ABSTRACT
Tularemia is caused by the Gram-negative bacterial pathogen Francisella tularensis. Infection of macrophages and their subsequent death are believed to play important roles in the progression of disease. Because complement is a particularly effective opsonin for Francisella, we asked whether complement-dependent uptake of F. tularensis strain SCHU S4 affects the survival of primary human macrophages during infection. Complement component C3 was found to be an essential opsonin in human serum not only for greatly increased uptake of SCHU S4 but also for the induction of macrophage death. Single-cell analysis also revealed that macrophage death did not require a high intracellular bacterial burden. In the presence of C3, macrophage death was observed at 24 h postinfection in a quarter of the macrophages that contained only 1 to 5 bacterial cells. Macrophages infected in the absence of C3 rarely underwent cell death, even when they contained large numbers of bacteria. The need for C3, but not extensive replication of the pathogen, was confirmed by infections with SCHU S4 ΔpurMCD, a mutant capable of phagosome escape but of only limited cytosolic replication. C3-dependent Francisella uptake alone was insufficient to induce macrophage death, as evidenced by the failure of the phagosome escape-deficient mutant SCHU S4 ΔfevR to induce cell death despite opsonization with C3. Together, these findings indicate that recognition of C3-opsonized F. tularensis, but not extensive cytosolic replication, plays an important role in regulating macrophage viability during intracellular infections with type A F. tularensis.
KEYWORDS: C3, Francisella tularensis, cell death, complement, macrophage
INTRODUCTION
Francisella tularensis is the causative agent of the zoonotic life-threatening disease tularemia. Among the subspecies responsible for disease in human beings, F. tularensis subsp. tularensis (type A) is the most virulent and causes high morbidity and mortality when delivered via the respiratory route (1–4). Very low minimum infectious doses have been reported with pulmonary challenge in humans (2, 5). In mice, alveolar macrophages are among the earliest cells infected following respiratory challenge (6–8). F. tularensis rapidly disrupts the phagosome and enters the cytosol of cultured macrophages, where it replicates to high intracellular numbers (9–11). Likewise, phagocytic cell death and bacteremia follow infection of mice with F. tularensis and lead to secondary colonization and pathology in the spleen, liver, and draining lymph nodes (12–15). Indeed, one of the hallmark histopathological features of disseminated tularemia caused by the F. tularensis subsp. tularensis is the appearance of infected clusters of macrophages and myeloid cells (microgranulomas), which rapidly transform into necrotic foci (13–16). Infection of macrophages with the laboratory strain F. tularensis subsp. tularensis SCHU S4 does not appear to activate caspase-1-mediated pyroptosis (17, 18), as has been reported for Francisella novicida strain U112 (19, 20). F. novicida infections in humans are exceedingly rare and are most often associated with immunocompromised humans (21). In contrast, strains of F. tularensis, including type A strains, that are pathogenic in otherwise healthy humans have been found to induce caspase-3-mediated apoptotic death in mouse and human macrophages (14, 15, 22–25).
The uptake of F. tularensis by macrophages can be mediated by a number of opsonic receptors, including the mannose receptor, the scavenger receptor, IgG-Fc receptors, nucleolin, and complement receptors CR3 and CR4 (26–33). Opsonization of F. tularensis with C3 results in particularly efficient phagocytosis by human macrophages (26, 28, 29, 32).
Several reports have suggested that the outcome of macrophage infection with F. tularensis is influenced by the receptor that mediates pathogen uptake (26, 27, 30, 32, 34). Clemens et al. (34) reported that the morphology of uptake of two F. tularensis live vaccine strain O-antigen mutants by human macrophages differed when they were opsonized with C7-depleted serum or with heat-inactivated human serum (HI-HS). They suggested that C3 opsonization of O-antigen mutants mediates greater physical interaction between the pathogen and macrophage surfaces, altering the morphology of phagocytosis. Geier and Celli (30) reported that CR3-mediated uptake of strain SCHU S4 by mouse bone marrow-derived macrophages resulted in somewhat impaired phagosome escape compared to that with nonopsonized bacteria. Dai et al. (32) reported that CR3-mediated uptake of serum-opsonized SCHU S4, but not nonopsonized SCHU S4, inhibited Toll-like receptor 2 (TLR2)-dependent cytokine production, the phosphorylation of ERK1/2 and p38 mitogen-activated protein (MAP) kinases, and the activation of NF-κB in human macrophages. These findings indicate that the responses to C3-opsonized F. tularensis can be significantly different from those initiated by nonopsonized bacteria.
Given the importance of C3-mediated uptake in shaping the macrophage response to F. tularensis, we have asked whether C3 opsonization of F. tularensis subsp. tularensis alters macrophage survival. Specifically, we wished to determine whether opsonization of strain SCHU S4 with complement influenced cell death induction in infected primary human monocyte-derived macrophages (MDM).
RESULTS
Complement component C3 promotes uptake of SCHU S4 and induction of human macrophage death following Francisella infection.
As an initial test of the effects of heat-sensitive serum components, SCHU S4 bacteria were opsonized in medium containing either fresh-frozen human serum (HS) or heat-inactivated human serum (HI-HS) and used to infect human MDM. The uptake of bacteria and induction of macrophage death (measured as lactate dehydrogenase [LDH] release into culture supernatants) increased with increasing HS concentration (Fig. 1A). Both half-maximum uptake and LDH release in HS were achieved at multiplicities of infection (MOIs) of 20 to 30 (see Fig. S1 in the supplemental material). Heat inactivation of HS significantly reduced bacterial uptake to that seen in the absence of serum (data not shown) and diminished macrophage death to levels observed in uninfected cells (Fig. 1B). To determine whether the death-promoting effects of HS simply reflected greater initial bacterial uptake than occurred in the presence of HI-HS, MDM were infected under the two opsonization conditions at MOIs that yielded comparable initial bacterial burdens (Fig. 1C). Despite comparable numbers of intracellular bacteria throughout the course of infection, significantly higher levels of cell death were seen in cultures infected in the presence of HS than in those infected in the presence of HI-HS.
FIG 1.
Heat-labile components in human serum promote SCHU S4 uptake and the induction of macrophage death. (A) MDM were infected with SCHU S4 (MOI = 230) in the presence of various concentrations of HS. Bacterial burdens and LDH release were measured at 3 and 24 h p.i. *, significant differences compared to samples infected in 7.5% HS at the same time p.i. This experiment was performed twice. (B) MDM were infected with SCHU S4 (MOI = 80) in the presence of 7.5% HS or HI-HS. *, significant differences in bacterial burden between groups or significant differences in LDH release compared to uninfected MDM at each time point. The results shown are representative of three independent experiments. (C) Bacterial burdens and LDH release were measured at the indicated times p.i. in HS at an MOI of 7 or in HI-HS at an MOI of 730. *, significant differences comparing HS to HI-HS cultures. Results are representative of three independent experiments.
