Abstract
Introduction
This study investigated the curcumin effect on the cerebral aneurysm. Apoptosis is known to play a fundamental role in the pathogenesis of a cerebral aneurysm. Therefore, we investigated the effect of curcumin on apoptosis of smooth muscle cells of a cerebral aneurysm‐induced male albino rats.
Methods
In this study, the cerebral aneurysm has been induced in the male albino rats by the CaCl2 administration. After cerebral aneurysm induction, smooth muscle cells were isolated. Cells were treated with curcumin (25 & 50 mg/kg bwt) for 48 hr.
Results
Curcumin reduced altered mitochondrial morphology significantly, evidenced through fluorescence and confocal study. Curcumin treatment reduced the expression of p53, caspase‐3, and bax/bxl‐2 ratio significantly. Curcumin treatment also reversed the cellular architecture of smooth muscle cell wall significantly. Fluorescence and the confocal study confirmed the reduction in apoptosis in a cerebral aneurysm‐induced smooth muscle cells of male albino rats.
Conclusion
Taking all these data together, it may suggest that the curcumin could significantly reduce the CaCl2‐induced cerebral aneurysm through the inhibition of cell apoptosis in the cells.
Keywords: a cerebral aneurysm, apoptosis, caspase‐3, curcumin, rats
1. INTRODUCTION
Curcumin is a principal curcuminoid of turmeric, which comes under the family of ginger. Turmeric present as desmethoxycurcumin and bis‐desmethoxycurcumin forms. Natural phenols provide the yellow color to turmeric and exist as 1, 3‐diketo and enol forms. The keto form is weaker than enol form (Manolova, Deneva, Antonov, Momekova, & Lambov, 2014). Curcumin has been reported to have antioxidant and anti‐inflammatory effects (Biswas, McClure, Jimenez, Megson, & Rahman, 2005; Dutta, Padhye, Priyadarsini, & Newton, 2005; Lim et al., 2005; Weber, Hunsaker, Abcouwer, Deck, & Vander Jagt, 2005). Thiyagarajan & Sharma (2004) have reported that the immunomodulatory, anti‐inflammatory, antioxidant, and neuroprotective effects. In Chinese traditional medicine, the curcumin has been used to treat mental stress and hypochondriac distensive mania and pain. The development of aneurysms has been reduced following curcumin treatment (Parodi, Mao, Ennis, Pagano, & Thompson, 2006). Curcumin has been reported to act against ischemia reperfusion injury (Fiorillo et al., 2008) and dopaminergic neuronal cell death (Yu et al., 2010). The potential therapeutic effect of curcumin against thoracic and abdominal aortic has been reported (Fan et al., 2012; Hao, Chen, Wang, Dong, & Yang, 2014).
An aneurysm is a condition that often leads to death by rupture and dissection (Jones, Spinale, & Ikonomidis, 2009). A cerebral aneurysm is defined as a cerebrovascular disorder in which weakness in the wall of a cerebral artery and vein causes ballooning of the blood vessel and a localized dilation. Aneurysms in the posterior circulation region have a greater risk of rupture. The basilar artery aneurysms account only 3–5% of total cerebral aneurysms. However, most of the common aneurysms located in the posterior circulation (Brisman, Song, & Newell, 2006). A detailed information and mechanism of cerebral aneurysm formation may provide active therapeutic strategies. There are several studies that have reported that the c‐Jun N‐terminal kinase plays a notable role in the prevention of cerebral artery and abdominal aortic aneurysms (Yoshimura et al., 2005). The c‐Jun N‐terminal kinase signaling pathway involves through apoptosis of vascular walls (Takagi, Ishikawa, Nozaki, Yoshimura, & Hashimoto, 2002). However, curcumin protection against cell apoptosis in a cerebral aneurysm is yet to be investigated. Therefore, this study was aimed to investigate the curcumin on apoptosis in a cerebral aneurysm‐induced male albino rats.
2. MATERIALS AND METHODS
2.1. Materials
Curcumin (≥80%) and other reagent used in this study were purchased from Sigma‐Aldrich (St. Louis, MO, USA). Mito Tracker Red, Hoechst 33258, and primary antibodies of p53, bax, bcl‐2, and caspase‐3 (Source: mouse) were purchased from Santa Cruz Biotechnology, Inc (Dallas, TX, USA). Smooth muscle cells were isolated according to Leik et al. (Wu, Hofman, & Zlokovic, 2003).
2.2. Animals
Male albino rats were obtained from the Shangai Animal House, China (180–200 g). The animal was maintained in well‐cleaned polypropylene cages at standard experimental condition (25 ± 0.5°C, relative humidity 60 ± 5%, and a photoperiod of 12 hr/day). All the animals were treated according to internationally accepted ethical procedures.
2.3. Experimental groups
Rats were divided into three groups containing six each.
Group I: Control (an aortic aneurysm)
Group II: Curcumin (10 mg/kg bwt)
Group III: Curcumin (20 mg/kg bwt)
2.4. Induction of a cerebral aneurysm
Application of CaCl2 has been reported to increase thickening of the cerebral wall in rats. Formation of a cerebral aneurysm was achieved following arterial application of CaCl2 in rats. Rats were anesthetized with pentobarbital sodium (40 mg/kg bwt) and connected to a rodent ventilator. Respiratory rate was set at 100 strokes/min in ventilator with tidal volume (3 ml). The cerebral artery was exposed and treated perivascularly with a CaCl2 solution (0.5 mol/L) for 20 min with use of presoaked gauze applicator (1.0 × 0.5 × 0.2 cm3). Sham‐operated animals were treated with saline for 20 min. After exposure, the gauze applicator was removed, and animals were housed in a temperature‐controlled room under normal 12‐hr light/12‐hr dark laboratory conditions with free access to chow and water (Fan et al., 2012).
2.5. Isolation of smooth muscle cells
Smooth muscle cells were isolated from rats with and without cerebral aneurysms. The aneurysm specimen was surgically removed and transferred to PBS with antibiotics (penicillin and streptomycin). Smooth muscle cells were isolated according to Leik and Willey (Wu et al., 2003).
