Abstract
Background
Normothermic machine perfusion (NMP) is an alternative strategy for preserving kidneys donated after cardiac death (DCD). The relative efficacy of prolonged NMP compared to hypothermic machine perfusion (HMP) in DCD kidneys with moderate ischemic injury is undetermined. This study compares NMP and HMP kidney preservation in a porcine DCD model.
Methods
Ten porcine kidneys underwent NMP or HMP preservation following 45 minutes of warm ischemia and 5 hours of cold ischemia. After 8 hours of machine preservation, hemodynamic stability, renal function, perfusate biomarkers, and histologic integrity were assessed in a simulated reperfusion model.
Results
Upon simulated reperfusion, no differences were observed in oxygen consumption, urine production, creatinine clearance, fractional excretion of sodium, proteinuria, and perfusate levels of lactate dehydrogenase and aspartate aminotransferase. Resistance was no different after 30 minutes of simulated reperfusion. NMP kidneys demonstrated increased histologic vacuolization after preservation and greater loss of tubular integrity after simulated reperfusion. Perfusate levels of alkaline phosphatase (AP) and gamma glutamyltransferase (GGT) were higher in NMP kidneys during preservation, but upon simulated reperfusion, AP and GGT levels were higher in HMP-preserved kidneys. Peak AP and GGT during HMP simulated reperfusion were over 14 times higher than peak AP and GGT during NMP preservation.
Conclusions
NMP provided comparable preservation of renal function as HMP and minimized AP and GGT release upon reperfusion.
Keywords: Organ preservation, Kidney transplantation, Perfusion, Donation after cardiac death, Normothermia, Swine
Introduction
The number of patients requiring kidney transplantation is growing faster than the supply of available organs.1 Efforts to compensate for this shortfall have led to a greater reliance on marginal quality grafts, including organs donated after cardiac death (DCD).1,2 Such grafts are more frequently discarded3 and recipients experience higher rates of delayed graft function compared to kidneys donated after brain death (DBD).4 New graft preservation modalities beyond the clinical standards of static cold storage (SCS) and hypothermic machine perfusion (HMP) could optimize utilization and outcomes of these marginal organs.
Normothermic machine perfusion (NMP) is an organ preservation technology that sustains organ grafts at physiologic temperatures. In the first clinical trial of kidney NMP, extended criteria donor kidneys that underwent 1 hour of NMP after a period of SCS had a reduced rate of delayed graft function compared to kidneys preserved with SCS alone.5 Extending the duration of NMP offers certain advantages including minimizing ischemic and hypothermic exposure and enabling physiologic organ assessment and pharmacologic interventions.
Prolonged NMP has been attempted previously. Metcalfe et al. preserved DCD porcine kidneys using NMP for 16 hours following a short 8-minute period of warm ischemia.6 Post-preservation renal function was comparable to HMP-preserved controls. More recently, Kaths et al. demonstrated the feasibility of 10 hours of kidney NMP in DBD porcine kidneys.7 Subsequently, this NMP model was compared to SCS in DCD porcine kidneys after 30 minutes of warm ischemia.8
For marginal quality DCD kidneys, the relative efficacy of prolonged NMP compared to HMP is unknown. This is an important comparison since the Eurotransplant Machine Perfusion Trial showed that HMP provided superior preservation compared to SCS in DCD kidneys,9 although a similar trial in the United Kingdom found no difference between these two modalities.10 To address this knowledge gap, we designed a study to compare NMP and HMP kidney preservation in a porcine DCD model. Our hypothesis was that NMP-preserved kidneys would demonstrate lower vascular resistance, better renal function, and less histologic damage in a simulated reperfusion model.
Methods
Kidney Procurement
Kidneys (n=10) and autologous blood of domestic pigs were obtained from local slaughterhouses, which were practicing in accordance to governing regulations.11 As previously described in translational experiments,12–15 animals were rendered unconscious and killed by exsanguination through a carotid artery and jugular vein incision, thus initiating the warm ischemic period. 2 L of blood were collected in a heparinized container (60,000 IU), transferred to citrated blood bags, and stored on ice.
Kidney pairs were provided to researchers en-bloc approximately 15 minutes after exsanguination. The left or right kidney was selected randomly for preservation, and the contralateral kidney was discarded. Gerota’s fascia and perinephric fat were removed, and the renal artery, renal vein, and ureter were isolated and cannulated. The kidney was placed within an organ isolation bag. After 45 minutes of warm ischemia, the kidney was flushed through the renal artery cannula from an arterial pressure of 100 cm H2O with 340 mL of 4°C histidine-tryptophan-ketoglutarate solution (Essential Pharmaceuticals LLC, Newtown, PA) supplemented with 2000 IU/L of heparin. Kidneys were stored in a cooler on ice for transport. After five hours of SCS, kidneys were assigned to either the NMP group (n=5) or HMP group (n=5) for a preservation time of 8 hours.8,16
Preservation Phase
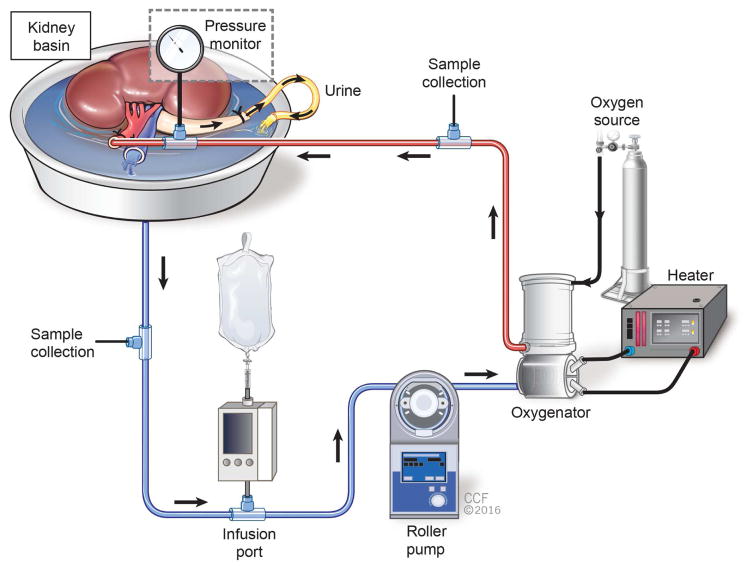
Kidneys were preserved during both NMP and HMP with a perfusion system consisting of an organ chamber, a roller pump (Sarns 8000 Roller Pump, Terumo), and an oxygenator/heat exchanger (Affinity, Medtronic), all connected by tubing (Fig. 1). Sampling ports were included before (venous) and after (arterial) the oxygenator/heat exchanger. An infusion line for supplemental drugs was connected to venous tubing. Ureteral outflow drained directly into the perfusate reservoir except when temporarily diverted into an external collection container for sampling purposes.
