Abstract
Background
The increase in serum estradiol (E2) concentrations during the follicular phase becomes the index of oocyte maturation in vivo. When ovarian stimulation is performed to hypogonadotropic hypogonadism (HH) patients with only follicle stimulating hormone (FSH), proper increase in serum E2 concentrations is not observed. Even if oocytes are obtained, which usually have low fertilization rate. In this report, we would like to present an unique case, in which under low E2 concentrations and without luteinizing hormone (LH) administration, numerous mature oocytes could be obtained and a healthy baby delivered.
Case presentation
During controlled ovarian stimulation (COS) with only recombinant follicular stimulating hormone (rFSH) administrations, a 26-year-old Japanese woman with hypothalamic amenorrhea (i.e., hypogonadotropic hypogonadism) developed numerous follicles despite low serum E2, 701 pg/ml, and high progesterone (P4) concentrations, 2.11 ng/ml, on the day of induced ovulation. However, 33 cumulus-oocyte complexes (COCs) were successfully obtained; following the embryo culture, four early embryos and six blastocysts were cryopreserved. This patient received hormone replacement therapy (HRT), during which one of six cryopreserved blastocysts was thawed and transferred into the uterine lumen. The patient became pregnant from the first transfer, went through her pregnancy without any complications, and delivered a healthy male baby in the 39th week. Low E2 concentrations in follicular fluids (FFs) are suggestive that aromatase and/or 17β-hydroxysteroid dehydrogenase (17β-HSD) could be low.
Conclusions
Serum E2 concentrations may not be the most important index for oocyte maturation during COS, and suggested that oocyte maturation was in progress even under low serum E2 and high P4 conditions. Even if serum E2 concentrations did not properly increase, numerous mature oocytes could be obtained, resulting in the birth of a healthy baby.
Electronic supplementary material
The online version of this article (10.1186/s12884-017-1510-6) contains supplementary material, which is available to authorized users.
Keywords: Low estradiol, High progesterone, Luteinizing hormone, Hypogonadotropic hypogonadism, Hypothalamic amenorrhea, Follicular fluids, Controlled ovarian stimulation, Assisted reproductive technology
Background
20–50 pg/ml serum estradiol (E2) concentrations are detected in the early follicular phase and menstruation, while concentrations over 200 pg/ml are detected in the late follicular phase and pre-ovulation [1]. The increase in serum E2 concentrations is indicative of follicular development and the important index in oocyte maturation. Hypogonadotropic hypogonadism (HH) patients exhibit low serum follicle stimulating hormone (FSH) and luteinizing hormone (LH) concentrations, resulting in negligible estrogen activity [2–4]. When a HH patient is treated with FSH alone during the controlled ovarian stimulation (COS), low serum E2 concentrations and the low fertilization rate are observed. However, human menopausal gonadotropin (HMG) containing FSH and LH activity is administered, serum E2 concentrations and fertilization rate are increased [2]. Administration of exogenous LH to HH patients is required to increase in serum E2 concentrations properly and to obtain adequate oocytes quality, resulting in delivery of healthy babies [2, 3].
Here, we report a case of a HH Japanese woman with daily administration of recombinant FSH (rFSH) alone, who had low serum E2 and high progesterone (P4) concentrations on the day of induced ovulation, but 33 cumulus-oocyte complexes (COCs) were collected. From these oocytes, good quality embryos were obtained and cryopreserved after in vitro fertilization (IVF) or intra cytoplasmic sperm injection (ICSI). A healthy male baby from one of the cryopreserved blastocysts was delivered.
Case presentation
Patient’s medical history
The patient, a 26-year-old Japanese woman, had been nulligravid. The first menstruation occurred at age 13 and had menstrual cycles regularly thereafter. At 21 years old, the patient experienced amenorrhea, resulting from 12 kg weight loss in two months. At another hospital, beginning at 23 years old, this patient had received oral E2 and P4 replacement therapy for two years. The patient was married at 26 years old and decided to undergo fertility treatment at our hospital. The patient’s body mass index (BMI) was 19.2 kg/m2 and her feminine characteristics appeared normal. The diameter of right ovary was 26.0 mm and left ovary was 29.1 mm. Serum FSH and LH concentrations were 3.2 mIU/ml and 0.5 mIU/ml, respectively. Her endocrine test results were within reference ranges of our hospital except for triiodothyronine (T3) and prolactin (PRL) (Additional file 1).The patient was diagnosed with hypothalamic amenorrhea following luteinizing hormone-releasing hormone (LH-RH) loading test.
