan-Meier plots of G. mellonella survival after injection with strain CFT073 (UPEC) resulted in higher mortality than injection with the attenuated ABU strain 83972. Mock-injected larvae were used as controls (**, P < 0.005; ***, P < 0.0005). (B) To determine the bacterial survival rate, the E. coli load in the infected larvae was measured at several time points postinfection. For each time point, homogenates of 10 larvae were plated individually on E. coli selective ECD agar plates. The results are shown as a box-whisker plot, with the box representing values from the lower to the upper quartile and the whiskers representing the range. Each experiment was carried out three times with 10 larvae/treatment.
We also considered whether the mortality of G. mellonella larvae was associated with the survival and multiplication of E. coli in vivo. Infected larvae were homogenized in lysogeny broth (LB) and then plated onto E. coli selective ECD agar (Sigma-Aldrich, Hamburg, Germany) and incubated at 37°C for 48 h. We observed a rapid decline in the CFU count for all strains 1 h postinjection, indicating that the G. mellonella constitutive immune response was able to reduce the bacterial load efficiently (Fig. 1B). The CFU count 1 h postinjection was higher for ABU strain 83972 than for UPEC strain CFT073, indicating that the ABU strain can suppress the induction of a strong initial bactericidal innate immune response. However, at later time points (24 and 72 h), the ABU and UPEC strains showed similar CFU counts. In contrast, the survival of strain D31 continuously declined over the experimental period, confirming that the bacteria were efficiently cleared from the larvae.
Transcriptional reprogramming of G. mellonella innate immunity by the UPEC and ABU strains.
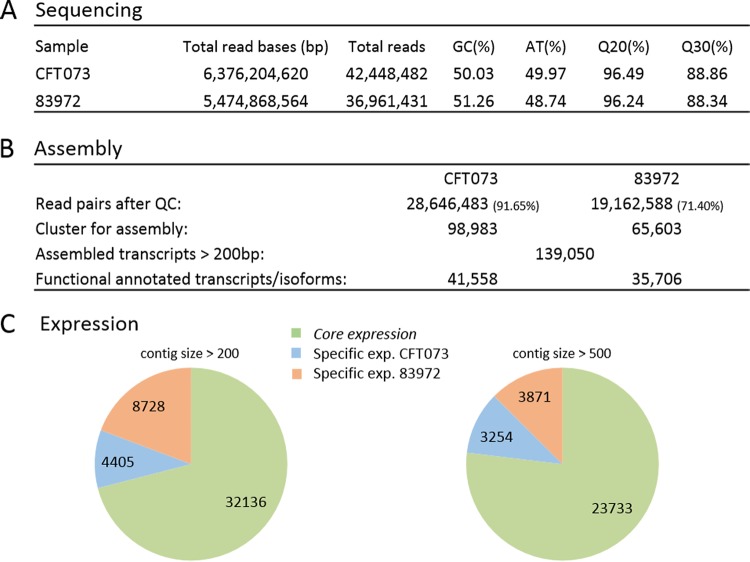
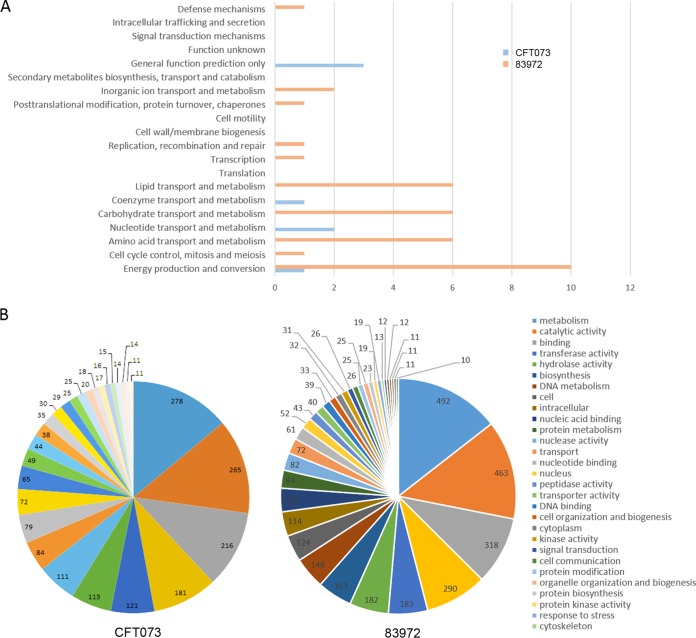
We investigated the impacts of the two strains on gene expression in the infected larvae by comparative Illumina RNA sequencing. The analysis of both libraries allowed us to assemble nearly 140,000 transcripts longer than 200 bp, among which 41,558 UPEC strain transcripts and 35,706 ABU strain transcripts could be functionally annotated (Fig. 2A and B). After false discovery rate (FDR) correction of 0.10, we identified more than 4,000 G. mellonella transcripts that were significantly expressed specifically in response to the UPEC strain, whereas more than 8,500 transcripts were significantly expressed specifically in response to the ABU strain (Fig. 2C). We characterized these strain-specific differences by annotating the transcripts based on their molecular functions. The sequencing reads were mapped to the available genome sequences of model insect species, such as Manduca sexta, Bombyx mori, and Drosophila melanogaster. The molecular functions of the ABU-specific transcripts included energy production and conversion, metabolism, DNA repair, transcription, and defense mechanisms (Fig. 3A and B). The same transcripts were also expressed in response to the UPEC strain, but they were much less abundant, indicating strain-specific differences in G. mellonella gene expression levels. We focused on the transcripts related to innate immunity and epigenetic regulation.
FIG 2.
Sequencing and assembly statistics from the G. mellonella transcriptome following infection with CFT073 (UPEC) and 83972 (ABU). (A and B) Raw sequencing results used for the assembly. (C) Core- and strain-specific expressed contigs of different sizes.
FIG 3.
COG annotations and GO assignments for the assembled sequences from larvae infected with CFT073 (UPEC) or 83972 (ABU). (A) All putative genes were aligned by BLAST to the COG database (protein identity, >50%; protein coverage, >75%), and genes expressed specifically for each condition are plotted on the x axis. (B) GO functional categories were assigned according to the molecular functions using BLAST2GO. Values indicate the numbers of classified genes.
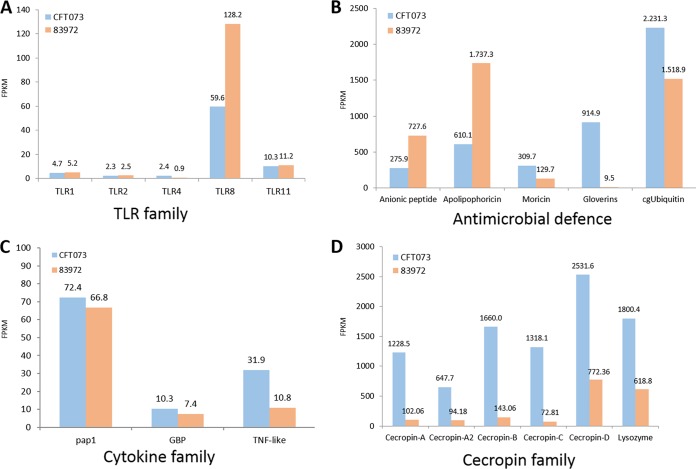
Toll-like receptors (TLRs) play an important role in the recognition of UPEC and ABU strains in humans. We observed the expression of TLR1, TLR2, TLR4, TLR8, and TLR11 transcripts in G. mellonella larvae challenged with either strain (Fig. 4A). However, TLR4 was more strongly induced in larvae infected with E. coli CFT073 (here referred to as UPEC larvae), whereas TLR1, TLR2, TLR8, and TLR11 were more strongly induced in larvae infected with E. coli 83972 (here referred to as ABU larvae).
