Abstract
BACKGROUND: Adrenocortical carcinoma (ACC) is a relatively rare, but aggressive type of cancer, which affects both children and adults. OBJECTIVE: Small non-coding RNAs (sncRNAs) play important roles and may serve as biomarkers for disease diagnosis, prognosis and treatment. METHODS: In our study, we sought to identify sncRNAs associated with malignant adrenal tumors. We obtained publicly available, small RNA sequencing data derived from 45 ACC and 30 benign tumors arising from the cortex of the adrenal gland, adrenocortical adenomas (ACA), and compared their sncRNA expression profiles. RESULTS: First, we remapped small RNA-seq to miRBase version 21 to check expression of miRNAs and found 147 miRNAs were aberrantly expressed (p<0.05) in ACC samples compared to ACA samples. Pathway analysis of differentially expressed miRNAs revealed p53 signaling pathways to be profoundly affected in ACC samples. Further examination for other types of small RNAs revealed 16 piRNAs, 48 lncRNAs and 19 sn/snoRNAs identified in ACC samples. Conclusions: Our data analysis suggests that publically available resources can be mined for biomarker development and improvements in-patient care; however, further research must be performed to correlate tumor grade with gene expression.
Keywords: Adrenocortical, ACC, data mining, genomics, miRNA, piRNA, sncRNA, snoRNA.
Introduction
Adrenocortical carcinoma (ACC) is a rare type of cancer which affects both children and adults at a rate of 0.72 persons per million (300-500 people diagnosed every year). It is estimated that only 1/3 of cases are confined to the adrenal gland at diagnosis, which substantially impacts prognosis 1. Most tumors are sporadic; however, they can be associated with Beckwith-Wiedemann, Li-Fraumeni and multiple endocrine neoplasia type I. They are more common in woman. Most ACC patients present with steroid hormone excess or obesity; however, approximately 20% present incidentally without associated symptoms 2. They can be difficult to differentiate from adenomas, particularly for tumors in the marginal category 3. ACCs are often large, 10-14 cm, tumors and may have areas of necrosis. Diagnostic work-up includes biochemical tests for steroid hormone levels and CT scan or MRI. The Weiss system is the most common pathological assessment used to determine risk of malignancy for an adrenal tumor. It includes high nuclear grade, mitotic rate >5/50 HPF, atypical mitotic figures, clear cells >25% tumor, diffuse architecture >1/3 tumor, necrosis, venous invasion, sinusoid invasion, and capsular invasion. Three or more features are highly suggestive of malignancy 1. There are two subtypes of ACC: oncocytic and myxoid. Treatments for ACC at stages I-III include surgical resection and mitotane therapy. Mitotane is the only FDA approved drug to treat metastatic adrenal cancer 2. ACC has an unfavorable prognosis with median survival for all types being 3.2 years and disease free survival being one year 1. Even with advances in genetics, radical surgical resection is still the only potential cure for ACC. Previous studies revealed significant alterations in IGF2 overexpression in both adenomas and ACC 3, 4, but nothing is known about sncRNA expression in ACCs.
Scientific progress in next generation sequencing (NGS) has helped profile the whole transcriptomic expression of diseased biological systems at the molecular level and increased our knowledge. Small RNAs are non-coding RNAs (sncRNA) consisting of 17-250 nucleotides in length that may have an essential role in disease development 5. A nearly comprehensive repertoire of small RNAs i.e. miRNAs (17-22 nucleotides) 6, piRNAs (26-33 nucleotides) 7, lncRNAs (more than 200 nucleotides) 8, and small nuclear/nucleolar RNAs (70-120 nucleotides) 9 has been collected and their roles in disease development analyzed via NGS. Understanding miRNAs role in transcriptional regulation has been a large focus over the past decade. Furthermore, piwi-interacting RNAs (piRNA), the largest class of the small non-coding RNA family, are involved in epigenetic and post transcriptional regulation, but their other functions are still unknown 10. Long non-coding RNAs (lncRNA) are a diverse class of RNAs believed to have a functional role; however, their biological relevance has not been established 11. The earliest and most highly conserved class of sncRNAs present in eukaryotes are snoRNAs, which carry the essential role of modification and processing of ribosomal RNAs (rRNA), transfer RNAs (tRNA) and small nuclear RNAs (snRNA) 12. C/D snoRNAs and box H/ACA snoRNAs, which primarily differ in sequence and structure, are two well-known classes of snoRNAs 12.
The present study focuses on in-depth analysis of small RNA sequencing data obtained from ACC patient samples compared to benign adrenocortical tumors as controls. We aimed to identify differential molecular signature expressions in whole small RNA groups. Furthermore, we sought to detect the most predominant patterns of non-coding RNA expression along with their corresponding molecular pathways that may be involved in the development of ACC and could serve as biomarkers for this type of cancer.
Materials and Methods
Samples and Data Assembly
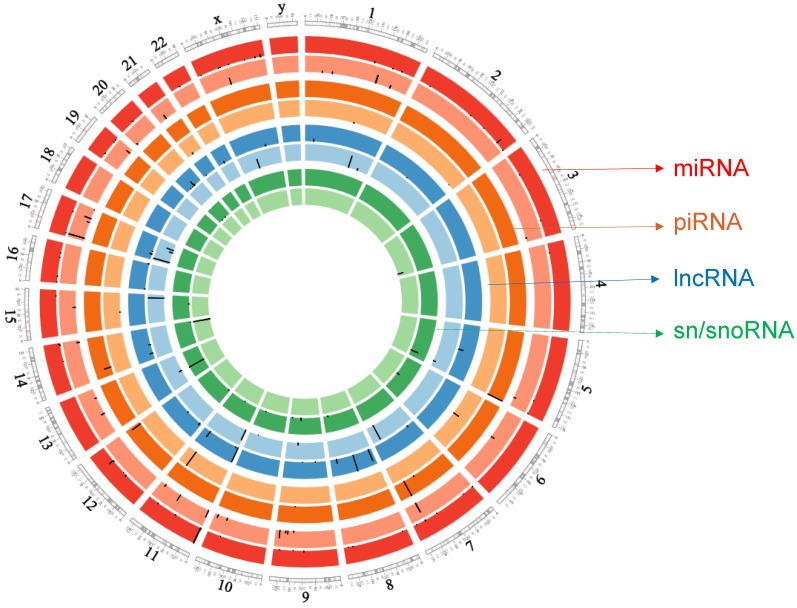
ACC small RNA sequencing raw sample datasets (Bioproject number: PRJNA213475; GEO: GSE49279) were downloaded from the NIH bioproject 13, which included 45 adrenocortical tumors and 30 adrenocortical adenoma (control) samples. Assie et al. reported clinical information about the patients 13. Small RNA libraries were constructed using multiplexed miRNAs from each RNA sample by 3' adapter ligation, 5' primer annealing, 5' adapter ligation, and reverse transcription along with PCR amplification 13, 14 followed by sequencing on an Illumina HiSeq 2000 sequencer. Raw files were downloaded as sequence raw archive (SRA) files and then converted to FASTQ, using the SRA toolkit version 2.5.7. PartekFlow® software, version 5.0 (Partek, Inc., St. Louis, MO, USA) to assemble data. Converted FASTQ files were uploaded to the PartekFlow® server and remapped to human genome hg19. Transcript abundances were determined and expression values were represented using reads per million (RPM) mapped reads which normalizes for sequencing depth. All small RNA with expression RPM values >1 in at least 10% of the samples were considered robustly expressed and used for further analysis with total reads kept at a minimum of 1000 in our data analysis to avoid less expressing sncRNAs. Expression matrices were aligned to clinicopatholgical features to compare miRNA, piRNA, lncRNA, and sn/snoRNA levels for association with ACC clinical samples. Statistical analyses were performed using the non-parametric Mann-Whitney U test followed by false discovery rate (FDR <0.05) correction with the Benjamin-Hochberg method, using a default p-value <0.05 for statistical significance 15. FDR is used to help control incorrect rejection of the null hypothesis. A Circos plot 16 was generated for differential expression of all small RNAs.
Assembly of miRNA, piRNA, lncRNA and snoRNA Annotations
Small non-coding RNA sequencing data was trimmed and aligned to the whole human genome hg19, and BWA-0.7.12 aligner (BWA-MEM) with a few modifications (mismatch penalty 2, gap open penalty 6, clipping penalty 4, and alignment score cutoff 15) for short read mapping. miRNAs were annotated from miRBase version 21 (http://www.mirbase.org/), which contains more than 1900 high confidence miRNAs 17. piRNA data was generated and annotated from piRBase (http://regulatoryrna.org/database/piRNA), which is manually curated with a focus on piRNA functional analysis 18. lncRNAs were quantified using reference annotation LNCipedia (http://www.lncipedia.org) version 3.1, downloaded from all coordinates relative to the hg19 reference genome 19. Gencode version 19 annotation file (www.gencodegenes.org), which provides comprehensive information on human small non-coding RNAs with specific regards to small nuclear and nucleolar RNAs, was used to annotate total small RNA (including miRNA, piRNA, snRNA, scRNA, snoRNA, piRNA, tRF3, tRF5, tRNA, and rRNA).
Biological Processes and Gene Network Visualization by MetaCore
Analysis of biological pathway interactions of small RNA expression was performed with MetaCore pathway analysis of differentially expressed genes (Thomson Reuters, New York, NY) 20 using p<0.05 (ACC vs control). Differentially regulated gene lists were used to build functional gene networks and generate disease biomarkers and GO terms (Data analyzed by Gene Arrays, Entity of Vedic Research, Inc., New York, USA).
Statistical Analysis
All experiments calculated FDR (<0.05) and p-values using paired student's t test, with p <0.05 considered statistically significant. Additionally, the Benjamin-Hochberg multiple testing adjustment method was applied to all small RNA sequencing studies and pathway analysis.