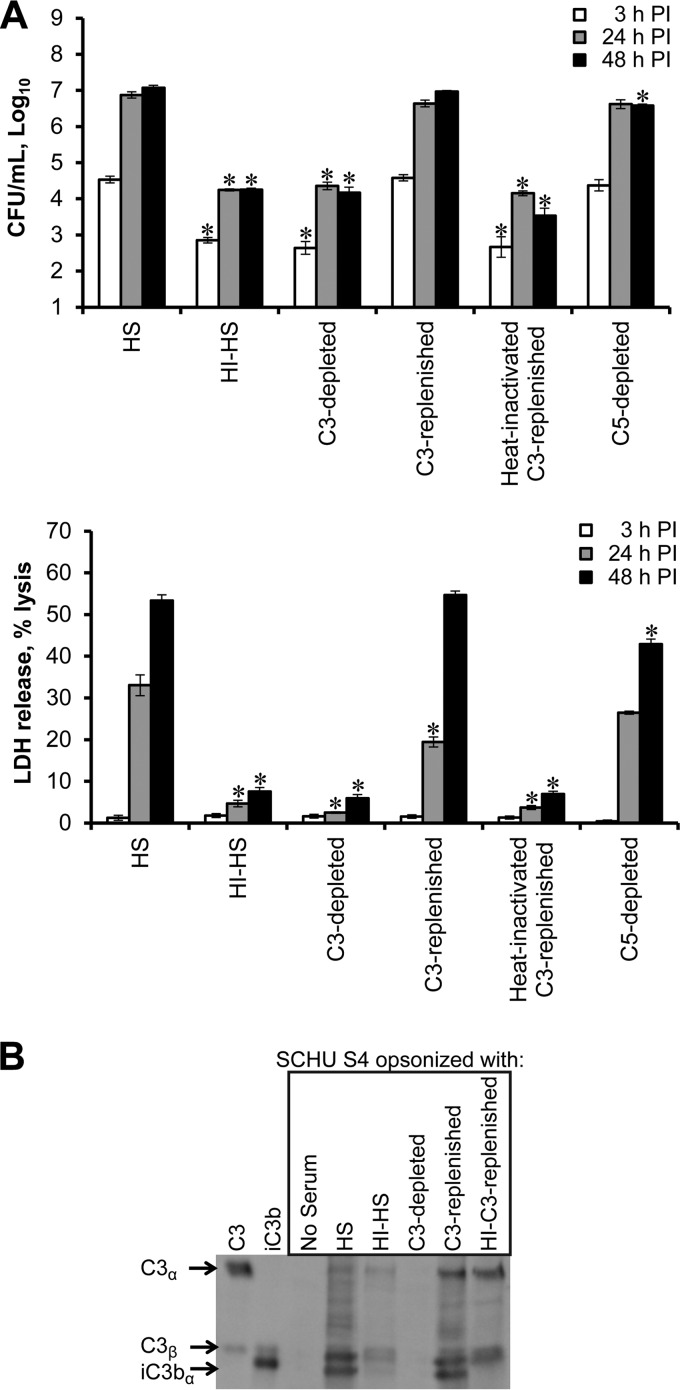
The heat-sensitive opsonic effect of serum on Francisella has been associated with the action of C3 peptides, namely, iC3b, produced during complement activation and deposited on the bacterial surface (35, 36). To determine the role of serum C3 in MDM death during Francisella infection, SCHU S4 bacteria were opsonized in HS, C3-depleted HS, or control C5-depleted HS and used to infect MDM (Fig. 2A). Following gentamicin treatment, all cultures were maintained in autologous HI-HS-containing medium to limit the opsonin effects to the uptake phase of infection. Serum lacking C3 failed to enhance bacterial uptake or promote macrophage death, resulting in an infection equivalent to that seen in HI-HS. The addition of purified C3 protein to replenish C3-depleted serum restored its ability to enhance uptake of Francisella and promote macrophage death. Heat inactivation of the C3-replenished serum reversed this effect. Depletion of C5 resulted in intracellular bacterial numbers and cell death levels similar to those observed in HS-containing cultures. These findings provide strong evidence that both bacterial uptake and the induction of macrophage death during infection with HS-opsonized SCHU S4 are C3 dependent.
FIG 2.
Complement component C3 mediates enhanced bacterial uptake and macrophage death induction. MDM were infected with SCHU S4 (MOI = 80) in the presence of the indicated sera. *, significant differences in bacterial burden or LDH release when comparing test sera to HS at the indicated times p.i. The results shown are representative of three independent experiments. (B) Deposition of C3 peptides on SCHU S4 (8 × 107 bacteria per lane) was detected via Western blotting. Purified C3 and iC3b proteins served as markers. These results are representative of three independent experiments.
As has been previously shown (35, 36), opsonization of SCHU S4 with HS deposited iC3b on the surface of SCHU S4, which was not seen in the absence of serum (Fig. 2B). Minimal C3 deposition on bacteria was observed after treatment with HI-HS, with only minor conversion to iC3b. Opsonization with C3-depleted serum failed to deposit any detectable C3 peptides. Replenishing C3-depleted serum with purified C3 restored the deposition of iC3b on the surface of SCHU S4. Whereas some C3b deposition occurred with heat-inactivated C3-replenished serum, there was no conversion to iC3b. This indicated that a strong correlation existed between the deposition of iC3b on the surface of SCHU S4 and the ability of opsonized bacteria to promote macrophage death.
High levels of intracellular F. tularensis alone are neither sufficient nor necessary for the induction of C3-dependent macrophage death.
We next asked whether C3 promoted cell death solely by promoting high cytosolic bacterial burdens. Infections of MDM with SCHU S4 were initiated in HS or C3-depleted HS after adjusting the MOIs to achieve equivalent initial bacterial burdens under the two conditions (Fig. 3). Despite similar intracellular bacterial numbers throughout the course of infection, MDM infected with HS-opsonized SCHU S4 showed a significantly higher level of cell death than MDM infected with bacteria opsonized with C3-depleted HS (Fig. 3A).
FIG 3.
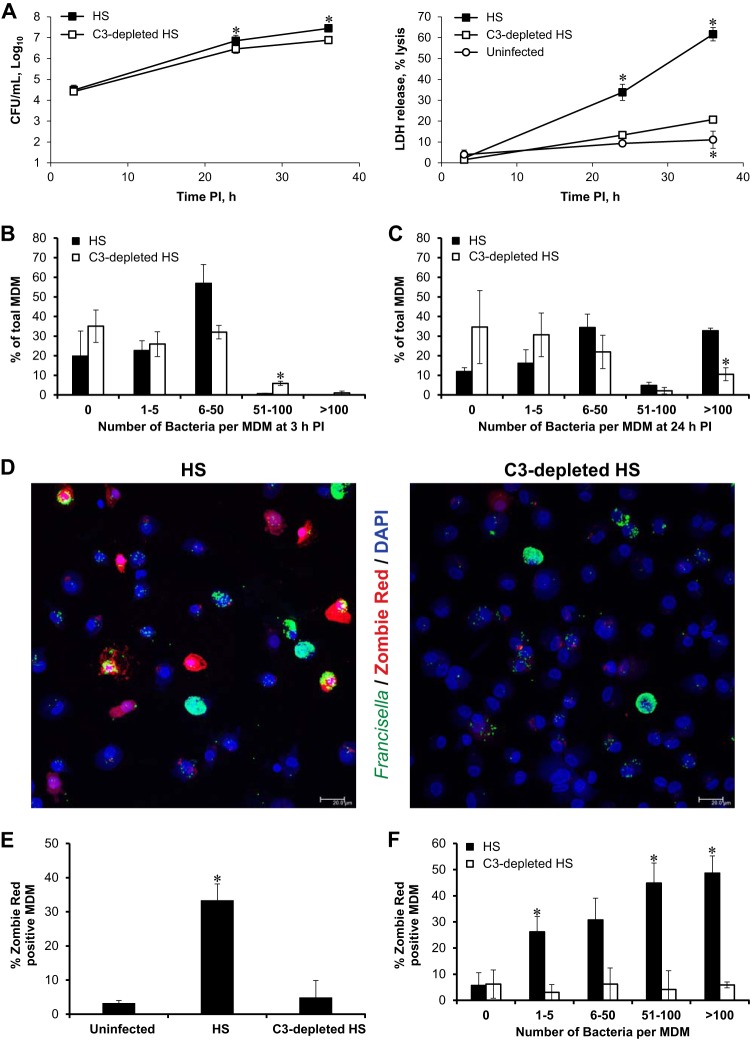
Macrophage death following Francisella infection requires C3 but not a high intracellular bacterial burden. To ensure similar levels of bacterial uptake, MDM were infected at a low MOI with SCHU S4 in the presence of HS or at a high MOI with SCHU S4 in the presence of C3-depleted serum. The results shown are averages from 3 independent experiments for which the average MOI was 24 in HS and 890 in C3-depleted HS. (A) *, significant differences in bacterial burden between the two uptake conditions or in LDH release compared to MDM infected in C3-depleted serum at each time point. (B to F) MDM on coverslips were stained with Zombie Red (red) and rabbit antiserum to Francisella (green) and then mounted in Prolong Gold with DAPI (blue). Individual MDM were enumerated based on their bacterial load and incidence of Zombie Red-positive staining for each uptake condition. (B and C) Relative frequency distributions for bacterial load for 3 h p.i. (B) and 24 h p.i. (C). *, significant differences in the percentage of MDM containing the indicated range of bacterial counts comparing HS and C3-depleted HS. (D) Representative images of Zombie Red-stained MDM infected in HS or C3-depleted HS. Scale bars, 20 μm. (E) Percentages of Zombie Red-positive cells found under each condition at 24 h p.i. *, significant differences compared to uninfected MDM. (F) *, significant differences between the percentage of Zombie Red-positive cells in HS and C3-depleted HS found in each bacterial load category at 24 h p.i.