Both oviducts and the uterine lumen in this patient were morphologically normal when examined with hysterosalpingography (HSG). The husband’s semen quality appeared normal and fell within the World Health Organization criteria 2010 [5]. Through the examination by transvaginal ultrasonography, over five antral follicles (i.e., 5–10 mm) each were observed in both ovaries. Serum Anti-Müllerian Hormone (AMH) concentrations were 10.8 ng/ml, from which the ovarian reserve was judged to be functioning [6–9]. To undergo planned infertility treatment for this couple, who lived in separately, the husband’s sperm were cryopreserved.
In our country, however, recombinant LH (rLH) is not yet available, and HMG and human chorionic gonadotropin (hCG) must be injected intramuscularly only by a medical doctor or nurse. Due to her work assignment and work loads, she was not able to receive daily administrations of HMG or hCG, therefore, self-administration of rFSH was only what we could offer for this patient during the COS period. Antral follicle counts (AFCs) and AMH concentrations of the patient were at high risk of ovarian hyperstimulation syndrome (OHSS) [10, 11]. Therefore, gonadotropin releasing hormone (GnRH) antagonist regimen was planned to avoid rapidly rising serum E2 concentrations [12, 13], only if proper increase in serum E2 concentrations were observed and dominant follicles reached over 12–14 mm in diameter.
First ovarian stimulation
When this patient experienced withdrawal bleeding following daily administration of oral contraceptive (OC) for 14 days, measurable follicles were not observed in either ovary on day 3 (Fig. 1). On the same day, COS was initiated with daily administration of 225 IU rFSH. Follicular developments and increased P4 concentrations were observed, but E2 concentrations were low on day 11 (Table 1). GnRH antagonist regimen was not performed because proper increase in serum E2 concentrations was not observed. Although both ovaries had over 30 follicles of 15–19 mm on day 13, serum E2 concentrations were low at 484 pg/ml and P4 concentrations were high at 2.09 ng/ml. COS was canceled due to the hormone concentration which did not reflect those of numerous follicular developments.
Fig. 1.
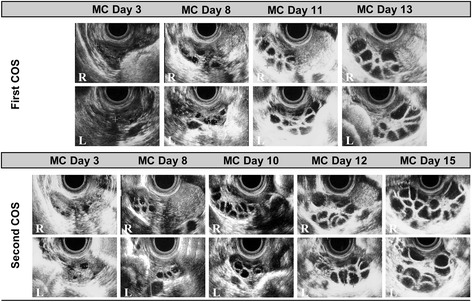
Follicular development following controlled ovarian stimulation (COS). Ovarian response was monitored through the use of transvaginal ultrasonography during COS. During the first COS, measurable follicles were not observed in either ovary on day 3 of the menstrual cycle (MC). On the same day, COS was initiated with daily administration of 225 international unit (IU) recombinant follicle stimulating hormone (rFSH). Follicular development and increased progesterone (P4) concentrations were observed, but estradiol (E2) concentrations were low on day 11. Although the right (R) and left (L) ovaries had over 30 follicles of 15–19 mm on day 13, serum E2 concentrations were low at 484 pg/ml and P4 concentrations were high at 2.09 ng/ml. COS was canceled due to the hormone concentration which did not reflect those of numerous follicular developments. From the day when the first COS was canceled, the patient took 10 mg synthetic progesterone (SP) orally for the next 14 days. The patient experienced withdrawal bleeding, and several small follicles were observed in both ovaries on day 3. From the same day, the daily dosage of 175 IU rFSH was administered for the first 5 days, followed by 200 IU for the remaining treatment period. Similar to the response seen at the first COS, high serum P4 concentrations, 2.11 ng/ml, were observed; however, serum E2 concentrations, 701 pg/ml, did not reflect those of numerous follicular developments
Table 1.
