Abstract
The overall goal of this work is to introduce nerve cuff electrodes into upper extremity hand grasp systems. The first challenge is to develop a nerve cuff electrode that can selectively activate multiple hand functions from common upper extremity peripheral nerves. The Flat Interface Nerve Electrode (FINE) has shown selective stimulation capability in animal trials. The FINE wraps around the nerve and gently reshapes the nerve and aligns the fascicles within the nerve. Our hypothesis is that the FINE can selectively stimulate multi-fascicular nerves in the human upper extremity resulting in selective hand function. To assess the ability of the FINE to produce control of a hand with many degrees of freedom, we have tested the FINE in nonhuman primates. Fascicular organization and fascicle count are important factors to consider when determining electrode placement. The proximal nerve is an attractive electrode location to access both extrinsic and intrinsic muscles in the upper extremity. A challenge with the nonhuman primate model is that the nonhuman primate median and ulnar nerves both have uni-fascicular regions proximally. The human proximal median and ulnar nerves have an encouraging anatomy of multi-fasciculated nerves with redundant fascicles that may result in more selective hand function than is capable in the nonhuman primate.
I. INTRODUCTION
The overall goal of this work is to introduce nerve cuff electrodes into upper extremity hand grasp systems. To be a feasible technology for a hand grasp neuroprosthesis, a peripheral nerve electrode must activate multiple hand functions from a common upper extremity peripheral nerve. Currently available upper extremity neural prostheses incorporate muscle based electrodes [4]. Nonselective nerve-cuff electrodes have been employed proximally for activation of shoulder and arm muscles [5]. The spiral cuff electrode [2] and the Flat Interface Nerve Electrode (FINE) [11] have been tested for implementation in a standing neuroprosthesis.
The Flat Interface Nerve Electrode (FINE) is an extraneural electrode designed to gently reshape the nerve [12]. By reshaping the nerve, the FINE increases the surface area and access to the central fascicles. Chronic studies have indicated that small applied pressures do not result in apparent histological or physiological change [7]. The FINE has been shown to be selective in acute cat experiments [6], [9].
Fascicular organization is important for achieving selective function from peripheral nerve stimulation. Jabaley et al. [3] demonstrated that the fascicles maintain an organization over the length of the upper arm. Brushart used horseradish peroxidase to trace nerve fibers along a monkey median nerve and found that the axons remained in a close group even though the fascicles intermingled [1]. Qualitative anatomy is available describing the fascicle arrangement in humans and monkeys, but detailed quantitative fascicular anatomy required for electrode design is limited. Preliminary data has shown that the success of the electrodes depends on the organization of the fascicles. Nerve diameter, fascicle size distributions along the nerve, fascicle counts, and organization of fascicles are necessary to determine the dimensions, geometry, and location of a peripheral nerve electrode in a hand grasp system.
The nonhuman primate model was selected to demonstrate the capability of the FINE to produce selective hand function in a highly articulated hand. The human hand is a complicated organ with 22 degrees of freedom [15] and an opposable thumb with an additional 5 degrees of freedom. The nonhuman primate model is suitable because nonhuman primates are able to independently move their fingers [16] and most nonhuman primates have an opposable thumb. The number of extrinsic and intrinsic muscles controlling the arm, the distribution of nerve branches in the upper extremity, and the motor point distribution in the extrinsic muscles are similar in the human and nonhuman primates [17]. Based on this analysis, the nonhuman primate seems to be an appropriate model to test the hypothesis that the FINE can selectively stimulate multi-fascicular nerves resulting in selective hand function.
II. METHODS
A. Histology and Anatomical Studies
Nerves from nonhuman primates and human cadavers were carefully dissected for anatomical measurements. The branches of the muscles of interest were labeled with suture for later identification. The dissected nerves were then preserved in 10% formalin. The extracted nerves were cut perpendicular to the long axis of the nerve into 5 mm sections. Marking dyes were used to conserve the nerve orientation. These nerve sections were embedded in paraffin and cut at 1 mm intervals into10 μm thick sections. The slices were stained with methylene blue, staining the myelin and perineurium in the nerve cross sections (Figure 1).
Figure 1.