Because LDH release measures death among a population of cells and the intracellular burden likely varies among individual macrophages, a single-cell microscopic analysis of bacterial load and cell viability was performed. MDM monolayers were infected in HS or C3-depleted HS with MOIs that yielded comparable initial burdens. Inspection of MDM monolayers at 3 h postinfection (p.i.) revealed no significant differences in the overall percentage of infected cells under these two infection conditions. The two infection conditions also yielded similar relative frequency distributions of the numbers of bacteria per MDM following uptake at 3 h p.i. (Fig. 3B).
The replication of the pathogen within macrophages is illustrated in Fig. 3A and by comparing the relative frequency distribution at 3 h p.i. to that at 24 h p.i. (Fig. 3B and C). Macrophages containing more than 100 bacteria each were observed under both infection conditions at 24 h p.i. (Fig. 3C). The percentage of uninfected cells did not decrease greatly from 3 to 24 h p.i. under either condition, indicating that secondary infection rates were low in these cultures. Infections initiated in HS and C3-depleted HS maintained similar relative frequency distributions of intracellular bacteria at 24 h p.i., with the exception that there were more macrophages with greater than 100 bacteria under HS than under C3-depleted HS infection conditions (Fig. 3C).
Despite these similar bacterial burdens, only cultures infected in the presence of C3 showed significant levels of macrophage death as measured by positive cytosolic staining with the fixable viability dye Zombie Red (Fig. 3D and E). Analysis of single cell death events in C3-opsonized cultures was particularly informative. Over 25% of MDM that contained only 1 to 5 bacteria were dead at 24 h p.i. (Fig. 3D and F), indicating that high bacterial burdens were not necessary for cell death induction. Conversely, approximately half of the MDM that contained more than 100 bacteria each remained viable at 24 h p.i. (Fig. 3D, left panel, and F). Although MDM death during infection with C3-opsonized bacteria correlated with the number of intracellular bacteria, cell death in these cultures did not require a high bacterial burden, nor did a large number of intracellular bacteria ensure that macrophage death would result. Additionally, cell death in infected HS cultures was rarely observed in MDM that lacked bacteria, indicating that bystander cell death was a rare event.
In comparison to HS cultures, MDM infected in the absence of C3 showed a similar range of bacterial burdens at 24 h p.i. (Fig. 3C) but little evidence of cell death (Fig. 3D, right panel, and E). Even in macrophages that contained very high bacterial loads (>100 bacteria per MDM) following infection in C3-depleted HS, the frequency of cell death was not above that observed in uninfected cells (Fig. 3F).
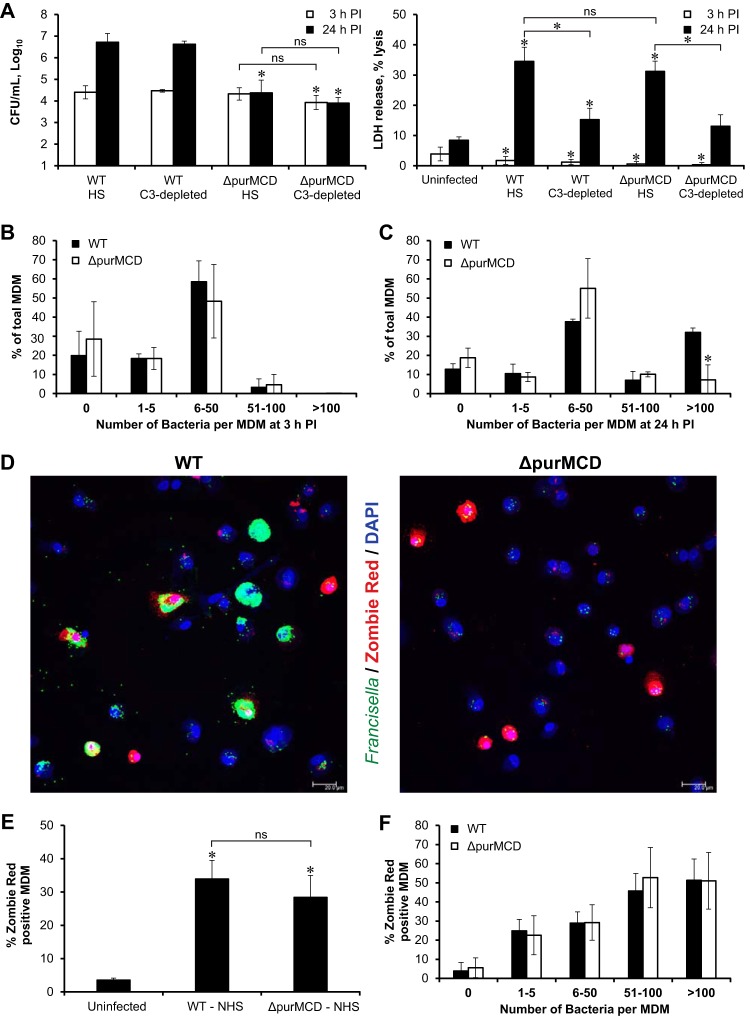
These findings led to the prediction that a mutant F. tularensis strain with limited capacity for cytosolic replication would still induce C3-dependent macrophage death. To test this hypothesis, infections of MDM with the replication-deficient SCHU S4 ΔpurMCD mutant were compared to wild-type (WT) SCHU S4 infections (Fig. 4). Deletion of the purMCD genes, which are required for purine synthesis, has no effect on the ability of SCHU S4 to escape the phagosome in mouse (37) and human (see Fig. S2B and C in the supplemental material) macrophages but has been shown to limit cytosolic replication (37, 38). Exposure of SCHU S4 ΔpurMCD to HS deposited iC3b on the bacterial surface, just as it did with WT SCHU S4 (Fig. S2A). There was also no difference in the uptake of the WT and ΔpurMCD bacteria in HS-containing cultures (Fig. 4A). As with WT SCHU S4, increasing the MOI in C3-depleted HS resulted in equivalent initial bacterial burdens for SCHU S4 ΔpurMCD in HS and C3-depleted HS (Fig. 4A). While the ΔpurMCD mutant failed to replicate to the same extent as WT bacteria, it did induce the same level of macrophage death in a C3-dependent manner (Fig. 4A).
FIG 4.
Replication-deficient mutant SCHU S4 ΔpurMCD induces wild-type levels of C3-dependent macrophage death. MDM were infected with WT or ΔpurMCD SCHU S4 at a low MOI in the presence of HS or at a high MOI in the presence of C3-depleted serum. The results shown are averages from 3 independent experiments. The average MOI for WT was 22 in HS and 890 in C3-depleted HS. The average MOI for SCHU S4 ΔpurMCD was 17 in HS and 672 in C3-depleted HS. (A) *, significant differences in bacterial burden compared to the WT in HS or significant differences in LDH release compared to uninfected MDM at each time point. ns, not significant. (B to F) MDM infected in HS were stained with Zombie Red (red) and rabbit antiserum to Francisella (green) and then mounted in Prolong Gold with DAPI (blue). Individual MDM infected with either WT or ΔpurMCD SCHU S4 were enumerated based on their bacterial load and incidence of Zombie Red-positive staining. (B and C) Relative frequency distributions for bacterial load for 3 h p.i. (B) and 24 h p.i. (C). *, significant differences between the percentage of WT SCHU S4- and SCHU S4 ΔpurMCD-infected macrophages containing the indicated range of bacterial counts. (D) Representative images of Zombie Red-stained MDM infected with either WT SCHU S4 or SCHU S4 ΔpurMCD in the presence of HS. Scale bars, 20 μm. (E) Percentage of Zombie Red-positive cells found at 24 h p.i. under each infection condition. *, significant differences compared to uninfected MDM. ns, not significant. (F) There were no significant differences between the percentages of Zombie Red-positive cells in WT SCHU S4- and SCHU S4 ΔpurMCD-infected MDM found in each bacterial load category at 24 h p.i.
