Abstract
Introduction
Autoantibodies to the alpha‐amino‐3‐hydroxy‐5‐methyl‐4‐isoxazolepropionic acid (AMPA) receptor and N‐methyl‐d‐aspartate (NMDA) receptor are known to be the causes of autoimmune encephalitis particularly limbic encephalitis. The involvement of the peripheral nervous system is rarely reported.
Methods
We analyzed the serial nerve conduction studies of a previously reported case of anti‐AMPA receptor encephalitis, who was presented with conscious disturbance and quadriplegia. Initial nerve conduction studies (NCS) revealed motor axonal polyneuropathy with active denervation. We also performed systematic review of similar cases with overlapped peripheral neuropathy and glutamate receptor encephalitis through Embase, PubMed, and MEDLINE.
Results
Follow‐up NCS of the patient with anti‐AMPA receptor encephalitis found reverse of the acute neuropathy, which was compatible with clinical recovery of quadriplegia. The systematic review identified 10 cases with overlapping peripheral neuropathy with anti‐AMPA or NMDA receptor encephalitis. Motor or sensorimotor neuropathies were more common than pure sensory neuropathies. Anti‐Hu, anti‐amphiphysin, or anti‐gnaglioside antibodies coexisted in some cases and might be associated with the peripheral symptoms.
Conclusions
Both anti‐AMPA and anti‐NMDA receptor encephalitis could overlap with acute peripheral neuropathy. It is important to consider peripheral symptoms and perform diagnostic tests.
Keywords: anti‐AMPA receptor encephalitis, anti‐NMDA receptor encephalitis, glutamate receptor encephalitis, peripheral nervous system, polyneuropathy
1. INTRODUCTION
Alpha‐amino‐3‐hydroxy‐5‐methyl‐4‐isoxazolepropionic acid (AMPA) and N‐methyl‐d‐aspartate (NMDA) receptors are two major glutamate receptors and are antigens of patients with autoimmune limbic encephalitis (Lai et al., 2009; Dalmau et al., 2008). B‐cell immunity and antibody‐mediated neuronal dysfunction are pathogenic of these glutamate receptor encephalitis (Peng et al., 2014; Dalmau, Lancaster, Martinez‐Hernandez, Rosenfeld, & Balice‐Gordon, 2011), while role of T‐cell immunity seem to be minor (Liba et al., 2016). Although NMDA and AMPA receptors are two major excitatory synaptic proteins in the central nervous system (CNS), these glutamate receptors also distribute peripherally (Coggeshall & Carlton, 1998). Theoretically, the pathogenic circulating antibodies are able to affect not only the CNS but also the peripheral nervous system (PNS). However, the involvement of the PNS has rarely been emphasized. Our study described serial nerve conduction studies (NCS) of a previously reported patient with anti‐AMPA receptor encephalitis (Wei et al., 2013) and systematically reviewed the peripheral neuropathy in glutamate receptor encephalitis.
2. MATERIALS AND METHODS
2.1. Clinical presentations and initial NCS of anti‐AMPA receptor encephalitis
In 2012, a 30‐year‐old pregnant woman developed headache (day 1), memory impairment, fever and confusion (day 7), unsteady gait (day 9), quadriparesis (day 11), and myoclonic seizure (day 13). The study of cerebrospinal fluid (CSF) showed that eosinophilic pleocytosis with elevated protein and IgG index (protein 112 mg/dl; white blood cell 70 cells/μl; IgG index 7.38). She was initially treated with antimicrobial agents (acyclovir between day 8 and 19, and albendazole between day 10 and 13) and intravenous dexamethasone (between day 8 and 18). She developed status epilepticus (day 13). She received intravenous anesthesia for status epilepticus (midazolam infusion from day 13 to 15 and propofol infusion from day 13 to 14), and antiepileptic drugs (phenytoin between day 13 and 16, levetiracetam between day 13 and 65, lamotrigine between day 14 and 19, and topiramate between day 19 and 56). Seizure was well controlled without recurrence.
Immunoactivity assay using human embryonic kidney‐293 cells, which were transfected with neuronal surface antigen‐containing plasmids, revealed antibodies to GluA2 subunit of AMPA receptor in both serum and CSF (Wei et al., 2013). She received plasmapheresis (between day 17 and 26), plasma exchange (between day 38 and 46), and methylprednisolone pulse therapy (between day 35 and 36, 52 and 55). However, the patient became stuporous and had quadriplegia, areflexia, and silent plantar responses since intensive care unit (ICU) admission (day 13). The first NCS on day 35 revealed reduced amplitudes of compound muscle action potential (CMAP) in bilateral median, ulnar, tibial, and deep peroneal nerves (Table 1, column 1 m). There was neither conduction block nor reduced conduction velocity of motor nerve conduction. Sensory nerve conduction was intact. The patient was likely to have a motor‐predominant polyneuropathy.
Table 1.