FIG 4.
Expression of selected innate-immunity-related genes in larvae infected with CFT073 (UPEC) or 83972 (ABU). Reference genes for each family were extracted from the UniProt database and aligned using BLASTX with identity of at least 50% to the reference protein and coverage of at least 75%. Expression levels of TLR family genes (A), antimicrobial defense genes (B), cytokine family genes (C), and cecropin family genes (D) are displayed as bar charts of FPKM values retrieved by Cuffdiff analysis.
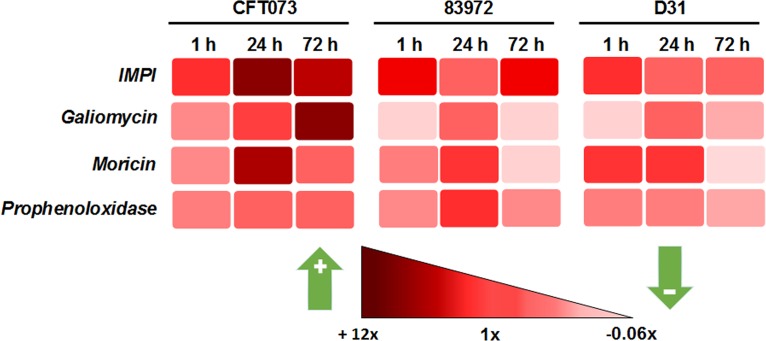
Antimicrobial peptides (AMPs) are key components of insect innate immunity against bacterial and fungal pathogens. We observed the selective expression of different AMPs and cytokine-like proteins in the UPEC and ABU larvae (Fig. 4B to D). Genes encoding phenoloxidase-activating proteinase 1 (PAP1), growth-blocking peptides (GBPs), and tumor necrosis factor (TNF) were induced more strongly in the UPEC larvae than in the ABU larvae (Fig. 4C). Moricins, cgUbiquitin, and gloverins were strongly induced in the UPEC larvae, whereas anionic peptides and apolipophoricin were strongly induced in the ABU larvae (Fig. 4B). All genes of the cecropin family, as well as lysozyme, were strongly and consistently induced in the UPEC larvae (Fig. 4D). The expression of some of these AMPs was confirmed by reverse transcription (RT)-PCR. The expression of galiomycin, moricin, insect metalloprotease inhibitor (IMPI), and prophenoloxidase was stronger in the UPEC larvae than in the ABU larvae (Fig. 5). The same genes were also transiently expressed in larvae that were injected with the nonpathogenic K-12 strain D31 (here referred to as K-12 larvae).
FIG 5.
Expression of antimicrobial peptide and prophenoloxidase genes in the infected larvae. The expression of the IMPI, galiomycin, moricin, and prophenoloxidase genes was assayed in UPEC (CFT073), ABU (83972), and K-12 (D31) larvae by quantitative real-time RT-PCR. Basal expression in the infected larvae was calculated as a fold change relative to mock-injected control larvae and normalized to the 18S rRNA housekeeping gene. The color gradient indicates fold changes in gene expression, with increasing red intensity representing upregulation and decreasing red intensity representing downregulation. The figure is a representation of the mean ΔΔCT values of three independent experiments with similar results.
Transcriptional regulation of epigenetic markers in UPEC and ABU larvae.
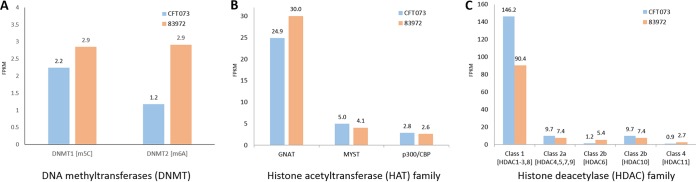
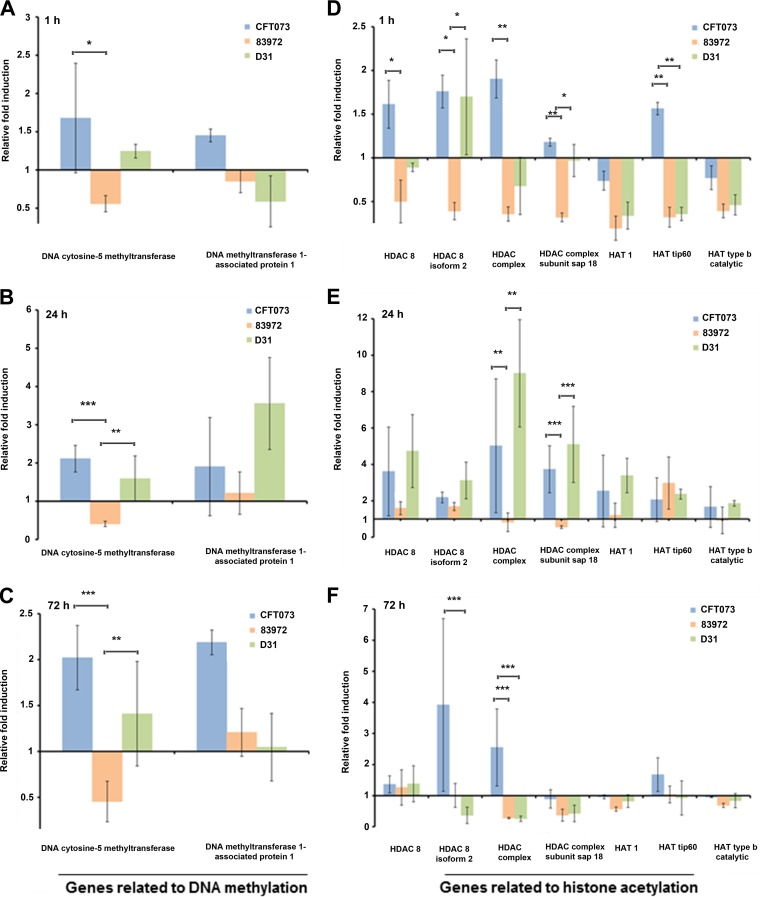
We investigated the expression of DNA methyltransferases (DNMTs), histone deacetylases (HDACs), and histone acetyltransferases (HATs) in the UPEC and ABU larvae to determine whether the different strains of bacteria induced different epigenetic markers. There are three major DNMTs that methylate genomic DNA in eukaryotes, namely, DNMT1, DNMT3a, and DNMT3b. DNMT2 shares strong sequence homology with the other DNMTs, but its role in DNA methylation remains unclear. We detected transcripts representing DNMT1 and DNMT2, but not DNMT3a or DNMT3b, in the infected larvae. The levels of DNMT1 and DNMT2 mRNAs were higher in the ABU larvae than in the UPEC larvae (Fig. 6A). Compared to mock-injected controls, genes encoding DNA methyltransferase 1-associated protein 1 and DNA cytosine 5-methyltransferase were induced in the UPEC larvae but suppressed in the ABU larvae (Fig. 7A to C). For example, the DNA cytosine 5-methyltransferase gene in ABU larvae was significantly downregulated compared to mock-injected controls after 24 h (P < 0.01) and 72 h (P < 0.01), but the expression profile was reversed in UPEC larvae after 24 h (P < 0.05). The expression of the gene differed significantly between the tested strains (P < 0.001), but no such differences were observed on different days postinjection. In contrast, differences in the expression of the DNA methyltransferase 1-associated protein 1 gene were significant only when we compared the expression levels on different days postinjection (P < 0.05).