2.6. Cell culture
Smooth muscle cells were obtained from rats with and without cerebral aneurysms. Cells were supplemented with DMEM (Dulbecco's Modified Eagle's medium), 10% FBS (Fetal Bovine Serum), and 1% antibiotics (penicillin–streptomycin) for the growth under standard conditions.
2.7. Mitochondrial dysfunction
To study apoptosis induction potential of curcumin by disrupting the mitochondrial membrane, we observed the changes in mitochondrial morphology following curcumin treatment. Cells were cultured at a density of 4000 cells/well and grown in the culture medium. Cells were treated with curcumin (25 and 50 mg/kg bwt) for 48 hr. At the end of 48 hr, cells were washed with PBS and exposed to 40 nmol/L Mito Tracker Red for 30 min at 37°C, and then cells were washed and exposed to 5 μg/ml of Hoechst 33258 for 25 min at room temperature. Cells were washed with PBS and viewed under a fluorescent microscope (Axiovert 2000; Carl Zeiss, Germany). Hoechst 33258 staining visualizes the cell nuclei, and Mito Tracker Red stains the mitochondria allowing visualizing alteration in mitochondrial structure due to the curcumin treatment (Moktan & Raucher, 2012).
2.8. Fluorescence microscopy
To study apoptosis induction potential of curcumin, we observed the changes in mitochondrial and cytoplasmic morphology following curcumin treatment. Cells were cultured at a density of 4000 cells/well and grown in the culture medium. Cells were treated with curcumin (25 and 50 mg/kg bwt) for 48 hr. At the end of 48 hr, cells were washed with PBS and cells were prepared according to Muthuraman et al. (2016) and viewed under a fluorescent microscope (Axiovert 2000; Carl Zeiss).
2.9. Confocal microscopy
To study apoptosis induction potential of curcumin, we observed the changes in mitochondrial and cytoplasmic morphology following curcumin treatment. Cells were cultured at a density of 4000 cells/well and grown in the culture medium. Cells were treated with curcumin (25 and 50 mg/kg bwt) for 48 hr. At the end of 48 hr, cells were washed with PBS and cells were prepared according to Muthuraman et al. (2016) and viewed under a confocal microscope (Olympus, Germany).
2.10. ROS measurement
To study ROS‐inducing potential of curcumin, we determined the ROS level after curcumin treatment. Cells were cultured at a density of 4000 cells/well and grown in the culture medium. In our preliminary study (data not shown), cells were treated with different concentrations of curcumin including 5, 10, 15, 20, 25, 30, 35, 40, 45, and 50 mg/kg bwt for 48 hr. We observed a significant effect on ROS induction at doses between 25 and 50 mg/kg bwt of curcumin. Therefore, we selected this range of concentrations for our study. Cells were treated with curcumin (25 and 50 mg/kg bwt) for 48 hr. At the end of 48 hr, cells were washed with PBS and cells were prepared according to Muthuraman et al. (2016) and viewed under a fluorescent microscope (Axiovert 2000; Carl Zeiss).
2.11. qPCR
Total RNA was isolated from the control and treated groups. The qPCR was performed using a cDNA equivalent of 10 ng of total RNA. The qPCR experiment was carried out using a cDNA with primers specific for p53, bcl‐2 bax, caspase‐3, and a housekeeping gene GAPDH (Table 1). SYBR Green Master Mix was used according to the manufacturers’ instructions (CFX Connect‐Real‐Time PCR Detection System) (Muthuraman, Ramkumar, & Kim, 2014).
Table 1.
A list of primers used in this study
| Gene | Primers | Sequence |
|---|---|---|
| p53 | Forward | 5′‐TAACAGTTCCTGCATGGGCGGC‐3′ |
| Reverse | 5′‐ AGGACAGGCACAAACACGCACC‐3′ | |
| bax | Forward | 5′‐TGG AGCTGCAGAGGATGATTG‐3′ |
| Reverse | 5′‐GAAGTTGCCGTCAGAAAACATG‐3′ | |
| Caspase‐3 | Forward | 5′‐TTAATAAAGGTATCCATGGAGAACACT‐3′ |
| Reverse | 5′‐TTAGTGATAAAAATAGAGTTCTTTTGTGAG‐3′ | |
| GAPDH | Forward | 5′‐GGTCACCAGGGCTGCTTTT‐3′ |
| Reverse | 5′‐ATCTCGCTCCTGGAAGATGGT‐3′ |
2.12. Western blot analysis
Cells were washed with PBS with lysis buffer. The equal amounts of lysate protein samples were investigated SDS‐polyacrylamide gel electrophoresis, and PVDF membrane was used for transferring, and nonspecific binding has been blocked with use of Tris‐buffered saline‐Tween buffer that contained 5% nonfat dry milk. Mouse monoclonal p53, bax, bcl‐2, and caspase‐3 and horseradish peroxidase (HRP) conjugated goat antirabbit IgG for 1 hr. Antibodies were used 1:500 ratio in this study. The protein levels of p53, bax, bcl‐2, and caspase‐3 were determined by using enhanced chemiluminescence method (Muthuraman, Jeongeun, & Eunjung, 2014).
2.13. Immunohistochemistry
Immunohistochemical staining experiment was done using the streptavidin–biotin complex method. The primary antibodies were diluted (1:400) and were applied to the prepared sections. Then, sections were incubated at 4°C for overnight. The immune complex was viewed with use of 3, 30‐diaminobenzidine. The slide was counterstained with hematoxylin. The section was dehydrated in alcohols and covered with the use of coverslips (Muthuraman, Senthilkumar, & Srikumar, 2009).
2.14. Statistical analysis
All the experimental values were expressed as mean ± SEM. The difference between control and treated groups was compared using Student's t‐test. A p < .05 was considered statistically significant.
