Abstract
Background
Despite treatment with therapeutic hypothermia (TH), infants who survive hypoxic ischemic (HI) encephalopathy (HIE) have persistent neurologic abnormalities at school age. Protection by TH against HI brain injury is variable in both humans and animal models. Our current pre-clinical model of HI and TH displays this variability of outcomes in neuropathological and neuroimaging endpoints with some sexual-dimorphism. The detailed behavioral phenotype of this model is unknown as well as whether there is sexual-dimorphism in certain behavioral domains. Brain derived neurotrophic factor (BDNF) supports neuronal cell survival and repair but may also be a marker of injury. Here, we characterize behavioral deficits after HI and TH stratified by sex, as well as late changes in BDNF and its correlation with memory impairment.
Methods
HI was induced in C57BL6 mice at p10 (modified Vannucci model). Mice were randomized to TH (31 °C) or normothermia (NT, 36 °C) for 4 h after HI. Controls were sham anesthesia-exposed age and sex-matched littermates. Between p16 and p39, growth was followed and behavioral testing was performed including: reflexes (air righting, forelimb grasp and negative geotaxis), sensorimotor, learning and memory skills (open field, balance beam, adhesive removal, Y-maze tests and object location task [OLT]). Correlations between mature BDNF levels in forebrain at p42 memory outcomes were studied.
Results
Both male and female HI mice had a ~8 to 12% lower growth rate (gr/day) than shams (p≤0.01) by p39. TH ameliorated this growth failure in female but not in male mice. In female mice HI injury prolonged the time spent in the periphery (open field) at p36 (p=0.004), regardless of treatment. TH prevented motor impairments on the balance beam and in adhesive removal tests in male and female mice, respectively (p≤0.05). Male and female HI mice visited the new arm of the Y-maze 12.5% (p≤0.05) and 10% (p=0.03) less often than shams, respectively. Male HI mice also had 35% lower exploratory preference score than sham (p≤0.001) in OLT. TH did not prevent memory impairments found with Y maze testing or OLT in either sex (p=0.01) at p26. At p42, BDNF levels in the forebrain ipsilateral to the HI insult were 1.7 to 2 fold higher than BDNF levels in sham forebrain and TH did not prevent this increase. Higher BDNF levels in the forebrain ipsilateral to the insult correlated with worse performance in the Y-maze in both sexes and in OLT in male mice (p=0.01).
Conclusions
TH provides benefit in specific domains of behavior following neonatal HI. In general, these benefits accrued to both males and females but not in all areas. In some domains, such as memory, no benefit of TH was found. Late differences in individual BDNF levels may explain some of these findings.
Keywords: growth rate, primitive reflexes, memory deficits, sensory-motor deficits, astrocytes
INTRODUCTION
Hypoxic-ischemic (HI) brain injury is a major cause of morbidity and mortality among full-term neonates [1–4]. Therapeutic hypothermia (TH) has become standard of care for those neonates meeting criteria for moderate to severe HI encephalopathy (HIE). However, despite the use of TH, [1,3–9] up to 55% of HIE survivors still have persistent neurologic abnormalities at school age, including 43% with significant disabilities [3,8,10–14]. While the responses to TH after HI brain injury are variable in humans, details about specific neurobehavioral domains protected by TH are limited. Except for motor outcomes, large randomized clinical trials (RCTs) were not designed to answer questions about specific neurobehavioral endpoints [7,12,15–17]. Nevertheless, RCTs provide evidence that while deficits in motor domains are attenuated by TH after HIE, deficits in memory and learning domains may persist despite TH [18]. Some preclinical mouse models of HI, including ours, emulate some facets of the injury produced by HIE and the variable protection afforded by TH in humans [19–21].
Sexual dimorphism documented in preclinical models of HI may contribute to the variable injury and response to treatment [19,21–24]. However, the importance of sex as a biological variable influencing HIE outcomes has been sparsely studied in humans [25–27]. While males appear to be at higher risk for cerebral palsy and worse development after perinatal brain injury, including HIE; [25,26,28–30] sexual dimorphism in responses to TH is not characterized. Growth failure in neonates suffering of HIE has been previously described, [31], but the effect of TH to ameliorate growth disturbance is not known nor is any possible influence of sex.
Neurotrophins, specifically brain derived neurotrophic factor (BDNF), are essential to promote synaptic plasticity within the hippocampus and prefrontal cortex, [32] brain regions essential for memory consolidation [33–37]. BDNF also plays a pivotal role in supporting cell survival and tissue repair after HI injury via binding to tropomyosin receptor kinase B (TrkB) [38–41]. Alternatively, mature BDNF may facilitate cell death via binding to the ubiquitous p75 neurotrophin receptor (p75ntr) [42]. While BDNF deficits in the hippocampus and cortex occur in both sexes by 96 h after neonatal HI in the mouse [39], BDNF changes at late time points after HI and TH are not known. Sex-specific changes in late BDNF levels after HI injury may explain the variability in behavioral responses to TH [19,21,24]. Here, we characterize i) growth and behavioral deficits after HI and TH, ii) the influence of sex on those outcomes, iii) the late changes of BDNF in injured and uninjured brain regions, and iv) the possible individual influence of changes in BDNF on memory in a p10 mouse model.
METHODS AND MATERIALS
Animals
One hundred and thirty-seven C57BL6 pups were used in this study. The mortality rate for this model was 15% (52% male and 48% female). Sixty percent of these deaths occurred during the hypoxia phase of the experiment, and 40% occurred after the end of TH / normothermia (NT) phase of the experiment [25% due to the results of brain injury (1 TH / 4 NT), and the remaining 15% because of maternal cannibalism].
Animal experimental protocols were approved by the Institutional Animal Care and Use Committee at Johns Hopkins University-School of Medicine and carried out with standards of care and housing in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, US Department of Health and Human Services 85–23, 2011.
Hypoxic Ischemic Injury and Therapeutic Hypothermia
HI was induced in C57BL6 mice (Charles River Laboratories, Wilmington, MA) on postnatal day (p) 10 using the modified Vannucci model for mice [43] with permanent unilateral right carotid artery ligation, followed by 45 min of hypoxia (FiO2 = 0.08) 1h after ligation. During surgery, which lasted up to 5 min, mice were anesthetized with inhaled isoflurane (3% for induction, followed by 1% maintenance) followed by a 5 to 10 min period of recovery until mice recovered spontaneous ambulation. Isoflurane combined with nitrous oxide as used in this protocol produces analgesia, sedation and muscle relaxation. Animals from each litter were randomized to TH (31 °C) or NT (36 °C) for 4 h after HI as previously reported by our group.[19]. Four hours of TH to 31°C following HI brain injury at p10 provides significant cortical, hippocampal and striatal neuroprotection assessed by MRI [19]. Following the TH/NT phase, pups were returned to the dam. Mice assigned to the control group were exposed to inhaled isoflurane on p10 for five minutes at similar concentrations as described above for the TH and NT groups. Age-matched littermates were used as age/ sex-matched anesthesia-exposed sham controls. Mice were monitored daily for at least 45 min to assess their activity, grooming and ability to access food and water.
Behavioral testing
Testing was conducted in a quiet experimental room between 2 to 7 pm, under low light conditions (80–110 lm) for all testing except the balance beam. In balance beam testing a bright light was used as an avoidance stimulus to promote motion along the beam toward the dark box at the other end. Timing and exposure to low lumen light for testing were used to recreate dusk (first part of dark phase), the period at which mice initiate their most intense activity [44]. Each behavioral test was performed within similar time frames and the order of testing of mice within the litters (6 pups per litter, 2 litters per experimental session) was randomly assigned. After a 30 min of habituation in the experimental room, mice were weighed on day of injury, and at the beginning and end of each testing week. Following initial evaluation of reflexes at p16, behavioral testing was performed between p22 and p36 (Fig. 1), based on minimal age in which the animals were considered developmentally ready to perform the tasks. Most tasks were recorded using video, except for the adhesive removal task and reflex evaluation. Videos were reviewed and scored by two team members blinded to mouse groups (SA and NK) and discrepancies were resolved by two other team members (JD and FJN). Following behavioral testing, mice were returned to the dam.
Figure 1.
Timeline representation of experimental protocol
Reflexes
The appearance of age appropriate motor skills (balance, coordination and strength) develop by p21 in mice. We chose to test animals at p16 during the period when reflexes appear and at p26 to evaluate for extinction of primitive reflexes [45]. Specifically, we evaluated i) negative geotaxis ii) air righting and iii) forelimb grasp.
Negative geotaxis to evaluate labyrinthine reflex, strength, and coordination. Pups were placed facing downwards on a 45° incline. Time to right upward on the incline was measured; a time over 30s was considered a failure.
Air righting to evaluate postural righting and coordination. Pups were suspended supine, 13cm over a soft surface and dropped. Positive air righting was counted if the pup landed prone.
Forelimb grasp to evaluate strength. Pups were placed on wire suspended over soft surface; time in seconds of forelimb grasp was measured.
Open Field
Open field activity evaluates locomotion and pivoting behavior of rodents [46]. Open field is also used to assess anxiety behavior and is a general assessment of animal basal locomotor activity and exploration [47]. The open field arena consists of a plastic square chamber (40 x 30 cm), divided into central and peripheral areas. Animals were placed in the center of the arena, and left to explore freely for a total of 5 min. Parameters analyzed included total time spent in the center and the periphery, and percent time moving, resting and grooming.
Balance Beam
Balance beam performance evaluates motor coordination and balance as previously reported [48]. Performance was measured by quantifying the time it took the mouse to cross the beam and the number of paw slips that occurred. The apparatus consisted of 3 different raised wood beams (12 mm in width, 6 mm in width and a 6 mm round beams) 80 cm in length and placed 50 cm above the table top. The finish (safe point) consisted of a black box at the end of the beam filled with nesting material. A bright light placed above the start point was used as an aversive stimulus. Animals were given three trials to cross each beam after the training day. A maximum of 60 seconds was allowed for the cross. Parameters analyzed included: time to cross beam in seconds at each trial, number and side of hind paws slips.
Adhesive Removal
Adhesive removal evaluates somatosensory and motor function or deficits in rodents [49]. Tactile responses were measured by recording the time of initial contact with the adhesive and how much time it took the animal to remove the adhesive from each side. Two small round pieces of adhesive tape (6 ± 0.5 mm) were applied to the each of the mouse’s front paw with equal pressure, alternating paw placement order. Mice were then placed in a testing box and a maximum of 60 seconds was allowed for the mice to remove the adhesive. First contact and total time for removal time from each paw were analyzed.