Figure 1.
Preservation and simulated reperfusion schematic. Urine drained directly to kidney basin except when sampling urine for analysis. Oxygen source refers to a mixture of 95% oxygen and 5% carbon dioxide during preservation and 100% oxygen during simulated reperfusion.
The NMP perfusate was a modification of the perfusate employed by Kaths et al.7 It consisted of 22.5 g of bovine serum albumin, 322 mL of Krebs-Henseleit Buffer, 282 mL of lactated Ringer’s solution, 44 mL of H2O, and 202 mL of washed red blood cells (Sequestra 1000, Medtronic). The circuit was primed with perfusate, which was supplemented with 1 g of ampicillin, 1 g of cefotaxime, 2000 IU of heparin, 10 mg of dexamethasone, and 3 mL of calcium gluconate (10%). The perfusate was infused with 500 IU/hr of heparin, 5 U/hr of insulin lispro, 0.05 g/hr of amino acids, and 0.25 mg/hr of verapamil. The target mean arterial pressure was 40 mmHg.17 The oxygenator was supplied with 95% oxygen and 5% carbon dioxide at an initial flow rate of 0.3 L/min. To achieve a pH of 7.3 – 7.5, sodium bicarbonate was supplemented and the gas flow rate was adjusted as needed. A heater was set to achieve a perfusate temperature of 37°C.7
HMP was performed at 4°C in a cold room, and no gas was supplied.18,19 The circuit was primed with Belzer’s Machine Perfusion Solution (Bridge to Life Ltd, Columbia, SC) and supplemented with 750 mg of cefuroxime, 2000 IU of heparin, 10 mg of dexamethasone, and 40 U of insulin lispro. The target mean arterial pressure was 30 mmHg.
Simulated Reperfusion Phase
Following preservation, all kidneys were flushed 150 mL of histidine-tryptophan-ketoglutarate solution at 4°C, and after a five-minute room temperature exposure, kidneys were transferred to a secondary circuit for a two-hour reperfusion phase to simulate transplantation.20 This circuit contained the same components as above. The perfusate consisted of 450 mL of lactated Ringer’s and 450 mL of autologous whole blood.21 This was supplemented with heparin (2000 IU), cefuroxime 750 mg, creatinine 22.5 mg, and calcium gluconate 3.6 mL, and infused with heparin 500 IU/hr and insulin lispro 5 U/hr. 100% oxygen gas was provided,20 and the gas flow rate was adjusted every 30 minutes to target a pH of 7.3 – 7.5.22 Temperature was set to 37°C and mean arterial pressure was 85 mmHg.23
Outcome Measures
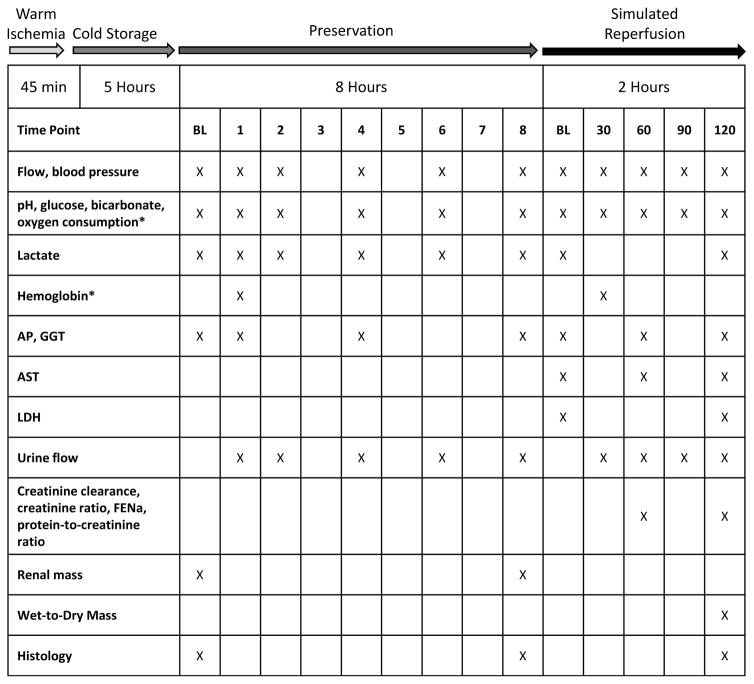
Hemodynamic, perfusate, urinary, and histologic markers were measured during preservation and simulated reperfusion (see Figure 2 for sampling schedule).
Figure 2.
Study design schematic demonstrating the experimental phases and sampling schedule. BL – baseline; AP – alkaline phosphatase; GGT – gamma glutamyltransferase; AST – aspartate aminotransferase; LDH – lactate dehydrogenase; FENa-fractional excretion of sodium. * Indicates that during the preservation phase, hemoglobin and oxygen consumption were measured in NMP only.
Hemodynamics
Mean renal artery pressure and flow rate were monitored continuously and recorded as indicated (Fig. 2). Vascular resistance was calculated as mean pressure/flow.
Blood gases, Electrolytes, and Hemoglobin
Arterial and venous pH, oxygen pressure, oxygen saturation, glucose, bicarbonate, and lactate were measured using an i-STAT Handheld Blood Analyzer (Abbott Point of Care, Princeton, NJ). Glucose was adjusted to 100 – 200 mg/dL by supplementing dextrose 50%. Glucose and pH were not adjusted during HMP preservation per clinical practice.
Hemoglobin was measured during preservation (NMP only) and simulated reperfusion (Marshfield Labs, Marshfield, WI). Oxygen content (mL O2 / dL perfusate) was calculated as 1.34 x hemoglobin concentration (g/dL) x oxygen saturation (%) + 0.0031 x partial pressure of oxygen. Oxygen consumption (mL O2 / minute) was calculated as the difference between arterial and venous oxygen content, multiplied by flow. Oxygen consumption could not be calculated for the HMP preservation phase in the absence of oxygenation.