Serial nerve conduction studies and electromyography
| Time from onset | Right | Left | Ref. | Right | Left | Ref. | Right | Left | Ref. | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 m | 2 m | 2y | 1 m | 2 m | 2y | 1 m | 2 m | 2y | 1 m | 2 m | 2y | 1 m | 2 m | 2y | 1 m | 2 m | 2y | |||||
| Sensory nerve conduction study | Onset latency (ms) | Amplitude (mV) | Conduction velocity (m/s) | |||||||||||||||||||
| Median | 1.7 | 1.0 | 1.8 | <3.5 | 32.3 | 34 | 25.7 | >13 | 71 | 50 | 65 | >50 | ||||||||||
| Ulnar | 1.8 | 1.6 | 1.6 | 1.6 | <3.1 | 50 | 35.7 | 41 | 55.5 | >12 | 60 | 63 | 73 | 63 | >50 | |||||||
| Sural | 2.5 | 3.1 | 2.3 | 2.9 | <3.6 | 17.0 | 9.6 | 16.6 | 19.8 | >9 | 56 | 45 | 61 | 48 | >38 | |||||||
| Motor nerve conduction study | Onset latency (ms) | Amplitude (mV) | Conduction velocity (m/s) | |||||||||||||||||||
| Median | Wrist | 2.9 | 2.7 | 2.9 | 2.8 | 2.7 | <4.2 | 2.8 | 4.3 | 3.0 | 3.7 | 10.7 | >6 | |||||||||
| Elbow | 6.7 | 6.5 | 6.6 | 6.3 | 2.8 | 3.1 | 2.8 | 10.6 | 57.9 | 55 | 59.5 | 61 | >50 | |||||||||
| Ulnar | Wrist | 2.4 | 2.1 | 2.3 | 1.8 | 2.0 | <3.4 | 2.0 | 6.0 | 3.3 | 5.3 | 13.4 | >5.5 | |||||||||
| Below elbow | 5.8 | 5.2 | 5.2 | 5.2 | 0.99 | 6.1 | 2.3 | 13.4 | ||||||||||||||
| Above elbow | 7.6 | 6.7 | 6.6 | 7.0 | 0.68 | 5.9 | 2.0 | 12.8 | 55.6 | 67 | 71.4 | 56 | >50 | |||||||||
| Deep peroneal | Ankle | 4.2 | 4.1 | 4.3 | 3.9 | 3.1 | 3.9 | <5.5 | 1.5 | 0.8 | 0.4 | 0.98 | 0.5 | 1.6 | >2 | |||||||
| Below fibular‐head | 10.8 | 10.7 | 11.5 | 10.2 | 9.9 | 19.3 | 0.78 | 0.6 | 0.5 | 0.89 | 0.4 | 0.8 | ||||||||||
| Above fibular‐head | 12.6 | 13.0 | 13.6 | 12.2 | 12.4 | 22.1 | 0.25 | 0.2 | 0.5 | 0.7 | 0.6 | 0.6 | 44.4 | 43 | 40 | 40 | 40 | 36 | >40 | |||
| Tibial | Ankle | 2.9 | 3.6 | 3.9 | 3.3 | 3.5 | 3.2 | <7 | 11.3 | 9.1 | 15.9 | 7.0 | 4.6 | 15.1 | >4 | |||||||
| Popliteal fossa | 9.8 | 11.4 | 12.0 | 10.2 | 11.2 | 11.1 | 9.7 | 7.5 | 10.8 | 6.7 | 4.5 | 9.4 | 50.7 | 49 | 47 | 47 | 49 | 49 | >40 | |||
| Electromyography a | Fibrillations | Positive sharp waves | Recruitment | |||||||||||||||||||
| First dorsal interosseous m | +++ | +++ | ↓ | |||||||||||||||||||
| Biceps m | + | ↓ | ||||||||||||||||||||
| Anterior tibialis m | ++ | ++ | ↓ | |||||||||||||||||||
| Vastus medialis m | ↓ | |||||||||||||||||||||
| Late responses | Latency (ms) | |||||||||||||||||||||
| Median F‐wave | 23.4 | 31.5 | 25.4 | 24.8 | <29 | |||||||||||||||||
| Ulnar F‐wave | 23.1 | 23.4 | 22.3 | <30 | ||||||||||||||||||
| Deep peroneal F‐wave | NR | NR | NR | NR | <50 | |||||||||||||||||
| Tibial F‐wave | 45.5 | 45.7 | 43.8 | 45.0 | <51 | |||||||||||||||||
| H‐reflex | NR | NR | NR | 29.7 | <31 | |||||||||||||||||
Ref., referential normal value; m, month; y, year; NR, no response. The numbers listed in bold were abnormal data. The gray numbers in italic were normal values for reference.
The nerve conduction studies (NCS) revealed diffusely decreased amplitudes of motor nerve conductions and poor responses of F‐wave and H‐reflex in the first 2 months (column 1 m and 2 m). There is normal sensory conduction. The electromyography performed at the second month showed fibrillations and positive sharp waves with severely reduced recruitment and normal motor unit potentials of tested muscles, which suggested an active denervation of motor neuropathy (column 2 m). In the first month, the patient was unconscious, quadriplegic with generalized areflexia. At the second‐year follow‐up, she was alert and oriented. Muscle power of all limbs returned to full. The NCS 2 years after onset revealed only peroneal neuropathy (column 2y).
The motor unit potentials of electromyography were normal.