Y maze test
The Y maze test evaluates memory in rodents, based on their tendency to explore new environments [50]. Rodents normally prefer to investigate a new arm of the maze rather than returning to one that was previously visited. Several brain areas including the hippocampus, septum, basal forebrain and prefrontal cortex are involved in this task [51]. The apparatus consisted of three identical plexiglas arms interconnected in a 120° angle to an equilateral triangular center compartment. Phase 1 of the Y-maze assesses working memory. Mice were placed in the center of the maze and allowed to explore the maze. Arm alternations were counted over a 5-min testing period and calculated as a percentage of the total arm entries. Spontaneous alternations performance (SAP), a triplet of three successive different arm visits and estimates good working memory was assessed along with the total number of arm visits. Three days after phase 1, phase 2 was performed to assess spatial and recognition memory. In the acquisition phase, one of the arms of the maze was closed by a vertical wall (novel arm); thus, animals were only able to explore the two open arms for a total of 5 min. After a delay of 30 min, all the arms were opened and mice were allowed to explore for 5 minutes. Percentage of entries to the novel arm was analyzed in the retention phase.
Object location task
Object location task (OLT) evaluates spatial and working memory, based on the tendency of rodents to discriminate new environments and/or objects [52,53]. A day of habituation to the arena, is followed the next day by a 5 min period to explore 4 objects placed in the arena and a delayed phase (30 min later) to explore the objects again after two of the objects have been switched in location. The arena was the same used for open field testing. All objects were of similar size but different in color, texture and material. Encounters of the mice in closed proximity (<1cm) to the object were deemed exploratory if the nose of the mouse was pointing towards the object and the mouse was looking or sniffing at the object. Parameters analyzed included: percentage time exploring objects in familiar location and percentage time exploring object in novel location. Times in seconds were used to calculate exploratory preference (EP) score in percentage (EP score = time exploring object in novel location *100/ total time exploring all objects) [54] and discrimination index (DI) [DI= (time exploring the novel object – the familiar object)*100/ total time exploring all objects].[55] The latter two scores measure discriminatory behavior in exploratory time between novel and familiar objects. For the EP score, a percentage >50% indicates good working memory and <50% impaired memory; for DI a positive score indicates more time spent with novel object, a negative score more time spent with familiar object and zero score no preference for either object.[53]
BDNF ELISA
BDNF protein levels were measured from fresh forebrain tissue obtained when mice were sacrificed at p42. Each sample included tissue from cortex and hippocampus (forebrain) and samples were taken ipsilateral and contralateral to the injury from NT and TH treated HI mice. A similar tissue sample was obtained from p42 sham anesthesia-exposed control mice. Protein homogenates were prepared as previously described [56] and protein concentrations determined using the Bradford assay [57]. Mouse sandwich ELISA method was used according to manufacturer’s instructions (Biosensis Pty ltd. Thebarton, SA- Australia). The kit consists of a pre-coated mouse monoclonal anti-mature BDNF capture antibody, with a detection range of 2 to 2000 pg/ml and no detectable cross-reactivity with other cytokines. Samples were clarified by centrifugation at 10 000 x g at 4°C for 2 min, then 50μl (202 ± 62.5μg protein) were incubated for 45 min at 37°C in the pre-coated plate, and then exposed to biotinylated anti-mouse BDNF antibody for 30 min at 37°C. Following washes, avidin-biotin-peroxidase complex working solution was applied for 30 min at 37°C. TMB (3,3',5,5' Tetramethyl- benzidine buffer) developing agent was added to each well after washes and after 8 min of incubation at 37°C, TMB stop solution was applied. Plates were read at 450 nm in a microplate reader (Biorad, Hercules, CA) within 30 min after stop solution was applied and analyzed using a linear regression model. Results were adjusted to total loaded protein per well (pg of BDNF/ mg of protein).
Western Blots
For western blot analysis of phospho-TrkB (pTrkB) and TrkB samples of forebrain were used. Protein homogenates were prepared in homogenization buffer with phosphatase and protease inhibitors and 20% (w/v) glycerol. Concentrations were determined using the Bradford assay.[57] Thirty μg-aliquots of homogenized protein were diluted 3:1 (v:v) in 4X loading buffer under reducing conditions and loaded into 4–20% mini-protean TGX polyacrylamide precast protein gels (Biorad Inc,, Hercules, CA). Protein was transferred to nitrocellulose membrane using TransBlot Turbo Midi-size (Biorad Inc), stained with Ponceau S, blocked with 2.5% nonfat dry milk with 0.1% Tween-20 in 50 mM Tris buffered saline (TBST, 50mM Tris/HCl and 150 mM NaCl, pH 7.4) except for pTrkB (Thermo Fisher PA5-36695) which was blocked in 2.5% bovine serum albumin (BSA). Nitrocellulose blots were incubated overnight at 4°C with primary antibodies at 1:500 (pTrkB) and 1:200 (TrkB). Tenμg of human TrkB transfected 293T cell lysate (sc-113925; Santa Cruz Biotechnology, Inc., Santa Cruz, CA) showing a band at 145 kDa and at 95 kDa was used for identification of TrkB. After exposure to each primary antibody, membranes were washed with TBST, exposed to secondary antibodies for 1h and then developed using enhanced chemiluminescence (Clarity Western ECL Substrate, Biorad Inc.). To quantify protein immunoreactivity optical density (OD) was determined with iVision Software adjusted for background. The reliability of sample loading and protein transfer was verified by staining nitrocellulose membranes with Ponceau S before immunoblotting and use of β-actin as a loading control.
Antibodies
pTrkB: mouse polyclonal antibody raised against phosphorylation site Tyrosine 515 (Thermo Fisher PA5-3669). There was no cross reactivity with other Trk receptors. The antibody detects bands between 145-95 kDa (1 μg/ mL). TrkB: rabbit polyclonal antibody raised against amino acids 160-340 of TrkB of human origin (sc-8316) with no cross reactivity with other Trk receptors. The antibody detects TrkB bands between 145-95 kDa (1 μg/ mL).
Immunohistochemistry
Glial fibrillary acid protein (GFAP) IHC was used to assess morphological changes in astrocytes suggesting persistent activation at p42. For GFAP IHC, mice were killed with an overdose of isoflurane and exsanguinated with cold 0.1 M PBS (pH 7.4) via intra-cardiac perfusion. Brains were perfusion fixed with 4% paraformaldehyde/0.1 M PBS for 30 min at 4 ml/min. Tissues were cryoprotected with graded immersion in 15% and then 30% sucrose in PBS until the tissue sank, frozen and stored at -80°C until cut at 50 μ on a freezing microtome.[56] Floating IHC for GFAP was performed as previously described[56] with whole rabbit antisera anti-GFAP antibody (DAKO North America, Carpinteria, CA, Cat# Z0334 at 1:1 000). Goat anti-rabbit antibody (1:200) was used as the secondary antibody and DAB as the chromagen.
Statistical analysis
Statistical analysis was performed using one-way ANOVA with Tamhane post-hoc analysis stratified by sex. Significance was assigned to a p < 0.05. Correlations between BDNF levels and behavioral outcomes were analyzed using Spearman Rho. The analysis design only allows comparison within treatment groups of the same sex and age.
RESULTS
Neonatal HI affected subsequent growth
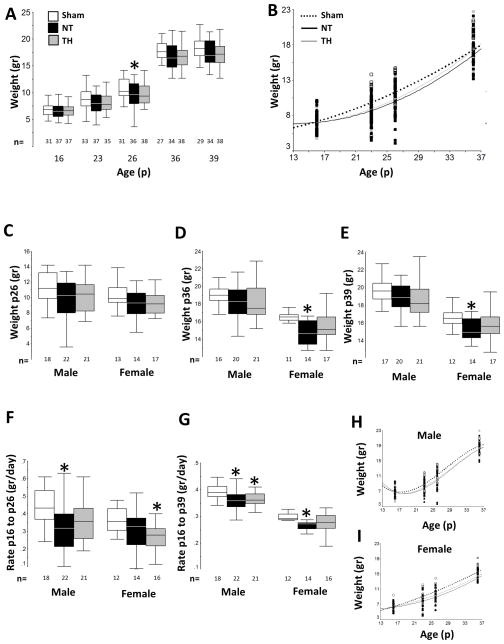
Mice were weighed routinely between p16 to p39, n=18–21/group male and n=15–17/group female. At p26 NT mice weighed 13% less than shams (ANOVA, p=0.04; p=0.04 vs. sham; Fig 2A), whereas TH and sham groups were not different. Scatterplot of weight vs. age showed the greatest differences at p26 (Fig 2B). However, when stratified by sex, the weights of male and female mice at p26 did not differ (Fig 2C). Differences by treatment groups and sex emerged by p36 after weaning from dam. Female NT mice weighed 11% less than sham at p36 (p=0.002, Fig 2D) and 9 % less than sham at p39 (p=0.01, Fig 2E). There were no differences between sham and TH groups at either p36 or p39. Prior to weaning, significant differences in the rate of growth were also observed in male mice. NT treated male mice grew slower (ANOVA p=0.01; p=0.02 vs. sham) by 0.2 grams/ day less than sham Fig 2F). Female mice treated with TH after HI also grew slower than sham mice up to p26 (ANOVA p=0.04; p=0.03 Fig 2F). However, after weaning TH female mice caught up to be no different from shams when growth was assessed for the entire 23-day time period (Fig 2G). In contrast, slow growth persisted in HI injured male mice after weaning with neither NT nor TH male mice growing at a rate comparable to shams overall (Fig 2G). Overall growth curves from male and female mice are shown in Fig 2H and 2I.
Figure 2. Neonatal HI, treatment with TH and growth.
