Abstract
Agriculture in the United States employs youth ages ten and older in work environments with high pesticide levels. Younger children in rural areas may also be affected by indirect pesticide exposures. The long-term effects of pesticides on health and development are difficult to assess and poorly understood. Yet, epidemiologic studies suggest associations with cancer as well as cognitive deficits. We report a practical and cost-effective approach to assess environmental pesticide exposures and their biological consequences in children. Our approach combines silicone wristband personal samplers and DNA damage quantification from hair follicles, and was tested as part of a community-based participatory research (CBPR) project involving ten Latino children from farmworker households in North Carolina. Our study documents high acceptance among Latino children and their caregivers of these noninvasive sampling methods. The personal samplers detected organophosphates, organochlorines, and pyrethroids in the majority of the participants (70%, 90%, 80%, respectively). Pesticides were detected in all participant samplers, with an average of 6.2 ± 2.4 detections/participant sampler. DNA damage in epithelial cells from the sheath and bulb of plucked hairs follicles was quantified by immunostaining 53BP1-labled DNA repair foci. This method is sensitive, as shown by dose response analyses to γ radiations where the lowest dose tested (0.1 Gy) led to significant increased 53BP1 foci density. Immunolabeling of DNA repair foci has significant advantages over the comet assay in that specific regions of the follicles can be analyzed. In this cohort of child participants, significant association was found between the number of pesticide detections and DNA damage in the papilla region of the hairs. We anticipate that this monitoring approach of bioavailable pesticides and genotoxicity will enhance our knowledge of the biological effects of pesticides to guide education programs and safety policies.
Keywords: Silicone wristbands, pesticides, children and adolescents, environmental justice, genotoxicity, health outcomes predictions
Graphical Abstract
1. Introduction
Modern agriculture relies on insecticides, fungicides and herbicides to which farmworkers and residents in rural areas are repeatedly exposed. Children in farmworker households are exposed to pesticides from different sources, including drift of applications in neighboring fields, residues brought home by farmworkers, and residential applications [1–3], which may have adverse health effects.
Organic pesticides are suspected of increasing the risk for severe health conditions, including asthma, neurologic deficits, and cancer [4, 5]. In contrast to acute toxicities from high dose exposures, the long-term effects of chronic lower-dose pesticide exposures on health and development are difficult to assess and therefore poorly understood [6], in particular for migrant and seasonal Latino workers and their families who rely on temporary employment in farming. For these populations, longitudinal studies tracking health conditions with long latencies, such as cancers, are challenging and scarce. Retrospective studies with medical or insurance records are also complicated due to scattered medical documentation and the high proportion of uninsured migrant and seasonal workers [7, 8]. For children, invasive biomonitoring approaches are not acceptable, which complicates studies on pesticide exposures and health for this age group.
Using personal sampling devices and biomarkers to assess environmental exposures and predict their impact on health is a practical and cost-effective approach, complementary to long-term observational studies. Pesticides, including the widely used organophosphate insecticides, induce DNA damage [9, 10], which is a common pathological characteristic of neurological disorders [11–13] and which increases cancer risk. Pesticide exposures are indeed associated with increased prevalence of multiple malignancies including lung [14], bladder [15, 16], non-Hodgkin lymphoma [17, 18], multiple myeloma [19] and acute myeloid leukemia [20] in adults. Although the effects of chronic pesticide exposure in children and adolescents remain poorly understood, increased childhood leukemia and brain tumors have been reported in rural areas or in association with parental and household pesticide exposures [21–24].
Measuring the DNA-damaging effects of pesticides provides a mechanistic link between environmental exposures and health outcomes. Yet, it is impossible to accurately recreate in vitro or in animal models the complex mixtures of pesticides and pesticide metabolites to which farmworkers and their children are exposed, nor relevant exposure doses, times, and frequencies. Therefore, approaches to directly quantify DNA damage levels from study participants and to correlate these measures with exposure data are needed. Hair collection is minimally invasive, and can be done for most children and virtually anywhere since the procedure does not require a medical setting. Plucked hair follicles contain stem cells [25, 26], which give rise to proliferating epithelial cells in the sheath of anaphase hairs. Mutagen’s effect on hair follicles may therefore parallel damage to the epithelium in other organs, relevant to carcinoma initiation. Plucked hair follicles have been used to characterize therapeutic responses in clinical trials for anticancer agents [27, 28] and isolated follicular cells have been used to monitor radiation-induced DNA damage by comet assay [29]. DNA damage can also be detected by immunostaining for DNA repair factors or chromatin modifications such as phosphorylated histone H2AX (γH2AX), which form distinct foci in nuclei [30]. The DNA repair factor 53BP1 is continuously expressed in the cell nucleus and rapidly accumulates at sites of DNA damage, specifically at highly deleterious DNA double-strand breaks [31]. 53BP1 repair foci are very distinct and straightforward to enumerate [32], and are therefore frequently used to quantify DNA damage. Importantly, cells without DNA damage have pan-nuclear 53BP1 signals and can therefore be identified as such. In contrast, absence of labeling for γH2A or other posttranslational protein modifications can either be interpreted as absence of DNA damage or unsuccessful staining.