Results and Discussion
miRNA Expression in Adrenocortical Tumor Samples
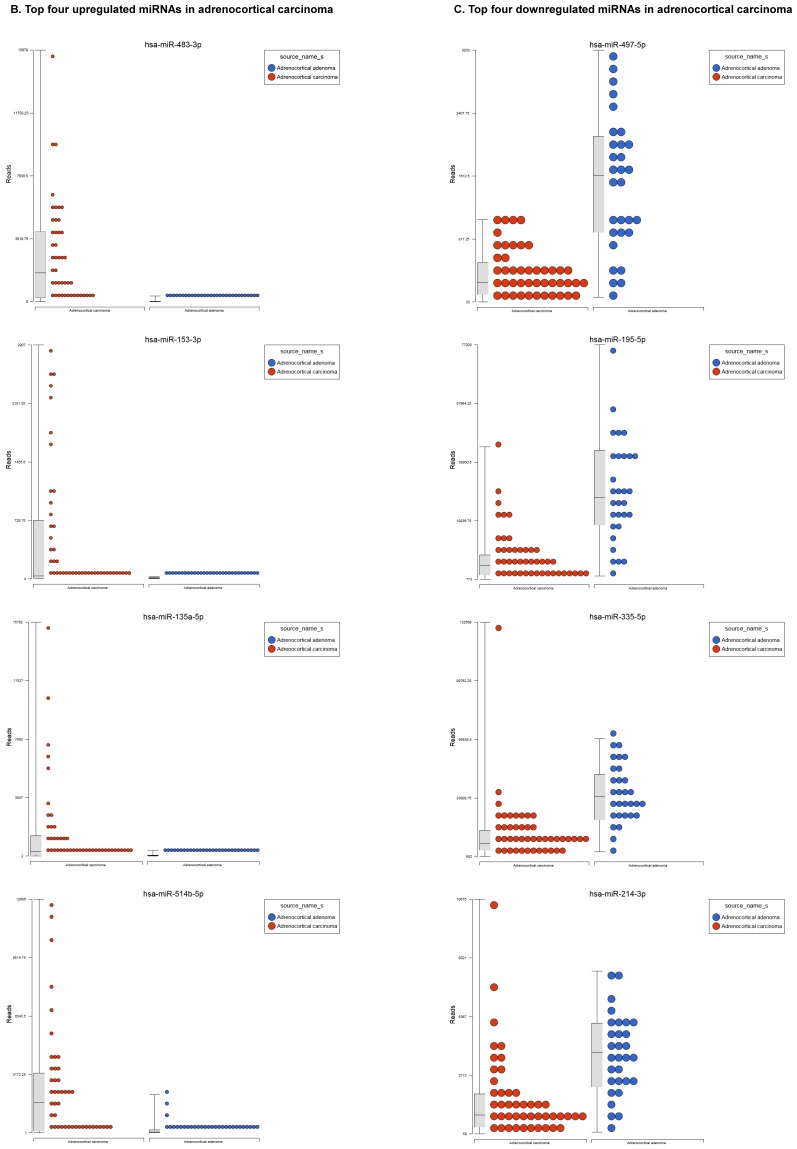
For miRNA data analysis, the raw data from patients with ACC and adjacent normal tissue samples (control) were downloaded from GEO series accession number GSE49279, converted to FASTQ files and uploaded to the PartekFlow server. 147 miRNAs enriched in ACC vs control samples (99 upregulated and 48 downregulated) were statistically significant (p<0.05). Using stringent statistical analysis (p<0.001) to create hierarchical clustering, we found 70 dysregulated miRs (p<0.001; Figure 1A) of which 50 were upregulated and 20 were downregulated. Figures 1B and 1C show the top four upregulated/downregulated miRNAs, respectively. Our miRNA findings were in line with that originally published by Assie et al., 13 i.e. the top upregulated microRNA was miR-483 (3p and 5p) in ACC (128 and 118 fold with p<2x10-9 and p<1x10-8 respectively,) along with miR-153 (41 fold, p<2x10-6), miR-135 (37 fold, p<2x10-8), miR-514 (16 fold, p<2x10-6), and miR-210 (16 fold, p<4x10-9) (Figure 1B). While miR-497 (4 fold, p<3x10-10), miR-195 (3.6 fold, p<1x10-9), miR-335 (3 fold, p<1x10-9), miR-214 (2.7 fold, p<1x10-6), and miR-199 (2.5 fold, multiple forms identified, p<9x10-6) were substantially downregulated in ACC samples (Figure 1C).
Figure 1.
Differential expression of miRNAs in ACC tissue samples: A heatmap view of differentially expressed miRNAs in adrenocortical carcinoma tissue samples (p<0.05; Figure 1A). Read counts of the top four statistically upregulated (Figure 1B) and downregulated (Figure 1C) miRNAs in ACC are shown by dot plots and box plots.
miR-483 was one of the highest expressed, with two forms including 3p and 5p (128 and 118 fold, Figure 1B), which is located within intron two of the insulin growth factor-2 (IGF2) locus 21, 22. miR-483-3p is reportedly over expressed in various cancers and its expression was shown to indicate poor prognosis in pancreatic ductal adenocarcinoma 22-24. Additionally, miR-483-5p was elevated in serum samples of multiple myeloma 25 and over expressed in adrenocortical tumors 26. miR-153 has oncogenic function in prostate cancer tissue, with knockdown of miR-153 shown to upregulate tumor suppressor genes 27. In the present study, miR-153 was drastically upregulated in ACC compared to ACA samples (41 fold, Figure 1B), which is consistent with previous studies of other cancers. miR-135 is over expressed in ACC clinical samples (37 fold), which was also reported in malignant melanoma 28. Over expression of miR-135 has also been shown to play a role in cellular proliferation, tumorogenicity and cell cycle progression in melanoma cells by directly targeting the FOXO tumor suppressor family genes 28. Previous studies also show miR-135 is involved in osteogenic differentiation by targeting bone formation related pathway signaling components 29. We have observed abrupt expression of miR-135 in our previous study on triple negative breast cancer 30. One of the top five non-coding RNAs in the current study, miR-514, a member of a cluster of miRNAs on chromosome X, is 16 fold overexpressed in ACC (Figure 1B). The previous studies indicate that miR-514 is overexpressed in malignant melanoma 31, however, it was downregulated in metastatic renal cell carcinoma 32. Another miRNA that is overexpressed in ACC is miR-210, which is in line with previous findings of its expression in plasma of patients with adrenocortical tumors 26. The expression of miR-497 is linked to a tumor suppressor gene and is the most downregulated microRNA in the ACC samples (4 fold). miR-497 plays an important role in IGF1-R expression and activation of PI3K/AKT signaling. Previous studies also noticed downregulation of miR-497 in various cancers 33-35. miR-195 is in the miR-15 family and is also downregulated in our samples as well as many other cancer types 35. Over expression of miR-195 targets FASN, BCL2 and HMGCR. It increases apoptosis in breast cancer cells 36 and inhibits cell proliferation in colorectal, hepatocellular and thyroid cancers 37-39. miR-335 is a tumor initiator but metastasis suppressor miRNA, located on chromosome 7q32.2. It is deleted in human breast cancer patients and also dysregulated in ovarian cancer reoccurrences 40; however, there are contradictory reports in the literature, where it was shown as a suppressor in some and initiator in others 41. In our data analysis of ACC, miR-335 is downregulated and acting as a tumor suppressor. miR-214 is a vertebrate specific microRNA, reportedly downregulated in cervical cancer 42 and upregulated in pancreatic cancer 43. miR-199 is one of the top dysregulated miRNAs with multiple transcripts observed in our ACC samples. This miRNA also co-exists with miR-214, which was also downregulated in our samples. Additionally, it is downregulated in hepatocellular cancer 44, 45, ovarian cancer 46 and triple negative breast cancer 47.
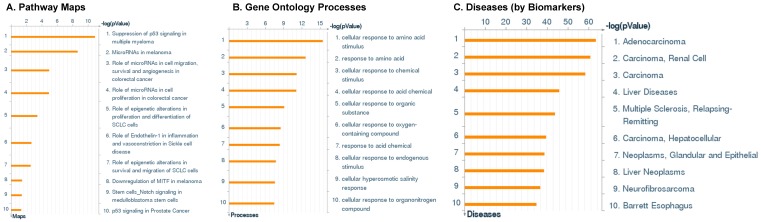
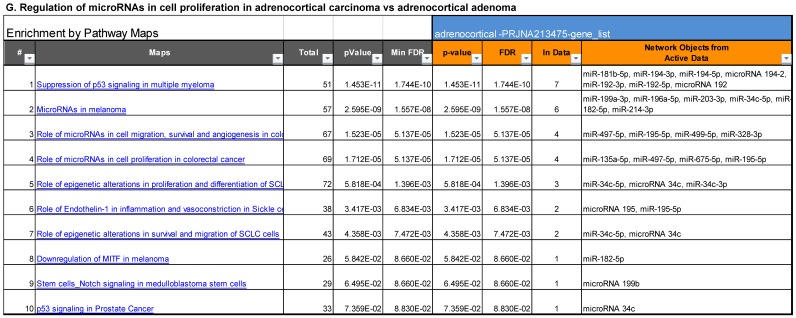
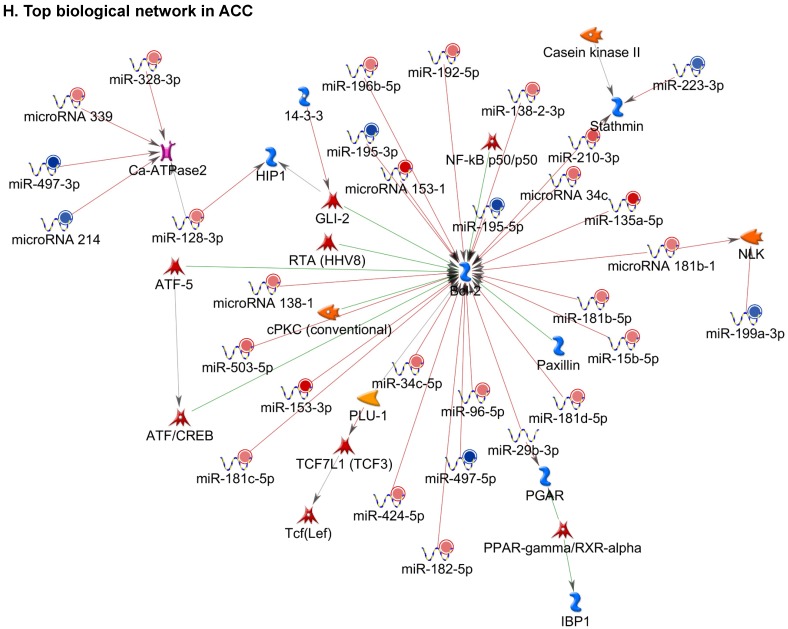
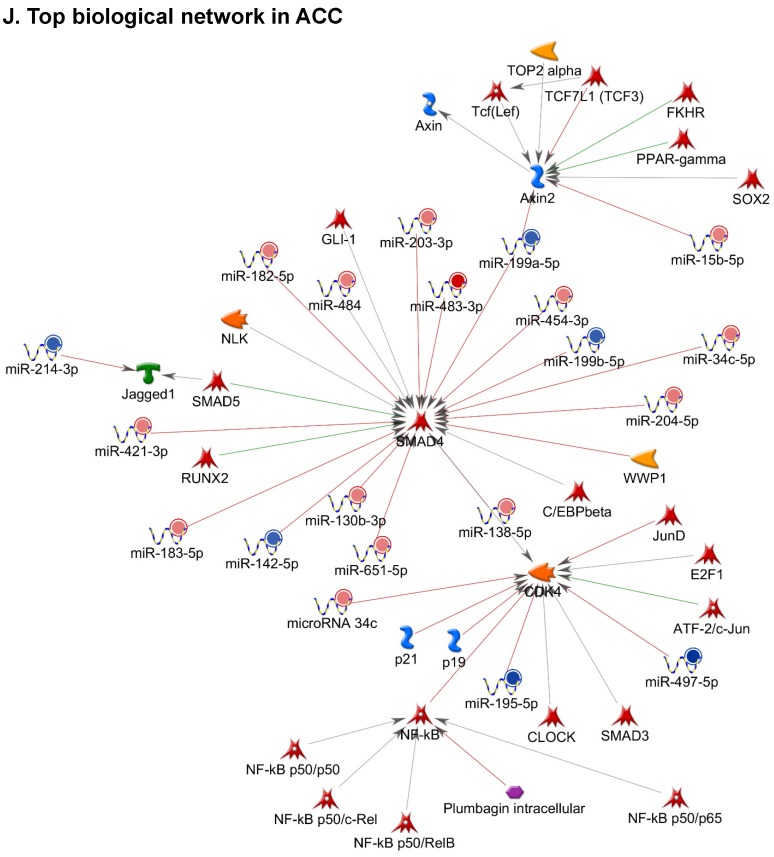
Following miRNA expression analysis using MetaCore software, we looked at various biological pathways to identity the molecular signature of the most affected and crucial signaling in ACC pathogenesis. Pathway analysis of differentially expressed biological pathways in ACC compared to ACA tissue samples clearly showed involvement of signaling molecules in various cancer types. p53 was the top in the signaling pathway and was suppressed (Figure 2A). Gene Ontology (GO) Biological Processes analysis revealed the response to amino acids was most significantly overrepresented (Figure 2B). The Disease Stages by Biomarkers analysis shows involvement of miRNAs in various cancer types (Figure 2C). Commonly enriched miRNAs in a pathway map showed the majority of the miRNAs were involved in various cancers (Figures 2D-G). Biological network analysis of involved miRNAs with the highest affected network processes (Bcl-2, VEGF-a, & SMAD4) in ACC were shown in Figures 2H-J, respectively.