Perfusate biomarkers
Alkaline phosphatase (AP) and gamma-glutamyltransferase (GGT) were measured (Marshfield Labs) during preservation and simulated reperfusion as markers of tubular injury,20,24,25 and lactate dehydrogenase (LDH) and aspartate aminotransferase (AST) were measured (Marshfield Labs) during simulated reperfusion as markers of cellular damage.8,26,27
Urine
To measure urinary function, the ureter outflow was temporarily diverted from the reservoir into a collection cup during sampling periods. After sampling, excess urine was returned to the perfusate reservoir. Urine production rate was regularly measured during preservation and simulated reperfusion. Urine creatinine, sodium, and protein were measured simultaneously with perfusate creatinine and sodium during simulated reperfusion (Marshfield Labs). Creatinine clearance was calculated as urine creatinine x urine production rate / perfusate creatinine. Fractional excretion of sodium (FENa) was calculated as urine sodium x serum creatinine / (serum sodium x urine creatinine) x 100. The urine-to-perfusate creatinine ratio and urine protein-to-creatinine ratio were also calculated. Urine dipstick analysis was performed at least once per simulated reperfusion phase to assess hematuria.
Histology
Cortical biopsies were taken at baseline, end-preservation, and end-simulated reperfusion, fixed in formalin, and stained with hematoxylin and eosin. Two blinded graders (WMB, MFB) evaluated specimens using a semi-quantitative scoring system. In each slide, five morphologic fields were evaluated. To characterize the histologic changes associated with NMP relative to HMP, end-preservation specimens were scored on five morphological parameters: tubular dilation, vacuolization, interstitial edema, epithelial shedding, and loss of tubular integrity (i.e. epithelial necrosis, destruction of basement membrane). The latter two criteria were again scored following simulated reperfusion to assess irreversible kidney injury. Samples were scored from 0 to 3 based on severity of the parenchymal injury parameter: 0-none, 1-mild, 2-moderate, and 3-severe.
Weight
Kidneys were weighed before and after preservation. Edema was quantified by comparing the fractional weight increase over the course of preservation (post-weight – pre-weight) / pre-weight. Analysis of total tissue water content was performed by measuring wet-to-dry weight ratios after simulated reperfusion. Tissue specimens were dried in an oven at 60°C for 24 hours and the weight ratio was calculated (wet – dry) / wet.
Statistical Analysis
Median and range are reported. Differences between groups were calculated using the Mann-Whitney U test using a level of significance P < 0.05. Pearson correlation coefficients were calculated for perfusate biomarkers. Histology scores between the two graders were averaged and inter-observer variability was compared by calculating a kappa with linear weighting for each parameter. Statistical analysis was carried out using Excel (Microsoft, Redmond, WA), JMP version 12.1.0 (SAS Institute Inc., Cary, NC), and SAS version 9.4 (SAS Institute, Cary, NC).
Results
Preservation
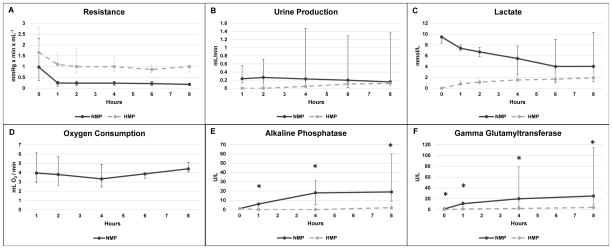
NMP perfusate hemoglobin was 4.4 (3.9 – 4.8) g/dL. Over 8 hours of preservation, mass increased by 49 (38 – 68) % in the NMP group and 33 (22 – 49) % the HMP group (P = 0.06). An initial parallel decrease in resistance was observed in both groups during the first hour of preservation, and by 8 hours median resistance was 0.18 (0.12 – 0. 24) mmHg x min x mL−1 in NMP and 1.00 (0.75 – 1.03) mmHg x min x mL−1 in HMP kidneys (Fig. 3A). Urine production rate at 8 hours was 0.15 (0.10 – 1.36) mL/min in NMP kidneys and 0.12 (0.04 – 0.20) mL/min in HMP kidneys (Fig. 3B). Lactate in the NMP group decreased from 9.5 (8.3 – 9.7) mmol/L at baseline to 4.0 (1.2 – 10.3) mmol/L at 8 hours, while HMP lactate increased from undetectable at baseline to 2.0 (1.7 – 3.0) mmol/L at 8 hours (Fig. 3C). Oxygen consumption was stable throughout NMP preservation, reaching 4.4 (4.1 – 5.1) mL O2/min (Fig. 3D).
Figure 3.
Functional parameters during preservation phase. Median ± range are reported. Significance of P < 0.05 between NMP and HMP is indicated with *. Statistical differences were calculated for alkaline phosphatase and gamma glutamyltransferase only.
Perfusate AP at the end of preservation was significantly higher in NMP than HMP (NMP 19 [9 – 60] U/L vs. HMP 2 [0 – 6] U/L, P = 0.008) (Fig. 3E). End-preservation perfusate GGT was also higher in NMP compared to HMP (NMP 25 [10 – 114] U/L vs. HMP 4 [1 – 8] U/L, P = 0.008) (Fig. 3F). NMP kidneys demonstrated a greater degree of epithelial vacuolization by the end of preservation compared to HMP kidneys (NMP 1.7 [0.9 – 2.3] vs. HMP 0 [0 – 0.5], P = 0.008). No significant difference was observed for tubular dilation, interstitial edema, epithelial shedding, and loss of tubular integrity (Table 1, Fig 4A–B).
Table 1.
End-preservation histology scores: histologic changes
| Criteria | NMP Score | HMP Score | P Value | Kappa |
|---|---|---|---|---|
| Tubular Dilation | 1.6 (0.1 – 2.3) | 0.9 (0.1 – 2.2) | 0.48 | 0.52 (0.36, 0.67) |
| Epithelial Vacuolization | 1.7 (0.9 – 2.3) | 0 (0 – 0.5) | 0.008 | 0.55 (0.39, 0.71) |
| Interstitial Edema | 0.5 (0 – 1) | 0.3 (0 – 0.5) | 0.42 | 0.45 (0.24, 0.66) |
| Epithelial Shedding | 1.1 (0.6 – 1.9) | 1.3 (1.2 – 1.9) | 0.13 | 0.33 (0.15, 0.52) |
| Loss of Tubular Integrity | 1.3 (0.1 – 2.7) | 0.5 (0.3 – 0.9) | 0.17 | 0.42 (0.22, 0.61) |
Histology scores are reported as median (range). Kappa coefficients are reported as point estimate (95% confidence interval).
