Abstract
Docetaxel is a third-generation chemotherapeutic drug that is widely used in the treatment of patients with non-small cell lung cancer (NSCLC). However, the majority of patients with NSCLC eventually acquire resistance to the treatment. In the present study, the mechanism of acquired resistance to docetaxel treatment in lung cancer cells was investigated. The three NSCLC cell lines, H1299 with wild-type epidermal growth factor receptor (EGFR), EGFR-mutant HCC4006 and HCC827, and experimentally established docetaxel-resistant (DR) cells, H1299-DR, HCC827-DR, and HCC4006-DR were used with stepwise increases in concentrations of docetaxel. It was demonstrated that the established cell lines showed resistance to docetaxel and EGFR-tyrosine kinase inhibitors (TKIs). Molecular analysis revealed that all of the resistant cell lines highly expressed ATP binding cassette subfamily B member 1 (ABCB1), which is also known as P-glycoprotein or MDR1. Furthermore, HCC827-DR and HCC4006-DR cells exhibited a cancer stem cell-like marker and epithelial-to-mesenchymal transition features, respectively. Elacridar (GF120918), a third-generation inhibitor of ABCB1, was able to overcome resistance to docetaxel. Additionally, knockdown of ABCB1 using small interfering RNA (si)-ABCB1 recovered sensitivity to docetaxel. However, elacridar and si-ABCB1 could not recover sensitivity to EGFR-TKIs in established resistant cells. The results of the present study revealed that docetaxel-resistant NSCLC cells also acquired cross-resistance to EGFR-TKI therapy through mechanisms other than ABCB1, that ABCB1 serves an important role in acquired resistance to docetaxel in lung cancer, and that combination therapy with elacridar can overcome ABCB1-mediated docetaxel resistance.
Keywords: non-small cell lung cancer, drug resistance, docetaxel, P-glycoprotein, ABCB1, elacridar
Introduction
Most non-small cell lung cancers (NSCLC) are already at an advanced stage when diagnosed and are therefore past the optimal timing for surgical resection (1). Seeking effective treatments for these types of cases is particularly important. Docetaxel is a semi-synthetic analogue of paclitaxel, which is widely used as a therapeutic agent in advanced NSCLC (2,3). Docetaxel shows survival benefit when it is used as single agent or in combination with other drugs including chemotherapeutics or vascular endothelial growth factor inhibitors (4–7). However, patients with NSCLC generally develop resistance to docetaxel, and the underlying mechanisms of acquired resistance to docetaxel are not fully understood (8).
The expression of the multidrug resistance (MDR) phenotype is a main mechanism involved in resistance to taxanes (9–12). ABCB1 (P-glycoprotein) is an ATP-binding cassette (ABC) drug pump (13), and is currently the most extensively studied MDR-related transporter protein (14,15), mediating the ATP-dependent efflux of a wide range of hydrophobic drugs such as taxanes (16–18). Several therapeutic agents targeting ABCB1 are available (18). Inhibitors of ABCB1, which reverse the ABCB1 efflux pump, have been studied for more than twenty years, and third-generation drugs, such as elacridar (GF120918), have been developed (9,19). They specifically and potently inhibit ABCB1 and generally do not alter the plasma pharmacokinetics of simultaneously administered antitumor agents, and therefore show potential for combined application with anticancer drugs to combat chemotherapeutic resistance (19–21).
Cellular phenotypes in addition to ABC molecules have been shown to be associated with multidrug resistance (22). Epithelial-mesenchymal transition (EMT) phenotype, loss of epithelial characteristics (E-cadherin), and acquisition of mesenchymal properties (vimentin, fibronectin, or N-cadherin), have been shown to play a crucial role in drug resistance of cancer cells against conventional therapeutics including taxane, vincristine, oxaliplatin, as well as epidermal growth factor receptor (EGFR)-targeted agents (23–25). Cancer stem cell (CSC) features also contribute to this drug resistance (26,27). CSCs drive both continued expansion of malignant cells and resistance to chemotherapy (28–30). However, CSCs in lung cancer remain a subject of ongoing research, and specific markers have not yet been identified. Jiang et al (31) reported that ALDH1 is a lung tumor stem cell-associated marker and that ALDH1-positive cells are highly resistant to chemotherapeutic drugs.
In the present study, we investigated the mechanisms of docetaxel resistance in NSCLC, and examined the effects of the ABCB1 inhibitor elacridar in combination with docetaxel in docetaxel-resistant lung cancer cells.