Following HI, injured mice grew slower than sham mice. TH ameliorated growth deficits only in female injured mice. Grouped data for weight (gr) from p16 to p39 are presented as box and whisker plot (A) and scatter plot (B). Sex-stratified weight data (gr) for p26 (C), p36 (D), and p39 (E), and growth rates (gr/day) from p16 to p26 (F), and from p16 to p39 (G) are represented as box and whiskers plots, while growth curves for males (H) and females (I) from p16 to p36 are represented as scatter plots. For box and whiskers plots, boxes represent the interquartile range (IQR) limited by the 25th and 75th percentile (lower and upper limit, respectively), line inside the box indicates the median and whiskers extend to 1.5 times the IQR. Outliers are not represented. White box or black discontinuous line represent shams, black box or black continuous line represents NT mice; and grey box or grey continuous line represent TH mice. *, p<0.05 vs. sham, one-way ANOVA (grouped or stratified by sex) with post-hoc analysis using Tamhane. Sample size (n) detailed below each box.
Early differences in negative geotaxis and later differences in open field behavior
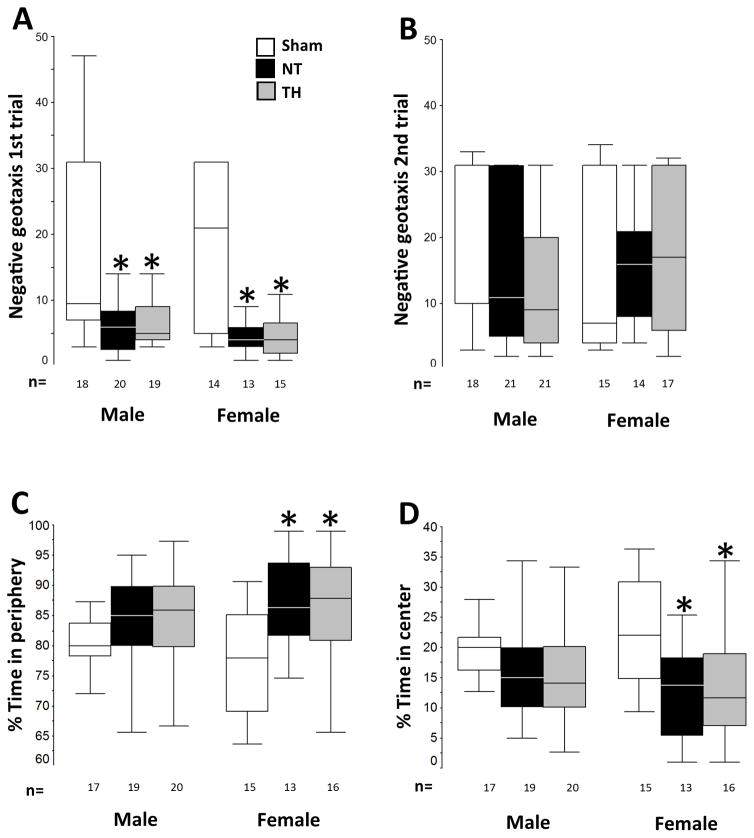
No differences for forelimb grasp or air righting were observed within treatment groups or when stratified by sex when assessed at p16 and p26. However, when negative geotaxis was assessed at p16, injured mice turned uphill 3 and 2.8 times faster than shams, respectively (ANOVA, p<0.001; sham vs. NT p<0.001, sham vs. TH p<0.001) (grouped data not shown). At p16, when stratified by sex, female HI mice turned uphill 2.3 to 2.9 times faster than shams (ANOVA, p=0.001; sham vs. NT p=0.01, sham vs. TH p=0.04), and male HI mice turned 3.6 to 4 times faster than male shams (ANOVA, p<0.001; sham vs. NT p=0.004, sham vs. TH p=0.002, Fig 3A). At p26, no differences between injured and uninjured mice were found in either sex or in grouped data, however, a greater degree of variability and longer times exploring downhill emerged in the sham animals (grouped data not shown). (Fig 3B). Open field performance at p36 unveiled a sex-specific anxious behavior. While no differences were documented in the percent time moving, resting and grooming, overall, HI injured mice spent more time exploring the periphery than the center of the field (ANOVA, p=0.001; p <0.05 vs. sham in all cases, grouped data not shown). Stratification by sex demonstrated that differences between uninjured and injured mice occurred significantly in female HI mice (ANOVA, p=0.004; sham vs. NT p=0.01 sham vs. TH p=0.02, Fig 3C and 3D). TH did not protect female mice against this behavior. Percent time in periphery and center was similar in male mice regardless of treatment.
Figure 3. Negative geotaxis and open field behavior.
Injured mice regardless of treatment or sex turned up hill in the negative geotaxis test. Only female injured mice spent more time in the periphery of the open field. Sex-stratified negative geotaxis data (in sec) at p16 (A) and p26 (B) and open field data (in %) for time in periphery (C) and center (D) are represented as box and whiskers plots, where boxes represent the interquartile range (IQR) limited by the 25th and 75th percentile (lower and upper limit, respectively), line inside the box indicates the median and whiskers extend to 1.5 times the IQR. Outliers are not represented. White boxes represent shams, black boxes represents NT mice; and grey boxes represent TH mice. *, p<0.05 vs. sham, one-way ANOVA (grouped or stratified by sex) with post-hoc analysis using Tamhane. Sample size (n) detailed below each box.
TH protected against motor deficits
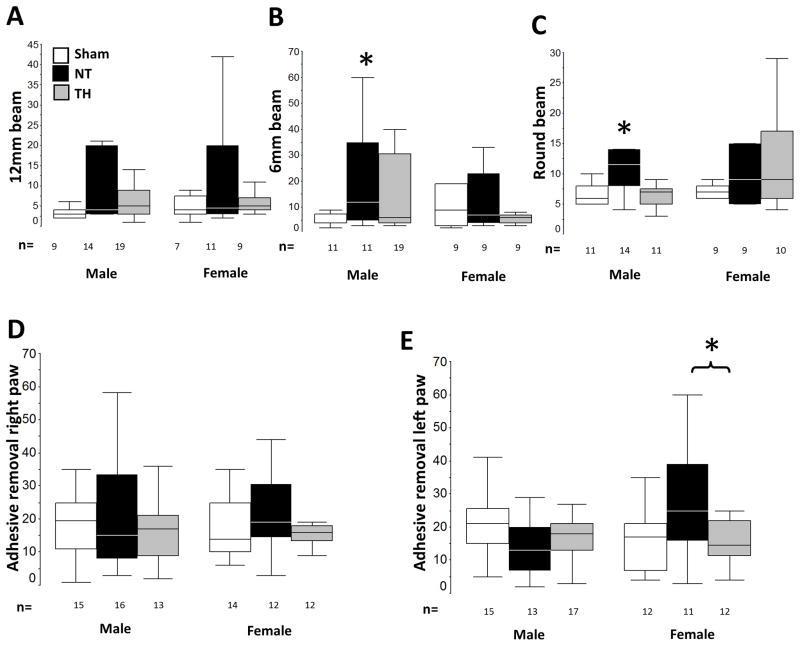
Motor outcomes were evaluated with balance beam and adhesive removal tests. As a whole, injured and uninjured mice performed similarly in the 12mm, 6mm and round beam assessments with differences only revealed when the results were stratified by sex. No differences were observed in either sex on the 12 mm beam (Fig 4A). However, male NT mice took approximately twice as long to cross the 6mm beam (ANOVA, p=0.05; p<0.05 vs. sham, Fig 4B) and the round beam (ANOVA, p=0.02; p<0.05 vs. sham, Fig 4C). TH improved the performance of male mice on both the 6mm and the round beams. Motor deficits were not found in female mice using the balance beam test, however, motor deficits were evident in injured female mice using the adhesive removal test. Once again, no differences in adhesive removal test were observed when all mice were compared together (grouped data not shown). With sex stratification it was evident that NT female mice took longer than those treated with TH to remove the adhesive from the left paw (contralateral to the injured brain) (ANOVA p-value 0.03, Fig 4D and 4E).
Figure 4. Motor outcomes and protection by TH.
TH protects against motor impairment in both male mice (balance beam performance) and female mice (adhesive removal test) following neonatal HI. Sex-stratified balance beam data (sec to cross) 12 mm (A), 6 mm (B) and round (C) beams at p24 and adhesive removal data (sec to remove) from right (C) and left (D) paw at p36 are represented as box and whiskers plots, where boxes represent the interquartile range (IQR) limited by the 25th and 75th percentile (lower and upper limit, respectively), line inside the box indicates the median and whiskers extend to 1.5 times the IQR. Outliers are not represented. White boxes represent shams, black boxes represents NT mice; and grey boxes represent TH mice. *, p<0.05 vs. sham, one-way ANOVA (grouped or stratified by sex) with post-hoc analysis using Tamhane. Sample size (n) detailed below each box.
TH was not protective against memory deficits found after neonatal HI
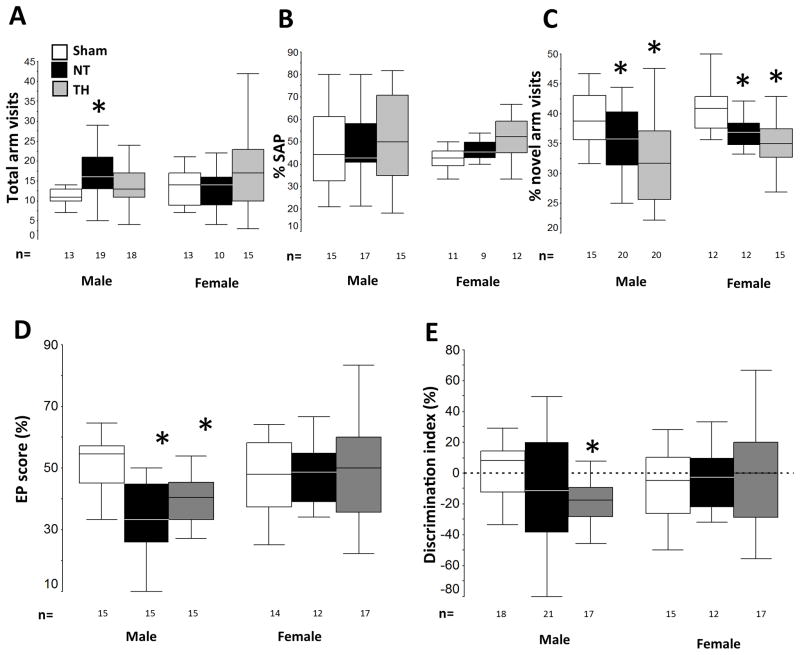
Y-maze test at p22 (phase 1) and p26 (phase 2) and OLT at p23 were used to evaluate memory. Grouped together, injured mice had similar number of visits to all arms and percentage of SAP as did uninjured mice during Y-maze phase 1. However, the percent of entrances to the novel arm during phase 2 in injured mice was 10% lower in NT and 17.5% lower in TH mice than in shams (ANOVA p<0.001; both p< 0.01 vs. sham, data not shown). When stratified by sex an additional difference emerged, male NT mice had 43% more visits to all arms of the maze (ANOVA p=0.005, p=0.04 vs. sham, Fig 5A) without any increase in the number of SAP (Fig 5B). Confirming the overall data on percent novel arm visits, the percentage of visits to novel arm in NT male and female mice were 12.5% (p=0.05) and 10% (p=0.03) lower than shams (ANOVA male p=0.002; ANOVA females p= 0.001, Fig 5C). TH did not improve the percentage of visits to the novel arm, which was 18% lower in males (p=0.002) and 15% lower in females (p=0.002) than in shams. When evaluating spatial and working memory by OLT, no differences were seen within the entire group. Again, when groups were stratified by sex, injured male mice performed worse than sham. Both NT and TH male mice had a 35% and 25% lower EP score than sham (ANOVA p<0.0001; NT p≤0.0001 and TH p = 0.0001, Fig 5D). No differences were observed within female mice. TH male mice also showed a −19.4 vs. +1.5 DI in sham mice (ANOVA p=0.05, p=0.003, Fig 5E)
Figure 5. Memory outcomes and TH.