FIG 6.
Expression of DNMT, HAT, and HDAC genes in larvae infected with CFT073 (UPEC) or 83972 (ABU). Reference genes for each family were extracted from the UniProt database and aligned using BLASTX with an identity of at least 50% to the reference protein and coverage of at least 75%. Gene expression levels of the DNMT (A), HAT (B), and HDAC (C) families are displayed as bar charts of FPKM values retrieved by Cuffdiff analysis.
FIG 7.
Transcriptional activation of genes encoding enzymes that regulate DNA methylation and histone acetylation in infected larvae. Shown is transcriptional activation of genes encoding DNA methyltransferase 1-associated protein 1 and DNA cytosine 5-methyltransferase (A to C) and HDAC8, HDAC8 isoform 2, HDAC complex, HDAC complex subunit sap18, HAT1, HAT tip60, and HAT type b catalytic (D to F) in larvae at time points 1, 24, and 72 h after exposure to E. coli strains CFT073 (UPEC), 83972 (ABU), and D31 (K-12). The expression levels are relative to those of the mock-injected larvae and were normalized to the 18S rRNA housekeeping gene. The data are means of the results of three independent experiments with standard deviations (*, P < 0.05; **, P < 0.01; ***, P < 0.001).
In the same set of samples, we identified four subclasses of HDACs and three subclasses of HATs in the infected larvae (Fig. 6B and C). HDAC class 1 comprises HDACs 1, 2, 3, and 8; class 2a comprises HDACs 4, 5, 7, and 9; class 2b contains HDACs 6 and 10; and class 4 contains HDAC 11. We observed upregulation of the class 1 and 2a HDACs in the UPEC larvae but a 5-fold upregulation of HDAC6 (class 2b) in the ABU larvae. HDAC11 (class 4) was upregulated 3-fold in the ABU larvae compared to the UPEC larvae (Fig. 6C). Gcn5-related N-acetyltransferases (GNATs) were slightly upregulated in the ABU larvae, whereas the MYST and p300/CBP family genes were not differentially expressed in our experiments (Fig. 6B).
We confirmed the expression of selected genes encoding HDACs and HATs in the UPEC and ABU larvae by RT-PCR (Fig. 7D to F). The expression levels of HDAC8 (P < 0.01), HDAC8 isoform 2 (P < 0.01), HDAC complex (P < 0.001), HDAC complex subunit sap18 (P < 0.001), HAT1 (P < 0.05), and HAT tip60 (P < 0.01) varied between the strains. Gene expression also differed significantly on different days postinjection for HDAC8 (P < 0.001), HDAC8 isoform 2 (P < 0.01), HDAC complex (P < 0.001), HDAC complex subunit sap18 (P < 0.001), HAT1 (P < 0.001), HAT type b catalytic (P < 0.01), and HAT tip60 (P < 0.001). Differential expression was most evident at the initial time point (1 h). In the UPEC larvae, genes such as those for HDAC8 isoform 2 (72 h; P < 0.01), HDAC complex (24 h; P < 0.01), and HDAC complex subunit sap18 (24 h, P < 0.001) were upregulated in comparison to the mock-injected control. In the ABU larvae, genes such as those for HDAC8 isoform 2 (1 h; P < 0.05), HDAC complex (1 h, P < 0.05; 72 h, P < 0.01), HDAC complex subunit sap18 (1 h, P < 0.001; 72 h, P < 0.01), HAT1 (1 h; P < 0.001), HAT tip60 (1 h, P < 0.01; 24 h, P < 0.001), and HAT type b catalytic (1 h; P < 0.05) were either transiently expressed or downregulated in comparison to the mock-injected control. In the K-12 larvae, genes such as those for HDAC8 (24 h; P < 0.001), HDAC8 isoform 2 (24 h, P < 0.05; 72 h, P < 0.01), HDAC complex (24 h, P < 0.001; 72 h, P < 0.01), HDAC complex subunit sap18 (24 h, P < 0.001; 72 h, P < 0.01), HAT1 (1 h, P < 0.05; 24 h, P < 0.05), and HAT tip60 (1 h, P < 0.01; 24 h, P < 0.01) were differentially expressed in comparison to the mock-injected control. Genes encoding the HDAC8 isoforms, HDAC complex subunit sap18, HAT1, HAT tip60, and HAT type b catalytic were upregulated in the UPEC larvae but downregulated in the ABU larvae (Fig. 7D to F). HDACs and HATs were similarly induced in the K-12 larvae, but the expression levels of the HDAC complex subunit and HAT tip60 were significantly higher in the UPEC larvae.
Strain-specific regulation of DNA/RNA methylation and histone acetylation in the infected larvae.