2.2. Systematic literature reviews of peripheral neuropathy in glutamate receptor encephalitis
We conducted systematic reviews of three main databases of medical literatures in English: Embase®, PubMed®, and MEDLINE®. Two searching strategies were applied. First, we searched literatures by combining keywords of glutamate receptor encephalitis and of peripheral neuropathies: (“glutamate receptor encephalitis” OR “AMPA receptor encephalitis” OR “NMDA receptor encephalitis” OR “anti‐N‐methyl‐D‐aspartate receptor encephalitis”) AND (“peripheral nerve” OR “polyneuropathy” OR “peripheral neuropathy” OR “Miller Fisher syndrome” OR “chronic inflammatory demyelinating polyneuropathy” OR “Guillain Barre syndrome” OR “demyelinating neuropathy” OR “axonal neuropathy” OR “nerve conduction” OR “electromyography”). Because narrations of patients' symptoms and signs were commonly covered in contents of case series or cohort studies, the second part of searching used combination of three sets of keywords to find out clinical studies of antineuronal surface antigen encephalitis: (“neuronal surface” OR “synapse” OR “synaptic” OR “anti‐neuronal surface” OR “AMPA receptor” OR “anti‐AMPA” OR “NMDA receptor” OR “anti‐NMDA” OR “N‐methyl‐D‐aspartate receptor” OR “glutamate receptor”) AND (“antibody” OR “autoantibody”) AND (“encephalitis” OR “encephalopathy”). Then, we further narrowed the results down by Boolean logic: NOT (“case report” OR “abstract” OR “conference” OR “review article”) NOT (“animal” OR “rat” OR “mouse” OR “in vivo” OR “in vitro” OR “cell line”). The results from Embase, PubMed, and MEDLINE were merged to remove duplicates and underwent full‐text review. The cases with coexisting peripheral neuropathy were collected (Figure 1).
Figure 1.
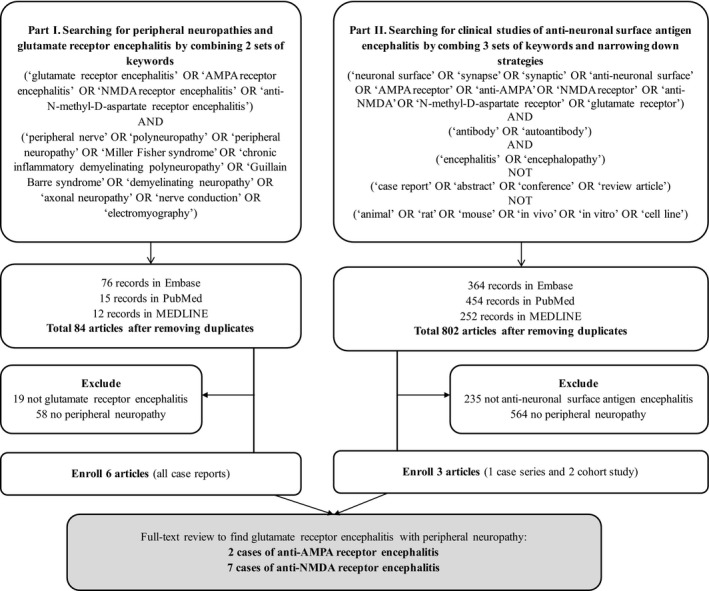
Flowchart of systematic reviews: peripheral neuropathies in patients with glutamate receptor encephalitis. AMPA, alpha‐amino‐3‐hydroxy‐5‐methyl‐4‐isoxazolepropionic acid; NMDA, N‐methyl‐d‐aspartate
This study had been approved by the Institutional Review Board of Chang Gung Memorial Hospital and underwent with patient's consensus.
3. RESULTS
3.1. Serial NCS and clinical follow‐up of the case
The patient received aggressive immunotherapy after diagnosis of anti‐AMPA receptor encephalitis. Her consciousness gradually improved, but quadriparesis and hyporeflexia remained. Therefore, the second NCS and electromyography (EMG) were arranged 2 months after symptom onset (Table 1, column 2 m). Motor amplitudes were reduced, and peroneal F waves and H‐reflex were absent. There is neither conduction block, prolonged latency, nor abnormal sensory conduction. EMG study showed various degree of fibrillations and positive sharp waves with severely reduced recruitment, and normal motor unit potentials in bilateral dorsal interosseous, biceps, and anterior tibialis muscles, suggesting active denervation of diffuse motor axonal neuropathy. She kept receiving immunotherapy and rehabilitation. Her muscle strength returned to normal 1 year after onset. Neuropsychological assessments also revealed significant improvement but remaining mild cognitive impairment (Mini‐Mental State Examination 26/30; impaired in recall 0/3 and orientation 9/10). She only took low‐dose prednisolone 5 mg, bisoprolol 2.5 mg, and amantadine 200 mg per day. The NCS performed 2 years after onset was much improved with only residual peroneal neuropathy (Table 1, column 2y).
3.2. Differential diagnoses of the peripheral neuropathy in this case
3.2.1. Myelopathy
Cervical magnetic resonance imaging study was unremarkable.
3.2.2. Metabolic, nutritional, inflammatory, and drug‐induced neuropathies
The laboratory studies did not find diabetes mellitus, renal function impairment (creatinine 0.31 mg/dl; reference 0.44–1.03 mg/dl), abnormal thyroid function (free‐T4 0.96 ng/dl; reference 0.76–1.64 ng/dl), vitamin B12 deficiency (223.2 pg/ml; reference 211–946 pg/ml), porphyria (porphobilinogen 1.44 mg/day; reference 0–2 mg/day), paraproteinemia (negative result in serum protein electrophoresis and immunofixation electrophoresis), vasculitis (negative for antineutrophil cytoplasmic antibody), hepatitis C virus, human immunodeficiency virus infection, syphilis, or heavy metal intoxication by serum tests of lead <0.6 μg/L (reference <23 μg/L), cadmium 1.5 μg/L (reference <2.6 μg/L), mercury <0.9 μg/L (reference <10 μg/L), and arsenic 19.35 μg/g (reference <100 μg/g). She did not have alcohol consumption habit, previous history of polyneuropathy, or hereditary neuropathy in her family. There was also no exposure history to offending agents of drug‐induced neuropathies. Her disease course was similar to that of acute motor axonal neuropathy (AMAN). Although anti‐ganglioside antibodies were not checked in this patient, she did not have common anticipating events of AMAN, such as diarrhea or upper respiratory tract infection. Therefore, she was not likely to have metabolic, nutritional, inflammatory, or drug‐induced neuropathy.