Transition in serum steroid hormone concentrations and follicular development during controlled ovarian stimulation
| First COS | |||||
| MC day | MC Day 3 | MC Day 8 | MC Day 11 | MC Day 13 | |
| (rFSH administration) | (0 IU) | (1125 IU) | (1800 IU) | (2250 IU) | |
| E2 | 10a | – | 219 | 484 | |
| (pg/ml) | |||||
| LH | 0.1a | – | 0.1a | 0.1a | |
| (mIU/ml) | |||||
| FSH | 0.1 | – | – | – | |
| (mIU/ml) | |||||
| P4 | – | – | 1.05 | 2.09 | |
| (ng/ml) | |||||
| Follicular diameter (mm) | NM | 6–8 | 9–12 | 15–19 | |
| Second COS | |||||
| MC day | MC Day 3 | MC Day 8 | MC Day 10 | MC Day 12 | MC Day 15 |
| (rFSH administration) | (0 IU) | (875 IU) | (1225 IU) | (1625 IU) | (2225 IU) |
| E2 | 10a | – | 60 | 201 | 701 |
| (pg/ml) | |||||
| LH | 0.1a | – | 0.1a | 0.1a | 0.1a |
| (mIU/ml) | |||||
| FSH | 0.2 | – | – | – | – |
| (mIU/ml) | |||||
| P4 | – | – | 0.40 | 0.53 | 2.11 |
| (ng/ml) | |||||
| Follicular diameter (mm) | 6–8 | 6–8 | 10 | 13–15 | 14–22 |
Note: COS controlled ovarian stimulation, MC menstrual cycle, IU international units, NM not measurable, bar no data, E 2 estradiol, LH luteinizing hormone, FSH follicle stimulating hormone, P 4 progesterone
aunder detection limit
Second controlled ovarian stimulation
From the day when the first COS was canceled, the patient took 10 mg synthetic progesterone (SP) orally for the next 14 days. The patient experienced withdrawal bleeding, and several small follicles were observed in both ovaries on day 3 (Fig. 1). From the same day, the daily dosage of 175 IU rFSH was administered for the first 5 days, followed by 200 IU for the remaining treatment period. Similar to the response seen at the first COS, high serum P4 concentrations were observed; however, serum E2 concentrations were not increased on day 15 (Table 1).
During COS, therefore, we advised this patient that assisted reproductive technology (ART) might fail due to her unusual serum steroid hormone concentrations, low E2 and high P4. At the same time, we also informed the patient that ART might succeed because serum AMH concentrations were adequate and numerous follicles were developed in response to rFSH. The patient and husband voluntarily agreed to go through ART in understanding the procedure with informed consent. On the same day, the ovulation was induced with 5000 IU hCG.
IVF/ICSI, embryo culture and cryopreservation
35 h after the hCG administration, follicles were pricked with an oocyte pick-up (OPU) needle transvaginally. Follicular Fluids (FFs) from the largest follicle were aspirated, which were frozen for the subsequent hormone analysis. Sperm that had been cryopreserved were thawed, which had characteristics of 44.0 × 106/ml and 9.5% motility. It was previously reported that oocytes from low serum E2 concentrations of a HH patient have decreased fertilization rate with IVF [2]. To obtain as many fertilized oocytes with sufficient quality frozen-thawed sperm, in addition to IVF, ICSI was also performed. 18 and 15 COCs were subjected to IVF and ICSI, respectively. IVF-COCs were inseminated with 5.0 × 104 motile sperm/COC. After 16–18 h after IVF and ICSI, fertilization was determined by the presence of two pronuclei (PN). Fertilized oocytes were cultured in the cleavage medium for the initial 3 days, and embryos were then cultured in the blastocyst medium until blastocyst formation. Embryo cryopreservation was performed with an ultra-rapid vitrification kit.
Hormone treatment, frozen-thawed blastocyst transfer and clinical pregnancy
Frozen-thawed blastocyst transfer (FBT) was performed following hormone replacement treatment (HRT) due to hypothalamic amenorrhea [14, 15]. After the patient experienced withdrawal bleeding by daily administration of OC for 14 days, daily administration of oral 2.0 mg E2 was initiated on day 3. Dosage of E2 was increased to 3.0 mg/day on day 6 and 4.0 mg/day on day 8. On day 11, serum E2 concentrations reached to 215 pg/ml and endometrial thickness was 12.0 mm, both of which exceeded our hospital standards of over 200 pg/ml serum E2 concentrations and endometrial thickness over 8.0 mm. The ovulation day (Ovd) was estimated to be on day 12 of the menstrual cycle. From the next day, luteal function was supported by daily administration of 30 mg oral SP, followed by 125 mg i.m. SP in every 5 days, which was continued until eight weeks of pregnancy.