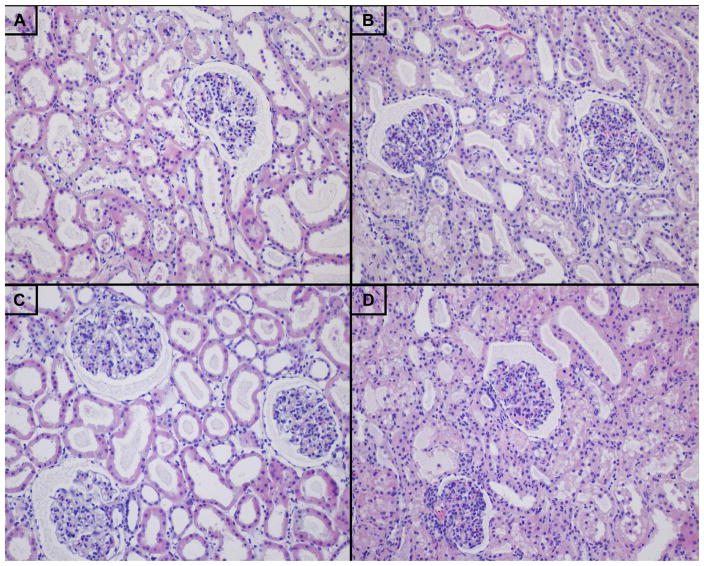
Figure 4.
Photomicrographs of cortical biopsies, hematoxylin and eosin staining, 20x. A) End-preservation HMP, B) End-preservation NMP, C) End-simulated reperfusion HMP, and D) End-simulated reperfusion NMP.
Simulated Reperfusion
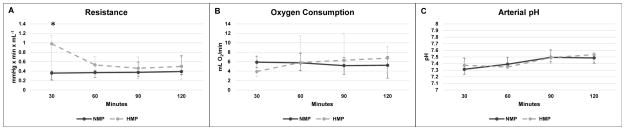
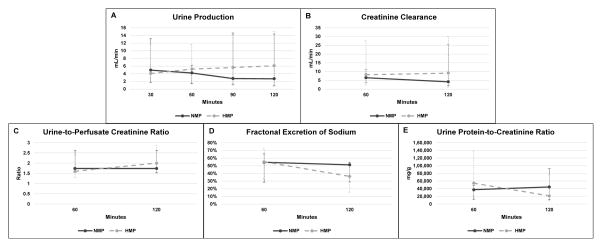
At the start of simulated reperfusion, perfusate hemoglobin concentration was 7.8 (5.7 – 9.2) g/dL in NMP and 7.4 (5.9 – 8.0) g/dL in HMP (P = 0.65). Resistance in NMP-preserved kidneys was significantly lower at 30 minutes of simulated reperfusion (NMP 0.36 [0.21 – 0.40] mmHg x min x mL−1 vs. HMP 0.98 [0.40 – 1.15] mmHg x min x mL−1, P = 0.008), but resistance converged to similar values for the rest of simulated reperfusion (Fig. 5A). Oxygen consumption was no different between groups (Fig 5B). Arterial pH is reported in Figure 5C. There was no difference in renal function parameters including urine production rate, creatinine clearance, urine-to-perfusate creatinine ratio, and FENa throughout simulated reperfusion (Fig. 6A–D). Hematuria (3+ in all cases) and proteinuria were observed in both groups, but there was no difference in the urinary protein-to-creatinine ratio at 60 minutes (P = 0.06) or 120 minutes of simulated reperfusion (P = 0.55) (Fig. 6E). Wet-to-dry ratio was 7.0 (6.5 – 7.7) in the NMP group and 6.3 (5.3 – 7.3) in HMP kidneys (P = 0.10).
Figure 5.
Vascular resistance, oxygen consumption, and arterial pH during simulated reperfusion. Median ± range are reported. Significance of P < 0.05 between NMP and HMP is indicated with *.
Figure 6.
Renal function parameters during simulated reperfusion. Median ± range are reported. There were no significant differences at any time point.
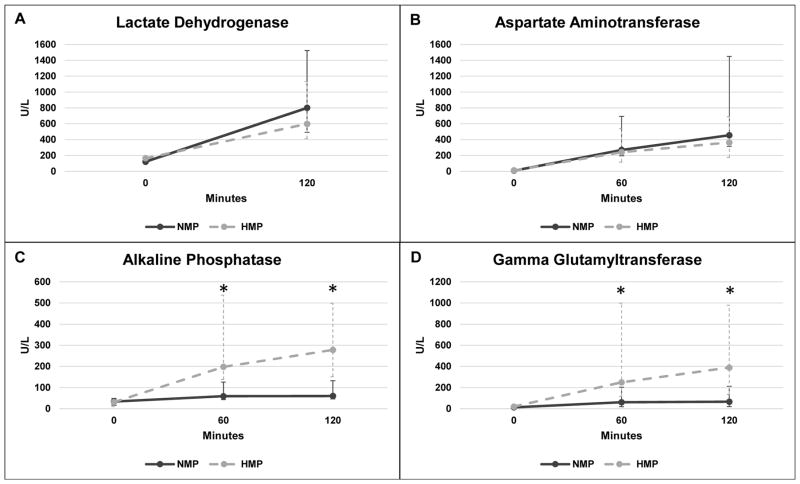
There was no difference between end-simulated reperfusion LDH (NMP 800 [492 – 1523] U/L vs. HMP 598 [411 – 1130] U/L, P = 0.69, Fig. 7A) or AST (NMP 455 [314 – 1450] U/L vs. HMP 364 [176 – 689] U/L, P = 0.42, Fig. 7B). Perfusate AP was higher in the HMP kidneys at 60-minute (NMP 59 [43 – 126] U/L vs. HMP 198 [138 – 537] U/L, P = 0.008) and 120-minute time points (NMP 60 [46 – 132] U/L vs. HMP 278 [151 – 499] U/L, P = 0.008, Fig 7C). Perfusate GGT was higher in HMP kidneys at both 60 minutes (NMP 61 [19 – 204] U/L vs. HMP 249 [117 – 998] U/L, P = 0.02) and 120 minutes (NMP 66 [20 – 212] U/L vs. HMP 389 [136 – 979] U/L, P = 0.02, Fig 7D). LDH at 120 minutes of simulated reperfusion positively correlated with 120-minute AST (R2 = 0.855, P < 0.001), but not GGT (P = 0.50) or AP (P = 0.89). 120-minute GGT positively correlated with 120-minute AP (R2 = 0.897, P < 0.001). On histologic analysis, NMP kidneys demonstrated a greater loss of tubular integrity (P = 0.04), but no significant difference in epithelial shedding was observed (Table 2, Fig 4C–D).
Figure 7.
Perfusate biomarkers during simulated reperfusion. Median ± range are reported. Significance of P < 0.05 between NMP and HMP is indicated with *.
Table 2.
