Abstract
Corneal immuno- and neuro-imaging approaches facilitate in vivo analyses of the cornea, including high-resolution imaging of corneal immune cells and nerves. This approach facilitates the analyses of underlying immune and nerve alterations not detected by clinical slit-lamp examination alone. In this review, we describe recent work performed in our translational ocular immunology center with a focus on ‘bench-to-bedside’ and ‘bedside-to-bench’ research. The ability to visualize dendritiform immune cells (DCs) in patients with laser in vivo confocal microscopy (IVCM), recently discovered in the central murine cornea, has allowed us to demonstrated their utility as a potential surrogate biomarker for inflammatory ocular surface diseases. This biomarker for inflammation allows the measurement of therapeutic efficacy of anti-inflammatory drugs and its utility as an endpoint in clinical trials with high inter-observer agreement. IVCM image analyses from our studies demonstrated a significant increase in DC density and size in ocular disease, a positive correlation between DC density and clinical signs and symptoms of disease and pro-inflammatory tear cytokines, and a strong negative correlation between DC density and subbasal nerve density. In conjunction with pre-clinical research investigating the inflammatory state in a partial or fully denervated cornea, our results indicated that corneal nerves are directly involved in the regulation of homeostasis and immune privilege in the cornea.
Keywords: In vivo confocal microscopy, immune privilege, corneal nerves, dendritic cells, neuro-immune crosstalk
INTRODUCTION
The cornea is the window to the foreign world and is an immune-privileged tissue. Corneal immune privilege is dependent, in part, on the absence or low expression of major histocompatibility complex (MHC) class I and II antigens, lack of blood vessels that limit access of the immune system to the cornea, lack of lymphatic vessels that prevent the egress and delivery of antigens and antigen-presenting cells (APC) to T cells in the draining lymph nodes (dLNs), the presence of anti-inflammatory molecules such as transforming growth factor-β, and the presence of Fas ligand, resulting in the apoptosis of infiltrating T cells.1–3 Historically, the cornea, with the exception of a population of intraepithelial MHC-II+ dendritic cells in the peripheral/limbal region, was thought to be a tissue devoid of bone marrow (BM)-derived cells.2, 4 This dogma was challenged at the turn of the century, following the identification of resident corneal APCs distributed throughout the central cornea during steady state.5–10
CORNEAL DENDRITIC CELLS IN CORNEAL IMMUNITY
Professional APCs in the naïve cornea include CD45+CD11c+ epithelial and stromal conventional dendritic cells (cDCs). During homeostasis, corneal cDCs are immature in the central cornea (MHC-II-, CD80-, and/or CD86-negative) and both immature and mature (MHC class II-, CD80-, and/or CD86-positive) in the peripheral cornea. In contrast, the density and maturation of cDCs increase throughout the cornea during inflammation.5–9
The important role of inflammation, corneal APCs, and cDCs in the pathogenesis of ocular surface diseases, including dry eye disease (DED), has been firmly established. It has been demonstrated that desiccation results in an inflammatory microenvironment and maturation of cDCs during the induction of DED, facilitating their migration to dLNs, subsequently activating T cells towards T helper (Th)1 and Th17 (autoreactive) subtypes.11–16 Similarly, an inflammatory response to noxious pathogens leads to inflammation, nerve damage, and other complications associated with infectious keratitis.17 Examples of clinically relevant ocular infections include herpes simplex virus (HSV) type 1, as well as Acanthamoeba and Pseudomonas aeruginosa. However, the use of clinical slit-lamp biomicroscopy alone cannot detect the underlying immune and nervous changes during the various severity grades of DED and infectious keratitis.
CLINICAL IMMUNO-IMAGING WITH IN VIVO CONFOCAL MICROSCOPY
The recent advent of laser in vivo confocal microscopy (IVCM), a non-invasive high-resolution real-time imaging device, allows layer-by-layer analysis of the corneal ultrastructure in both ocular health and disease, with images comparable to that of ex vivo histochemical techniques.18–20 From the two corneal confocal microscopes currently in clinical use, the Heidelberg Retinal Tomograph 3 with the Rostock Corneal Module (HRT3/RCM; Heidelberg Engineering GmBH, Heidelberg, Germany) laser IVCM allows for the visualization and differentiation of hyper-reflective dendritiform immune cells (DCs)21 and improved visualization of subbasal nerves22 as compared to the Confoscan 4 (NIDEK Co., Ltd., Aichi, Japan) (Fig. 1).18, 19, 23–26
Figure 1. Comparison of laser in vivo confocal microscopy (IVCM) with scanning light confocal microscopy.