3. RESULTS
3.1. Effect of curcumin mitochondrial membrane disruption
The changes in the morphology of mitochondria and nucleus in response to the treatment were assessed by the Mito Red and Hoechst 33258, respectively. Normal mitochondria appeared as an extended lace‐like network in control cells, whereas mitochondria were condensed and clumped in the cells of a cerebral aneurysm. Curcumin treatment significantly normalized the mitochondrial morphology toward control (Figure 1).
Figure 1.
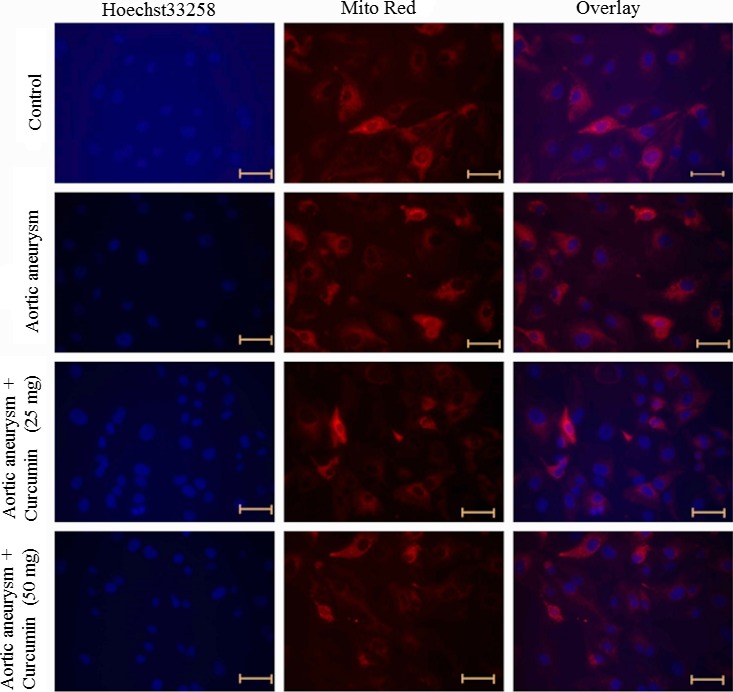
Effect of curcumin on mitochondrial morphology. Cells were exposed to the different concentration of curcumin and stained with Mito Tracker® Red and Hoechst 33258 to image the mitochondria and nuclei, respectively. Scale bar is 50 μm
3.2. Effect of curcumin on apoptosis in a cerebral aneurysm
The fluorescence microscopy examination was applied to determine the therapeutic effect of curcumin was involved in the inhibition of apoptosis. Viable cells appear as a green color with a nucleus, and fragmented green chromatin in the nucleus indicated apoptosis. The necrotic cells appear as a uniform bright orange nucleus. Cells incubated with curcumin for 48 hr showed reduced fragmented and condensed chromatin, the appearance of apoptotic cells, and fragmented nuclei (Figure 2).
Figure 2.
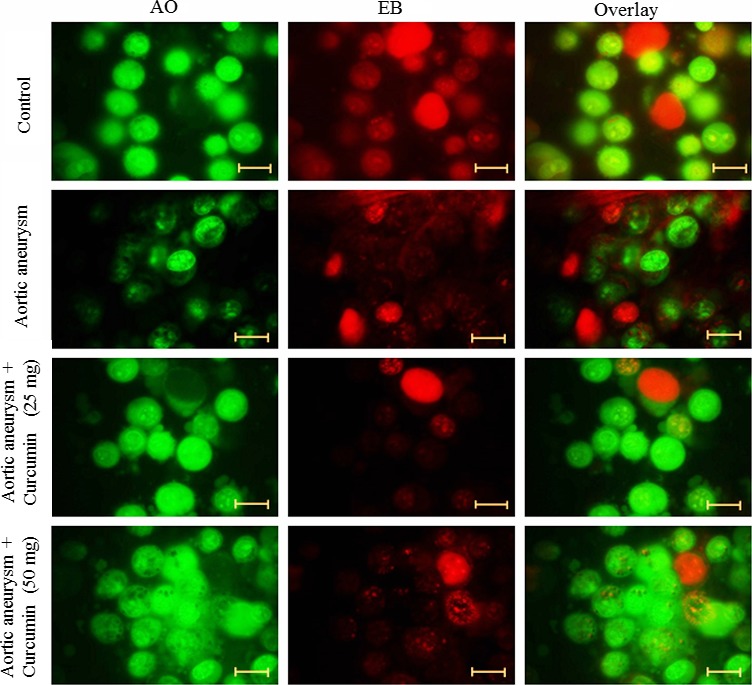
Effect of curcumin on apoptosis. Cells were exposed to the different concentration of curcumin and stained with acridine orange and ethidium bromide. Cells were viewed under a fluorescent microscope. Scale bar is 50 μm
Control cells contained smoother and bigger nucleus. Curcumin‐treated cells significantly reduced oval and fragmented granular masses in the nucleus, as well as decreased nuclear volume. The appearance of the nucleus with green color suggesting the induction of apoptosis in curcumin incubated cells (Figure 3).
Figure 3.