These relationships were confirmed by single-cell confocal analysis of cell death events revealed by Zombie Red staining. There was no difference at 3 h p.i. in the relative frequency distributions of bacteria per MDM between WT and ΔpurMCD SCHU S4 following uptake in HS (Fig. 4B). However, there was a significantly higher percentage of MDM infected with WT bacteria that achieved high bacterial burdens (>100 bacteria per MDM) at 24 h p.i. than those infected with SCHU S4 ΔpurMCD (Fig. 4C). Over 80% of the macrophages infected with C3-opsonized SCHU S4 ΔpurMCD contained fewer than 50 bacteria at 24 h p.i. Nonetheless, macrophages infected in the presence of HS with either WT or ΔpurMCD SCHU S4 showed equivalent high levels of cell death (Fig. 4D, E, and F). Similar to WT infections, nearly 25% of the MDM infected with just 1 to 5 ΔpurMCD bacteria were dead at 24 h p.i. (Fig. 4F). Thus, a mutant strain of SCHU S4 that lacked the ability to replicate to a high intracellular density was still able to promote host cell death when opsonized with C3-containing HS.
Complement-mediated uptake alone is not sufficient for the induction of macrophage death following Francisella infection.
To determine whether complement receptor engagement provides a sufficient signal for initiating MDM death, we opsonized the phagosome escape-deficient mutant SCHU S4 ΔfevR with HS or C3-depleted HS and used these bacteria to infect MDM. Deletion of the fevR gene eliminates the ability of SCHU S4 to escape the phagosome (39). Exposure of SCHU S4 ΔfevR or the complemented mutant to HS deposited iC3b on the bacterial surface, just as it did with WT SCHU S4 (Fig. S2A). Fluorescence confocal microscopy verified the expected intracellular localization of each strain at 8 h p.i. and demonstrated a persistent colocalization between SCHU S4 ΔfevR and the lysosomal marker LAMP-1 (Fig. S2B and C) (39). Complementation of the mutant with a fevR-expressing plasmid restored its ability to escape the phagosome.
Similar to the case for WT SCHU S4, the uptake of the ΔfevR mutant was greatly increased by opsonization with HS compared to C3-depleted HS, indicating that uptake of both strains was C3 dependent (Fig. 5). As expected, the escape-deficient mutant showed impaired intracellular replication, and this trait could be genetically complemented with a fevR-expressing plasmid. Although the SCHU S4 ΔfevR strain was taken up in a C3-dependent manner, it failed to induce MDM death (Fig. 5), indicating that complement receptor-mediated uptake by itself was not a sufficient signal for host cell death induction. Complementation with a fevR-expressing plasmid restored the ability of the mutant to access the cytosol and induce C3-dependent MDM death.
FIG 5.
The phagosome escape deficient mutant SCHU S4 ΔfevR fails to induce macrophage death despite C3 opsonization. MDM were infected with WT SCHU S4 or the mutant strains in the presence of HS or C3-depleted HS. The MOIs were as follows: WT, 87; ΔfevR strain, 86; ΔfevR/pfevR strain, 96. Significant differences (*) in bacterial burden and LDH release compared to the WT in HS are shown for 48 h p.i. The results shown are representative of three independent experiments.
Neither the presence of large numbers of cytosolic C3-deficient bacteria nor the initial uptake of C3-opsonized bacteria was by itself sufficient to induce MDM death. For this reason, we tested a combination of these signals (Fig. 6). MDM were first infected with WT bacteria in C3-depleted HS at a high MOI to achieve high cytosolic burdens. Following gentamicin treatment and removal of residual extracellular bacteria, the macrophages were infected with C3-opsonized WT or SCHU S4 ΔfevR bacteria, and MDM viability was measured. Challenging preinfected MDM with C3-opsonized WT SCHU S4 led to MDM death. Challenging the same preinfected macrophages with C3-opsonized SCHU S4 ΔfevR did not affect the viability of the macrophages. Thus, even in MDM that contained large numbers of intracellular F. tularensis, subsequent binding and uptake of C3-opsonized bacteria did not ensure macrophage death. Only when C3 peptides were present on the WT SCHU S4 strain did MDM death result.
FIG 6.
Engagement of cell surface complement receptors is not sufficient for the induction of macrophage death, despite high cytosolic bacterial load. MDM were first infected at a high MOI with WT SCHU S4 in the presence of C3-depleted HS. This was followed by a second infection with either WT or ΔfevR SCHU S4 in the presence of HS or C3-depleted HS. Significant differences (*) in bacterial burden or LDH release are shown compared to the primary infection of WT in C3-depelted serum alone (no secondary infection). ns, not significant. The results shown are averages from 3 independent experiments in which the average MOI for the primary infection with WT in C3-depleted HS was 1,889. The average MOI in the secondary infection was 189 for WT and 97 for ΔfevR SCHU S4.
DISCUSSION
Because C3 is important for the uptake of F. tularensis (26–29, 35, 36, 40) and macrophage death is a common consequence of Francisella infection (13–15, 19, 41), we undertook this study to determine whether complement component C3 contributes to the induction of cell death in primary human macrophages during type A F. tularensis infections. A number of findings reported here are consistent with bacterial surface-bound iC3b having a major role in initiating macrophage death during type A Francisella infections. Unlike HS, C3-depleted HS failed to promote cell death even when similar levels of bacterial uptake were attained by adjusting the MOIs. Uptake and cell death induction were fully restored when C3-depleted serum was supplemented with purified C3 protein. Replenishing C3-depleted serum also restored the deposition of iC3b on the surface of SCHU S4, and heat inactivation of C3-replenished serum abolished both macrophage death and iC3b deposition. In contrast, C5-depleted serum was similar to HS in its ability to promote both bacterial uptake and host cell death, indicating that this downstream complement component was not involved. Our findings are consistent with the conclusion that iC3b plays an important role in controlling the viability of infected human macrophages in addition to its well-characterized effect on the uptake of type A F. tularensis by these cells (26, 27, 29, 32). Though we have not directly examined other opsonins found in normal human serum, the results with C3-depleted serum suggest that they lack the ability to promote macrophage death following infection with type A F. tularensis.
The results of the current study predict that macrophage death would not occur following infection with type A F. tularensis in culture medium lacking complement activity. For example, opsonization with fetal bovine serum, which contains very low levels of C3 and little hemolytic complement activity (42), would not be expected to support this form of macrophage death.
We were surprised to find that cell death measured by single-cell analysis occurred independent of the presence of large numbers of cytosolic SCHU S4 bacteria. Rather, macrophage death appeared to be dependent solely on the presence of complement component C3 during initial infection. Cell death was rarely observed in macrophages infected in C3-depleted serum, whether the MDM contained a low or an extremely high bacterial load. Despite a correlation between higher bacterial burden and cell death frequency when SCHU S4 was opsonized with C3, over 25% of the macrophages that contained just 1 to 5 bacterial cells were dead at 24 h p.i., and approximately half of the MDM that contained more than 100 bacteria were still viable. These findings indicate that a high burden was neither sufficient nor necessary for C3-dependent macrophage death. Considered together, our results indicate that C3 is an important trigger for macrophage death independent of its role as an opsonin. Given that uninfected bystander cells in infected cultures did not die, even in the presence of C3, it seems reasonable to conclude that both infection and C3 are required to induce the death of a given macrophage.
The finding that C3-opsonized WT SCHU S4 could induce cell death without achieving high intracellular bacterial burdens led us to predict that a mutant strain with a limited capacity for intracellular replication would nonetheless behave like WT SCHU S4 as long as it bore iC3b. Both LDH release assays and single-cell microscopic analysis of macrophages infected with the cytosolic replication-deficient mutant SCHU S4 ΔpurMCD confirmed the prediction that a high bacterial burden resulting from extensive cytosolic replication is not required for macrophage death.
F. tularensis infections in macrophages proceed from bacterial uptake and escape from endocytic vesicles to a phase of significant cytosolic replication. Because the death of human macrophages infected with type A F. tularensis is delayed until after 15 h p.i. in vitro, it has been reasonable to assume that bacterial replication is required to signal macrophage death and bacterial release. The findings that minimally infected MDM frequently underwent cell death and that other MDM with high bacterial burdens remained viable at 24 h p.i. question this presumed connection between pathogen replication and cell death.