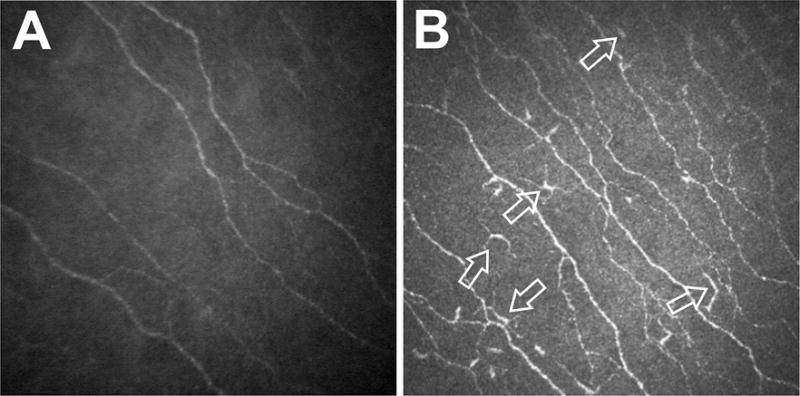
Representative standard IVCM images acquired from the central cornea of normal patients with a Confoscan 4 (A) and a Heidelberg Retinal Tomograph 3 with the Rostock Corneal Module (HRT3/RCM) (B) demonstrating the visualization of hyperreflective dendritiform immune cells (arrows in panel B) and improved visualization of the subbasal corneal nerve plexus by the HRT3/RCM.
Thus, with the availability of laser IVCM, alterations of the corneal immune system can now be assessed and quantified in the clinic, allowing for the assessment of inflammation, tailored therapeutic decision making, and the monitoring of disease progression and responses to therapy in corneal diseases, such as DED and infectious keratitis.20 In addition, although the cornea is the most densely innervated tissue of the body, in vivo assessments in patients have remained problematic. However, laser IVCM now facilitates in vivo assessment and quantification of corneal nerves (Fig. 2).18 Our initial findings in a prospective controlled study analyzing IVCM images from bacterial, fungal, and Acanthamoeba keratitis patients revealed a significantly higher epithelial DC density compared with normal control subjects.22 Interestingly, this increase in DC density was inversely correlated with corneal nerve density in these patients. More recent comparisons of standard 400 × 400 μm single images with wide-field composite images revealed no significant differences in the mean subbasal nerve and DC densities between the average values of three representative standard IVCM images and wide-field mapped composite images.27 Therefore, standard IVCM images in clinical studies are appropriate for the accurate measurements of cellular changes in the subbasal layer with a high inter-observer agreement in quantification (R = 0.997, P < 0.001).27 To demonstrate whether cellular DC changes are a sign of inflammation, we compared changes in DC density with pro-inflammatory tear cytokines in patients with bacterial keratitis. Alterations in DC density in patients with unilateral bacterial keratitis correlated positively with a bilateral increase in pro-inflammatory tear cytokines, including interleukin (IL)-1β and IL-6, in addition to ipsilateral changes in IL-8 and IL-17a.28
Figure 2. Laser in vivo confocal microscopy (IVCM) for the assessment of corneal nerves and dendritic cells.
