Abstract
Background
In recent years, the commercial importance of changes in muscle function of broiler chickens and of the corresponding effects on meat quality has increased. Furthermore, broilers are more sensitive to heat stress during transport and at high ambient temperatures than smaller egg-laying chickens. We hypothesised that heat stress would amplify muscle damage and expression of genes that are involved in such changes and, thus, lead to the identification of pathways and networks associated with broiler muscle and meat quality traits. Broiler and layer chickens were exposed to control or high ambient temperatures to characterise differences in gene expression between the two genotypes and the two environments.
Results
Whole-genome expression studies in breast muscles of broiler and layer chickens were conducted before and after heat stress; 2213 differentially-expressed genes were detected based on a significant (P < 0.05) genotype × treatment interaction. This gene set was analysed with the BioLayout Express3D and Ingenuity Pathway Analysis software and relevant biological pathways and networks were identified. Genes involved in functions related to inflammatory reactions, cell death, oxidative stress and tissue damage were upregulated in control broilers compared with control and heat-stressed layers. Expression of these genes was further increased in heat-stressed broilers.
Conclusions
Differences in gene expression between broiler and layer chickens under control and heat stress conditions suggest that damage of breast muscles in broilers at normal ambient temperatures is similar to that in heat-stressed layers and is amplified when broilers are exposed to heat stress. The patterns of gene expression of the two genotypes under heat stress were almost the polar opposite of each other, which is consistent with the conclusion that broiler chickens were not able to cope with heat stress by dissipating their body heat. The differentially expressed gene networks and pathways were consistent with the pathological changes that are observed in the breast muscle of heat-stressed broilers.
Electronic supplementary material
The online version of this article (doi:10.1186/s12711-017-0346-x) contains supplementary material, which is available to authorized users.
Background
Modern broiler chickens are characterised by relatively fast growth rate, greater muscle mass and better feed conversion ratio compared with layer and traditional chicken breeds [1, 2]. The carcasses of some broiler chickens show changes in the appearance of breast meat, such as a pale colour with reduced water holding capacity, or dark, firm and dry muscle with different functional properties [3]. More recently, white striping, which is characterised by white parallel striations in the direction of the muscle fibres and “wooden breast” muscles, have been reported [4, 5]. Elevated activity of creatine kinase and histopathological changes in affected muscles are suggestive of a degenerative myopathy [4, 6]. These changes have implications for meat quality and, potentially, have a significant economic cost. Several factors affect the proportion of affected carcasses, including different genetic background, growth rate, season, heat and transport stress, and abattoir practices [7–9].
Genetic variation in muscle and meat quality traits has been quantified [2, 10] but these traits usually involve measuring slaughtered sibs. Recent technological innovations have opened the way for genomic selection (GS) based on DNA markers (single nucleotide polymorphisms, SNPs) [11, 12]. Therefore, our objective was to identify genetic networks and pathways that might be useful for the detection of causal genetic factors that are involved in breast muscle and meat quality disorders of broiler chickens. It is also likely that the identified genetic factors would be helpful in updating the existing SNP chips to enable scientists to perform genomic selection for better muscle and meat quality in broilers.
Through the use of high-throughput microarray technology, it is possible to identify differentially-expressed genes as a result of a specific treatment [13]. In this study, we used microarray analysis to identify candidate genes that may contribute to differences in muscle damage between broilers and layers. Spontaneous and stress-induced myopathies in broiler skeletal muscles are exacerbated by heat stress [14, 15] and, thus, we compared gene expression profiles in the breast muscles of broiler and layer genotypes that were subjected to control or heat stress conditions. Our experimental strategy was based on the hypothesis that the expression of genes that are differentially expressed in broilers and layers under normal conditions is increased and therefore more easily detected after heat stress. However, it is often difficult to assign biological significance to the large number of genes that are detected in a microarray experiment. This problem can be solved when the differentially-expressed genes are organised via hierarchical clustering methods [16] and, for this purpose, we used BioLayout Express3D [17, 18] and Ingenuity Pathway Analysis (IPA) (http://www.ingenuity.com/). In addition, we compared the results from these analyses with those obtained with the DAVID [19, 20] (https://david.ncifcrf.gov/) and Reactome [21, 22] (http://reactome.org/) software using more recent databases.
Methods
Animals and husbandry
We used 40 male broiler chicks of a male line (Ross 308, Aviagen, Newbridge, UK) from a commercial hatchery and 74 layer chicks (White Leghorn) from a line maintained at the Roslin Institute. For the first 2 weeks, birds were reared in groups of 20 individuals until the layers had been sexed by a DNA method [23]. At 2 weeks of age, the birds were distributed to eight pens by sex and genotype, with each pen containing 12 male layers and nine or ten broilers, in a completely randomised design. The birds were provided with feed (a commercial layer starter diet) and water ad libitum and the daily photoperiod was 16 h light and 8 h darkness.
The birds were subjected to experimental treatments over four days from 42 to 46 days of age. On each day, we randomly selected two pens for each breed and the birds were transferred into four controlled environment chambers. On each day, we randomly selected four chambers, i.e. two for the heat treatment (32 °C, 75% relative humidity or RH) and two as controls (21 °C, 50% RH). Each chamber contained two crates with two male broilers or two male layers, with pens and crates confounded. The crates were placed on a wooden pallet and the order of the pairs (crates) in each room was randomised. Sixty-four birds were used in the experiment.
About 30 min before the birds were transferred to the chambers, the relevant chamber was turned on, such that it could reach the required temperature and humidity before birds were placed into the chamber for the following 2 h. Birds were introduced in each chamber at intervals of 45 min to allow for sampling of the birds.
After completing the 2-h treatment, birds were removed from the crate and rectal temperatures were measured using a thermistor probe (Model 612-849; RS Components Ltd., Corby, Northants, UK). Then, they were euthanized by an intravenous injection of sodium pentobarbitone into the wing vein and two tissue samples of 100–120 mg were taken from the left pectoral muscle and snap frozen in liquid nitrogen for subsequent RNA extraction.
RNA extraction and microarray experiment
Samples of breast muscle from male chickens were randomised prior to extraction of RNA using Trizol (Life Technologies, Paisley, UK) following the manufacturer’s recommended protocol. Briefly, the frozen tissue was homogenised in 1 ml of Trizol using the FastPrep® system with Lysing matrix D (MP Biochemicals). The phases were separated by addition of 200 µl of 2-bromo-chloro-propane (Sigma Aldrich) and centrifuged for 15 min. A 500-µl sample of the clear upper aqueous layer was transferred to a fresh tube and 500 µl of isopropanol was added. The samples were centrifuged for 30 min to pellet the RNA, which was washed twice with 70% ethanol before air-drying. The RNA was resuspended in 100 µl of RNAse-free water prior to quantification and quality assessment. All RNA samples had a RNA integrity number (RIN) value higher than 8.0, as determined by the Agilent Bioanalyser RNA 6000 Nano Chip. Samples were diluted to 50 ng/μl with deionised and RNAse-free water. Aliquots of 20 μl from each sample were used for pooling the two samples from each crate to obtain eight replicates for each breed × treatment combination.
Microarray hybridisation was completed in the ArkGenomics laboratory at the Roslin Institute (http://genomics.ed.ac.uk ). Total RNA was prepared for hybridisation to the Affymetrix chicken GeneChip array using the Affymetrix IVT express kit according to the manufacturer’s protocol. The generated cRNA was hybridised overnight to the cartridge arrays according to Affymetrix’s protocols. The cartridges were washed and stained in the Affymetrix fluidic station using the hybridisation, wash and stain kit from Affymetrix. After staining, the arrays were scanned with the Affymetrix GeneChip system 3000 scanner. The resultant CEL files were reviewed using the Expression Console software from Affymetrix.
Thrity-two Affymetrix chicken array chips (38.5K; each GeneChip included 38,535 probes) were used in the microarray experiment. After scanning, the CEL files were analysed in four batches of eight slides to obtain expression values in GenStat (www.vsni.co.uk/software/genstat). Each batch contained slides from birds treated on the same day. The Robust Multichip Average (RMA) algorithm [24] was used to extract the gene expression data.
Statistical analysis
The experiment was a 2 × 2 factorial design (breed × treatment), with day/chambers/crates as blocking factors. Standard analysis of variance methods was used to analyse body temperature and body weight using GenStat v13 (https://www.vsni.co.uk/software/genstat/). Transformation to natural logarithms was necessary to achieve normally distributed residuals of body weight.
For the analysis of differentially-expressed genes, we used a model with fixed effect terms for breed and treatment and their interaction. The normalised data were analysed by using Microarray One-Channel ANOVA in GenStat, with a model that included breed × treatment as treatment structure and the hierarchical structure of day/chamber/breed as blocking factor. Genes that showed a significant breed × treatment interaction (P < 0.05) were used for subsequent investigation because they were expected to be most relevant for genetic differences between broilers and layers in response to heat stress. Based on these ANOVA results, the false discovery rate (FDR) was calculated for three probability values (P < 0.05, <0.01 and <0.001) for the effects of treatment, breed, and their interaction. FDR was calculated using the Mixture Model of GenStat and the maximum number of iteration cycles was set to 300.