A number of published studies using receptor-blocking antibodies or small interfering RNA (siRNA) knockdown techniques have implicated complement receptor CR3 and its ligand iC3b in the uptake by human MDM of both F. tularensis subsp. holarctica and F. tularensis subsp. tularensis (26, 29, 31, 32, 35, 36). For this reason, we next asked whether signaling through complement receptors played an essential role in MDM death following infection with type A Francisella. Similar to the case for WT SCHU S4, the SCHU S4 ΔfevR phagosome escape-deficient mutant was taken up by MDM in a C3-dependent fashion. However, unlike the WT, the mutant failed to induce macrophage death, despite the presence of iC3b on its surface. This suggested that complement receptor engagement, although important for bacterial uptake, was not a sufficient signal for macrophage death induction.
We then asked whether the combination of complement receptor binding of C3-opsonized SCHU S4 and cytosolic F. tularensis constituted sufficient signals for the cell death response. MDM were first infected with a high MOI of WT SCHU S4 in the absence of C3 and then challenged with either C3-opsonized WT SCHU S4 or C3-opsonized SCHU S4 ΔfevR, both of which were capable of binding to complement receptors for uptake in a C3-dependent fashion. Secondary infection with C3-opsonized WT bacteria induced death as expected. Secondary infection with C3-opsonized SCHU S4 ΔfevR failed to induce cell death, despite WT levels of uptake that were C3 dependent. Reversing the order of these two infections did not alter the results (data not shown). Thus, the presence of large numbers of cytosolic SCHU S4 lacking surface iC3b plus engagement of cell surface complement receptors was not sufficient to induce macrophage death, even when the two signals were combined. The fact that FevR controls the expression of a number of genes in F. tularensis (43, 44) does not alter the fact that surface complement receptor engagement, even in the presence of cytosolic bacteria, did not signal for cell death. Only when MDM were infected with WT SCHU S4 bearing surface iC3b did macrophage death result.
Apoptosis is a form of programmed cell death which is noninflammatory and nonlytic, whereas pyroptosis is associated with the assembly of inflammasomes, the activation of inflammatory caspases (e.g., caspase-1), the release of the proinflammatory cytokines interleukin-1β (IL-1β) and IL-18, and cell lysis (45, 46). Francisella novicida has been reported to activate inflammasomes in both mouse and human cells (18–20, 47). Type A F. tularensis does not appear to activate inflammasomes (17, 18). Rather, virulent type A F. tularensis has been more closely associated with macrophage apoptosis, as evidenced by the expression of activated caspase-3 in tissues of infected mice (14).
CR3-mediated uptake of serum-opsonized SCHU S4 by human MDM has been reported to inhibit TLR2-induced NF-κB activation and nuclear translocation, as well as to suppress the release of proinflammatory cytokines, including IL-1β (32). Consistent with this report, we found that, unlike the proinflammatory F. novicida U112 strain (18, 19), neither C3-opsonized WT SCHU S4 nor C3-opsonized ΔpurMCD SCHU S4 stimulated significant IL-1β release (see Fig. S3 in the supplemental material). Thus, our findings extend earlier evidence (17, 18, 32) and suggest that C3-opsonized F. tularensis also fails to induce macrophage death by pyroptosis. It may indeed inhibit aspects of this proinflammatory form of cell death.
It is known that F. tularensis is very responsive to its environment (48). For example, growth of F. tularensis in different bacterial media can alter the virulence of the bacterium, both in vitro and in mice (49–52). Additionally, F. tularensis is capable of responding to increased concentrations of spermine (52), limiting concentrations of iron (53), and changes to mammalian temperatures (54), which is understood to reflect its adaptation to the host and intramacrophage environment. It is possible that C3 deposition, the binding of factor H (35), or C3b inactivation leads F. tularensis to alter its expression of important virulence properties.
It is also possible that C3 activation alters an essential aspect of the pathogen-host cell interaction. Clemens et al. (34) reported a different form of looping phagocytosis by macrophages infected with the F. tularensis live vaccine strain opsonized with C3 than was seen with nonopsonized bacteria. Geier and Celli (30) have reported that uptake of C3-opsonized SCHU S4 via CR3 in mouse bone marrow-derived macrophages delayed and partially decreased phagosomal escape by the bacterium compared to its intracellular trafficking in the absence of the receptor. Additional studies will be necessary to determine whether either of these effects contributes to the high level of cell death seen in infections with C3-opsonized type A F. tularensis.
The experiments with sequentially infected macrophages (Fig. 6) suggest that MDM death may require the entry of bacteria bearing C3 peptides into the cytosol. Tam et al. (55) reported that infection of a variety of nonimmune cells with a number of microbes capable of carrying C3 peptides into the cytosol led to the activation of signaling pathways that were not activated in the absence of C3 opsonization. The results of an array of experiments, including transfection of C3-coated beads, were consistent with the conclusion that cytosolic C3 activated intracellular signaling pathways associated with host defense. Although the authors postulated the existence of a cytosolic C3-sensing system similar to the TRIM21 system, which recognizes cytosolic IgG (56, 57), no such sensor has yet been described. These findings add to a growing list of potential intracellular functions associated with complement (55, 58) and may have relevance to the pathogenesis of a number of intracellular microbes capable of reaching the cytosol.
Although the mechanism by which C3 facilitates the death of human macrophages infected with type A Francisella remains unclear, the results presented here clearly demonstrate that complement component C3 can play a pivotal role in determining the fate of human macrophages during Francisella infections. As Francisella will likely encounter the complement system during in vivo infections, a better understanding of complement-Francisella interactions is needed. Like Francisella, other intracellular microbial pathogens are likely to activate the complement system prior to host cell entry and may utilize C3 to regulate important features of disease pathogenesis.
MATERIALS AND METHODS
Ethics statement.
Protocols for the collection and handling of the blood and identifying data from healthy human donors were approved by University of Kansas Medical Center Institutional Review Board. The University of Kansas Medical Center is an approved select agent entity (registration no. C20070606-0662) responsible for oversight of all the research with type A F. tularensis strains described here.
Reagents.
Histopaque-1077, gentamicin, protease inhibitor cocktail (P8340), and RPMI 1640 medium with l-glutamine were obtained from Sigma-Aldrich (St. Louis, MO). 10× Dulbecco's phosphate-buffered saline (DPBS) and a solution containing both penicillin (10,000 U/ml) and streptomycin (10,000 μg/ml) (17-602E) were obtained from Lonza (Walkersville, MD). Human fibronectin was purchased from Corning (Bedford, MA). Chocolate agar plates were obtained from Remel (Lenexa, KS). Rabbit antiserum to F. tularensis was purchased from Becton, Dickinson and Company (Sparks, MD). Alexa Fluor 488-conjugated donkey anti-rabbit IgG and the Zombie Red fixable viability kit were purchased from BioLegend (San Diego, CA). Horseradish peroxidase (HRP)-conjugated rabbit anti-goat IgG (ZyMax 81-1620) and ProLong Gold Antifade with DAPI (4′,6′-diamidino-2-phenylindole) were obtained from Life Technologies (Eugene, OR). HyGLO Quick Spray chemiluminescent HRP substrate was from Denville Scientific (Holliston, MA). Pooled human AB serum was from Atlanta Biologicals (Flowery Branch, GA). Commercial human serum (HS) with intact complement activity and C3-depleted HS were purchased from either Quidel (San Diego, CA) or Complement Technology, Inc. (Tyler, TX). C3-depleted serum was verified to be devoid of C3 via Western blotting. C5-depleted HS and goat polyclonal antiserum to human C3 (A304) were purchased from Quidel. Purified C3 protein (A113c) and purified iC3b protein (A115) were purchased from Complement Technology, Inc. To prepare C3-replenished HS, purified C3 protein was added to C3-depleted HS to obtain a final concentration of 1 mg/ml C3.
To prepare autologous HS, whole blood was allowed to clot for 30 min at 37°C and then centrifuged at 530 × g for 10 min at 4°C. The serum fraction was immediately collected, centrifuged again, and stored as aliquots at −80°C. When required, heat-inactivated HS (HI-HS) was prepared immediately prior to use by incubation at 56°C for 30 min.
Bacterial strains.
Wild-type (WT) F. tularensis type A strain SCHU S4 was provided by Kevin King (Midwest Research Institute, Kansas City, MO), prepared under National Institute of Allergy and Infectious Diseases, National Institutes of Health, contract SHHSM266200400002C. SCHU S4 mutant strains used in these experiments were kindly provided by Catharine Bosio (Hamilton, MT) with permission of Jean Celli (Pullman, WA). Mutant strains SCHU S4 ΔfevR (Francisella effector of virulence regulation, ΔFTT0383) (44), the complemented SCHU S4 ΔfevR/pfevR (Δ0383pFNLTP60383) (44), and SCHU S4 ΔpurMCD (37) have been described previously. Francisella novicida strain U112 was kindly provided by Lee-Ann Allen (Iowa City, IA).