Following neonatal HI, TH does not protect against memory deficits assessed by Y-maze and object location test (OLT) in either sex. Sex-stratified Y-maze data for total number of visits (phase 1, A) and percent SAP (phase 1, B) and percent visit to novel arm (phase 2, C) at p22-p26 and OLT data for EP score (D) and discrimination index (E) at p23 are represented as box and whiskers plots, where boxes represent the interquartile range (IQR) limited by the 25th and 75th percentile (lower and upper limit, respectively), line inside the box indicates the median and whiskers extend to 1.5 times the IQR. Outliers are not represented. White boxes represent shams, black boxes represents NT mice; and grey boxes represent TH mice. *, p<0.05 vs. sham, one-way ANOVA (grouped or stratified by sex) with post-hoc analysis using Tamhane. Sample size (n) detailed below each box.
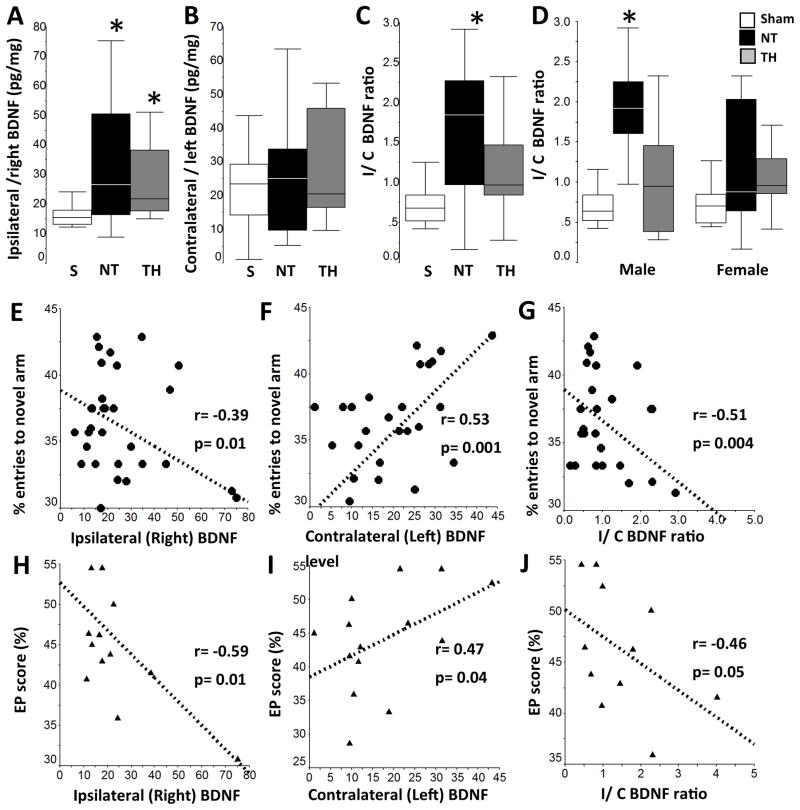
Late changes in BDNF expression correlated with memory outcomes
Since memory outcomes are linked to changes in BDNF, [35–38,58] we evaluated if injury and treatment group correlated with forebrain BDNF levels at p42, n=7–11/group. In NT mice, BDNF level in the hemisphere ipsilateral to the injury was 2-fold higher than the level in sham mice (ANOVA, p=0.02; p=0.03 vs. sham, Fig 6A). Similarly, in TH mice BDNF level in the ipsilateral hemisphere was 1.7-fold higher than sham (p=0.02 vs. sham). No differences were documented in BDNF levels in the contralateral hemisphere (Fig 6B). Ratio of BDNF levels in the ipsilateral vs. contralateral (I/C ratio) hemispheres of NT mice was 2.2-fold higher than shams (ANOVA p = 0.004, p=0.009 vs. sham, Fig 6C). When stratified by sex no differences were seen for ipsilateral or contralateral BDNF level, but male NT mice had a 2.8-fold higher I/C ratio than sham (ANOVA p-value =0.005, p=0.004 vs. sham, Fig 6D). Correlation of BDNF levels with memory outcomes, including percent entries to novel arm (Y-maze phase 2) and EP score (OLT) was performed on an individual basis. Male or female mice with higher BDNF levels in the ipsilateral hemisphere (Fig 6E and 6H), lower levels in the contralateral hemisphere (Fig 6F and 6I), and greater I/C ratios (Fig 6G and 6J) had worse performance in memory tests. Increased BDNF levels in the hemisphere ipsilateral to the injury in HI mice did not result in increased phosphorylation of TrkB (data not shown).
Figure 6. Late BDNF changes after HI.
Higher BDNF levels observed in the forebrain of the hemisphere ipsilateral to the injury correlates with worse memory outcomes regardless of treatment. Grouped BDNF data for ipsilateral (A), contralateral (B) and ipsilateral/ contralateral (I/C) ratio (C) and sex-stratified I/C ratio (D) are represented as box and whiskers plots, where boxes represent the interquartile range (IQR) limited by the 25th and 75th percentile (lower and upper limit, respectively), line inside the box indicates the median and whiskers extend to 1.5 times the IQR. Outliers are not represented. White boxes represent shams, black boxes represent NT mice, and grey boxes represent TH mice. *, p<0.05 vs. sham, one-way ANOVA (grouped or stratified by sex) with post-hoc analysis using Tamhane (n=14 per group for panels A, B and C, and n = 7 per group/ per sex for panel D). Subject-by subject correlation between BDNF levels in ipsilateral hemisphere (E, H), contralateral hemisphere (F, I) and I/C ratio (G, J) vs. % entries to novel arm (Y-maze phase 2, group data) and EP score (OLT, male mice). Analysis by Spearman Rho. r, correlation coefficient. Significance determined by p<0.05. Representative immunoblots for pTrkB, TrkB and loading control with β-actin (n=5/group) shows no significant receptor activation despite BDNF increase in hemisphere ipsilateral to injury.
Astrocyte activation at late stages after HI brain injury
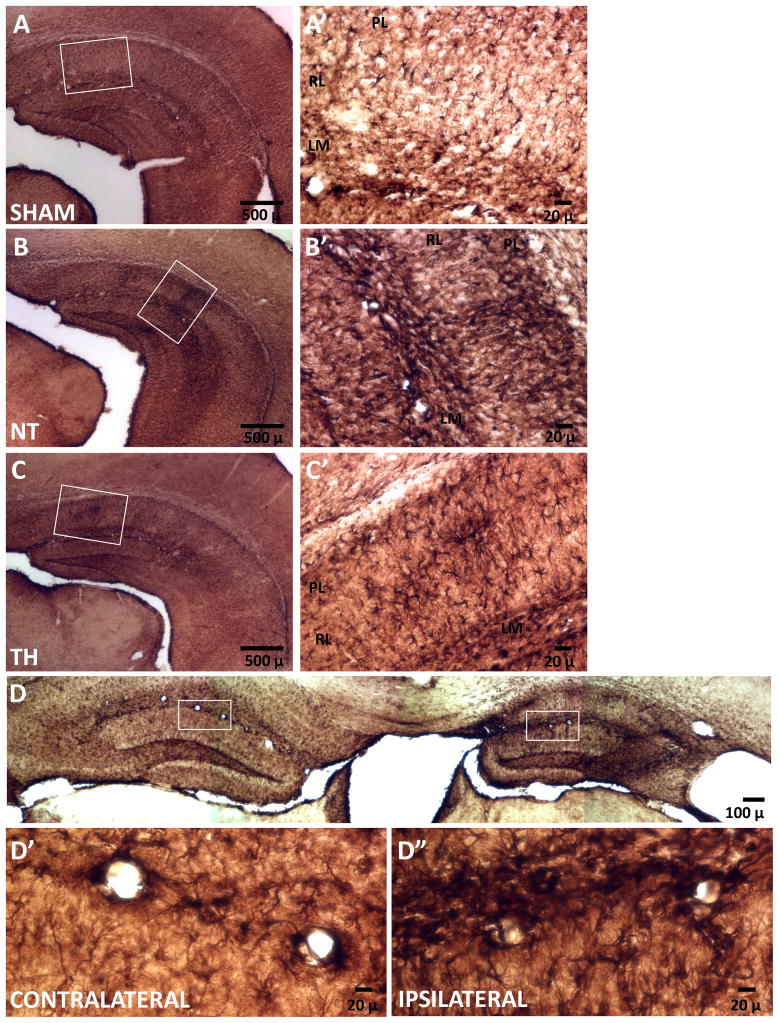
Activated astrocytes are a significant source of BDNF after injury, [59,60] thus we investigated if astrocyte activation persisted by p42, time at which BDNF changes were documented. GFAP immunostaining of brain coronal sections from sham mice at p42 revealed quiescent astrocytes with small soma and thin processes dispersed throughout the cortex and mostly in the lacunosum moleculare layer of the hippocampus (Fig 7A). In contrast, GFAP staining from injured mice treated with NT revealed areas of persistent/late astrogliosis (Fig 7B). In injured mice treated with NT, large numbers of hypertrophic and densely GFAP-stained astrocytes with thick and abundant processes were visualized throughout the hippocampus, particularly in the lacunosum moleculare and pyramidal cell layers with areas of glial scarring observed in the radiatum layer (Fig 7B’). In mice treated with TH morphologically reactive astrocytes were present but less abundant and the area of scaring in the radiatum was smaller (Fig 7C). GFAP staining of the hemispheres ipsilateral and contralateral to the HI injury are shown (Fig 7D) again demonstrating the marked amount of gliosis evident in the ipsilateral hippocampus even in this relatively moderately injured animal. Abundant reactive astrocytes in a perivascular location are seen at higher power in the ipsilateral hemisphere of injured mice (Fig 7D’ vs 7D”).