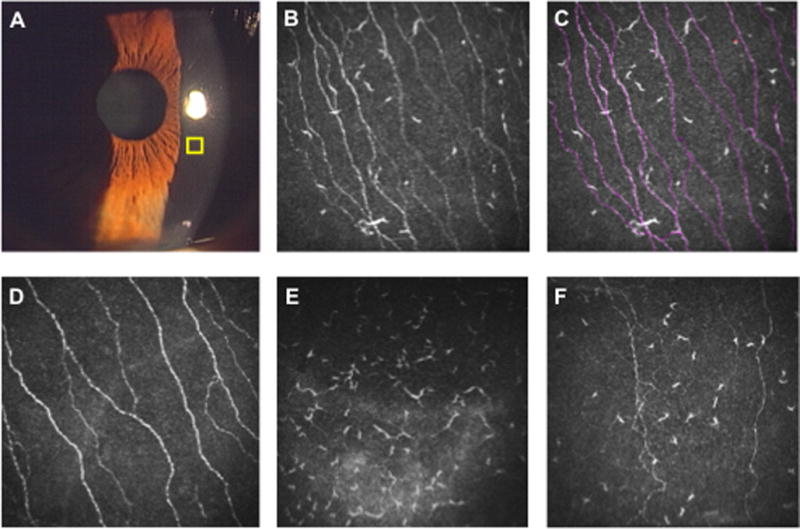
Slit lamp microscopy is the gold standard for anterior segment examination to assess corneal infiltration, edema, corneal epithelial defects, and cells/flare in the anterior chamber (A). However, slit-lamp examination does not allow assessment of corneal nerves or dendritiform immune cells (DCs). Using laser IVCM, high-resolution images of the corneal cellular structures can be obtained in an area of 400 × 400 μm (yellow square). (B) IVCM image of a cornea with allergic conjunctivitis. (C) Using ImageJ/Neuron J, corneal nerve/DC density can be quantified. Corneal nerves are traced semi-automatically with Neuron J software (red lines). (D) IVCM image of a normal cornea. (E) IVCM image of an eye with microbial keratitis. Note the increased density of DCs, while the corneal nerve branches could not be detected. (F) IVCM image of the contralateral eye of a patient with unilateral microbial keratitis. Nerve density is decreased and DC density is increased compared with normal cornea.
To assess the degree of immune activation and inflammation in patients with DED, we initially investigated differential changes in DC density and morphological parameters in patients with subtypes of DED.29 Our results demonstrated that DC density, but not DC morphology, was significantly higher in aqueous-deficient subtype compared with the evaporative subtype. Furthermore, aqueous-deficient DED with underlying systemic immune diseases had significantly higher DC density and morphological alterations compared to non-immunologic diseases, suggesting that DC changes reflect the degree of immune activation and inflammation, and may thus be used for clinical practice and clinical trials.29
The subsequent assessment of clinical DC density by IVCM in a recent phase IV randomized trial in patients with evaporative DED demonstrated a significant correlation between corneal DC density and clinical signs of corneal fluorescein staining and symptoms as measured by the ocular surface disease index (OSDI), highlighting the utility of DC density as a potential surrogate biomarker for ocular surface inflammation.30 This clinical trial also demonstrated that alterations in DC density and morphology as endpoint measurements by IVCM could be used successfully to determine the anti-inflammatory therapeutic efficacy of topical loteprednol compared with artificial tears.31 Thus, in addition to its utility as an endpoint in clinical trials, image-guided evaluation allows for personalized treatment based on cellular alterations specific to each patient. How resident immune cells are regulated in the homeostatic cornea, and how this regulation is lost in keratitis, remains an area of investigation.
CLINICAL NEURO-IMAGING WITH IN VIVO CONFOCAL MICROSCOPY
Corneal nerve dysfunction is the pathophysiological basis of many ocular surface diseases arising from DED,26, 32 surgery,33 diabetic neuropathy,34–37 contact lens wear, corneal neuralgia,38 corneal neuropathy-induced photoallodynia,39 and small fiber neuropathy.40 Of particular interest has been the correlation of subbasal corneal nerves to their function, given their pivotal role in the regulation of corneal sensation, maintenance of epithelial integrity, as well as proliferation and the promotion of wound healing. Our analyses of corneal IVCM images in patients with infectious keratitis revealed a significant decrease in nerve density, number of nerve branches, number of main nerve trunks, and total number of nerves in keratitis patients infected with bacteria, fungi, and Acanthamoeba.22 Moreover, we recently reported that while the corneal subbasal nerve plexus regenerates during the first 6 months following the resolution of infectious keratitis and cessation of therapy, it does not fully recover to baseline levels.41 Notably, the decrease in nerve density in patients with infectious keratitis resulted in an inverse correlation with DC density.22 Furthermore, in separate studies, we reported that the contralateral unaffected eyes of patients with unilateral infectious keratitis, including herpes simplex, herpes zoster, Acanthamoeba, fungal and bacterial keratitis, showed a bilateral decrease in the subbasal nerve plexus, compared with controls.19, 42, 43 Finally, DC density in the contralateral unaffected eyes was significantly increased compared with controls, albeit not as dramatic as in affected eyes with acute infectious keratitis. The strong correlation between the clinical density of subbasal nerves and DCs suggests a potential direct interaction between the immune and nervous systems in the cornea.