The present study focused on children from farmworker households in North Carolina, USA. The goal of the study was to test a combined approach to quantify pesticide exposure detected with wristband samplers with noninvasive DNA damage measurements based on immunostaining for DNA repair foci in participant hair follicles.
2. Materials and Methods
2.1. Study participants and data collection
Study participants included ten Latino children age 7–9, recruited in collaboration with our community partner, North Carolina (NC) Farmworkers Project, in the area of Benson, NC. A $45 incentive was provided for participation. Written parental permission and child assent was obtained for each participant. Parents completed an interview providing background information for each child. The child was then given a wristband and instructed to wear it for one week. Hair samples were pulled from the scalp of the participants using flat tweezers. Immediately after collection, hair samples were incubated for 20 min in fixative (4% formaldehyde; Sigma cat# HT5011; 5 ml in 30 ml screw-cap jars); then transferred into phosphate buffer saline (PBS) supplemented with 50 mM glycine. Hair samples were kept at 4°C and were analyzed within 3 days of collection. We determined that storage in PBS-glycine up to 72h did not affect results (but that prolonged incubation in fixative was detrimental). From the ten participants, 45 hairs were collected, but only 20 (44%) passed visual inspection in the laboratory (i.e. had a visible follicle). Hair collection was therefore the bottleneck of this study, and the collection procedure will be improved for future investigations. Cognitive testing was also conducteded with the study participants, but is not reported here. This study was approved by the Institutional Review Board at Wake Forest School of Medicine (protocol number IRB00037775).
2.2. Pesticide assessment with PSDs
Exposures were measured using wristband personal sampling devices [33] worn by the participants. Each wristband was cleaned of particulate matter by rinsing twice with 18 MΩ*cm water and once with isopropanol. To assess recovery during extraction of chemicals from wristbands, surrogate standards (TCMX, PCB-100, and PCB-209) were pipetted onto wristbands immediately before extraction. For extraction, each wristband was set in 100 ml of ethyl acetate in an amber glass jar for at least twelve hours at ambient temperature. The ethyl acetate (extract) was removed and the extraction was repeated with new ethyl acetate for two hours. For each wristband, the two extracts were combined and reduced to 1 ml. Aliquots of the wristband extracts were spiked with the internal standard p,p′-dibromooctafluorobiphenyl and analyzed with gas chromatography. Extracts were diluted 1:10 to reduce analytical interferences of background lipids and siloxanes. Wristband extracts were quantitatively analyzed for 72 pesticides and pesticide degradation products (listed in Supplementary Table S1) on a dual column gas chromatograph (Agilent 6890N) with dual micro-electron capture detection (GC-μECD, or “ECD”) as described previously [34]. Detection limits were determined as described previously [34]. Of 72 pesticides, 50 are classified as insecticides, 12 as herbicides, and ten as fungicides. Our method reflected interest in insecticides for their potential human health impacts and includes 41 organochlorines (OC), 15 organophosphates (OP), seven pyrethroids, three phenylpyrazoles, and one neonicotinoid. To ensure that the samplers contained no measurable pesticide at the beginning of the exposure assessment period, the same extraction and analysis was conducted with unused wristband samplers before each group of experiments. Concentrations of pesticides in wristband extracts were corrected for dilution, surrogate recovery, and mass of the wristband. The amounts detected were divided by the time during which each sampler was worn, yielding time-weighted average concentration values of 72 pesticides, expressed in ng/g wristband.
Quality assurance/quality control: Sample handling, analysis, and quantitation were performed as defined by laboratory data quality objectives and standard operating procedures. Surrogate standard compounds accounted for any loss during extraction and analysis of PSDs. Instrument blanks and continuing calibration checks were analyzed regularly during chromatography. Samples were randomized for chemical analysis, and data was collected blind to the sample identity.