Figure 7. Late activation of astrocytes.
Representative microphotographs of sham (A), NT (B) and TH (C) mice and details (A’, B’, and C’) in hippocampal CA1 region. Large numbers of hypertrophic and densely GFAP-stained astrocytes primarily throughout the hippocampus of injured mice treated with NT. Active astrocytes are mostly seen around glial scaring in injured mice treated with TH. Comparative GFAP staining of the hemispheres ipsilateral and contralateral to the HI injury are shown (D). In contrast to contralateral staining, abundant reactive astrocytes were visualized in the ipsilateral hemisphere of injured mice (D’ and D”). Scale bars are shown. LM, lacunosum moleculare layer; PL, pyramidal cell layer; RL, radiatum layer.
DISCUSSION
In this preclinical mouse model, exposure to HI produced a variable degree of deficits in growth, and in motor, memory, and other behavioral domains. Using a TH protocol previously described by our group [19], we showed that TH provided a variable degree of neuroprotection to specific domains of behavior, often, in a sex-specific manner. Furthermore, a late surge in mature BDNF in the hemisphere ipsilateral to the injury correlated with deficits in working memory. The source of late BDNF may be from persistently activated astrocytes as previously suggested [60,61]. Overall, these findings were consistent with evolving data from clinical trials [4–6,14,15,62–64], making this preclinical model a potent tool for investigation of neonatal HI and TH.
The effect of TH on functional outcomes following neonatal HI is inconsistent among pre-clinical rodent models. Although, rodents injured by HI manifest cognitive impairments, attention deficits, and increased impulsivity and compulsivity [65–67]; the protection reported by TH is contradictory [19,21,24,68–74]. These contradictions may arise from the lack of proper standardization in behavioral testing in immature rodents, the variable protocols of TH used, and the effects of other biological variables such as sex and growth. Our protocol of TH attenuates cortical and hippocampal injury at 8 and 20 days after neonatal HI at p10 (full-term equivalent), while providing limited protection of memory domains [19], which is consistent with findings in humans [18]. Furthermore, the sexual dimorphism documented in our model of HI and TH matches that reported in other experimental models of perinatal brain injury [19,21,24,74].
Because poor growth in the setting of neonatal HI brain injury may be both a cause and a consequence of neurologic and behavioral impairments, we analyzed the growth of the mice following HI and TH for a month after injury. A marked variability in weight was observed in the overall group. Males were heavier than females after weaning and this added to the overall variability. When growth rate was stratified by sex, differences emerged. Both injured males and female mice had poorer weight gain after weaning, and only female mice treated with TH recovered. The reason for the differences in growth after HI is unclear, possibly reflecting abnormal suck-swallow patterns often seen in the clinic [31] or other neuromotor, neuroendocrine or behavioral deficits [75]. The relative protection against growth failure afforded to females by TH may be explained by the difference in outcomes documented using the balance beam and adhesive removal testing to assess motor balance/coordination and sensorimotor processing, respectively [48,49]. While TH after HI preserved sensorimotor processing in female mice, TH preserved motor balance and coordination in male mice. Since intact sensorimotor processing is necessary for development of exploratory behaviors, including food seeking [76], we hypothesize that these very refined differences in protection of various motor domains with TH may account for sexual dimorphism in growth seen in our experiments.
Early assessment of sensorimotor deficit by measuring primitive reflexes in neonates who suffer birth asphyxia is predictive of cerebral palsy and developmental disability [77]. Because sensorimotor reflexes also evaluate behavioral and functional development in neonatal mice, we chose to assess them based on age of physiologic acquisition [45]. While no difference in air righting and forelimb grasp were observed, in negative geotaxis testing HI injured mice turned uphill faster than shams in both sexes regardless of treatment. It is controversial but possible that uninjured mice display a more positive geotaxis (downward movement) due to exploratory behavior instead of a true negative geotaxis response with possible anxiety as observed in injured mice. These findings are in agreement with observations by Motz [78], who disagrees with the use of negative geotaxis as a measurement of sensory function in rodent pups, and describes the phenomenon of “positive” geotaxis in which rodents normally orient downhill [78,79]. Additionally, injured female mice in this model display more anxiety-like behavior in the open field performance, and TH does not provide protection in this testing domain either. Although mice have a natural tendency to explore the periphery more than the unprotected center areas while in the open field, mice with anxiety like behavior spent even more time in the periphery than the center than did shams [80,81]. Congruent with these findings, a higher prevalence of anxiety disorders and increased impulsivity and compulsivity characterize HIE survivors [82].
Clinical trials using TH for birth asphyxia have consistently documented a decrease in the incidence of motor impairments at age 18 months and again at 6–7 years [1,3–5,62,83,84]. Except for motor outcomes, large RCTs were not designed to answer questions about specific neurobehavioral endpoints. In agreement with these clinical trial results, our mouse model of 4 h of TH following HI brain injury also showed better motor outcomes in the treated mice than NT mice at both p24 and p36. Additionally, we found differences in motor impairment using sex-stratification and two different tests. While male NT mice took twice as long to cross the 6 mm and round balance beams, female NT mice took almost twice as long to remove the adhesive from the left paw (contralateral to the injured hemisphere). Regardless of the test, TH provided protection against motor impairment in both sexes. Differences in results in the balance beam and adhesive removal tests may provide further understanding of how each sex displays motor deficits. While success in crossing the beam requires motor strength, coordination and balance [48], success in removing the adhesive also requires somatosensory recognition [49]. Whether differences in coordination and somatosensory recognition after injury and TH between sexes explain the differences in the sensitivity of these tests to show motor deficits in males and females, is not known.
Despite TH, learning and memory impairments persist at age 6 to 7 years after birth asphyxia [1,2,15,18]. Similarly, deficits in spatial recognition and working memory were documented in HI mice of both sexes despite treatment with TH. All injured mice visited the novel arm of the Y maze less often than sham mice. In addition, injured NT male mice had more overall arm visits than shams. Increased number of total arm visits may be indicative of hyperactive behavior previously described in rodents exposed to HI injury [65,67], but performance in the Y-maze is not designed to evaluate hyperactivity. Similarly, during OLT performance, injured male mice had a negative DI score compared to shams implying impaired memory associated with anxiety like behavior. Sex differences in the ability to discriminate objects has been hypothesized before [85–87] in many species including humans and rodents, with females having a greater ability to recognize different objects. In the present study only injured male mice displayed an impaired ability to recognized differences in object location.
BDNF levels in the cortex and hippocampus (forebrain) acutely increase by 24h after HI injury in the mouse and then decreases by 96h after injury.[39] Similar findings are documented in a middle cerebral artery occlusion stroke model in rats.[88] Changes in BDNF occurring at late stages after neonatal HI injury have not been documented previously. Thirty days after HI, BDNF levels in the injured forebrain were two times higher than those in sham forebrain, and TH did not have any effect on this increase. Neither the source nor the significance of this late elevation of BDNF in the ipsilateral brain is known. BDNF may be used by the neurons to improve their survival and thus promote recovery after injury [58], and may also be a surrogate for persistent ongoing injury or activation of astrocytes.[61,89] Although BDNF level in the forebrain contralateral to the injury was unchanged by HI exposure, the lack of compensatory BDNF upregulation and thus a deficit relative to the ipsilateral side may be detrimental. Altered ipsilateral vs. contralateral BDNF levels may signal disrupted interhemispheric connectivity, well described in models of unilateral stroke.[90–92] Disrupted integrity of the corpus callosum in patients with deficient BDNF levels and memory impairment substantiates the importance of interhemispheric connectivity and the effects of BDNF produced remote from the region of injury.[93] The present data suggest that a similar physiology may be occurring in the present model given that individual mice with higher I/C BDNF ratios had worse performance in the Y-maze memory test and in the OLT, while those with higher contralateral BDNF had better outcomes. Because these BDNF changes were not associated with changes in TrkB phosphorylation, we speculate that the association with memory impairments may be mediated by the binding of BDNF or pro-BDNF to p75Ntr [39,42]. These findings coincided temporally with persistent activation of astrocytes occurring over a month after the HI injury in the present model. Whether activated astrocytes were the source for the increase BDNF level measured in the forebrain ipsilateral to the injury is unclear.
We acknowledge several limitations in this study. An imaging correlate was lacking, limiting the assessment of overall neuropathology and white matter integrity, both variables that affect neuro-behavior. However, studies by our group and others have shown that findings on neuropathology do not necessarily predict which subjects will develop neurobehavioral impairments [19,94,95]. In the present study evaluation of feeding pattern and fluid/food intake were not performed, limiting the interpretation of the growth data. However, all animals had access to food and water AD LIBITUM, even while in the testing room and they were monitored daily for at least 45 min to ascertain accessibility despite potential motor impairments. Testing of young mice remains a challenge and there is no clear standardization. In fact, our shams performed poorer than expected compared to adult mice on some tests. Nevertheless, we were still able to detect differences attributable to injury and treatment. Lastly, we were unable to compare outcomes between sexes or between time points. Instead, we are only able to accurately document differences between groups of the same sex, and at the same age or period.
In summary, the variable injury and incomplete neuroprotection by TH presented here was consistent with clinical data from large RCTs [4–6,14,15,62–64] and with several other experimental models of HI and TH.[19,20,96] Similarly, the growth deficits following HI injury and TH were similar to those described in HIE survivors who developed cerebral palsy [97]. The sexual dimorphism documented in our study was consistent with that reported in other experimental models of perinatal injury [19,21,24,74]. BDNF changes in those mice performing worse after HI, regardless of treatment, may provide information about the importance of BDNF at late time points after perinatal brain injury. Our results confirmed that sex-stratification is necessary in some assessments of outcome following neonatal HI. Our findings that abnormalities in certain behavioral domains were unresponsive to TH support the relevance of this model for preclinical testing of adjuvant therapies for neonatal HI.