EXPERIMENTAL EVIDENCE OF CORNEAL NEURO-IMMUNE CROSSTALK
The neural control of ocular immune privilege was initially proposed by Streilein et al.44 Neuropeptides, such as α-melanocyte-stimulating hormone and vasoactive intestinal peptide (VIP) are typically released from nerve termini. In addition to their role as neurotransmitters, these peptides are immunomodulatory and are present in the cornea.44 Neuropeptides suppress pro-inflammatory cytokine production by leukocytes and other resident cells,44–48 and participate in maintaining inflammation at a minimum in the cornea.44 Corneal nerve damage is common clinically and may occur after surgical procedures, infectious keratitis, diabetes, and inflammatory diseases, such as DED, resulting in complete or partial neurotrophic keratopathy (NTK). However, the exact relationship between the immune and nervous systems in the cornea in relation to adaptive immunity of corneal transplantation remains poorly understood.
Our previous clinical data indicated that corneal nerves may be directly involved in the regulation of the immune system and immune privilege in the cornea. Although the role of peripheral nerves in the regulation of neuropeptides and innate immunity has been demonstrated in other peripheral sites,44–48 the regulation of the adaptive immune system and the maintenance of immune privilege by peripheral nerves has not been reported. Thus, we next developed several animal models to investigate the potential immunomodulatory roles of corneal nerves.
We initially developed a corneal denervation model using a lateral conjunctival approach of transecting the ciliary nerves, resulting in a complete loss of sensory innervation.49 Our pilot studies demonstrated that sensory nerve denervation lead to a significant increase in the expression of vascular adhesion molecules, and increased adhesion of cDC rolling and sticking in vivo—required for active migration into the cornea—translating to an influx of bone marrow-derived cells, including cDCs, into the peripheral and central cornea as early as 24 hours after surgery.50 This was followed by an increase in the secretion of pro-inflammatory cytokines including IL-1β, IL-6, and IL-17, and increased hemangiogenesis and lymphangiogensis.50 Furthermore, analyses of cDC kinetics after complete or partial central ciliary nerve axotomy, but not peripheral nerve axotomy, and corneal denervation in isogeneic and allogeneic corneal transplantations, revealed a significant increase in cDC motility in the cornea and ipsilateral dLNs.51 Moreover, axotomy prior to corneal transplantation resulted in the rejection of all grafts and a positive donor specific delayed type hypersensitivity response in mice at 2 weeks compared to a lack of response in mice after allogeneic corneal transplantation without prior axotomy.
Recently, Paunicka et al. demonstrated that a circular corneal incision in contralateral eyes lead to a marked upregulation of neuropeptides such as substance P, calcitonin gene-related peptide, and VIP,58 suggesting the sympathetic regulation of neuropeptides in the cornea. Clinically, NTK,59 which is often refractory to therapy, may result in deceased visual acuity, corneal scarring/melting, necessitating corneal transplantation. However, it is well-established that corneal transplants in NTK recipients have a very high rate of graft rejection, although corneal allografts typically have an exceptionally high acceptance rate that often exceeds 85%.60 While the low success rate in patients with NTK has been associated with poor ocular surface, our data suggest that deregulation or loss of corneal nerves may result in the loss of immune privilege and increased inflammation. Taken together, our findings indicate that the peripheral nervous system plays a critical role in maintaining corneal immune privilege, where the loss of corneal innervation results in the immediate breakdown of immune privilege. Thus, neuro-regenerative treatments might provide a new avenue for the treatment of immune and inflammatory diseases, as re-innervation regulation of the nervous system would then result in the subsequent regulation of the immune response, such as in corneal transplantation and DED. In summary, our translational approach to ocular immunology has resulted in a rapid and novel characterization/dissection of corneal neuro-immune crosstalk.