2.3. Hair follicle irradiation
For validation, hairs collected from adult volunteers were plucked from the scalp, resected to approx. 2 cm fragments including the follicular region, and transferred into DMEM medium in 35 mm dishes. A 137Cs irradiator (JL Sherperd MarkI, Model 68A) was used to deliver 0.1, 0.5, 1, or 3 Gy or ionizing radiations. Controls were mock-irradiated. Hair samples were left to recover for 1h in a humidified cell culture incubator (37°C; 5% CO2) before analysis by immunostaining.
2.4. Immunostaining of follicular cells and DNA repair foci quantification
Hairs were visually inspected for presence of the follicle and transferred into 35 mm dishes. All incubation steps (except those with antibodies) were done with gentle agitation. After fixation and washes with PBS-glycine (3× 20 min), hairs were incubated 2h in blocking buffer [10% goat serum in immunofluorescence buffer (IF; 130 mM NaCl, 13.2 mM Na2HPO4, 3.5 mM NaH2PO4, 0.1% bovine serum albumin, 0.05% NaN3, 0.2% Triton X-100, and 0.05% Tween 20)]. Antibody dilutions made in blocking buffer (100 μl) were added to glass slides, at the center of 5 cm2 squares drawn with a hydrophobic PAP pen (to prevent leakage). Hair follicles were then immersed in the antibody solution, covered with parafilm, and incubated overnight at 4°C in humidified chambers. Hairs were washed with IF buffer (3× 20 min; 2 ml in 35 mm dishes), incubated with fluorescently labeled secondary antibodies (as above, but 1h at room temperature), washed with IF buffer (3× 20 min), and stained with 4′,6-Diamidino-2-phenylindole dihydrochloride (DAPI; 0.5 μg/ml dilution in PBS; 10 min). After rinsing with IF buffer, hairs were mounted on microscopy slides using ProLong Gold Antifade (ThermoFisher). Primary antibodies to detect DNA damage were 53BP1 (Abcam, Ab36823, 2 μg/ml) and γH2AX (Ser139; Millipore, clone JBW301, 1 μg/ml). To ensure proper immunolabeling of the follicular cells, dual staining was performed with a constitutively expressed nuclear protein (NuMA), which served as staining control. NuMA antibody (B1C11, 1:2) was a gift from Dr. Jeffrey Nickerson, UMass, Worcester, USA. Ki67 antibodies (ThermoFisher, PA5-19462, 1:1000) were used as proliferation marker. Secondary antibodies were highly cross-adsorbed goat anti-mouse and anti-rabbit IgGs labeled with Alexa Fluor dyes AF488 or AF568 (1:300 dilutions; ThermoFisher). Images were taken using a Zeiss CLSM710 confocal microscope, using a 20x objective. Efforts were made to image similar regions of the tip and proximal segments of the sheath and at similar tissue depths between participants. 53BP1 repair foci were quantified by visual scoring. Quantification was performed blind to the pesticide exposure assessment.
2.5. Statistical analyses
Statistical analysis was performed using Prism (GraphPad Software Inc.). Comparisons were performed using Kruskal-Wallis testing. A P value < 0.05 was considered significant.
3. Results
3.1. Acceptability of the wristband pesticide samplers and hair collection in Latino children from farmworker communities
A protocol combining monitoring of pesticide exposures using wristband samplers, and DNA damage analysis from plucked hair follicles was established and tested on 10 Latino children ages 7–9 (average age 8.4; 6 boys and 4 girls) from farmworker households in North Carolina. Participant characteristics are listed in Table 1. All participants agreed to wear the wristband samplers. Although participants were instructed to wear the wristbands for 7 days, there was a range of 7–14 days that the wristbands were actually worn.
Table 1.
Characteristics of the study participants.
| Participant ID | Wristband worn (days) | Hairs collected (N) | Immunostaining results of DNA damage | |
|---|---|---|---|---|
| Hair sheath | Hair tip | |||
| 100 | 7 | 5 | X | X |
| 101 | 7 | 5 | X | X |
| 102 | 10 | 6 | X | |
| 103 | 7 | 6 | X | |
| 104 | 7 | 4 | ||
| 105 | 9 | 3 | ||
| 106 | 8 | 3 | X | |
| 107 | 8 | 5 | ||
| 108 | 14 | 5 | X | |
| 109 | 8 | 3 | X | X |
3.2. Pesticide exposures detected with personal passive sampling devices
Pesticides were detected in all participants, with 6.2 ± 2.4 (2 – 10) detections on average. The majority of participants had detections for OP (7/10), OC (9/10), and pyrethroids (8/10). A summary of detections is shown in Table 2.