Cluster analysis in BioLayout Express3D
Gene annotations were downloaded from the NetAffx analysis centre of Affymetrix (http://www.affymetrix.com/analysis/index.affx; downloaded 15 December 2016). Expression values for the selected subset of genes/probes were unlogged, entered into BioLayout Express3D (BLE, http://www.biolayout.org/) and analysed using a Pearson correlation threshold of 0.80. Clusters were viewed in the Class Viewer, after running the Markov Clustering Algorithm (MCL). For cluster size, a minimum threshold of four genes/probes per cluster was selected to limit the size of the smallest clusters [25]. Selected clusters were identified on the basis of a clear difference in expression pattern of the genes between treatments (control vs. heat treatment) and breeds. For functional analyses, clusters were combined into ‘categories’ on the basis of similarity in mean expression pattern across breeds and treatments.
Analysis of pathways and networks in IPA
The gene expression data for each of the six selected categories were combined into a single Excel sheet for analysis in Ingenuity Pathway Analysis (IPA, http://www.ingenuity.com/products/ipa) of the four breed × treatment combinations (broiler control, BC; broiler heat stress, BH; layer control, LC and layer heat stress, LH). The lists of genes for each category were analysed in IPA by using Fisher’s exact test to identify biological functions and pathways that were enriched in the dataset using the ‘Core Analysis’ function of the IPA program. Genes were mapped against the ‘Tissues and Cell Lines’ available in the Ingenuity Pathway Analysis Knowledge Base (IPAKB). Because information in the IPA originates mainly from mammals (human, mouse and rat), the submitted lists of genes were mapped against all available species and changes to avian terminology, e.g. neutrophil to heterophil, were made. For network generation, we set a threshold of 35 molecules per network and 25 networks per analysis. Both direct and indirect relationships of molecules were considered.
Additional analyses of pathways and networks
To reconfirm the initial results, we repeated the analyses on pathways and networks with more recent databases. We used two software programs, i.e. DAVID (https://david.ncifcrf.gov/) and Reactome (http://reactome.org/), both accessed on 2nd July 2017. Further information is in Additional file 1: Table S1.
Results
Differentially-expressed genes
The Affymetrix Genechips were filtered for expression levels higher than 1, which reduced the number of probes from 38,535 to 19,038. The results of the ANOVA for the filtered set of genes are in Table 1. The false discovery rate (FDR) for statistically significant genes (P < 0.05) was less than 31.5% for the treatment × breed interaction, 44% for treatment, and 3% for breed. A total of 2213 genes were differentially expressed among the four treatment comparisons. The numbers of differentially-regulated genes that overlapped between the two treatments are in Fig. 1. We found 1361 upregulated genes in the comparison between BH and BC, of which 1316 (97%) were shared with downregulated genes in the comparison between LH and LC. Similarly, we found 852 downregulated genes in the comparison between BH and BC, of which 753 (88%) were shared with upregulated genes in the comparison between LH and LC.
Table 1.
Number of significant genes for treatment, breed, and breed × treatment interaction at different levels of significance
| Significance (P<) | Treatment (heat-stress vs. control) | Breed (broiler vs. layer) | Breed × treatment interaction |
|---|---|---|---|
| 0.001 | 107 | 5208 | 93 |
| 0.01 | 617 | 8182 | 635 |
| 0.05 | 1922 | 10,733 | 2213 |
Fig. 1.
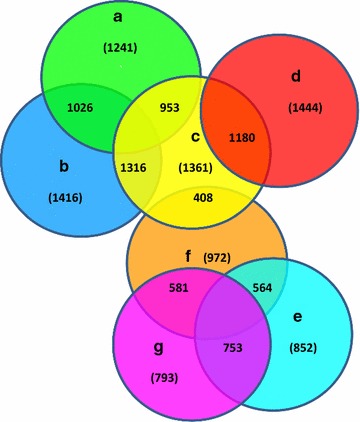
Differentially-regulated genes that overlap between two treatments. a Downregulated genes in broiler controls (BC) versus layer controls (LC). b Downregulated genes in heat-stressed layers (LH) compared with control layers (LC). c Upregulated genes in heat-stressed broilers (BH) compared with control broilers (BC). d Upregulated genes in heat-stressed broilers (BH) compared with heat-stressed layers (LH). e Downregulated genes in heat-stressed broilers (BH) compared with control broilers (BC). f Upregulated genes in broiler controls (BC) compared with layer controls (LC). g Upregulated genes in heat-stressed layers (LH) compared with layer controls (LC). The number in parentheses in each circle is the total number of differentially-expressed genes
Categorisation of candidate genes on the basis of their biological functions
Based on their biological function, genes that were differentially expressed for the breed × treatment interaction were divided into 12 categories (Table 2). More than 43% (959) of the genes had no gene ontology (GO) term for a biological process or function. These genes fell in two major groups: 424 genes had no known function and 534 genes were not involved in a known biological process.
Table 2.
Significant differentially-expressed genes for breed × treatment interaction (P < 0.05) grouped by function
| Group | Biological functions | Number of genes |
|---|---|---|
| 1 | Transcripts with no known gene name | 424 |
| 2 | Genes with no GO terms for biological functions | 534 |
| 3 | Signal transduction | 130 |
| 4 | Stress-related response, inflammatory, angiogenesis, apoptotic, and proteolytic functions | 334 |
| 5 | Metabolic, and catabolic processes | 190 |
| 6 | Inter and intracellular transport of proteins, ions, and muscle contraction | 162 |
| 7 | Cellular proliferation, and organ development | 142 |
| 8 | Transcription and translation | 138 |
| 9 | Protein phosphorylation, dephosphorylation, modification, and folding | 95 |
| 10 | Signal transduction | 92 |
| 11 | DNA damage, repair, metabolism, and catabolic processes | 60 |
| 12 | Cytoskeleton organization and polymerization of filaments | 42 |
Comparisons of genes within and between breed and treatment significant for interaction
The selected genes were further divided into up- and downregulated patterns of gene expression for different comparisons within- and between-breed and treatment. Of the 54 clusters, 21 were selected for further analysis on the basis of their clear expression pattern, which included 509 genes that were grouped into six distinct categories (Table 3) according to the nature of their expression patterns corresponding to the (statistically significant) interactions of heat stress and genotype (Fig. 2). The expression values of the genes in category I were higher for broilers than for layers. Heat-stress resulted in a further increase in expression levels for broiler but a decrease for layers, compared to their respective controls. In the case of category II, the expression level of genes was higher for broilers than layers under control temperatures (as for category I). However, after heat-stress expression levels were lower in broilers compared with control broilers and conversely, higher in layers compared with LC. Expression values of category III genes were substantially higher for LC than BC whereas heat-stress resulted in further increases in gene expression in layers and decreases in broilers. Category IV genes were upregulated in BH compared with BC, whereas they were upregulated in LC compared with LH. Expression of category V genes was low in LC compared with all other groups and genes were upregulated in LH compared with LC, whereas they were downregulated in BH compared with BC. In the case of category VI genes, expression values for control layers were higher than for the respective broilers. After treatment, the expression of these genes increased in broilers but decreased in layers. Each of the six patterns of gene expression were analysed separately in IPA and significant (P < 0.05) pathways and networks were identified (see Additional file 1: Tables S2, S3, S4, S5, S6, S7). The set of genes which were filtered out by BioLayout Express (i.e. genes with a correlation coefficient of less than 0.80) was analysed in IPA separately, using the same procedure, to determine significant pathways and networks for this gene set, as shown in Additional file 1: Table S8.
Table 3.
Numbers of genes, pathways and networks associated with different categories of genes based on function (see Fig. 2)
| Category | Genesa | Pathways | Networks | Selected | ||
|---|---|---|---|---|---|---|
| Pathways | Networks | Functions | ||||
| I | 180 | 35 | 23 | 9 | 5 | Stress response, cellular damage, connective tissue and muscle disorders |
| II | 74 | 40 | 7 | 12 | 4 | Cellular development, anti-apoptotic, anti-inflammatory and anti-stress functions |
| III | 55 | 3 | 9 | 2 | 0 | Anti-apoptotic, anti-oxidant, anti-inflammatory, energy production |
| IV | 13 | 9 | 0 | 10 | 0 | Stress, inflammatory, tissue damage, anti-oxidative, wound healing |
| V | 7 | 9 | 4 | 5 | 0 | Inflammation, immune functions, oxidative stress, phospholipid degradation |
| VI | 16 | 7 | 4 | 3 | 0 | Cell death, inflammatory and immune response, dellular development, haematopoiesis |
aGenes mapped to corresponding identifiers
Fig. 2.