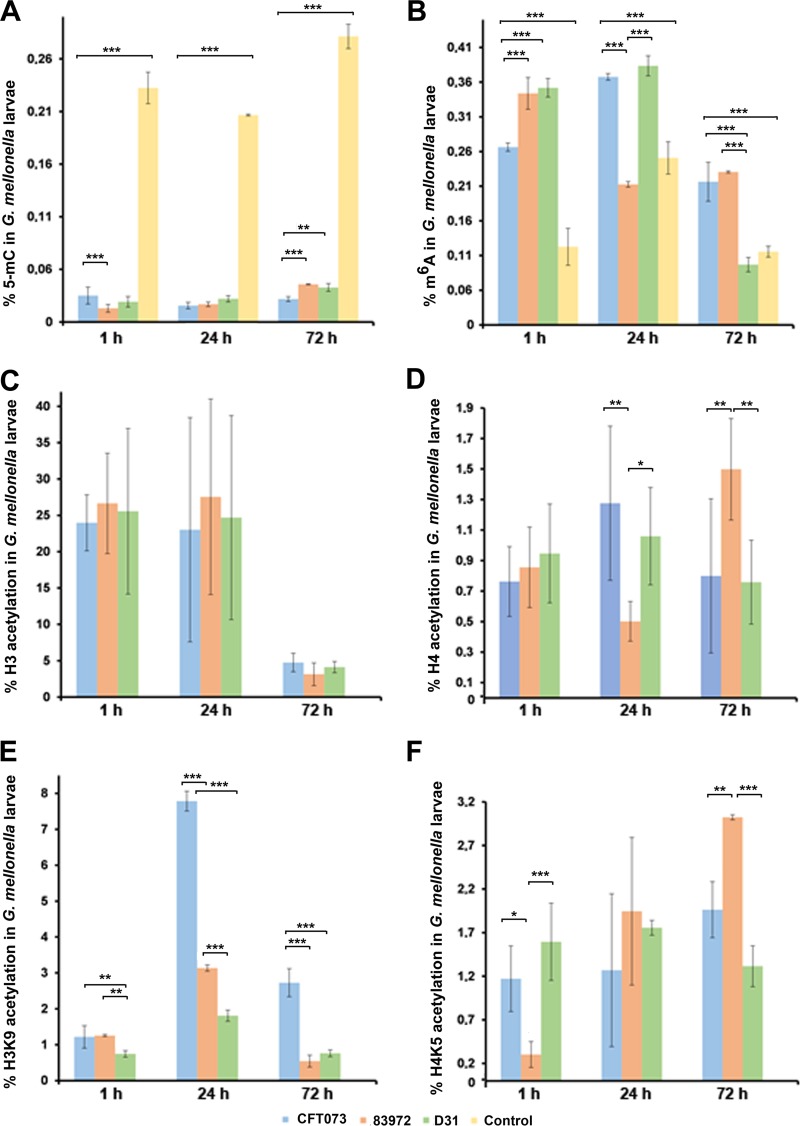
We complemented the transcriptional analysis of DNMTs, HDACs and HATs by characterizing the DNA methylation and histone acetylation status of the UPEC and ABU larvae. DNA, RNA, and histones were isolated from whole larvae injected with the three strains of E. coli for the antibody-based detection of global DNA and RNA methylation and histone acetylation (Fig. 8A to F). DNA methylation levels differed among the tested strains (P < 0.05) and also when we compared different days postinjection (P < 0.001). The levels of 5-methylcytosine in the infected larvae were significantly lower than in mock-injected control larvae (P < 0.001) (Fig. 8A). DNA methylation was also less abundant in the UPEC larvae than in the ABU and K-12 larvae, but there was little difference between the ABU and K-12 larvae (Fig. 8A).
FIG 8.
Epigenetic changes in infected larvae. Global DNA methylation (A), global m6A RNA methylation (B), and global histone H3 (C) and H4 (D), H3K9 (E), and H4K5 (F) acetylation levels in G. mellonella larvae following exposure to E. coli strains CFT073 (UPEC), 83972 (ABU), and D31 (K-12). The data are means of the results of three independent experiments with standard deviations (*, P < 0.05; **, P < 0.01; ***, P < 0.001).
We observed considerable differences in the levels of DNA and RNA methylation in the infected larvae. RNA methylation levels differed among the tested strains (P < 0.01) and also when we compared different days postinjection (P < 0.001). The abundance of methylated adenosine residues (m6A) in RNA was significantly higher in the infected larvae than in mock-injected controls (P < 0.001) (Fig. 8B). There was little difference in the abundance of m6A residues between the ABU and K-12 larvae, but this residue was significantly more abundant in the UPEC larvae than in the ABU larvae at least until the 24-h time point (P < 0.001). However, the abundance of the residue was significantly higher in the K-12 larvae than in the UPEC larvae immediately after injection (P < 0.001).
We selected histones H3, H3K9, H4, and H4K5 to investigate global histone acetylation levels because these markers are involved in the transcriptional control of immunity in mammals, although their specific roles in insect immunity are unclear (Fig. 8C to F). The acetylation levels of histones H3, H3K9, and H4K5 were differentially regulated during the course of infection and compared to the mock-injected control, and H3K9 acetylation was also strain specific (P < 0.001). We observed significant differences in the acetylation of histones H4, H3K9, and H4K5 between the UPEC and ABU larvae (Fig. 8D to F). For example, 24 h postinfection, we observed a significant increase in H3K9 (P < 0.001) and H4 (P < 0.01) acetylation in the UPEC larvae compared to the ABU larvae, but this trend had reversed by the 72-h time point with significant deficits in the levels of H4 (P < 0.01) and H4K5 (P < 0.01), but not H3K9 (P < 0.001), acetylation in the UPEC larvae compared to the ABU larvae. The acetylation of the selected histone markers was more consistent in the K-12 larvae during the experiment.
DISCUSSION
Commensal microbes may cause significant harm if the innate immune system is compromised. The attenuated commensal-like ABU E. coli strain 83972 can therefore avoid destructive inflammatory responses in humans by suppressing RNA polymerase-independent gene expression, and the strain has been isolated from patients more likely to develop pyelonephritis (8, 32). However, TLR4/type 1 interferon (IFN)-dependent immune suppression by the ABU strain does not interfere with NFKB activation (32). This signifies that transcriptional control in humans is required to prevent commensals from acquiring virulence. Because gene expression is regulated by epigenetic mechanisms, we proposed that DNA methylation and histone acetylation may regulate the type and magnitude of innate immune response in the host to discriminate between commensal ABU and UPEC strains. This may favor immune system plasticity because epigenetic mechanisms can reversibly regulate gene expression on demand, in contrast to permanent genetic mutations. The investigation of epigenetics has provided great insight into the basis of host-pathogen interactions, but little is known about the epigenetic aspects of UPEC infections. To bridge this gap, we infected G. mellonella larvae with the genetically classified E. coli strains CFT073 (UPEC) and 83972 (ABU). G. mellonella larvae provide a reliable and ethically acceptable model host for the epigenetic analysis of host-pathogen interactions at 37°C (20).
The infection of G. mellonella larvae with human-pathogenic bacteria causes the differential regulation of histone acetylation compared to uninfected larvae (24). Larval longevity is extended by inhibiting HDACs despite the severe tissue damage and septic shock (24). G. mellonella can differentiate microbial effectors from endogenous danger/alarm signals by stimulating cellular and humoral immune responses (33). Here, we found that the response to UPEC and ABU E. coli strains involves not only different innate-immunity-related genes, but also different epigenetic mechanisms that regulate their expression levels. Despite the poor survival of the UPEC and ABU strains inside the larvae, UPEC strain CFT073 was more lethal than ABU strain 83972. Even in the complex G. mellonella microbiome, virulence attenuation could help the ABU strain moderate the induction of innate immunity. This provides survival benefits to the ABU strain and helps it to outcompete the UPEC strain in growth but does not affect pathogenicity, as can be seen during bacteriuria in humans (34).