A cryopreserved blastocyst was thawed with a thawing kit. FBT was performed transvaginally using an embryo transfer (ET) catheter under transabdominal ultrasound guidance. Serum hCG concentrations were determined 7 days after FBT and clinical pregnancy was confirmed with a transvaginal ultrasonography.
Results of ART and Pregnancy
IVF/ICSI and frozen-thawed blastocyst transfer
Among 33 COCs collected, 2PN rates were 77.8% (14/18) from IVF and 73.3% (11/15) from ICSI. Following the embryo culture, two early embryos (i.e., 7–10 blastomeres, culture on day 3) and five blastocysts were found from the IVF, and two early embryos and one blastocyst from the ICSI, all of which were cryopreserved. At day 5 from Ovd at the HRT cycle, one frozen-thawed blastocyst from the IVF, classified 5AA according to Gardner’s method [16], was transvaginally transferred into the uterine lumen.
Pregnancy outcome and hormone concentrations throughout pregnancy period
Serum hCG concentrations, 87.4 mIU/ml, were detected on day 7 following the FBT procedure. The presence of a germinal sac was confirmed on the third day of the fifth week (5w3d) and fetal heartbeats were detected on the first day of the seventh week (7w1d) during her pregnancy period. In this patient, serum steroid hormone concentrations transitioned as expected (Table 2), and went through her pregnancy without any complications. The patient gave birth to a baby boy via vaginal delivery at the beginning of the 39th week (39w0d). The male baby without anomalies was weighed at 3288 g and scaled 9 points on Apgar Score.
Table 2.
Hormone concentrations throughout her pregnancy period
| estradiol (pg/ml) | androstenedione (ng/ml) | testosterone (ng/ml) | DHEA-S (μg/dl) | |
|---|---|---|---|---|
| 15w2d | 8,410 | 7.2 | 0.95 | 111 |
| 19w6d | 14,500 | 8.5 | 1.09 | 114 |
| 30w2d | 37,300 | 12.0 | 1.26 | 105 |
Note: w week, d day, DHEA-S dehydroepiandrosterone sulfate
Steroid hormone concentrations and its hormonal precursor/hormone ratios in follicular fluids
Steroid hormone concentrations in this patient’s FFs were compared to those of infertile patients (Inf-) with similar serum E2 concentrations. These women, Inf-A and Inf-B, whose informed consents had been obtained and OPU performed in our hospital were both 700 ± 50 pg/ml serum E2 concentrations on the day of induced ovulation.
On day 4 of the menstrual cycle, these infertile women received daily administration of 100 mg clomifene citrate (CC) for 5 days. Follicular diameters were 25.2 mm and 18.1 mm for Inf-A and 22.8 mm for Inf-B on the day of induced ovulation. From the same day, serum hormone concentrations for Inf-A and Inf-B were 692 pg/ml and 745 pg/ml E2, 10.5 mIU/ml and 8.0 mIU/ml LH, and 0.40 ng/ml and 0.29 ng/ml P4, respectively. Ovulation was induced with the administration of 600 μg GnRH agonist, followed by the same procedures of OPU, IVF and FFs cryopreservation. However, no oocyte was collected from Inf-A, while one oocyte was collected from Inf-B, but its development ceased after fertilization.
In this patient, P4, dehydroepiandrosterone sulfate (DHEA-S) and androstenedione (A2) concentrations in FFs did not differ from those of Inf-A and Inf-B, but estrone (E1), testosterone (T) and E2 concentrations were lower than those of Inf-A and Inf-B (Fig. 2). Although the T/E2 ratio did not differ, the A2/E1, A2/T and E1/E2 ratios of the patient were lower than those of the infertile women (Table 3).
Fig. 2.