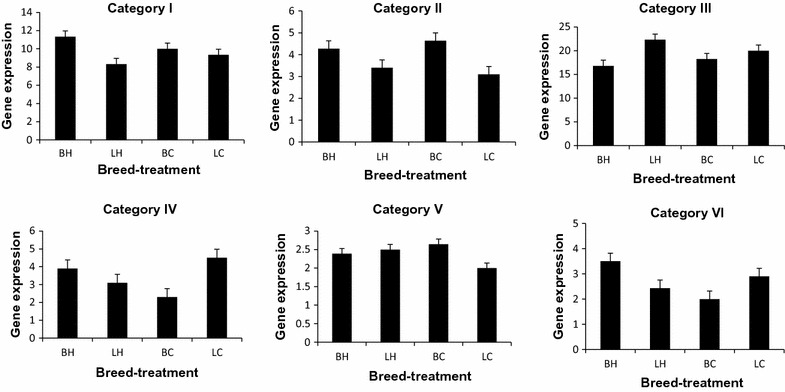
Mean expression levels of genes with significant breed × treatment interaction (P < 0.05) grouped into six categories (categories I–VI). Each graph has four bars and each bar represents one group. BC broiler control, BH heat-stressed broiler, LC layer control, LH heat-stressed layer
Body weight and rectal temperature
Mean rectal temperatures for the control and heat-stressed conditions were 41.0 and 43.7 °C, respectively, in broilers, and 42.0 and 42.3 °C, respectively, in layers [standard error of difference (SED) 0.15 between breed and 0.14 between treatments]. The increase in rectal temperature in the heat-stressed birds was significantly larger in broilers (2.6 °C) than in layers (0.3 °C) which resulted in a significant breed × treatment interaction (P < 0.001). Average body weights (back-transformed) of broiler and layer males were 8.38 (4384 g) and 6.54 (693 g), respectively (SED 0.017, P < 0.001).
Discussion
Phenotypic responses validate experimental treatments
The large increase in rectal body temperature for broilers compared with layers is consistent with early reports in the literature [15, 26]. The results confirm the difficulty that broiler chickens have in coping with high ambient temperatures and other stressors, such as shackling, that may ultimately lead to detrimental consequences for both muscle function and meat quality [27, 28]. The results confirm that the heat treatment had the expected effect on the metabolism of broiler chickens and that the response in broilers was greater than in layers.
Microarray analysis
The microarray results showed large differences between broilers and layers. Nevertheless, comparatively few significant genes (107, 617 and 1922 at P < 0.001, P < 0.01 and P < 0.05, respectively) were differentially-expressed in the comparison between treatments, which indicated that the differences in gene expression between heat-stressed and control birds were not as large as those between breeds. The number of upregulated genes in BH compared with BC (97%) that were shared with downregulated genes in the LH and LC comparison, and the number of downregulated genes in the former (BH vs. BC) compared with the latter (LH vs. LC) (88%), suggest that changes in gene expression in response to heat-stress are opposite in broilers compared to layers, which is consistent with the conclusion that broiler chickens do not manage heat stress appropriately. Furthermore, differential gene expression in breast muscles of BC and LH compared with LC, separately, involved a similar set of genes, which suggests that, in terms of gene expression, control broilers are similar to heat-stressed layers. We found that 1026 downregulated genes overlapped in the comparison of LH vs. LC (71%) and BC versus LC (83%) and likewise 753 genes were common/overlapped in the set of upregulated genes in the LH vs. LC (95%) comparison and in the list of downregulated genes in BH versus BC (88%) comparison. Taken together, these results are consistent with the physiological changes and muscle disorders that were reported for broiler chickens reared at conventional temperatures [1, 4].
The 2213 genes that were differentially-expressed for the breed × treatment interaction term were classified into categories according to their function and the biological processes in which they are involved. For 424 transcripts (19.2% of the total), we found no gene symbol and no gene name, which indicates that many genes involved in heat-stress induced responses in chicken skeletal muscle are not characterised to date. Similarly, the second largest group of genes, representing 13.1% of the significant genes, had no GO term for a biological function at the time the GO terms for this gene set were retrieved from the NetAffx Analysis Centre of Affymetrix (http://www.affymetrix.com/estore/analysis/index.affx, re-accessed 15th December 2016).
About 15% of the 2213 genes, which were significant (P < 0.05) for breed × treatment interaction, are directly involved in stress-related response, inflammatory, angiogenesis, apoptotic, and proteolytic functions, which is consistent with the physiological changes in broiler muscle caused by heat-stress [15, 29]. Similarly, 4% of the genes are involved in signal transduction and are associated with various biological processes, including oxidative stress, inflammation, muscle contraction, glycogen metabolism, and the concentrations of intracellular ions [30–34], and 7.3% are involved in inter and intracellular transport of proteins associated with muscle contraction and muscle damage-related functions [35–37]. Other smaller categories of genes are involved in cellular proliferation, development and DNA damage repair.
Stress is known to accelerate metabolic rate, mainly through carbohydrate metabolism to produce larger amounts of energy and facilitate “fight or flight” responses [38–40]; about 6% of all 2213 genes were involved in metabolic and catabolic functions. The cytoskeleton is required for cell shape and motility and is involved in cell division [41, 42]. It has been suggested that the genes in Group 11 (Table 2) have a role in the movement and division of leukocytes, such as heterophils and macrophages, as secondary mediators of the genes in Groups 3, 4 and 5 to shape the stress and inflammatory response to heat stress. Of all the significant genes for breed × treatment interaction, 49 encode proteins located in the mitochondria and about 200 affect the cell membrane directly. These results suggest that damage to mitochondria and cell membrane are potentially important components of heat-stress induced pathogenesis in chicken breast muscles.
Taken together, these results suggest a picture of stress responses, inflammation, oxidative stress, and tissue damage, which is consistent with histological and physiological changes in broiler breast muscle [1]. Confirmatory evidence was also reported in a recent IPA analysis of differentially-expressed genes in “wooden breast” and control broiler muscles [43].
IPA analysis
Heat stress in broilers led to further increases in the expression of category I genes of the α-adrenergic signalling network (see Additional file 1: Table S2), which are involved in glycogenolysis under stressful conditions to provide energy for muscle contraction. However, stress hormones are also known to alter the activities of immune cells and lead to the production of various pro-inflammatory cytokines and chemokines [44, 45]. In agreement with these findings, genes of several chemokine pathways were also present in this category, which are involved in cytokine signalling, tissue damage and related functions (see Additional file 1: Table S2). Upregulation of these pathways in control broilers indicates that breast muscles in broilers at conventional ambient temperatures show physiological and functional changes that are further exacerbated by exposure to heat stress. However, the upregulated vascular endothelial growth factor (VEGF) signalling pathway is also a significant mediator of hypoxia-induced angiogenesis and is usually upregulated in hypoxia-like situations. Upregulation of this pathway in control broilers compared with layers suggests that broiler muscle cells were under hypoxic-stress even under control conditions. The reason for this may lie in the larger size of muscle fibres in broilers and an inadequate capillary supply, which are, in turn, considered to induce metabolic stress due to the larger diffusion distances for nutrients, metabolites and waste products [1]. This is consistent with reports that thermal stress leads to oxidative stress and muscle damage, as indicated by higher plasma creatine kinase activity [26, 46–48]. Upregulation of the nuclear factor erythroid 2-related factor 2 (NRF2)-mediated oxidative stress response pathway may be a protective measure to minimise the damaging effects of heat stress on anti-oxidant functions [49–51].
Expression of category II genes was highest in BC and decreased after heat stress. Upregulation of protein synthesis and angiogenic pathways in BC is logical, in the sense, that broilers have substantially higher growth rates and larger body mass than layers [52, 53]. Exposure to heat stress resulted in downregulation of these pathways in broilers, which is consistent with the negative effects of heat stress on growth-related traits [54]. Conversely, inflammatory and anti-inflammatory pathways were upregulated in layers after heat stress, possibly as a mechanism to protect the body from tissue damage. However, these results are in agreement with the physiological data (body temperature) from the current study that show that the increase in body temperature was much smaller in layers than in broilers. Consistent with this, Sandercock et al. [14] reported that the effects of heat stress on body temperature and plasma creatine kinase activity were much smaller in layers than in broilers. Similarly, the extent of heat stress induced oxidative stress in skeletal muscles was much smaller in layers than in broilers [55].
Hypoxia is known to decrease the efficiency of oxidative phosphorylation [56] and, thus, the downregulation of this pathway in broilers (Additional file 1: Table S4) could be due to hypoxia-like conditions in skeletal muscles. In contrast to our finding, Toyomizu et al. [57] reported that oxidative phosphorylation in skeletal muscles was much more efficient in broilers than in layers at 14–28 days of age when body weights were about 1.0 and 0.2 kg for broilers and layers, respectively. This greater efficiency of oxidative phosphorylation in broilers at that age is a logical outcome of selection for rapid growth. In the present study, broilers at 6 weeks of age were over 3.5 kg heavier than 28-day layers and the occurrence of an hypoxia-like situation in their muscles is consistent with a higher muscle to capillary ratio and larger diffusion distances for nutrients and metabolic wastes [1]. Consistent with this conclusion, some angiogenic pathways in category I that are involved in hypoxia-induced angiogenesis, such as the VEGF signalling pathway, were upregulated in broilers.
Category VI genes, such as Janus kinase 1 (JAK1), Janus kinase 2 (JAK2) and tyrosine kinase 2 (TYK2) were up-regulated in BH compared with BC and have a role in wound healing and tissue regeneration [58], in agreement with categories I and IV genes, which indicates that muscle damage is much more important in heat-stressed broilers than BC, LC and LH. However, these pathways were downregulated in LH compared with LC, possibly because upregulation of survival-related pathways reduced damaging effects in LH [59].