Figure 2.
Enrichment analysis of microRNA for Pathway Maps, Gene Ontology, Disease by Biomarker, and Network processes in ACC. MetaCore pathway analysis of miRNA expression differences between ACC and ACA samples. Differentially expressed miRNA data were uploaded to MetaCore servers and the most significantly affected pathways were analyzed. A: Pathway Maps: Canonical pathway analysis showed most of the miRNAs that were affected involved the oncogenic signature. B: GO Biological Process: The most affected Biological Processes involved cellular responses to amino acid synthesis. C: Disease status: Significantly affected miRNAs in disease status included adeno and renal cell carcinoma. D-F: Enrichment analysis of top pathway: The top three signaling pathways involved in colorectal cancers, analyzed by MetaCore software. In up‑ward thermometers, red indicates up‑regulated signals and down‑ward (blue) indicates down‑regulated expression levels of genes. G: Top 10 pathways enriched along with network objectives from the active miRNA data. H-J: Biological network analysis: miRNA differentially expressed data were analyzed for the biological networks involved in ACC, top three regulated biological networks are shown in I, J and K. This is a variant of the shortest paths algorithm with main parameters of enrichment with enriched miRNAs prioritized based on the number of fragments of canonical pathways on the networks. Up‑regulated genes are marked with red circles and down‑regulated with blue circles. The 'checkerboard' color indicates mixed expression for the gene between files or between multiple tags for the same gene.
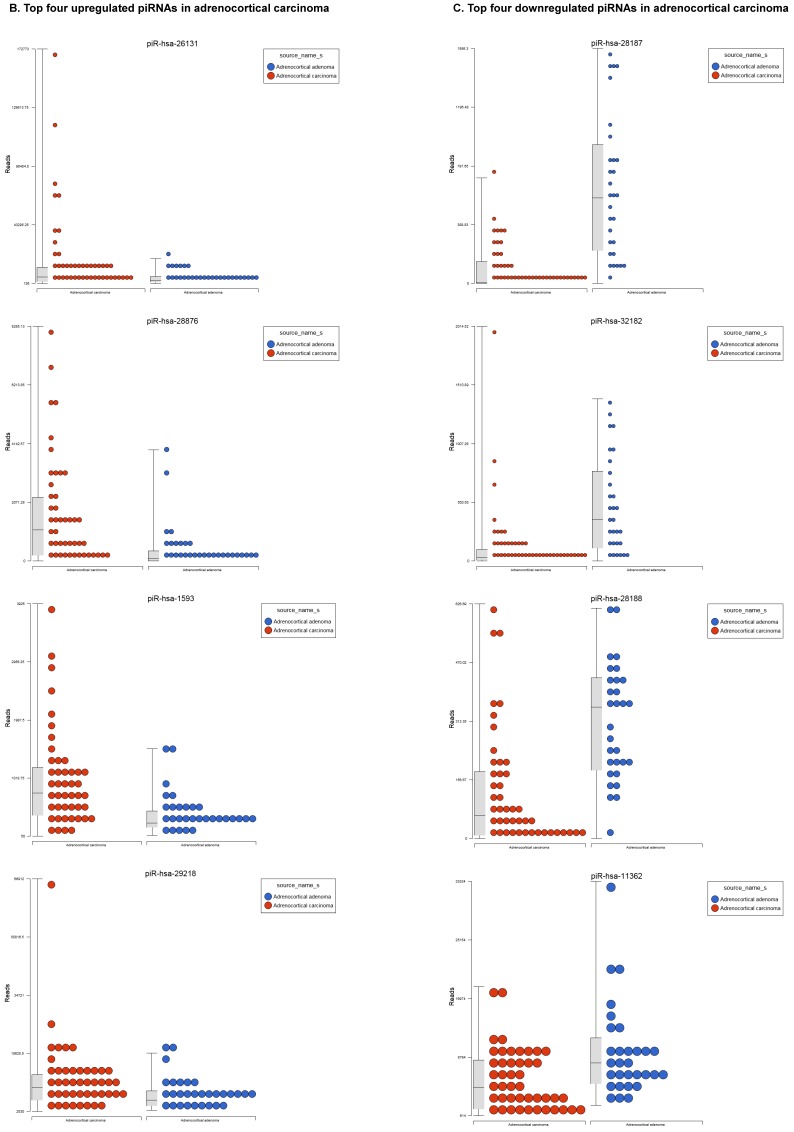
Differentially Expressed piRNAs in Adrenocortical Carcinoma
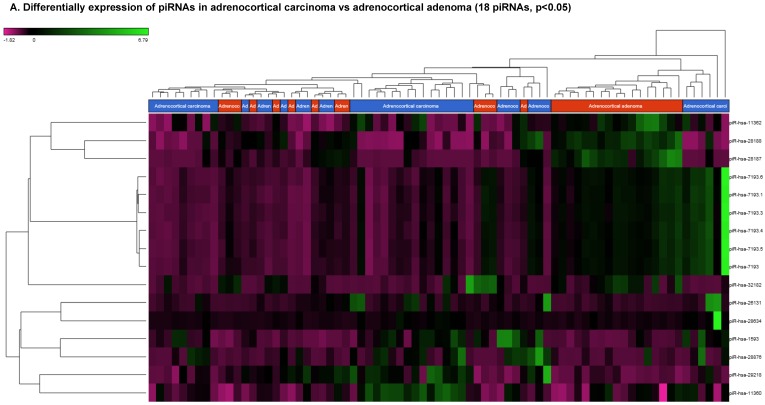
Piwi-interacting RNAs are the largest class of endogenous non-coding small RNAs. Recently, they have been shown to play important biological roles as RNA silencers. Piwi-proteins form RNA-protein complexes and are required for both epigenetic and post-transcriptional gene silencing of retrotransposons and other genetic elements in germ line cells, particularly during spermatogenesis 48. Aligning small RNA sequencing data and remapping with piRBase annotation that contains thousands of piRNAs, more than 16 differentially expressed piRNAs that were statistically significant (p < 0.05; Figure 3A-C) in ACC compared to normal adjacent tissue samples were identified. Out of 16, six piRNAs were upregulated and 10 were downregulated. Several groups have directed their attention to understanding the biological and epigenetic functions of piRNAs, since detection of piRNAs in cancer correlates with poorer prognostics and clinical outcomes, suggesting they play an important functional role in cancer biogenesis. There is an ongoing effort to utilize piRNAs for developing diagnostic, prognostic and therapeutic tools, but the gap in our understanding of mechanisms underlying piRNA biogenesis and function must be filled 49. We listed the top four upregulated piRNAs (Figure 3B) i.e. piR-26131 (4.3 fold, p<0.0001), piR-28876 (4 fold, p<0.00004), piR-28634 (3.2 fold, p<0.003), piR-1593 (2.6 fold, p<0.0003), and piR-29218 (1.6 fold, p<0.0003) and downregulated piRNAs (Figure 3C) piR-28187 (7 fold, p<0.0000007), piR-32182 (3.3 fold, p<0.00002), piR-28188 (2.7 fold, p<0.00006), piR-11362 (1.7 fold, p<0.0003), and piR-7193 (1.2 fold, p<0.002) in ACC compared to ACA control tissue samples. Recent studies also reported the involvement of specific piRNAs in different cancers. For example, piR-4987, piR-20365, piR-20485, piR-20582, and piR-932 are involved in breast cancer 50, 51; piR-823 and piR651 in gastric cancer 30, 52-54. piR-32051, piR-39894 and piR-43607 are upregulated and piR-38756, piR-57125 and piR-30924 downregulated in renal cancer 55. Additionally, piR-Hep1 is involved in liver cancer 56; piR-017061 downregulated in pancreatic cancer 57; piR-823 downregulated in multiple myeloma, piR-L-163 downregulated in lung cancer 58; and piR-59056, piR-32105 and piR-58099 in colon cancer 59.
Figure 3.
Differential expression of piRNAs in ACC samples: piRNA analysis revealed 18 piRNAs statistically significant that are shown as hierarchical clustering (p≤0.05; Figure 3A), with 6 piRNAs upregulated (Figure 3B) and 10 downregulated (Figure 3C).