Table 2.
Pesticides (ng/g wristband) detected using personal wristband samplers.
| Class | Pesticide | Participant Identification | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 100 | 101 | 102 | 103 | 104 | 105 | 106 | 107 | 108 | 109 | ||
| Organophosphate | Chlorpyrifos (DL=0.444) | 30.2 | 64.9 | 202 | 35.8 | 86.6 | |||||
|
| |||||||||||
| Ethion (DL=1.28) | 6.27 | ||||||||||
|
| |||||||||||
| Parathion-ethyl (DL=1.57) | 33.0 | ||||||||||
|
| |||||||||||
| Prophos (DL=5.42) | 57.1 | ||||||||||
|
| |||||||||||
| Organochlorine | 4,4′-DDE (DL=0.356) | 13.8 | 27.6 | ||||||||
|
| |||||||||||
| α-Chlordane (DL=0.249) | 8.41 | 10.4 | 6.12 | 12.6 | 34.6 | 28.3 | 26.8 | 5.41 | |||
|
| |||||||||||
| γ-Chlordane (DL=0.241) | 5.29 | 10.2 | 6.61 | 4.02 | 16.7 | 14.3 | 5.51 | ||||
|
| |||||||||||
| Dieldrin (DL=0.134) | 27.8 | ||||||||||
|
| |||||||||||
| Heptachlor (DL=0.612) | 4.39 | 1.96 | |||||||||
|
| |||||||||||
| Isodrin (DL=0.0732) | 59.5 | 50.5 | |||||||||
|
| |||||||||||
| trans-Nonachlor (DL=0.263) | 8.85 | 5.22 | 5.54 | 2.73 | 10.5 | 5.02 | 13.9 | 3.4 | |||
|
| |||||||||||
| Pyrethroid | Bifenthrin (DL=0.807) | 1400 | |||||||||
|
| |||||||||||
| Cypermethrin (DL=1.24) | 52.4 | 212 | 114 | 138 | |||||||
|
| |||||||||||
| Deltamethrin (DL=2.68) | 23.5 | 19.7 | 71.0 | ||||||||
|
| |||||||||||
| cis-Permethrin (DL=0.178) | 172 | 249 | 38.8 | 74.4 | |||||||
|
| |||||||||||
| trans-Permethrin (DL=0.310) | 398 | 490 | 254 | 534 | |||||||
|
| |||||||||||
| Residential Insecticide | Fipronil-sulfide (DL=0.754) | 160 | 24.0 | 44.6 | 19.7 | 39.0 | |||||
|
| |||||||||||
| Fipronil-sulfone (DL=0.715) | 34.4 | ||||||||||
|
| |||||||||||
| Residential Herbicide | Diallate I (DL=5.10) | 24.9 | |||||||||
|
| |||||||||||
| Simazine (DL=5.14) | 595 | ||||||||||
Blank spaces indicate below detection limit.