Acknowledgments
The authors thank Mrs. Rosie Silva for their administrative assistance. Experiments and investigators were funded in part by grants from the National Institute of Health (RO1HD070996, RO1HD086058 - FJN) and by the Sutland-Pakula Endowment for neonatal Research (RC-V). The authors are grateful for the work of Dr. Jennifer Burnsed to develop the TH model in p10 mice. The authors declare no conflict of interest related to the data presented in the manuscript.
References
- 1.Shankaran S. Outcomes of hypoxic-ischemic encephalopathy in neonates treated with hypothermia. Clin Perinatol. 2014;41:149–159. doi: 10.1016/j.clp.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 2.Natarajan G, Shankaran S, Laptook AR, Pappas A, Bann CM, McDonald SA, Das A, Higgins RD, Hintz SR, Vohr BR Extended Hypothermia Subcommittee of the Eunice Kennedy Shriver National Institute of Child H, Human Development Neonatal Research N. Apgar scores at 10 min and outcomes at 6–7 years following hypoxic-ischaemic encephalopathy. Arch Dis Child Fetal Neonatal Ed. 2013;98:F473–479. doi: 10.1136/archdischild-2013-303692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shankaran S, Pappas A, McDonald SA, Vohr BR, Hintz SR, Yolton K, Gustafson KE, Leach TM, Green C, Bara R, Petrie Huitema CM, Ehrenkranz RA, Tyson JE, Das A, Hammond J, Peralta-Carcelen M, Evans PW, Heyne RJ, Wilson-Costello DE, Vaucher YE, Bauer CR, Dusick AM, Adams-Chapman I, Goldstein RF, Guillet R, Papile LA, Higgins RD Eunice Kennedy Shriver NNRN. Childhood outcomes after hypothermia for neonatal encephalopathy. N Engl J Med. 2012;366:2085–2092. doi: 10.1056/NEJMoa1112066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Edwards AD, Brocklehurst P, Gunn AJ, Halliday H, Juszczak E, Levene M, Strohm B, Thoresen M, Whitelaw A, Azzopardi D. Neurological outcomes at 18 months of age after moderate hypothermia for perinatal hypoxic ischaemic encephalopathy: synthesis and meta-analysis of trial data. BMJ. 2010;340:c363. doi: 10.1136/bmj.c363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burton VJ, Gerner G, Cristofalo E, Chung SE, Jennings JM, Parkinson C, Koehler RC, Chavez-Valdez R, Johnston MV, Northington FJ, Lee JK. A pilot cohort study of cerebral autoregulation and 2-year neurodevelopmental outcomes in neonates with hypoxic-ischemic encephalopathy who received therapeutic hypothermia. BMC Neurol. 2015;15:209. doi: 10.1186/s12883-015-0464-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eicher DJ, Wagner CL, Katikaneni LP, Hulsey TC, Bass WT, Kaufman DA, Horgan MJ, Languani S, Bhatia JJ, Givelichian LM, Sankaran K, Yager JY. Moderate hypothermia in neonatal encephalopathy: efficacy outcomes. Pediatr Neurol. 2005;32:11–17. doi: 10.1016/j.pediatrneurol.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 7.Gluckman PD, Wyatt JS, Azzopardi D, Ballard R, Edwards AD, Ferriero DM, Polin RA, Robertson CM, Thoresen M, Whitelaw A, Gunn AJ. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet. 2005;365:663–670. doi: 10.1016/S0140-6736(05)17946-X. [DOI] [PubMed] [Google Scholar]
- 8.Shankaran S, Laptook AR, Ehrenkranz RA, Tyson JE, McDonald SA, Donovan EF, Fanaroff AA, Poole WK, Wright LL, Higgins RD, Finer NN, Carlo WA, Duara S, Oh W, Cotten CM, Stevenson DK, Stoll BJ, Lemons JA, Guillet R, Jobe AH National Institute of Child H, Human Development Neonatal Research N. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005;353:1574–1584. doi: 10.1056/NEJMcps050929. [DOI] [PubMed] [Google Scholar]
- 9.Shankaran S, Pappas A, Laptook AR, McDonald SA, Ehrenkranz RA, Tyson JE, Walsh M, Goldberg RN, Higgins RD, Das A, Network NNR. Outcomes of safety and effectiveness in a multicenter randomized, controlled trial of whole-body hypothermia for neonatal hypoxic-ischemic encephalopathy. Pediatrics. 2008;122:e791–798. doi: 10.1542/peds.2008-0456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kurinczuk JJ, White-Koning M, Badawi N. Epidemiology of neonatal encephalopathy and hypoxic-ischaemic encephalopathy. Early Hum Dev. 2010;86:329–338. doi: 10.1016/j.earlhumdev.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 11.Graham EM, Ruis KA, Hartman AL, Northington FJ, Fox HE. A systematic review of the role of intrapartum hypoxia-ischemia in the causation of neonatal encephalopathy. Am J Obstet Gynecol. 2008;199:587–595. doi: 10.1016/j.ajog.2008.06.094. [DOI] [PubMed] [Google Scholar]
- 12.Jacobs S, Hunt R, Tarnow-Mordi W, Inder T, Davis P. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database Syst Rev. 2007:CD003311. doi: 10.1002/14651858.CD003311.pub2. [DOI] [PubMed] [Google Scholar]
- 13.Gonzalez FF, Ferriero DM. Neuroprotection in the newborn infant. Clin Perinatol. 2009;36:859–880. vii. doi: 10.1016/j.clp.2009.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoehn T, Hansmann G, Buhrer C, Simbruner G, Gunn AJ, Yager J, Levene M, Hamrick SE, Shankaran S, Thoresen M. Therapeutic hypothermia in neonates. Review of current clinical data, ILCOR recommendations and suggestions for implementation in neonatal intensive care units. Resuscitation. 2008;78:7–12. doi: 10.1016/j.resuscitation.2008.04.027. [DOI] [PubMed] [Google Scholar]
- 15.Azzopardi D, Brocklehurst P, Edwards D, Halliday H, Levene M, Thoresen M, Whitelaw A The TOBY Study. Whole body hypothermia for the treatment of perinatal asphyxial encephalopathy: a randomised controlled trial. BMC Pediatr. 2008;8:17. doi: 10.1186/1471-2431-8-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jacobs SE. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: Multicentre randomised trial. J Pediatr. 2005;147:122–123. doi: 10.1016/j.jpeds.2005.04.047. [DOI] [PubMed] [Google Scholar]
- 17.Simbruner G, Mittal RA, Rohlmann F, Muche R neo.n EnTP. Systemic hypothermia after neonatal encephalopathy: outcomes of neo.nEURO. network RCT. Pediatrics. 2010;126:e771–778. doi: 10.1542/peds.2009-2441. [DOI] [PubMed] [Google Scholar]
- 18.de Vries LS, Cowan FM. Evolving understanding of hypoxic-ischemic encephalopathy in the term infant. Seminars in pediatric neurology. 2009;16:216–225. doi: 10.1016/j.spen.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 19.Burnsed JC, Chavez-Valdez R, Hossain MS, Kesavan K, Martin LJ, Zhang J, Northington FJ. Hypoxia-ischemia and therapeutic hypothermia in the neonatal mouse brain--a longitudinal study. PLoS One. 2015;10:e0118889. doi: 10.1371/journal.pone.0118889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carlsson Y, Wang X, Schwendimann L, Rousset CI, Jacotot E, Gressens P, Thoresen M, Mallard C, Hagberg H. Combined effect of hypothermia and caspase-2 gene deficiency on neonatal hypoxic-ischemic brain injury. Pediatr Res. 2012;71:566–572. doi: 10.1038/pr.2012.15. [DOI] [PubMed] [Google Scholar]
- 21.Reinboth BS, Koster C, Abberger H, Prager S, Bendix I, Felderhoff-Muser U, Herz J. Endogenous hypothermic response to hypoxia reduces brain injury: Implications for modeling hypoxic- ischemic encephalopathy and therapeutic hypothermia in neonatal mice. Exp Neurol. 2016;283:264–275. doi: 10.1016/j.expneurol.2016.06.024. [DOI] [PubMed] [Google Scholar]
- 22.Northington FJ, Chavez-Valdez R, Graham EM, Razdan S, Gauda EB, Martin LJ. Necrostatin decreases oxidative damage, inflammation, and injury after neonatal HI. J Cereb Blood Flow Metab. 2011;31:178–189. doi: 10.1038/jcbfm.2010.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Waddell J, Hanscom M, Shalon Edwards N, McKenna MC, McCarthy MM. Sex differences in cell genesis, hippocampal volume and behavioral outcomes in a rat model of neonatal HI. Exp Neurol. 2016;275(Pt 2):285–295. doi: 10.1016/j.expneurol.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith AL, Garbus H, Rosenkrantz TS, Fitch RH. Sex differences in behavioral outcomes following temperature modulation during induced neonatal hypoxic ischemic injury in rats. Brain sciences. 2015;5:220–240. doi: 10.3390/brainsci5020220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gupta SK, Sarmah BK, Tiwari D, Shakya A, Khatiwada D. Clinical Profile of Neonates with Perinatal Asphyxia in a Tertiary Care Hospital of Central Nepal. JNMA J Nepal Med Assoc. 2014;52:1005–1009. [PubMed] [Google Scholar]
- 26.Hayes BC, McGarvey C, Mulvany S, Kennedy J, Geary MP, Matthews TG, King MD. A case-control study of hypoxic-ischemic encephalopathy in newborn infants at >36 weeks gestation. Am J Obstet Gynecol. 2013;209:29e21–29e19. doi: 10.1016/j.ajog.2013.03.023. [DOI] [PubMed] [Google Scholar]
- 27.Yeargin-Allsopp M, Van Naarden Braun K, Doernberg NS, Benedict RE, Kirby RS, Durkin MS. Prevalence of cerebral palsy in 8-year-old children in three areas of the United States in 2002: a multisite collaboration. Pediatrics. 2008;121:547–554. doi: 10.1542/peds.2007-1270. [DOI] [PubMed] [Google Scholar]