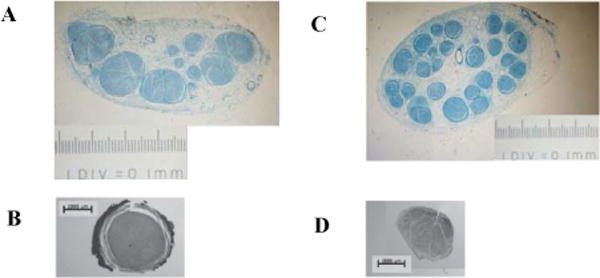
Images are shown with the same scale. A, Histological cross section of the proximal human median nerve. B, Histological cross section of the proximal nonhuman primate median nerve. C, Histological cross section of the distal human median. D, Histological cross section of the distal nonhuman primate median nerve.
Images of the slides were captured using a Zeiss Model microscope with a 1×, 10×, or 40× objective (Figure 1). The images were measured with ImageJ [13] and analyzed with custom MATLAB (2007a, The MathWorks, Natick, MA) scripts.
B. Electrode Development
The electrode dimensions (Figure 2) were based on the anatomical measurements. The height, h, of the electrode was chosen to be between the diameter of the 2nd and 3rd largest fascicles. The width, w, was chosen to make the cross sectional area of the opening, w × h, to be 1.4 times the nerve cross sectional area. The wall thickness was chosen after calculating the pressure exerted on the largest fascicle, Df, in the nerve cross section according to previously validated methods [12]. The length along the nerve was selected to be 6 mm.
Figure 2.
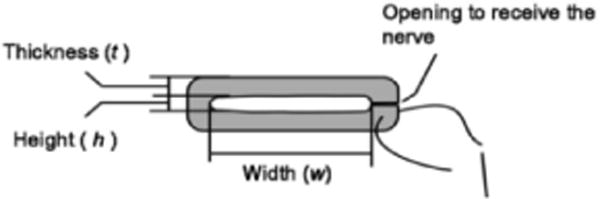
A diagram of the electrode dimension parameters [12].
The electrode body was made with MED-4870 (Nusil, Carpinteria, CA). The contacts were cut from 25 μm thick platinum foil and then welded to 7-strand 316LVM stainless-steel, PFA-coated wires from Fort Wayne Metals Research Corp.
C. Stimulation Recruitment
Two different FINE configurations, one for the ulnar nerve and one for the median nerve, both proximally and distally on each nerve, were tested on three terminal Rhesus Macaques, based on previously developed methods [19].
Bipolar needle electromyography (EMG) electrodes were inserted into hand muscles innervated by the ulnar and median nerves. The EMG signals were recorded using programmable amplifiers (Cambridge Electronic Design). The EMG data was filtered with a low pass filter of 1000 Hz and sampled at 5000 kHz. A custom-built external stimulator (Crishtronics, Cleveland, OH) applied stimulation to the different contacts on the electrode. A custom MATLAB software package controlled the data recording and the stimulator output for pulse amplitude modulation and pulse width modulation recruitment. The muscle twitch was quantified by the rectified, 40 ms-windowed, integrated EMG response to the stimulation. The recruitment curves of each muscle were normalized to the maximum recruitment for the given muscle over all trials within the session.
D. Anatomically based Finite Element Models
A modeling paradigm has been developed to predict recruitment selectivity of the FINE [11]. In MATLAB, the nerve cross sections were traced and imported into Ansoft (Pittsburgh, PA), an FEM software package, to create a 3-dimensional representation of the nerve. The epineurium, perineurium, and endoneurium were assigned electrical conductances, as previously described [14] (Figure 3).
Figure 3.
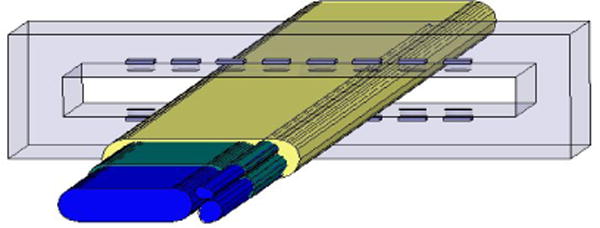
An FEM model of a cross section in the median proximal nerve from a nonhuman primate. The blue, green, and yellow correspond to the endoneurium (material inside fascicle), perineurium (tissue that wraps around the fascicle) and epineurium (outer nerve layer) respectively.