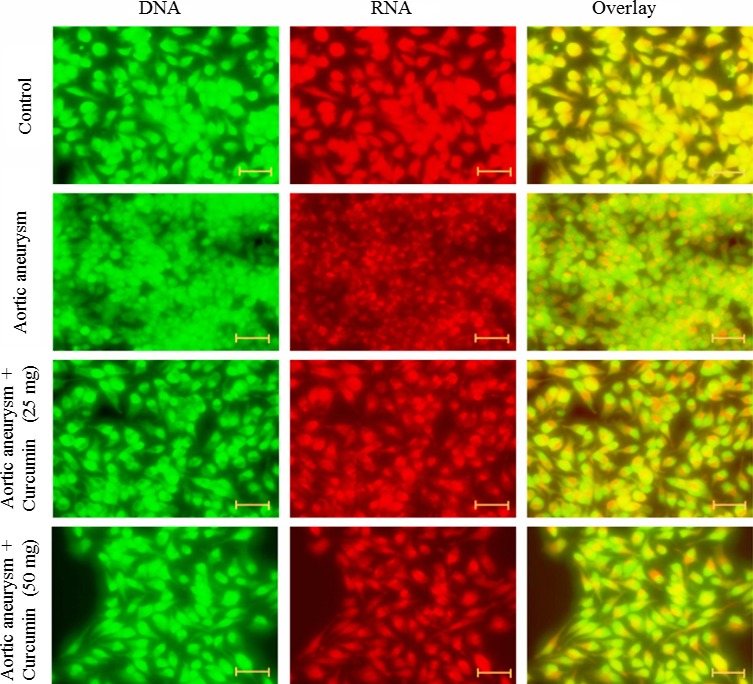
Effect of curcumin on apoptosis. Cells were exposed to the different concentration of curcumin and stained with acridine orange. Cells were viewed under a confocal microscope. Scale bar is 50 μm
3.3. Effect of curcumin on ROS production of an aortic aneurysm
ROS level was determined in the cells incubated with curcumin using fluorescent probe DCFH‐DA dye. DCF fluorescence intensity was decreased in the curcumin‐treated cells in a dose‐dependent relationship manner (Figure 4).
Figure 4.
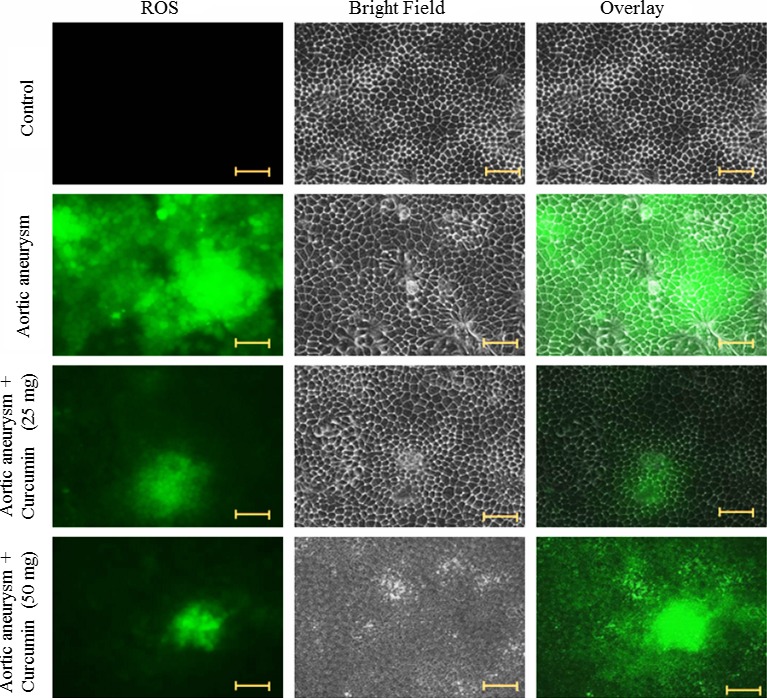
Effect of curcumin on ROS levels. Cells were exposed to the different concentration of curcumin and stained with DCF‐DA. Cells were viewed under a fluorescent microscope. Scale bar is 50 μm
3.4. Effect of curcumin on apoptotic‐related gene expressions
We quantitated apoptotic marker gene mRNA expression by qPCR to confirm apoptosis. Cells incubated with different concentrations of curcumin showed significant changes in the p53, bax, bcl‐2, and caspase‐3 mRNA expressions. The p53 mRNA expression was increased 0.34‐ and 0.51‐fold at 25 and 50 mg/kg bwt of curcumin exposure, respectively (Figure 5a). Caspase‐3 mRNA expression was increased 0.45‐ and 0.73‐fold at 25 and 50 mg/kg bwt of curcumin exposure, respectively (Figure 6a). The bax mRNA expression was increased 0.31‐ and 0.60‐fold at 25 and 50 mg/kg bwt of curcumin exposure, respectively (Figure 7a). The mRNA expression of bcl‐2 was significantly reduced 0.24‐ and 0.46‐fold following 25 and 50 mg/kg bwt of curcumin exposure, respectively (Figure 8a).
Figure 5.
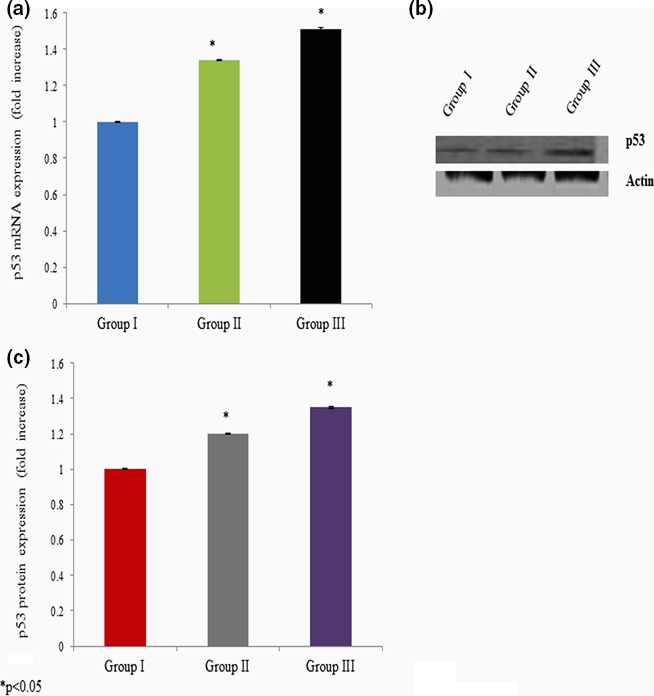
Effect of curcumin on p53 mRNA and protein expression. Cells were exposed to the different concentration of curcumin and qPCR and Western blot analysis. The mRNA expression was expressed as fold change (a), protein expression was given as band (b), and “c” represents the densitometry values of “b”
Figure 6.
