ABSTRACT
Clostridium difficile, the main cause of diarrhea in hospitalized patients, produces toxins A (TcdA) and B (TcdB), which affect intestinal epithelial cell survival, proliferation, and migration and induce an intense inflammatory response. Transforming growth factor β (TGF-β) is a pleiotropic cytokine affecting enterocyte and immune/inflammatory responses. However, it has been shown that exposure of intestinal epithelium to a low concentration of TcdA induces the release of TGF-β1, which has a protective effect on epithelial resistance and a TcdA/TGF-β signaling pathway interaction. The activation of this pathway in vivo has not been elucidated. The aim of this study was to investigate the role of the TGF-β1 pathway in TcdA-induced damage in a rat intestinal epithelial cell line (IEC-6) and in a mouse model of an ileal loop. TcdA increased the expression of TGF-β1 and its receptor, TβRII, in vitro and in vivo. TcdA induced nuclear translocation of the transcription factors SMAD2/3, a hallmark of TGF-β1 pathway activation, both in IEC cells and in mouse ileal tissue. The addition of recombinant TGF-β1 (rTGF-β) prevented TcdA-induced apoptosis/necrosis and restored proliferation and repair activity in IEC-6 cells in the presence of TcdA. Together, these data show that TcdA induces TGF-β1 signaling pathway activation and suggest that this pathway might play a protective role against the effect of C. difficile-toxin.
KEYWORDS: Clostridium difficile, toxin A, TGF-β, SMAD, SMAD transcription factors
INTRODUCTION
Clostridium difficile is a Gram-positive, spore-forming bacterium responsible for infectious diarrhea and pseudomembranous colitis with significant morbidity and mortality (1). C. difficile infection (CDI) usually affects elderly (>65 years of age) hospital patients who have received broad-spectrum antimicrobial treatment (2–4). Pathogenicity is dependent on the presence of one or both of two closely related toxins named toxin A (TcdA) and toxin B (TcdB). These toxins are responsible for gastrointestinal illnesses with a wide spectrum of severity, ranging from mild diarrhea to pseudomembranous colitis, which may progress to toxic megacolon, sepsis, and death (5).
In animal models, the exposure of ileal loops to C. difficile TcdA produces an intense inflammatory response characterized by mucosal disruption, fluid accumulation, edema, mast cell degranulation, epithelial cell death, and severe neutrophil infiltration (6–9). In addition, TcdA stimulates the release of endogenous mediators of inflammation, including tumor necrosis factor alpha (TNF-α), interleukin 1 (IL-1), IL-8, platelet activating factor, and leukotriene B4, which results in further interruption of the intestinal tight junction barrier (10–13). It has been suggested that anti-inflammatory cytokines, such as IL-10 and transforming growth factor β (TGF-β), may attenuate or protect against intestinal inflammation by preserving tight junction barrier function (14, 15).
TGF-β is a multifunctional cytokine that regulates cell growth, adhesion, and differentiation (16–19). TGF-β signals are transmitted via a cell surface receptor complex, the TβRII and TβRI/Alk5 heterodimer. TGF-β binds to TβRII, which, in turn, recruits, transphosphorylates, and activates TβRI, thereby achieving cross-membrane signaling to the inside of the cell (20, 21). TGF-β canonic signaling is triggered by the phosphorylation of transcription factors of the SMAD family of proteins, SMAD2 and SMAD3, followed by recruitment of SMAD4, thus leading to the nuclear translocation of the SMAD2-3/SMAD4 complex and activation of TGF-activated genes. It has been reported that intestinal mucosal cells express TGF-β (22, 23).
Johal and collaborators (24) showed that a low concentration of TcdA of Clostridium difficile induces the release of TGF-β1 by the human intestinal epithelial T84 cell line, suggesting a protective effect of TGF-β against C. difficile infection. However, the C. difficile toxin A/TGF-β signaling pathway interaction and the activation of the TGF-β pathway in vivo have not yet been elucidated. The present study investigated the in vitro and in vivo effects of TcdA on TGF-β1 pathway activation and characterized the role of this cytokine in those effects.
RESULTS
TcdA induces activation of the TGF-β signaling pathway in vivo.
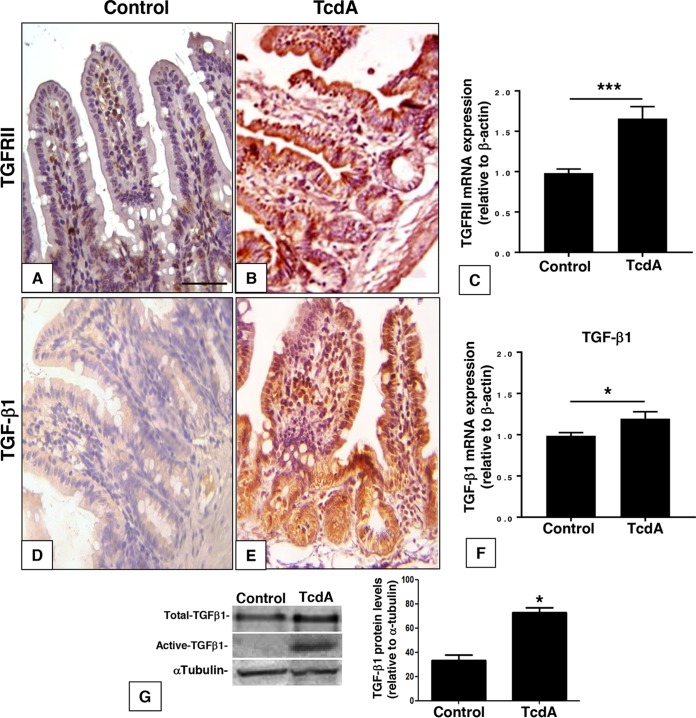
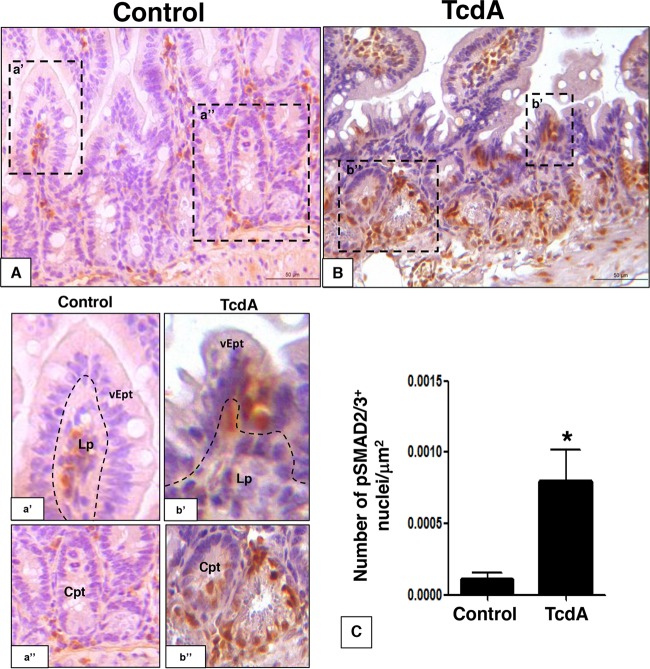
To investigate the effect of TcdA on the level and distribution of TGF-β1 and TGF-β type 2 receptor (TGFRII) in vivo, we injected TcdA (10 μg) into the mouse ileal loop. Injection of TcdA led to an increase in the immunostaining for TGF-β1 in intestinal crypts, villus epithelial cells, and lamina propria cells and for TGFRII mainly in villus epithelial cells (Fig. 1A, B, D, and E). There was an upregulation of TGFRII and TGF-β1 mRNA in the ileal tissue by 70% and 20%, respectively (Fig. 1C and F). In addition, the TcdA-induced increase in TGF-β1 mRNA was followed by a similar increase at the protein level for both the total and active forms of TGF-β1 in the ileal loop tissue (Fig. 1G). The increase in TGFRII and TGF-β1 gene expression came together with the activation of the TGF-β1/SMAD signaling pathway in the mouse ileal tissue. TcdA increased nuclear immunostaining of SMAD2/3 in the villus, crypt epithelial cells, and lamina propria cells 7-fold (Fig. 2), suggesting SMAD2/3 translocation. These results suggest that the intestinal tissue responds to C. difficile TcdA by activating the TGF-β1/SMAD2/3 signaling pathway in vivo.
