Abstract
Daily HIV preexposure prophylaxis (PrEP) is an effective form of HIV protection that remains unknown and inaccessible for many people in the United States despite receiving federal approval over five years ago.
PrEP is supported by the public health community, but forgoing condoms while taking PrEP has proven controversial; this controversy may be contributing to the lag in PrEP uptake. We argue that limiting PrEP access based on anticipated or actual sexual behavior contradicts the goals of public health research and practice and is not scientifically justified.
As evidence for the effectiveness of novel forms of biomedical HIV protection emerges, public health professionals need to accept new definitions of “protected sex” and ensure that their personal values do not override empirical evidence when determining public health priorities.
Mounting evidence for the effectiveness of oral HIV preexposure prophylaxis (PrEP)1 has generated broad support for its implementation within public health discourse. However, the use of PrEP is more controversial as a sole form of sexual protection, without concomitant condom use. Some scholars2 and health practitioners3 have expressed concerns about the health implications of sexual risk compensation, or increased sexual risk-taking behavior because of a perceived decrease in HIV susceptibility when taking PrEP. In an era in which PrEP awareness and access are already constrained, particularly among socially marginalized groups who stand to benefit the most,4 this value-driven reluctance to provide and promote PrEP may have dangerous consequences. In this commentary, we question the merit of limiting PrEP based on individuals’ anticipated or actual sexual behavior, considering the goals of public health research and practice and drawing on empirical evidence.
GOALS OF RESEARCH AND PRACTICE
The primary reason why denying PrEP to people who engage in condomless sex is unwarranted is that it is incompatible with the goals of public health research and clinical care. The American Public Health Association identifies evidence-based health promotion and disease prevention as fundamental objectives of the field of public health (https://www.apha.org/what-is-public-health). Public health researchers should seek to provide factual information to understand and improve the health of people and communities. Accordingly, public health researchers need to be responsible in reporting and interpreting scientific data pertaining to PrEP users’ sexual behavior and health and recognize the implications of research and commentary for public opinion, public policy, and public health.
Two recent scientific publications demonstrate how unfounded conclusions could diminish programmatic support and access to PrEP. One meta-analysis5 reported that men who have sex with men (MSM) using PrEP were 25 times more likely to acquire gonorrhea, 11 times more likely to acquire chlamydia, and 45 times more likely to acquire syphilis than MSM not using PrEP, concluding, “We must ensure that our efforts fighting one public health crisis do not lead to another.”5(p2252) However, the authors arrived at this conclusion by comparing two groups with differing sexually transmitted infection (STI) testing frequencies and differing study entry criteria: (1) a group of PrEP users drawn mainly from PrEP studies in which eligibility necessitated recently reported, high-risk behavior or STIs; and (2) a group of non-PrEP users largely consisting of MSM participating in other studies with less risky behavioral criteria. Although the authors acknowledged these issues as study limitations, this study was uncritically cited by the president of the AIDS Healthcare Foundation as scientific evidence supporting his position against PrEP “being widely promoted as a public health strategy” (https://www.aidshealth.org/#/archives/28203).
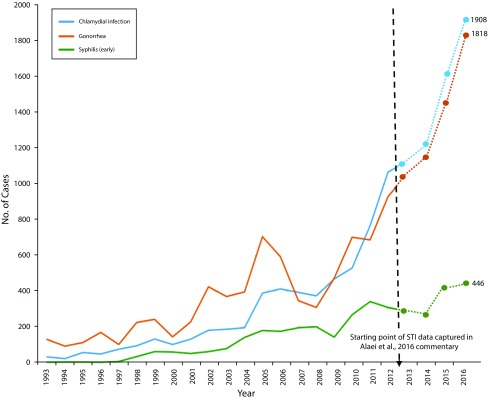
In a separate scholarly commentary,2 researchers similarly raised the specter that public health could be “inadvertently threatened” as a result of PrEP being “erroneously interpreted as a substitute for safe sex practices” and leading to “careless” condom use.2(p2755) The authors cited rising rates of STIs among MSM since the approval of PrEP in the United States but neglected to fully contextualize these trends as part of a preexisting pattern of rising STI rates that predated the approval of PrEP (Figure 1). A link to this publication was posted on the HIVguidelines.org Facebook page, a publicly visible online resource for clinicians and other service providers (https://www.facebook.com/hivguidelines/posts/10154466253547200). As illustrated by these examples, conclusions based on flawed or incomplete information could undermine the goals of public health. That is not to say that PrEP use is unrelated to condom choices and the spread of other STIs (see Supporting Evidence for further discussion); rather, our point is that it is imperative that reports of PrEP-related health outcomes be scientifically sound and appropriately contextualized.