Dynamic changes in host gene expression precede the colonization of G. mellonella larvae and humans by UPEC and ABU E. coli strains. The ABU strain induced more transcripts than the UPEC strain (8,728 compared to 4,405), and this involved the differential regulation of TLR gene expression. The TLR4 gene was suppressed and the TLR11 gene was induced by the ABU strain, whereas the opposite profile was observed following infection with the UPEC strain. TLR4 controls the initial mucosal response to UPEC in murine models, preventing the development of asymptomatic bacteriuria (10), whereas TLR11 helps to prevent UTIs, given that mice lacking this receptor are highly susceptible to UPEC kidney infections (35). Our findings suggest that different TLRs play a prominent role in the recognition of ABU and UPEC strains in G. mellonella. TLR signaling ultimately affects DNA methylation and histone acetylation to influence the downstream expression of genes encoding AMPs and cytokines.
Insect genomes are sparsely methylated compared to those of mammals, and little is known about the role of DNA methylation in innate immunity (22, 36). The increase in DNA methylation and the differential regulation of DNMT genes in UPEC larvae within 1 h of infection contrasted with the profile observed in ABU larvae. At the same time point, we also observed a relatively high level of RNA methylation in the ABU larvae compared to the UPEC larvae. We found that the differential regulation of DNA and RNA methylation correlated with infection by the UPEC and ABU strains of E. coli in the G. mellonella larvae. Different levels of DNA and RNA methylation have also been reported during tumor metastasis, which supports similar findings in our insect model following UPEC and ABU infection (37). In human uroepithelial cells, infection with UPEC caused an increase in DNMT activity and CpG methylation, which downregulates the expression of the G1 cell cycle inhibitor CDKN2A (17). Commensal bacteria can downregulate TLR4 expression by DNA methylation in intestinal epithelial cells (38). In contrast, the functional significance of N6-methyladenosine (m6A) modification in mRNAs is largely unknown (39).
In addition to DNA and RNA methylation, we studied the modification of histones because they are the key protein component of chromatin, and histone modifications influence gene expression. The acetylation of lysine residues on core histones, such as H3 and H4, is a reversible posttranslational modification catalyzed by HATs. This generates a chromatin structure that promotes DNA accessibility and gene expression. The process is reversed by HDACs. Histone acetylation in G. mellonella differed qualitatively (spectrum of histone markers) and quantitatively (level of HAT/HDAC gene expression and abundance of the corresponding modified histones) following infection with the ABU and UPEC strains. The transcriptional induction of HDACs correlates with lower levels of H3 and H4 acetylation, with the exception of H4K5 acetylation, which specifically increased within 1 h of infection with the UPEC strain (40). After 24 h, we observed the hyperacetylation of histone H3K9, supporting similar observations in a mammalian model for Staphylococcus aureus infection (41). The abundance of the histone marker was reversed or transiently regulated in the ABU and K-12 larvae. Our data provide the first evidence of epigenetic remodeling by the ABU E. coli strain 83972, including differences in the expression levels of epigenetic markers compared to UPEC infection.
Histone acetylation induces the expression of innate-immunity genes encoding products such as AMPs (but not inflammatory cytokines) in cells challenged with E. coli (42). We found that insects can selectively express AMPs and cytokine-like proteins when challenged with UPEC and ABU strains. For example, peptides with strong antibacterial activity, such as lysozymes, cecropins, gloverin, IMPI, galiomycin, and moricin, were strongly induced in the UPEC larvae (43–49), whereas anionic peptides and apolipophoricin, with weaker antimicrobial activity, were strongly induced in the ABU larvae. These data confirm that the ABU E. coli strain 83972 specifically downregulates AMPs with strong antibacterial activities to facilitate bacterial survival and prolonged infection in G. mellonella.
We therefore conclude that major aspects of the innate immune response to infections by uropathogenic and commensal-like E. coli strains are conserved between G. mellonella and humans. Using complementary transcriptomics, enzyme immunoassays, and bioinformatics approaches, we found that the larvae responded differently to UPEC and commensal-like ABU infections and that the overall response was epigenetically regulated by DNA/RNA methylation and histone acetylation. The differential expression of prominent AMP genes shows that strong antimicrobial activities are induced when larvae are infected by UPEC but not ABU E. coli strains. Taken together, our results emphasize the importance of host epigenetic regulation of the innate immune response, allowing the discrimination of uropathogenic and commensal E. coli strains.
MATERIALS AND METHODS
Bacterial strains, insects, and culture media.
Bacterial cultures of UPEC strain CFT073, ABU E. coli strain 83972, and E. coli K-12 strain D31 were maintained aerobically in LB medium (Carl Roth, Karlsruhe, Germany) at 37°C and on LB agar plates. For long-term storage, bacteria were frozen at −80°C in LB medium supplemented with 30% glycerol. G. mellonella larvae at their sixth developmental stage were obtained from Fauna Topics Zoobedarf Zucht und Handels GmbH, Marbach am Neckar, Germany. The larvae were maintained as previously described (28).
G. mellonella injection and CFU counts.
We used logarithmic-growth-phase bacterial cultures in 10 ml LB for injection experiments. The bacterial inocula were washed and serially diluted in 0.09% NaCl, and 10-μl aliquots of 105-CFU/ml cultures were injected into larvae through the left proleg using 1-ml disposable syringes and 0.4- by 20-mm needles mounted on a microapplicator, as previously described (28). Larvae that received a mock injection with an empty needle were treated as controls. Larvae were considered dead after incubation at 37°C when they showed no movement in response to touch after injection.
Survival of E. coli strains CFT073, 83972, and D31 in G. mellonella was determined by injecting larvae with a dose of 1 × 103 CFU/larva, followed by incubation at 37°C. Larvae were homogenized in LB medium 1, 24, and 72 h postinjection. The homogenates were plated onto E. coli selective ECD agar (Sigma-Aldrich, Hamburg, Germany), and colonies were counted after incubation at 37°C for 48 h. The E. coli colonies were confirmed when they tested indole positive with Kovac's reagent (Sigma-Aldrich) following the manufacturer's protocol.
Extraction of genomic DNA, RNA, and histones.
Larvae injected with E. coli strain CFT073, 83972, or D31 and mock-injected controls were crushed to fine powder under liquid nitrogen at three different time points (1, 24, and 72 h postinjection). Three replicate samples were collected per time point, and each of the triplicate samples comprised at least five whole larvae. DNA was isolated using a DNeasy blood and tissue kit (Qiagen, Hilden, Germany) according to the manufacturer's recommendations.
Similarly, total RNA was prepared by collecting samples after crushing under liquid nitrogen and extracting the RNA using an RNeasy minikit (Qiagen) according to the manufacturer's recommendations. RNA concentrations were determined by spectrophotometry.
Histones were extracted on ice for 5 min using an EpiQuik total histone extraction kit (EpiGentek, Farmingdale, NY, USA) in three volumes of the supplied extraction buffer with glycerol, following the manufacturer's recommendations. The supernatant was mixed with 100% trichloroacetic acid (TCA) and incubated on ice for 30 min. The precipitate was collected after centrifugation for 10 min at 13,523 × g and 4°C, washed twice with acetone, and dissolved in water. The histone protein concentration was estimated using the bicinchoninic acid (BCA) method, and the extract was aliquoted and stored at −80°C.
Measurement of global changes in DNA and RNA methylation.