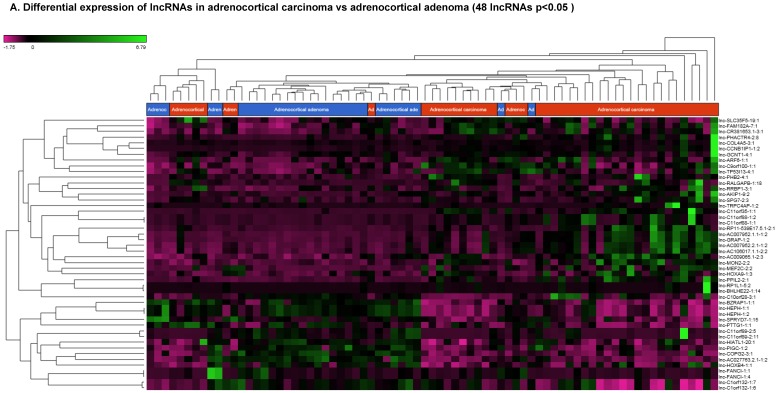
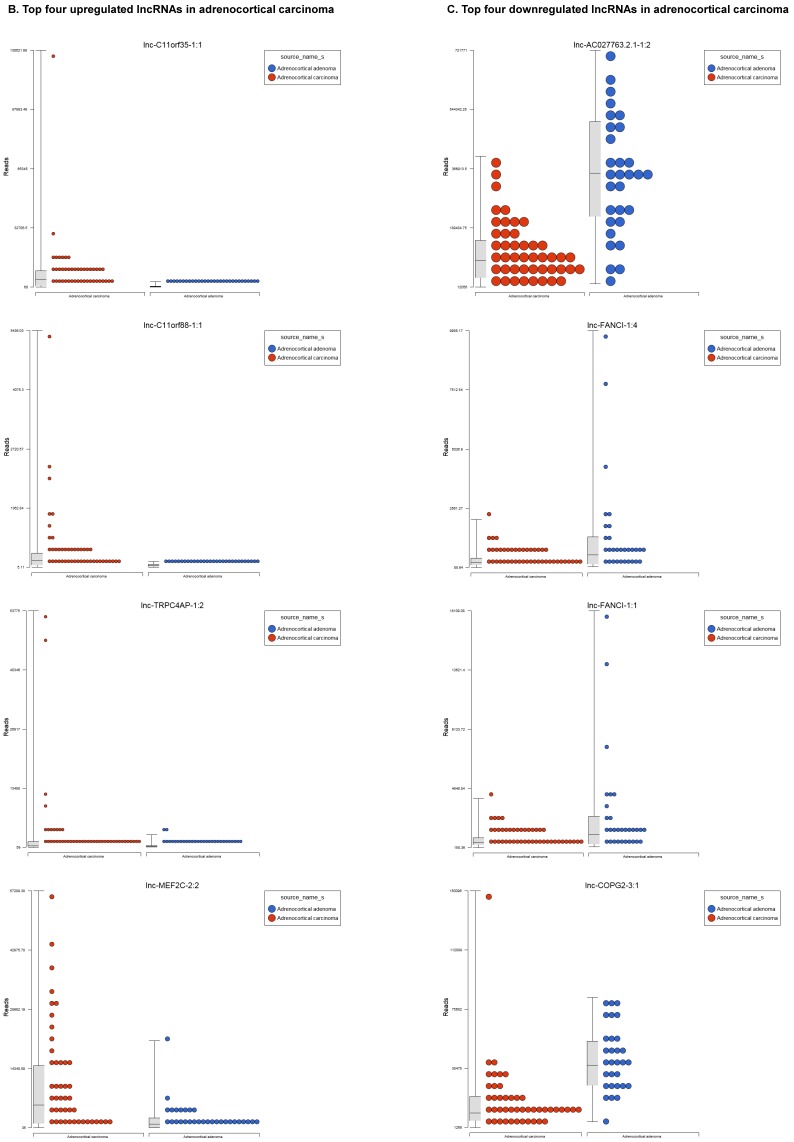
Differentially Expressed Long Non-coding RNAs in ACC Tissue Samples
Long non-coding RNAs (lncRNAs) are the newest and least described class of sncRNAs. They are larger than 200 nucleotides and non-conserved among species 48. They have tissue specific expression in a regulated manner correlated with distinct groups of genes that affect cellular function 60 and can act as a tumor suppressor or promoter 61-63. Remapping small RNA sequencing data to identify differentially expressed lncRNAs in ACC tissue samples showed 48 lncRNAs (p<0.05; Figure 4A) were significantly expressed where 31 lncRNAs were upregulated including the top five listed here: lnc-BHLHHE22-1:14 (16.7 fold, p<0.0007), lnc-C11orf35-1:1 (15.7 fold, p<0.000000001), lnc-RP1L1-5:2 (12.6 fold, p<0.0001), lnc-C11orf88-1:1 (7.2 fold, p<0.000006), and lnc-TRPC4AP-1:2 (6 fold, p<0.0005; Figure 4B) and 17 were downregulated. The top five downregulated lncRNAs were: lnc-AC027763.2.1-1:2 (3.1 fold, p<0.0000005), lnc-FANCI-1 (3 fold, p<0.008), lnc-COPG2-3:1 (2.9 fold, p<0.0000000003), lnc-PIGC-1:2 (2.5 fold, p<0.000002), and lnc-C11orf89-2 (2.3 fold, p<0.000000001; Figure 4C). lncRNAs are a diverse group of transcripts with various mechanisms and are differentially expressed in many diseases including cancer 62.
Figure 4.
Differential expression of lncRNAs in ACC samples: A: Dendrogram view of 48 lncRNAs that were statistically significant (p≤0.05) as a hierarchical tree. Top four upregulated lncRNAs shown in Figure 4B and top four downregulated in Figure 4C.
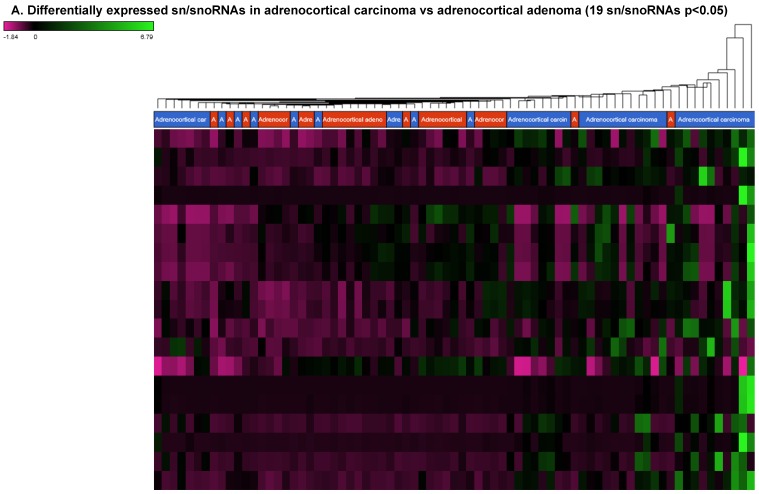
Differentially Expressed snRNA and snoRNAs in Adrenocortical Carcinoma Tissue Samples
Small nuclear RNAs are another class of RNAs that localize within the nucleus of eukaryotic cells 48 and are involved in pre-mRNA processing. For processing, they are always associated with a group of specific proteins and these complexes are called small nuclear ribonucleoproteins (snRNP). The small nucleolar RNAs (snoRNAs) are another subclass of snRNAs that localize in the nucleolus and play a role in maturation of RNA molecules through chemical modifications targeting mainly rRNAs, tRNAs and snRNAs 48. Using the GenCode database, which contains most of the curated small RNAs in order to specifically look for snRNAs and snoRNAs, we remapped aligned reads. We identified nine snRNAs (p<0.05; Figure 5A), all were upregulated and 10 snoRNAs (p<0.05; Figure 5A), of which six were upregulated and the remaining four downregulated (Figures 5B and 5C). The top five upregulated snRNAs were RNU2-6P (86 fold, p<0.00002), RNU2-48P (39 fold, p<0.00001), RNU2-36P (14.2 fold, p<0.00008), RNU2-61P (13.2 fold, p<0.001), and RNU7-1 (3.6 fold, p<0.000001). U1 small nuclear RNA is a multigene family located on the small arm of chromosome one and U1 snRNA pseudogenes are present throughout the genome 64. Many of the snRNAs which are upregulated in ACC samples indicated that these snRNAs may be involved in oncogenesis; however, further validation and investigation is required for them to be used as biomarkers. Most of the upregulated snRNAs belong to RNU2 family members in our analysis; although previous studies have shown the RNU family of snRNAs are expressed in pancreatic, colorectal and lung cancers, no concrete evidence is available to define the function of RNU2 snRNAs 65.
Figure 5.
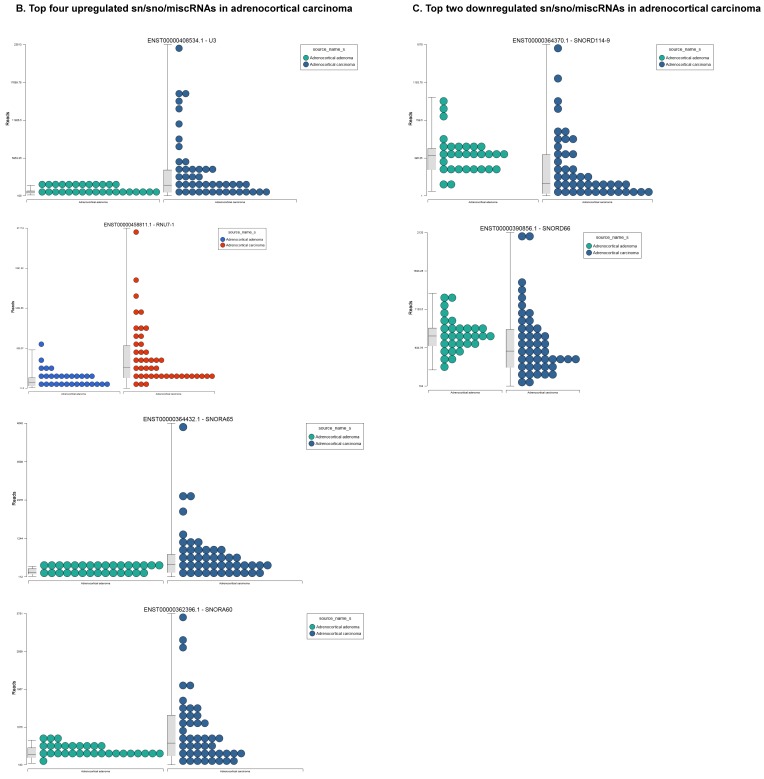
Differential expression of snRNAs or snoRNAs in ACC samples: small RNA-seq data was analyzed for snRNAs or snoRNAs with GenCode annotation and we found 19 sn/snoRNAs (p<0.05; Figure 5A), shown in hierarchical clustering. Top four upregulated (Figure 5B) and top two downregulated (Figure 5C) sn/snoRNAs are shown.