FIGURE 1—
Number of Cases of Sexually Transmitted Infections (STIs) Among Men Who Have Sex With Men (MSM): King County, WA, 2016
Note. The rising STI rates reported as an extension of previous trends when earlier data are included. A 2016 commentary by Alaei et al.2 presented STI incidence data from 2013–2016 to implicate preexposure prophylaxis (PrEP) uptake in the rising rates of STIs among MSM in King County, WA. STI rates increased between 2013 and 2016, but rates have been rising since 1997, with a pattern of accelerated growth beginning in 2008, 4 years before the approval of PrEP by the United States Food and Drug Administration.
Source. Reproduced from the 2013 King County Sexually Transmitted Diseases Epidemiology Report, with subsequent extrapolated data points obtained from the 2015 and 2016 fourth quarter reports (http://www.kingcounty.gov/depts/health/communicable-diseases/hiv-std/patients/epidemiology.aspx).
With respect to clinical practice goals, the American Medical Association states, “The health and well-being of patients depends on a collaborative effort between patient and physician in a mutually respectful alliance” (https://www.ama-assn.org/sites/default/files/media-browser/code-of-medical-ethics-chapter-1.pdf), a patient-centered philosophy espoused by other health authorities. Both PrEP and condoms protect against HIV, and although simultaneous use offers the most comprehensive sexual health protection (because PrEP will not protect against other STIs and pregnancy), their separate use still offers independent HIV prevention benefits. That is, PrEP operates by interfering with viral replication once the HIV virus has entered the body, and although condoms may add an initial line of defense by acting as a barrier to viral entry, forgoing condoms does not invalidate the effectiveness of PrEP in preventing HIV transmission when used as prescribed. Health care providers should supply comprehensive information concerning the relative effectiveness of PrEP and condoms so that patients can make educated decisions about these aspects of their sexual health. PrEP users, like all people, may make conscious and informed choices to forgo condoms to attain desired benefits (e.g., pleasure, conception), avoid potential harms (e.g., adverse partner reactions), or both. Some individuals may deliberately choose to engage in condomless sex when using PrEP, even when fully cognizant of the risks involved. Others may have limited choice or power to control condom use (e.g., in an abusive relationship), and PrEP may offer a more feasible mode of HIV prevention. A client-centered approach to care requires respecting the autonomy, values, and life circumstances of patients in their health decision-making, and this principle ought to be applied to the delivery of PrEP-related services.
Providing PrEP offers secondary health benefits: accessing PrEP may prompt more regular STI screening and sexual health counseling than previously received and present a gateway to other forms of health care. Enhanced screening of PrEP users could lead to earlier detection of STIs, resulting in more immediate treatment and limitation of STI spread to partners. Education about other STIs, including asymptomatic infections, as part of PrEP-related care may also provide newfound motivation for condom use. In addition, PrEP may yield psychosocial benefits, such as reduced HIV-related anxiety.6
Concern about risk compensation is not a sound rationale for denying PrEP, especially because of the lack of evidence for associated adverse outcomes (reviewed in Supporting Evidence). Asking providers to make individual judgments about the likelihood of their patients engaging in risk compensation behavior would likely exacerbate inequalities in PrEP access, as suggested by previous research indicating that racism and heterosexism may manifest in such judgments.7,8 Even if a patient reports engaging in risk compensation behavior, this does not constitute a reason to withhold PrEP. Ultimately, the focus should be on PrEP adherence rather than risk compensation, because PrEP is protective if consistently used. In addition, penalizing the behavioral decisions of individual patients for the perceived good of the broader population2 is inconsistent with the professional obligation of providers to act in the best interest of their patients and violates expectations of patients when entering into a treatment relationship.