FIG 1.
C. difficile TcdA injection enhances TGF-β1 and TGFRII expression in ileal loop epithelium. TcdA (10 μg/loop) increased TβRII and TGF-β1 labeling intensity (B and E) compared to that in the control group (PBS at 0.1 ml/loop) (A and D) and enhanced TGFRII and TGF-β1 mRNA expression in ileal loop tissue compared to that in the control (C and F). Total and active TGF-β1 protein levels were also increased in the presence of TcdA in ileum loop tissue (G). *, P < 0.05, and ***, P < 0.001, compared to the value for the control (Student's t test). Scale bar: 100 μm.
FIG 2.
C. difficile TcdA injection promotes TGF-β1/SMAD signaling activation in ileal loop epithelium. TcdA injection (10 μg/loop) promoted TGF-β1 signaling activation by SMAD2/3 nuclear translocation in ileal loop tissue (B and C) compared to that in the control group (A and C). The increase in SMAD2/3 nuclear accumulation was evident in epithelial (vEpt), crypt epithelium (Cpt), and lamina propria (Lp) layers (dashed box b') compared to that in the control (dashed box a'). *, P < 0.05 (Student's t test). Scale bar: 100 μm.
TcdA induces the activation of TGF-β1 signaling pathway in vitro.
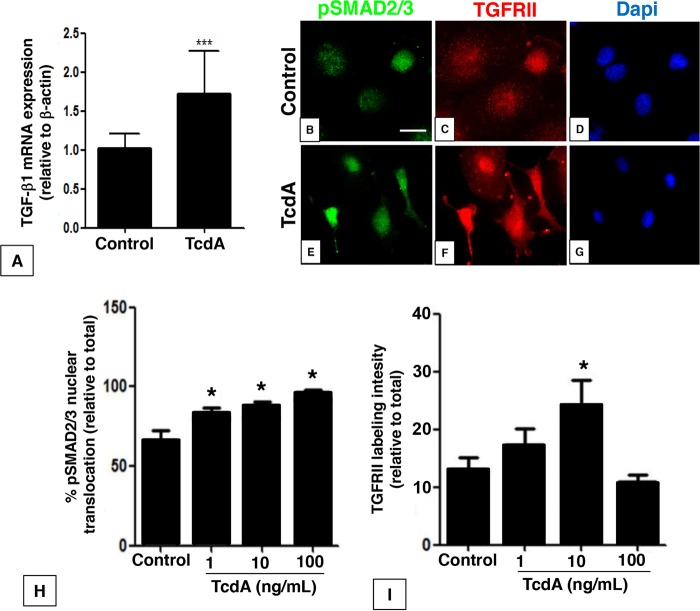
To access the mechanism involved in TGF-β1 signaling activation in response to TcdA, we used a rat intestinal epithelial cell line, IEC-6. Culture of IEC-6 cells in the presence of TcdA (10 ng/ml) increased the expression of TGF-β1 mRNA by 70% (Fig. 3A). Treatment of IEC-6 cells with increasing concentrations of TcdA (1, 10, and 100 ng/ml) similarly enhanced the number of phosphoSMAD2/3-positive cells, with no additive effects among the three different concentrations (Fig. 3E and H) compared to the effects in the control group (Fig. 3B). In parallel, 10 ng/ml of TcdA also increased TGFRII labeling intensity in IEC-6 cells (Fig. 3F and I) compared to that in the control group (Fig. 3C). These effects of TcdA were associated with a decreased number of cells (Fig. 3G) compared to that in the control (Fig. 3D), as observed by 4′,6-diamidino-2-phenylindole (DAPI) staining. These results suggest that TcdA can promote TGF-β1/SMAD2/3 signaling pathway activation in intestinal epithelial cells in vitro.
FIG 3.
C. difficile TcdA promotes TGF-β1 secretion and signaling activation in IEC-6 cells. Treatment of IEC-6 cells with TcdA (10 ng/ml) for 24 h enhanced TGF-β1 mRNA expression (A), followed by induction of SMAD2/3 phosphorylation and nuclear translocation (E and H) compared to control (B), increased TGFRII labeling intensity in IEC-6 cells (F and I) compared to control (C) in a concentration-dependent manner, and decreased number of cells (G) compared to control (D) as observed by DAPI stain. *, P < 0.05; ***, P < 0.0001 by analysis of variance (ANOVA) and Bonferroni’s test. Scale bar: 20 mm.
TGF-β1 protects IEC-6 cells from TcdA-induced apoptosis and necrosis.
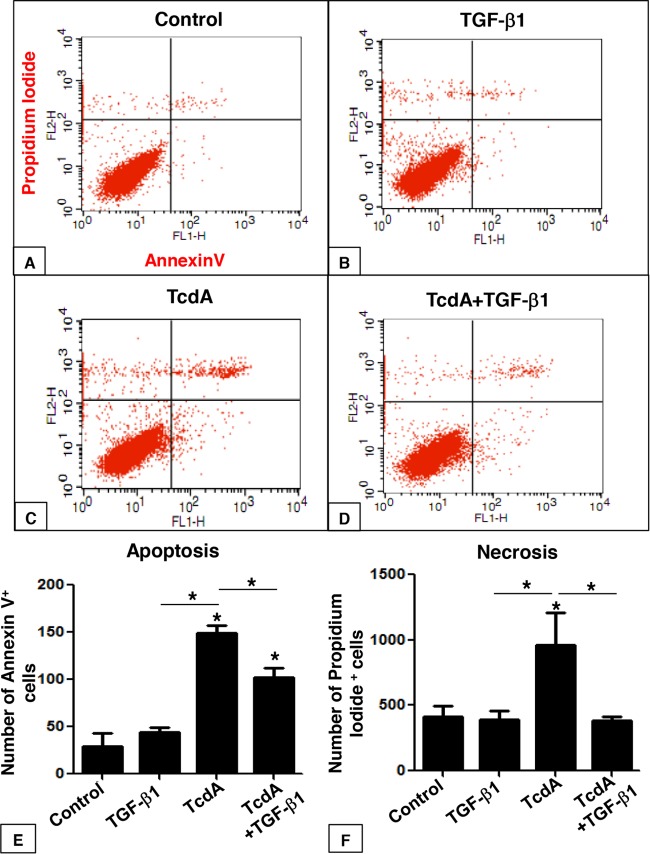
C. difficile infection promotes several deleterious effects in intestinal tissue, which includes intense inflammatory responses characterized by mucosal disruption, edema, epithelial cell death, neutrophil infiltration, and the release of mediators of inflammation (6–13, 25). However, little is known about the role of TGF-β1 in C. difficile infection establishment and/or tissue recovery. We sought to investigate the effect of TcdA in IEC-6 death and the protective property of TGF-β1 against the effect of this toxin. IEC-6 cells were treated with TcdA (10 ng/ml) for 24 h, followed by flow cytometry-based quantification of annexin V- and propidium iodide-stained cells. Early apoptotic cells were positive for annexin V-fluorescein isothiocyanate (FITC) only (lower right quadrant), and necrotic or late apoptotic cells were positive for both annexin V-FITC and propidium iodide (upper right and left quadrants). TcdA led to a significant increase in the incidence of IEC-6 apoptosis (Fig. 4C and E) and necrosis (Fig. 4C and F) compared to the control group (Fig. 4A). Treatment of IEC-6 cells with TGF-β1 (10 ng/ml) did not affect the number of apoptotic and necrotic cells (Fig. 4B, E, and F). However, concomitant incubation of TcdA (10 ng/ml) and TGF-β1 (10 ng/ml) partially reduced apoptosis and completely prevented necrosis induction by TcdA (Fig. 4D, E, and F). Immunofluorescence labeling for activated caspase 3, a specific marker for apoptosis, corroborated the flow cytometric analysis (see Fig. S1 in the supplemental material). Exposure of IEC-6 cells to TcdA (10 ng/ml) for 24 h increased the number of caspase 3-positive cells 20-fold (Fig. S1C). Treatment of IEC-6 cells with TGF-β1 (10 ng/ml) alone did not affect the number of caspase 3-positive cells (Fig. S1C), whereas concomitant incubation of TGF-β1 (10 ng/ml) with TcdA significantly prevented TcdA-caspase 3-induced activation (Fig. S1). These results suggest that TGF-β1 is able to rescue cells from TcdA-induced apoptosis and necrosis, potentially protecting intestinal epithelial cells from TcdA-induced cell death.