Acknowledgments
Source of Funding
This study received the following financial support: NIH R01-EY022695 (PH), NIH R21-EY025393 (PH), and Tufts Medical Center Institutional Support (PH).
Footnotes
Conflict of Interest
The authors have no conflicts of interest to declare.
References
- 1.Abi-Hanna D, Wakefield D, Watkins S. HLA antigens in ocular tissues. I. In vivo expression in human eyes. Transplantation. 1988;45(3):610–613. doi: 10.1097/00007890-198803000-00021. [DOI] [PubMed] [Google Scholar]
- 2.Streilein JW. Regional immunity and ocular immune privilege. Chem Immunol. 1999;73:11–38. doi: 10.1159/000058741. [DOI] [PubMed] [Google Scholar]
- 3.Niederkorn JY. The immune privilege of corneal grafts. J Leukoc Biol. 2003;74(2):167–171. doi: 10.1189/jlb.1102543. [DOI] [PubMed] [Google Scholar]
- 4.Gillette TE, Chandler JW, Greiner JV. Langerhans cells of the ocular surface. Ophthalmology. 1982;89(6):700–711. doi: 10.1016/s0161-6420(82)34737-5. [DOI] [PubMed] [Google Scholar]
- 5.Hamrah P, Zhang Q, Liu Y, et al. Novel characterization of MHC class II-negative population of resident corneal Langerhans cell-type dendritic cells. Invest Ophthalmol Vis Sci. 2002;43(3):639–646. [PubMed] [Google Scholar]
- 6.Hamrah P, Liu Y, Zhang Q, et al. The corneal stroma is endowed with a significant number of resident dendritic cells. Invest Ophthalmol Vis Sci. 2003;44(2):581–589. doi: 10.1167/iovs.02-0838. [DOI] [PubMed] [Google Scholar]
- 7.Hamrah P, Liu Y, Zhang Q, et al. Alterations in corneal stromal dendritic cell phenotype and distribution in inflammation. Arch Ophthalmol. 2003;121(8):1132–1140. doi: 10.1001/archopht.121.8.1132. [DOI] [PubMed] [Google Scholar]
- 8.Nakamura T, Ishikawa F, Sonoda KH, et al. Characterization and distribution of bone marrow-derived cells in mouse cornea. Invest Ophthalmol Vis Sci. 2005;46(2):497–503. doi: 10.1167/iovs.04-1154. [DOI] [PubMed] [Google Scholar]
- 9.Hamrah P, Dana MR. Corneal antigen-presenting cells. Chem Immunol Allergy. 2007;92:58–70. doi: 10.1159/000099254. [DOI] [PubMed] [Google Scholar]
- 10.Brissette-Storkus CS, Reynolds SM, Lepisto AJ, et al. Identification of a novel macrophage population in the normal mouse corneal stroma. Invest Ophthalmol Vis Sci. 2002;43(7):2264–2271. [PMC free article] [PubMed] [Google Scholar]
- 11.Chauhan SK, El Annan J, Ecoiffier T, et al. Autoimmunity in dry eye is due to resistance of Th17 to Treg suppression. J Immunol. 2009;182(3):1247–1252. doi: 10.4049/jimmunol.182.3.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.El Annan J, Chauhan SK, Ecoiffier T, et al. Characterization of effector T cells in dry eye disease. Invest Ophthalmol Vis Sci. 2009;50(8):3802–3807. doi: 10.1167/iovs.08-2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chauhan SK, Dana R. Role of Th17 cells in the immunopathogenesis of dry eye disease. Mucosal Immunol. 2009;2(4):375–376. doi: 10.1038/mi.2009.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barabino S, Chen Y, Chauhan S, et al. Ocular surface immunity: homeostatic mechanisms and their disruption in dry eye disease. Prog Retin Eye Res. 2012;31(3):271–285. doi: 10.1016/j.preteyeres.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pflugfelder SC, Corrales RM, de Paiva CS. T helper cytokines in dry eye disease. Exp Eye Res. 2013;117:118–125. doi: 10.1016/j.exer.2013.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yagci A, Gurdal C. The role and treatment of inflammation in dry eye disease. Int Ophthalmol. 2014;34(6):1291–1301. doi: 10.1007/s10792-014-9969-x. [DOI] [PubMed] [Google Scholar]