Public health ethics call for interventions to reduce morbidity or mortality, to be based on empirical science, and to appropriately balance burdens and benefits.9 As an HIV prevention intervention, PrEP achieves these ethical standards. Withholding PrEP on the grounds of actual or anticipated risk compensation prioritizes personal values that threaten public health ethics. As stated in a recent ethics commentary on PrEP, “the healthcare worker’s obligations are to the patient’s health, not to their own sexual morality.”10(p272) Providers do not typically withhold other preventive care because of anticipated risk compensation: contraception is not withheld because of anticipated condomless sex, and statins are not withheld for fear of dietary excesses. To withhold PrEP based on assumed or actual risk compensation would constitute unjustified exceptionalism.10,11
SUPPORTING EVIDENCE
Further supporting our stance that risk compensation is not a valid reason for restricting PrEP access is the lack of scientific evidence that either PrEP or PrEP-associated changes in risk behavior cause significant harm to public health. We offer several examples from the published literature to date; however, this is not intended to be a comprehensive review of PrEP-related behavior change or health outcomes and we refer the reader to another resource1 for further review.
Because of the recency of the United States Food and Drug Administration’s approval of PrEP (2012) and the slow uptake of PrEP in the ensuing years, there are limited aggregate data on “real-world” PrEP users’ sexual behavior and health while taking PrEP. Clinical trial results have collectively revealed minimal risk compensation among participants, with some trials even reporting increased condom use on average.1 However, in recent years, select open-label studies have documented more condomless sex over time (e.g., Victorian Pre-Exposure Prophylaxis [VicPrEP] project12) or relative to a deferred control group (e.g., Pre-exposure Prophylaxis to Prevent the Acquisition of HIV-1 Infection [PROUD] study13).
The limited data gathered outside of research settings suggest that most real-world PrEP users continue their previous pattern of condom use (or nonuse) after initiating PrEP.14,15 Many PrEP users are strong candidates for PrEP precisely because they do not use condoms to begin with, and they do not change their condom practices upon initiating PrEP. Nonetheless, subsets of PrEP users have reported both increased risk-taking and, to a lesser extent, decreased risk-taking.14,15 For example, at a health care center based in San Francisco, California (Strut), 53% of PrEP patients reported the same amount of condomless sex, 34% reported more, and 9% reported less seven months after initiating PrEP.15
Assessing population-level trends in PrEP-related risk compensation is challenging because PrEP was introduced in an era when the HIV prevention landscape was shifting in other ways. Condom use was already decreasing, and new information about the effectiveness of antiretroviral therapy in preventing transmission was emerging. Thus, there are multiple causes of population-level decreases in condom use, and it would be overly simplistic to attribute condom use trends to PrEP uptake alone.