Materials and methods
Cell lines and reagents
Three NSCLC cell lines (H1299, HCC827, and HCC4006) were used in this study, including one wild-type EGFR cell line (H1299) and two EGFR-mutant cell lines HCC827 (exon19del E746-A750) and HCC4006 (exon19del L747-E749). These cell lines were obtained from Adi F. Gazdar, MD (Hamon Center for Therapeutic Oncology Research and Department of Pathology, University of Texas Southwestern Medical Center at Dallas, Dallas, TX). These cell lines were proven to have individual genetic origins by using the Powerplex 1.2 system (Promega, Madison, WI, USA) at the University of Texas Southwestern Medical Center at Dallas. All cell lines were cultured in RPMI-1640 media supplemented with 10% fetal bovine serum. They were grown in a humidified incubator with 5% CO2 at 37°C. Docetaxel-resistant sublines (H1299-DR, HCC4006-DR, and HCC827-DR) were established by their parental cells which were cultured with stepwise escalation of concentrations of docetaxel from 0.1 to 100 nmol/l for about 9 months.
Docetaxel, gefitinib, afatinib, and AZD9291 were purchased from Selleck Chemicals (Selleck Chemicals, Houston, TX, USA). Elacridar and Tween 80 (polysorbate 80) were purchased from Sigma, Inc. (Sigma-Aldrich, St Louis, MO, USA).
Determination of cell proliferation
Cells were seeded into 96-well plates at a density of 2×103 cells/well with or without drugs for 72 h and the sensitivities to the drugs were determined by using a modified MTS assay with CellTiter 96 Aqueous One Solution Reagent (Promega), as previously described (32). The anti-proliferative effects are shown as IC50, which is the concentration of the drug required to inhibit cell proliferation by 50%.
Western blot analysis
Resistant cells were cultured in 6 cm dishes for 24 h, and then treated with dimethyl sulfoxide (DMSO) as control, 100 nM docetaxel, and 100 nM docetaxel combined with 0.25 µg/ml elacridar for 48 h. The total cell lysates were extracted with lysis buffer, a mixture of RIPA buffer, phosphatase inhibitor cocktails 2 and 3 (Sigma-Aldrich), and Complete Mini (Roche, Basel, Switzerland). The primary antibodies were as follows: Monoclonal anti-PARP (46D11) (Catalog #9532), anti-E-cadherin (24E10) (#3195), anti-vimentin (D21H3) (#5741), and anti-ABCB1/MDR1 (E1Y7B) (#13342) (Cell Signaling Technology, Beverly, MA, USA). Monoclonal anti-actin antibody (#MAB1501R), used as an equal loading control, was purchased from Merck Millipore (Billerica, MA, USA). The following secondary antibodies were used: Goat anti-rabbit (#sc-2030) or anti-mouse (#sc-2031) immunoglobulin G (IgG)-conjugated horseradish peroxidase (Santa Cruz Biotechnology, Dallas, TX). To detect specific signals, the membranes were examined using the ECL Prime Western Blotting Detection System (GE Healthcare, Amersham, UK) and LAS-3000 (Fujifilm, Tokyo, Japan).
mRNA and siRNA expression analysis by qRT-PCR
Total RNA was extracted by using RNeasy Mini Kit (Qiagen, Valencia, CA, USA) and transcribed into cDNA using High-Capacity cDNA Reverse Transcription Kits (Applied Biosystems, Foster City, CA, USA) according to the manufacturer's instructions. mRNA expression analysis by quantitative reverse transcription PCR (qRT-PCR) was conducted on cDNA by using TaqMan probes and the TaqMan Universal PCR Master Mix (Applied Biosystems). PCR amplification was conducted on an ABI StepOne Real-Time PCR Instrument (Applied Biosystems) and gene expression was calculated using the comparative CT method. Three replicates per sample were assayed for each gene. To quantify the relative changes in gene expression, the (−ΔΔCQ) method was used and reactions were normalized to endogenous control gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) expression levels in mRNA expression analysis.
Transient transfection
Cells were reverse-transfected with 10 nM small interfering RNAs (siRNAs) mixed with Lipofectamine RNAiMAX (Invitrogen, Carlsbad, CA, USA). The validated siRNAs specific for ABCB1 (si-ABCB1#1, 5-CGA UAC AUG GUU UUC CGA U-3; si-ABCB1#2, 5-GGC UUG CUG UAA UUA CCC A-3) and nonspecific scrambled siRNAs (si-Scr) were purchased from Applied Biosystems. Twenty-four hours after reverse-transfection, the transfected-cells could be used for further experiment according to the manufacturer's protocol. The indicated drugs were added, and cell viability was measured by using the MTS assay after an additional 72 h or made the RNA and protein extraction for real-time PCR and western blot detection.