- 17.Hazlett LD, Hendricks RL. Reviews for immune privilege in the year 2010: immune privilege and infection. Ocul Immunol Inflamm. 2010;18(4):237–243. doi: 10.3109/09273948.2010.501946. [DOI] [PubMed] [Google Scholar]
- 18.Cruzat A, Pavan-Langston D, Hamrah P. In vivo confocal microscopy of corneal nerves: analysis and clinical correlation. Semin Ophthalmol. 2010;25(5–6):171–177. doi: 10.3109/08820538.2010.518133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hamrah P, Cruzat A, Dastjerdi MH, et al. Corneal sensation and subbasal nerve alterations in patients with herpes simplex keratitis: an in vivo confocal microscopy study. Ophthalmology. 2010;117(10):1930–1936. doi: 10.1016/j.ophtha.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qazi Y, Aggarwal S, Hamrah P. Image-guided evaluation and monitoring of treatment response in patients with dry eye disease. Graefes Arch Clin Exp Ophthalmol. 2014;252(6):857–872. doi: 10.1007/s00417-014-2618-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Knickelbein JE, Buela KA, Hendricks RL. Antigen-presenting cells are stratified within normal human corneas and are rapidly mobilized during ex vivo viral infection. Invest Ophthalmol Vis Sci. 2014;55(2):1118–1123. doi: 10.1167/iovs.13-13523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cruzat A, Witkin D, Baniasadi N, et al. Inflammation and the nervous system: the connection in the cornea in patients with infectious keratitis. Invest Ophthalmol Vis Sci. 2011;52(8):5136–5143. doi: 10.1167/iovs.10-7048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosenberg ME, Tervo TM, Muller LJ, et al. In vivo confocal microscopy after herpes keratitis. Cornea. 2002;21(3):265–269. doi: 10.1097/00003226-200204000-00006. [DOI] [PubMed] [Google Scholar]
- 24.Zhivov A, Stave J, Vollmar B, et al. In vivo confocal microscopic evaluation of Langerhans cell density and distribution in the normal human corneal epithelium. Graefes Arch Clin Exp Ophthalmol. 2005;243(10):1056–1061. doi: 10.1007/s00417-004-1075-8. [DOI] [PubMed] [Google Scholar]
- 25.Efron N. Contact lens-induced changes in the anterior eye as observed in vivo with the confocal microscope. Prog Retin Eye Res. 2007;26(4):398–436. doi: 10.1016/j.preteyeres.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 26.Alhatem A, Cavalcanti B, Hamrah P. In vivo confocal microscopy in dry eye disease and related conditions. Semin Ophthalmol. 2012;27(5–6):138–148. doi: 10.3109/08820538.2012.711416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kheirkhah A, Muller R, Mikolajczak J, et al. Comparison of Standard Versus Wide-Field Composite Images of the Corneal Subbasal Layer by In Vivo Confocal Microscopy. Invest Ophthalmol Vis Sci. 2015;56(10):5801–5807. doi: 10.1167/iovs.15-17434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamaguchi T, Calvacanti BM, Cruzat A, et al. Correlation between Human Tear Cytokine Levels and Cellular Corneal Changes in Patients with Bacterial Keratitis by In Vivo Confocal Microscopy. Invest Ophthalmol Vis Sci. 2014 doi: 10.1167/iovs.14-15411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kheirkhah A, Dohlman TH, Amparo F, et al. Effects of corneal nerve density on the response to treatment in dry eye disease. Ophthalmology. 2015;122(4):662–668. doi: 10.1016/j.ophtha.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qazi Y, Kheirkhah A, Dohlman TH, et al. Corneal Dendritic Cells as a Surrogate Biomarker of Therapeutic Efficacy in Dry Eye-Associated Corneal Inflammation. Invest Ophthalmol Vis Sci. 2015;56(7):291–291. [Google Scholar]