Different contacts were activated with 1 mA cathodic current stimulation. The capacitance in the tissue and at the electrode interface may be assumed to be purely resistive. The generated voltage potentials throughout the nerve were calculated by Ansoft. The results were imported into MATLAB. Axonal diameter distribution [20] and 100 random positions of axons within the fascicles were specified in MATLAB and applied to a linear approximation method. We scaled the applied unit current amplitude to calculate the voltage fields. The linear approximation, based on features of the voltage field, was used to calculate the thresholds of axonal activation [18].
III. RESULTS
A. Anatomy
The human median nerve and nonhuman primate median nerve followed similar fascicle count trends. A notable difference in the human anatomy is that the human median nerve had more fascicles than the nonhuman primate nerve. The human median nerve was multi-fasciculated throughout all regions where the non human primate had uni-fascicular nerve regions. This observation was also seen in the ulnar nerve. The uni-fascicular nerve regions in the nonhuman primate are not an optimal location for the fascicle selective cuff placement.
B. Stimulation
Stimulation in the nonhuman primate selectively produced pronation and recruitment of several extrinsic muscles (Figure 5). Stimulation by a cuff at a proximal location in the median nerve from Monkey 2 was selective for pronator (Figure 6). When the cuff was shifted slightly distal, a different recruitment pattern was seen (Figure 7).
Figure 5.
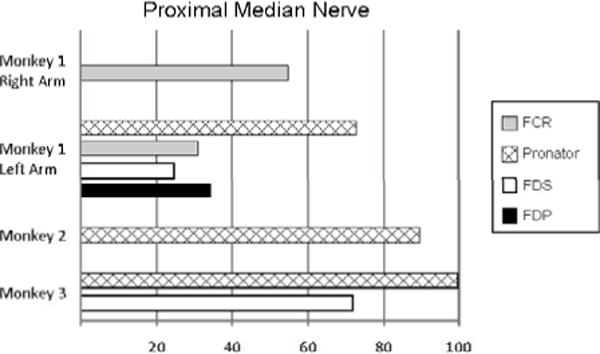
The selectivity values displayed in the bar graph are the largest normalized recruitment values from a single contact. The selectivity values were the normalized values of muscle activation before 10% of another muscle was activated.
Figure 6.
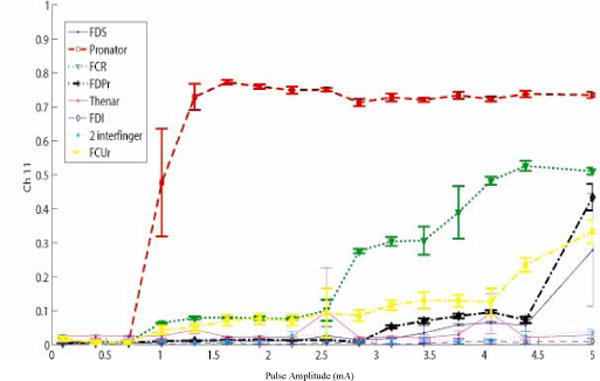
Pronator was selective in monkey 2 from the median proximal nerve before moving the cuff.
Figure 7.
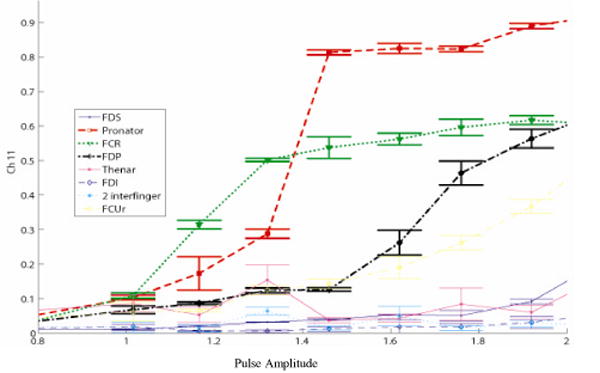
A different recruitment pattern was seen in monkey 2 when the cuff was shifted slightly distal (compared to Figure 6).
C. Modeling
Finite element models were created from histology at two different proximal regions of the nonhuman primate median nerve. The cross section in Figure 8 shows a single, large fascicle. Stimulation from one contact resulted in axon activation throughout the large fascicle. Based on a different nerve cross-sections, another model with three fascicles was constructed (Figures 9–11). Selective activation of all three fascicles was achieved from activation of three different contacts.