Results
All established cell lines showed resistance not only to docetaxel but also to EGFR-tyrosine kinase inhibitors
In order to understand the mechanisms of resistance to docetaxel in NSCLC, we first established docetaxel-resistant cell lines. Three cell lines (H1299, HCC827, and HCC4006) were exposed to docetaxel using the stepwise escalation method. Then, the resistance of the established cell lines to docetaxel was confirmed by an MTS assay (Table I). The IC50 values against docetaxel of all the established docetaxel-resistant cells were significantly higher compared with their corresponding parental cells. Moreover, the results were confirmed by western blot analysis for expression of the apoptosis marker, cleaved PARP, and showed that docetaxel treatment effectively induced apoptosis in parental cells, but not in resistant cells (Fig. 1).
Table I.
IC50 values against each drug of parental and docetaxel resistant cell lines.
| Cell Line | EGFR-mutation | Docetaxel (nM) | Gefitinib (µM) | Afatinib (µM) | AZD9291 (µM) |
|---|---|---|---|---|---|
| H1299 | Wild-type | 3.2 | >10 | 4.3 | 5.8 |
| H1299-DR | Wild-type | 272.1 | >10 | 5.7 | 5.5 |
| HCC827 | Exon 19 del (E746-A750) | 0.3 | 0.0005 | <0.001 | <0.001 |
| HCC827-DR | Exon 19 del (E746-A750) | >1000 | 5.0 | 1.6 | 2.5 |
| HCC4006 | Exon 19 del (L747–A750, P ins) | 0.02 | 0.031 | 0.002 | 0.025 |
| HCC4006-DR | Exon 19 del (L747–A750, P ins) | >1000 | 8.3 | 2.7 | 3.1 |
Figure 1.
Docetaxel treatment effectively induced apoptosis in parental cells, but not in docetaxel-resistant cells. Cells were treated with DMSO or 100 nM of docetaxel for 48 h. Then, lysates were subjected to western blot analysis.
Next, we explored sensitivity to EGFR-tyrosine kinase inhibitors (TKIs) (gefitinib, afatinib, and AZD9291) both in parental and resistant cell lines. As shown in Table I, the docetaxel-resistant cells with the EGFR mutation showed greater resistance to EGFR-TKIs than their parental cells. The wild-type EGFR cell lines, H1299 parental and H1299-DR, were both insensitive to EGFR-TKI treatment. Although we also treated cells with chemotherapeutic agents such as cisplatin and pemetrexed, no obvious differences between the parental and resistant cells were observed (data not shown).
All resistant cells overexpressed ABCB1, and HCC827-DR and HCC4006-DR exhibited a CSC-like marker and EMT features
Overexpression of ABCB1 is known to be the most common mechanism of cellular resistance to cytotoxic agents. Thus, to explore the mechanism of resistance to docetaxel, expression of ABCB1, ALDH1, and EMT-related markers was examined by real-time PCR and Western blotting in both parental and resistant cell lines. As shown in Fig. 2A, docetaxel-resistant cells highly expressed ABCB1. Moreover, HCC827-DR overexpressed ALDH1, showing a CSC-like marker. To further investigate whether the acquisition of docetaxel resistance induced specific molecular changes consistent with EMT, western blot analysis was performed. As shown in Fig. 2B, lower expression of E-cadherin (epithelial marker) at the protein level was observed in HCC4006-DR cells compared to parental HCC4006 cells.
Figure 2.
Docetaxel-resistant cells highly expressed ABCB1. (A) The gene expression of ABCB1 and ALDH1 were determined by real-time PCR in both parental and docetaxel-resistant cells. (B) The protein expression of E-cadherin and vimentin were detected by western blot analysis.