- 31.Qazi Y, Kheirkhah A, Dohlman TH, et al. Relative Efficacy of Loteprednol (Lotemax®) vs. Loteprednol-Tobramycin (Zylet®) on Corneal and Conjunctival Immune Response in Treatment of Meibomian Gland Dysfunction (MGD)-Associated Ocular Surface Inflammation: In Vivo Confocal Microscopy Results of a Phase IV Randomized Clinical Trial. Invest Ophthalmol Vis Sci. 2014;55(13):45–45. [Google Scholar]
- 32.Benitez del Castillo JM, Wasfy MA, Fernandez C, et al. An in vivo confocal masked study on corneal epithelium and subbasal nerves in patients with dry eye. Invest Ophthalmol Vis Sci. 2004;45(9):3030–3035. doi: 10.1167/iovs.04-0251. [DOI] [PubMed] [Google Scholar]
- 33.Linna TU, Vesaluoma MH, Perez-Santonja JJ, et al. Effect of myopic LASIK on corneal sensitivity and morphology of subbasal nerves. Invest Ophthalmol Vis Sci. 2000;41(2):393–397. [PubMed] [Google Scholar]
- 34.Rosenberg ME, Tervo TM, Immonen IJ, et al. Corneal structure and sensitivity in type 1 diabetes mellitus. Invest Ophthalmol Vis Sci. 2000;41(10):2915–2921. [PubMed] [Google Scholar]
- 35.Efron N. The Glenn A. Fry award lecture 2010: Ophthalmic markers of diabetic neuropathy. Optom Vis Sci. 2011;88(6):661–683. doi: 10.1097/OPX.0b013e3182171020. [DOI] [PubMed] [Google Scholar]
- 36.Chen DK, Frizzi KE, Guernsey LS, et al. Repeated monitoring of corneal nerves by confocal microscopy as an index of peripheral neuropathy in type-1 diabetic rodents and the effects of topical insulin. J Peripher Nerv Syst. 2013;18(4):306–315. doi: 10.1111/jns5.12044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leppin K, Behrendt AK, Reichard M, et al. Diabetes mellitus leads to accumulation of dendritic cells and nerve fiber damage of the subbasal nerve plexus in the cornea. Invest Ophthalmol Vis Sci. 2014;55(6):3603–3615. doi: 10.1167/iovs.14-14307. [DOI] [PubMed] [Google Scholar]
- 38.Theophanous C, Jacobs DS, Hamrah P. Corneal Neuralgia after LASIK. Optom Vis Sci. 2015;92(9):e233–40. doi: 10.1097/OPX.0000000000000652. [DOI] [PubMed] [Google Scholar]
- 39.Aggarwal S, Kheirkhah A, Cavalcanti BM, et al. Autologous Serum Tears for Treatment of Photoallodynia in Patients with Corneal Neuropathy: Efficacy and Evaluation with In Vivo Confocal Microscopy. Ocul Surf. 2015;13(3):250–262. doi: 10.1016/j.jtos.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bucher F, Schneider C, Blau T, et al. Small-Fiber Neuropathy Is Associated With Corneal Nerve and Dendritic Cell Alterations: An In Vivo Confocal Microscopy Study. Cornea. 2015;34(9):1114–1119. doi: 10.1097/ICO.0000000000000535. [DOI] [PubMed] [Google Scholar]
- 41.Muller RT, Abedi F, Cruzat A, et al. Degeneration and Regeneration of Subbasal Corneal Nerves after Infectious Keratitis: A Longitudinal In Vivo Confocal Microscopy Study. Ophthalmology. 2015;122(11):2200–2209. doi: 10.1016/j.ophtha.2015.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hamrah P, Cruzat A, Dastjerdi MH, et al. Unilateral herpes zoster ophthalmicus results in bilateral corneal nerve alteration: an in vivo confocal microscopy study. Ophthalmology. 2013;120(1):40–47. doi: 10.1016/j.ophtha.2012.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cruzat A, Schrems WA, Schrems-Hoesl LM, et al. Contralateral Clinically Unaffected Eyes of Patients With Unilateral Infectious Keratitis Demonstrate a Sympathetic Immune Response. Invest Ophthalmol Vis Sci. 2015;56(11):6612–6620. doi: 10.1167/iovs.15-16560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Streilein JW, Okamoto S, Sano Y, et al. Neural control of ocular immune privilege. Ann N Y Acad Sci. 2000;917:297–306. doi: 10.1111/j.1749-6632.2000.tb05396.x. [DOI] [PubMed] [Google Scholar]
- 45.Streilein JW, Bradley D. Analysis of immunosuppressive properties of iris and ciliary body cells and secretary products. Invest Ophthalmol Vis Sci. 1991;32:2700–2710. [PubMed] [Google Scholar]