Global DNA methylation was measured using a MethylFlash methylated DNA quantification kit (EpiGentek). Ninety-six-well plates were coated with DNA isolated from whole animal larvae previously injected with E. coli strain CFT073, 83972, or D31 (100 ng per sample) or mock-injected controls. The methylated DNA was detected with capture and detection antibodies by measuring the absorbance at 450 nm using a microplate reader (BioTek Instruments, Inc., Winooski, VT, USA). The relative percentage of methylated DNA in infected larvae was estimated using the formula provided in the manufacturer's protocol (EpiGentek).
Global RNA methylation was measured using an EpiQuik m6A RNA methylation quantification kit (EpiGentek). Ninety-six-well plates were coated with RNA isolated from whole animal larvae previously injected with E. coli strain CFT073, 83972, or D31 (200 ng per sample) or mock-injected controls. The methylated adenosine residues (N6) in RNA were detected with capture and detection antibodies by measuring the absorbance at 450 nm using a microplate reader (BioTek). The relative percentage of methylated RNA in infected larvae was estimated using the formula provided in the manufacturer's protocol (EpiGentek).
Measurement of global histone H3, H3K9, H4, and H4K5 acetylation.
Global histone H3, H3K9, H4, and H4K5 acetylation levels in larvae injected with E. coli strain CFT073, 83972, or D31 (or mock-injected controls) were determined using the EpiQuik global histone H3, H3K9, H4, H4K5 acetylation assay kit (EpiGentek) following the manufacturer's recommendations. Briefly, the strip wells were coated with histone proteins (200 ng), and acetylated histone H3/H3K9/H4/H4K5 was detected with a high-affinity antibody. The ratio in infected and uninfected larvae was estimated using a horseradish peroxidase (HRP)-conjugated secondary antibody, and the colorimetric signal was quantified by measuring the absorbance at 450 nm.
RNA extraction, library preparation, and sequencing.
Total RNA from G. mellonella larvae was collected 24 h after the injection of E. coli strain CFT073 or 83972 using the miRNeasy minikit (Qiagen). Library preparation and sequencing were conducted by LC Sciences (Houston, TX, USA). Briefly, poly(A)+ mRNA was isolated using oligo(dT) beads and fragmented into small pieces. Double-stranded cDNA was then synthesized with random-hexamer primers (Illumina, Inc., San Diego, CA, USA). The cDNA fragments were subjected to an end repair process, followed by phosphorylation and adapter ligation. The amplified and purified PCR products were used to create the final cDNA libraries, which were sequenced using an Illumina Hiseq 2500 instrument.
Illumina sequencing, transcriptome assembly, and analysis.
Paired-end reads (two; 100 nucleotides [nt]) were acquired from the Illumina Hiseq 2500 with an error rate of <0.001 for 88% of the bases. The quality of the G. mellonella reads was checked using fastQC v0.11.4, and the sequences were trimmed using Trimmomatic v0.36 (parameters: slidingwindow, 4, 5; leading, 5; trailing, 5; minlen, 25). Sequences shorter than 25 bp were discarded. The transcriptome was assembled de novo using Trinity v2.3.2. Various assembly combinations were analyzed by Transrate v1.0.3, and the resulting transcripts were aligned with the NCBI NR database using a BLASTX search with an E value cutoff of 10−4. The BLAST hits were processed using Blast2GO software to classify transcripts into Gene Ontology (GO) term categories, including molecular function, biological process, and cellular component. Additionally, transcripts were translated to all six reading frames using transeq (EMBOSS package) and aligned by BLAST to the Clusters of Orthologous Groups (COG) database with a minimum protein identity of 50% and a protein coverage of at least 75% and less than 125%.
Gene expression levels and the significance of differential expression were calculated by pairwise comparisons in Cuffdiff, which is part of the Cufflinks package v2.2.1. Cuffdiff was used with geometric normalization and threshold criteria for an FDR of 0.01. Expression levels were presented as log2-fold change of fragments per kilobase per million mapped reads (FPKM)-normalized count data.
In order to identify innate-immunity-related genes, reviewed proteins from the UniProt database representing reference sequences for AMPs, cytokines, HATs, HDACs, DNMTs, and the TLR family were stored in a local database (see Table S1 in the supplemental material). NCBI BLAST, with a minimum protein identity of 50% and protein coverage of at least 75% but less than 125%, was used to determine the expressed homologs based on the database built from UniProt references.
RT-PCR.
Total RNA was used for the preparation of cDNA with the First Stand cDNA synthesis kit (Thermo Fisher Scientific, Darmstadt, Germany). The quantity of cDNA was determined by spectrophotometry. Quantitative real-time RT-PCR was performed with the CFX 96 real-time PCR system (Bio-Rad Laboratories, Inc., Hercules, CA, USA) using the SensiMix SYBR No-Rox kit (Bioline, Luckenwalde, Germany). We used 50 ng of cDNA per reaction to amplify the HAT, HDAC, DNMT, and AMP genes using the primers shown in Table S2 in the supplemental material. The PCR program for gene amplification comprised an initial activation step at 95°C for 10 min, denaturation at 95°C for 15 s, annealing at 56°C for 15 s, and extension at 72°C for 15 s. The program was repeated for 39 cycles.
Data analysis.
All experiments except RNA sequencing were performed a minimum of three times. For Fig. 1A, survival curves were plotted using the Kaplan-Meier method in GraphPad Prism v7.03 for Windows (GraphPad Software, San Diego, CA, USA). Survival differences were calculated using the log rank test, and P values were adjusted for multiple comparisons using the Bonferroni method. For Fig. 7 and 8, statistical analysis was carried out using R v3.4.0 (6 March 2017) (https://www.R-project.org). We carried out parametric two-factorial analysis of variance (ANOVA) on the strains and days postinfection (with interactions) after transforming the data to obtain approximate homoscedastic normality (controlled by exploratory data analysis). Subsequent multiple pairwise comparisons (with a single-step method to adjust P values) among strains on different days postinfection, or between each strain and the corresponding mock-injected control on different days postinfection were performed using the R package “multicomp” version 1.4-6 (50).
Accession number(s).
The sequence data from this study have been submitted to the NCBI Sequence Read Archive (SRA) under accession number SRX2727977. The BioProject is available under accession number PRJNA382478.
Supplementary Material
ACKNOWLEDGMENTS
We acknowledge Cosima Palm and Regina Zweigert for technical support, Henrike Schmidtberg for critical reading, and Richard M. Twyman for professional editing of the manuscript. We thank Gerrit Eichner (Mathematical Institute, Justus-Liebig University) for providing excellent statistical analysis support.
K.M. and U.D. acknowledge funding provided by the German Research Foundation (MU 4008/1-1 and CRC 1009/2, B05, respectively). A.V. acknowledges generous funding by the Hessen State Ministry of Higher Education, Research and the Arts (HMWK) via the LOEWE Center for Insect Biotechnology and Bioresources. The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
We have no financial conflict of interest.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/IAI.00336-17.