FIG 4.
TGF-β1 protects C. difficile TcdA-treated IEC-6 cells from apoptosis and necrosis. IEC-6 cells were incubated for 24 h in culture medium (control) with TcdA (10 ng/ml), TGF-β1 (10 ng/ml), or TcdA plus TGF-β1 (10 ng/ml) and stained with FITC-conjugated annexin V and propidium iodide (PI), followed by flow cytometry analysis. Density plots with PI versus annexin V-FITC show that viable cells have low annexin V-FITC and low PI staining (lower left quadrant), apoptotic cells have high annexin V-FITC and low PI staining (lower right quadrant), and necrotic cells have high PI and annexin V-FITC staining (upper quadrant). The numbers of apoptotic and necrotic cells were increased in the presence of TcdA compared to those in the control (A, C, E, and F). Concomitant addition of TGF-β1 partially rescued cells from apoptosis (D and E) and totally rescued cells from necrosis (D and F). The addition of TGF-β1 alone did not affect these events (B, E, and F). *, P < 0.05 (ANOVA and Bonferroni's test).
TGF-β1 protects IEC-6 cells from the antiproliferative and antimigratory effects of TcdA.
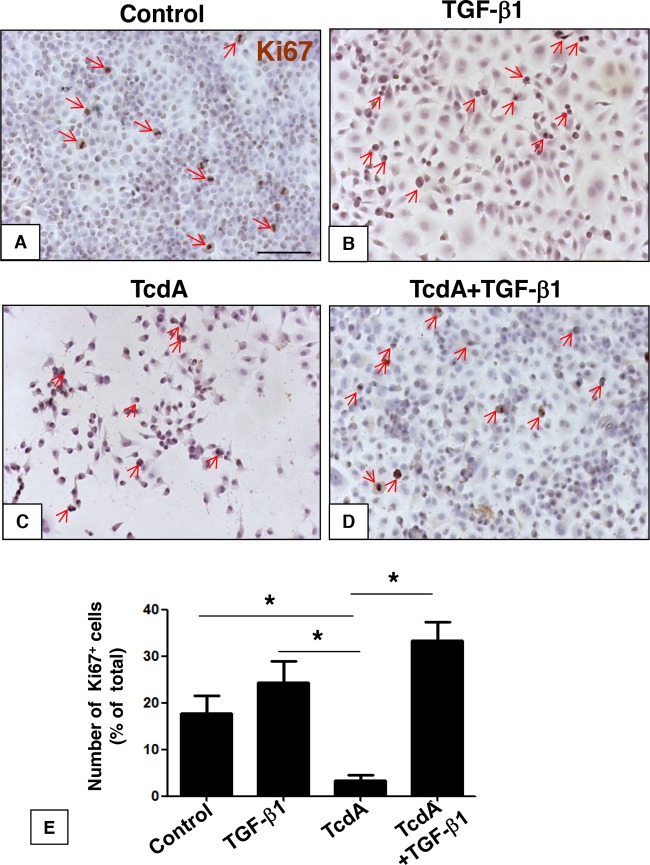
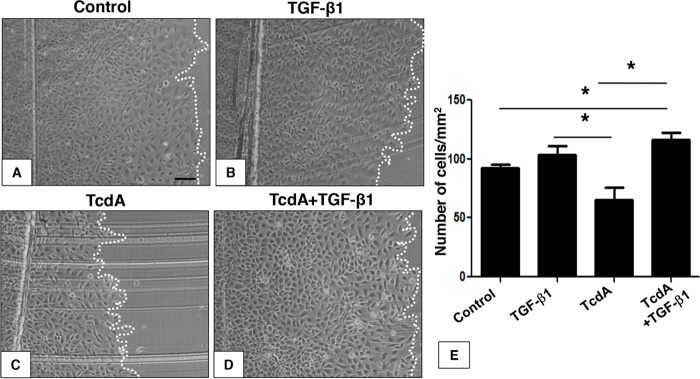
Intestinal epithelial cell restitution or cellular repair after injury (wound healing) requires both cell proliferation and migration (26). We sought to investigate the effect of TGF-β1 in TcdA-induced impairment of proliferation and migration. To do this, we used a scratch wound assay in which confluent IEC-6 cell cultures were scratched with a razor blade at the midline, extending to the right side of the well. The intention of the scrape is to reproduce an epithelial damage model and measure repair as a result of cell migration from the injured site during early phases and proliferation during late phases (26). Exposure of IEC-6 cells to TcdA (10 ng/ml) for 24 h decreased the number of Ki67+ proliferative cells by 90% (Fig. 5C and E). However, concomitant addition of TGF-β1 (10 ng/ml) significantly prevented the antiproliferative effect induced by TcdA (10 ng/ml) (Fig. 5D and E). The reduction of the proliferation rate of IEC-6 cells by TcdA was also followed by a 35% decrease in the cell repair rate 24 h after the scratch (Fig. 6C and E). However, concomitant addition of TGF-β1 and TcdA for 24 h significantly prevented this event (Fig. 6D and E). The addition of TGF-β1 (10 ng/ml) alone did not affect the repair (Fig. 6B and E). Since the addition of TGF-β1 alone to the IEC-6 cell culture did not significantly affect either proliferation (Fig. 5B and E) or repair (Fig. 6B and E), these results suggest that TGF-β1 might have a role in cellular repair, rescuing the cells from the deleterious antimigratory and antiproliferative effects induced by TcdA in vitro.
FIG 5.