3.2.3. Paraneoplastic syndrome
The surveillance for malignancy included gynecological sonography, breast sonography, pelvis MRI, CSF cytology, peripheral blood smear, tumor markers (CA199 3.12 U/ml, CA153 12.8 U/ml, CEA <0.50 ng/ml, AFP 11.9 ng/ml, SCC 1.70 ng/ml, CA125 487.7 U/ml possibly related to endometriosis, beta HCG 144,559 mIU/ml during pregnancy), and contrast‐enhanced chest computed tomography (after termination of pregnancy). However, no malignant tumor was found.
3.2.4. Critical illness neuropathy and critical illness myopathy
Critical illness neuropathy (CIN) and critical illness myopathy (CIM) are difficult to be distinguished from other acute neuropathies by NCS and EMG studies. Clinical history and laboratory exclusion of other causes are essential. Several predisposing factors are highly correlated to CIN and CIM, including sepsis, multiple organ failure, acute respiratory distress syndrome, ICU admission, and prolonged neuromuscular blocking or sedative agents (Dyck & Thomas, 2005; Katirji, 2002; Hermans, De Jonghe, Bruyninckx, & Van den Berghe, 2008). Although the patient had ICU admission and short‐term midazolam and propofol infusion, gait disturbance and quadriparesis developed before seizure and ICU admission. The absence of sepsis and multiple organ failure suggested low risk of CIN. Most of CINs are axonal type with sensorimotor (60%–71%), followed by pure motor (19%–40%) and pure sensory (0%–10%) pattern (Khan, Harrison, Rich, & Moss, 2006; Zifko, Zipko, & Bolton, 1998). According to the serial NCS, the patient's pure motor neuropathy was the less common type of CIN. Serum creatine kinase (CK) of this patient was normal (160 U/L; reference 20–180 U/L; day 13). Although CIM is usually non‐necrotizing myopathy with limited CK elevation (Hermans et al., 2008), the normal motor unit potentials and severely reduced recruitment of EMG suggested neuropathy rather than myopathy of our patient (Table 1, column 2 m). Therefore, CIN and CIM were less likely to be the cause of the patient's weakness.
3.3. Results of systematic review of glutamate receptor encephalitis and peripheral neuropathy
Through the protocol of systematic review (Figure 1), part I searching yielded 76 records in Embase, 15 in PubMed, and 12 in MEDLINE. Full‐text review of 84 merged articles found six case reports of peripheral neuropathy in anti‐NMDA receptor encephalitis (Pohley et al., 2015; Hatano et al., 2011; Tojo et al., 2011; Ishikawa et al., 2013; Pruss, Hoffmann, Stenzel, Saschenbrecker, & Ebinger, 2014; Samejima et al., 2010). One report describing a case with severe axonal neuropathy 33 months before the detection of NMDA receptor antibodies was excluded due to difficulty in identifying the correlation between neuropathy and encephalitis (Köhler et al., 2012). Part II review found 364 records in Embase, 454 in PubMed, and 252 in MEDLINE. One case of anti‐NMDA receptor encephalitis (Byun et al., 2016) and two cases of anti‐AMPA receptor encephalitis were identified (Zekeridou, McKeon, & Lennon, 2016; Hoftberger et al., 2015).
Table 2 summarized the symptoms and NCS/EMG findings of our patient and the nine cases from systematic reviews. Pure motor or motor‐predominant neuropathies were relative common among these cases (4 motor or motor predominant, 3 sensorimotor, 1 pure sensory; Table 2). Response to immunotherapy and reverse of neuropathy were found by serial NCS in at least 2 over 9 cases.
Table 2.