3.3. Optimization and validation of DNA damage detection in hair follicles
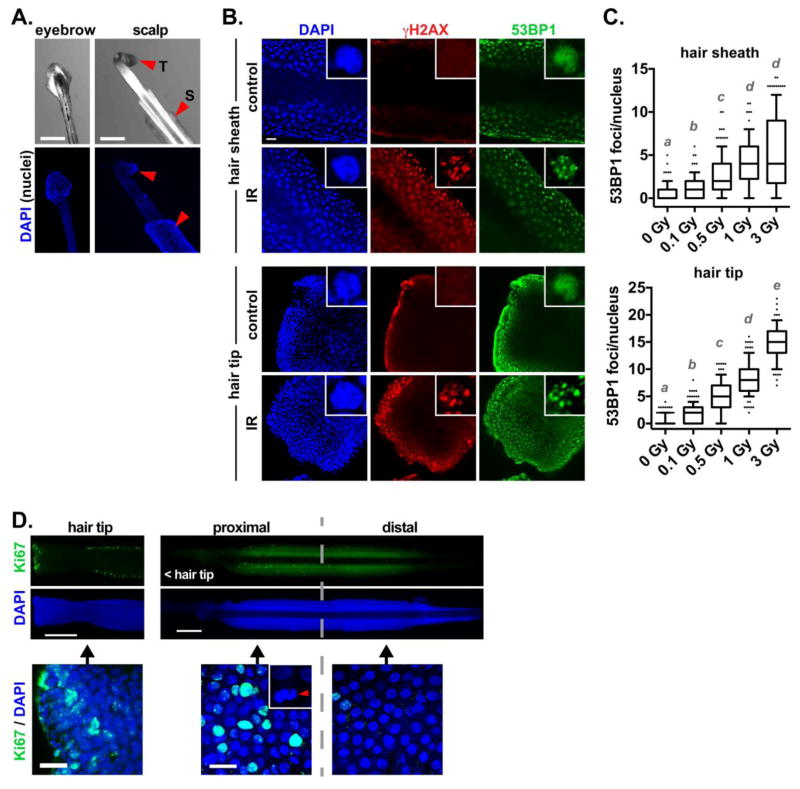
Follicular cells were found associated with the tip and the sheath of plucked hairs from the scalp and the eyebrow from adult volunteers (Fig. 1A). Although most plucked eyebrow hairs had associated cells, scalp hairs were used because (1) they have a longer anagene (active growth) phase characterized with proliferating cells, and (2) the community partners agreed that scalp was preferable to eyebrow. After eliminating the hairs that broke during collection (visual inspection), it was determined that the majority of the plucked hairs had cells at the sheath (92%; 149/162) and at the tip, near the papilla (60%; 89/162).
Fig. 1.
Detection of DNA breaks in plucked hair follicles. (A) Bright field images and nuclei staining (DAPI) in hairs from the eyebrows and the scalp. Arrowheads indicate the outer sheath region (S) and hair tip (T). (B) Confocal microscopy images of 53BP1 and γH2AX staining in the sheath (top) or tip (bottom) of hairs plucked from the scalp, and either irradiated (IR, 3 Gy) or left untreated (control). Single cell nuclei are shown in the insets. (C) Quantification of 53BP1 foci per nucleus cross-section as a function of IR doses. Different letters indicate significant differences (P < 0.01; Kruskal-Wallis and Dunn’s multiple comparison test, N > 100 nuclei from 5 different hairs). (D) Proliferation of hair follicular cells. Ki67 staining of a hair tip and sheath is shown. A mitotic figure is displayed in the inset. Scale bars, 200 μm (A, D [top]) or 20 μm (B and D [bottom]).
To test our ability to measure DNA damage, hairs from the adult volunteers were irradiated and left to recover for 1h in culture medium at 37°C - a time sufficient for the activation of the DNA damage response in epithelial cells [32]. As expected, a robust and consistent increase in the number of DNA repair foci labeled by the DNA repair factor 53BP1 was measured in irradiated hairs. γH2AX foci were also detected after radiation exposure (Fig. 1B). Detection sensitivity was determined with a radiation dose response. Doses as low as 0.1 Gy, which produce no known deterministic effects [35], led to significant increased 53BP1 foci (Fig. 1C). A similar result was obtained for cells at the tip of the hairs (Fig. 1B–C). As expected, immunostaining for the Ki67 proliferation marker indicated the presence of proliferating cells in hair follicles [36]. Most proliferative cells were located towards the proximal region of the hair sheath, where mitotic figures were also observed, and at the tip of the hair (Fig. 1D).