TGF-β1 prevents IEC-6 cell proliferation deficits induced by C. difficile TcdA. IEC-6 cells were incubated for 24 h in culture medium (control) with TcdA (10 ng/ml), TGF-β1 (10 ng/ml), or TcdA plus TGF-β1 (10 ng/ml). TcdA treatment decreased the number of proliferative Ki67+ cells, and concomitant treatment with TGF-β1 rescued the decrease in TcdA-induced proliferation (C, D, and E). TGF-β1 alone did not affect proliferation (B and E). *, P < 0.05 (ANOVA and Bonferroni's test). Scale bar: 100 μm.
FIG 6.
TGF-β1 rescues IEC-6 cell monolayers from migration deficits induced by C. difficile TcdA treatment. After scratch wound assay performance on IEC-6 monolayers, cells were incubated for 24 h in culture medium (control) with TcdA (10 ng/ml), TGF-β1 (10 ng/ml), or TcdA plus TGF-β1 (10 ng/ml). After 24 h, TcdA cell monolayer migration deficits persisted (C and E); however, TGF-β1 concomitant incubation rescued cells from TcdA-induced migration deficits (D and E). TGF-β1 alone did not affect cell migration (B and E). *, P < 0.05 (ANOVA and Bonferroni's test). Scale bar: 10 μm.
DISCUSSION
In this study, we demonstrated that the TcdA of C. difficile induces TGF-β1 mRNA expression, both in mouse ileal loop tissue and in rat intestinal epithelial cells (IEC-6), thus resulting in protein expression in ileal tissue. This effect was associated with increased expression of TGFRII as well as phosphorylation and nuclear translocation of SMAD2/3, a hallmark of TGF-β1 signaling activation. Taken together, these findings strongly suggest that TcdA induces activation of the TGF-β1 pathway through canonical SMAD signaling. Accordingly, it was previously demonstrated that previous exposure to low concentrations of TcdA (<10 ng/ml) also induced expression of TGF-β1 by T84 cells, a human colon adenocarcinoma cell line (24). The present report adds new insight about the intracellular mechanism of activation of the TGF-β1 pathway in a nontransformed rat small intestinal epithelial cell line with characteristics of crypt epithelial cells and demonstrates that this cytokine is also expressed and produced in response to TcdA in vivo in a well-established TcdA-induced enteritis model in mice (27).
We previously demonstrated that TcdA induces apoptosis in T84 cells through the inactivation of Rho and activation of caspases 3, 6, and 9 and Bid, causing mitochondrial damage and the release of cytochrome c (28). In addition, using the same cell line employed in the present study (IEC-6), we showed that 100 ng/ml of TcdA induces cell death characteristic of apoptosis and necrosis and that a concentration of 10 ng/ml induces cell death in a lower percentage of cells with apoptotic characteristics. Thus, in the present work, we used 10 ng/ml of TcdA to demonstrate that the simultaneous incubation of IEC-6 cells with TGF-β1 reduces TcdA-induced cell death and the activation of caspase 3. Taking into account that proapoptotic activity is involved in the disruption of intestinal mucosa, TGF-β1 might have an important protective function in intestinal tissue against TcdA-induced damage.
The antiapoptotic effect found in this study is consistent with earlier reports showing that TGF-β1 increases mRNA expression of cellular inhibitor of apoptosis protein 2 (cIAP2) in Caco2 cells (human colonic cell line), which could explain the antiapoptotic effects of TGF-β1 (29). Additionally, TGF-β1 protects a cell line from human colorectal cancer (DLD-1) from cell death induced by hydrogen peroxide (H2O2) by increasing expression of glutathione peroxidase 1 (GPx-1); this event was mediated by activation of the TGF-β1 signaling pathway by type I receptor (TβRI)/SMAD2/ERK1/2/HIF-1α protein association and activation (30). However, there are contradictory data showing that TGF-β1 might also be involved in apoptosis induction of cold-related stress in rat small intestine, although the mechanisms by which TGF-β1 induces apoptosis in this model were not fully understood (31). It is therefore clear that although TGF-β1 plays an important role in the survival of intestinal epithelial cells, the results may vary according to the type of cell and the experimental model. Our data, however, are consistent with those of Johal et al. (24), who demonstrated that pretreatment of T84 cells with TGF-β makes epithelial monolayers more resistant to the barrier-disrupting effect of continuous exposure to a low concentration of TcdA, suggesting a protective effect of TGF-β. Accordingly, pretreatment of intestinal epithelial cells (T84) with TGF-β has also been shown to ameliorate the barrier-disrupting effects of Cryptosporidium parvum (31). Additionally, regarding the role of TGF-β1 as a protective factor against TcdA-induced apoptosis, our results also demonstrated that TGF-β1 prevents decreased proliferation and repair impairment in IEC-6 cells induced by TcdA, as reported previously (32).
Although some evidence implicates TGF-β1 in the regulation of proliferation and migration of nonepithelial cells, the role of this pathway in these events in intestinal epithelial cells has not been fully described. A study with IEC-6 cells suggested that TGF-β1, in addition to being associated with the regulation of differentiation, is also associated with the proliferation of these cells through SMAD-dependent or independent pathways (33). As demonstrated by Bezerra Lima et al. (34), TcdA promotes inhibition of the Wnt/β-catenin pathway, a critical pathway for renewal of intestinal epithelium. Unfortunately, the meaning of these antiproliferative effects induced by TcdA in the pathogenesis of Clostridium difficile-associated disease (CDAD) is not yet fully understood. However, inhibition of intestinal epithelial renewal by C. difficile toxins is probably linked to the promotion of diarrhea in patients suffering from CDAD (35). It is well known that TGF-β and Wnt/β-catenin are involved in many developmental processes, and changes in both signaling pathways are associated with pathological conditions. TGF-β signaling activates β-catenin through SMAD3 (36). SMAD3 interacts with β-catenin and increases its nuclear translocation and signaling (37). In addition, LEF1/β-catenin and T cell form (TCF) form a complex with SMAD3 or SMAD4 (38) and activate expression of specific genes. These data suggest that Wnt/β-catenin could mediate events downstream of TGF-β1 receptor binding. However, the relationship between TGF-β1 and activation of Wnt/β-catenin/LEF1/TCF in preventing the antiproliferative effect of TcdA needs to be clarified.
As previously mentioned, TGF-β1 also increases the expression of cIAP2, a protein responsible for the inhibition of apoptosis, and this, in turn, increases wound healing of the intestine (29). It is known that epithelial ulcer closure occurs by a reciprocal action between the epithelial cells and the underlying stroma and involves a number of growth factors, including TGF-β1 (39). Using IEC-6 cells, McCormack et al. (26) determined that wound healing occurs shortly after the induction of injury, with migration of cells from the lesion edges moving to the wound area, thus initiating the process of restitution or cellular repair. In a second stage, 12 to 16 h after injury, proliferation starts and is completed after 1 to 2 days (26). In this study, we investigated the repair at 24 h. Our results suggest that both migration and proliferation contribute to TGF-β1-induced repair. Thus, it is possible that TGF-β1 acts in two ways: (i) by promoting cell proliferation and/or (ii) through its indirect effect on the migration process (29, 40). It is noteworthy, however, that in many systems, TGF-β1 has an antiproliferative effect. Thus, it is possible that TGF-β1 may not directly affect the proliferation/migration of IEC-6 cells and instead may prevent TcdA injury in epithelial cells rather than impact proliferation/migration of these cells in a physiological context.