Beyond the limited evidence for widespread risk compensation, there is a lack of evidence that risk compensation results in net harm. PrEP is highly effective in preventing HIV; to date, after tens of thousands of person-years of PrEP use, only three cases of breakthrough HIV infection have been documented among PrEP users with verified daily adherence.16–18 Condoms, like PrEP, are an imperfect form of protection, and many people do not or cannot use them correctly and consistently.19 Thus, the protective benefit of PrEP when taken as prescribed likely outweighs the HIV risk added by reducing condom use.20 This supposition is supported by a modeling study that estimated HIV protection among Black MSM based on empirically derived estimates of effectiveness rates for PrEP (based on Iniciativa Profilaxis Pre-Exposición [iPrEx] clinical trial) and condoms (based on two prospective clinical trials). The modeling study suggested that a man who has sex with men would derive as much or more HIV protection from PrEP as he would from condoms, even if he reduced or discontinued his condom use, so long as he adhered to his PrEP regimen.20
Further evidence that PrEP use might offset reduced condom use comes from two San Francisco−based health care settings. In one setting (Kaiser Permanente), 41% of the subset of PrEP patients surveyed six months after initiating PrEP reported reducing their condom use. However, none of the 657 PrEP patients, most of whom were MSM, seroconverted.14 Similarly, in the previously referenced second setting (Strut), despite 34% of the subset sampled reporting reduced condom use seven months after initiating PrEP, none of the nearly 1200 MSM PrEP patients seroconverted.15
Not only is there a lack of evidence that PrEP-related risk compensation increases HIV infection, but there is a theory to suggest that PrEP-related risk compensation could counterintuitively reduce the spread of HIV at the population level. A recent modeling study21 concluded that PrEP-related decreases in condom use among PrEP users with moderate-to-high adherence could amplify the population-level reduction in HIV incidence achieved with PrEP through its impact on PrEP uptake. That is, to the extent that PrEP use by some members of a sexual network resulted in greater condomless sex within the network, network members not already taking PrEP might become candidates for PrEP based on such behavior and start taking PrEP themselves, which could result in enhanced network-level HIV protection.21
With respect to STI incidence, the primary concern expressed by public health professionals is the potential for PrEP use to increase incident STIs indirectly through risk compensation, because PrEP users may feel more comfortable engaging in condomless sex. In longitudinal studies, changes in incident STIs have been inconsistent. Although increases in rates of some STIs have been documented over time with PrEP use (e.g., Kaiser Permanente cohort22), STI incidence has been stable or oscillated over time among other cohorts of PrEP users (e.g., HIV Prevention Trials Network [HPTN] 07323 and the Demo Project24).
Reported rates of STIs among PrEP users have been relatively high (e.g., 30% after six months of PrEP use among the Kaiser Permanente cohort14) and concurrent trends in STI diagnoses and PrEP uptake have been observed in some groups.2 However, it is essential that caution be exercised in interpreting such reports. Condomless sex is a behavioral criterion for PrEP eligibility, so it is unsurprising that the number of STIs diagnosed among PrEP users is high compared with the general population. STI incidence among current PrEP users could be even higher if these individuals did not access PrEP, because those who became HIV-infected without protection might have heightened biological susceptibility to other STIs.25 Other factors potentially contributing to a positive correlation between rates of STI diagnosis and PrEP uptake include PrEP being sought by people who would not otherwise present for STI screening and for whom STIs would otherwise go undiagnosed, and STI rates already rising because of other causes (Figure 1). STIs other than HIV are an important public health issue. However, most are curable or manageable, and PrEP-related medical care offers opportunities for early STI diagnosis, immediate STI treatment, and ongoing sexual health education.
CONCLUSIONS
Condoms have long been considered a mainstay and gold standard for HIV prevention among sexually active individuals, and sex without condoms, which at one time was synonymous with the term “unprotected sex,” has consequently been stigmatized as risky and irresponsible. Yet, as other methods of prevention emerge and demonstrate comparable or superior HIV protection to condoms, the public health community needs to disentangle personal values around condoms from public health priorities, allowing science to dictate practice and policy. PrEP is effective. Sex among PrEP users is therefore protected from HIV, whether or not condoms are used. It is backward-looking to allow the fear of condomless sex to hinder PrEP access.
The behavioral impact of PrEP outside of clinical settings merits continued study, and evidence is ever accumulating. However, science to date suggests that PrEP-related risk compensation occurs among a minority of PrEP users and is not associated with widespread adverse health consequences. By contrast, there is increasing evidence for the beneficial effect of PrEP on sexual health with respect to HIV prevention, and reported breakthrough infections have been rare. In addition, PrEP offers valuable secondary benefits.
PrEP has great potential for improving public health. Yet, that potential can only be realized if people have access to it. If risk compensation becomes a valid basis for limiting PrEP access, this invites inequality and undermines the transformative possibilities of PrEP. We wholeheartedly support continued research on attitudes, behaviors, and health outcomes associated with PrEP use and believe that STI counseling and management are integral to the delivery of PrEP. However, restricting access to PrEP based on reported condom intentions or (non)use is fundamentally misaligned with the mission of public health and lacks empirical justification.