End-simulated reperfusion histology scores: irreversible injury
| Criteria | NMP Score | HMP Score | P Value | Kappa |
|---|---|---|---|---|
| Epithelial Shedding | 1.0 (0.5 – 1.6) | 0.7 (0 – 0.9) | 0.13 | 0.49 (0.29, 0.68) |
| Loss of Tubular Integrity | 0.9 (0.2 – 2.9) | 0.2 (0 – 0.5) | 0.04 | 0.50 (0.29, 0.71) |
Scores are reported as median (range). Kappa coefficients are reported as point estimate (95% confidence interval).
Discussion
This study describes the efficacy of kidney preservation using 8 hours of NMP relative to HMP control in a porcine DCD model, and the results suggest that the two modalities provide a comparable level of preservation. While both methods yielded similar hemodynamic and renal function, AP and GGT biomarker levels suggest that NMP may minimize cellular injury. However, histologic assessment revealed a greater degree of tubular injury in NMP-preserved kidneys.
Kootstra et al. pioneered the development of warm ex-vivo perfusion for marginal kidney preservation by designing a nutrient-rich acellular perfusate, which contained an artificial oxygen carrier and sustained kidneys subnormothermically at 32°C. In a DCD model (120 minutes warm ischemia), canine kidneys that underwent 18 hours of subnormothermic perfusion had lower post-transplant serum creatinine than those that underwent 18 hours of HMP.17 This model awaits clinical application.
The Nicholson group has compared renal NMP and HMP. In one study, DCD porcine kidneys (8 minutes of warm ischemia) were preserved with NMP or HMP for 16 hours. Post-preservation urine-to-plasma concentration ratios were higher for creatinine and lower for sodium in NMP kidneys, while no other differences in renal function were reported.6 In another study, DCD porcine kidneys (10 minutes of warm ischemia) underwent 16 hours of SCS followed by 2 hours of either NMP or HMP. NMP resulted in higher AST, ATP:ADP ratio, and cytoplasmic vacuolization. There were no significant differences in creatinine clearance, FENa, resistance, or perfusate von Willebrand factor.28
Kaths et al. demonstrated the efficacy of prolonged NMP using a Steen Solution-based perfusate, supplemented with lactated Ringer’s solution, water, and red blood cells.7 Steen Solution, which was originally developed for ex-vivo lung perfusion,29,30 contains an extracellular electrolyte solution, dextran 40, and human albumin.31 In DBD porcine kidneys, 8 hours of NMP yielded similar post-transplantation renal function as SCS.16 In DCD porcine kidneys (30 minutes of warm ischemia), NMP resulted in lower serum creatinine, blood urea nitrogen, potassium, and neutrophil gelatinase-associated lipocalin and higher creatinine clearance at various points during the first 7 days post-transplantation compared to SCS.8 When preservation was extended to 16 hours, kidneys perfused for this entire period demonstrated improved creatinine clearance and less apoptosis compared to those perfused for 0, 1, and 8 hours.32 This NMP preservation protocol has not been compared to HMP.
Similar to Kaths et al.,7 our perfusate contained a sub-physiologic protein concentration. Our previous experience with an iso-oncotic albumin-based perfusate yielded oliguria and proteinaceous casts.33 To avoid cast formation, we lowered the perfusate’s oncotic pressure by reducing the albumin concentration to promote the passage of filtrate across the glomerular basement membrane. Since low oncotic pressure promotes extracellular edema, we counteracted this effect by reducing hydrostatic pressure to a mean arterial pressure of 40 mmHg. Similarly, Brasile et al.’s model featured a hydrostatic pressure of 50/30 mmHg in the setting of a perfusate containing 30 g/L of bovine serum albumin.17
To model the resuscitation of marginal quality kidneys, this DCD model included 45 minutes of warm ischemia, which is longer than the 30-minute warm ischemic period of two recent NMP studies in DCD porcine kidneys.8,32 It also exceeds the maximum warm ischemic duration of two clinical trials comparing the use of HMP and SCS in DCD kidneys (38 and 35 minutes in Jochmans et al.34 and Watson et al.,10 respectively). Experimental logistics necessitated 5 hours of subsequent cold ischemia. The 8-hour preservation duration was modeled after Kaths et al.’s initial studies comparing NMP vs. SCS,8,16 and the 2-hour simulated reperfusion duration was adopted from Hoyer et al.,20 though various renal simulated reperfusion models last between 1 and 3 hours.35,36
By recirculating urine in the perfusate, this study deviates from other kidney NMP and simulated reperfusion protocols. We chose to recirculate urine out of concern that replacing proteinuric and hematuric urine with lactated Ringer’s solution would gradually deplete oncotic pressure and oxygen carrying capacity. Furthermore, the homeostatic necessity of urine is uncertain in the machine perfusion environment in which the kidney is isolated from neural and hormonal determinants of urine content. While recirculating urine precludes the potentially beneficial excretion of metabolic waste, urine is not diverted during clinical HMP, thus calling into question the need to excrete these metabolites.
NMP and HMP kidneys demonstrated similar vascular resistance and renal function during simulated reperfusion. The initial resistance difference during simulated reperfusion was likely due to slower rewarming in HMP kidneys.37 This difference dissipated after 30 minutes, suggesting that both modalities similarly preserve vascular function. We observed proteinuria and impaired creatinine clearance and FENa in both groups, and this is commonly seen in renal perfusion studies.20,38–40 Since warm and cold ischemia have been shown to reduce renal function23,41 and we observed similar renal function in both groups, we speculate that the antecedent warm and cold ischemia may have contributed to this functional deficit. Proving this would require testing NMP and HMP in a DBD model.
Enzymatic changes are another clue to cellular destruction. Perfusate levels of LDH and AST have been used to gauge the success of kidney perfusion.7 In this study, LDH closely correlated with AST, while GGT correlated with AP, but there was no correlation across the two pairs. Unlike GGT and AP, LDH and AST levels fluctuate in the setting of minimal hemolysis.42,43 Thus, hemolysis may distort the ability of LDH and AST to predict renal cellular destruction when using blood-based perfusates.
GGT and AP are expressed in proximal tubule brush borders and their urinary expression has been linked to kidney injury.25 Since urine produced by the kidneys was directed into the perfusate, accumulation of these enzymes within the perfusate may reflect tubular injury. Higher AP and GGT levels were seen during NMP preservation compared to HMP, but this was reversed during simulated reperfusion. Notably, at the end of simulated reperfusion, HMP AP and GGT levels were over 14 and 15 times higher than levels seen at the end of NMP preservation, respectively. This suggests that HMP may delay the progression of inevitable kidney injury, which ultimately manifests once reperfusion occurs, and that NMP may limit the extent of this injury.