Recent reports indicate snoRNAs play a role in regulation of cell fate and tumorigenesis 66. We identified 10 differentially affected snoRNAs in ACC samples (p<0.05), where only four (snoRD114-9, snoRD114-12, snoRD66, and snoRD114-22) were downregulated (Figure 5B) while the remaining six were upregulated (U3, snoRA65, snoRD60, snoRD17, and snoRD114-21). The snoRD113, snoRD114 and snoRD116 cluster region is becoming an important molecular target 67. snoRD114 belongs to the C/D box class of snoRNAs which we reported remarkable downregulation of in triple negative breast cancer 30. Additionally, snoRD114 is overexpressed in acute promyelocytic leukemia (APL) 68, suggesting a tissue specific action of this cluster of snoRNAs. At the moment, we do not understand the biological and clinical implication of this finding; however, it is anticipated that a better understanding of these molecules will lead to finding a prognostic marker or novel drug target for specific diseases. snoRNA U3 is a member of the C/D class of snoRNAs, which are thought to guide rRNA cleavage and is upregulated in our analysis 69. snoRD66, located in chromosome region 3q27.1, is thought to guide the methylation of 18S rRNAs. snoRD66 overexpression was observed in non-small cell lung cancer and COPD 70.
Further validation of large clinical samples is needed to confirm the biological function of dysregulated small non-coding RNAs identified in this study and to develop future prognostic, diagnostic and therapeutic biomarkers for ACC.
Conclusion
Unbiased and sensitive detection of non-coding RNAs in disease and normal cellular biology has been enabled through the development of high-throughput sequencing technologies. Small RNAs are thought to play a crucial role in disease pathogenesis. Currently, our understanding is limited mainly to the biological role of small RNAs and little is known about the expression pattern of non-coding RNAs in pathological conditions. Identifying the specific molecular gene signature in distinct disease states will allow for better diagnosis and optimization of treatment modalities. In summary, the present study analyzed all sncRNA sequencing data from ACC and ACA tissue samples for the first time. We identified several differentially regulated miRNAs, piRNAs, lncRNAs, and sn/snoRNAs in ACC that could serve as new biomarkers for easy and early disease detection while offering new therapeutic targets. Specifically, miR-483, miR-153, miR-135, miR-514, lnc-BHLHHE22-1:14, lnc-C11orf351:1, lnc-RP1L1-5:2, RNU2-6P, RNU2-48P, RNU2-36P, and RNU2-61P showed greater than 10 fold expression in ACC versus ACA samples. piR-26131 and piR-28876 showed four fold overexpression in ACC samples. Further research is needed to correlate these specific sncRNAs with tumor grade and prognosis. Our investigation demonstrates a comprehensive screening of non-coding RNA signature using publicly available NGS resources. Our approach seeks to maximize the utilization of established datasets to understand the biological role of non-coding RNAs in tumorigenesis, disease prognosis and treatment outcomes.
Figure 6.
Circos plot incorporating differential expressions of all small non-coding RNAs affected in ACC. Chromosome and bands are listed in chromosomal positions of small RNAs expression in ACC vs ACA tissue samples. Outermost ring is miRNA, then piRNA, lncRNAs, and sn/snoRNA with darker and lighter background colors representing upregulated and downregulated genes respectively.
Table 1.
Differential expression of miRNAs enriched in ACC samples.
| microRNA ID | Chromosome | Fold change | P-value |
|---|---|---|---|
| hsa-miR-483-3p | 11 | 128.57 | 2.05E-09 |
| hsa-miR-483-5p | 11 | 118.42 | 1.11E-08 |
| hsa-miR-153-3p | 2 | 41.54 | 2.63E-06 |
| hsa-miR-135a-5p | 12 | 37.63 | 2.34E-08 |
| hsa-miR-514b-5p | X | 16.28 | 2.52E-06 |
| hsa-miR-210-3p | 11 | 15.95 | 4.19E-09 |
| hsa-miR-510-5p | X | 15.63 | 5.36E-04 |
| hsa-miR-509-3p | X | 14.88 | 2.76E-05 |
| hsa-miR-509-5p | X | 14.08 | 7.77E-04 |
| hsa-miR-514b-3p | X | 13.00 | 2.00E-06 |
| hsa-miR-196a-5p | 12 | 12.84 | 2.26E-07 |
| hsa-miR-513c-5p | X | 12.80 | 1.39E-05 |
| hsa-miR-513b-5p | X | 12.77 | 1.13E-04 |
| hsa-miR-514a-5p | X | 12.59 | 5.55E-05 |
| hsa-miR-508-5p | X | 11.63 | 5.51E-05 |
| hsa-miR-508-3p | X | 10.95 | 5.29E-05 |
| hsa-miR-203a | 14 | 10.76 | 1.11E-04 |
| hsa-miR-514a-3p | X | 10.52 | 2.55E-05 |
| hsa-miR-507 | X | 10.27 | 6.07E-06 |
| hsa-miR-503-5p | X | 9.94 | 1.44E-12 |
| hsa-miR-513c-3p | X | 9.17 | 1.00E-04 |
| hsa-miR-204-5p | 9 | 8.78 | 2.00E-06 |
| hsa-miR-509-3-5p | X | 8.14 | 3.06E-04 |
| hsa-miR-499a-5p | 20 | 7.63 | 2.74E-03 |
| hsa-miR-183-5p | 7 | 7.16 | 1.40E-04 |
| hsa-miR-34c-5p | 11 | 6.57 | 1.69E-05 |
| hsa-miR-9-5p | 1 | 6.49 | 6.88E-06 |
| hsa-miR-542-3p | X | 6.46 | 4.42E-10 |
| hsa-miR-192-5p | 11 | 6.19 | 7.82E-07 |
| hsa-miR-194-5p | 1 | 6.17 | 1.89E-06 |
| hsa-miR-9-3p | 1 | 5.87 | 2.41E-06 |
| hsa-miR-542-5p | X | 5.03 | 2.50E-09 |
| hsa-miR-450a-5p | X | 4.91 | 1.38E-08 |
| hsa-miR-450b-5p | X | 4.86 | 5.29E-09 |
| hsa-miR-424-3p | X | 4.37 | 5.31E-08 |
| hsa-miR-96-5p | 7 | 4.36 | 2.46E-03 |
| hsa-miR-182-5p | 7 | 4.31 | 1.75E-03 |
| hsa-miR-3529-3p | 15 | 4.22 | 9.59E-03 |
| hsa-miR-138-5p | 16 | 3.92 | 2.33E-07 |
| hsa-miR-4662a-5p | 8 | 3.63 | 9.76E-04 |
| hsa-miR-421 | X | 3.27 | 3.41E-08 |
| hsa-miR-130b-3p | 22 | 3.02 | 4.12E-07 |
| hsa-miR-196b-5p | 7 | 2.63 | 5.81E-03 |
| hsa-miR-181d-5p | 19 | 2.53 | 6.53E-04 |
| hsa-miR-181b-5p | 1 | 2.44 | 2.60E-07 |
| hsa-miR-181c-5p | 19 | 2.26 | 8.10E-03 |
| hsa-miR-1301-3p | 2 | 2.24 | 1.54E-02 |
| hsa-miR-98-5p | X | 2.24 | 3.74E-03 |
| hsa-miR-454-3p | 17 | 2.17 | 5.08E-06 |
| hsa-miR-15b-3p | 3 | 2.16 | 5.66E-07 |
| hsa-miR-339-5p | 7 | 2.15 | 1.93E-04 |
| hsa-miR-424-5p | X | 2.13 | 8.67E-03 |
| hsa-miR-181c-3p | 19 | 2.12 | 1.99E-02 |
| hsa-miR-940 | 16 | 2.08 | 3.00E-06 |
| hsa-miR-128-3p | 2 | 2.08 | 1.63E-05 |
| hsa-miR-1307-3p | 10 | 2.06 | 2.38E-05 |
| hsa-miR-484 | 16 | 2.04 | 3.16E-06 |
| hsa-let-7i-5p | 12 | 2.04 | 1.27E-04 |
| hsa-miR-651-5p | X | 2.01 | 3.55E-03 |
| hsa-miR-328-3p | 16 | 2.01 | 6.90E-04 |
| hsa-miR-148b-3p | 12 | 1.98 | 7.93E-06 |
| hsa-miR-149-5p | 2 | 1.97 | 2.52E-03 |
| hsa-miR-339-3p | 7 | 1.94 | 2.08E-05 |
| hsa-miR-324-5p | 17 | 1.94 | 1.75E-02 |
| hsa-miR-99b-5p | 19 | 1.92 | 5.84E-05 |
| hsa-miR-181a-2-3p | 9 | 1.91 | 1.09E-04 |