Considering the data presented here, TGF-β1 has emerged as a potential candidate to be administered to patients suffering from the disease induced by Clostridium difficile. Accordingly, a study shows that oral administration of TGF-β1 in neonatal rat pups with necrotizing enterocolitis (NEC) can potentially be used against gastrointestinal damage, as it reduces NF-κB and inhibits IL-6 expression and gamma interferon (IFN-γ) (41). In addition, TGF-β administration in patients with inflammatory bowel disease could potentially be beneficial. However, as shown here for C. difficile TcdA in ileal tissue in patients with intestinal inflammation, such as Crohn's disease and ulcerative colitis, TGF-β levels were already increased and immune cells did not respond to exogenous TGF-β (41, 42) due to excess SMAD7, an inhibitory protein of the TGF-β pathway. The presence of inhibitory proteins, such as SMAD7, in inflamed intestine in vivo could be an explanation to why the endogenous concentration of TGF-β produced in response to TcdA is not sufficient to protect cells from toxin damage. On the other hand, administration of exogenous doses of TGF (this study) protected IEC-6 cells from TcdA damage. Reinforcing this hypothesis, others have previously shown that administration of an antisense oligonucleotide that inhibits the action of SMAD7 (GED0301) has been promising in Crohn's disease (43).
Taken together, our results demonstrate that the TGF-β1 pathway is activated by TcdA and suggest that this pathway protects intestinal epithelial tissue against the harmful effects of C. difficile TcdA. However, the mechanisms by which TGF-β1 protects the intestine from the harmful effects of TcdA have not yet been fully clarified. Our data suggest that modulation of this pathway may have an impact on toxin effects in patients with severe or recurrent CDAD.
MATERIALS AND METHODS
Cell line culture and toxin A.
Rat intestinal jejunal crypt cells (IEC-6) were obtained from Banco de Células do Rio de Janeiro (BCRJ) and cultured in Dulbecco's modified Eagle's medium/F-12 (DMEM/F-12; Gibco Thermo Fisher Scientific) supplemented with 10% fetal bovine serum (FBS), 5 mg of bovine insulin (Sigma-Aldrich), 50 IU/ml of penicillin, and 100 μg/ml of streptomycin (Invitrogen Molecular Probes) at 37°C in a 5% CO2 incubator. For in vitro experiments, toxin A (TcdA) from Clostridium difficile was purchased from Sigma-Aldrich (St. Louis, MO; C3977-2UG). For in vivo experiments, TcdA proteins were obtained from supernatants of C. difficile strain VPI 10463 grown in a dialysis system culture and purified as described previously (44). Native TcdA was purified by anion-exchange chromatography, followed by gel filtration chromatography. The purity of the toxins was determined by SDS-PAGE and mass spectrometry.
Immunocytochemistry.
Immunostaining was performed, as described previously (45), to investigate the effect of TcdA on TGFRII and phosphorylated SMAD2/3 (pSMAD2/3) distribution in IEC-6 cells. Briefly, the cells were seeded overnight at 3 × 105 cells/well in a 24-well plate. Subconfluent cultures were left untreated (control) or incubated with different treatments: (i) 10 ng/ml TcdA, (ii) TGF-β1 (10 ng/ml), or (iii) TcdA plus TGF-β1 (both at 10 ng/ml) in standard DMEM/F-12. The concentration of toxin A was chosen based on previous data (24) indicating that preincubation of T84 cells with concentrations equal to or less than 10 ng/ml of TcdA increased the release of TGF-β1. Additionally, Brito and colleagues (32) showed that 100 ng/ml of TcdA induced death in IEC-6 cells characteristic of apoptosis and necrosis, and a concentration of 10 ng/ml induced death in a lower percentage of cells. After 24 h, cells were fixed for 15 min with 4% paraformaldehyde in phosphate-buffered saline (PBS; pH 7.6), washed with PBS, and permeabilized with 0.1% Triton X-100 for 5 min. Samples were then blocked for 1 h with PBS containing 5% bovine serum albumin (BSA) and incubated for 1 h at room temperature with the following primary antibodies: rabbit anti-phospho-SMAD2/3 (1:50; Santa Cruz Biotechnology) and rabbit anti-TGRII (1:100; Santa Cruz Biotechnology). Samples were then incubated for 1 h at room temperature with anti-rabbit secondary antibodies conjugated with Alexa Fluor 488 (1:400; Invitrogen Molecular Probes) and/or Alexa Fluor 546 (1:1,000; Invitrogen Molecular Probes). Slides were mounted with Vectashield mounting medium with 4′,6-diamidino-2-phenylindole (DAPI; Vector Laboratories, Burlingame, CA). Images were captured using a Nikon Eclypse TE2000-S fluorescence optical microscope. The total number of cells in each random field was quantified by counting DAPI-stained nuclei, and the percentage of cells stained for the different markers in each random field was determined. To analyze TGF-β1/SMAD pathway activation, number of cells with SMAD2/3 nuclear translocation was quantified and expressed as the percentage of total cells (measured by DAPI staining). At least 5 fields per slide in 3 slides per condition were analyzed. At least three independent experiments were performed. Levels of TGFRII in IEC-6 cells were quantified by measuring the intensity of TGFRII immunostaining in IEC-6 cell cultures. To do that, TGFRII immunostaining was quantified under different experimental conditions, using integrated density values generated with the ImageJ program (National Institutes of Health, USA). Arbitrary values for labeling intensity were expressed in relation to the number of cells in the field (DAPI staining). At least 5 fields were analyzed per condition and at least three independent experiments were performed.
Proliferation assay.
Ki67 is a nuclear protein that is tightly linked to the cell cycle and is a marker of cell proliferation. Proliferation was performed as described previously (49). Briefly, the cells were seeded overnight at 3 × 105/well in a 24-well plate. Subconfluent cultures were left untreated (control) or incubated with different treatments: (i) 10 ng/ml of TcdA, (ii) TGF-β1 (10 ng/ml), or (iii) TcdA plus TGF-β1 (both at 10 ng/ml) in standard DMEM/F-12. After 24 h, cells were fixed for 15 min with 4% paraformaldehyde in PBS (pH 7.6), washed with PBS, and permeabilized with 0.1% Triton X-100 for 5 min. Samples were then blocked for 1 h with PBS containing 5% bovine serum albumin and incubated for 1 h at room temperature with the following primary antibody: rabbit anti-Ki67 (Santa Cruz Biotechnology). After wash, the slides were incubated with Dako-labeled polymer (Envision Flex no. K4010; Dako) for 1 h. Slides were mounted, and Ki67 samples were stained with the chromogen 3,3-diaminobenzidine (DAB; Dako), counterstained with Mayer's hematoxylin, and then mounted in Faramount mounting medium (Dako). Images were captured using a Leica DM 2000. Cells positive for Ki67 were counted in 10 fields per slide in 4 slides per treatment. At least three independent experiments were performed.
Flow cytometric analysis of apoptosis and necrosis.
Apoptosis and necrosis were assessed by flow cytometry. The ApoAlert annexin V kit (BDR Biotech) was used to detect phosphatidylserine on the reverse membrane surface of apoptotic cells. Propidium iodide was used to detect nucleic acids inside necrotic and late apoptotic cells. IEC-6 cells were seeded in 6-well plates at a concentration of 106/well and incubated for 24 h. Then the cells were treated as described above for an additional 24 h. After this period, cells were washed twice with PBS, trypsinized (0.05% trypsin; Sigma-Aldrich), collected by centrifugation, and doubly stained with fluorescein isothiocyanate-conjugated annexin V and propidium iodide (both from BDR Biotech). The samples were then analyzed on a Coulter EPICS XL flow cytometer (EasyCyte; Guava Technologies).