3.4. DNA damage analysis in hairs collected from the children study participants
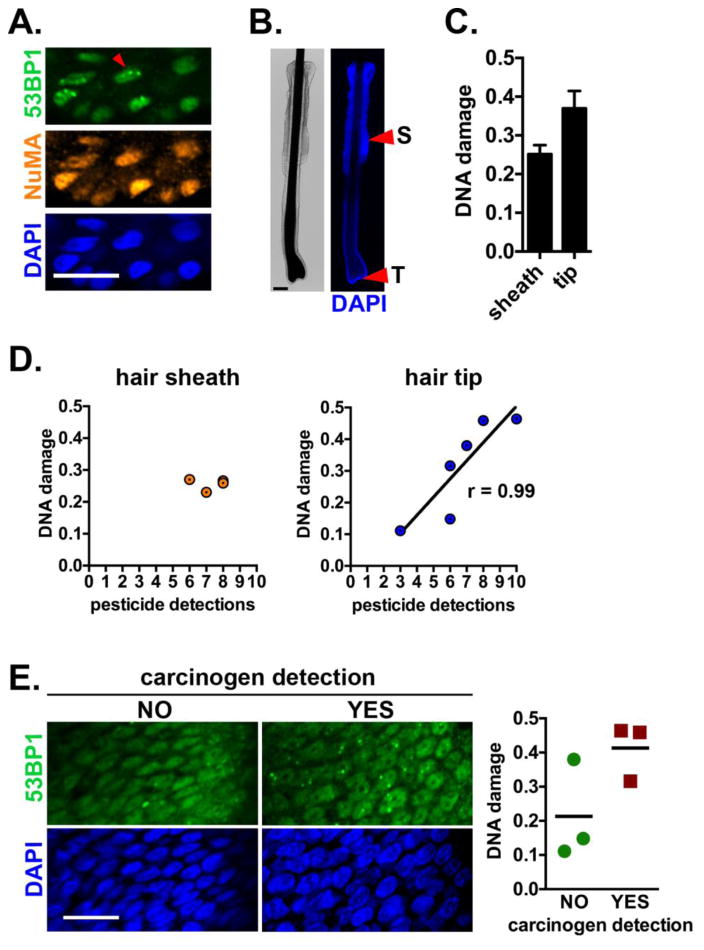
After establishing feasibility to detect DNA damage with high sensitivity in specific regions of plucked hairs, the same optimized staining protocol was tested with hairs from Latino children enrolled in our study. Hairs from seven participants passed visual inspection and were processed for 53BP1 immunostaining and imaged by confocal microscopy (Fig. 2A). DNA damage quantification in the sheath and tip was possible for four and six participants, respectively (Table 1, Fig. 2B). The majority of cells had pan-nuclear 53BP1 signals, indicative of a lack of DNA double-strand breaks. On average, cells from the tip had more DNA damage compared to cells from the sheath (Fig. 2C). No association was computed between DNA damage in hair sheaths and the number of pesticide exposures, whereas the number of exposures positively correlated with DNA damage in the papilla region, at the tip of the hairs (Fig. 2D; Spearman r = 0.9856, P = 0.0056). A subset of the pesticides identified in the study participants are known carcinogens or reproductive toxins according to the State of California Environmental Protection Agency (Cal/EPA; Supplementary Table S1). Children for whom at least one of these pollutants was detected had on average higher DNA damage levels detected in the hair papilla region compared to children without detection of these chemicals (0.41 ± 0.0.08 and 0.21 ± 0.15, respectively), but this difference was not significant (P = 0.109). No difference in DNA damage was found between males and females.
Fig. 2.
Association between pesticide exposures and DNA damage in children hair follicles. (A) Staining of a child participant hair with antibodies against 53BP1 (DNA damage) and NuMA (staining control). The arrowhead points to a nucleus with DNA repair foci. Nuclei were counterstained with DAPI. (B) Illustration of the sheath (S) and tip (T) regions of a scalp hair follicle plucked from a participant. (C) DNA damage (average number of 53BP1 foci/nucleus cross-section ± SEM) in the sheath and tip regions. (D) DNA damage in hair sheaths or at hair tips, plotted against the number of pesticides detected with PSD in each participant. (E) Confocal images of 53BP1 staining (left) and DNA damage quantification (right) in participants with or without detection of pesticides described as carcinogenic by Cal/EPA. Individual values are plotted and means are indicated. Scale bars, 20 μm.
4. Discussion
Farmworkers involved in tending or picking crops are routinely exposed to a broad range of residual pesticides [37–40], as are their family members [1, 41–43]. The present exploratory study in partnership with a Latino farmworker community validated a new approach to study pesticide exposures and their biological effects in children. The high acceptance rate of the procedures in this study confirms enthusiasm for this newly validated approach, and highlights the capacity and potential for integrating basic science research methodology with CBPR methodology among rural Latino communities [44]. In addition, immunostaining of plucked hair follicles proved to be a sensitive, reliable, and non-invasive, procedure – provided that sufficient hair samples are collected.