REFERENCES
- 1.Dobrindt U, Wullt B, Svanborg C. 2016. Asymtomatic bacteriuria as a model to study the coevolution of hosts and bacteria. Pathogens 5:e21. doi: 10.3390/pathogens5010021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shariff VAA, Shenoy MS, Yadav T, Radhakrishna M. 2013. The antibiotic susceptibility patterns of uropathogenic Escherichia coli, with special reference to the fluoroquinolones. J Clin Diagn Res 7:1027–1030. doi: 10.7860/JCDR/2013/4917.3038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lloyd AL, Henderson TA, Vigil PD, Mobley HL. 2009. Genomic islands of uropathogenic Escherichia coli contribute to virulence. J Bacteriol 191:3469–3481. doi: 10.1128/JB.01717-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leimbach A, Hacker J, Dobrindt U. 2013. E. coli as an all-rounder: the thin line between commensalism and pathogenicity. Curr Top Microbiol Immunol 358:3–32. doi: 10.1007/82_2012_303. [DOI] [PubMed] [Google Scholar]
- 5.Zdziarski J, Svanborg C, Wullt B, Hacker J, Dobrindt U. 2008. Molecular basis of commensalism in the urinary tract: low virulence or virulence attenuation? Infect Immun 76:695–703. doi: 10.1128/IAI.01215-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zdziarski J, Brzuszkiewicz E, Wullt B, Liesegang H, Biran D, Voigt B, Gronberg-Hernandez J, Ragnarsdottir B, Hecker M, Ron EZ, Daniel R, Gottschalk G, Hacker J, Svanborg C, Dobrindt U. 2010. Host imprints on bacterial genomes—rapid, divergent evolution in individual patients. PLoS Pathog 6:e1001078. doi: 10.1371/journal.ppat.1001078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ambite I, Lutay N, Stork C, Dobrindt U, Wullt B, Svanborg C. 2016. Bacterial suppression of RNA polymerase II-dependent host gene expression. Pathogens 5:e49. doi: 10.3390/pathogens5030049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lutay N, Ambite I, Gronberg Hernandez J, Rydstrom G, Ragnarsdottir B, Puthia M, Nadeem A, Zhang J, Storm P, Dobrindt U, Wullt B, Svanborg C. 2013. Bacterial control of host gene expression through RNA polymerase II. J Clin Invest 123:2366–2379. doi: 10.1172/JCI66451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gronberg-Hernandez J, Sunden F, Connolly J, Svanborg C, Wullt B. 2011. Genetic control of the variable innate immune response to asymptomatic bacteriuria. PLoS One 6:e28289. doi: 10.1371/journal.pone.0028289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ragnarsdottir B, Samuelsson M, Gustafsson MC, Leijonhufvud I, Karpman D, Svanborg C. 2007. Reduced Toll-like receptor 4 expression in children with asymptomatic bacteriuria. J Infect Dis 196:475–484. doi: 10.1086/518893. [DOI] [PubMed] [Google Scholar]
- 11.Hagberg L, Hull R, Hull S, McGhee JR, Michalek SM, Svanborg Eden C. 1984. Difference in susceptibility to gram-negative urinary tract infection between C3H/HeJ and C3H/HeN mice. Infect Immun 46:839–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Godaly G, Ambite I, Puthia M, Nadeem A, Ho J, Nagy K, Huang Y, Rydstrom G, Svanborg C. 2016. Urinary tract infection molecular mechanisms and clinical translation. Pathogens 5:E24. doi: 10.3390/pathogens5010024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Godaly G, Ambite I, Svanborg C. 2015. Innate immunity and genetic determinants of urinary tract infection susceptibility. Curr Opin Infect Dis 28:88–96. doi: 10.1097/QCO.0000000000000127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jaenisch R, Bird A. 2003. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nat Genet 33(Suppl):245–254. doi: 10.1038/ng1089. [DOI] [PubMed] [Google Scholar]
- 15.Bierne H, Hamon M, Cossart P. 2012. Epigenetics and bacterial infections. Cold Spring Harb Perspect Med 2:a010272. doi: 10.1101/cshperspect.a010272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hamon MA, Batsche E, Regnault B, Tham TN, Seveau S, Muchardt C, Cossart P. 2007. Histone modifications induced by a family of bacterial toxins. Proc Natl Acad Sci U S A 104:13467–13472. doi: 10.1073/pnas.0702729104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tolg C, Sabha N, Cortese R, Panchal T, Ahsan A, Soliman A, Aitken KJ, Petronis A, Bagli DJ. 2011. Uropathogenic E. coli infection provokes epigenetic downregulation of CDKN2A (p16INK4A) in uroepithelial cells. Lab Invest 91:825–836. doi: 10.1038/labinvest.2010.197. [DOI] [PubMed] [Google Scholar]
- 18.Carey AJ, Tan CK, Ipe DS, Sullivan MJ, Cripps AW, Schembri MA, Ulett GC. 2016. Urinary tract infection of mice to model human disease: practicalities, implications and limitations. Crit Rev Microbiol 42:780–799. doi: 10.3109/1040841X.2015.1028885. [DOI] [PubMed] [Google Scholar]
- 19.Abraham SN, Miao Y. 2015. The nature of immune responses to urinary tract infections. Nat Rev Immunol 15:655–663. doi: 10.1038/nri3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mukherjee K, Twyman RM, Vilcinskas A. 2015. Insects as models to study the epigenetic basis of disease. Prog Biophys Mol Biol 118:69–78. doi: 10.1016/j.pbiomolbio.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 21.Vilcinskas A. 2011. Insects emerge as valuable model hosts to explore virulence. Virulence 2:376–378. doi: 10.4161/viru.2.5.18289. [DOI] [PubMed] [Google Scholar]
- 22.Vilcinskas A. 2016. The role of epigenetics in host-parasite coevolution: lessons from the model host insects Galleria mellonella and Tribolium castaneum. Zoology 119:273–280. doi: 10.1016/j.zool.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 23.Joop G, Vilcinskas A. 2016. Coevolution of parasitic fungi and insect hosts. Zoology 119:350–358. doi: 10.1016/j.zool.