Figure 8.
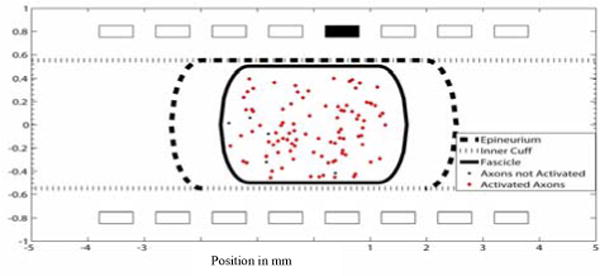
A uni-fascicle cross section of the nonhuman primate median nerve. A cathodic current with .1 msec pulse width and .2 mA pulse amplitude was applied to contact 5. Axon that fired (red diamonds) and axons that did not fire (black circles) are shown.
Figure 9.
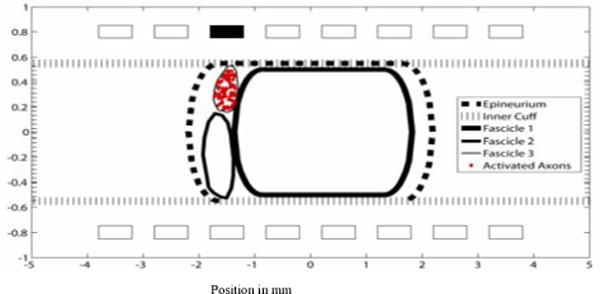
A cross section of the nonhuman primate median nerve. A cathodic current with .02 msec pulse width and .1 mA pulse amplitude was applied to contact 3. Only axons that were activated are shown.
Figure 11.
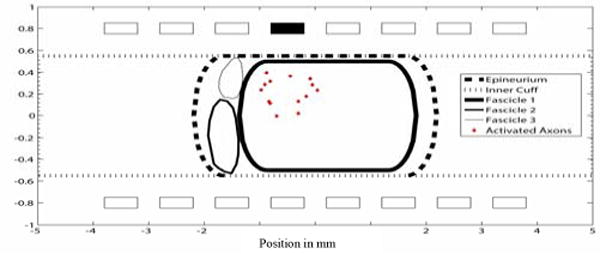
A multi-fascicle cross section of the nonhuman primate median nerve. Contact 4 was able to activate the largest fascicle with a cathodic current of .02 msec pulse width and .1 mA pulse amplitude. Only axons that were activated are shown (red diamonds).
IV. DISCUSSION
The nonhuman primate was thought to be an appropriate model for testing a human hand grasp system because of their highly articulated hands. Although the nonhuman primate upper extremity anatomy has similar features to the human upper extremity anatomy, internal nerve structure is different which limits conclusions from the nonhuman primate. The human nerve is multi-fasciculated but in the nonhuman primate nerve, there are uni-fascicular nerve regions. Human nerves have more fascicles than the nonhuman primate nerves but have a similar number of muscles which indicates that the human nerve has multiple fascicles innervating a particular muscle. Redundant fascicles to a particular muscle allow for an increased opportunity to stimulate that particular muscle.
Axon organization in the nerve is necessary to produce selective hand function from nerve cuff stimulation. Axons tend to stay in a group throughout the monkey to the proximal level [1]. Axonal groups can be selectively stimulated in multi-fasciculated nerves by stimulating the individual fascicles in which they are contained [11]. In Figure 4, the proximal fascicle count changed rapidly over a distance of a cm. Figure 7 demonstrates how moving the cuff in the proximal region of the median nerve slightly distal results in a different recruitment order.
Figure 4.
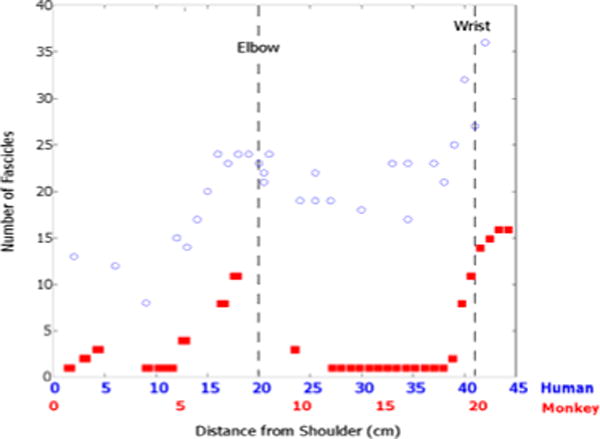
The fascicle count trends are similar in the human (blue circles) and the nonhuman primate (red squares). The nonhuman primate and the human primate fascicle counts were normalized to the elbow.