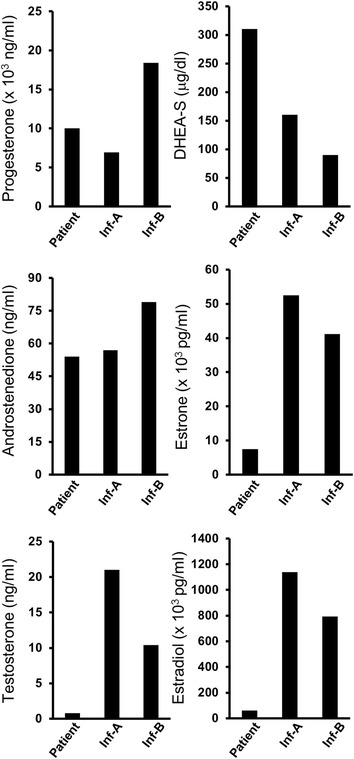
Comparison of steroid hormone concentrations of this patient to those of infertile patients (Inf-), Inf-A and Inf-B. On day 4 of the menstrual cycle, these infertile women received daily administration of 100 mg clomifene citrate (CC) for 5 days. Follicular diameters were 25.2 mm and 18.1 mm for Inf-A and 22.8 mm for Inf-B on the day of induced ovulation. From the same day, serum hormone concentrations for Inf-A and Inf-B were 692 pg/ml and 745 pg/ml E2, 10.5 mIU/ml and 8.0 mIU/ml LH, and 0.40 ng/ml and 0.29 ng/ml P4, respectively. Ovulation was induced with 600 μg gonadotropin releasing hormone (GnRH) agonist, followed by oocyte pick-up (OPU), in vitro fertilization (IVF) and follicular fluids (FFs) cryopreservation in the same manner. However, no oocyte was collected from Inf-A, while one oocyte was collected from Inf-B, but its development ceased after fertilization. Although progesterone (P4), dehydroepiandrosterone sulfate (DHEA-S) and androstenedione (A2) concentrations in FFs of this patient did not differ from those of Inf-A and Inf-B, estrone (E1), testosterone (T) and estradiol (E2) concentrations were lower than those of Inf-A and Inf-B
Table 3.
Hormonal Precursor/Hormone Ratios
| A2/E1 | T/E2 | A2/T | E1/E2 | |
|---|---|---|---|---|
| Enzyme involved | Aromatase | Aromatase | 17β-HSD | 17β-HSD |
| Patient | 138.6 | 76,875.0 | 0.015 | 8.2 |
| Inf-A | 920.7 | 54,285.7 | 0.368 | 21.7 |
| Inf-B | 521.1 | 76,346.2 | 0.132 | 19.3 |
Note: Under bar denotes significantly lower in ratio than that of Inf-A and Inf-B
E 1 estrone, E 2 estradiol, A 2 androstenedione, T testosterone, 17β-HSD 17β-hydroxysteroid dehydrogenase
Discussion
Here we report that a woman with hypothalamic amenorrhea became pregnant and delivered a healthy baby boy. During the follicular phase, the patient had low serum E2 and high P4 concentrations, followed by 33 COCs collection, to which IVF and ICSI were performed, and good early embryos and blastocysts were obtained. Although we carefully monitored this patient, her serum E2 concentrations quickly decreased and the ovaries were returned to the normal size 17 days after the first COS cancellation. She had similar condition at the time of the second COS cancellation, but symptoms of OHSS such abdominal pain and bloating, nausea, diarrhea and sudden weight increase were not observed. Observations in which concentrations of E1, T and E2 in FFs of this patient were found to be lower than those of the infertile women. When the ratios of steroid hormone concentrations of the patient were compared with those of the infertile women, the A2/E1, A2/T and E1/E2 ratios of the patient were lower than those of the infertile women. It appears that E2 was not sufficiently synthesized from A2. Low E2 concentrations in FFs suggest that aromatase and/or 17β-hydroxysteroid dehydrogenase (17β-HSD) levels and/or activities were low. It was possible that her hormone profile during COS could have been due to hereditary, her hormone profile was monitored throughout to the course of pregnancy, and that her hormone concentrations proceeded as healthy pregnancy. Both the patient and the child are healthy, and she delivered a female baby as second child through the use of a cryopreserved blastocyst obtained from the ART previously performed.
The increase in serum E2 concentrations during the follicular phase becomes the index of oocyte maturation in vivo; however, oocyte development in ovary undergoes even under E2 synthesis inhibition. It was reported that 7–16 mature oocytes were obtained under 500 pg/ml serum E2 concentrations on the day of induced ovulation, when COS with aromatase inhibitor was performed to women with breast cancer [17–19], and that number of mature oocytes and fertilization rates were not inferior to spontaneous COS [17, 19]. It has been noted that addition of E2 to the in vitro maturation (IVM) medium is not required [20, 21], suggesting that molecules other than E2 could be more important than steroid conditions [21–23].