- 28.Jarvis S, Glinianaia SV, Arnaud C, Fauconnier J, Johnson A, McManus V, Topp M, Uvebrant P, Cans C, Krageloh-Mann I Registers ScoECP. Case gender and severity in cerebral palsy varies with intrauterine growth. Archives of disease in childhood. 2005;90:474–479. doi: 10.1136/adc.2004.052670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ment LR, Vohr BR, Makuch RW, Westerveld M, Katz KH, Schneider KC, Duncan CC, Ehrenkranz R, Oh W, Philip AG, Scott DT, Allan WC. Prevention of intraventricular hemorrhage by indomethacin in male preterm infants. J Pediatr. 2004;145:832–834. doi: 10.1016/j.jpeds.2004.07.035. [DOI] [PubMed] [Google Scholar]
- 30.Reiss AL, Kesler SR, Vohr B, Duncan CC, Katz KH, Pajot S, Schneider KC, Makuch RW, Ment LR. Sex differences in cerebral volumes of 8-year-olds born preterm. J Pediatr. 2004;145:242–249. doi: 10.1016/j.jpeds.2004.04.031. [DOI] [PubMed] [Google Scholar]
- 31.Slattery J, Morgan A, Douglas J. Early sucking and swallowing problems as predictors of neurodevelopmental outcome in children with neonatal brain injury: a systematic review. Dev Med Child Neurol. 54:796–806. doi: 10.1111/j.1469-8749.2012.04318.x. [DOI] [PubMed] [Google Scholar]
- 32.Lipska BK, Khaing ZZ, Weickert CS, Weinberger DR. BDNF mRNA expression in rat hippocampus and prefrontal cortex: effects of neonatal ventral hippocampal damage and antipsychotic drugs. Eur J Neurosci. 2001;14:135–144. doi: 10.1046/j.1460-9568.2001.01633.x. [DOI] [PubMed] [Google Scholar]
- 33.Figurov A, Pozzo-Miller LD, Olafsson P, Wang T, Lu B. Regulation of synaptic responses to high-frequency stimulation and LTP by neurotrophins in the hippocampus. Nature. 1996;381:706–709. doi: 10.1038/381706a0. [DOI] [PubMed] [Google Scholar]
- 34.Lu B, Gottschalk W. Modulation of hippocampal synaptic transmission and plasticity by neurotrophins. Prog Brain Res. 2000;128:231–241. doi: 10.1016/S0079-6123(00)28020-5. [DOI] [PubMed] [Google Scholar]
- 35.Aarse J, Herlitze S, Manahan-Vaughan D. The requirement of BDNF for hippocampal synaptic plasticity is experience-dependent. Hippocampus. 2016;26:739–751. doi: 10.1002/hipo.22555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Briz V, Liu Y, Zhu G, Bi X, Baudry M. A novel form of synaptic plasticity in field CA3 of hippocampus requires GPER1 activation and BDNF release. The Journal of cell biology. 2015;210:1225–1237. doi: 10.1083/jcb.201504092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Graves AR, Moore SJ, Spruston N, Tryba AK, Kaczorowski CC. Brain-derived neurotrophic factor differentially modulates excitability of two classes of hippocampal output neurons. Journal of neurophysiology. 2016;116:466–471. doi: 10.1152/jn.00186.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Almli CR, Levy TJ, Han BH, Shah AR, Gidday JM, Holtzman DM. BDNF protects against spatial memory deficits following neonatal hypoxia-ischemia. Exp Neurol. 2000;166:99–114. doi: 10.1006/exnr.2000.7492. [DOI] [PubMed] [Google Scholar]
- 39.Chavez-Valdez R, Martin LJ, Razdan S, Gauda EB, Northington FJ. Sexual dimorphism in BDNF signaling after neonatal hypoxia-ischemia and treatment with necrostatin-1. Neuroscience. 2014;260:106–119. doi: 10.1016/j.neuroscience.2013.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Johnston MV. Plasticity in the developing brain: implications for rehabilitation. Dev Disabil Res Rev. 2009;15:94–101. doi: 10.1002/ddrr.64. [DOI] [PubMed] [Google Scholar]
- 41.Madri JA. Modeling the neurovascular niche: implications for recovery from CNS injury. J Physiol Pharmacol. 2009;60(Suppl 4):95–104. [PubMed] [Google Scholar]
- 42.Foltran RB, Diaz SL. BDNF isoforms: a round trip ticket between neurogenesis and serotonin? J Neurochem. 2016;138:204–221. doi: 10.1111/jnc.13658. [DOI] [PubMed] [Google Scholar]
- 43.Graham EM, Sheldon RA, Flock DL, Ferriero DM, Martin LJ, O'Riordan DP, Northington FJ. Neonatal mice lacking functional Fas death receptors are resistant to hypoxic-ischemic brain injury. Neurobiol Dis. 2004;17:89–98. doi: 10.1016/j.nbd.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 44.Roedel A, Storch C, Holsboer F, Ohl F. Effects of light or dark phase testing on behavioural and cognitive performance in DBA mice. Laboratory animals. 2006;40:371–381. doi: 10.1258/002367706778476343. [DOI] [PubMed] [Google Scholar]
- 45.Hill JMLM, Stone MM. Developmental Milestones in the newborn mouse. 1. Totowa, New Jersey: Humana Press; 2007. [Google Scholar]
- 46.Bailey KRCJ. Anxiety-Related Behavior in Mice. 2. Boca Raton, Florida: CRC Press/ Taylor &Francis; 2009. [Google Scholar]
- 47.Prut L, Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur J Pharmacol. 2003;463:3–33. doi: 10.1016/s0014-2999(03)01272-x. [DOI] [PubMed] [Google Scholar]
- 48.Luong TN, Carlisle HJ, Southwell A, Patterson PH. Assessment of motor balance and coordination in mice using the balance beam. J Vis Exp. doi: 10.3791/2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bouet V, Boulouard M, Toutain J, Divoux D, Bernaudin M, Schumann-Bard P, Freret T. The adhesive removal test: a sensitive method to assess sensorimotor deficits in mice. Nat Protoc. 2009;4:1560–1564. doi: 10.1038/nprot.2009.125. [DOI] [PubMed] [Google Scholar]
- 50.Bird CM, Bisby JA, Burgess N. The hippocampus and spatial constraints on mental imagery. Front Hum Neurosci. 6:142. doi: 10.3389/fnhum.2012.00142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Save E, Poucet B. Role of the parietal cortex in long-term representation of spatial information in the rat. Neurobiol Learn Mem. 2009;91:172–178. doi: 10.1016/j.nlm.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 52.Carlini VP, Ghersi M, Gabach L, Schioth HB, Perez MF, Ramirez OA, Fiol de Cuneo M, de Barioglio SR. Hippocampal effects of neuronostatin on memory, anxiety-like behavior and food intake in rats. Neuroscience. 197:145–152. doi: 10.1016/j.neuroscience.2011.09.040. [DOI] [PubMed] [Google Scholar]
- 53.Antunes M, Biala G. The novel object recognition memory: neurobiology, test procedure, and its modifications. Cogn Process. 13:93–110. doi: 10.1007/s10339-011-0430-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jablonski SA, Schreiber WB, Westbrook SR, Brennan LE, Stanton ME. Determinants of novel object and location recognition during development. Behav Brain Res. 2013;256:140–150. doi: 10.1016/j.bbr.2013.07.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vogel-Ciernia A, Wood MA. Examining object location and object recognition memory in mice. Curr Protoc Neurosci. 2014;69:8 31 31–17. doi: 10.1002/0471142301.ns0831s69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Northington FJ, Koehler RC, Traystman RJ, Martin LJ. Nitric oxide synthase 1 and nitric oxide synthase 3 protein expression is regionally and temporally regulated in fetal brain. Brain Res Dev Brain Res. 1996;95:1–14. doi: 10.1016/0165-3806(96)00051-x. [DOI] [PubMed] [Google Scholar]
- 57.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 58.Bath KG, Lee FS. Variant BDNF (Val66Met) impact on brain structure and function. Cogn Affect Behav Neurosci. 2006;6:79–85. doi: 10.3758/cabn.6.1.79. [DOI] [PubMed] [Google Scholar]
- 59.Saha RN, Liu X, Pahan K. Up-regulation of BDNF in astrocytes by TNF-alpha: a case for the neuroprotective role of cytokine. J Neuroimmune Pharmacol. 2006;1:212–222. doi: 10.1007/s11481-006-9020-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bejot Y, Prigent-Tessier A, Cachia C, Giroud M, Mossiat C, Bertrand N, Garnier P, Marie C. Time-dependent contribution of non neuronal cells to BDNF production after ischemic stroke in rats. Neurochem Int. 2011;58:102–111. doi: 10.1016/j.neuint.2010.10.019. [DOI] [PubMed] [Google Scholar]
- 61.Yu W, Zhu H, Wang Y, Li G, Wang L, Li H. Reactive Transformation and Increased BDNF Signaling by Hippocampal Astrocytes in Response to MK-801. PLoS One. 10:e0145651. doi: 10.1371/journal.pone.0145651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Azzopardi D, Strohm B, Marlow N, Brocklehurst P, Deierl A, Eddama O, Goodwin J, Halliday HL, Juszczak E, Kapellou O, Levene M, Linsell L, Omar O, Thoresen M, Tusor N, Whitelaw A, Edwards AD, Group TS. Effects of hypothermia for perinatal asphyxia on childhood outcomes. N Engl J Med. 2014;371:140–149. doi: 10.1056/NEJMoa1315788. [DOI] [PubMed] [Google Scholar]
- 63.Eicher DJ, Wagner CL, Katikaneni LP, Hulsey TC, Bass WT, Kaufman DA, Horgan MJ, Languani S, Bhatia JJ, Givelichian LM, Sankaran K, Yager JY. Moderate hypothermia in neonatal encephalopathy: safety outcomes. Pediatr Neurol. 2005;32:18–24. doi: 10.1016/j.pediatrneurol.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 64.Gardiner J, Wagh D, McMichael J, Hakeem M, Rao S. Outcomes of hypoxic ischaemic encephalopathy treated with therapeutic hypothermia using cool gel packs - experience from Western Australia. Eur J Paediatr Neurol. 2014;18:391–398. doi: 10.1016/j.ejpn.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 65.Miguel PM, Schuch CP, Rojas JJ, Carletti JV, Deckmann I, Martinato LH, Pires AV, Bizarro L, Pereira LO. Neonatal hypoxia-ischemia induces attention-deficit hyperactivity disorder-like behavior in rats. Behav Neurosci. 2015;129:309–320. doi: 10.1037/bne0000063. [DOI] [PubMed] [Google Scholar]