| hsa-miR-625-5p | 14 | 1.88 | 2.29E-02 |
| hsa-miR-769-5p | 19 | 1.86 | 7.50E-04 |
| hsa-miR-598-3p | 8 | 1.86 | 2.11E-03 |
| hsa-miR-99b-3p | 19 | 1.81 | 1.97E-04 |
| hsa-miR-106b-3p | 7 | 1.79 | 1.56E-03 |
| hsa-let-7d-5p | 9 | 1.78 | 1.24E-02 |
| hsa-miR-671-5p | 7 | 1.71 | 2.31E-03 |
| hsa-miR-652-3p | X | 1.69 | 4.57E-04 |
| hsa-miR-331-5p | 12 | 1.66 | 4.91E-05 |
| hsa-miR-145-3p | 5 | 1.64 | 1.40E-02 |
| hsa-miR-143-3p | 5 | 1.60 | 5.09E-03 |
| hsa-miR-188-5p | X | 1.60 | 6.79E-03 |
| hsa-miR-30d-5p | 8 | 1.59 | 4.89E-03 |
| hsa-miR-331-3p | 12 | 1.57 | 3.42E-03 |
| hsa-miR-193b-3p | 16 | 1.50 | 1.86E-02 |
| hsa-miR-425-5p | 3 | 1.50 | 6.43E-04 |
| hsa-miR-181a-5p | 1 | 1.49 | 2.41E-03 |
| hsa-miR-340-3p | 5 | 1.47 | 1.36E-04 |
| hsa-miR-10b-5p | 2 | 1.46 | 4.36E-03 |
| hsa-miR-25-3p | 7 | 1.44 | 9.02E-04 |
| hsa-miR-93-5p | 7 | 1.43 | 3.97E-03 |
| hsa-miR-10b-3p | 2 | 1.41 | 8.41E-03 |
| hsa-miR-340-5p | 5 | 1.36 | 2.09E-02 |
| hsa-let-7f-5p | 9 | 1.36 | 4.03E-03 |
| hsa-miR-185-5p | 22 | 1.34 | 1.81E-02 |
| hsa-miR-191-5p | 3 | 1.30 | 1.63E-03 |
| hsa-miR-106b-5p | 7 | 1.25 | 2.16E-02 |
| hsa-miR-493-5p | 14 | 1.24 | 2.17E-02 |
| hsa-miR-337-3p | 14 | 1.13 | 1.97E-02 |
| hsa-miR-493-3p | 14 | 1.08 | 8.92E-03 |
| hsa-miR-381-3p | 14 | 1.08 | 1.51E-02 |
| hsa-miR-218-5p | 4 | 1.02 | 6.99E-03 |
| hsa-miR-337-5p | 14 | 1.00 | 1.23E-02 |
| hsa-miR-382-3p | 14 | -1.00 | 1.71E-02 |
| hsa-miR-338-3p | 17 | -1.05 | 1.92E-02 |
| hsa-miR-136-5p | 14 | -1.10 | 1.13E-02 |
| hsa-miR-379-5p | 14 | -1.12 | 5.69E-03 |
| hsa-miR-27b-3p | 9 | -1.17 | 2.05E-02 |
| hsa-miR-370-3p | 14 | -1.19 | 8.09E-03 |
| hsa-miR-23b-3p | 9 | -1.20 | 1.03E-02 |
| hsa-miR-28-5p | 3 | -1.23 | 4.66E-03 |
| hsa-miR-654-3p | 14 | -1.24 | 3.46E-03 |
| hsa-miR-103b | 20 | -1.25 | 3.86E-03 |
| hsa-miR-103a-3p | 20 | -1.25 | 3.86E-03 |
| hsa-miR-377-5p | 14 | -1.28 | 3.12E-03 |
| hsa-miR-30e-3p | 1 | -1.32 | 1.67E-03 |
| hsa-miR-125b-5p | 11 | -1.32 | 1.81E-02 |
| hsa-miR-101-3p | 1 | -1.34 | 1.88E-03 |
| hsa-miR-17-3p | 13 | -1.34 | 2.26E-04 |
| hsa-miR-100-5p | 11 | -1.40 | 1.26E-02 |
| hsa-miR-146a-5p | 5 | -1.40 | 5.38E-03 |
| hsa-miR-381-5p | 14 | -1.41 | 3.61E-03 |
| hsa-miR-16-5p | 13 | -1.41 | 9.50E-04 |
| hsa-miR-146b-5p | 10 | -1.44 | 4.37E-03 |
| hsa-miR-345-5p | 14 | -1.45 | 1.77E-04 |
| hsa-miR-455-3p | 9 | -1.45 | 2.42E-03 |
| hsa-miR-29c-3p | 1 | -1.46 | 5.80E-04 |
| hsa-miR-10a-5p | 17 | -1.49 | 1.72E-04 |
| hsa-miR-190a-5p | 15 | -1.52 | 5.47E-04 |
| hsa-miR-29c-5p | 1 | -1.54 | 8.41E-05 |
| hsa-miR-32-3p | 9 | -1.58 | 1.39E-03 |
| hsa-miR-4484 | 10 | -1.58 | 2.49E-03 |
| hsa-miR-15a-5p | 13 | -1.67 | 1.78E-07 |
| hsa-miR-30a-3p | 6 | -1.68 | 7.43E-04 |
| hsa-miR-125b-2-3p | 21 | -1.72 | 1.09E-05 |
| hsa-let-7c-5p | 21 | -1.75 | 3.69E-04 |
| hsa-miR-150-5p | 19 | -1.82 | 1.06E-04 |
| hsa-miR-99a-5p | 21 | -1.82 | 1.06E-05 |
| hsa-miR-142-3p | 17 | -1.91 | 1.64E-07 |
| hsa-miR-223-3p | X | -2.28 | 1.22E-09 |
| hsa-miR-708-5p | 11 | -2.29 | 3.41E-07 |
| hsa-miR-142-5p | 17 | -2.31 | 7.17E-05 |
| hsa-miR-675-5p | 11 | -2.41 | 1.91E-09 |
| hsa-miR-199a-3p | 1 | -2.49 | 3.51E-06 |
| hsa-miR-199b-3p | 9 | -2.50 | 3.53E-06 |
| hsa-miR-199a-5p | 1 | -2.54 | 9.62E-06 |
| hsa-miR-214-3p | 1 | -2.60 | 1.38E-06 |
| hsa-miR-335-3p | 7 | -2.73 | 1.30E-08 |
| hsa-miR-335-5p | 7 | -2.96 | 1.07E-09 |
| hsa-miR-195-5p | 17 | -3.69 | 1.10E-09 |
| hsa-miR-497-5p | 17 | -4.28 | 3.40E-10 |
Table 2.
Differential expression of piRNAs enriched in ACC samples.
| piRNA ID | Chromosome | Fold change | P-value |
|---|---|---|---|
| piR-hsa-26131 | 7 | 4.32 | 1.52E-03 |
| piR-hsa-28876 | 5 | 4.17 | 4.04E-04 |
| piR-hsa-28634 | 12 | 3.19 | 1.27E-03 |
| piR-hsa-1593 | 14 | 2.61 | 3.28E-04 |
| piR-hsa-29218 | 7 | 1.59 | 2.00E-03 |
| piR-hsa-11360 | 9 | 1.40 | 2.27E-03 |
| piR-hsa-7193 | 15 | -1.23 | 4.37E-03 |
| piR-hsa-7193 | 15 | -1.24 | 4.08E-03 |
| piR-hsa-7193 | 1 | -1.25 | 4.45E-03 |
| piR-hsa-7193 | 2 | -1.26 | 2.62E-03 |
| piR-hsa-7193 | 1 | -1.26 | 2.78E-03 |
| piR-hsa-7193 | 15 | -1.27 | 2.68E-03 |
| piR-hsa-11362 | 21 | -1.76 | 3.03E-04 |
| piR-hsa-28188 | 11 | -2.72 | 6.77E-04 |
| piR-hsa-32182 | 6 | -3.34 | 2.46E-04 |
| piR-hsa-28187 | 11 | -7.06 | 7.12E-07 |
Table 3.
Differential expression of lncRNAs enriched in ACC samples.
| lncRNA ID | Chromosome | Fold change | P-value |
|---|---|---|---|
| lnc-BHLHE22-1:14 | 8 | 16.75 | 7.29E-04 |
| lnc-C11orf35-1:1 | 11 | 15.66 | 1.56E-09 |
| lnc-RP1L1-5:2 | 8 | 12.58 | 1.74E-04 |
| lnc-C11orf88-1:1 | 11 | 7.24 | 6.07E-06 |
| lnc-C11orf88-1:2 | 11 | 7.23 | 6.06E-06 |
| lnc-TRPC4AP-1:2 | 20 | 6.10 | 5.25E-03 |
| lnc-MEF2C-2:2 | 5 | 5.84 | 7.55E-06 |
| lnc-RP11-539E17.5.1-2:1 | 8 | 5.23 | 3.21E-05 |
| lnc-CCNB1IP1-1:2 | 14 | 4.24 | 1.01E-06 |
| lnc-PHACTR4-2:8 | 1 | 3.64 | 3.18E-06 |
| lnc-PHB2-4:1 | 12 | 3.58 | 3.76E-06 |
| lnc-AC007952.1.1-1:2 | 17 | 3.45 | 1.63E-06 |
| lnc-GRAP-1:2 | 17 | 3.30 | 1.88E-06 |
| lnc-ARF6-1:1 | 14 | 3.18 | 1.59E-04 |
| lnc-PPIL2-2:1 | 22 | 2.98 | 3.31E-07 |
| lnc-COL4A5-3:1 | X | 2.96 | 3.57E-03 |
| lnc-RALGAPB-1:18 | 20 | 2.55 | 6.06E-04 |
| lnc-HOXA9-1:3 | 7 | 2.55 | 7.89E-03 |
| lnc-AKIP1-9:2 | 11 | 2.53 | 2.39E-04 |
| lnc-SPG7-2:3 | 16 | 2.40 | 1.04E-04 |
| lnc-AC007952.2.1-1:2 | 17 | 2.36 | 1.64E-05 |
| lnc-AC106017.1.1-2:2 | 17 | 2.17 | 1.97E-05 |
| lnc-AC009065.1-2:3 | 16 | 2.10 | 1.82E-06 |
| lnc-MON2-2:2 | 12 | 2.04 | 1.21E-04 |
| lnc-RRBP1-3:1 | 20 | 1.81 | 3.62E-04 |
| lnc-FAM182A-7:1 | 20 | 1.75 | 7.02E-06 |
| lnc-CR381653.1-3:1 | 21 | 1.73 | 3.78E-04 |
| lnc-SLC35F5-19:1 | 2 | 1.55 | 5.76E-03 |
| lnc-TP53I13-4:1 | 17 | 1.54 | 4.16E-03 |
| lnc-GCNT1-4:1 | 9 | 1.52 | 1.22E-02 |
| lnc-C9orf100-1:1 | 9 | 1.50 | 1.81E-03 |
| lnc-C10orf28-3:1 | 10 | -1.08 | 8.35E-03 |
| lnc-C1orf132-1:7 | 1 | -1.32 | 4.41E-03 |
| lnc-C1orf132-1:6 | 1 | -1.33 | 3.63E-03 |
| lnc-HIATL1-20:1 | 9 | -1.34 | 1.50E-04 |
| lnc-PTTG1-1:1 | 5 | -1.37 | 7.09E-03 |
| lnc-SPRYD7-1:15 | 13 | -1.41 | 8.04E-04 |
| lnc-HOXB4-1:1 | 17 | -1.50 | 1.35E-04 |
| lnc-BZRAP1-1:1 | 17 | -1.94 | 1.76E-07 |
| lnc-HEPH-1:1 | X | -2.18 | 1.12E-08 |
| lnc-HEPH-1:2 | X | -2.18 | 1.42E-08 |
| lnc-C11orf89-2:5 | 11 | -2.30 | 8.03E-09 |
| lnc-C11orf89-2:11 | 11 | -2.40 | 1.16E-09 |
| lnc-PIGC-1:2 | 1 | -2.52 | 2.49E-06 |
| lnc-COPG2-3:1 | 7 | -2.94 | 5.57E-10 |
| lnc-FANCI-1:1 | 15 | -2.97 | 8.52E-03 |
| lnc-FANCI-1:4 | 15 | -2.99 | 8.54E-03 |
| lnc-AC027763.2.1-1:2 | 17 | -3.11 | 5.87E-08 |
Table 4.