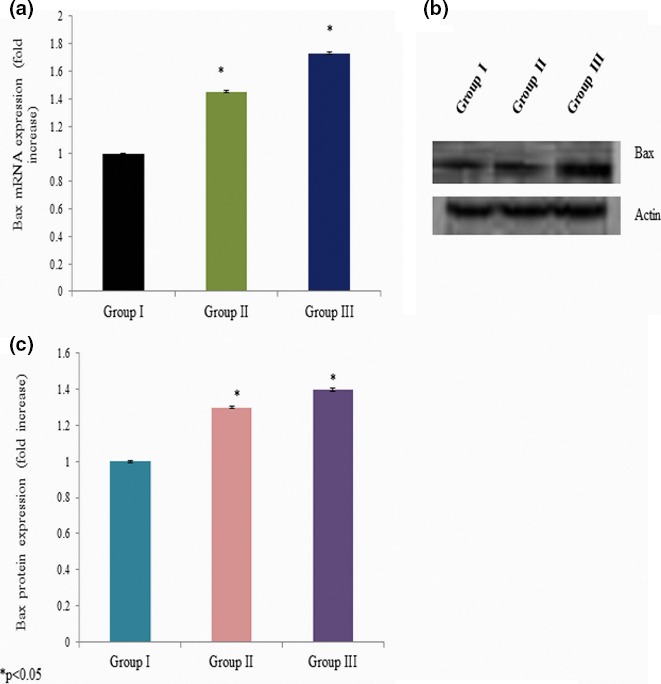
Effect of curcumin on caspase‐3 mRNA and protein expression. Cells were exposed to the different concentration of curcumin and qPCR and Western blot analysis. The mRNA expression was expressed as fold change (a), protein expression was given as band (b), and “c” represents the densitometry values of “b”
Figure 7.
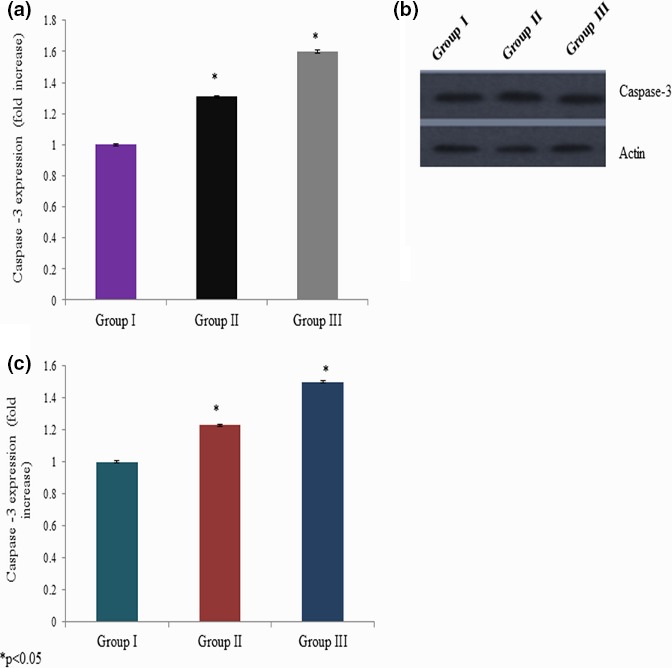
Effect of curcumin on bax mRNA and protein expression. Cells were exposed to the different concentration of curcumin and qPCR and Western blot analysis. The mRNA expression was expressed as fold change (a), protein expression was given as band (b), and “c” represents the densitometry values of “b”
Figure 8.
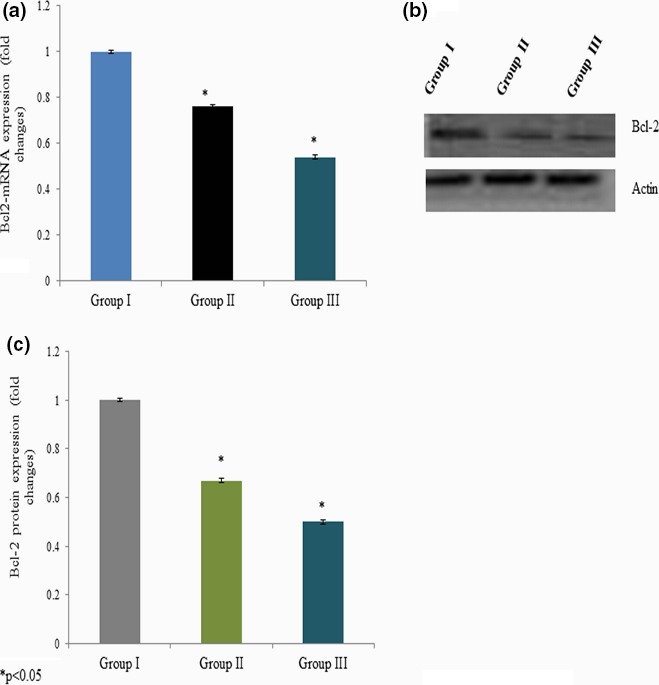
Effect of curcumin on bcl‐2 mRNA and protein expression. Cells were exposed to the different concentration of curcumin and qPCR and Western blot analysis. The mRNA expression was expressed as fold change (a), protein expression was given as band (b), and “c” represents the densitometry values of “b”
3.5. Effect of curcumin on protein expression
We quantitated apoptotic marker gene expression by Western blot analysis to confirm the effect of curcumin on apoptosis. Cells exposed to various concentrations of curcumin showed significant changes in p53, bax, bcl‐2, and caspase‐3 protein expressions. The p53 protein expression was increased 0.2‐ and 0.35‐fold at 25 and 50 mg/kg bwt of curcumin exposure, respectively (Figure 5b,c). Caspase‐3 protein expression was increased 0.3‐ and 0.4‐fold at 25 and 50 mg/kg bwt of curcumin exposure, respectively (Figure 6b,c). The bax protein expression was increased 0.23‐ and 0.5‐fold at 25 and 50 mg/kg bwt of curcumin exposure, respectively (Figure 7b,c). The protein expression of bcl‐2 was significantly reduced 0.33‐ and 0.5‐fold following 25 and 50 mg/kg bwt of curcumin exposure, respectively (Figure 8b,c).