Based on anatomy we suspect that we can achieve selective hand function in multi-fascicular regions of the human upper extremity nerves. The human femoral nerve, larger than nerves in the human and nonhuman upper extremity, has been selectively activated using the FINE [11]. Thus, the FINE is capable of producing selective activation at a scale larger than the nonhuman primate nerves.
Subfascicle selectivity has also been shown [6] but it is more difficult to achieve. The voltage field from the simulations in the endoneurium, within the fascicle, is approximately constant which limits the ability to selectively activate axons within a fascicle. Stimulation at one contact in Figure 8 activated axons throughout the fascicle. Axons in a multi-fasciculated model were selectively activated in different fascicles from stimulation in a single contact (Figures 9–11).
V. CONCLUSION
Axons in the uni-facular nerve in the nonhuman primate proximal region are difficult to selectively activate. Cuff placement at a multi-fascicular nerve region results in selective activation. Human nerves have an encouraging anatomy to promote selective stimulation in the proximal region of the nerve because they are multi-fasciculated and have redundant fascicles to a particular muscle. Anatomical measurements and anatomically based computer simulations of human upper extremity nerves will be applied to implement this potentially viable technology in a human upper extremity hand grasp system.
Figure 10.
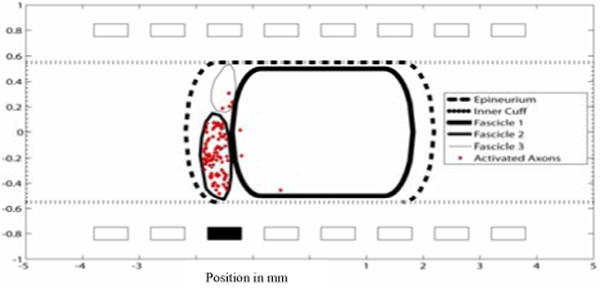
A cross section of the nonhuman primate median nerve. A cathodic current with .05 msec pulse width and .1 mA pulse amplitude was applied to contact 11. Only axons that were activated are shown.
Acknowledgments
The project described was supported by Grant Number R21 NS058705 from the National Institute of Neurological Disorders and Stroke. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disorders and Stroke or the National Institutes of Health. The work of N. Brill was supported by the National Institutes of Health training grant #FT32EB004314.