Elacridar, a third-generation ABCB1 inhibitor, overcomes docetaxel resistance, but not to EGFR-TKIs resistance
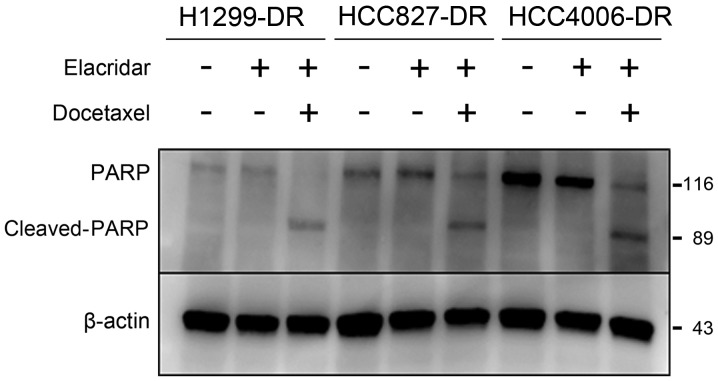
Having identified that ABCB1 is overexpressed in docetaxel-resistant cells, we examined whether suppression of ABCB1 leads to improved docetaxel resistance. We chose elacridar to inhibit the drug pump function of ABCB1 by competitively combining with ABCB1. Elacridar markedly recovered sensitivity to docetaxel, but not to gefitinib (Table II). The IC50 value of H1299-DR was 9.4 nM at 0.25 µg/ml of elacridar, which was higher than that of parental cells (3.2 nM); thus elacridar only partially recovered the sensitivity to docetaxel. These cells may contain a resistance mechanism other than ABCB1. The expression of the apoptosis marker cleaved-PARP increased in the resistant cells only when both docetaxel and elacridar were administered in combination (Fig. 3). These results suggest that elacridar restored the sensitivity to docetaxel.
Table II.
IC50 values against docetaxel or gefitinib of docetaxel-resistant cell lines, with or without elacridar treatment.
| Docetaxel (nM) | Gefitinib (µM) | |||
|---|---|---|---|---|
| Elacridar (0.25 µg/ml) | − | + | − | + |
| H1299DR | 272.1 | 9.4 | >10 | >10 |
| HCC827DR | >1000 | 21.9 | 5.0 | 1.6 |
| HCC4006DR | >1000 | 0.3 | 8.3 | 4.7 |
Figure 3.
Elacridar recovered sensitivity to docetaxel. Docetaxel resistant cells were treated with DMSO, elacridar (0.25 µg/ml) only, or elacridar (0.25 µg/ml) combined with 100 nM of docetaxel for 48 h, and lysates were subjected to western blot analysis with the indicated antibodies.
siRNA-mediated knockdown of ABCB1 sensitized resistant cells to docetaxel
To further investigate the function of ABCB1 in the docetaxel resistance mechanism, siRNA-mediated suppression of ABCB1 expression was examined in the docetaxel resistant cell lines. The efficacy of ABCB1 knockdown was confirmed by real-time PCR and Western blot analysis (Fig. 4A and B); siRNA-mediated knockdown of ABCB1 partially sensitized resistant cells to docetaxel (Fig. 4C and Table III). In contrast, ABCB1 depletion did not restore gefitinib sensitivity in docetaxel-resistant cells (Table III).
Figure 4.
RNAi-mediated knockdown of ABCB1 modulates the drug sensitivity to docetaxel. (A) The siRNA-mediated suppression of ABCB1 expressions were confirmed by real-time PCR. (B) Seventy-two h after treatment by two different siRNAs against ABCB1 (si-ABCB1#1 or si-ABCB1#2) or scrambled control si-RNA (si-Scr), total cell lysates were harvested and examined by western blot analysis. (C) Cell viabilities against docetaxel with or without siRNA mediated ABCB1 suppression were determined by MTS assay.
Table III.
IC50 values against docetaxel or gefitinib of docetaxel resistant cell lines, with or without si-RNA mediated knockdown of ABCB1.