Systematic literature reviews of cases with overlapped glutamate receptor encephalitis and peripheral neuropathy
| Type of antibodies | Reference | Age/sex | CNS symptoms | PNS symptoms | Type of neuropathy by NCS/EMG | Existing of additional antibodies | Tested anti‐neuronal and paraneoplastic antibodies | Tumor | Immunotherapy | Response to treatment |
|---|---|---|---|---|---|---|---|---|---|---|
| Anti‐AMPA receptor | Hoftberger et al. (2015) | 72/M | Short‐term memory loss, ataxia, insomnia, psychotic features | Sensory polyneuropathy | S+/M−, D?/A? | Amphiphysin | NMDAR, AMPAR, GABAbR, LGI1, CASPR2, Hu, Yo, Ri, Ma1/2, Tr, amphiphysin, SOX1, ZIC4, GAD65, AK5, Homer3 | Lung cancer | Steroid | Poor, died of cancer |
| Zekeridou et al. (2016) | NA | Encephalopathy with limbic or cortical manifestations, insomnia | Peripheral neuropathy, rigidity | NA | NMDAR, AMPAR, GABAbR, GABAaR, VGKC, CASPR2, mGluR1, mGluR5, GlyR, AQP4, AChR | Thymoma | NA | NA | ||
| Wei et al. (2013) (This case) | 30/F | Short‐term memory loss, ataxia, confusion, coma | Quadriplegia, areflexia |
S−/M+, D‐/A+ Reversible |
NMDAR, AMPAR, GABAbR, LGI1, CASPR2, GAD65 | None | Steroid/PE/PP/Azathioprine | Good | ||
| Anti‐NMDA receptor | Samejima et al. (2010) | 75/M | Memory loss, ophthalmoplegia, seizure | Distal weakness and muscle atrophy of limbs | S+/M+ motor predominant, D+/A+ | Hu | NMDAR, Hu, Yo, Ri, Ma, CV2, amphiphysin | Lung cancer | IVIg | Poor, died of cancer |
| Hatano et al. (2011) | 23/F | Ophthalmoplegia, nystagmus, ataxia, orolingual dyskinesia, psychosis | Ataxia, areflexia, atypical Miller–Fisher syndrome. | NA | GQ1b, GT1a | NMDAR, GQ1b, GT1a (complete panel not listed) | None | Steroid/IVIg | Good | |
| Tojo et al. (2011) | 19/M | Psychomotor agitation, orolingual dyskinesia, status epilepticus | Preceding Guillain‐Barré Syndrome | S−/M+, D+/A− | NMDAR, GM1, GQ1b | None | Steroid/IVIg | Partial | ||
| Ishikawa et al. (2013) | 26/F | Ophthalmoplegia, confusion, hypoventilation, dysautonomia, | Flaccid paraplegia |
S−/M+, D?/A? Reversible |
NMDAR, VGKC, GM1, GD1a, GD1b, GQ1b, GT1a | Ovarian teratoma | Steroid/IVIg | Good | ||
| Pruss et al. (2014) | 75/M | Agitation, hyperkinetic movement, fever, respiratory failure | Lower limb predominant ascending pain and numbness, difficulties of walking | S+/M+, D+/A+ | NMDAR, AMPAR, GABAbR, LGI1, CASPR2, GlyR, AQP4, Tr, Hu, Yo, Ri, Ma/Ta, amphiphysin, GAD65, MAG, myelin | None | Steroid/IVIg/PE/rituximab | Partial | ||
| Pohley et al. (2015) | 32/M | Headache, hallucination, ataxia, ophthalmoplegia, anisocoria, seizure | Lower limb hyperesthesia and areflexia. Myositis with myalgia and muscle atrophy | S+/M+, D+/A− | Hu | NMDAR, Hu (Euroimmun, complete panel not listed) | Primary mediastinal seminoma | Steroid/IVIg/PP | Poor | |
| Byun et al. (2016) | 67/M | Visual hallucination, ataxia | Polyneuropathy pattern | NA | Hu | NMDAR, AMPAR, GABAbR, LGI1, CASPR2, Hu, Yo, Ri, Ma2, CV2/CRMP5, Amphiphysin | NA | NA | NA |
AMPA, alpha‐amino‐3‐hydroxy‐5‐methyl‐4‐isoxazolepropionic acid; NMDA, N‐methyl‐d‐aspartate; M, male; F, female; NA, not available; NCS, nerve conduction study; EMG, electromyography; S, sensory; M, motor; D, demyelinating; A, axonal; NMDAR, NMDA receptor; AMPAR, AMPA receptor; GABAB R, γ‐aminobutyric acid B receptor; GABAAR, γ‐aminobutyric acid A receptor; LGI1, leucine‐rich glioma inactivated protein 1; CASPR2, contactin‐associated protein‐like 2; VGKC, voltage‐gated potassium channel; ZIC, zinc finger protein; GAD65, 65 kDa glutamic acid decarboxylase; mGluR, metabotropic glutamate receptor; GlyR, glycine receptor; AQP4, aquaporin‐4; AchR, acetylcholine receptor; MAG, myelin‐associated glycoprotein; IVIg, intravenous immunoglobulin; PE, plasma exchange; PP, plasmapheresis.
A total of three cases of anti‐AMPA receptor encephalitis (2 from literature review and 1 from our case) and seven cases of anti‐NMDA receptor encephalitis were identified.
4. DISCUSSIONS
The serial NCS/EMG findings of our patient suggested that acute reversible motor axonal polyneuropathy could coexist with acute anti‐AMPA receptor encephalitis. We also found nine similar cases combining acute neuropathies with acute anti‐AMPA or NMDA receptor encephalitis in systematic literature review. Anti‐NMDA receptor encephalitis may also overlap with other diseases. Demyelinating diseases including neuromyelitis optica spectrum disorder (NMOSD) (Titulaer et al., 2014), brain stem encephalitis, leukoencephalopathy following herpes simplex encephalitis, and acquired demyelination syndrome could overlap with anti‐NMDA receptor encephalitis (Hacohen et al., 2014). Coexisting antibodies including antibodies against aquaporin‐4 (AQP4) and myelin oligodendrocyte glycoprotein (MOG) were found in the patients who had overlapping anti‐NMDA receptor encephalitis and NMOSD (Titulaer et al., 2014). From our systematic literature review, anti‐Hu antibody was found to be the coexisting antibody in three cases and was assumed to be the paraneoplastic antibody in two of them (Pohley et al., 2015; Samejima et al., 2010). The clinical manifestations of these cases were typical for anti‐Hu antibody associated neuropathy (Camdessanche et al., 2002). Besides, antibodies against amphiphysin was noted in another case with anti‐AMPA receptor encephalitis and was assumed to be the paraneoplastic antibodies of lung cancer (Hoftberger et al., 2015; Saiz et al., 1999). Antibodies to ganglioside were also found in another reported case with anti‐NMDA receptor encephalitis (Hatano et al., 2011). However, this case probably had overlapped Miller–Fisher syndrome (Kaida et al., 2006). In summary, the presence of coexisting antibodies may indicate an overlapping syndrome. It is important to arrange diagnostic tests like NCS/EMG in anti‐AMPA and anti‐NMDA receptor encephalitis patients when peripheral nervous system involvement is suspected. It is also important to arrange comprehensive work‐ups of possible malignancy when these coexisting antibodies are well‐known paraneoplastic antibodies.