Because serum P4 concentrations of the patient were higher than those in the infertile women, accumulation of P4 during COS was suspected. However, P4 concentrations in FFs did not differ between these patients, suggesting that P4 was not accumulated. In COS, infertile patients with numerous developing follicles tend to have increased serum P4 concentrations over 1.0 ng/ml. In this patient, increase in serum E2 concentrations did not reflect those of numerous follicular developments, possibly due to low aromatase and/or 17β-HSD, while serum P4 concentrations were sufficiently increased.
This case revealed that even if serum E2 concentrations were lower than those in the common practice, mature oocytes could be obtained and a healthy baby delivered. In the recent report, it was shown that mature oocytes are obtained even if COS was initiated in various phase of women’s menstrual cycle [24]. Together, these results suggest that E2 synthesis during COS and oocyte maturation could be more flexible than is commonly accepted.
Conclusions
Serum E2 concentrations may not be the most important index for oocyte maturation during COS, and suggested that oocyte maturation was in progress even under low serum E2 and high P4 conditions. Even if serum E2 concentrations did not properly increase, numerous mature oocytes could be obtained, resulting in the birth of a healthy baby.
Acknowledgements
We thank Makio Shozu, M.D., Ph.D. (Department of Reproductive Medicine, Graduate School of Medicine, Chiba University), Naomi Kashiwazaki, Ph.D. (Laboratory of Animal Reproduction, Department of Veterinary Medicine, Azabu University), Katsuhiko Hayashi, Ph.D. (Department of Developmental Stem Cell Biology, Faculty of Medical Sciences, Kyushu University), Mariko Shirota, Ph.D. (Laboratory of Comparative Toxicology, Department of Veterinary Medicine, Azabu University), Kazuya Kusama, Ph.D. (Animal Resource Science Center, Graduate School of Agricultural and Life Sciences, The University of Tokyo), Naoki Maedomari, Ph.D. (Sora-no mori Clinic), Katsuyoshi Fujiwara, Ph.D. (St. Marianna University School of Medicine Hospital), Takayuki Ishikawa, Ph.D. and Robert Moriarty for their valuable comments and support. We would like to thank this patient for her understanding and cooperation during her COS and pregnancy.
Funding
None
Availability of data and materials
Not applicable.
Abbreviations
- 17β-HSD
17β-hydroxysteroid dehydrogenase
- A2
Androstenedione
- AFCs
Antral follicle counts
- AMH
Anti-Müllerian Hormone
- ART
Assisted reproductive technology
- BMI
Body mass index
- CC
Clomifene citrate
- COCs
Cumulus-oocyte complexes
- COS
Controlled ovarian stimulation
- DHEA-S
Dehydroepiandrosterone sulfate
- E1
Estrone
- E2
Estradiol
- ET
Embryo transfer
- FBT
Frozen-thawed blastocyst transfer
- FFs
Follicular Fluids
- FSH
Follicle stimulating hormone
- GnRH
Gonadotropin releasing hormone
- hCG
Human chorionic gonadotropin
- HH
Hypogonadotropic hypogonadism
- HMG
Human menopausal gonadotropin
- HRT
Hormone replacement treatment
- HSG
Hysterosalpingography
- ICSI
Intra cytoplasmic sperm injection
- Inf-
Infertile patients
- IVF
In vitro fertilization
- IVM
In vitro maturation
- LH
Luteinizing hormone
- LH-RH
Luteinizing hormone-releasing hormone
- OC
Oral contraceptive
- OHSS
Ovarian hyperstimulation syndrome
- OPU
Oocyte pick-up
- Ovd
Ovulation day
- P4
Progesterone
- PN
Pronuclei
- PRL
Prolactin
- rFSH
Recombinant FSH
- rLH
Recombinant LH
- SP
Synthetic progesterone
- T
Testosterone
- T3
Triiodothyronine
Additional file
Endocrine tests of the patient for infertility screening. (XLSX 9 kb)
Authors’ contributions
K.M., an embryologist and infertility counselor, performed the IVF, cryopreservation, FBT, designed the experiments and wrote this case report. K.I. discussed for this case report and contributed to the manuscript. C.H. was observation managing the patient. All authors approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any images and clinical data. A copy of the written consent is available for reviewer by the editor of this journal.