Scratch wound assay.
IEC-6 cells were seeded in 6-well plates at a concentration of 106/well and allowed to grow until total confluence. The confluent cells were scratched with a razor blade at the midline, extending to the right side of the well, as described elsewhere (26). After the scrape or “wounding,” the wells were washed with PBS twice, and the medium was changed to fresh medium containing TcdA (10 ng/ml), TGF-β1 (10 ng/ml), or TcdA (10 ng/ml) plus TGF-β1 (10 ng/ml). The control cells were incubated with medium alone. Migrated cells were observed under a magnification of ×100 after 12 and 24 h using an Olympus 1 × 71 inverted microscope with a QImaging camera. Images were acquired using QCapture Pro.5.1 software, and cells were counted in 10 fields/well.
Animals.
C57BL/6 mice, weighing 25 to 30 g, were housed in temperature-controlled rooms under 12-h light-dark cycles. The animals received water and food ad libitum. Surgical procedures and animal treatments were conducted in accordance with the guidelines for institutional and animal care and use of the Federal University of Ceará, Brazil. All procedures involving animals were approved by the Committee on the Ethical Treatment of Research Animals of the Federal University of Ceará.
Induction of intestinal inflammation.
The TcdA-induced enteritis mouse model was used as described previously (46). Briefly, mice were fasted overnight but with free access to water. Prior to surgery, mice were anesthetized with ketamine and xylazine (100 mg/kg and 10 mg/kg intramuscularly, respectively). Through a midline laparotomy, a 4-cm ileal loop was ligated and injected with either 0.1 ml of PBS (pH 7.4; control) or PBS containing TcdA (10 μg). The abdomen was sutured, and the animals were allowed to regain consciousness. Four hours after administration of TcdA or PBS, the mice were sacrificed. Intestinal loops were removed and the loop length and weight were measured. The intestinal loop tissues were processed accordingly for analysis using different techniques, including immunohistochemistry, Western blotting, and quantitative real-time reverse transcription-PCR (qPCR).
Immunohistochemistry.
Immunohistochemistry assays to evaluate TGF-β1, TGRII, and pSMAD2/3 distribution were performed with the ileal tissues using the avidin-biotin-peroxidase method (47) in formalin-fixed, paraffin-embedded tissue sections (4 μm thick) mounted on poly-l-lysine-coated microscope slides. Briefly, the sections were deparaffinized and rehydrated through xylene and graded alcohols. Afterward, sections were blocked twice with endogenous peroxidase (10 min each) with 3% (vol/vol) hydrogen peroxide and washed in PBS. Sections were incubated overnight at 4°C with rabbit anti-TGF-β1 antibody (1:100; R&D Systems), rabbit anti-TGRII (1:100; Santa Cruz), and rabbit anti-pSMAD2/3 (1:50; Santa Cruz Biotechnology). All the antibodies were diluted in PBS plus BSA. After a washing, the slides were incubated with avidin-biotin-horseradish peroxidase conjugated for 30 min, according to the protocol of the manufacturer (Santa Cruz Biotechnology). TGF-β1, TGFRII, and pSMAD2/3 were visualized with DAB after 2 min of incubation. Negative controls were obtained by omitting primary antibodies. Slides were counterstained, dehydrated in a graded alcohol series, cleared in xylene, and mounted in Faramount mounting medium (Dako Cytomation).
qPCR.
Quantitative real-time PCR (qPCR) analysis of mRNA for TGF-β1 and TGFRII was performed for IEC-6 cells and mouse ileal tissue. For culture assays, IEC-6 cells were seeded overnight at 6 × 105/well in a six-well plate, and subconfluent cultures were incubated with or without 10 ng/ml of TcdA in standard DMEM/F-12 medium for 24 h. The ileal tissues were treated with TcdA or PBS as described previously. The RNA was extracted using TRIzol (Invitrogen, USA) according to the protocol provided by the manufacturer. The quality of the RNA was analyzed by a 260/280 ratio and quantified by UV absorption using a NanoDrop ND-1000 (Thermo Fisher Scientific). Two micrograms of total RNA was reverse transcribed with a high-capacity cDNA reverse transcription kit according to the manufacturer's instructions (Applied Biosystems). The primers used in this assay were as follows: for TGF-β1, TAC CAT GCC AAC TTC TGT CTG GG A (forward)/ATG TTG GAC AAC TGC TCC ACC TTG (reverse), and for TGRII, ACT GTC CAC TTG CGA CAA CCA GA A (forward)/AGA AGC GGC ATC TTC CAG AGT GAA (reverse). The following β-actin primers were used as an endogenous control: TGG ATC GGT TCC ATC CTG G (forward)/GCA GCT CAG TAA CAG TCC GCC TAG A (reverse). qPCR was performed using a SYBR green PCR master mix, including Ampli Taq Gold polymerase (Applied Biosystems). Reactions were performed on an ABI Prism 7500 real-time PCR system (Applied Biosystems). The relative expression levels of genes were calculated using the threshold cycle (2−ΔΔCT) method (48). The amount of target genes expressed in a sample was normalized to the average of the endogenous control.
Immunoblotting assays.
Ileal tissue samples (100 mg) were homogenized in lysis buffer plus protease inhibitor tablet (Sigma-Aldrich). The protein concentration was determined using the Bradford assay. Sample proteins (50 μg) were loaded onto a range of polyacrylamide gels (8 to 15%) for electrophoresis and transferred onto nitrocellulose membrane by electroblotting under wet conditions (Mini Trans blot; Bio-Rad). The membranes were incubated overnight at 4°C individually with the following antibodies: anti-TGF-β1/2 and anti-α-tubulin (Santa Cruz Biotechnology) at dilutions of 1:200 and 1:1,000, respectively. The membranes were then incubated with secondary antibody conjugated with horseradish peroxidase (Santa Cruz Biotechnology) for 2 h at room temperature at a 1:1,000 dilution. For measurement, the chemiluminescence system was visualized using the ChemiDocTM XRS+ system (Bio-Rad, Life Technologies), and the resulting bands were analyzed and quantified by ImageJ (National Institutes of Health). The α-tubulin gene was used as the housekeeping gene.
Statistical analysis.
All data are expressed as means ± standard errors of the means (SEM). The difference between multiple groups were evaluated by one-way analysis of variances (ANOVA) followed by Bonferroni's test using GraphPad Prism (GraphPad, San Diego, CA). Student's t test was used to compare the means of two groups. A P value of <0.05 was considered to indicate significant differences. All experiments were done in triplicate, and all results represent means from at least 3 independent experiments.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by PRONEX/FUNCAP/CNPq of Brazil through grant PR2-0101-00060.01.00/15.
C.M.T.-V. performed experiments and analyzed the data; A.A.Q.A.S. performed experiments and contributed to data analysis and paper writing; J.S. contributed to data analysis, interpretation, and paper writing; M.M. performed experiments and contributed to data analysis; A.P.B.A. performed experiments and contributed to data analysis; C.Q-G. performed the toxin purification, contributed reagents, and contributed to data analysis and manuscript revision; D.L-U. performed the toxin purification and contributed to paper revision; R.F.C.L. contributed to data analysis, interpretation, and paper writing; D.A.F. performed experiments; F.C.A.G. designed the experiments and contributed to data analysis, interpretation, and paper writing; and G.A.D.C.B. designed the experiments and contributed to data analysis, interpretation, and paper writing.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/IAI.00430-17.