For histologic analysis, we structured the end-preservation scoring system to determine how each preservation method affected cortical histology, while the end-simulated reperfusion scoring system compared the extent of irreversible histologic damage. Recovery from acute kidney injury requires the clearance of tubular debris44 and the migration of viable cells along the basement membrane.45 Therefore, of the five morphologic criteria evaluated during preservation, tubular debris and loss of tubular architecture were selected as the most likely to reflect irrecoverable injury after simulated reperfusion.
Histologic analysis revealed vacuolization in NMP kidneys after preservation and superior maintenance of tubular integrity in HMP kidneys after simulated reperfusion. Vacuolization in NMP-preserved kidneys has been previously described,7 but the clinical significance of this finding is uncertain.46 The higher degree of tubular integrity loss in NMP kidneys may mean that NMP is more damaging than HMP. On the other hand, warm and cold ischemia cause proximal tubular and glomerular injury.47,48 In clinical transplantation, this injury is revealed upon reperfusion, at which point sufficient energy and biochemical substrates are available to fuel cellular processes like apoptosis.47 Since reperfusion occurs earlier in NMP, this expedites the manifestation of preexisting injury, which would remain occult during the metabolically dormant HMP. Determining whether the histologic insult occurred before or during preservation requires a non-slaughterhouse DBD model without warm or cold ischemia.
The main limitation of our study is the use of organs from slaughterhouse animals. The uncontrolled living conditions and exposures prior to sacrifice may contribute to variability in kidney function. Additionally, a portion of the warm ischemia time occurs extracorporeally at room temperature. Nonetheless, this model has a number of advantages. Exsanguination serves as a reproducible mechanism of death by hemorrhagic shock. Slaughterhouse organs enable low-cost testing of new hypotheses on large animals, which is an otherwise relatively expensive model. Utilization of this widely available resource reduces the need for laboratory animals in early pre-clinical studies. Other groups have successfully conducted perfusion experiments with slaughterhouse organs including kidneys,14,49 livers, 50,51 hearts,15,52 and lungs.12,13,53
Additional limitations include the lack of both an SCS control and an autotransplantation assessment phase. Future studies should evaluate NMP, HMP, and SCS in a highly controlled setting. While autotransplantation is the gold standard for kidney assessment, simulated reperfusion enables immediate and controlled measurement of kidney function.35 Furthermore, a slight alkalotic trend was observed in both experimental arms during simulated reperfusion, suggesting that mixed oxygen and carbon dioxide gas may be a preferable gas for that setting.
Further mechanistic insight could be gleaned from assessing additional markers of renal injury, including glutathione-S-transferase, heart-type fatty acid binding protein, and neutrophil gelatinase-associated lipocalin.27 Future studies should address these limitations, optimize oncotic and hydrostatic pressures, extend the preservation duration, and minimize warm and cold ischemia prior to preservation to clarify the potentially confounding role of pre-preservation ischemic injury.
Conclusions
This is the first study to explore prolonged NMP and HMP in kidneys exposed to an extended period of warm ischemia. Our results suggest that 8-hour NMP using a blood, electrolyte, and albumin-based perfusate provides comparable preservation of DCD kidneys as an HMP control. By providing adequate preservation along with increased opportunities for organ assessment and intervention, this technology offers promise as an alternative to hypothermic kidney preservation.
Acknowledgments
The authors would like to thank Dr. Nina Dvorina for her histotechnical expertise and Dr. Amy Nowacki for statistical assistance. Histidine-tryptophan-ketoglutarate solution was provided at no cost by Essential Pharmaceuticals LLC (Newtown, PA). Belzer’s Machine Perfusion Solution was provided at no cost by Bridge to Life Ltd (Columbia, SC).
Funding
This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (grant number T32DK007470) and the Cleveland Clinic Foundation Research Programs Committees (grant number 2012-1060).
Footnotes
Disclosures
The authors report no proprietary or commercial interest in any product mentioned or concept discussed in this article.
Author Contributions:
MFB, QL, EDP, DAG, and CQ designed the study. MFB, BS, and PD conducted the experiments. MFB, QL, TO, WMB, and CQ performed data analysis and interpretation. MFB drafted the manuscript. All authors edited and approved the final manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Matas AJ, Smith JM, Skeans MA, et al. OPTN/SRTR 2013 Annual Data Report: kidney. Am J Transplant. 2015;15(Suppl 2):1–34. doi: 10.1111/ajt.13195. [DOI] [PubMed] [Google Scholar]
- 2.Matas AJ, Smith JM, Skeans MA, et al. OPTN/SRTR 2012 Annual Data Report: kidney. Am J Transplant. 2014;14(Suppl 1):11–44. doi: 10.1111/ajt.12579. [DOI] [PubMed] [Google Scholar]
- 3.Callaghan CJ, Harper SJF, Saeb-Parsy K, et al. The discard of deceased donor kidneys in the UK. Clin Transplant. 2014;28(3):345–353. doi: 10.1111/ctr.12319. [DOI] [PubMed] [Google Scholar]
- 4.Gagandeep S, Matsuoka L, Mateo R, et al. Expanding the donor kidney pool: utility of renal allografts procured in a setting of uncontrolled cardiac death. Am J Transplant. 2006;6(7):1682–1688. doi: 10.1111/j.1600-6143.2006.01386.x. [DOI] [PubMed] [Google Scholar]
- 5.Nicholson ML, Hosgood SA. Renal transplantation after ex vivo normothermic perfusion: the first clinical study. Am J Transplant. 2013;13(5):1246–1252. doi: 10.1111/ajt.12179. [DOI] [PubMed] [Google Scholar]
- 6.Metcalfe MS, Waller JR, Hosgood SA, Shaw M, Hassanein W, Nicholson ML. A paired study comparing the efficacy of renal preservation by normothermic autologous blood perfusion and hypothermic pulsatile perfusion. Transplant Proc. 2002;34(5):1473–1474. doi: 10.1016/s0041-1345(02)02935-4. [DOI] [PubMed] [Google Scholar]
- 7.Kaths JM, Spetzler VN, Goldaracena N, et al. Normothermic Ex Vivo Kidney Perfusion for the Preservation of Kidney Grafts prior to Transplantation. J Vis Exp. 2015;(101):e52909. doi: 10.3791/52909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaths JM, Echeverri J, Chun YM, et al. Continuous normothermic ex vivo kidney perfusion improves graft function in donation after circulatory death pig kidney transplantation. Transplantation. 2016 Jul; doi: 10.1097/TP.0000000000001343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jochmans I, Moers C, Ploeg R, Pirenne J. To perfuse or not to perfuse kidneys donated after cardiac death. Am J Transplant. 2011;11(2):409–410. doi: 10.1111/j.1600-6143.2010.03350.x. [DOI] [PubMed] [Google Scholar]
- 10.Watson CJE, Wells AC, Roberts RJ, et al. Cold machine perfusion versus static cold storage of kidneys donated after cardiac death: a UK multicenter randomized controlled trial. Am J Transplant. 2010;10(9):1991–1999. doi: 10.1111/j.1600-6143.2010.03165.x. [DOI] [PubMed] [Google Scholar]
- 11.Ohio Rev. Code § 945.01 Humane Methods of Slaughtering Livestock.