ACKNOWLEDGMENTS
S. K. Calabrese and K. H. Mayer have received compensation for their efforts in developing and delivering medical education related to PrEP from the Connecticut Department of Public Health and District of Columbia Department of Health (S. K. C.) and New England AIDS Education and Training Center and UptoDate, Inc. (K. H. M.). K. H. Mayer has conducted research with unrestricted project support from Gilead Sciences, Merck, and ViiV Healthcare. S. K. Calabrese and K. Underhill were supported by Award Numbers K01-MH103080 and K01-MH093273, respectively, from the National Institute of Mental Health (NIMH). Additional support was provided through the District of Columbia Center for AIDS Research (P30-AI117870) and Harvard University Center for AIDS Research (P30-AI060354), both of which are funded by the National Institutes of Health (NIH).
Note. The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the NIMH or the NIH.
Footnotes
See also Landers and Kapadia, p. 1534.
REFERENCES
- 1.Fonner VA, Dalglish SL, Kennedy CE et al. Effectiveness and safety of oral HIV pre-exposure prophylaxis (PrEP) for all populations: a systematic review and meta-analysis. AIDS. 2016;30(12):1973–1983. doi: 10.1097/QAD.0000000000001145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alaei K, Paynter CA, Juan S-C, Alaei A. Using preexposure prophylaxis, losing condoms? Preexposure prophylaxis promotion may undermine safe sex. AIDS. 2016;30(18):2753–2756. doi: 10.1097/QAD.0000000000001262. [DOI] [PubMed] [Google Scholar]
- 3.Doblecki-Lewis S, Jones D. Community federally qualified health centers as homes for HIV preexposure prophylaxis: perspectives from South Florida. J Int Assoc Provid AIDS Care. 2016;15(6):522–528. doi: 10.1177/2325957416661422. [DOI] [PubMed] [Google Scholar]
- 4. Bush S, Magnuson D, Rawlings MK, Hawkins T, McCallister S, Mera Giler R. Racial characteristics of FTC/TDF for preexposure prophylaxis (PrEP) users in the US. Presented at: ASM Microbe; June 16–20, 2016; Boston, MA.
- 5.Kojima N, Davey DJ, Klausner JD. Pre-exposure prophylaxis for HIV infection and new sexually transmitted infections among men who have sex with men. AIDS. 2016;30(14):2251–2252. doi: 10.1097/QAD.0000000000001185. [DOI] [PubMed] [Google Scholar]
- 6.Koester K, Amico RK, Gilmore H et al. Risk, safety and sex among male PrEP users: Time for a new understanding. Cult Health Sex. 2017 doi: 10.1080/13691058.2017.1310927. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 7.Calabrese SK, Earnshaw VA, Underhill K, Hansen NB, Dovidio JF. The impact of patient race on clinical decisions related to prescribing HIV pre-exposure prophylaxis (PrEP): assumptions about sexual risk compensation and implications for access. AIDS Behav. 2014;18(2):226–240. doi: 10.1007/s10461-013-0675-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Calabrese SK, Mayer KH, Krakower DS, et al. Adverse implications of heterosexism for PrEP clinical decision-making and considerations regarding provider education. Presented at: 11th International Conference on HIV Treatment and Prevention Adherence; May 9–11, 2016; Fort Lauderdale, FL.