Contributor Information
N. Brill, Case Western Reserve University (phone: 216-368-8615
K. Polasek, Case Western Reserve University (phone: 216-368-8541
E. Oby, Northwestern University (phone: 312-503-7353
C. Ethier, Northwestern University (phone: 312-503-7353
L. Miller, L. Miller is with Northwestern University (phone: 312-503-7353
D. Tyler, Case Western Reserve University (phone: 216-368-0319.
References
- 1.Brushart TME. Central course of digital axons within median nerve of Macaca mulatta. J Comp Neurol. 1991;311:197–209. doi: 10.1002/cne.903110203. [DOI] [PubMed] [Google Scholar]
- 2.Fisher LE, Miller ME, et al. Preliminary Evaluation of a Neural Prosthesis for Standing after Spinal Cord Injury with Four Contact Nerve Cuff Electrodes for Quadriceps Stimulation; 28th IEEE EMBS Annual International Conference; New York City, USA. 2006. [DOI] [PubMed] [Google Scholar]
- 3.Jabaley ME, Wallace WH, Heckler FR. Internal topography of major nerves of the forearm and hand: a current view. J Hand Surg [Am] 1980;5:1–18. doi: 10.1016/s0363-5023(80)80035-9. [DOI] [PubMed] [Google Scholar]
- 4.Keith MW, Peckham PH, Thorpe GB, Buckett JR, Stroh KC, Menger V. Functional neuromuscular stimulation neuroprotheses for the tetrplegic hand. Clin Orthop. 1988;233:25–33. [PubMed] [Google Scholar]
- 5.Kirsch R. Development of a neuroprosthesis for restoring arm and hand function via functional electrical stimulation following high cervical SCI. Conf Proc IEEE Eng Med Biol Soc. 2005;4:4142–4144. doi: 10.1109/IEMBS.2005.1615375. [DOI] [PubMed] [Google Scholar]
- 6.Leventhal DK, Durand DM. Subfascicle stimulation selectivity with the flat interface nerve electrode. Ann Biomed Eng. 2003;31:643–652. doi: 10.1114/1.1569266. [DOI] [PubMed] [Google Scholar]
- 7.Levanthal DK, Cohen M, Durand DM. Chronic histological effects of the flat interface nerve electrode. J Neural Eng. 2006;3:102–13. doi: 10.1088/1741-2560/3/2/004. [DOI] [PubMed] [Google Scholar]
- 8.Stoffel A. Beitrage zu einer reationellen Nervenchirurgie. Munchen Med Wehnschr. 1913;60:175–179. [Google Scholar]
- 9.Sweeney JD, Ksienski DA, Mortimer JT. A nerve cuff technique for selective excitation of peripheral nerve trunk regions. IEEE Trans Biomed Eng. 1990 Jul;37:706–715. doi: 10.1109/10.55681. [DOI] [PubMed] [Google Scholar]
- 10.Tyler DJ, Durand DM. Functionally selective peripheral nerve stimulation with a flat interface nerve electrode. IEEE Trans Neural Syst Rehabil Eng. 2002;10:294–303. doi: 10.1109/TNSRE.2002.806840. [DOI] [PubMed] [Google Scholar]
- 11.Schiefer M, Triolo R, Tyler D. A Model of Selective Activation of the Femoral Nerve With a Flat Interface Nerve Electrode for a Lower Extremity Neuroprosthesis. IEEE Neural Systems and Rehab ENG. 2008 Apr;16(2) doi: 10.1109/TNSRE.2008.918425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tyler DJ, Durand D. Chronic Response of the Rat Sciatic Nerve to the Flat Interface Nerve Electrode. Annals of Biomedical Engineering. 2003 Jun;31(6) doi: 10.1114/1.1569263. [DOI] [PubMed] [Google Scholar]
- 13.Rasband WS. ImageJ. U.S. National Institutes of Health; Bethesda, Maryland, USA: pp. 1997–2005. http://rsb.info.nih.gov/ij/ [Google Scholar]
- 14.Choi AQ, Cavanaugh JK, Durand DM. Selectivity of multiple-contact nerve cuff electrodes: a simulation analysis. IEEE Transactions on Biomedical Engineering. 2001;48:165, 72. doi: 10.1109/10.909637. [DOI] [PubMed] [Google Scholar]
- 15.Agur AMR, Lee MJ. Grant’s Atlas of Anatomy. 10th. Lippincott Williams and Wilkins; 1999. [Google Scholar]
- 16.Schiever MH. Individuated movements of rhesus monkeys: means of quantifying the independence of digits. Journal of Neurophysiology. 1991;65:1381–1391. doi: 10.1152/jn.1991.65.6.1381. [DOI] [PubMed] [Google Scholar]
- 17.Liu J, Lau HK, Pereira BP, et al. Terminal nerve branch entries (motor points) of forearm muscles: a comparative study between monkey and human. Acta Anat. 1996;155:41–9. doi: 10.1159/000147788. [DOI] [PubMed] [Google Scholar]
- 18.Izad O. Unpublished master’s dissertation. Case Western Reserve University; Cleveland, Ohio: 2009. Computationally Efficient Method in Predicting Axonal Excitation. [Google Scholar]
- 19.Polasek K, et al. Human Nerve Stimulation Thresholds and Selectivity using a multi-contact nerve cuff electrode. IEEE Trans Neural Syst Rehabil Eng. 2007;15(1):76–82. doi: 10.1109/TNSRE.2007.891383. [DOI] [PubMed] [Google Scholar]
- 20.Buchthal F, Carlsen F, Behse F. Schmidt–Lanterman clefts: a morphometric study in human sural nerve. Am J Anat. 1987;180:156–60. doi: 10.1002/aja.1001800205. [DOI] [PubMed] [Google Scholar]