Differential expression of sn/snoRNAs enriched in ACC samples.
| Gene ID | Gene type | Chromosome | Fold change | P-value |
|---|---|---|---|---|
| RNU2-6P | snRNA | 13 | 86.03 | 2.72E-05 |
| RNU2-48P | snRNA | 5 | 39.16 | 1.62E-05 |
| RNU2-36P | snRNA | 9 | 14.21 | 8.90E-05 |
| RNU2-61P | snRNA | 6 | 13.24 | 1.02E-03 |
| U3 | snoRNA | 9 | 5.56 | 9.35E-06 |
| U3 | snoRNA | 8 | 5.23 | 3.58E-05 |
| RNU7-1 | snRNA | 12 | 3.66 | 1.20E-06 |
| SNORA65 | snoRNA | 9 | 2.59 | 3.11E-05 |
| RNU2-2P | snRNA | 11 | 2.50 | 7.92E-04 |
| SNORA60 | snoRNA | 20 | 2.15 | 2.63E-03 |
| SNORD17 | snoRNA | 20 | 1.81 | 3.80E-04 |
| RNU2-59P | snRNA | 10 | 1.77 | 5.00E-03 |
| RNU5A-1 | snRNA | 15 | 1.53 | 2.64E-03 |
| RNU5B-1 | snRNA | 15 | 1.53 | 3.80E-03 |
| SNORD114-21 | snoRNA | 14 | 1.12 | 1.70E-02 |
| SNORD114-22 | snoRNA | 14 | -1.02 | 7.05E-03 |
| SNORD66 | snoRNA | 3 | -1.20 | 1.32E-02 |
| SNORD114-12 | snoRNA | 14 | -1.24 | 4.46E-03 |
| SNORD114-9 | snoRNA | 14 | -1.62 | 1.75E-04 |
Acknowledgments
We thank Gene Arrays (An entity of Vedic Research, Inc., USA) for MetaCore data analysis free of charge. This work was supported by the Department of Surgery at Penn State Milton S. Hershey Medical Center.
References
- 1.Erickson LA, Rivera M, Zhang J. Adrenocortical Carcinoma: Review and Update. Advances in Anatomic Pathology. 2014;21:151–9. doi: 10.1097/PAP.0000000000000019. [DOI] [PubMed] [Google Scholar]
- 2.Libé R. Adrenocortical carcinoma (ACC): diagnosis, prognosis, and treatment. Frontiers in Cell and Developmental Biology. 2015;3:45. doi: 10.3389/fcell.2015.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Özata DM, Caramuta S, Velázquez-Fernández D, Akçakaya P, Xie H, Höög A. et al. The role of microRNA deregulation in the pathogenesis of adrenocortical carcinoma. Endocrine-Related Cancer. 2011;18:643–55. doi: 10.1530/ERC-11-0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cecchetto G, Ganarin A, Bien E, Vorwerk P, Bisogno G, Godzinski J, Outcome and prognostic factors in high-risk childhood adrenocortical carcinomas: A report from the European Cooperative Study Group on Pediatric Rare Tumors (EXPeRT) Pediatric Blood & Cancer; 2016. n/a-n/a. [DOI] [PubMed] [Google Scholar]
- 5.Eddy SR. Non-coding RNA genes and the modern RNA world. Nat Rev Genet. 2001;2:919–29. doi: 10.1038/35103511. [DOI] [PubMed] [Google Scholar]
- 6.Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75:843–54. doi: 10.1016/0092-8674(93)90529-y. [DOI] [PubMed] [Google Scholar]
- 7.Sai Lakshmi S, Agrawal S. piRNABank: a web resource on classified and clustered Piwi-interacting RNAs. Nucleic Acids Research. 2008;36:D173–D7. doi: 10.1093/nar/gkm696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cao J. The functional role of long non-coding RNAs and epigenetics. Biological Procedures Online. 2014;16:11. doi: 10.1186/1480-9222-16-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scott MS, Ono M, Yamada K, Endo A, Barton GJ, Lamond AI. Human box C/D snoRNA processing conservation across multiple cell types. Nucleic Acids Research. 2011;40:3676–88. doi: 10.1093/nar/gkr1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thomson T, Lin H. The Biogenesis and Function PIWI Proteins and piRNAs: Progress and Prospect. Annual review of cell and developmental biology. 2009;25:355–76. doi: 10.1146/annurev.cellbio.24.110707.175327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Derrien T, Johnson R, Bussotti G, Tanzer A, Djebali S, Tilgner H. et al. The GENCODE v7 catalog of human long noncoding RNAs: Analysis of their gene structure, evolution, and expression. Genome Res. 2012;22:1775–89. doi: 10.1101/gr.132159.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scott MS, Ono M. From snoRNA to miRNA: Dual function regulatory non-coding RNAs. Biochimie. 2011;93:1987–92. doi: 10.1016/j.biochi.2011.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Assie G, Letouze E, Fassnacht M, Jouinot A, Luscap W, Barreau O. et al. Integrated genomic characterization of adrenocortical carcinoma. Nature genetics. 2014;46:607–12. doi: 10.1038/ng.2953. [DOI] [PubMed] [Google Scholar]
- 14.Vigneault F Ter-Ovanesyan D Alon S Eminaga S Christodoulou DC Seidman JG et al. High-throughput multiplex sequencing of miRNA Current protocols in human genetics / editorial board, Jonathan L Haines [et al]2012. 011: Unit-11.1210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Firmino N, Martinez VD, Rowbotham DA, Enfield KS, Bennewith KL, Lam WL. HPV status is associated with altered PIWI-interacting RNA expression pattern in head and neck cancer. Oral Oncol. 2016;55:43–8. doi: 10.1016/j.oraloncology.2016.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krzywinski M, Schein J, Birol İ, Connors J, Gascoyne R, Horsman D. et al. Circos: An information aesthetic for comparative genomics. Genome Research. 2009;19:1639–45. doi: 10.1101/gr.092759.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kozomara A, Griffiths-Jones S. miRBase: annotating high confidence microRNAs using deep sequencing data. Nucleic Acids Research. 2014;42:D68–D73. doi: 10.1093/nar/gkt1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang P, Si X, Skogerbø G, Wang J, Cui D, Li Y. et al. piRBase: a web resource assisting piRNA functional study. Database. 2014;2014:bau110. doi: 10.1093/database/bau110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Volders P-J, Verheggen K, Menschaert G, Vandepoele K, Martens L, Vandesompele J. et al. An update on LNCipedia: a database for annotated human lncRNA sequences. Nucleic Acids Research. 2015;43:D174–D80. doi: 10.1093/nar/gku1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sehgal K, Guo X, Koduru S, Shah A, Lin A, Yan X. et al. Plasmacytoid dendritic cells, interferon signaling, and FcgammaR contribute to pathogenesis and therapeutic response in childhood immune thrombocytopenia. Sci Transl Med. 2013;5:193ra89. doi: 10.1126/scitranslmed.3006277. [DOI] [PubMed] [Google Scholar]
- 21.Fu H, Tie Y, Xu C, Zhang Z, Zhu J, Shi Y. et al. Identification of human fetal liver miRNAs by a novel method. FEBS letters. 2005;579:3849–54. doi: 10.1016/j.febslet.2005.05.064. [DOI] [PubMed] [Google Scholar]
- 22.Veronese A, Lupini L, Consiglio J, Visone R, Ferracin M, Fornari F. et al. Oncogenic role of miR-483-3p at the IGF2/483 locus. Cancer research. 2010;70:3140–9. doi: 10.1158/0008-5472.CAN-09-4456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kong D, Piao Y-S, Yamashita S, Oshima H, Oguma K, Fushida S. et al. Inflammation-induced repression of tumor suppressor miR-7 in gastric tumor cells. Oncogene. 2012;31:3949–60. doi: 10.1038/onc.2011.558. [DOI] [PubMed] [Google Scholar]
- 24.Wang W, Zhao LJ, Tan Y-X, Ren H, Qi Z-T. Identification of deregulated miRNAs and their targets in hepatitis B virus-associated hepatocellular carcinoma. World J Gastroenterol. 2012;18:5442–53. doi: 10.3748/wjg.v18.i38.5442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qu X, Zhao M, Wu S, Yu W, Xu J, Xu J. et al. Circulating microRNA 483-5p as a novel biomarker for diagnosis survival prediction in multiple myeloma. Medical Oncology. 2014;31:219. doi: 10.1007/s12032-014-0219-x. [DOI] [PubMed] [Google Scholar]
- 26.Szabo DR, Luconi M, Szabo PM, Toth M, Szucs N, Horanyi J. et al. Analysis of circulating microRNAs in adrenocortical tumors. Lab Invest. 2014;94:331–9. doi: 10.1038/labinvest.2013.148. [DOI] [PubMed] [Google Scholar]
- 27.Wu Z, He B, He J, Mao X. Upregulation of miR-153 promotes cell proliferation via downregulation of the PTEN tumor suppressor gene in human prostate cancer. The Prostate. 2013;73:596–604. doi: 10.1002/pros.22600. [DOI] [PubMed] [Google Scholar]
- 28.Ren J-W, Li Z-J, Tu C. MiR-135 post-transcriptionally regulates FOXO1 expression and promotes cell proliferation in human malignant melanoma cells. International Journal of Clinical and Experimental Pathology. 2015;8:6356–66. [PMC free article] [PubMed] [Google Scholar]
- 29.Xu S, Cecilia Santini G, De Veirman K, Vande Broek I, Leleu X, De Becker A. et al. Upregulation of miR-135b Is Involved in the Impaired Osteogenic Differentiation of Mesenchymal Stem Cells Derived from Multiple Myeloma Patients. PLOS ONE. 2013;8:e79752. doi: 10.1371/journal.pone.0079752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koduru S, Tiwari A, Leberfinger A, Hazard S, Kawasawa Y, Mahajan M. et al. A Comprehensive NGS Data Analysis of Differentially Regulated miRNAs, piRNAs, lncRNAs and sn/snoRNAs in Triple Negative Breast Cancer. Journal of Cancer. 2017;8:578–96. doi: 10.7150/jca.17633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stark MS, Bonazzi VF, Boyle GM, Palmer JM, Symmons J, Lanagan CM. et al. miR-514a regulates the tumour suppressor NF1 and modulates BRAFi sensitivity in melanoma. Oncotarget. 2015;6:17753–63. doi: 10.18632/oncotarget.3924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wotschofsky Z, Busch J, Jung M, Kempkensteffen C, Weikert S, Schaser KD. et al. Diagnostic and prognostic potential of differentially expressed miRNAs between metastatic and non-metastatic renal cell carcinoma at the time of nephrectomy. Clinica Chimica Acta. 2013;416:5–10. doi: 10.1016/j.cca.2012.11.010. [DOI] [PubMed] [Google Scholar]