Another reasonable anatomical explanation of our patient's presentation might be motor neuron dysfunction due to delayed late response with pure motor involvement. Both NMDA receptors (Spalloni, Nutini, & Longone, 2013) and AMPA receptors (Tomiyama et al., 1996) are not only widespread in the neocortex, but also are distributed in anterior horn cells of the spinal cord. Antibody‐mediated damage on motor neurons had been found in autopsy of a case with anti‐NMDA receptor encephalitis (Tuzun et al., 2009). AMPA receptor GluA2 subunit RNA editing error also played roles in developing motor neuron disease (Hideyama et al., 2012). Therefore, motor neuron injury in acute anti‐AMPA receptor encephalitis could be another possible cause of motor paresis. Immunohistological statins or animal studies are warranted in the future for further confirmation.
To date, concurrent motor paresis or peripheral presentations had rarely been described in anti‐AMPA receptor encephalitis. (Lai et al., 2009) only reported one patient with rigidity due to coexisting stiff‐person syndrome and another with gait disturbance in a case series of 10 patients. Besides, anticipating Guillain–Barré syndrome (GBS) had also been reported in patients with anti‐NMDA receptor encephalitis (Tojo et al., 2011; Pruss et al., 2014). Moreover, GBS may also develop concurrently with acute disseminated encephalomyelitis (Bernard, Riou, Rosenblatt, Dilenge, & Poulin, 2008), Bickerstaff's brainstem encephalitis (Odaka et al., 2003; Han et al., 2012), and combined central and peripheral demyelination (Ogata et al., 2016; Cortese et al., 2016). In clinical practice, peripheral symptoms may be masked by severe CNS dysfunctions (Joubert et al., 2015). Therefore, localization of motor weakness requires comprehensive history taking, neurological examination, assistance of electrophysiological tools, and neuroimaging. However, differentiating overlapping syndromes from patients with concurrent CNS and PNS disorders is still challenging. We wish our study will help to improve the detection of PNS involvement in glutamate receptor encephalitis in the future.
This literature review was based on previously published reports. Due to the rarity of this disease, the majority of articles were case reports and the clinical correlations were difficultly studied. For example, although patients with underlying malignancies (e.g., two lung cancers and one seminoma in Table 2) had poor outcomes in our review, registry studies with larger patient numbers in the future may be needed to examine the statistical correlation. Second, the literature searching was based on full‐text or abstract in English and publications in other languages could also be missed. Third, some paraneoplastic autoantibodies including anti‐Hu antibody were not examined in this patient, which may lead to bias of our results.
5. CONCLUSIONS
PNS and CNS presentations could overlap in anti‐AMPA receptor and anti‐NMDA receptor encephalitis. It is important to arrange proper diagnostic tests when peripheral neuropathy is considered.
CONFLICT OF INTEREST
All authors declare that there's no conflict of interest existed.
ACKNOWLEDGMENTS
None.