Stocks of bacterial strains were prepared by growth in supplemented Mueller-Hinton broth and stored as aliquots at −80°C as previously described (59). Complemented SCHU S4 ΔfevR/pfevR was grown in the presence of 10 μg/ml kanamycin, and SCHU S4 ΔpurMCD was grown in the presence of 2.5% fetal bovine serum and 1.0% Proteose Peptone. At 2 to 3 days prior to infection, bacterial stocks were rapidly thawed and streaked for isolation on chocolate agar. Inoculated chocolate agar plates were incubated at 37°C with 5% CO2 for 2 to 3 days. For growth of complemented SCHU S4 ΔfevR/pfevR, chocolate agar plates were supplemented with 25 μg of kanamycin. On the day of infection, 1 to 6 colonies were suspended in RPMI, and the indicated serum was added as a source of opsonins at a concentration of 7.5% (vol/vol), unless otherwise noted. No difference in bacterial viability was noted for WT or mutant strains following opsonization with HS, HI-HS, or C3-depleted HS.
MDM.
For the preparation of monocyte-derived macrophages (MDM), peripheral blood mononuclear cells were purified as described by Clemens et al. (10) from venous blood of heathy human donors without a history of tularemia. Diluted, heparinized blood was centrifuged (530 × g for 26 min at 25°C) over Histopaque-1077. Cells at the plasma-Histopaque interface were collected, washed, and incubated in Teflon beakers (Savillex, Eden Prairie, MN) at 37°C in a humid atmosphere with 5% CO2 for 5 to 7 days in RPMI medium containing 20% pooled human AB serum, 100 units/ml penicillin, and 100 μg/ml streptomycin. Supernatant containing nonadherent cells was then removed, and adherent MDM were eluted in RPMI containing 10 mM HEPES following incubation of the beakers on ice for 30 min. MDM were >93% positive for CD14 and >93% positive for CD11b by flow cytometry. Fifty thousand MDM were cultured per well in 96-well plates and incubated overnight to allow for adherence. Alternatively, 2 × 105 MDM were plated in 24-well plates with or without fibronectin-coated coverslips. Nonadherent cells were removed the next day prior to infection.
Infection.
Two- to 3-day colonies of individual SCHU S4 strains were suspended in RPMI, and serum was added to a concentration of 7.5% (unless otherwise noted). Bacteria were opsonized for 30 to 60 min at 37°C and then used to infect MDM in medium containing like serum. Following 2 h of infection at 37°C, the medium was removed and replaced with like medium containing 50 μg/ml gentamicin for 1 h to kill residual extracellular bacteria. All cultures were then washed 3 times in RPMI with 10 mM HEPES and placed in medium containing 7.5% autologous HI-HS to minimize reinfection of the monolayer and restrict differences in opsonization to the uptake phase.
The multiplicity of infection (MOI) was determined following opsonization by dilution plating on chocolate agar. Macrophage bacterial burdens were determined by lysing MDM in 0.01% sodium dodecyl sulfate (SDS) in sterile water for 1 min followed by dilution in DPBS and plating on chocolate agar.
Measurement of macrophage death.
The CytoTox 96 nonradioactive cytotoxicity assay (Promega, Madison, WI) was used to determine the percent lactate dehydrogenase (LDH) release. Culture supernatants were collected at the indicated times postinfection (p.i.) and stored at 4°C until assayed. LDH release was calculated as a proportion of total LDH released upon lysis of uninfected cell monolayers with detergent supplied in the assay kit. Each supernatant was assayed in triplicate.
Single-cell analysis by confocal microscopy.
MDM on fibronectin-coated coverslips were infected as described above. For identification of dying macrophages at 24 h p.i., coverslips were stained with the Zombie Red fixable viability amine-reactive dye according to the manufacturer's instructions. Zombie Red was used at a concentration of 1:100 in DPBS for 20 min at room temperature, protected from light. The coverslips were then washed twice with kit wash buffer and once with DPBS. Coverslips were treated with 4% paraformaldehyde in DPBS for 1 h and then stored at 4°C in 70% ethanol. After verifying sterility, coverslips were washed in DPBS and incubated at room temperature for 1 h with a rabbit antiserum to Francisella. Following DPBS washes, coverslips were stained with Alexa Fluor 488-conjugated donkey anti-rabbit IgG in DPBS. Coverslips were mounted in ProLong Gold with DAPI and imaged using the ACS APO 40×/1.15 numerical aperture oil objective of a Leica TCS SPE confocal microscope. Images are presented as maximum projections of 1-μm Z-series stacks spanning the entire monolayer.
For at least 200 individual macrophages per sample, the presence or absence of Zombie Red staining and the number of bacteria within each cell were recorded. Only those MDM with diffuse cytosolic Zombie Red staining were scored as positive.
C3 deposition.
Deposition of C3 peptides onto the surface of SCHU S4 strains was monitored by Western blotting following a slight modification of the procedure described by Clay et al. (36). Several 2- to 3-day colonies of bacteria were suspended in RPMI medium containing 7.5% serum in 1.5-ml polypropylene microcentrifuge tubes (Fisher Scientific, Pittsburgh, PA) that had been preblocked with 1% bovine serum albumin (BSA). Bacterial inputs were enumerated by serial dilution plating to ensure equal loading for gel electrophoresis. Following 1 h of opsonization at 37°C, bacterial suspensions were centrifuged at 12,000 × g for 5 min at 4°C. The bacteria were washed twice and resuspended in DPBS containing a protease inhibitor cocktail. Following heat treatment (97°C for 5 min) in Laemmli buffer, samples were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) on 7.5% acrylamide gels (Bio-Rad, Hercules, CA) and transferred to Immobilon-P polyvinylidene fluoride membranes (Millipore, Billerica, MA). Membranes were blocked with 3% BSA in Tris-buffered saline containing 0.05% Tween 20 (TBS-T), which was also used for antibody dilutions. Membranes were then incubated with goat polyclonal antiserum to human C3 for 2 h at room temperature and washed 5 times in TBS-T. The membranes were then incubated in HRP-conjugated rabbit anti-goat antiserum for 1 h at room temperature and then washed 5 times in TBS-T. Chemiluminescent HRP substrate was used for detection.
Statistical analysis.
Error bars in graphed data represent standard deviations of triplicate measurements. Data were analyzed using a Student's t test when comparing 2 groups or an analysis of variance (ANOVA) followed by Tukey's post hoc test when comparing 3 or more groups. A P value of <0.001 was selected for comparisons within a single representative experiment and a P value of <0.01 for comparisons of means of three independent experiments. Significant differences are indicated with an asterisk. In some cases, differences between two indicated groups are identified as not significant (ns).
Supplementary Material
ACKNOWLEDGMENTS
We thank Catharine Bosio and Jean Celli for providing the mutant SCHU S4 strains used in this study. We also thank Lee-Ann Allen for providing Francisella novicida strain U112. We also acknowledge the contributions of Alexandra Machen in characterizing the expression of CD14 and CD11b by MDM populations.
This work was supported by NIAID grant AI093921 from the National Institutes of Health and by a Lied Basic Science Grant from the University of Kansas Medical Center Research Institute to M.J.P. S.R.B. was supported by a Biomedical Research Training Program grant from the University of Kansas Medical Center.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/IAI.00424-17.