| Docetaxel (nM) | Gefitinib (µM) | |||||
|---|---|---|---|---|---|---|
| si-Scr | si-ABCB1#1 | si-ABCB1#2 | si-Scr | si-ABCB1#1 | si-ABCB1#2 | |
| H1299-DR | 590.0 | 25.8 | 12.6 | >10 | >10 | >10 |
| HCC827-DR | 679.4 | 301.4 | 382.2 | 1.2 | 0.67 | 0.43 |
| HCC4006-DR | 995.8 | 290.2 | 328.6 | 5.7 | 6.0 | 4.2 |
Discussion
In the present study, we established docetaxel-resistant NSCLC cell lines and analyzed their resistance mechanisms in order to understand the biological mechanisms of chemo-resistance in NSCLC cells and to identify reversion opportunities. Several mechanisms for resistance to taxanes have already been reported, such as MDR, CSC-like, and EMT-related marker overexpression (9,25,31,33). The main mechanisms involved in resistance to taxanes are expression of the MDR phenotype and the alteration of their cellular target, namely the tubulin/microtubule system (11,34). Several putative mechanisms of resistance, including alteration of the signaling pathways, altered regulation of the cell cycle, and altered control of apoptosis and cell death signals, have also been described (10,12). Our study confirmed that ABCB1, an MDR molecule, was overexpressed in experimentally established cells. Overexpression of ABCB1 was one of the causes of docetaxel resistance, confirmed by using elacridar and siRNA against ABCB1. However, restoration of sensitivity to docetaxel by ABCB1 siRNA was not perfect. This may be due to incomplete suppression of ABCB1 gene expression in our system, and we therefore inferred that overexpression of ABCB1 is not the only mechanism of docetaxel and multidrug resistance.
We tested EMT- and CSC-related markers in the resistant cell lines because we have recently reported that a gefitinib-resistant cell line was also resistant to docetaxel and exhibited both EMT features and CSC properties (32). E-cadherin and vimentin are EMT-related markers (25), and the expression levels of E-cadherin were slightly lower in HCC4006-DR than in parental cells. The functional implications of EMT include enhanced mobility, invasion, and resistance to apoptotic stimuli (35,36). Moreover, tumor cells can acquire CSC features, secondary tumor initiating, and chemo-resistance properties through EMT (37–39). CSCs, which generate tumors with self-renewal and differentiation abilities, are believed to be highly resistant to conventional chemotherapies owing to various crucial features, including high expression of ABC transporter proteins (40) and ALDH activity (41). HCC827-DR cells overexpressed ALDH1, a CSC-like feature. ABC transporters, including ABCB1, have also been reported to be implicated in promoting CSC-like properties (42).
Notably, EGFR-mutant docetaxel-resistant cells showed cross-resistance to EGFR-TKIs. To our knowledge, we found first that docetaxel treatment led to EGFR-TKIs resistance in EGFR-mutant NSCLC cells. However, ABCB1 expression was not related to sensitivity to EGFR-TKI because elacridar or siRNA against ABCB1 had no effect on sensitivity to EGFR-TKI. We were unable to clarify the mechanism of resistance to EGFR-TKI because well-known mechanisms, such as T790M mutation or MET amplification, were not observed in these cell lines. Although HCC4006-DR cells did not show increased expression of other CSC-like markers, we recently reported a similar observation, that EGFR-TKI resistant cells exhibiting both EMT features and CSC properties with overexpression of ABCB1 were resistant to anti-microtuble agents (32). Mizuuchi et al (43) recently reported similar findings that acquired EGFR-TKI resistance cells became more resistant to anti-microtuble agents. They suggested that this ‘collateral’ cross-resistance to EGFR-TKIs and anti-microtubule agents resulted from two distinct mechanisms, both of which were thought to be a cause of or to result from EMT. Further investigation is required to clarify detailed mechanisms causing this cross-resistance.
Based on our results, elacridar showed a strong effect on docetaxel-resistant NSCLC cells. Elacridar, a third-generation inhibitor of ABCB1, is a non-competitive inhibitor that functions by changing the active allosteric site of ABCB1 (19,44). It shows a relatively minor influence on other members of the ABC family and on the pharmacokinetics of the anti-tumor drugs in clinical use (45).
In conclusion, we have demonstrated that docetaxel-resistant NSCLC cells showed multi-resistance to docetaxel as well as EGFR-TKIs. Molecular analyses showed that all of these resistant cell lines highly expressed ABCB1, and ABCB1 played an important role in acquired resistance to docetaxel in lung cancer. Furthermore, HCC827-DR and HCC4006-DR cells exhibited a CSC-like marker and EMT features, respectively. Thus, elacridar could overcome resistance to docetaxel, suggesting that development of ABCB1 inhibitors show great promise for clinical use to overcome multi-drug resistance.
Acknowledgements
We thank Ms. Fumiko Isobe (Department of Thoracic, Breast and Endocrinological Surgery, Okayama University Graduate School of Medicine, Dentistry and Pharmaceutical Sciences, Okayama, Japan) for her technical assistance.