Conclusions
The experimental paradigm of combining genetic and environmental differences was successful in identifying a limited number of pathways and networks that underlie muscle function and meat quality. Our findings provide new insights into the genetics and pathogenesis of muscle damage induced by heat stress through the identification of previously unknown pathways and networks. Importantly, our study also showed that the gene expression pattern for breast muscle of broiler chickens that were raised under a conventional (control) temperature was similar to that of heat-stressed layers and that the expression of these genes was further enhanced in heat-stressed broilers. These results provide a resource for the identification of candidate genes for muscle function and meat quality, which we will use in an accompanying paper to determine statistically significant associations of SNPs with muscle and meat quality traits in chicken.
Authors’ contributions
IZ assisted with the experiment, collated the data, carried out the RNA extraction, analysed the microarray data, conducted the bioinformatics analyses, evaluated the pathways and networks and wrote the draft manuscript. DJK participated in data and bioinformatics analyses and interpretation and helped to draft the manuscript. PMH devised the experiment, assisted with data collection, supervised and coordinated the research and helped to draft the manuscript. All authors read and approved the final manuscript.
Acknowledgements
The authors are grateful for assistance from the staff of the poultry unit at the Roslin Institute, to Caroline McCorquodale for assistance in the analysis of the microarray data and to Alison Downing of Edinburgh Genomics, The University of Edinburgh, for conducting the microarray hybridisations.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
The microarray data supporting the results of this article are available in the ArrayExpress database (www.ebi.ac.uk/arrayexpress) under accession number E-MTAB-5011 (http://www.ebi.ac.uk/arrayexpress/experiments/E-MTAB-5011).
Consent for publication
Not applicable.
Ethics approval and consent to participate
All animal husbandry and procedures were conducted under legislation to protect the welfare of animals and were licensed after local ethical review under the Government licence (Project Licence PIL 60/3815).
Funding
Edinburgh Genomics is partly supported through core Grants from NERC (R8/H10/56), MRC (MR/K001744/1) and BBSRC (BB/J004243/1). The broiler chicks were kindly donated by Aviagen Ltd (Newbridge, UK). Funding for IZ was provided by the Higher Education Commission, Government of Pakistan through a PhD scholarship to IZ. The Roslin Institute is supported by a BBSRC Institute Strategic Programme Grant and this research was conducted under programme grant ISP1 (BB/J004316/1).
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional file
Additional file 1: Table S1. Comparison of pathways analyses of microarray (gene expression) data on IPA, DAVID, and REACTOME. Table S2. Pathways selected from category I cluster analysis. Table S3. Pathways selected from category II cluster analysis. Table S4. Pathways selected from category III cluster analysis. Table S5. Pathways selected from category IV cluster analysis. Table S6. Pathways selected from category V cluster analysis. Table S7. Pathways selected from category VI cluster analysis. Table S8. Selected pathways from IPA analysis of Biolayout filtered genes.
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1186/s12711-017-0346-x) contains supplementary material, which is available to authorized users.
Contributor Information
Imran Zahoor, Email: imran.zahoor@uvas.edu.pk.
Dirk-Jan de Koning, Email: dj.de-koning@slu.se.
Paul M. Hocking, Email: paul.hocking@roslin.ed.ac.uk
References
- 1.MacRae VE, Mahon M, Gilpin S, Sandercock DA, Mitchell MA. Skeletal muscle fibre growth and growth associated myopathy in the domestic chicken (Gallus domesticus) Br Poult Sci. 2006;47:264–272. doi: 10.1080/00071660600753615. [DOI] [PubMed] [Google Scholar]
- 2.Hocking PM, Sandercock DA, Wilson S, Fleming RH. Quantifying genetic (co)variation and effects of genetic selection on tibial bone morphology and quality in 37 lines of broiler, layer and traditional chickens. Br Poult Sci. 2009;50:443–450. doi: 10.1080/00071660903110927. [DOI] [PubMed] [Google Scholar]
- 3.Sheard PR, Hughes SI, Jaspal MH. Colour, pH and weight changes of PSE, normal and DFD breast fillets from British broilers treated with a phosphate-free, low salt marinade. Br Poult Sci. 2012;53:57–65. doi: 10.1080/00071668.2012.655707. [DOI] [PubMed] [Google Scholar]
- 4.Kuttappan VA, Shivaprasad HL, Shaw DP, Valentine BA, Hargis BM, Clark FD, McKee SR, Owens CM. Pathological changes associated with white striping in broiler breast muscles. Poult Sci. 2013;92:331–338. doi: 10.3382/ps.2012-02646. [DOI] [PubMed] [Google Scholar]
- 5.Petracci M, Mudalal S, Bonfiglio A, Cavani C. Occurrence of white striping under commercial conditions and its impact on breast meat quality in broiler chickens. Poult Sci. 2013;92:1670–1675. doi: 10.3382/ps.2012-03001. [DOI] [PubMed] [Google Scholar]
- 6.Kuttappan VA, Huff GR, Huff WE, Hargis BM, Apple JK, Coon C, Owens CM. Comparison of hematologic and serologic profiles of broiler birds with normal and severe degrees of white striping in breast fillets. Poult Sci. 2013;92:339–345. doi: 10.3382/ps.2012-02647. [DOI] [PubMed] [Google Scholar]
- 7.Petracci M, Bianchi M, Cavani C. The European perspective on pale, soft, exudative conditions in poultry. Poult Sci. 2009;88:1518–1523. doi: 10.3382/ps.2008-00508. [DOI] [PubMed] [Google Scholar]
- 8.Bailey RA, Watson KA, Bilgili SF, Avendano S. The genetic basis of pectoralis major myopathies in modern broiler chicken lines. Poult Sci. 2015;94:2870–2879. doi: 10.3382/ps/pev304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trocino A, Piccirillo A, Birolo M, Radaelli G, Bertotto D, Filiou E, Petracci M, Xiccato G. Effect of genotype, gender and feed restriction on growth, meat quality and the occurrence of white striping and wooden breast in broiler chickens. Poult Sci. 2015;94:2996–3004. doi: 10.3382/ps/pev296. [DOI] [PubMed] [Google Scholar]
- 10.Le Bihan-Duval E, Berri C, Baeza E, Millet N, Beaumont C. Estimation of the genetic parameters of meat characteristics and of their genetic correlations with growth and body composition in an experimental broiler line. Poult Sci. 2001;80:839–843. doi: 10.1093/ps/80.7.839. [DOI] [PubMed] [Google Scholar]
- 11.Meuwissen THE, Hayes BJ, Goddard ME. Prediction of total genetic value using genome-wide dense marker maps. Genetics. 2001;157:1819–1829. doi: 10.1093/genetics/157.4.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kranis A, Gheyas AA, Boschiero C, Turner F, Yu L, Smith S, Talbot R, Pirani A, Brew F, Kaiser P, Hocking PM, Fife M, Salmon N, Fulton J, Strom TM, Haberer G, Weigend S, Preisinger R, Gholami M, Qanbari S, Simianer H, Watson KA, Woolliams JA, Burt DW. Development of a high density 600K SNP genotyping array for chicken. BMC Genomics. 2013; 14. [DOI] [PMC free article] [PubMed]
- 13.Whitworth G. An introduction to microarray data analysis and visualization. Methods Mol Biol. 2010;470:19–50. doi: 10.1016/S0076-6879(10)70002-1. [DOI] [PubMed] [Google Scholar]
- 14.Sandercock DA, Hunter RR, Mitchell MA, Hocking PM. Thermoregulatory capacity and muscle membrane integrity are compromised in broilers compared with layers at the same age or body weight. Br Poult Sci. 2006;47:322–329. doi: 10.1080/00071660600732346. [DOI] [PubMed] [Google Scholar]
- 15.Sandercock DA, Hunter RR, Nute GR, Mitchell MA, Hocking PM. Acute heat stress-induced alterations in blood acid-base status and skeletal muscle membrane integrity in broiler chickens at two ages: Implications for meat quality. Poult Sci. 2001;80:418–425. doi: 10.1093/ps/80.4.418. [DOI] [PubMed] [Google Scholar]