4. DISCUSSION
In the last decades, the anti‐inflammatory and anticancer activity of curcumin has been reported (Wongcharoen & Phrommintikul, 2009), and safety of curcumin consumption and use in clinical treatment has been shown (Chainani‐Wu, 2003). Recently, the curcumin has been reported to reduce aneurysmal degeneration (Parodi et al., 2006). However, the mechanism of curcumin action on cerebral aneurysm yet to be investigated. Therefore, this study examined the key mechanism of curcumin action on cerebral aneurysm using animal cell model. Our experimental data suggest that the curcumin treatment could significantly reduce cerebral degeneration. Curcumin induced damage in the mitochondrial and nuclear genomes in a dose‐dependent manner. However, the mitochondrial damage was greater than nuclear damage. The possible mechanism of curcumin for its effect underlies through the increased ROS and lipid peroxidation. DNA damage was not found at the low level of curcumin treatment. However, it induced DNA damage and oxidative stress at a higher level. Therefore, the nuclear and DNA damage could be the starting point before curcumin‐induced cell death (Jun, Li, Hui‐Min, Yong, & Lai‐Fu, 2006).
The JNK is a mitogen‐activated protein kinase known to play a fundamental role in the development of aneurysms (Yoshimura et al., 2005). The upregulated JNK phosphorylation leads to the destruction of the aorta through the reduction in biosynthetic enzymes and extracellular matrix degeneration. In our experiment, we have used CaCl2 to induce a cerebral aneurysm in the male albino rats. The reduction in JNK expression could be helpful to reduce the severity of an aortic aneurysm in the male albino rats. Apoptosis in the cerebral wall has been directly associated with cerebral elastin damage and aortic dilation. The degree of apoptosis in the cells is a crucial mechanism for the prevention and management of an aneurysm (Jones et al., 2009).
The induction of apoptosis has been associated with activation of JNK protein kinase (Xia, Dickens, Raingeaud, Davis, & Greenberg, 1995) and directly associated with phosphorylation of c‐Jun (Force, Pombo, Avruch, Bonventre, & Kyriakis, 1996). Apoptosis in the vessel wall cells has been associated with JNK/c‐Jun pathway in a cerebral aneurysm (Takagi, Ishikawa, Nozaki, Yoshimura, & Hashimoto, 1996). The apoptosis has been reduced in the human smooth muscle cells during the blockage of JNK activation (Han et al., 2010). We studied the effect of curcumin on apoptosis by using fluorescence and confocal microscopy. Apoptosis was significantly reduced in the brain cells after curcumin treatment. Curcumin treatment also reduced JNK expression and reduced the ischemia reperfusion–induced cell death (Fiorillo et al., 2008). Caspase‐3 is an autocatalytic enzyme that plays a key role in apoptosis process. The reduced intracranial aneurysm has been associated with increased caspase‐3 expression (Guo et al., 2007), and our data also agreed on these findings.
The expression of p53, bax, and bcl‐2 had been directly participating in the apoptosis process (Hsu et al., 2011). The ratio of bax/bcl‐2 is very crucial for the determination of cell survival and death (Xu, Liu, Liu, Lu, & Pang, 2011). Curcumin‐induced apoptosis particularly involves the mitochondria‐mediated pathway in different cancer cells. Curcumin induces apoptosis‐like changes in thymocytes, whereas it inhibits cell proliferation in normal cell types (Karunagaran, Rashmi, & Kumar, 2005). Curcumin induces apoptosis through the mitochondria‐mediated apoptotic pathway in HT‐29 cells (Wang, Qi, Zheng, & Wu, 2009). Curcumin‐induced apoptosis by downregulating anti‐apoptotic Bcl‐2 and upregulating proapoptotic Bax, thereby decreasing the Bcl‐2/Bax ratios. Our result about Bcl‐2/Bax ratio is in agreement with previous studies (Shi et al., 2006; Song et al., 2005). Therefore, to confirm the role of curcumin in apoptosis, we further quantitated the expression of p53, bax, and bcl‐2. CaCl2‐induced cerebral aneurysm had reduced bax expression and increased bcl‐2 expression. However, curcumin treatment significantly reversed these expression levels in the cells of male albino rats. Taking all these data together, the curcumin treatment significantly protects cells of a cerebral aneurysm.
In other hands, curcumin has been shown to have antioxidant and anti‐inflammatory effects. The chronic inflammation and accelerated oxidative stress could play a key role in prevention and management of a cerebral aneurysm (Ramos‐Mozo et al., 2011). Curcumin treatment has been shown to reduce NF‐kb activation during inflammatory events. Also, curcumin has been reported as a good free radical scavenger by reducing DNA damage and lipid peroxidation (Srivastava, Singh, Dubey, Misra, & Khar, 2011). Curcumin has been reported to induce apoptosis by the activation of caspases through the mitochondrial signaling pathway in melanoma cells (Jiang, Jiang, Li, & Zheng, 2015).
In conclusion, our experimental results show that the curcumin treatment could reduce pathogenesis of CaCl2‐induced cerebral aneurysm of brain cells through inhibition of mitochondrial apoptosis process. Furthermore, our experimental result may suggest regular consumption of curcumin could significantly reduce a cerebral aneurysm.
CONFLICT OF INTEREST
None declared.