- 12.Okamoto T, Chen F, Zhang J, et al. Establishment of an ex vivo lung perfusion model using non-heart-beating large pigs. Transplant Proc. 2010;42(5):1598–1601. doi: 10.1016/j.transproceed.2010.03.140. [DOI] [PubMed] [Google Scholar]
- 13.Okamoto T, Chen F, Zhang J, et al. Comparison of extracellular-type-Kyoto solution and Perfadex as a preservation solution in a pig ex vivo lung perfusion model: impact of potassium level. Transplant Proc. 2011;43(5):1525–1528. doi: 10.1016/j.transproceed.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 14.Grosse-Siestrup C, Unger V, Fehrenberg C, et al. A model of isolated autologously hemoperfused porcine slaughterhouse kidneys. Nephron. 2002;92(2):414–421. doi: 10.1159/000063298. [DOI] [PubMed] [Google Scholar]
- 15.Görge G, Erbel R, Dobbertin A, Hänggi M, Hake U, Meyer J. Isolated in-vitro perfusion of pig hearts obtained from the abattoir: an alternative to animal experiments? Eur Heart J. 1994;15(6):851–857. doi: 10.1093/oxfordjournals.eurheartj.a060594. [DOI] [PubMed] [Google Scholar]
- 16.Kaths JM, Echeverri J, Goldaracena N, et al. Eight Hour Continuous normothermic ex vivo kidney perfusion is a safe preservation technique for kidney transplantation: a new opportunity for the storage, assessment and repair of kidney grafts. Transplantation. 2016;100(9):1862–1870. doi: 10.1097/TP.0000000000001299. [DOI] [PubMed] [Google Scholar]
- 17.Brasile L, Stubenitsky BM, Booster MH, et al. Overcoming severe renal ischemia: the role of ex vivo warm perfusion. Transplantation. 2002;73(6):897–901. doi: 10.1097/00007890-200203270-00011. [DOI] [PubMed] [Google Scholar]
- 18.Lindell SL, Muir H, Brassil J, Mangino MJ. Hypothermic machine perfusion preservation of the DCD kidney: machine effects. J Transplant. 2013;2013:802618. doi: 10.1155/2013/802618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moers C, Smits JM, Maathuis M-HJ, et al. Machine perfusion or cold storage in deceased-donor kidney transplantation. N Engl J Med. 2009;360(1):7–19. doi: 10.1056/NEJMoa0802289. [DOI] [PubMed] [Google Scholar]
- 20.Hoyer DP, Gallinat A, Swoboda S, et al. Subnormothermic machine perfusion for preservation of porcine kidneys in a donation after circulatory death model. Transpl Int. 2014;27(10):1097–1106. doi: 10.1111/tri.12389. [DOI] [PubMed] [Google Scholar]
- 21.Hosgood S, Harper S, Kay M, Bagul A, Waller H, Nicholson ML. Effects of arterial pressure in an experimental isolated haemoperfused porcine kidney preservation system. Br J Surg. 2006;93(7):879–884. doi: 10.1002/bjs.5381. [DOI] [PubMed] [Google Scholar]
- 22.Brodie D, Bacchetta M. Extracorporeal membrane oxygenation for ARDS in adults. N Engl J Med. 2011;365(20):1905–1914. doi: 10.1056/NEJMct1103720. [DOI] [PubMed] [Google Scholar]
- 23.Hosgood SA, Bagul A, Yang B, Nicholson ML. The relative effects of warm and cold ischemic injury in an experimental model of nonheartbeating donor kidneys. Transplantation. 2008;85(1):88–92. doi: 10.1097/01.tp.0000296055.76452.1b. [DOI] [PubMed] [Google Scholar]
- 24.Ward JP. Gamma-glutamyl transpeptidase. A sensitive indicator of renal ischaemic injury in experimental animals and renal homograft rejection in man. Ann R Coll Surg Engl. 1975;57(5):248–261. [PMC free article] [PubMed] [Google Scholar]
- 25.de Geus HRH, Betjes MG, Bakker J. Biomarkers for the prediction of acute kidney injury: a narrative review on current status and future challenges. Clin Kidney J. 2012;5(2):102– 108. doi: 10.1093/ckj/sfs008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harper SJF, Hosgood SA, Waller HL, et al. The effect of warm ischemic time on renal function and injury in the isolated hemoperfused kidney. Transplantation. 2008;86(3):445–451. doi: 10.1097/TP.0b013e31817fe0cd. [DOI] [PubMed] [Google Scholar]
- 27.Hoogland ERP, de Vries EE, Christiaans MHL, Winkens B, Snoeijs MGJ, van Heurn LWE. The value of machine perfusion biomarker concentration in DCD kidney transplantations. Transplantation. 2013;95(4):603–610. doi: 10.1097/TP.0b013e31827908e6. [DOI] [PubMed] [Google Scholar]
- 28.Bagul A, Hosgood SA, Kaushik M, Kay MD, Waller HL, Nicholson ML. Experimental renal preservation by normothermic resuscitation perfusion with autologous blood. Br J Surg. 2008;95(1):111–118. doi: 10.1002/bjs.5909. [DOI] [PubMed] [Google Scholar]
- 29.Wierup P, Haraldsson A, Nilsson F, et al. Ex vivo evaluation of nonacceptable donor lungs. Ann Thorac Surg. 2006;81(2):460–466. doi: 10.1016/j.athoracsur.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 30.Cypel M, Rubacha M, Yeung J, et al. Normothermic ex vivo perfusion prevents lung injury compared to extended cold preservation for transplantation. Am J Transplant. 2009;9(10):2262–2269. doi: 10.1111/j.1600-6143.2009.02775.x. [DOI] [PubMed] [Google Scholar]
- 31.Van Raemdonck D, Neyrinck A, Cypel M, Keshavjee S. Ex-vivo lung perfusion. Transpl Int. 2015;28(6):643–656. doi: 10.1111/tri.12317. [DOI] [PubMed] [Google Scholar]
- 32.Kaths JM, Cen JY, Chun YM, et al. Continuous normothermic ex vivo kidney perfusion is superior to brief normothermic perfusion following static cold storage in donation after circulatory death pig kidney transplantation. Am J Transplant. 2016 doi: 10.1111/ajt.14059. [DOI] [PubMed] [Google Scholar]