- 16.Lim WK, Wang K, Lefebvre C, Califano A. Comparative analysis of microarray normalization procedures: effects on reverse engineering gene networks. Bioinformatics (Oxford, England) 2007;23:i282–i288. doi: 10.1093/bioinformatics/btm201. [DOI] [PubMed] [Google Scholar]
- 17.Freeman TC, Goldovsky L, Brosch M, van Dongen S, Mazière P, Grocock RJ, Freilich S, Thornton J, Enright AJ. Construction, visualisation, and clustering of transcription networks from microarray expression data. PLoS Comput Biol. 2007;3:2032–2042. doi: 10.1371/journal.pcbi.0030206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Theocharidis A, van Dongen S, Enright AJ, Freeman TC. Network visualization and analysis of gene expression data using BioLayout Express(3D) Nat Protoc. 2009;4:1535–1550. doi: 10.1038/nprot.2009.177. [DOI] [PubMed] [Google Scholar]
- 19.Huang DW, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 20.Huang DW, Sherman BT, Lempicki RA. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009;37:1–13. doi: 10.1093/nar/gkn923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fabregat A, Sidiropoulos K, Garapati P, Gillespie M, Hausmann K, Haw R, Jassal B, Jupe S, Korninger F, McKay S, Matthews L, May B, Milacic M, Rothfels K, Shamovsky V, Webber M, Weiser J, Williams M, Wu GM, Stein L, Hermjakob H, D’Eustachio P. The reactome pathway knowledgebase. Nucleic Acids Res. 2016;44:D481–D487. doi: 10.1093/nar/gkv1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Croft D, Mundo AF, Haw R, Milacic M, Weiser J, Wu GM, Caudy M, Garapati P, Gillespie M, Kamdar MR, Jassal B, Jupe S, Matthews L, May B, Palatnik S, Rothfels K, Shamovsky V, Song H, Williams M, Birney E, Hermjakob H, Stein L, D’Eustachio P. The reactome pathway knowledgebase. Nucleic Acids Res. 2014;42:D472–D477. doi: 10.1093/nar/gkt1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clinton M, Haines L, Belloir B, McBride D. Sexing chick embryos: a rapid and simple protocol. Br Poult Sci. 2001;42:134–138. doi: 10.1080/713655025. [DOI] [PubMed] [Google Scholar]
- 24.Irizarry RA, Hobbs B, Collin F, Beazer-Barclay YD, Antonellis KJ, Scherf U, Speed TP. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics. 2003;4:249–264. doi: 10.1093/biostatistics/4.2.249. [DOI] [PubMed] [Google Scholar]
- 25.Theocharidis A, Dongen SV, Enright AJ, Freeman TC. Network visualization and analysis of gene expression data using BioLayout Express 3D. Nat Protoc. 2009;4:1535–1550. doi: 10.1038/nprot.2009.177. [DOI] [PubMed] [Google Scholar]
- 26.Mitchell MA, Sandercock DA. Increased hyperthermia induced skeletal muscle damage in fast growing broiler chickens? Poult Sci. 1995;74:74. [Google Scholar]
- 27.Debut M, Berri C, Arnould C, Guemene D, Sante-Lhoutellier V, Sellier N, Baeza E, Jehl N, Jego Y, Beaumont C, Le Bihan-Duval E. Behavioural and physiological responses of three chicken breeds to pre-slaughter shackling and acute heat stress. Br Poult Sci. 2005;46:527–535. doi: 10.1080/00071660500303032. [DOI] [PubMed] [Google Scholar]
- 28.Petracci M, Bianchi M, Cavani C. Pre-slaughter handling and slaughtering factors influencing poultry product quality. Worlds Poult Sci J. 2010;66:17–26. doi: 10.1017/S0043933910000024. [DOI] [Google Scholar]
- 29.Sandercock DA, Mitchell MA. Myopathy in broiler chickens: a role for Ca2+-activated phospholipase A(2)? Poult Sci. 2003;82:1307–1312. doi: 10.1093/ps/82.8.1307. [DOI] [PubMed] [Google Scholar]
- 30.Allen SJ, Crown SE, Handel TM. Chemokine: receptor structure, interactions, and antagonism. Ann Rev Immunol. 2007; 787–820. [DOI] [PubMed]
- 31.Amir R, Argoff CE, Bennett GJ, Cummins TR. The role of sodium channels in chronic inflammatory and neuropathic pain. J Pain. 2006;7:S1–S29. doi: 10.1016/j.jpain.2006.01.444. [DOI] [PubMed] [Google Scholar]
- 32.Ashton AW, Ware GM, Kaul DK, Ware JA. Inhibition of tumor necrosis factor alpha-mediated NF kappa B activation and leukocyte adhesion, with enhanced endothelial apoptosis, by G protein-linked receptor (TP) ligands. J Biol Chem. 2003;278:11858–11866. doi: 10.1074/jbc.M210766200. [DOI] [PubMed] [Google Scholar]
- 33.Baker OJ, Camden JM, Rome DE, Seye CI, Weisman GA. P2Y(2) nucleotide receptor activation up-regulates vascular cell adhesion molecular-1 expression and enhances lymphocyte adherence to a human submandibular gland cell line. Mol Immunol. 2008;45:65–75. doi: 10.1016/j.molimm.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lattin J, Zidar DA, Schroder K, Kellie S, Hume DA, Sweet MJ. G-protein-coupled receptor expression, function, and signaling in macrophages. J Leukoc Biol. 2007;82:16–32. doi: 10.1189/jlb.0107051. [DOI] [PubMed] [Google Scholar]
- 35.Buraei Z, Yang J. The beta subunit of voltage-gated Ca2+ channels. Physiol Rev. 2010;90:1461–1506. doi: 10.1152/physrev.00057.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Allen DG. Skeletal muscle function: role of ionic changes in fatigue, damage and disease. Clin Exp Pharmacol Physiol. 2004;31:485–493. doi: 10.1111/j.1440-1681.2004.04032.x. [DOI] [PubMed] [Google Scholar]
- 37.Allen DG, Gervasio OL, Yeung EW, Whitehead NP. Calcium and the damage pathways in muscular dystrophy. Can J Physiol Pharmacol. 2010;88:83–91. doi: 10.1139/Y09-058. [DOI] [PubMed] [Google Scholar]
- 38.Shini S, Kaiser P. Effects of stress, mimicked by administration of corticosterone in drinking water, on the expression of chicken cytokine and chemokine genes in lymphocytes. Stress. 2009;12:388–399. doi: 10.1080/10253890802526894. [DOI] [PubMed] [Google Scholar]
- 39.Shini S, Shini A, Huff GR. Effects of chronic and repeated corticosterone administration in rearing chickens on physiology, the onset of lay and egg production of hens. Physiol Behav. 2009;98:73–77. doi: 10.1016/j.physbeh.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 40.Kaiser P, Zhiguang W, Lisa R, Mark F, Gibson M, Poh T-Y, Agim S, Wayne B, Shaniko S. Prospects for understanding immune-endocrine interactions in the chicken. Gen Comp Endocrinol. 2009;163:83–91. doi: 10.1016/j.ygcen.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 41.Krupp M, Weinmann A, Galle PR, Teufel A. Actin binding LIM protein 3 (abLIM3) Int J Mol Med. 2006;17:129–133. [PubMed] [Google Scholar]
- 42.Maekawa M, Ishizaki T, Boku S, Watanabe N, Fujita A, Iwamatsu A, Obinata T, Ohashi K, Mizuno K, Narumiya S. Signaling from Rho to the actin cytoskeleton through protein kinases ROCK and LIM-kinase. Science. 1999;285:895–898. doi: 10.1126/science.285.5429.895. [DOI] [PubMed] [Google Scholar]
- 43.Mutryn MF, Brannick EM, Fu W, Lee WR, Abasht B. Characterization of a novel chicken muscle disorder through differential gene expression and pathway analysis using RNA-sequencing. BMC Genomics. 2015;16. [DOI] [PMC free article] [PubMed]
- 44.Shini S, Huff GR, Shini A, Kaiser P. Understanding stress-induced immunosuppression: exploration of cytokine and chemokine gene profiles in chicken peripheral leukocytes. Poult Sci. 2010;89:841–851. doi: 10.3382/ps.2009-00483. [DOI] [PubMed] [Google Scholar]
- 45.Shini S, Shini A, Kaiser P. Cytokine and chemokine gene expression profiles in heterophils from chickens treated with corticosterone. Stress. 2010;13:185–194. doi: 10.3109/10253890903144639. [DOI] [PubMed] [Google Scholar]
- 46.Mujahid A, Yoshiki Y, Akiba Y, Toyomizu M. Superoxide radical production in chicken skeletal muscle induced by acute heat stress. Poult Sci. 2005;84:307–314. doi: 10.1093/ps/84.2.307. [DOI] [PubMed] [Google Scholar]
- 47.Mujahid A, Sato K, Akiba Y, Toyomizu M. Acute heat stress stimulates mitochondrial superoxide production in broiler skeletal muscle, possibly via downregulation of uncoupling protein content. Poult Sci. 2006;85:1259–1265. doi: 10.1093/ps/85.7.1259. [DOI] [PubMed] [Google Scholar]