- 33.Guo ST, Jiang CC, Wang GP, Li YP, Wang CY, Guo XY. et al. MicroRNA-497 targets insulin-like growth factor 1 receptor and has a tumour suppressive role in human colorectal cancer. Oncogene. 2013;32:1910–20. doi: 10.1038/onc.2012.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Furuta M, Kozaki K-i, Tanimoto K, Tanaka S, Arii S, Shimamura T. et al. The Tumor-Suppressive miR-497-195 Cluster Targets Multiple Cell-Cycle Regulators in Hepatocellular Carcinoma. PLOS ONE. 2013;8:e60155. doi: 10.1371/journal.pone.0060155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li D, Zhao Y, Liu C, Chen X, Qi Y, Jiang Y. et al. Analysis of MiR-195 and MiR-497 expression, regulation and role in breast cancer. Clinical cancer research. 2011;17:1722–30. doi: 10.1158/1078-0432.CCR-10-1800. [DOI] [PubMed] [Google Scholar]
- 36.Singh R, Yadav V, kumar S, Saini N. MicroRNA-195 inhibits proliferation, invasion and metastasis in breast cancer cells by targeting FASN, HMGCR, ACACA and CYP27B1. Scientific Reports. 2015;5:17454. doi: 10.1038/srep17454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang F, Jiang C, Sun Q, Yan F, Wang L, Fu Z. et al. mir-195 is a key regulator of raf1 in thyroid cancer. OncoTargets and therapy. 2015;8:3021. doi: 10.2147/OTT.S90710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang L, Qian L, Li X, Yan J. MicroRNA-195 inhibits colorectal cancer cell proliferation, colony-formation and invasion through targeting CARMA3. Molecular medicine reports. 2014;10:473–8. doi: 10.3892/mmr.2014.2178. [DOI] [PubMed] [Google Scholar]
- 39.Xu T, Zhu Y, Xiong Y, Ge YY, Yun JP, Zhuang SM. MicroRNA-195 suppresses tumorigenicity and regulates G1/S transition of human hepatocellular carcinoma cells. Hepatology. 2009;50:113–21. doi: 10.1002/hep.22919. [DOI] [PubMed] [Google Scholar]
- 40.Png KJ, Yoshida M, Zhang XH-F, Shu W, Lee H, Rimner A. et al. MicroRNA-335 inhibits tumor reinitiation and is silenced through genetic and epigenetic mechanisms in human breast cancer. Genes & development. 2011;25:226–31. doi: 10.1101/gad.1974211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cao J, Cai J, Huang D, Han Q, Yang Q, Li T. et al. miR-335 represents an invasion suppressor gene in ovarian cancer by targeting Bcl-w. Oncology reports. 2013;30:701–6. doi: 10.3892/or.2013.2482. [DOI] [PubMed] [Google Scholar]
- 42.Yang Z, Chen S, Luan X, Li Y, Liu M, Li X. et al. MicroRNA-214 is aberrantly expressed in cervical cancers and inhibits the growth of HeLa cells. IUBMB Life. 2009;61:1075–82. doi: 10.1002/iub.252. [DOI] [PubMed] [Google Scholar]
- 43.Zhang XJ, Ye H, Zeng CW, He B, Zhang H, Chen YQ. Dysregulation of miR-15a and miR-214 in human pancreatic cancer. Journal of Hematology & Oncology. 2010;3:46. doi: 10.1186/1756-8722-3-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Callegari E, Elamin BK, D'Abundo L, Falzoni S, Donvito G, Moshiri F. et al. Anti-Tumor Activity of a miR-199-dependent Oncolytic Adenovirus. PLOS ONE. 2013;8:e73964. doi: 10.1371/journal.pone.0073964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guo W, Qiu Z, Wang Z, Wang Q, Tan N, Chen T. et al. MiR-199a-5p is negatively associated with malignancies and regulates glycolysis and lactate production by targeting hexokinase 2 in liver cancer. Hepatology. 2015;62:1132–44. doi: 10.1002/hep.27929. [DOI] [PubMed] [Google Scholar]
- 46.Kinose Y, Sawada K, Nakamura K, Sawada I, Toda A, Nakatsuka E. et al. The hypoxia-related microRNA miR-199a-3p displays tumor suppressor functions in ovarian carcinoma. Oncotarget. 2015;6:11342–56. doi: 10.18632/oncotarget.3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen J, Shin VY, Siu MT, Ho JCW, Cheuk I, Kwong A. miR-199a-5p confers tumor-suppressive role in triple-negative breast cancer. BMC Cancer. 2016;16:887. doi: 10.1186/s12885-016-2916-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Morceau F, Chateauvieux S, Gaigneaux A, Dicato M, Diederich M. Long and Short Non-Coding RNAs as Regulators of Hematopoietic Differentiation. International Journal of Molecular Sciences. 2013;14:14744–70. doi: 10.3390/ijms140714744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moyano M, Stefani G. piRNA involvement in genome stability and human cancer. Journal of Hematology & Oncology. 2015;8:38. doi: 10.1186/s13045-015-0133-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huang G, Hu H, Xue X, Shen S, Gao E, Guo G. et al. Altered expression of piRNAs and their relation with clinicopathologic features of breast cancer. Clinical and Translational Oncology. 2013;15:563–8. doi: 10.1007/s12094-012-0966-0. [DOI] [PubMed] [Google Scholar]
- 51.Zhang H, Ren Y, Xu H, Pang D, Duan C, Liu C. The expression of stem cell protein Piwil2 and piR-932 in breast cancer. Surgical Oncology. 2013;22:217–23. doi: 10.1016/j.suronc.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 52.Cheng J, Deng H, Xiao B, Zhou H, Zhou F, Shen Z. et al. piR-823, a novel non-coding small RNA, demonstrates in vitro and in vivo tumor suppressive activity in human gastric cancer cells. Cancer Letters. 2012;315:12–7. doi: 10.1016/j.canlet.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 53.Cui L, Lou Y, Zhang X, Zhou H, Deng H, Song H. et al. Detection of circulating tumor cells in peripheral blood from patients with gastric cancer using piRNAs as markers. Clinical Biochemistry. 2011;44:1050–7. doi: 10.1016/j.clinbiochem.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 54.Koduru S, Tiwari A, Hazard S, Mahajan M, Ravnic D. Exploration of small RNA-seq data for small non-coding RNAs in Human Colorectal Cancer. Journal of Genomics. 2017;5:16–31. doi: 10.7150/jgen.18856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Busch J, Ralla B, Jung M, Wotschofsky Z, Trujillo-Arribas E, Schwabe P. et al. Piwi-interacting RNAs as novel prognostic markers in clear cell renal cell carcinomas. Journal of Experimental & Clinical Cancer Research: CR. 2015;34:61. doi: 10.1186/s13046-015-0180-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee JH, Schütte D, Wulf G, Füzesi L, Radzun H-J, Schweyer S. et al. Stem-cell protein Piwil2 is widely expressed in tumors and inhibits apoptosis through activation of Stat3/Bcl-XL pathway. Human Molecular Genetics. 2006;15:201–11. doi: 10.1093/hmg/ddi430. [DOI] [PubMed] [Google Scholar]
- 57.Müller S, Raulefs S, Bruns P, Afonso-Grunz F, Plötner A, Thermann R. et al. Next-generation sequencing reveals novel differentially regulated mRNAs, lncRNAs, miRNAs, sdRNAs and a piRNA in pancreatic cancer. Mol Cancer. 2015;14:94. doi: 10.1186/s12943-015-0358-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mei Y, Wang Y, Kumari P, Shetty AC, Clark D, Gable T. et al. A piRNA-like small RNA interacts with and modulates p-ERM proteins in human somatic cells. Nature Communications. 2015;6:7316. doi: 10.1038/ncomms8316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ng KW, Anderson C, Marshall EA, Minatel BC, Enfield KSS, Saprunoff HL. et al. Piwi-interacting RNAs in cancer: emerging functions and clinical utility. Molecular Cancer. 2016;15:1–13. doi: 10.1186/s12943-016-0491-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Guttman M, Amit I, Garber M, French C, Lin MF, Feldser D. et al. Chromatin signature reveals over a thousand highly conserved large non-coding RNAs in mammals. Nature. 2009;458:223–7. doi: 10.1038/nature07672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Huarte M, Guttman M, Feldser D, Garber M, Koziol MJ, Kenzelmann-Broz D. et al. A large intergenic non-coding RNA induced by p53 mediates global gene repression in the p53 response. Cell. 2010;142:409–19. doi: 10.1016/j.cell.2010.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Huarte M. The emerging role of lncRNAs in cancer. Nat Med. 2015;21:1253–61. doi: 10.1038/nm.3981. [DOI] [PubMed] [Google Scholar]
- 63.Zheng GXY, Do BT, Webster DE, Khavari PA, Chang HY. Dicer-microRNA-Myc circuit promotes transcription of hundreds of long noncoding RNAs. Nat Struct Mol Biol. 2014;21:585–90. doi: 10.1038/nsmb.2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.O'Reilly D, Dienstbier M, Cowley SA, Vazquez P, Drożdż M, Taylor S. et al. Differentially expressed, variant U1 snRNAs regulate gene expression in human cells. Genome research. 2013;23:281–91. doi: 10.1101/gr.142968.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Köhler J, Schuler M, Gauler TC, Nöpel-Dünnebacke S, Ahrens M, Hoffmann A-C. et al. Circulating U2 small nuclear RNA fragments as a diagnostic and prognostic biomarker in lung cancer patients. Journal of Cancer Research and Clinical Oncology. 2016;142:795–805. doi: 10.1007/s00432-015-2095-y. [DOI] [PubMed] [Google Scholar]
- 66.Chen L, Han L, Wei J, Zhang K, Shi Z, Duan R, SNORD76, a box C/D snoRNA, acts as a tumor suppressor in glioblastoma. Scientific reports; 2015. p. 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bieth E, Eddiry S, Gaston V, Lorenzini F, Buffet A, Conte Auriol F. et al. Highly restricted deletion of the SNORD116 region is implicated in Prader-Willi Syndrome. Eur J Hum Genet. 2015;23:252–5. doi: 10.1038/ejhg.2014.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liuksiala T, Teittinen KJ, Granberg K, Heinaniemi M, Annala M, Maki M. et al. Overexpression of SNORD114-3 marks acute promyelocytic leukemia. Leukemia. 2014;28:233–6. doi: 10.1038/leu.2013.250. [DOI] [PubMed] [Google Scholar]
- 69.Cléry A, Senty-Ségault V, Leclerc F, Raué HA, Branlant C. Analysis of Sequence and Structural Features That Identify the B/C Motif of U3 Small Nucleolar RNA as the Recognition Site for the Snu13p-Rrp9p Protein Pair. Molecular and cellular biology. 2007;27:1191–206. doi: 10.1128/MCB.01287-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liao J, Yu L, Mei Y, Guarnera M, Shen J, Li R. et al. Small nucleolar RNA signatures as biomarkers for non-small-cell lung cancer. Molecular Cancer. 2010;9:198. doi: 10.1186/1476-4598-9-198. - [DOI] [PMC free article] [PubMed] [Google Scholar]