Wei Y‐C, Huang C‐C, Liu C‐H, Kuo H‐C, Lin J‐J. Peripheral neuropathy in limbic encephalitis with anti‐glutamate receptor antibodies: Case report and systematic literature review. Brain Behav. 2017;7:e00779 https://doi.org/10.1002/brb3.779
REFERENCES
- Bernard, G. , Riou, E. , Rosenblatt, B. , Dilenge, M. E. , & Poulin, C. (2008). Simultaneous Guillain‐Barre syndrome and acute disseminated encephalomyelitis in the pediatric population. Journal of Child Neurology, 23(7), 752–757. [DOI] [PubMed] [Google Scholar]
- Byun, J. I. , Lee, S. T. , Jung, K. H. , Sunwoo, J. S. , Moon, J. , Kim, T. J. , … Lee, S. K . (2016). Prevalence of antineuronal antibodies in patients with encephalopathy of unknown etiology: Data from a nationwide registry in Korea. Journal of Neuroimmunology, 293, 34–38. [DOI] [PubMed] [Google Scholar]
- Camdessanche, J. P. , Antoine, J. C. , Honnorat, J. , Vial, C. , Petiot, P. , Convers, P. , & Michel, D. (2002). Paraneoplastic peripheral neuropathy associated with anti‐Hu antibodies. A clinical and electrophysiological study of 20 patients. Brain, 125(Pt 1), 166–175. [DOI] [PubMed] [Google Scholar]
- Coggeshall, R. E. , & Carlton, S. M. (1998). Ultrastructural analysis of NMDA, AMPA, and kainate receptors on unmyelinated and myelinated axons in the periphery. Journal of Comparative Neurology, 391(1), 78–86. [DOI] [PubMed] [Google Scholar]
- Cortese, A. , Franciotta, D. , Alfonsi, E. , Visigalli, N. , Zardini, E. , Diamanti, L ., … Marchioni, E . (2016). Combined central and peripheral demyelination: Clinical features, diagnostic findings, and treatment. Journal of the Neurological Sciences, 363, 182–187. [DOI] [PubMed] [Google Scholar]
- Dalmau, J. , Gleichman, A. J. , Hughes, E. G. , Rossi, J. E. , Peng, X. , Lai, M. , … Lynch, D. R . (2008). Anti‐NMDA‐receptor encephalitis: Case series and analysis of the effects of antibodies. The Lancet Neurology, 7(12), 1091–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalmau, J. , Lancaster, E. , Martinez‐Hernandez, E. , Rosenfeld, M. R. , & Balice‐Gordon, R. (2011). Clinical experience and laboratory investigations in patients with anti‐NMDAR encephalitis. Lancet Neurology, 10(1), 63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyck, P. J. , & Thomas, P. K. (2005). Peripheral neuropathy, 4th ed. (pp. 2027–2028). Philadelphia: Saunders. [Google Scholar]
- Hacohen, Y. , Absoud, M. , Hemingway, C. , Jacobson, L. , Lin, J. P. , Pike, M ., … Lim, M . (2014). NMDA receptor antibodies associated with distinct white matter syndromes. Neurology(R) Neuroimmunology & Neuroinflammation, 1(1), e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han, C. , Wang, Y. , Jia, J. , Ji, X. , Fredrickson, V. , & Ding, Y. , … Sun, Y. X . (2012). Bickerstaff's brainstem encephalitis, Miller Fisher syndrome and Guillain‐Barre syndrome overlap in an asthma patient with negative anti‐ganglioside antibodies. BMC Research Notes, 5, 295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatano, T. , Shimada, Y. , & Kono, A. , Kubo, S. , Yokoyama, K. , Yoritaka, A ., … Hattori, N . (2011). A typical Miller Fisher syndrome associated with glutamate receptor antibodies. BMJ Case Reports, doi:10.1136/bcr.08.2010.3228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermans, G. , De Jonghe, B. , Bruyninckx, F. , & Van den Berghe, G. (2008). Clinical review: Critical illness polyneuropathy and myopathy. Critical Care, 12(6), 238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hideyama, T. , Yamashita, T. , Aizawa, H. , Tsuji, S. , Kakita, A. , Takahashi, H. , & Kwak, S. (2012). Profound downregulation of the RNA editing enzyme ADAR2 in ALS spinal motor neurons. Neurobiology of Disease, 45(3), 1121–1128. [DOI] [PubMed] [Google Scholar]
- Hoftberger, R. , van Sonderen, A. , Leypoldt, F. , Houghton, D. , Geschwind, M. , Gelfand, J. , … Dalmau, J . (2015). Encephalitis and AMPA receptor antibodies: Novel findings in a case series of 22 patients. Neurology, 84(24), 2403–2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa, Y. , Ikeda, K. , Murata, K. , Hirayama, T. , Takazawa, T. , Yanagihashi, M. , … Iwasaki, Y . (2013). Ophthalmoplegia and flaccid paraplegia in a patient with anti‐NMDA receptor encephalitis: A case report and literature review. Internal Medicine, 52(24), 2811–2815. [DOI] [PubMed] [Google Scholar]
- Joubert, B. , Kerschen, P. , Zekeridou, A. , Desestret, V. , Rogemond, V. , Chaffois, M. O. , … Honnorat, J . (2015). Clinical spectrum of encephalitis associated with antibodies against the alpha‐amino‐3‐hydroxy‐5‐methyl‐4‐isoxazolepropionic acid receptor: Case series and review of the literature. JAMA Neurology, 72(10), 1163–1169. [DOI] [PubMed] [Google Scholar]
- Kaida, K. , Kanzaki, M. , Morita, D. , Kamakura, K. , Motoyoshi, K. , Hirakawa, M. , & Kusunoki, S. (2006). Anti‐ganglioside complex antibodies in Miller Fisher syndrome. Journal of Neurology, Neurosurgery and Psychiatry, 77(9), 1043–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katirji, B. (2002). Neuromuscular disorders in clinical practice (pp. 1305–1306). Boston: Butterworth‐Heinemann. [Google Scholar]