- 48.Mujahid A, Pumford NR, Bottje W, Nakagawa K, Miyazawa T, Akiba Y, Toyomizu M. Mitochondrial oxidative damage in chicken skeletal muscle induced by acute heat stress. Journal of Poultry Science. 2007;44:439–445. doi: 10.2141/jpsa.44.439. [DOI] [Google Scholar]
- 49.Alamdari N, Smith IJ, Aversa Z, Hasselgren PO. Sepsis and glucocorticoids upregulate p300 and downregulate HDAC6 expression and activity in skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2010;299:R509–R520. doi: 10.1152/ajpregu.00858.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Frost RA, Lang CH. Skeletal muscle cytokines: regulation by pathogen-associated molecules and catabolic hormones. Curr Opin Clin Nutr Metab Care. 2005;8:255–263. doi: 10.1097/01.mco.0000165003.16578.2d. [DOI] [PubMed] [Google Scholar]
- 51.Vaarmann A, Fortin D, Veksler V, Momken I, Ventura-Clapier R, Garnier A. Mitochondrial biogenesis in fast skeletal muscle of CK deficient mice. Biochim Biophys Acta-Bioenerg. 2008;1777:39–47. doi: 10.1016/j.bbabio.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 52.Sandercock DA, Nute GR, Hocking PM. Quantifying the effects of genetic selection and genetic variation for body size, carcass composition, and meat quality in the domestic fowl (Gallus domesticus) Poult Sci. 2009;88:923–931. doi: 10.3382/ps.2008-00376. [DOI] [PubMed] [Google Scholar]
- 53.Griffin HD, Goddard C. Rapidly growing broiler (meat-type) chickens: their origin and use for comparative studies of the regulation of growth. Int J Biochem. 1994;26:19–26. doi: 10.1016/0020-711X(94)90190-2. [DOI] [PubMed] [Google Scholar]
- 54.Khan RU, Naz S, Nikousefat Z, Tufarelli V, Javdani M, Rana N, Laudadio V. Effect of vitamin E in heat-stressed poultry. Worlds Poult Sci J. 2011;67:469–477. doi: 10.1017/S0043933911000511. [DOI] [Google Scholar]
- 55.Mujahid A, Akiba Y, Toyomizu M. Acute heat stress induces oxidative stress and decreases adaptation in young white leghorn cockerels by downregulation of avian uncoupling protein. Poult Sci. 2007;86:364–371. doi: 10.1093/ps/86.2.364. [DOI] [PubMed] [Google Scholar]
- 56.Cerretelli P, Gelfi C. Energy metabolism in hypoxia: reinterpreting some features of muscle physiology on molecular grounds. Eur J Appl Physiol. 2011;111:421–432. doi: 10.1007/s00421-010-1399-5. [DOI] [PubMed] [Google Scholar]
- 57.Toyomizu M, Kikusato M, Kawabata Y, Azad MAK, Inui E, Amo T. Meat-type chickens have a higher efficiency of mitochondrial oxidative phosphorylation than laying-type chickens. Comp Biochem Physiol A Mol Integr Physiol. 2011;159:75–81. doi: 10.1016/j.cbpa.2011.01.020. [DOI] [PubMed] [Google Scholar]
- 58.Flammer JR, Dobrovolna J, Kennedy MA, Chinenov Y, Glass CK, Ivashkiv LB, Rogatsky I. The type I interferon signaling pathway is a target for glucocorticoid inhibition. Mol Cell Biol. 2010;30:4564–4574. doi: 10.1128/MCB.00146-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dogra C, Changotra H, Mohan S, Kumar A. Tumor necrosis factor-like weak inducer of apoptosis inhibits skeletal myogenesis through sustained activation of nuclear factor-kappa B and degradation of MyoD protein. J Biol Chem. 2006;281:10327–10336. doi: 10.1074/jbc.M511131200. [DOI] [PubMed] [Google Scholar]
- 60.Caveggion E, Continolo S, Pixley FJ, Stanley ER, Bowtell DDL, Lowell CA, Berton G. Expression and tyrosine phosphorylation of Cbl regulates macrophage chemokinetic and chemotactic movement. J Cell Physiol. 2003;195:276–289. doi: 10.1002/jcp.10236. [DOI] [PubMed] [Google Scholar]
- 61.Kedzierska K, Ellery P, Mak J, Lewin SR, Crowe SM, Jaworowski A. HIV-1 down-modulates gamma signaling chain of Fc gamma R in human macrophages: a possible mechanism for inhibition of phagocytosis. J Immunol. 2002;168:2895–2903. doi: 10.4049/jimmunol.168.6.2895. [DOI] [PubMed] [Google Scholar]
- 62.Lee WL, Cosio G, Ireton K, Grinstein S. Role of CrkII in Fcgamma receptor-mediated phagocytosis. J Biol Chem. 2007;282:11135–11143. doi: 10.1074/jbc.M700823200. [DOI] [PubMed] [Google Scholar]
- 63.Lehmann DM, Seneviratne AM, Smrcka AV. Small molecule disruption of G protein beta gamma subunit signaling inhibits neutrophil chemotaxis and inflammation. Mol Pharmacol. 2008;73:410–418. doi: 10.1124/mol.107.041780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Omori K, Ohira T, Uchida Y, Ayilavarapu S, Batista EL, Yagi M, Iwata T, Liu H, Hasturk H, Kantarci A, Van Dyke TE. Priming of neutrophil oxidative burst in diabetes requires preassembly of the NADPH oxidase. J Leukoc Biol. 2008;84:292–301. doi: 10.1189/jlb.1207832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Appleman LJ, van Puijenbroek AA, Shu KM, Nadler LM, Boussiotis VA. CD28 costimulation mediates down-regulation of p27kip1 and cell cycle progression by activation of the PI3K/PKB signaling pathway in primary human T cells. J Immunol. 2002;168:2729–2736. doi: 10.4049/jimmunol.168.6.2729. [DOI] [PubMed] [Google Scholar]
- 66.Borchers MT, Ansay T, DeSalle R, Daugherty BL, Shen H, Metzger M, Lee NA, Lee JJ. In vitro assessment of chemokine receptor-ligand interactions mediating mouse eosinophil migration. J Leukoc Biol. 2002;71:1033–1041. [PubMed] [Google Scholar]
- 67.Pease JE. Asthma, allergy and chemokines. Curr Drug Targets. 2006;7:3–12. doi: 10.2174/138945006775270204. [DOI] [PubMed] [Google Scholar]
- 68.Stojkov NJ, Janjic MM, Bjelic MM, Mihajlovic AI, Kostic TS, Andric SA. Repeated immobilization stress disturbed steroidogenic machinery and stimulated the expression of cAMP signaling elements and adrenergic receptors in Leydig cells. Am J Physiol Endocrinol Metab. 2012;302:E1239–E1251. doi: 10.1152/ajpendo.00554.2011. [DOI] [PubMed] [Google Scholar]
- 69.Dhakshinamoorthy S, Jain AK, Bloom DA, Jaiswal AK. Bach1 competes with Nrf2 leading to negative regulation of the antioxidant response element (ARE)-mediated NAD(P)H: quinone oxidoreductase 1 gene expression and induction in response to antioxidants. J Biol Chem. 2005;280:16891–16900. doi: 10.1074/jbc.M500166200. [DOI] [PubMed] [Google Scholar]
- 70.Karkkainen MJ, Petrova TV. Vascular endothelial growth factor receptors in the regulation of angiogenesis and lymphangiogenesis. Oncogene. 2000;19:5598–5605. doi: 10.1038/sj.onc.1203855. [DOI] [PubMed] [Google Scholar]
- 71.Lu X, Masic A, Li Y, Shin Y, Liu Q, Zhou Y. The PI3K/Akt pathway inhibits influenza A virus-induced Bax-mediated apoptosis by negatively regulating the JNK pathway via ASK1. J Gen Virol. 2010. [DOI] [PubMed]
- 72.Lee C, Liu QH, Tomkowicz B, Yi Y, Freedman BD, Collman RG. Macrophage activation through CCR5- and CXCR4-mediated gp120-elicited signaling pathways. J Leukoc Biol. 2003;74:676–682. doi: 10.1189/jlb.0503206. [DOI] [PubMed] [Google Scholar]
- 73.Oh JE, So KS, Lim SJ, Kim MY. Induction of apoptotic cell death by a ceramide analog in PC-3 prostate cancer cells. Arch Pharm Res. 2006;29:1140–1146. doi: 10.1007/BF02969305. [DOI] [PubMed] [Google Scholar]
- 74.Woodcock J. Sphingosine and ceramide signalling in apoptosis. IUBMB Life. 2006;58:462–466. doi: 10.1080/15216540600871118. [DOI] [PubMed] [Google Scholar]
- 75.Cao Q, Kim JH, Richter JD. CDK1 and calcineurin regulate Maskin association with eIF4E and translational control of cell cycle progression. Nat Struct Mol Biol. 2006;13:1128–1134. doi: 10.1038/nsmb1169. [DOI] [PubMed] [Google Scholar]
- 76.Karlsson HK, Nilsson PA, Nilsson J, Chibalin AV, Zierath JR, Blomstrand E. Branched-chain amino acids increase p70S6k phosphorylation in human skeletal muscle after resistance exercise. Am J Physiol Endocrinol Metab. 2004;287:E1–E7. doi: 10.1152/ajpendo.00430.2003. [DOI] [PubMed] [Google Scholar]
- 77.Jivotovskaya AV, Valásek L, Hinnebusch AG, Nielsen KH. Eukaryotic translation initiation factor 3 (eIF3) and eIF2 can promote mRNA binding to 40S subunits independently of eIF4G in yeast. Mol Cell Biol. 2006;26:1355–1372. doi: 10.1128/MCB.26.4.1355-1372.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Park JS, Gamboni-Robertson F, He Q, Svetkauskaite D, Kim JY, Strassheim D, Sohn JW, Yamada S, Maruyama I, Banerjee A, Ishizaka A, Abraham E. High mobility group box 1 protein interacts with multiple Toll-like receptors. Am J Physiol Cell Physiol. 2006;290:C917–C924. doi: 10.1152/ajpcell.00401.2005. [DOI] [PubMed] [Google Scholar]