Bo L‐J, Miao Z, Wang Z‐F, Zhang K‐Z, Gao Z. A study on effect of curcumin on anticerebral aneurysm in the male albino rats. Brain Behav. 2017;7:e00729 https://doi.org/10.1002/brb3.729
REFERENCES
- Biswas, S. K. , McClure, D. , Jimenez, L. A. , Megson, I. L. , & Rahman, I. (2005). Curcumin induces glutathione biosynthesis and inhibits NF‐kappaB activation and interleukin‐8 release in alveolar epithelial cells: Mechanism of free radical scavenging activity. Antioxidants & Redox Signaling, 7, 32–41. [DOI] [PubMed] [Google Scholar]
- Brisman, J. L. , Song, J. K. , & Newell, D. W. (2006). Cerebral aneurysms. The New England Journal of Medicine, 355, 928–939. [DOI] [PubMed] [Google Scholar]
- Chainani‐Wu, N. (2003). Safety and anti‐inflammatory activity of curcumin: A component of turmeric (Curcuma longa). Journal of Alternative and Complementary Medicine, 9, 161–168. [DOI] [PubMed] [Google Scholar]
- Dutta, S. , Padhye, S. , Priyadarsini, K. I. , & Newton, C. (2005). Antioxidant and antiproliferative activity of curcumin semicarbazone. Bioorganic & Medicinal Chemistry Letters, 15, 2738–2744. [DOI] [PubMed] [Google Scholar]
- Fan, J. , Li, X. , Yan, Y. W. , Tian, X. H. , Hou, W. J. , Tong, H. , & Bai, S. L. (2012). Curcumin attenuates rat thoracic aortic aneurysm formation by inhibition of the c‐Jun N‐terminal kinase pathway and apoptosis. Nutrition, 28, 1068–1074. [DOI] [PubMed] [Google Scholar]
- Fiorillo, C. , Becatti, M. , Pensalfini, A. , Cecchi, C. , Lanzilao, L. , & Donzelli, G. (2008). Curcumin protects cardiac cells against ischemia–reperfusion injury: Effects on oxidative stress, NF‐kappaB, and JNK pathways. Free Radical Biology and Medicine, 45, 839–846. [DOI] [PubMed] [Google Scholar]
- Force, T. , Pombo, C. M. , Avruch, J. A. , Bonventre, J. V. , & Kyriakis, J. M. (1996). Stress‐activated protein kinases in cardiovascular disease. Circulation Research, 78, 947–953. [DOI] [PubMed] [Google Scholar]
- Guo, F. , Li, Z. , Song, L. , Han, T. , Feng, Q. , & Guo, Y. (2007). Increased apoptosis and cysteinyl aspartate‐specific protease‐3 gene expression in the human intracranial aneurysm. Journal of Clinical Neuroscience, 14, 550–555. [DOI] [PubMed] [Google Scholar]
- Han, Y. , Wu, G. , Deng, J. , Tao, J. , Guo, L. , & Tian, X. (2010). A Cellular repressor of E1A‐stimulated genes inhibits human vascular smooth muscle cell apoptosis via blocking P38/JNK MAP kinase activation. Journal of Molecular and Cellular Cardiology, 48, 1225–1235. [DOI] [PubMed] [Google Scholar]
- Hao, Q. Q. , Chen, X. , Wang, X. Y. , Dong, B. , & Yang, C. H. (2014). Curcumin attenuates angiotensin II‐induced abdominal aortic aneurysm by inhibition of inflammatory response and ERK signaling pathways. Evidence‐Based Complementary and Alternative Medicine, 2014, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu, J. H. , Wu, J. R. , Liou, S. F. , Chen, H. M. , Dai, Z. K. , & Chen, I. J. (2011). Labedipinedilol‐A prevents lysophosphatidylcholine‐induced vascular smooth muscle cell death through reducing reactive oxygen species production and antiapoptosis. Atherosclerosis, 217, 379–386. [DOI] [PubMed] [Google Scholar]
- Jiang, A. J. , Jiang, G. , Li, L. T. , & Zheng, J. N. (2015). Curcumin induces apoptosis through the mitochondrial pathway and caspases activation in human melanoma cells. Molecular Biology Reports, 42, 267–275. [DOI] [PubMed] [Google Scholar]
- Jones, J. A. , Spinale, F. G. , & Ikonomidis, J. S. (2009). Transforming growth factor‐beta signaling in thoracic aortic aneurysm development: A paradox in pathogenesis. Journal of Vascular Research, 46, 119–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun, C. , Li, J. , Hui‐Min, Z. , Yong, L. , & Lai‐Fu, Z. (2006). Mitochondrial and Nuclear DNA Damage Induced by Curcumin in Human Hepatoma G2 Cells. Toxicological Sciences, 91, 476–483. [DOI] [PubMed] [Google Scholar]
- Karunagaran, D. , Rashmi, R. , & Kumar, T. R. (2005). Induction of apoptosis by curcumin and its implications for cancer therapy. Current Cancer Drug Targets, 5, 117–129. [DOI] [PubMed] [Google Scholar]
- Lim, C. S. , Jin, D. Q. , Mok, H. , Oh, S. J. , Lee, J. U. , Hwang, J. K. , … Han, J. S. (2005). Antioxidant and anti‐inflammatory activities of xanthorrhizol in hippocampal neurons and primary cultured microglia. Journal of Neuroscience Research, 82, 831–838. [DOI] [PubMed] [Google Scholar]
- Manolova, Y. , Deneva, V. , Antonov, L. , Momekova, D. , & Lambov, N. (2014). The effect of the water on the curcumin tautomerism: A quantitative approach. Spectrochimica Acta, 132A(1), 815–820. [DOI] [PubMed] [Google Scholar]
- Moktan, S. , & Raucher, D. (2012). Anticancer activity of proapoptotic peptides are highly improved by thermal targeting using elastin‐like polypeptides. International Journal of Peptide Research and Therapeutics, 18, 227–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthuraman, P. , Enkhtaivan, G. , Baskar, V. , Bhupendra, M. , Rafi, N. , Bong Yeon, J. , & Kim, D. H. (2016). Time and concentration dependent therapeutic potential of silver nanoparticles in cervical carcinoma cells. Biological Trace Element Research, 170, 309–319. [DOI] [PubMed] [Google Scholar]