REFERENCES
- 1.Stuart BM, Pullen RL. 1945. Tularemic pneumonia: review of American literature and report of 15 additional cases. Am J Med Sci 210:223–236. doi: 10.1097/00000441-194508000-00013. [DOI] [Google Scholar]
- 2.McCrumb FR. 1961. Aerosol infection of man with pasteurella tularensis. Bacteriol Rev 25:262–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dennis DT, Inglesby TV, Henderson DA, Bartlett JG, Ascher MS, Eitzen E, Fine AD, Friedlander AM, Hauer J, Layton M, Lillibridge SR, McDade JE, Osterholm MT, O'Toole T, Parker G, Perl TM, Russell PK, Tonat K, Working Group on Civilian Biodefense. 2001. Tularemia as a biological weapon: medical and public health management. JAMA 285:2763–2773. doi: 10.1001/jama.285.21.2763. [DOI] [PubMed] [Google Scholar]
- 4.Feldman KA, Enscore RE, Lathrop SL, Matyas BT, McGuill M, Schriefer ME, Stiles-Enos D, Dennis DT, Petersen LR, Hayes EB. 2001. An outbreak of primary pneumonic tularemia on Martha's Vineyard. N Engl J Med 345:1601–1606. doi: 10.1056/NEJMoa011374. [DOI] [PubMed] [Google Scholar]
- 5.Saslaw S, Eigelsbach HT, Prior JA, Wilson HE, Carhart S. 1961. Tularemia vaccine study. II. Respiratory challenge. Arch Intern Med 107:702–714. [DOI] [PubMed] [Google Scholar]
- 6.Hall JD, Woolard MD, Gunn BM, Craven RR, Taft-Benz S, Frelinger JA, Kawula TH. 2008. Infected-host-cell repertoire and cellular response in the lung following inhalation of Francisella tularensis Schu S4, LVS, or U112. Infect Immun 76:5843–5852. doi: 10.1128/IAI.01176-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roberts LM, Tuladhar S, Steele SP, Riebe KJ, Chen CJ, Cumming RI, Seay S, Frothingham R, Sempowski GD, Kawula TH, Frelinger JA. 2014. Identification of early interactions between Francisella and the host. Infect Immun 82:2504–2510. doi: 10.1128/IAI.01654-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steiner DJ, Furuya Y, Jordan MB, Metzger DW. 3 April 2017. Protective role for macrophages in respiratory Francisella tularensis infection. Infect Immun doi: 10.1128/IAI.00064-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Golovliov I, Baranov V, Krocova Z, Kovarova H, Sjostedt A. 2003. An attenuated strain of the facultative intracellular bacterium Francisella tularensis can escape the phagosome of monocytic cells. Infect Immun 71:5940–5950. doi: 10.1128/IAI.71.10.5940-5950.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clemens DL, Lee BY, Horwitz MA. 2004. Virulent and avirulent strains of Francisella tularensis prevent acidification and maturation of their phagosomes and escape into the cytoplasm in human macrophages. Infect Immun 72:3204–3217. doi: 10.1128/IAI.72.6.3204-3217.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chong A, Wehrly TD, Nair V, Fischer ER, Barker JR, Klose KE, Celli J. 2008. The early phagosomal stage of Francisella tularensis determines optimal phagosomal escape and Francisella pathogenicity island protein expression. Infect Immun 76:5488–5499. doi: 10.1128/IAI.00682-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Conlan JW, KuoLee R, Shen H, Webb A. 2002. Different host defences are required to protect mice from primary systemic vs pulmonary infection with the facultative intracellular bacterial pathogen, Francisella tularensis LVS. Microb Pathog 32:127–134. doi: 10.1006/mpat.2001.0489. [DOI] [PubMed] [Google Scholar]
- 13.Conlan JW, Chen W, Shen H, Webb A, KuoLee R. 2003. Experimental tularemia in mice challenged by aerosol or intradermally with virulent strains of Francisella tularensis: bacteriologic and histopathologic studies. Microb Pathog 34:239–248. doi: 10.1016/S0882-4010(03)00046-9. [DOI] [PubMed] [Google Scholar]
- 14.Wickstrum JR, Bokhari SM, Fischer JL, Pinson DM, Yeh HW, Horvat RT, Parmely MJ. 2009. Francisella tularensis induces extensive caspase-3 activation and apoptotic cell death in the tissues of infected mice. Infect Immun 77:4827–4836. doi: 10.1128/IAI.00246-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parmely MJ, Fischer JL, Pinson DM. 2009. Programmed cell death and the pathogenesis of tissue injury induced by type A Francisella tularensis. FEMS Microbiol Lett 301:1–11. doi: 10.1111/j.1574-6968.2009.01791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rasmussen JW, Cello J, Gil H, Forestal CA, Furie MB, Thanassi DG, Benach JL. 2006. Mac-1+ cells are the predominant subset in the early hepatic lesions of mice infected with Francisella tularensis. Infect Immun 74:6590–6598. doi: 10.1128/IAI.00868-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dotson RJ, Rabadi SM, Westcott EL, Bradley S, Catlett SV, Banik S, Harton JA, Bakshi CS, Malik M. 2013. Repression of inflammasome by Francisella tularensis during early stages of infection. J Biol Chem 288:23844–23857. doi: 10.1074/jbc.M113.490086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghonime MG, Mitra S, Eldomany RA, Wewers MD, Gavrilin MA. 2015. Inflammasome priming is similar for Francisella species that differentially induce inflammasome activation. PLoS One 10:e0127278. doi: 10.1371/journal.pone.0127278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mariathasan S, Weiss DS, Dixit VM, Monack DM. 2005. Innate immunity against Francisella tularensis is dependent on the ASC/caspase-1 axis. J Exp Med 202:1043–1049. doi: 10.1084/jem.20050977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Henry T, Brotcke A, Weiss DS, Thompson LJ, Monack DM. 2007. Type I interferon signaling is required for activation of the inflammasome during Francisella infection. J Exp Med 204:987–994. doi: 10.1084/jem.20062665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kingry LC, Petersen JM. 2014. Comparative review of Francisella tularensis and Francisella novicida. Front Cell Infect Microbiol 4:35. doi: 10.3389/fcimb.2014.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Santic M, Pavokovic G, Jones S, Asare R, Kwaik YA. 2010. Regulation of apoptosis and anti-apoptosis signalling by Francisella tularensis. Microbes Infect 12:126–134. doi: 10.1016/j.micinf.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pierini R, Juruj C, Perret M, Jones CL, Mangeot P, Weiss DS, Henry T. 2012. AIM2/ASC triggers caspase-8-dependent apoptosis in Francisella-infected caspase-1-deficient macrophages. Cell Death Differ 19:1709–1721. doi: 10.1038/cdd.2012.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Doyle CR, Pan JA, Mena P, Zong WX, Thanassi DG. 2014. TolC-dependent modulation of host cell death by the Francisella tularensis live vaccine strain. Infect Immun 82:2068–2078. doi: 10.1128/IAI.00044-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lai XH, Sjostedt A. 2003. Delineation of the molecular mechanisms of Francisella tularensis-induced apoptosis in murine macrophages. Infect Immun 71:4642–4646. doi: 10.1128/IAI.71.8.4642-4646.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clemens DL, Lee BY, Horwitz MA. 2005. Francisella tularensis enters macrophages via a novel process involving pseudopod loops. Infect Immun 73:5892–5902. doi: 10.1128/IAI.73.9.5892-5902.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Balagopal A, MacFarlane AS, Mohapatra N, Soni S, Gunn JS, Schlesinger LS. 2006. Characterization of the receptor-ligand pathways important for entry and survival of Francisella tularensis in human macrophages. Infect Immun 74:5114–5125. doi: 10.1128/IAI.00795-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pierini LM. 2006. Uptake of serum-opsonized Francisella tularensis by macrophages can be mediated by class A scavenger receptors. Cell Microbiol 8:1361–1370. doi: 10.1111/j.1462-5822.2006.00719.x. [DOI] [PubMed] [Google Scholar]