Glossary
Abbreviations
- NSCLC
non-small cell lung cancer
- MDR
multidrug resistance
- ABC
ATP-binding cassette
- EMT
epithelial-to-mesenchymal transition
- EGFR
epidermal growth factor receptor
- CSC
Cancer stem cell
- IC50
50% inhibitory concentrations
- DR
docetaxel-resistant
- TKI
tyrosine kinase inhibitor
- GAPDH
gene glyceraldehyde-3-phosphate dehydrogenase
- Scr
scrambled
References
- 1.Wu L, Chang W, Zhao J, Yu Y, Tan X, Su T, Zhao L, Huang S, Liu S, Cao G. Development of autoantibody signatures as novel diagnostic biomarkers of non-small cell lung cancer. Clin Cancer Res. 2010;16:3760–3768. doi: 10.1158/1078-0432.CCR-10-0193. [DOI] [PubMed] [Google Scholar]
- 2.Rigas JR, Kelly K. Current treatment paradigms for locally advanced non-small cell lung cancer. J Thorac Oncol. 2007;2:S77–S85. doi: 10.1097/01.JTO.0000269735.21209.bc. (Suppl 2) [DOI] [PubMed] [Google Scholar]
- 3.Choy H, Pyo H, Kim JS, MacRae R. Role of taxanes in the combined modality treatment of patients with locally advanced non-small cell lung cancer. Expert Opin Pharmacother. 2001;2:963–974. doi: 10.1517/14656566.2.6.963. [DOI] [PubMed] [Google Scholar]
- 4.Wakelee H, Ramalingam S, Belani CP. Docetaxel in advanced non-small cell lung cancer. Expert Rev Anticancer Ther. 2005;5:13–24. doi: 10.1586/14737140.5.1.13. [DOI] [PubMed] [Google Scholar]
- 5.Stinchcombe TE. Recent advances in the treatment of non-small cell and small cell lung cancer. F1000Prime Rep. 2014;6:117. doi: 10.12703/P6-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garon EB, Ciuleanu TE, Arrieta O, Prabhash K, Syrigos KN, Goksel T, Park K, Gorbunova V, Kowalyszyn RD, Pikiel J, et al. Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (REVEL): A multicentre, double-blind, randomised phase 3 trial. Lancet. 2014;384:665–673. doi: 10.1016/S0140-6736(14)60845-X. [DOI] [PubMed] [Google Scholar]
- 7.Yoh K, Hosomi Y, Kasahara K, Yamada K, Takahashi T, Yamamoto N, Nishio M, Ohe Y, Koue T, Nakamura T, et al. A randomized, double-blind, phase II study of ramucirumab plus docetaxel vs. placebo plus docetaxel in Japanese patients with stage IV non-small cell lung cancer after disease progression on platinum-based therapy. Lung Cancer. 2016;99:186–193. doi: 10.1016/j.lungcan.2016.07.019. [DOI] [PubMed] [Google Scholar]
- 8.Lage H. An overview of cancer multidrug resistance: A still unsolved problem. Cell Mol Life Sci. 2008;65:3145–3167. doi: 10.1007/s00018-008-8111-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Myer MS, Joone G, Chasen MR, van Rensburg CE. The chemosensitizing potential of GF120918 is independent of the magnitude of P-glycoprotein-mediated resistance to conventional chemotherapeutic agents in a small cell lung cancer line. Oncol Rep. 1999;6:217–218. [PubMed] [Google Scholar]
- 10.Galletti E, Magnani M, Renzulli ML, Botta M. Paclitaxel and docetaxel resistance: Molecular mechanisms and development of new generation taxanes. ChemMedChem. 2007;2:920–942. doi: 10.1002/cmdc.200600308. [DOI] [PubMed] [Google Scholar]
- 11.Orr GA, Verdier-Pinard P, McDaid H, Horwitz SB. Mechanisms of Taxol resistance related to microtubules. Oncogene. 2003;22:7280–7295. doi: 10.1038/sj.onc.1206934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luqmani YA. Mechanisms of drug resistance in cancer chemotherapy. Med Princ Pract. 2005;14:S35–S48. doi: 10.1159/000086183. (Suppl 1) [DOI] [PubMed] [Google Scholar]
- 13.Gottesman MM, Ambudkar SV. Overview: ABC transporters and human disease. J Bioenerg Biomembr. 2001;33:453–458. doi: 10.1023/A:1012866803188. [DOI] [PubMed] [Google Scholar]
- 14.Sarkadi B, Homolya L, Szakács G, Váradi A. Human multidrug resistance ABCB and ABCG transporters: Participation in a chemoimmunity defense system. Physiol Rev. 2006;86:1179–1236. doi: 10.1152/physrev.00037.2005. [DOI] [PubMed] [Google Scholar]