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 24.Mukherjee K, Fischer R, Vilcinskas A. 2012. Histone acetylation mediates epigenetic regulation of transcriptional reprogramming in insects during metamorphosis, wounding and infection. Front Zool 9:25. doi: 10.1186/1742-9994-9-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alghoribi MF, Gibreel TM, Dodgson AR, Beatson SA, Upton M. 2014. Galleria mellonella infection model demonstrates high lethality of ST69 and ST127 uropathogenic E. coli. PLoS One 9:e101547. doi: 10.1371/journal.pone.0101547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Williamson DA, Mills G, Johnson JR, Porter S, Wiles S. 2014. In vivo correlates of molecularly inferred virulence among extraintestinal pathogenic Escherichia coli (ExPEC) in the wax moth Galleria mellonella model system. Virulence 5:388–393. doi: 10.4161/viru.27912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ciesielczuk H, Betts J, Phee L, Doumith M, Hope R, Woodford N, Wareham DW. 2015. Comparative virulence of urinary and bloodstream isolates of extra-intestinal pathogenic Escherichia coli in a Galleria mellonella model. Virulence 6:145–151. doi: 10.4161/21505594.2014.988095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mukherjee K, Hain T, Fischer R, Chakraborty T, Vilcinskas A. 2013. Brain infection and activation of neuronal repair mechanisms by the human pathogen Listeria monocytogenes in the lepidopteran model host Galleria mellonella. Virulence 4:324–332. doi: 10.4161/viru.23629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Danis-Wlodarczyk K, Vandenheuvel D, Jang HB, Briers Y, Olszak T, Arabski M, Wasik S, Drabik M, Higgins G, Tyrrell J, Harvey BJ, Noben JP, Lavigne R, Drulis-Kawa Z. 2016. A proposed integrated approach for the preclinical evaluation of phage therapy in Pseudomonas infections. Sci Rep 6:28115. doi: 10.1038/srep28115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chung JH, Bhat A, Kim CJ, Yong D, Ryu CM. 2016. Combination therapy with polymyxin B and netropsin against clinical isolates of multidrug-resistant Acinetobacter baumannii. Sci Rep 6:28168. doi: 10.1038/srep28168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vogel H, Altincicek B, Glockner G, Vilcinskas A. 2011. A comprehensive transcriptome and immune-gene repertoire of the lepidopteran model host Galleria mellonella. BMC Genomics 12:308. doi: 10.1186/1471-2164-12-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wagenlehner FM, Naber KG. 2012. Editorial commentary: asymptomatic bacteriuria—shift of paradigm. Clin Infect Dis 55:778–780. doi: 10.1093/cid/cis541. [DOI] [PubMed] [Google Scholar]
- 33.Altincicek B, Berisha A, Mukherjee K, Spengler B, Rompp A, Vilcinskas A. 2009. Identification of collagen IV derived danger/alarm signals in insect immunity by nanoLC-FTICR MS. Biol Chem 390:1303–1311. doi: 10.1515/BC.2009.128. [DOI] [PubMed] [Google Scholar]
- 34.Roos V, Ulett GC, Schembri MA, Klemm P. 2006. The asymptomatic bacteriuria Escherichia coli strain 83972 outcompetes uropathogenic E. coli strains in human urine. Infect Immun 74:615–624. doi: 10.1128/IAI.74.1.615-624.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang D, Zhang G, Hayden MS, Greenblatt MB, Bussey C, Flavell RA, Ghosh S. 2004. A Toll-like receptor that prevents infection by uropathogenic bacteria. Science 303:1522–1526. doi: 10.1126/science.1094351. [DOI] [PubMed] [Google Scholar]
- 36.Glastad KM, Hunt BG, Yi SV, Goodisman MA. 2011. DNA methylation in insects: on the brink of the epigenomic era. Insect Mol Biol 20:553–565. doi: 10.1111/j.1365-2583.2011.01092.x. [DOI] [PubMed] [Google Scholar]
- 37.Huang W, Qi CB, Lv SW, Xie M, Feng YQ, Huang WH, Yuan BF. 2016. Determination of DNA and RNA methylation in circulating tumor cells by mass spectrometry. Anal Chem 88:1378–1384. doi: 10.1021/acs.analchem.5b03962. [DOI] [PubMed] [Google Scholar]
- 38.Takahashi K, Sugi Y, Nakano K, Tsuda M, Kurihara K, Hosono A, Kaminogawa S. 2011. Epigenetic control of the host gene by commensal bacteria in large intestinal epithelial cells. J Biol Chem 286:35755–35762. doi: 10.1074/jbc.M111.271007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meyer KD, Jaffrey SR. 2014. The dynamic epitranscriptome: N6-methyladenosine and gene expression control. Nat Rev Mol Cell Biol 15:313–326. doi: 10.1038/nrm3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang Z, Wang M, Eisel F, Tchatalbachev S, Chakraborty T, Meinhardt A, Bhushan S. 2016. Uropathogenic Escherichia coli epigenetically manipulate host cell death pathways. J Infect Dis 213:1198–1207. doi: 10.1093/infdis/jiv569. [DOI] [PubMed] [Google Scholar]
- 41.Modak R, Das Mitra S, Vasudevan M, Krishnamoorthy P, Kumar M, Bhat AV, Bhuvana M, Ghosh SK, Shome BR, Kundu TK. 2014. Epigenetic response in mice mastitis: role of histone H3 acetylation and microRNA(s) in the regulation of host inflammatory gene expression during Staphylococcus aureus infection. Clin Epigenetics 6:12. doi: 10.1186/1868-7083-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fischer N, Sechet E, Friedman R, Amiot A, Sobhani I, Nigro G, Sansonetti PJ, Sperandio B. 2016. Histone deacetylase inhibition enhances antimicrobial peptide but not inflammatory cytokine expression upon bacterial challenge. Proc Natl Acad Sci U S A 113:E2993–E3001. doi: 10.1073/pnas.1605997113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reigstad CS, Hultgren SJ, Gordon JI. 2007. Functional genomic studies of uropathogenic Escherichia coli and host urothelial cells when intracellular bacterial communities are assembled. J Biol Chem 282:21259–21267. doi: 10.1074/jbc.M611502200. [DOI] [PubMed] [Google Scholar]
- 44.Zitzmann J, Weidner T, Czermak P. 2017. Optimized expression of the antimicrobial protein Gloverin from Galleria mellonella using stably transformed Drosophila melanogaster S2 cells. Cytotechnology 69:371–389. doi: 10.1007/s10616-017-0068-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Freitak D, Schmidtberg H, Dickel F, Lochnit G, Vogel H, Vilcinskas A. 2014. The maternal transfer of bacteria can mediate trans-generational immune priming in insects. Virulence 5:547–554. doi: 10.4161/viru.28367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guilhelmelli F, Vilela N, Albuquerque P, Derengowski LDS, Silva-Pereira I, Kyaw CM. 2013. Antibiotic development challenges: the various mechanisms of action of antimicrobial peptides and of bacterial resistance. Front Microbiol 4:353. doi: 10.3389/fmicb.2013.00353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mylonakis E, Podsiadlowski L, Muhammed M, Vilcinskas A. 2016. Diversity, evolution and medical applications of insect antimicrobial peptides. Philos Trans R Soc Lond B Biol Sci 371:20150290. doi: 10.1098/rstb.2015.0290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vilcinskas A. 2010. Coevolution between pathogen-derived proteinases and proteinase inhibitors of host insects. Virulence 1:206–214. doi: 10.4161/viru.1.3.12072. [DOI] [PubMed] [Google Scholar]
- 49.Cytrynska M, Mak P, Zdybicka-Barabas A, Suder P, Jakubowicz T. 2007. Purification and characterization of eight peptides from Galleria mellonella immune hemolymph. Peptides 28:533–546. doi: 10.1016/j.peptides.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 50.Hothorn T, Bretz F, Westfall P. 2008. Simultaneous inference in general parametric models. Biom J 50:346–363. doi: 10.1002/bimj.200810425. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.