Competing interests
All the author’s declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
Electronic supplementary material
The online version of this article (10.1186/s12884-017-1510-6) contains supplementary material, which is available to authorized users.
Contributor Information
Kaori Matsumoto, Phone: 81-3-3972-8111, Email: matsumoto.kaori10@nihon-u.ac.jp.
Kazuhiko Imakawa, Phone: 81-299-45-8952, Email: akaz@mail.ecc.u-tokyo.ac.jp.
Chuyu Hayashi, Phone: 81-3-3972-8111, Email: hayashi.chuyu@nihon-u.ac.jp.
References
- 1.Erickson GF. An analysis of follicle development and ovum maturation. Semin Reprod Med. 1986;4(3):233–254. doi: 10.1055/s-2007-1022504. [DOI] [Google Scholar]
- 2.Balasch J, Miró F, Burzaco I, Casamitjana R, Civico S, Ballescá JL, et al. The role of luteinizing hormone in human follicle development and oocyte fertility: evidence from in-vitro fertilization in a woman with long-standing hypogonadotrophic hypogonadism and using recombinant human follicle stimulating hormone. Hum Reprod. 1995;10(7):1678–1683. doi: 10.1093/oxfordjournals.humrep.a136154. [DOI] [PubMed] [Google Scholar]
- 3.The European Recombinant Human LH Study Group Recombinant human luteinizing hormone (LH) to support recombinant human follicle-stimulating hormone (FSH)-induced follicular development in LH- and FSH-deficient anovulatory women: a dose-finding study. J Clin Endocrinol Metab. 1998;83(5):1507–1514. doi: 10.1210/jcem.83.5.4770. [DOI] [PubMed] [Google Scholar]
- 4.Silveira LF, Latronico AC. Approach to the patient with hypogonadotropic hypogonadism. J Clin Endocrinol Metab. 2013;98(5):1781–1788. doi: 10.1210/jc.2012-3550. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization . WHO laboratory manual for the examination and processing of human semen. 5. Geneva: WHO; 2010. Department of Reproductive Health and Research. [Google Scholar]
- 6.van Rooij IA, Broekmans FJ, te Velde ER, Fauser BC, Bancsi LF, de Jong FH, et al. Serum anti-Müllerian hormone levels: a novel measure of ovarian reserve. Hum Reprod. 2002;17(12):3065–3071. doi: 10.1093/humrep/17.12.3065. [DOI] [PubMed] [Google Scholar]
- 7.Hsu A, Arny M, Knee AB, Bell C, Cook E, Novak AL, et al. Antral follicle count in clinical practice: analyzing clinical relevance. Fertil Steril. 2011;95(2):474–479. doi: 10.1016/j.fertnstert.2010.03.023. [DOI] [PubMed] [Google Scholar]
- 8.Styer AK, Toth TL. Antral follicle count in clinical practice: building the bridge from ovarian reserve to in vitro fertilization outcome. Fertil Steril. 2011;95(2):480–481. doi: 10.1016/j.fertnstert.2010.11.054. [DOI] [PubMed] [Google Scholar]
- 9.Nelson SM, Klein BM, Arce JC. Comparison of antimüllerian hormone levels and antral follicle count as predictor of ovarian response to controlled ovarian stimulation in good-prognosis patients at individual fertility clinics in two multicenter trials. Fertil Steril. 2015;103(4):923–930. doi: 10.1016/j.fertnstert.2014.12.114. [DOI] [PubMed] [Google Scholar]
- 10.Kwee J, Elting ME, Schats R, McDonnell J, Lambalk CB. Ovarian volume and antral follicle count for the prediction of low and hyper responders with in vitro fertilization. Reprod Biol Endocrinol. 2007;5:9. doi: 10.1186/1477-7827-5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.La Marca A, Sighinolfi G, Radi D, Argento C, Baraldi E, Artenisio AC, et al. Anti-Mullerian hormone (AMH) as a predictive marker in assisted reproductive technology (ART) Hum Reprod Update. 2010;16:113–130. doi: 10.1093/humupd/dmp036. [DOI] [PubMed] [Google Scholar]