REFERENCES
- 1.Burke KE, Lamont JT. 2014. Clostridium difficile infection: a worldwide disease. Gut Liver 8:1–6. doi: 10.5009/gnl.2014.8.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnson S, Clabots CR, Linn FV, Olson MM, Peterson LR, Gerding DN. 1990. Nosocomial Clostridium difficile colonisation and disease. Lancet 336:97–100. doi: 10.1016/0140-6736(90)91605-A. [DOI] [PubMed] [Google Scholar]
- 3.Simor AE. 2010. Diagnosis, management, and prevention of Clostridium difficile infection in long-term care facilities: a review. J Am Geriatr Soc 58:1556–1564. doi: 10.1111/j.1532-5415.2010.02958.x. [DOI] [PubMed] [Google Scholar]
- 4.Yuille S, Mackay WG, Morrison DJ, Tedford MC. 2015. Optimising gut colonisation resistance against Clostridium difficile infection. Clin Microbiol Infect Dis 34:2161–2166. doi: 10.1007/s10096-015-2479-6. [DOI] [PubMed] [Google Scholar]
- 5.Dobson G, Hickey C, Trinder J. 2003. Clostridium difficile colitis causing toxic megacolon, severe sepsis and multiple organ dysfunction syndrome. Intensive Care Med 29:1030. doi: 10.1007/s00134-003-1754-7. [DOI] [PubMed] [Google Scholar]
- 6.Humphrey CD, Condon CW, Cantey JR, Pittman FE. 1979. Partial purification of a toxin found in hamsters with antibiotic-associated colitis. Reversible binding of the toxin by cholestyramine. Gastroenterology 76:468–476. [PubMed] [Google Scholar]
- 7.Rehg JE. 1980. Cecal toxin(s) from guinea pigs with clindamycin-associated colitis, neutralized by Clostridium sordellii antitoxin. Infect Immun 27:387–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lima AA, Lyerly DM, Wilkins TD, Innes D, Guerrant RL. 1988. Effects of Clostridium difficile toxins A and B in rabbit small and large intestine in vivo and on cultured cells in vitro. Infect Immun 56:582–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lyerly DM, Krivan HC, Wilkins TD. 1988. Clostridium difficile: its disease and toxins. Clin Microbiol Rev 1:1–18. doi: 10.1128/CMR.1.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flegel WA, Muller F, Daubener W, Fischer HG, Hadding U, Northoff H. 1991. Cytokine response by human monocytes to Clostridium difficile toxin A and toxin B. Infect Immun 59:3659–3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pothoulakis C, Karmeli F, Kelly CP, Eliakim R, Joshi MA, O'Keane CJ, Castagliuolo I, LaMont JT, Rachmilewitz D. 1993. Ketotifen inhibits Clostridium difficile toxin A-induced enteritis in rat ileum. Gastroenterology 105:701–707. doi: 10.1016/0016-5085(93)90886-H. [DOI] [PubMed] [Google Scholar]
- 12.Bobak D, Gilmer L. 1994. Toxin A of Clostridium difficile is a potent activator of interleukin-8 synthesis by human neutrophils. Clin Res 42:150A. [Google Scholar]
- 13.Burakoff R, Zhao L, Celifarco AJ. 1995. Effects of purified Clostridium difficile toxin A on rabbit distal colon. Gastroenterology 109:348–354. doi: 10.1016/0016-5085(95)90320-8. [DOI] [PubMed] [Google Scholar]
- 14.Madsen K, Lewis SA, Tavernini MM, Hibbard J, Fedorak RN. 1997. Interleukin 10 prevents cytokine-induced disruption of T84 monolayer barrier integrity and limits chloride secretion. Gastroenterology 113:151–159. doi: 10.1016/S0016-5085(97)70090-8. [DOI] [PubMed] [Google Scholar]
- 15.Forsyth CB, Banan A, Farhadi A, Fields JZ, Tang Y, Shaikh M, Zhang LJ, Engen PA, Keshavarzian A. 2007. Regulation of oxidant-induced intestinal permeability by metalloprotease-dependent epidermal growth factor receptor signaling. J Pharmacol Exp Ther 321:84–97. doi: 10.1124/jpet.106.113019. [DOI] [PubMed] [Google Scholar]
- 16.Gomes FC, Sousa VO, Romão L. 2005. Emerging roles for TGF-beta1 in nervous system development. Int J Dev Neurosci 23:413–424. doi: 10.1016/j.ijdevneu.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 17.Massagué J. 2008. A very private TGF-beta receptor embrace. Mol Cell 29:149–150. doi: 10.1016/j.molcel.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 18.Stipursky J, Spohr TC, Sousa VO, Gomes FC. 2012. Neuron-astroglial interactions in cell-fate commitment and maturation in the central nervous system. Neurochem Res 37:2402–2418. doi: 10.1007/s11064-012-0798-x. [DOI] [PubMed] [Google Scholar]
- 19.Diniz LP, Tortelli V, Garcia MN, Araújo APB, Melo HM, Seixas da Silva GS, De Felice FG, Alves-Leon SV, de Souza JM, Romão LF, Castro NG, Gomes FCA. 2014. Astrocyte transforming growth factor beta 1 promotes inhibitory synapse formation via CaM kinase II signaling. Glia 62:1917–1931. doi: 10.1002/glia.22713. [DOI] [PubMed] [Google Scholar]
- 20.Derynck R, Feng XH. 1997. TGF-beta receptor signaling. Biochim Biophys Acta 1333:105–150. [DOI] [PubMed] [Google Scholar]
- 21.Shi Y, Massague J. 2003. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell 113:685–700. doi: 10.1016/S0092-8674(03)00432-X. [DOI] [PubMed] [Google Scholar]
- 22.Chung HL, Hwang JB, Park JJ, Kim SG. 2002. Expression of transforming growth factor β1, transforming growth factor type I and II receptors, and TNF-β in the mucosa of the small intestine in infants with food protein-induced enterocolitis syndrome. J Allergy Clin Immunol 109:150–154. doi: 10.1067/mai.2002.120562. [DOI] [PubMed] [Google Scholar]
- 23.Maheshwari A, Kelly DR, Nicola T, Ambalavanan N, Jain SK, Murphy-Ullrich J, Athar M, Shimamura M, Bhandari V, Aprahamian C, Dimmitt RA, Serra R, Ohls RK. 2011. TGF-β2 suppresses macrophage cytokine production and mucosal inflammatory responses in the developing intestine. Gastroenterology 140:242–253. doi: 10.1053/j.gastro.2010.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johal SS, Solomon K, Dodson S, Borriello SP, Mahida YR. 2004. Differential effects of varying concentrations of Clostridium difficile toxin A on epithelial barrier function and expression of cytokines. J Infect Dis 189:2110–2119. doi: 10.1086/386287. [DOI] [PubMed] [Google Scholar]
- 25.Al-Sadi R, Guo S, Ye D, Ma TY. 2013. TNF-α modulation of intestinal epithelial tight junction barrier is regulated by ERK1/2 activation of Elk-1. Am J Pathol 183:1871–1884. doi: 10.1016/j.ajpath.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McCormack SA, Viar MJ, Johnson LR. 1992. Migration of IEC-6 cells: a model for mucosal healing. Am J Physiol 263:G426–G435. [DOI] [PubMed] [Google Scholar]