- 46.Kawashima H, Prasad SA, Gregerson DS. Corneal endothelial cells inhibit T cell proliferation by blocking IL-2 production. J Immunol. 1994;153:1982–1989. [PubMed] [Google Scholar]
- 47.Hori J, Joyce NC, Streilein JW. Immune privilege and immunogenicity reside among different layers of the mouse cornea. Invest Ophthalmol Vis Sci. 2000;41:3032–3042. [PubMed] [Google Scholar]
- 48.Hamrah P, Hoskava Haskova Z, Taylor AW, et al. Local treatment of alpha melanocyte-stimulating hormone reduces corneal allorejection. Transplantation. 2009;88:180–187. doi: 10.1097/TP.0b013e3181ac11ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cruzat A, Witkin D, Baniasadi N, et al. Inflammation and the nervous system: the connection in the cornea in patients with infectious keratitis. Invest Ophthalmol Vis Sci. 2011;52:5136–5143. doi: 10.1167/iovs.10-7048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rochlitzer S, Veres TZ, Kuhne K, et al. The neuropeptide calcitonin gene-related peptide affects allergic airway inflammation by modulating dendritic cell function. Clin Exp Allergy. 2011;41(11):1609–1621. doi: 10.1111/j.1365-2222.2011.03822.x. [DOI] [PubMed] [Google Scholar]
- 51.Chiu IM, von Hehn CA, Woolf CJ. Neurogenic inflammation and the peripheral nervous system in host defense and immunopathology. Nat Neurosci. 2012;15(8):1063–1067. doi: 10.1038/nn.3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Voedisch S, Rochlitzer S, Veres TZ, et al. Neuropeptides control the dynamic behavior of airway mucosal dendritic cells. PloS one. 2012;7(9):e45951. doi: 10.1371/journal.pone.0045951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Riol-Blanco L, Ordovas-Montanes J, Perro M, et al. Nociceptive sensory neurons drive interleukin-23-mediated psoriasiform skin inflammation. Nature. 2014;510(7503):157–161. doi: 10.1038/nature13199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Talbot S, Abdulnour RE, Burkett PR, et al. Silencing Nociceptor Neurons Reduces Allergic Airway Inflammation. Neuron. 2015;87(2):341–354. doi: 10.1016/j.neuron.2015.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yamaguchi T, Turhan A, Harris DL, et al. Bilateral nerve alterations in a unilateral experimental neurotrophic keratopathy model: a lateral conjunctival approach for trigeminal axotomy. PloS one. 2013;8(8):e70908. doi: 10.1371/journal.pone.0070908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yamaguchi T, Harris DL, Higa K, et al. Neurogenic Immune Homeostasis: Peripheral Innervation Maintains Avascularity and Immune Privilege of the Cornea. ARVO Meeting Abstracts. 2015;56(4034) [Google Scholar]
- 57.Yamaguchi T, Hu K, Harris DL, et al. Intravital Multiphoton Microscopy of Corneas and Draining Lymph Nodes Shows Increased Velocity of Dendritic Cells after Corneal Transplantation and Directionality in Corneal Allografts. ARVO Meeting Abstracts. 2013;54(1288) [Google Scholar]
- 58.Paunicka KJ, Mellon J, Robertson D, et al. Severing corneal nerves in one eye induces sympathetic loss of immune privilege and promotes rejection of future corneal allografts placed in either eye. Am J Transplant. 2015;15:1490–501. doi: 10.1111/ajt.13240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bonini S, Rama P, Olzi D, Lambiase A. Neurotrophic keratitis. Eye (Lond) 2003;17:989–995. doi: 10.1038/sj.eye.6700616. [DOI] [PubMed] [Google Scholar]
- 60.Tan DT, Janardhanan P, Zhou H, et al. Penetrating keratoplasty in Asian eyes: the Singapore Corneal Transplant Study. Ophthalmology. 2008;115:975–982.e971. doi: 10.1016/j.ophtha.2007.08.049. [DOI] [PubMed] [Google Scholar]