- 12.Yovich J, Stanger J, Hinchliffe P. Targeted gonadotrophin stimulation using the PIVET algorithm markedly reduces the risk of OHSS. Reprod BioMed Online. 2012;24:281–292. doi: 10.1016/j.rbmo.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 13.Hill MJ, Chason RJ, Payson MD, Segars JH, Csokmay JM. GnRH antagonist rescue in high responders at risk for OHSS results in excellent assisted reproduction outcomes. Reprod BioMed Online. 2012;25(3):284–291. doi: 10.1016/j.rbmo.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muasher SJ, Kruithoff C, Simonetti S, Oehninger S, Acosta AA, Jones GS. Controlled preparation of the endometrium with exogenous steroids for the transfer of frozen-thawed pre-embryos in patients with anovulatory or irregular cycles. Hum Reprod. 1991;6(3):443–445. doi: 10.1093/oxfordjournals.humrep.a137355. [DOI] [PubMed] [Google Scholar]
- 15.Queenan JT, Jr, Veeck LL, Seltman HJ, Muasher SJ. Transfer of cryopreserved-thawed pre-embryos in a natural cycle or a programmed cycle with exogenous hormonal replacement yields similar pregnancy results. Fertil Steril. 1994;62(3):545–550. doi: 10.1016/S0015-0282(16)56943-X. [DOI] [PubMed] [Google Scholar]
- 16.Jansen R, Mortimer D. Towards reproductive certainty. New York: Parthenon; 1999. [Google Scholar]
- 17.Oktay K, Hourvitz A, Sahin G, Oktem O, Safro B, Cil A, et al. Letrozole reduces estrogen and gonadotropin exposure in women with breast cancer undergoing ovarian stimulation before chemotherapy. J Clin Endocrinol Metab. 2006;91(10):3885–3890. doi: 10.1210/jc.2006-0962. [DOI] [PubMed] [Google Scholar]
- 18.Sönmezer M, Türkçüoğlu I, Coşkun U, Oktay K. Random-start controlled ovarian hyperstimulation for emergency fertility preservation in letrozole cycles. Fertil Steril. 2011;95(6):2125–e9–11. doi: 10.1016/j.fertnstert.2011.01.030. [DOI] [PubMed] [Google Scholar]
- 19.Johnson LN, Dillon KE, Sammel MD, Efymow BL, Mainigi MA, Dokras A, et al. Response to ovarian stimulation in patients facing gonadotoxic therapy. Reprod BioMed Online. 2013;26(4):337–344. doi: 10.1016/j.rbmo.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tesarik J, Mendoza C. Nongenomic effects of 17 beta-estradiol on maturing human oocytes: relationship to oocyte developmental potential. J Clin Endocrinol Metab. 1995;80(4):1438–1443. doi: 10.1210/jcem.80.4.7714121. [DOI] [PubMed] [Google Scholar]
- 21.Hikabe O, Hamazaki N, Nagamatsu G, Obata Y, Hirao Y, Hamada N, et al. Reconstitution in vitro of the entire cycle of the mouse female germ line. Nature. 2016;539(7628):299–303. doi: 10.1038/nature20104. [DOI] [PubMed] [Google Scholar]
- 22.Goud PT, Goud AP, Qian C, Laverge H, Van der Elst J, De Sutter P, et al. In-vitro maturation of human germinal vesicle stage oocytes: role of cumulus cells and epidermal growth factor in the culture medium. Hum Reprod. 1998;13(6):1638–1644. doi: 10.1093/humrep/13.6.1638. [DOI] [PubMed] [Google Scholar]
- 23.Ben-Ami I, Komsky A, Bern O, Kasterstein E, Komarovsky D, Ron-El R. In vitro maturation of human germinal vesicle-stage oocytes: role of epidermal growth factor-like growth factors in the culture medium. Hum Reprod. 2011;26(1):76–81. doi: 10.1093/humrep/deq290. [DOI] [PubMed] [Google Scholar]
- 24.Cakmak H, Katz A, Cedars MI, Rosen MP. Effective method for emergency fertility preservation: random-start controlled ovarian stimulation. Fertil Steril. 2013;100(6):1673–1680. doi: 10.1016/j.fertnstert.2013.07.1992. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