- 33.Urcuyo D, Blum MF, Liu Q, et al. Development of a prolonged warm ex-vivo perfusion model for kidneys donated after cardiac death. Int J Artif Organs. doi: 10.5301/ijao.5000586. In press. [DOI] [PubMed] [Google Scholar]
- 34.Jochmans I, Moers C, Smits JM, et al. Machine perfusion versus cold storage for the preservation of kidneys donated after cardiac death: a multicenter, randomized, controlled trial. Ann Surg. 2010;252(5):756–764. doi: 10.1097/SLA.0b013e3181ffc256. [DOI] [PubMed] [Google Scholar]
- 35.Mangino M. Hypothermic Machine perfusion of kidneys. In: Uygun K, Lee CY, editors. Methods in Bioengineering: Organ Preservation and Reengineering. Artech House; 2011. pp. 35–57. [Google Scholar]
- 36.Patel M, Hosgood S, Nicholson ML. The effects of arterial pressure during normothermic kidney perfusion. J Surg Res. 2014;191(2):463–468. doi: 10.1016/j.jss.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 37.Levy MN. Oxygen consumption and blood flow in the hypothermic, perfused kidney. Am J Physiol. 1959;197:1111–1114. doi: 10.1152/ajplegacy.1959.197.5.1111. [DOI] [PubMed] [Google Scholar]
- 38.Hoyer DP, Gallinat A, Swoboda S, et al. Influence of oxygen concentration during hypothermic machine perfusion on porcine kidneys from donation after circulatory death. Transplantation. 2014;98(9):944–950. doi: 10.1097/TP.0000000000000379. [DOI] [PubMed] [Google Scholar]
- 39.Harper S, Hosgood S, Kay M, Nicholson M. Leucocyte depletion improves renal function during reperfusion using an experimental isolated haemoperfused organ preservation system. Br J Surg. 2006;93(5):623–629. doi: 10.1002/bjs.5324. [DOI] [PubMed] [Google Scholar]
- 40.Hosgood SA, Yang B, Bagul A, Mohamed IH, Nicholson ML. A comparison of hypothermic machine perfusion versus static cold storage in an experimental model of renal ischemia reperfusion injury. Transplantation. 2010;89(7):830–837. doi: 10.1097/TP.0b013e3181cfa1d2. [DOI] [PubMed] [Google Scholar]
- 41.Hosgood SA, Patel M, Nicholson ML. The conditioning effect of ex vivo normothermic perfusion in an experimental kidney model. J Surg Res. 2013;182(1):153–160. doi: 10.1016/j.jss.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 42.Lippi G, Salvagno GL, Montagnana M, Brocco G, Guidi GC. Influence of hemolysis on routine clinical chemistry testing. Clin Chem Lab Med. 2006;44(3):311–316. doi: 10.1515/CCLM.2006.054. [DOI] [PubMed] [Google Scholar]
- 43.Koseoglu M, Hur A, Atay A, Cuhadar S. Effects of hemolysis interferences on routine biochemistry parameters. Biochem Med. 2011;21(1):79–85. doi: 10.11613/bm.2011.015. [DOI] [PubMed] [Google Scholar]
- 44.Arai S, Kitada K, Yamazaki T, et al. Apoptosis inhibitor of macrophage protein enhances intraluminal debris clearance and ameliorates acute kidney injury in mice. Nat Med. 2016;22(2):183–193. doi: 10.1038/nm.4012. [DOI] [PubMed] [Google Scholar]
- 45.Bonventre JV, Yang L. Cellular pathophysiology of ischemic acute kidney injury. J Clin Invest. 2011;121(11):4210–4221. doi: 10.1172/JCI45161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Haas M, Sonnenday CJ, Cicone JS, Rabb H, Montgomery RA. Isometric tubular epithelial vacuolization in renal allograft biopsy specimens of patients receiving low-dose intravenous immunoglobulin for a positive crossmatch. Transplantation. 2004;78(4):549–556. doi: 10.1097/01.tp.0000137199.32333.03. [DOI] [PubMed] [Google Scholar]
- 47.Kosieradzki M, Rowiński W. Ischemia/reperfusion injury in kidney transplantation: mechanisms and prevention. Transplant Proc. 2008;40(10):3279–3288. doi: 10.1016/j.transproceed.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 48.Yin M, Currin RT, Peng X-X, Mekeel HE, Schoonhoven R, Lemasters JJ. Different patterns of renal cell killing after warm and cold ischemia. Ren Fail. 2002;24(2):147–163. doi: 10.1081/jdi-120004092. [DOI] [PubMed] [Google Scholar]
- 49.Unger V, Grosse-Siestrup C, Fehrenberg C, Fischer A, Meissler M, Groneberg DA. Reference values and physiological characterization of a specific isolated pig kidney perfusion model. J Occup Med Toxicol. 2007;2:1. doi: 10.1186/1745-6673-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grosse-Siestrup C, Pfeffer J, Unger V, et al. Isolated hemoperfused slaughterhouse livers as a valid model to study hepatotoxicity. Toxicol Pathol. 2002;30(6):749–754. doi: 10.1080/01926230290166841. [DOI] [PubMed] [Google Scholar]
- 51.Izamis M-L, Efstathiades A, Keravnou C, Leen EL, Averkiou MA. Dynamic contrast-enhanced ultrasound of slaughterhouse porcine livers in machine perfusion. Ultrasound Med Biol. 2014;40(9):2217–2230. doi: 10.1016/j.ultrasmedbio.2014.03.031. [DOI] [PubMed] [Google Scholar]
- 52.Schuster A, Grünwald I, Chiribiri A, et al. An isolated perfused pig heart model for the development, validation and translation of novel cardiovascular magnetic resonance techniques. J Cardiovasc Magn Reson. 2010;12:53. doi: 10.1186/1532-429X-12-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Grosse-Siestrup C, Fehrenberg C, von Baeyer H, Groneberg DA. Multiple-organ harvesting for models of isolated hemoperfused organs of slaughtered pigs. ALTEX. 2002;19(1):9–13. [PubMed] [Google Scholar]