- 29.Schulert GS, Allen LA. 2006. Differential infection of mononuclear phagocytes by Francisella tularensis: role of the macrophage mannose receptor. J Leukoc Biol 80:563–571. doi: 10.1189/jlb.0306219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Geier H, Celli J. 2011. Phagocytic receptors dictate phagosomal escape and intracellular proliferation of Francisella tularensis. Infect Immun 79:2204–2214. doi: 10.1128/IAI.01382-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schwartz JT, Barker JH, Long ME, Kaufman J, McCracken J, Allen LA. 2012. Natural IgM mediates complement-dependent uptake of Francisella tularensis by human neutrophils via complement receptors 1 and 3 in nonimmune serum. J Immunol 189:3064–3077. doi: 10.4049/jimmunol.1200816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dai S, Rajaram MV, Curry HM, Leander R, Schlesinger LS. 2013. Fine tuning inflammation at the front door: macrophage complement receptor 3-mediates phagocytosis and immune suppression for Francisella tularensis. PLoS Pathog 9:e1003114. doi: 10.1371/journal.ppat.1003114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barel M, Hovanessian AG, Meibom K, Briand JP, Dupuis M, Charbit A. 2008. A novel receptor-ligand pathway for entry of Francisella tularensis in monocyte-like THP-1 cells: interaction between surface nucleolin and bacterial elongation factor Tu. BMC Microbiol 8:145. doi: 10.1186/1471-2180-8-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clemens DL, Lee BY, Horwitz MA. 2012. O-antigen-deficient Francisella tularensis live vaccine strain mutants are ingested via an aberrant form of looping phagocytosis and show altered kinetics of intracellular trafficking in human macrophages. Infect Immun 80:952–967. doi: 10.1128/IAI.05221-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ben Nasr A, Klimpel GR. 2008. Subversion of complement activation at the bacterial surface promotes serum resistance and opsonophagocytosis of Francisella tularensis. J Leukoc Biol 84:77–85. doi: 10.1189/jlb.0807526. [DOI] [PubMed] [Google Scholar]
- 36.Clay CD, Soni S, Gunn JS, Schlesinger LS. 2008. Evasion of complement-mediated lysis and complement C3 deposition are regulated by Francisella tularensis lipopolysaccharide O antigen. J Immunol 181:5568–5578. doi: 10.4049/jimmunol.181.8.5568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chong A, Wehrly TD, Child R, Hansen B, Hwang S, Virgin HW, Celli J. 2012. Cytosolic clearance of replication-deficient mutants reveals Francisella tularensis interactions with the autophagic pathway. Autophagy 8:1342–1356. doi: 10.4161/auto.20808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pechous RD, McCarthy TR, Mohapatra NP, Soni S, Penoske RM, Salzman NH, Frank DW, Gunn JS, Zahrt TC. 2008. A Francisella tularensis Schu S4 purine auxotroph is highly attenuated in mice but offers limited protection against homologous intranasal challenge. PLoS One 3:e2487. doi: 10.1371/journal.pone.0002487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Long ME, Lindemann SR, Rasmussen JA, Jones BD, Allen LA. 2013. Disruption of Francisella tularensis Schu S4 iglI, iglJ, and pdpC genes results in attenuation for growth in human macrophages and in vivo virulence in mice and reveals a unique phenotype for pdpC. Infect Immun 81:850–861. doi: 10.1128/IAI.00822-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ben Nasr A, Haithcoat J, Masterson JE, Gunn JS, Eaves-Pyles T, Klimpel GR. 2006. Critical role for serum opsonins and complement receptors CR3 (CD11b/CD18) and CR4 (CD11c/CD18) in phagocytosis of Francisella tularensis by human dendritic cells (DC): uptake of Francisella leads to activation of immature DC and intracellular survival of the bacteria. J Leukoc Biol 80:774–786. doi: 10.1189/jlb.1205755. [DOI] [PubMed] [Google Scholar]
- 41.Lai XH, Golovliov I, Sjostedt A. 2001. Francisella tularensis induces cytopathogenicity and apoptosis in murine macrophages via a mechanism that requires intracellular bacterial multiplication. Infect Immun 69:4691–4694. doi: 10.1128/IAI.69.7.4691-4694.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Linscott WD, Triglia RP. 1981. The bovine complement system. Adv Exp Med Biol 137:413–430. [PubMed] [Google Scholar]
- 43.Brotcke A, Monack DM. 2008. Identification of fevR, a novel regulator of virulence gene expression in Francisella novicida. Infect Immun 76:3473–3480. doi: 10.1128/IAI.00430-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wehrly TD, Chong A, Virtaneva K, Sturdevant DE, Child R, Edwards JA, Brouwer D, Nair V, Fischer ER, Wicke L, Curda AJ, Kupko JJ 3rd, Martens C, Crane DD, Bosio CM, Porcella SF, Celli J. 2009. Intracellular biology and virulence determinants of Francisella tularensis revealed by transcriptional profiling inside macrophages. Cell Microbiol 11:1128–1150. doi: 10.1111/j.1462-5822.2009.01316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wallach D, Kang TB, Dillon CP, Green DR. 2016. Programmed necrosis in inflammation: toward identification of the effector molecules. Science 352:aaf2154. doi: 10.1126/science.aaf2154. [DOI] [PubMed] [Google Scholar]
- 46.Jorgensen I, Rayamajhi M, Miao EA. 2017. Programmed cell death as a defence against infection. Nat Rev Immunol 17:151–164. doi: 10.1038/nri.2016.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peng K, Broz P, Jones J, Joubert LM, Monack D. 2011. Elevated AIM2-mediated pyroptosis triggered by hypercytotoxic Francisella mutant strains is attributed to increased intracellular bacteriolysis. Cell Microbiol 13:1586–1600. doi: 10.1111/j.1462-5822.2011.01643.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hazlett KR, Cirillo KA. 2009. Environmental adaptation of Francisella tularensis. Microbes Infect 11:828–834. doi: 10.1016/j.micinf.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zarrella TM, Singh A, Bitsaktsis C, Rahman T, Sahay B, Feustel PJ, Gosselin EJ, Sellati TJ, Hazlett KR. 2011. Host-adaptation of Francisella tularensis alters the bacterium's surface-carbohydrates to hinder effectors of innate and adaptive immunity. PLoS One 6:te22335. doi: 10.1371/journal.pone.0022335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Carlson PE Jr, Carroll JA, O'Dee DM, Nau GJ. 2007. Modulation of virulence factors in Francisella tularensis determines human macrophage responses. Microb Pathog 42:204–214. doi: 10.1016/j.micpath.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hazlett KR, Caldon SD, McArthur DG, Cirillo KA, Kirimanjeswara GS, Magguilli ML, Malik M, Shah A, Broderick S, Golovliov I, Metzger DW, Rajan K, Sellati TJ, Loegering DJ. 2008. Adaptation of Francisella tularensis to the mammalian environment is governed by cues which can be mimicked in vitro. Infect Immun 76:4479–4488. doi: 10.1128/IAI.00610-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Carlson PE Jr, Horzempa J, O'Dee DM, Robinson CM, Neophytou P, Labrinidis A, Nau GJ. 2009. Global transcriptional response to spermine, a component of the intramacrophage environment, reveals regulation of Francisella gene expression through insertion sequence elements. J Bacteriol 191:6855–6864. doi: 10.1128/JB.00995-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Deng K, Blick RJ, Liu W, Hansen EJ. 2006. Identification of Francisella tularensis genes affected by iron limitation. Infect Immun 74:4224–4236. doi: 10.1128/IAI.01975-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Horzempa J, Carlson PE Jr, O'Dee DM, Shanks RM, Nau GJ. 2008. Global transcriptional response to mammalian temperature provides new insight into Francisella tularensis pathogenesis. BMC Microbiol 8:172. doi: 10.1186/1471-2180-8-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tam JC, Bidgood SR, McEwan WA, James LC. 2014. Intracellular sensing of complement C3 activates cell autonomous immunity. Science 345:1256070. doi: 10.1126/science.1256070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mallery DL, McEwan WA, Bidgood SR, Towers GJ, Johnson CM, James LC. 2010. Antibodies mediate intracellular immunity through tripartite motif-containing 21 (TRIM21). Proc Natl Acad Sci U S A 107:19985–19990. doi: 10.1073/pnas.1014074107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McEwan WA, Tam JC, Watkinson RE, Bidgood SR, Mallery DL, James LC. 2013. Intracellular antibody-bound pathogens stimulate immune signaling via the Fc receptor TRIM21. Nat Immunol 14:327–336. doi: 10.1038/ni.2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liszewski MK, Kolev M, Le Friec G, Leung M, Bertram PG, Fara AF, Subias M, Pickering MC, Drouet C, Meri S, Arstila TP, Pekkarinen PT, Ma M, Cope A, Reinheckel T, Rodriguez de Cordoba S, Afzali B, Atkinson JP, Kemper C. 2013. Intracellular complement activation sustains T cell homeostasis and mediates effector differentiation. Immunity 39:1143–1157. doi: 10.1016/j.immuni.2013.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wickstrum JR, Hong KJ, Bokhari S, Reed N, McWilliams N, Horvat RT, Parmely MJ. 2007. Coactivating signals for the hepatic lymphocyte gamma interferon response to Francisella tularensis. Infect Immun 75:1335–1342. doi: 10.1128/IAI.01203-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.