- 79.Holmlund U, Wähämaa H, Bachmayer N, Bremme K, Sverremark-Ekström E, Palmblad K. The novel inflammatory cytokine high mobility group box protein 1 (HMGB1) is expressed by human term placenta. Immunology. 2007;122:430–437. doi: 10.1111/j.1365-2567.2007.02662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yang H, Wang H, Czura CJ, Tracey KJ. The cytokine activity of HMGB1. J Leukoc Biol. 2005;78:1–8. doi: 10.1189/jlb.1104648. [DOI] [PubMed] [Google Scholar]
- 81.Chalfant CE, Spiegel S. Sphingosine 1-phosphate and ceramide 1-phosphate: expanding roles in cell signaling. J Cell Sci. 2005;118:4605–4612. doi: 10.1242/jcs.02637. [DOI] [PubMed] [Google Scholar]
- 82.Alemany R, van Koppen CJ, Danneberg K, Braak TM, Heringdorf MD. Regulation and functional roles of sphingosine kinases. Naunyn Schmiedebergs Arch Pharmacol. 2007;374:413–428. doi: 10.1007/s00210-007-0132-3. [DOI] [PubMed] [Google Scholar]
- 83.Kono M, Mi Y, Liu Y, Sasaki T, Allende ML, Wu YP, Yamashita T, Proia RL. The sphingosine-1-phosphate receptors S1P1, S1P2, and S1P3 function coordinately during embryonic angiogenesis. J Biol Chem. 2004;279:29367–29373. doi: 10.1074/jbc.M403937200. [DOI] [PubMed] [Google Scholar]
- 84.Liu X, Zhang Q-H, Yi G-H. Regulation of metabolism and transport of sphingosine-1-phosphate in mammalian cells. Mol Cell Biochem. 2012;363:21–33. doi: 10.1007/s11010-011-1154-1. [DOI] [PubMed] [Google Scholar]
- 85.Garcia-Echeverria C, Sellers WR. Drug discovery approaches targeting the PI3K/Akt pathway in cancer. Oncogene. 2008;27:5511–5526. doi: 10.1038/onc.2008.246. [DOI] [PubMed] [Google Scholar]
- 86.Hossain MA, Rosengren KJ, Haugaard-Jonsson LM, Zhang S, Layfield S, Ferraro T, Daly NL, Tregear GW, Wade JD, Bathgate RA. The A-chain of human relaxin family peptides has distinct roles in the binding and activation of the different relaxin family peptide receptors. J Biol Chem. 2008;283:17287–17297. doi: 10.1074/jbc.M801911200. [DOI] [PubMed] [Google Scholar]
- 87.Frost RA, Lang CH. Regulation of muscle growth by pathogen-associated molecules. J Anim Sci. 2008;86:E84–E93. doi: 10.2527/jas.2007-0483. [DOI] [PubMed] [Google Scholar]
- 88.Grant S. Cotargeting survival signaling pathways in cancer. J Clin Invest. 2008;118:3003–3006. doi: 10.1172/JCI36898E1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yokogami K, Wakisaka S, Avruch J, Reeves SA. Serine phosphorylation and maximal activation of STAT3 during CNTF signaling is mediated by the rapamycin target mTOR. Curr Biol. 2000;10:47–50. doi: 10.1016/S0960-9822(99)00268-7. [DOI] [PubMed] [Google Scholar]
- 90.Hamal KR, Wideman RF, Anthony NB, Erf GF. Differential gene expression of proinflammatory chemokines and cytokines in lungs of ascites-resistant and -susceptible broiler chickens following intravenous cellulose microparticle injection. Vet Immunol Immunopathol. 2010;133:250–255. doi: 10.1016/j.vetimm.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 91.Jaeschke H, Hasegawa T. Role of neutrophils in acute inflammatory liver injury. Liver Int. 2006;26:912–919. doi: 10.1111/j.1478-3231.2006.01327.x. [DOI] [PubMed] [Google Scholar]
- 92.Zhou HY, Shin EM, Guo LY, Youn UJ, Bae K, Kang SS, Zou LB, Kim YS. Anti-inflammatory activity of 4-methoxyhonokiol is a function of the inhibition of iNOS and COX-2 expression in RAW 264.7 macrophages via NF-kappaB, JNK and p38 MAPK inactivation. Eur J Pharmacol. 2008;586:340–349. doi: 10.1016/j.ejphar.2008.02.044. [DOI] [PubMed] [Google Scholar]
- 93.Vila-del Sol V, Díaz-Muñoz MD, Fresno M. Requirement of tumor necrosis factor alpha and nuclear factor-kappaB in the induction by IFN-gamma of inducible nitric oxide synthase in macrophages. J Leukoc Biol. 2007;81:272–283. doi: 10.1189/jlb.0905529. [DOI] [PubMed] [Google Scholar]
- 94.Willoughby DS, Wilborn CD. Estradiol in females may negate skeletal muscle myostatin mRNA expression and serum myostatin propeptide levels after eccentric muscle contractions. J Sports Sci Med. 2006;5:672–681. [PMC free article] [PubMed] [Google Scholar]
- 95.Enns DL, Tiidus PM. Estrogen influences satellite cell activation and proliferation following downhill running in rats. J Appl Physiol. 2008;104:347–353. doi: 10.1152/japplphysiol.00128.2007. [DOI] [PubMed] [Google Scholar]
- 96.Chen X-N, Zhu H, Meng Q-Y, Zhou J-N. Estrogen receptor-alpha and -beta regulate the human corticotropin-releasing hormone gene through similar pathways. Brain Res. 2008;1223:1–10. doi: 10.1016/j.brainres.2008.05.043. [DOI] [PubMed] [Google Scholar]
- 97.Szendroedi J, Phielix E, Roden M. The role of mitochondria in insulin resistance and type 2 diabetes mellitus. Nat Rev Endocrinol. 2012;8:92–103. doi: 10.1038/nrendo.2011.138. [DOI] [PubMed] [Google Scholar]
- 98.Masana MI, Dubocovich ML. Melatonin receptor signaling: finding the path through the dark. Sci STKE. 2001;2001: PE39. [DOI] [PubMed]
- 99.Ivanina T, Blumenstein Y, Shistik E, Barzilai R, Dascal N. Modulation of L-type Ca2+ channels by gbeta gamma and calmodulin via interactions with N and C termini of alpha 1C. J Biol Chem. 2000;275:39846–39854. doi: 10.1074/jbc.M005881200. [DOI] [PubMed] [Google Scholar]
- 100.Qin S, Stadtman ER, Chock PB. Regulation of oxidative stress-induced calcium release by phosphatidylinositol 3-kinase and Bruton’s tyrosine kinase in B cells. Proc Natl Acad Sci USA. 2000;97:7118–7123. doi: 10.1073/pnas.130198197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mujahid A, Furuse M. Central administration of corticotropin-releasing factor induces tissue specific oxidative damage in chicks. Comp Biochem Physiol A Mol Integr Physiol. 2008;151:664–669. doi: 10.1016/j.cbpa.2008.08.013. [DOI] [PubMed] [Google Scholar]
- 102.Thaxton JP, Stayer P, Ewing M, Rice J. Corticosterone in commercial broilers. J Appl Poul Res. 2005;14:745–749. doi: 10.1093/japr/14.4.745. [DOI] [Google Scholar]
- 103.Post J, Rebel JMJ, ter Huurne A. Physiological effects of elevated plasma corticosterone concentrations in broiler chickens. An alternative means by which to assess the physiological effects of stress. Poul Sci. 2003;82:1313–1318. doi: 10.1093/ps/82.8.1313. [DOI] [PubMed] [Google Scholar]
- 104.Sak K, Boeynaems JM, Everaus H. Involvement of P2Y receptors in the differentiation of haematopoietic cells. J Leukoc Biol. 2003;73:442–447. doi: 10.1189/jlb.1102561. [DOI] [PubMed] [Google Scholar]
- 105.Burnstock G. Purinergic signaling and vascular cell proliferation and death. Arterioscler Thromb Vasc Biol. 2002;22:364–373. doi: 10.1161/hq0302.105360. [DOI] [PubMed] [Google Scholar]
- 106.Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three Akts. Genes Dev. 1999;13:2905–2927. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- 107.Seino S, Shibasaki T. PKA-dependent and PKA-independent pathways for cAMP-regulated exocytosis. Physiol Rev. 2005;85:1303–1342. doi: 10.1152/physrev.00001.2005. [DOI] [PubMed] [Google Scholar]
- 108.Wehrens XH, Lehnart SE, Reiken S, Vest JA, Wronska A, Marks AR. Inaugural article: ryanodine receptor/calcium release channel PKA phosphorylation: a critical mediator of heart failure progression. Proc Natl Acad Sci USA. 2006;103:511–518. doi: 10.1073/pnas.0510113103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gardai S, Whitlock BB, Helgason C, Ambruso D, Fadok V, Bratton D, Henson PM. Activation of SHIP by NADPH oxidase-stimulated Lyn leads to enhanced apoptosis in neutrophils. J Biol Chem. 2002;277:5236–5246. doi: 10.1074/jbc.M110005200. [DOI] [PubMed] [Google Scholar]