- 9.Kass NE. An ethics framework for public health. Am J Public Health. 2001;91(11):1776–1782. doi: 10.2105/ajph.91.11.1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Venter F, Allais L, Richter M. Exposure ethics: does HIV pre-exposure prophylaxis raise ethical problems for the health care provider and policy maker? Bioethics. 2014;28(6):269–274. doi: 10.1111/bioe.12021. [DOI] [PubMed] [Google Scholar]
- 11.Calabrese SK, Magnus M, Mayer KH et al. “Support your client at the space that they’re in”: pre-exposure prophylaxis (PrEP) prescribers’ perspectives on PrEP-related risk compensation. AIDS Patient Care STDS. 2017;31(4):196–204. doi: 10.1089/apc.2017.0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lal L, Audsley J, Murphy D et al. Medication adherence, condom use and sexually transmitted infections in Australian PrEP users: interim results from the Victorian PrEP Demonstration Project. AIDS. 2017 doi: 10.1097/QAD.0000000000001519. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 13.McCormack S, Dunn DT, Desai M. Pre-exposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): effectiveness results from the pilot phase of a pragmatic open-label randomised trial. Lancet. 2016;387(10013):53–60. doi: 10.1016/S0140-6736(15)00056-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Volk JE, Marcus JL, Phengrasamy T et al. No new HIV infections with increasing use of HIV preexposure prophylaxis in a clinical practice setting. Clin Infect Dis. 2015;61(10):1601–1603. doi: 10.1093/cid/civ778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gibson S, Crouch P-C, Hecht J, et al. Eliminating barriers to increase uptake of PrEP in a community-based clinic in San Francisco. Presented at: International AIDS Conference (AIDS 2016); July 18–22, 2016; Durban, South Africa.
- 16.Knox DC, Anderson PL, Harrigan PR, Tan DH. Multidrug-resistant HIV-1 infection despite preexposure prophylaxis. N Engl J Med. 2017;376(5):501–502. doi: 10.1056/NEJMc1611639. [DOI] [PubMed] [Google Scholar]
- 17. Grossman H, Anderson P, Grant R, Gandhi M, Mohri H, Markowitz M. Newly acquired HIV-1 infection with multi-drug resistant (MDR) HIV-1 in a patient on TDF/FTC-based PrEP. Presented at: HIV Research for Prevention (HIVR4P); October 17–19, 2016; Chicago, IL.
- 18. Hoornenborg E, de Bree GJ. Acute infection with a wild-type HIV-1 virus in PrEP user with high TDF levels. Presented at: Conference on Retroviruses and Opportunistic Infections; February 13–16, 2017; Seattle, WA.
- 19.Crosby R, Sanders S, Yarber WL, Graham CA. Condom-use errors and problems: a neglected aspect of studies assessing condom effectiveness. Am J Prev Med. 2003;24(4):367–370. doi: 10.1016/s0749-3797(03)00015-1. [DOI] [PubMed] [Google Scholar]
- 20.Smith DK, Herbst JH, Rose CE. Estimating HIV protective effects of method adherence with combinations of preexposure prophylaxis and condom use among African American men who have sex with men. Sex Transm Dis. 2015;42(2):88–92. doi: 10.1097/OLQ.0000000000000238. [DOI] [PubMed] [Google Scholar]
- 21.Jenness SM, Sharma A, Goodreau S et al. Individual HIV risk versus population impact of risk compensation after HIV preexposure prophylaxis initiation among men who have sex with men. PLoS One. 2017;12(1):e0169484. doi: 10.1371/journal.pone.0169484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marcus JL, Hurley LB, Hare CB et al. Preexposure prophylaxis for HIV prevention in a large integrated health care system: adherence, renal safety, and discontinuation. J Acquir Immune Defic Syndr. 2016;73(5):540–546. doi: 10.1097/QAI.0000000000001129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hightow-Weidman L, Magnus M, Beauchamp G, et al. Incidence and correlates of STIs among Black men who have sex with men participating in a US PrEP study. Presented at: International AIDS Conference (AIDS 2016); July 18–22, 2016; Durban, South Africa.
- 24.Liu AY, Cohen SE, Vittinghoff E et al. Preexposure prophylaxis for HIV infection integrated with municipal- and community-based sexual health services. JAMA Intern Med. 2016;176(1):75–84. doi: 10.1001/jamainternmed.2015.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mayer KH, Venkatesh KK. Interactions of HIV, other sexually transmitted diseases, and genital tract inflammation facilitating local pathogen transmission and acquisition. Am J Reprod Immunol. 2011;65(3):308–316. doi: 10.1111/j.1600-0897.2010.00942.x. [DOI] [PMC free article] [PubMed] [Google Scholar]