- 66.Ten VS, Bradley-Moore M, Gingrich JA, Stark RI, Pinsky DJ. Brain injury and neurofunctional deficit in neonatal mice with hypoxic-ischemic encephalopathy. Behav Brain Res. 2003;145:209–219. doi: 10.1016/s0166-4328(03)00146-3. [DOI] [PubMed] [Google Scholar]
- 67.Mucci de DB, Fernandes FS, Souza Ados S, Sardinha FL, Soares-Mota M, Tavares do Carmo M. Flaxseed mitigates brain mass loss, improving motor hyperactivity and spatial memory, in a rodent model of neonatal hypoxic-ischemic encephalopathy. Prostaglandins Leukot Essent Fatty Acids. 97:13–19. doi: 10.1016/j.plefa.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 68.Hobbs CE, Oorschot DE. Neonatal rat hypoxia-ischemia: long-term rescue of striatal neurons and motor skills by combined antioxidant-hypothermia treatment. Brain Pathol. 2008;18:443–454. doi: 10.1111/j.1750-3639.2008.00146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dalen ML, Liu X, Elstad M, Loberg EM, Saugstad OD, Rootwelt T, Thoresen M. Resuscitation with 100% oxygen increases injury and counteracts the neuroprotective effect of therapeutic hypothermia in the neonatal rat. Pediatr Res. 71:247–252. doi: 10.1038/pr.2011.43. [DOI] [PubMed] [Google Scholar]
- 70.Thoresen M, Bagenholm R, Loberg EM, Apriccna F. The stress of being restrained reduces brain damage after a hypoxic-ischaemic insult in the 7-day-old rat. Neuroreport. 1996;7:481–484. doi: 10.1097/00001756-199601310-00025. [DOI] [PubMed] [Google Scholar]
- 71.Thoresen M, Bagenholm R, Loberg EM, Apricena F, Kjellmer I. Posthypoxic cooling of neonatal rats provides protection against brain injury. Arch Dis Child Fetal Neonatal Ed. 1996;74:F3–9. doi: 10.1136/fn.74.1.f3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hobbs C, Thoresen M, Tucker A, Aquilina K, Chakkarapani E, Dingley J. Xenon and hypothermia combine additively, offering long-term functional and histopathologic neuroprotection after neonatal hypoxia/ischemia. Stroke. 2008;39:1307–1313. doi: 10.1161/STROKEAHA.107.499822. [DOI] [PubMed] [Google Scholar]
- 73.Sabir H, Scull-Brown E, Liu X, Thoresen M. Immediate hypothermia is not neuroprotective after severe hypoxia-ischemia and is deleterious when delayed by 12 hours in neonatal rats. Stroke. 43:3364–3370. doi: 10.1161/STROKEAHA.112.674481. [DOI] [PubMed] [Google Scholar]
- 74.Dietz RM, Deng G, Orfila JE, Hui X, Traystman RJ, Herson PS. Therapeutic hypothermia protects against ischemia-induced impairment of synaptic plasticity following juvenile cardiac arrest in sex-dependent manner. Neuroscience. 325:132–141. doi: 10.1016/j.neuroscience.2016.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Robertson CM, Perlman M. Follow-up of the term infant after hypoxic-ischemic encephalopathy. Paediatrics & child health. 2006;11:278–282. [PMC free article] [PubMed] [Google Scholar]
- 76.Arakawa H, Erzurumlu RS. Role of whiskers in sensorimotor development of C57BL/6 mice. Behav Brain Res. 2015;287:146–155. doi: 10.1016/j.bbr.2015.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Futagi Y, Suzuki Y, Goto M. Clinical significance of plantar grasp response in infants. Pediatr Neurol. 1999;20:111–115. doi: 10.1016/s0887-8994(98)00103-9. [DOI] [PubMed] [Google Scholar]
- 78.Motz BA, Alberts JR. The validity and utility of geotaxis in young rodents. Neurotoxicol Teratol. 2005;27:529–533. doi: 10.1016/j.ntt.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 79.Kreider JC, Blumberg MS. Geotaxis and beyond: commentary on Motz and Alberts (2005) Neurotoxicol Teratol. 2005;27:535–537. doi: 10.1016/j.ntt.2005.06.002. author reply 543–534. [DOI] [PubMed] [Google Scholar]
- 80.Nguyen L, Bohlen J, Stricker J, Chahal I, Zhang H, Pistilli EE. Hippocampus-specific deficiency of IL-15Ralpha contributes to greater anxiety-like behaviors in mice. Metab Brain Dis. 2016 doi: 10.1007/s11011-016-9930-y. [DOI] [PubMed] [Google Scholar]
- 81.Yoshizaki K, Furuse T, Kimura R, Tucci V, Kaneda H, Wakana S, Osumi N. Paternal Aging Affects Behavior in Pax6 Mutant Mice: A Gene/Environment Interaction in Understanding Neurodevelopmental Disorders. PLoS One. 2016;11:e0166665. doi: 10.1371/journal.pone.0166665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Moster D, Lie RT, Markestad T. Joint association of Apgar scores and early neonatal symptoms with minor disabilities at school age. Arch Dis Child Fetal Neonatal Ed. 2002;86:F16–21. doi: 10.1136/fn.86.1.F16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shankaran S, McDonald SA, Laptook AR, Hintz SR, Barnes PD, Das A, Pappas A, Higgins RD Eunice Kennedy Shriver National Institute of Child H, Human Development Neonatal Research N. Neonatal Magnetic Resonance Imaging Pattern of Brain Injury as a Biomarker of Childhood Outcomes following a Trial of Hypothermia for Neonatal Hypoxic-Ischemic Encephalopathy. J Pediatr. 2015;167:987–993. e983. doi: 10.1016/j.jpeds.2015.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cao CQ, Li YN, Yang XM, Gong YG, Wang F, Li WG. Long-term clinical efficacy of mild hypothermia therapy in neonates with hypoxic-ischemic encephalopathy: a Meta analysis. Zhongguo Dang Dai Er Ke Za Zhi. 2015;17:122–127. [PubMed] [Google Scholar]
- 85.Bettis TJ, Jacobs LF. Sex-specific strategies in spatial orientation in C57BL/6J mice. Behav Processes. 2009;82:249–255. doi: 10.1016/j.beproc.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 86.Bettis T, Jacobs LF. Sex differences in object recognition are modulated by object similarity. Behav Brain Res. 233:288–292. doi: 10.1016/j.bbr.2012.04.028. [DOI] [PubMed] [Google Scholar]
- 87.Bettis TJ, Jacobs LF. Sex differences in memory for landmark arrays in C57BL/J6 mice. Anim Cogn. 16:873–882. doi: 10.1007/s10071-013-0619-x. [DOI] [PubMed] [Google Scholar]
- 88.Kokaia Z, Andsberg G, Martinez-Serrano A, Lindvall O. Focal cerebral ischemia in rats induces expression of P75 neurotrophin receptor in resistant striatal cholinergic neurons. Neuroscience. 1998;84:1113–1125. doi: 10.1016/s0306-4522(97)00579-4. [DOI] [PubMed] [Google Scholar]
- 89.Thomas AX, Cruz Del Angel Y, Gonzalez MI, Carrel AJ, Carlsen J, Lam PM, Hempstead BL, Russek SJ, Brooks-Kayal AR. Rapid Increases in proBDNF after Pilocarpine-Induced Status Epilepticus in Mice Are Associated with Reduced proBDNF Cleavage Machinery. eNeuro. :3. doi: 10.1523/ENEURO.0020-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhang B, Wang RZ, Lian ZG, Song Y, Yao Y. Experimental study on plasticity of proliferated neural stem cells in adult rats after cerebral infarction. Chin Med Sci J. 2006;21:184–188. [PubMed] [Google Scholar]
- 91.Almeida SR, Vicentini J, Bonilha L, De Campos BM, Casseb RF, Min LL. Brain Connectivity and Functional Recovery in Patients With Ischemic Stroke. J Neuroimaging. 2016 doi: 10.1111/jon.12362. [DOI] [PubMed] [Google Scholar]
- 92.Grefkes C, Fink GR. Connectivity-based approaches in stroke and recovery of function. Lancet Neurol. 2014;13:206–216. doi: 10.1016/S1474-4422(13)70264-3. [DOI] [PubMed] [Google Scholar]
- 93.Smolders R, Rijpkema M, Franke B, Fernandez G. BDNF Val66Met polymorphism interacts with sex to influence bimanual motor control in healthy humans. Brain Behav. 2012;2:726–731. doi: 10.1002/brb3.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bertolino G, De Araujo FL, Souza HC, Coimbra NC, De Araujo JE. Neuropathology and behavioral impairments after bilateral global ischemia surgery and exposure to static magnetic field: Evidence in the motor cortex, the hippocampal CA1 region and the neostriatum. Int J Radiat Biol. 2013;89:595–601. doi: 10.3109/09553002.2013.784422. [DOI] [PubMed] [Google Scholar]
- 95.Bertolino G, Dutra Souza HC, de Araujo JE. Neuropathology and behavioral impairments in Wistar rats with a 6-OHDA lesion in the substantia nigra compacta and exposure to a static magnetic field. Electromagn Biol Med. 2013;32:527–535. doi: 10.3109/15368378.2012.751394. [DOI] [PubMed] [Google Scholar]
- 96.Lin EP, Miles L, Hughes EA, McCann JC, Vorhees CV, McAuliffe JJ, Loepke AW. A combination of mild hypothermia and sevoflurane affords long-term protection in a modified neonatal mouse model of cerebral hypoxia-ischemia. Anesth Analg. 119:1158–1173. doi: 10.1213/ANE.0000000000000262. [DOI] [PubMed] [Google Scholar]
- 97.Vohr BR, Stephens BE, McDonald SA, Ehrenkranz RA, Laptook AR, Pappas A, Hintz SR, Shankaran S, Higgins RD, Das A Extended Hypothermia Follow-up Subcommittee of the NNRN. Cerebral palsy and growth failure at 6 to 7 years. Pediatrics. 2013;132:e905–914. doi: 10.1542/peds.2012-3915. [DOI] [PMC free article] [PubMed] [Google Scholar]