- 15.Higgins CF. Multiple molecular mechanisms for multidrug resistance transporters. Nature. 2007;446:749–757. doi: 10.1038/nature05630. [DOI] [PubMed] [Google Scholar]
- 16.Sharom FJ. ABC multidrug transporters: Structure, function and role in chemoresistance. Pharmacogenomics. 2008;9:105–127. doi: 10.2217/14622416.9.1.105. [DOI] [PubMed] [Google Scholar]
- 17.Li Y, Yuan H, Yang K, Xu W, Tang W, Li X. The structure and functions of P-glycoprotein. Curr Med Chem. 2010;17:786–800. doi: 10.2174/092986710790514507. [DOI] [PubMed] [Google Scholar]
- 18.Szakács G, Váradi A, Ozvegy-Laczka C, Sarkadi B. The role of ABC transporters in drug absorption, distribution, metabolism, excretion and toxicity (ADME-Tox) Drug Discov Today. 2008;13:379–393. doi: 10.1016/j.drudis.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 19.Palmeira A, Sousa E, Vasconcelos MH, Pinto MM. Three decades of P-gp inhibitors: Skimming through several generations and scaffolds. Curr Med Chem. 2012;19:1946–2025. doi: 10.2174/092986712800167392. [DOI] [PubMed] [Google Scholar]
- 20.Wu CP, Calcagno AM, Ambudkar SV. Reversal of ABC drug transporter-mediated multidrug resistance in cancer cells: Evaluation of current strategies. Curr Mol Pharmacol. 2008;1:93–105. doi: 10.2174/1874467210801020093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hubensack M, Müller C, Höcherl P, Fellner S, Spruss T, Bernhardt G, Buschauer A. Effect of the ABCB1 modulators elacridar and tariquidar on the distribution of paclitaxel in nude mice. J Cancer Res Clin Oncol. 2008;134:597–607. doi: 10.1007/s00432-007-0323-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kajiyama H, Shibata K, Terauchi M, Yamashita M, Ino K, Nawa A, Kikkawa F. Chemoresistance to paclitaxel induces epithelial-mesenchymal transition and enhances metastatic potential for epithelial ovarian carcinoma cells. Int J Oncol. 2007;31:277–283. [PubMed] [Google Scholar]
- 23.Min C, Eddy SF, Sherr DH, Sonenshein GE. NF-kappaB and epithelial to mesenchymal transition of cancer. J Cell Biochem. 2008;104:733–744. doi: 10.1002/jcb.21695. [DOI] [PubMed] [Google Scholar]
- 24.Sabbah M, Emami S, Redeuilh G, Julien S, Prévost G, Zimber A, Ouelaa R, Bracke M, De Wever O, Gespach C. Molecular signature and therapeutic perspective of the epithelial-to-mesenchymal transitions in epithelial cancers. Drug Resist Updat. 2008;11:123–151. doi: 10.1016/j.drup.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 25.Ren J, Chen Y, Song H, Chen L, Wang R. Inhibition of ZEB1 reverses EMT and chemoresistance in docetaxel-resistant human lung adenocarcinoma cell line. J Cell Biochem. 2013;114:1395–1403. doi: 10.1002/jcb.24481. [DOI] [PubMed] [Google Scholar]
- 26.Wu C, Alman BA. Side population cells in human cancers. Cancer Lett. 2008;268:1–9. doi: 10.1016/j.canlet.2008.03.048. [DOI] [PubMed] [Google Scholar]
- 27.Sugano T, Seike M, Noro R, Soeno C, Chiba M, Zou F, Nakamichi S, Nishijima N, Matsumoto M, Miyanaga A, et al. Inhibition of ABCB1 overcomes cancer stem cell-like properties and acquired resistance to MET inhibitors in non-small cell lung cancer. Mol Cancer Ther. 2015;14:2433–2440. doi: 10.1158/1535-7163.MCT-15-0050. [DOI] [PubMed] [Google Scholar]
- 28.O'Flaherty JD, Barr M, Fennell D, Richard D, Reynolds J, O'Leary J, O'Byrne K. The cancer stem-cell hypothesis: Its emerging role in lung cancer biology and its relevance for future therapy. J Thorac Oncol. 2012;7:1880–1890. doi: 10.1097/JTO.0b013e31826bfbc6. [DOI] [PubMed] [Google Scholar]