- Khan, J. , Harrison, T. B. , Rich, M. M. , & Moss, M. (2006). Early development of critical illness myopathy and neuropathy in patients with severe sepsis. Neurology, 67(8), 1421–1425. [DOI] [PubMed] [Google Scholar]
- Köhler, A. , Hofstadt‐van Oy, U. , Pötzl, U. , Wandinger, K. P. , Weis, J. , Oschmann, P. (2012). A 58‐year‐old female with encephalopathy and acute axonal and severe autonomic neuropathy of undetermined cause developing ovarian carcinoma and NMDA‐receptor antibodies 33 months later. Journal of Neurology, 259(1), S99–S100. [Google Scholar]
- Lai, M. , Hughes, E. G. , Peng, X. , Zhou, L. , Gleichman, A. J. , Shu, H. , … Dalmau, J. (2009). AMPA receptor antibodies in limbic encephalitis alter synaptic receptor location. Annals of Neurology, 65(4), 424–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liba, Z. , Kayserova, J. , Elisak, M. , Marusic, P. , Nohejlova, H. , Hanzalova, J. , … Sediva, A . (2016). Anti‐N‐methyl‐D‐aspartate receptor encephalitis: The clinical course in light of the chemokine and cytokine levels in cerebrospinal fluid. Journal of Neuroinflammation, 13(1), 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odaka, M. , Yuki, N. , Yamada, M. , Koga, M. , Takemi, T. , Hirata, K. , & Kuwabara, S. (2003). Bickerstaff's brainstem encephalitis: Clinical features of 62 cases and a subgroup associated with Guillain‐Barre syndrome. Brain, 126(Pt 10), 2279–2290. [DOI] [PubMed] [Google Scholar]
- Ogata, H. , Matsuse, D. , Yamasaki, R. , Kawamura, N. , Matsushita, T. , Yonekawa, T. , … Kira, J . (2016). A nationwide survey of combined central and peripheral demyelination in Japan. Journal of Neurology, Neurosurgery and Psychiatry, 87(1), 29–36. [DOI] [PubMed] [Google Scholar]
- Peng, X. , Hughes, E. G. , Moscato, E. H. , Parsons, T. D. , Dalmau, J. , & Balice‐Gordon, R. J. (2014). Cellular plasticity induced by anti‐AMPA receptor encephalitis antibodies. Annals of Neurology, 77(3), 381–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohley, I. , Roesler, K. , Wittstock, M. , Bitsch, A. , Benecke, R. , & Wolters, A. (2015). NMDA‐receptor antibody and anti‐Hu antibody positive paraneoplastic syndrome associated with a primary mediastinal seminoma. Acta Neurologica Belgica, 115(1), 81–83. [DOI] [PubMed] [Google Scholar]
- Pruss, H. , Hoffmann, C. , Stenzel, W. , Saschenbrecker, S. , & Ebinger, M. (2014). A case of inflammatory peripheral nerve destruction antedating anti‐NMDA receptor encephalitis. Neurology(R) Neuroimmunology & Neuroinflammation, 1(2), e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiz, A. , Dalmau, J. , Butler, M. H. , Chen, Q. , Delattre, J. Y. , De Camilli, P. , & Graus, F. (1999). Anti‐amphiphysin I antibodies in patients with paraneoplastic neurological disorders associated with small cell lung carcinoma. Journal of Neurology, Neurosurgery and Psychiatry, 66(2), 214–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samejima, S. , Tateishi, T. , Arahata, H. , Shigeto, H. , Ohyagi, Y. , & Kira, J. (2010). A case of anti‐Hu antibody‐ and anti‐GluR epsilon2 antibody‐positive paraneoplastic neurological syndrome presenting with limbic encephalitis and peripheral neuropathy. Rinsho Shinkeigaku, 50(7), 467–472. [DOI] [PubMed] [Google Scholar]
- Spalloni, A. , Nutini, M. , & Longone, P. (2013). Role of the N‐methyl‐d‐aspartate receptors complex in amyotrophic lateral sclerosis. Biochimica et Biophysica Acta, 1832(2), 312–322. [DOI] [PubMed] [Google Scholar]
- Titulaer, M. J. , Hoftberger, R. , Iizuka, T. , Leypoldt, F. , McCracken, L. , Cellucci, T ., … Dalmau, J . (2014). Overlapping demyelinating syndromes and anti‐N‐methyl‐D‐aspartate receptor encephalitis. Annals of Neurology, 75(3), 411–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tojo, K. , Nitta, K. , Ishii, W. , Sekijima, Y. , Morita, H. , Takahashi, Y. , … Ikeda, S . (2011). A young man with anti‐NMDAR encephalitis following Guillain‐Barre syndrome. Case Reports in Neurology, 3(1), 7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomiyama, M. , RodriguezPuertas, R. , Cortes, R. , Christnacher, A. , Sommer, B. , Pazos, A. , … Mengod, G . (1996). Differential regional distribution of AMPA receptor subunit messenger rnas in the human spinal cord as visualized by in situ hybridization. Neuroscience, 75(3), 901–915. [DOI] [PubMed] [Google Scholar]
- Tuzun, E. , Zhou, L. , Baehring, J. M. , Bannykh, S. , Rosenfeld, M. R. , & Dalmau, J. (2009). Evidence for antibody‐mediated pathogenesis in anti‐NMDAR encephalitis associated with ovarian teratoma. Acta Neuropathologica, 118(6), 737–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei, Y. C. , Liu, C. H. , Lin, J. J. , Lin, K. J. , Huang, K. L. , Lee, T. H. , … Huang, C. C . (2013). Rapid progression and brain atrophy in anti‐AMPA receptor encephalitis. Journal of Neuroimmunology, 261(1–2), 129–133. [DOI] [PubMed] [Google Scholar]
- Zekeridou, A. , McKeon, A. , & Lennon, V. A . (2016). Frequency of synaptic autoantibody accompaniments and neurological manifestations of thymoma. JAMA Neurology, 73(7): 853–859. [DOI] [PubMed] [Google Scholar]
- Zifko, U. A. , Zipko, H. T. , & Bolton, C. F. (1998). Clinical and electrophysiological findings in critical illness polyneuropathy. Journal of the Neurological Sciences, 159(2), 186–193. [DOI] [PubMed] [Google Scholar]