- Muthuraman, P. , Jeongeun, P. , & Eunjung, K. (2014). Aspartame downregulates 3T3‐L1 differentiation. Vitro Cellular & Developmental Biology‐Animal, 50, 851–857. [DOI] [PubMed] [Google Scholar]
- Muthuraman, P. , Ramkumar, K. , & Kim, D. H. (2014). Analysis of the dose‐dependent effect of zinc oxide nanoparticles on the oxidative stress and antioxidant enzyme activity in adipocytes. Applied Biochemistry and Biotechnology, 174, 2851–2863. [DOI] [PubMed] [Google Scholar]
- Muthuraman, P. , Senthilkumar, R. , & Srikumar, K. (2009). Alterations in beta‐islets of Langerhans in alloxan‐induced diabetic rats by marine Spirulina platensis. Journal of Enzyme Inhibition and Medicinal Chemistry, 24, 1253–1256. [DOI] [PubMed] [Google Scholar]
- Parodi, F. E. , Mao, D. , Ennis, T. L. , Pagano, M. B. , & Thompson, R. W. (2006). Oral administration of diferuloylmethane (curcumin) suppresses proinflammatory cytokines and destructive connective tissue remodeling in experimental abdominal aortic aneurysms. Annals of Vascular Surgery, 20, 360–368. [DOI] [PubMed] [Google Scholar]
- Ramos‐Mozo, P. , Madrigal‐Matute, J. , Martinez‐Pinna, R. , Blanco‐Colio, L. M. , Lopez, J. A. , & Camafeita, E. (2011). Proteomic analysis of polymorphonuclear neutrophils identifies catalase as a novel biomarker of abdominal aortic aneurysm: Potential implication of oxidative stress in abdominal aortic aneurysm progression. Arteriosclerosis, Thrombosis, and Vascular Biology, 31, 3011–3019. [DOI] [PubMed] [Google Scholar]
- Shi, M. , Cai, Q. , Yao, L. , Mao, Y. , Ming, Y. , & Ouyang, G. (2006). Antiproliferation and apoptosis induced by curcumin in human ovarian cancer cells. Cell Biology International, 30, 221–226. [DOI] [PubMed] [Google Scholar]
- Song, G. , Mao, Y. B. , Cai, Q. F. , Yao, L. M. , Ouyang, G. L. , & Bao, D. (2005). Curcumin induces human HT‐29 colon adenocarcinoma cell apoptosis by activating p53 and regulating apoptosis‐related protein expression. Brazilian Journal of Medical and Biological Research, 38, 1791–1798. [DOI] [PubMed] [Google Scholar]
- Srivastava, R. M. , Singh, S. , Dubey, S. K. , Misra, K. , & Khar, A. (2011). the Immunomodulatory and therapeutic activity of curcumin. International Immunopharmacology, 11, 331–341. [DOI] [PubMed] [Google Scholar]
- Takagi, Y. , Ishikawa, M. , Nozaki, K. , Yoshimura, S. , & Hashimoto, N. (1996). Increased expression of phosphorylated c‐Jun amino‐terminal kinase and phosphorylated c‐Jun in human cerebral aneurysms: The role of the c‐Jun amino‐terminal kinase/c‐Jun pathway in apoptosis of vascular walls. Neurosurgery, 51, 997–1002. [DOI] [PubMed] [Google Scholar]
- Takagi, Y. , Ishikawa, M. , Nozaki, K. , Yoshimura, S. , & Hashimoto, N. (2002). Increased expression of phosphorylated c‐Jun amino‐terminal kinase and phosphorylated c‐Jun in human cerebral aneurysms: The role of the c‐Jun amino‐terminal kinase/c‐Jun pathway in apoptosis of vascular walls. Neurosurgery, 51, 997–1002. [DOI] [PubMed] [Google Scholar]
- Thiyagarajan, M. , & Sharma, S. S. (2004). Neuroprotective effect of curcumin in middle cerebral artery occlusion induced focal cerebral ischemia in rats. Life Sciences, 74, 969–985. [DOI] [PubMed] [Google Scholar]
- Wang, J. , Qi, L. , Zheng, S. , & Wu, T. (2009). Curcumin induces apoptosis through the mitochondria‐mediated apoptotic pathway in HT‐29 cells. Journal of Zhejiang University Science, B10, 93–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber, W. M. , Hunsaker, L. A. , Abcouwer, S. F. , Deck, L. M. , & Vander Jagt, D. L. (2005). Anti‐oxidant activities of curcumin and related enones. Bioorganic & Medicinal Chemistry, 13, 3811–3820. [DOI] [PubMed] [Google Scholar]
- Wongcharoen, W. , & Phrommintikul, A. (2009). The protective role of curcumin in cardiovascular diseases. International Journal of Cardiology, 133, 145–151. [DOI] [PubMed] [Google Scholar]
- Wu, Z. , Hofman, F. M. , & Zlokovic, B. V. (2003). A simple method for isolation and characterization of mouse brain microvascular endothelial cells. Journal of Neuroscience Methods, 130, 53–63. [DOI] [PubMed] [Google Scholar]
- Xia, Z. , Dickens, M. , Raingeaud, J. , Davis, R. J. , & Greenberg, M. E. (1995). Opposing effects of ERK and JNK‐p38 MAP kinases on apoptosis. Science, 270, 1326–1331. [DOI] [PubMed] [Google Scholar]
- Xu, L. , Liu, J. T. , Liu, N. , Lu, P. P. , & Pang, X. M. (2011). Effects of Panax notoginseng saponins on proliferation and apoptosis of vascular smooth muscle cells. Journal of Ethnopharmacology, 137, 226–230. [DOI] [PubMed] [Google Scholar]
- Yoshimura, K. , Aoki, H. , Ikeda, Y. , Fujii, K. , Akiyama, N. , & Furutani, A. (2005). Regression of abdominal aortic aneurysm by inhibition of c‐Jun N‐terminal kinase. Nature Medicine, 11, 1330–1138. [DOI] [PubMed] [Google Scholar]
- Yu, S. , Zheng, W. , Xin, N. , Chi, Z. H. , Wang, N. Q. , & Nie, Y. X. (2010). Curcumin prevents dopaminergic neuronal death through inhibition of the c‐Jun N‐terminal kinase pathway. Rejuvenation Research, 13, 55–64. [DOI] [PubMed] [Google Scholar]