- 29.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 30.Berns A. Stem cells for lung cancer? Cell. 2005;121:811–813. doi: 10.1016/j.cell.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 31.Jiang F, Qiu Q, Khanna A, Todd NW, Deepak J, Xing L, Wang H, Liu Z, Su Y, Stass SA, Katz RL. Aldehyde dehydrogenase 1 is a tumor stem cell-associated marker in lung cancer. Mol Cancer Res. 2009;7:330–338. doi: 10.1158/1541-7786.MCR-08-0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shien K, Toyooka S, Yamamoto H, Soh J, Jida M, Thu KL, Hashida S, Maki Y, Ichihara E, Asano H, et al. Acquired resistance to EGFR inhibitors is associated with a manifestation of stem cell-like properties in cancer cells. Cancer Res. 2013;73:3051–3061. doi: 10.1158/0008-5472.CAN-12-4136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang YJ, Zhang YK, Kathawala RJ, Chen ZS. Repositioning of tyrosine kinase inhibitors as antagonists of ATP-binding cassette transporters in anticancer drug resistance. Cancers (Basel) 2014;6:1925–1952. doi: 10.3390/cancers6041925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krishna R, Mayer LD. Multidrug resistance (MDR) in cancer. Mechanisms, reversal using modulators of MDR and the role of MDR modulators in influencing the pharmacokinetics of anticancer drugs. Eur J Pharm Sci. 2000;11:265–283. doi: 10.1016/S0928-0987(00)00114-7. [DOI] [PubMed] [Google Scholar]
- 35.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scheel C, Weinberg RA. Phenotypic plasticity and epithelial-mesenchymal transitions in cancer and normal stem cells? Int J Cancer. 2011;129:2310–2314. doi: 10.1002/ijc.26311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hennessy BT, Gonzalez-Angulo AM, Stemke-Hale K, Gilcrease MZ, Krishnamurthy S, Lee JS, Fridlyand J, Sahin A, Agarwal R, Joy C, et al. Characterization of a naturally occurring breast cancer subset enriched in epithelial-to-mesenchymal transition and stem cell characteristics. Cancer Res. 2009;69:4116–4124. doi: 10.1158/0008-5472.CAN-08-3441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gal A, Sjöblom T, Fedorova L, Imreh S, Beug H, Moustakas A. Sustained TGF beta exposure suppresses Smad and non-Smad signalling in mammary epithelial cells, leading to EMT and inhibition of growth arrest and apoptosis. Oncogene. 2008;27:1218–1230. doi: 10.1038/sj.onc.1210741. [DOI] [PubMed] [Google Scholar]
- 39.Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dean M. ABC transporters, drug resistance, and cancer stem cells. J Mammary Gland Biol Neoplasia. 2009;14:3–9. doi: 10.1007/s10911-009-9109-9. [DOI] [PubMed] [Google Scholar]
- 41.Resetkova E, Reis-Filho JS, Jain RK, Mehta R, Thorat MA, Nakshatri H, Badve S. Prognostic impact of ALDH1 in breast cancer: A story of stem cells and tumor microenvironment. Breast Cancer Res Treat. 2010;123:97–108. doi: 10.1007/s10549-009-0619-3. [DOI] [PubMed] [Google Scholar]
- 42.Holohan C, Van Schaeybroeck S, Longley DB, Johnston PG. Cancer drug resistance: An evolving paradigm. Nat Rev Cancer. 2013;13:714–726. doi: 10.1038/nrc3599. [DOI] [PubMed] [Google Scholar]
- 43.Mizuuchi H, Suda K, Sato K, Tomida S, Fujita Y, Kobayashi Y, Maehara Y, Sekido Y, Nishio K, Mitsudomi T. Collateral chemoresistance to anti-microtubule agents in a lung cancer cell line with acquired resistance to erlotinib. PLoS One. 2015;10:e0123901. doi: 10.1371/journal.pone.0123901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goldman B. Multidrug resistance: Can new drugs help chemotherapy score against cancer? J Natl Cancer Inst. 2003;95:255–257. doi: 10.1093/jnci/95.4.255. [DOI] [PubMed] [Google Scholar]
- 45.Mayur YC, Peters GJ, Prasad VV, Lemo C, Sathish NK. Design of new drug molecules to be used in reversing multidrug resistance in cancer cells. Curr Cancer Drug Targets. 2009;9:298–306. doi: 10.2174/156800909788166619. [DOI] [PubMed] [Google Scholar]