- 27.Medeiros CA, Warren CA, Freire R, Vieira CA, Lima BB, Vale ML, Ribeiro RA, Souza MH, Brito GA. 2011. Role of the haem oxygenase/carbon monoxide pathway in Clostridium difficile toxin A-induced enteritis in mice. J Med Microbiol 60:1146–1154. doi: 10.1099/jmm.0.028910-0. [DOI] [PubMed] [Google Scholar]
- 28.Brito GA, Fujji J, Carneiro-Filho BA, Lima AA, Obrig T, Guerrant RL. 2002. Mechanism of Clostridium difficile toxin A-induced apoptosis in T84 cells. J Infect Dis 186:1438–1447. doi: 10.1086/344729. [DOI] [PubMed] [Google Scholar]
- 29.Seidelin JB, Larsen S, Linnemann D, Vainer B, Coskun M, Troelsen JT, Nielsen OH. 2015. Cellular inhibitor of apoptosis protein 2 controls human colonic epithelial restitution, migration, and Rac1 activation. Am J Physiol Gastrointest Liver Physiol 308(2):G92–G99. doi: 10.1152/ajpgi.00089.2014. [DOI] [PubMed] [Google Scholar]
- 30.Huang Y, Fang W, Wang Y, Yang W, Xiong B. 2012. Transforming growth factor-β1 induces glutathione peroxidase-1 and protects from H2O2-induced cell death in colon cancer cells via the Smad2/ERK1/2/HIF-1α pathway. Int J Mol Med 29:906–912. [DOI] [PubMed] [Google Scholar]
- 31.Roche JK, Martins CA, Cosme R, Fayer R, Guerrant RL. 2000. Transforming growth factor beta1 ameliorates intestinal epithelial barrier disruption by Cryptosporidium parvum in vitro in the absence of mucosal T lymphocytes. Infect Immun 68:5635–5644. doi: 10.1128/IAI.68.10.5635-5644.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brito GA, Carneiro-Filho B, Oriá RB, Destura RV, Lima AA, Guerrant RL. 2005. Clostridium difficile toxin A induces intestinal epithelial cell apoptosis and damage: role of Gln and Ala-Gln in toxin A effects. Dig Dis Sci 50:1271–1278. doi: 10.1007/s10620-005-2771-x. [DOI] [PubMed] [Google Scholar]
- 33.Yamada Y, Mashima H, Sakai T, Matsuhashi T, Jin M, Ohnishi H. 2013. Functional roles of TGF-β1 in intestinal epithelial cells through Smad-dependent and non-Smad pathways. Dig Dis Sci 58:1207–1217. doi: 10.1007/s10620-012-2515-7. [DOI] [PubMed] [Google Scholar]
- 34.Bezerra Lima B, Faria Fonseca B, da Graça Amado N, Moreira Lima D, Albuquerque Ribeiro R, Garcia Abreu J, de Castro Brito GA. 2014. Clostridium difficile toxin A attenuates Wnt/β-catenin signaling in intestinal epithelial cells. Infect Immun 82:2680–2687. doi: 10.1128/IAI.00567-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lica M, Schulz F, Schelle I, May M, Just I, Genth H. 2011. Difference in the biological effects of Clostridium difficile toxin B in proliferating and non-proliferating cells. Naunyn Schmiedebergs Arch Pharmacol 383:275–283. doi: 10.1007/s00210-010-0595-5. [DOI] [PubMed] [Google Scholar]
- 36.Labbé E, Lock L, Letamendia A, Gorska AE, Gryfe R, Gallinger S, Moses HL, Attisano L. 2007. Transcriptional cooperation between the transforming growth factor-beta and Wnt pathways in mammary and intestinal tumorigenesis. Cancer Res 67:75–84. doi: 10.1158/0008-5472.CAN-06-2559. [DOI] [PubMed] [Google Scholar]
- 37.Cheon SS, Wei Q, Gurung A, Youn A, Bright T, Poon R. 2006. Beta-catenin regulates wound size and mediates the effect of TGF-beta in cutaneous healing. FASEB J 20:692–701. doi: 10.1096/fj.05-4759com. [DOI] [PubMed] [Google Scholar]
- 38.Hussein SM, Duff EK, Sirard C. 2003. Smad4 and beta-catenin co-activators functionally interact with lymphoid-enhancing factor to regulate graded expression of Msx2. J Biol Chem 278:805–814. doi: 10.1074/jbc.M305472200. [DOI] [PubMed] [Google Scholar]
- 39.Hsu YC, Chen MJ, Yu YM, Ko SY, Chang CC. 2010. Suppression of TGF-β1/SMAD pathway and extracellular matrix production in primary keloid fibroblasts by curcuminoids: its potential therapeutic use in the chemoprevention of keloid. Arch Dermatol Res 302:717–724. doi: 10.1007/s00403-010-1075-y. [DOI] [PubMed] [Google Scholar]
- 40.Aoki CA, Borchers AT, Li M, Flavell RA, Bowlus CL, Ansari AA, Gershwin ME. 2005. Transforming growth factor beta (TGF-beta) and autoimmunity. Autoimmun Rev 4:450–459. doi: 10.1016/j.autrev.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 41.Monteleone G, Kumberova A, Croft NM, McKenzie C, Steer HW, MacDonald TT. 2001. Blocking Smad7 restores TGF-beta1 signaling in chronic inflammatory bowel disease. J Clin Invest 108:601–609. doi: 10.1172/JCI12821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Monteleone G, Pallone F, MacDonald TT. 2004. Smad7 in TGF-betamediated negative regulation of gut inflammation. Trends Immunol 25:513–517. doi: 10.1016/j.it.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 43.Monteleone G, Fantini MC, Onali S, Zorzi F, Sancesario G, Bernardini S, Calabrese E, Viti F, Monteleone I, Biancone L, Pallone F. 2012. Phase I clinical trial of Smad7 knockdown using antisense oligonucleotide in patients with active Crohn's disease. Mol Ther 20:870–876. doi: 10.1038/mt.2011.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pruitt RN, Chambers MG, Ng KK-S, Ohib MD, Lacy DB. 2010. Structural organization of the functional domains of Clostridium difficile toxins A and B. Proc Natl Acad Sci U S A 107:13467–13472. doi: 10.1073/pnas.1002199107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stipursky J, Gomes FC. 2007. TGF-beta1/SMAD signaling induces astrocyte fate commitment in vitro: implications for radial glia development. Glia 55:1023–1033. doi: 10.1002/glia.20522. [DOI] [PubMed] [Google Scholar]
- 46.Castagliuolo I, Riegler M, Pasha A, Nikulasson S, Lu B, Gerard C, Gerard NP, Pothoulakis C. 1998. Neurokinin-1 (NK-1) receptor is required in Clostridium difficile-induced enteritis. J Clin Invest 101:1547–1550. doi: 10.1172/JCI2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hsu SM, Raine LL. 1981. Protein A, avidin, and biotin in immunohistochemistry. J Histochem Cytochem 29:1349–1353. doi: 10.1177/29.11.6172466. [DOI] [PubMed] [Google Scholar]
- 48.Livak KJ, Schmittgen TD. 2001. Analyses of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 49.Skeff MA, Brito GAC, de Oliveira MG, Braga CM, Cavalcante MM, Baldim V, Holanda-Afonso RC, Silva-Boghossian CM, Colombo AP, Ribeiro RA, Moura-Neto V, Leitão RFC. 2014. S-Nitrosoglutathione accelerates recovery from 5-fluorouracil-induced oral mucositis. PLoS One 9:e113378. doi: 10.1371/journal.pone.0113378. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.