- 110.Yamasaki S, Saito T. Progress in allergy signal research on mast cells: signal regulation of multiple mast cell responses through FcepsilonRI. J Pharmacol Sci. 2008;106:336–340. doi: 10.1254/jphs.FM0070251. [DOI] [PubMed] [Google Scholar]
- 111.Balistreri CR, Caruso C, Grimaldi MP, Listi F, Vasto S, Orlando V, Campagna AM, Lio D, Candore G. CCR5 receptor—biologic and genetic implications in age-related diseases. In: Rattan SISAS (ed) Biogerontology: Mechanisms and Interventions, 2007; 162–72. [DOI] [PubMed]
- 112.Viejo-Borbolla A, Martinez-Martin N, Nel HJ, Rueda P, Martin R, Blanco S, Arenzana-Seisdedos F, Thelen M, Fallon PG, Alcami A. Enhancement of chemokine function as an immunomodulatory strategy employed by human herpesviruses. Plos Pathogens. 2012;8. [DOI] [PMC free article] [PubMed]
- 113.Sun L, Ye RD. Role of G protein-coupled receptors in inflammation. Acta Pharmacol Sin. 2012;33:342–350. doi: 10.1038/aps.2011.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Schwartz EA, Reaven PD. Lipolysis of triglyceride-rich lipoproteins, vascular inflammation, and atherosclerosis. Biochim Biophys Acta Mol Cell Biol Lipids. 1821;2012:858–866. doi: 10.1016/j.bbalip.2011.09.021. [DOI] [PubMed] [Google Scholar]
- 115.Ren GH, Takano T, Papillon J, Cybulsky AV. Cytosolic phospholipase A(2)-alpha enhances induction of endoplasmic reticulum stress. Biochim Biophys Acta Mol Cell Res. 1803;2010:468–481. doi: 10.1016/j.bbamcr.2010.01.020. [DOI] [PubMed] [Google Scholar]
- 116.Calandra T, Roger T. Macrophage migration inhibitory factor: a regulator of innate immunity. Nat Rev Immunol. 2003;3:791–800. doi: 10.1038/nri1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Roger T, David J, Glauser MP, Calandra T. MIF regulates innate immune responses through modulation of Toll-like receptor 4. Nature. 2001;414:920–924. doi: 10.1038/414920a. [DOI] [PubMed] [Google Scholar]
- 118.Daun JM, Cannon JG. Macrophage migration inhibitory factor antagonizes hydrocortisone-induced increases in cytosolic IkappaBalpha. Am J Physiol Regul Integr Comp Physiol. 2000;279:R1043–R1049. doi: 10.1152/ajpregu.2000.279.3.R1043. [DOI] [PubMed] [Google Scholar]
- 119.Santos LL, Dacumos A, Yamana J, Sharma L, Morand EF. Reduced arthritis in MIF deficient mice is associated with reduced T cell activation: down-regulation of ERK MAP kinase phosphorylation. Clin Exp Immunol. 2008;152:372–380. doi: 10.1111/j.1365-2249.2008.03639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ohkawara T, Takeda H, Miyashita K, Nishiwaki M, Nakayama T, Taniguchi M, Yoshiki T, Tanaka J, Takana J, Imamura M, Sugiyama T, Asaka M, Nishihira J. Regulation of Toll-like receptor 4 expression in mouse colon by macrophage migration inhibitory factor. Histochem Cell Biol. 2006;125:575–582. doi: 10.1007/s00418-005-0092-y. [DOI] [PubMed] [Google Scholar]
- 121.Spik I, Brénuchon C, Angéli V, Staumont D, Fleury S, Capron M, Trottein F, Dombrowicz D. Activation of the prostaglandin D2 receptor DP2/CRTH2 increases allergic inflammation in mouse. J Immunol. 2005;174:3703–3708. doi: 10.4049/jimmunol.174.6.3703. [DOI] [PubMed] [Google Scholar]
- 122.Nagata M, Saito K, Tsuchiya K, Sakamoto Y. Leukotriene D4 upregulates eosinophil adhesion via the cysteinyl leukotriene 1 receptor. J Allergy Clin Immunol. 2002;109:676–680. doi: 10.1067/mai.2002.122841. [DOI] [PubMed] [Google Scholar]
- 123.Yang CH, Murti A, Valentine WJ, Du Z, Pfeffer LM. Interferon{alpha} Activates NF-{kappa}B in JAK1-deficient Cells through a TYK2-dependent Pathway. J Biol Chem. 2005;280:25849–25853. doi: 10.1074/jbc.M413721200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Simoncic PD, Lee-Loy A, Barber DL, Tremblay ML, McGlade CJ. The T cell protein tyrosine phosphatase is a negative regulator of janus family kinases 1 and 3. Curr Biol. 2002;12:446–453. doi: 10.1016/S0960-9822(02)00697-8. [DOI] [PubMed] [Google Scholar]
- 125.Ning Y, Riggins RB, Mulla JE, Chung H, Zwart A, Clarke R. IFNgamma restores breast cancer sensitivity to fulvestrant by regulating STAT1, IFN regulatory factor 1, NF-kappaB, BCL2 family members, and signaling to caspase-dependent apoptosis. Mol Cancer Ther. 2010;9:1274–1285. doi: 10.1158/1535-7163.MCT-09-1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Varfolomeev EE, Ashkenazi A. Tumor necrosis factor: an apoptosis JuNKie? Cell. 2004;116:491–497. doi: 10.1016/S0092-8674(04)00166-7. [DOI] [PubMed] [Google Scholar]
- 127.Jiang S, Zu Y, Fu Y, Zhang Y, Efferth T. Activation of the mitochondria-driven pathway of apoptosis in human PC-3 prostate cancer cells by a novel hydrophilic paclitaxel derivative, 7-xylosyl-10-deacetylpaclitaxel. Int J Oncol. 2008;33:103–111. [PubMed] [Google Scholar]
- 128.Bots M, Medema JP. Granzymes at a glance. J Cell Sci. 2006;119:5011–5014. doi: 10.1242/jcs.03239. [DOI] [PubMed] [Google Scholar]
- 129.Ding S-Q, Li Y, Zhou Z-G, Wang C, Zhan L, Zhou B. Toll-like receptor 4-mediated apoptosis of pancreatic cells in cerulein-induced acute pancreatitis in mice. Hepatobiliary Pancreat Dis Int. 2010;9:645–650. [PubMed] [Google Scholar]
- 130.Barton GM, Medzhitov R. Toll-like receptor signaling pathways. Science. 2003;300:1524–1525. doi: 10.1126/science.1085536. [DOI] [PubMed] [Google Scholar]
- 131.Rolo AP, Palmeira CM. Diabetes and mitochondrial function: Role of hyperglycemia and oxidative stress. Toxicol Appl Pharmacol. 2006;212:167–178. doi: 10.1016/j.taap.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 132.Pinkoski MJ, Waterhouse NJ, Heibein JA, Wolf BB, Kuwana T, Goldstein JC, Newmeyer DD, Bleackley RC, Green DR. Granzyme B-mediated apoptosis proceeds predominantly through a Bcl-2-inhibitable mitochondrial pathway. J Biol Chem. 2001;276:12060–12067. doi: 10.1074/jbc.M009038200. [DOI] [PubMed] [Google Scholar]
- 133.Kim R, Emi M, Tanabe K. Caspase-dependent and -independent cell death pathways after DNA damage (Review) Oncol Rep. 2005;14:595–599. [PubMed] [Google Scholar]
- 134.Kuwano K, Yoshimi M, Maeyama T, Hamada N, Yamada M, Nakanishi Y. Apoptosis signaling pathways in lung diseases. Med Chem. 2005;1:49–56. doi: 10.2174/1573406053402497. [DOI] [PubMed] [Google Scholar]
- 135.Kuai J, Nickbarg E, Wooters J, Qiu Y, Wang J, Lin LL. Endogenous association of TRAF2, TRAF3, cIAP1, and Smac with lymphotoxin beta receptor reveals a novel mechanism of apoptosis. J Biol Chem. 2003;278:14363–14369. doi: 10.1074/jbc.M208672200. [DOI] [PubMed] [Google Scholar]
- 136.You RI, Chen MC, Wang HW, Chou YC, Lin CH, Hsieh SL. Inhibition of lymphotoxin-beta receptor-mediated cell death by survivin-DeltaEx3. Cancer Res. 2006;66:3051–3061. doi: 10.1158/0008-5472.CAN-05-2479. [DOI] [PubMed] [Google Scholar]
- 137.Shi C-S, Shenderov K, Huang N-N, Kabat J, Abu-Asab M, Fitzgerald KA, Sher A, Kehrl JH. Activation of autophagy by inflammatory signals limits IL-1 beta production by targeting ubiquitinated inflammasomes for destruction. Nat Immunol. 2012;13:U255–U274. doi: 10.1038/ni.2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sigala I, Zacharatos P, Toumpanakis D, Michailidou T, Noussia O, Theocharis S, Roussos C, Papapetropoulos A, Vassilakopoulos T. MAPKs and NF-kappa B differentially regulate cytokine expression in the diaphragm in response to resistive breathing: the role of oxidative stress. Am J Physiol Regul Integr Comp Physiol. 2011;300:R1152–R1162. doi: 10.1152/ajpregu.00376.2010. [DOI] [PubMed] [Google Scholar]
- 139.Yang CM, Luo SF, Hsieh HL, Chi PL, Lin CC, Wu CC, Hsiao LD. Interleukin-1 beta induces ICAM-1 expression enhancing leukocyte adhesion in human rheumatoid arthritis synovial fibroblasts: involvement of ERK, JNK, AP-1, and NF-kappa B. J Cell Physiol. 2010;224:516–526. doi: 10.1002/jcp.22153. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The microarray data supporting the results of this article are available in the ArrayExpress database (www.ebi.ac.uk/arrayexpress) under accession number E-MTAB-5011 (http://www.ebi.ac.uk/arrayexpress/experiments/E-MTAB-5011).