Pesticide exposures were measured using wristband personal sampling devices (PSD) containing membranes that adsorb a large range of organic chemicals from any media they contact [33]. The wristbands concentrate organic compounds over time, which improves detection limits and reflects episodic exposures. By sampling through passive diffusion into a hydrophobic polymer, such as polydimethylsiloxane (silicone) [45], PSDs mimic uptake of chemicals by cellular lipid membranes. The technology is highly sensitive in multiple environmental settings, and is versatile in that it can be adapted for personal sampling of a wide range of compounds, including numerous pesticides (organochlorines, organophosphates, pyrethroids; [34, 45, 46]). As a result, the wristbands in this study captured chemicals from inhalation and dermal routes of exposure but did not account for ingestion. As mixed-media samplers, the calculation of environmental concentrations is difficult, but wristbands can be used to evaluate relative exposures over the time worn [34, 45, 46]. Recent work showed concentrations of organophosphate flame retardants and polycyclic aromatic hydrocarbons (PAHs) in wristbands correlated with urinary metabolites of those compounds [47], which support a link between the ambient personal environment sampled by the wristbands and the internal environment.
As expected, select pesticides were detected in most participant wristbands, such as the semi-volatile insecticides α-chlordane and trans-nonachlor. Phased out of use in the US during the 1980s, both legacy OC insecticides were broadly used for residential and agricultural areas and are estimated to have half-lives of up to 20 years [48]. Exposures to other pesticides, namely the current-use pyrethroid insecticides cis- and trans-permethrin, displayed greater concentration variability between participant wristbands. Hence, each participant was exposed to a unique blend of pesticides during the collection time. The advantage of the passive wristband samplers used in this study is their cumulative readout of diverse environmental pollutants that enable comprehensive assessments of exposures. In contrast, urinary pesticide biomarkers are difficult to interpret due to the differential and often rapid metabolization and clearance of pesticides. The major limitation of our sampling method is that external rather than internal exposures to pesticides are measured. Although studies show correlation between wristband sampling and urine detections [47], there is a growing interest to integrate exposure detections with toxicology bioassays, as implemented here with DNA damage measures.
Cross-sectional studies have identified increased DNA damage in adult farmworkers and applicators compared to controls with lesser exposure to pesticides [49–52]. A few studies that quantified DNA damage in children indicate a correlation between OP exposure and DNA breaks [53, 54], which is associated with increased risk for most solid tumors and hematological malignancies [55–57]. Importantly, childhood and adolescence are viewed as windows of susceptibility for malignancies [58]. Our data indicate a possible association between pesticide detections and DNA damage levels in cells near hair papilla. In contrast to radiation used for assay validation (where each cell experiences a similar level of DNA damage), it is likely that pesticides and other toxicants in the blood stream predominantly affect cells from the tip of the hair, near the papilla irrigated by blood capillaries. Future studies based on the approach described herein will further assess associations between pesticide exposure and DNA damage in children. Model selection procedures may even identify subsets of pesticides or specific pesticide combinations causing DNA damage. This knowledge is important to (1) improve our understanding of environmental exposures and their effect on health and development; (2) inform changes needed in the organization of work to reduce occupational and para-occupational pesticide exposures; (3) help develop work safety education programs; and (4) provide a framework for public health policy regulations aimed at reducing health disparities between rural minority populations and the general US population.
Supplementary Material
Highlights.
DNA damage detection by 53BP1 immunostaining in plucked hair follicles
Silicone wristband personal sampling devices to quantify pesticide exposures
High acceptance of hair and pesticide sampling methods in children
Pesticide exposures associated with DNA damage in cells near the hair papilla
Acknowledgments
Funding
Funding was provided by a grant from the National Institute of Environmental Health Sciences (R01-ES008739). This work was further supported by Wake Forest University Health Sciences, through startup funds to P.-A.V., and by the Wake Forest Center for Molecular Signaling (CMS) via its imaging facility.
We thank our community partner (Episcopal Farmworker Ministry in Newton Grove NC), and Iliana Tenvooren, Monica Jenks, Alan Bergmann, and Richard Scott for technical assistance.
List of abbreviations
- CBPR
community-based participatory research
- DAPI
4′,6-Diamidino-2-phenylindole dihydrochloride
- OC
organochlorines
- OP
organophosphates
- PSD
personal sampling device
Footnotes
Conflict of interest
Kim Anderson, an author of this research, discloses a financial interest in MyExposome, Inc., which is marketing products related to the research being reported. The terms of this arrangement have been reviewed and approved by Oregon State University in accordance with its policy on research conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Quandt SA, Arcury TA, Rao P, Snively BM, Camann DE, Doran AM, Yau AY, Hoppin JA, Jackson DS. Agricultural and residential pesticides in wipe samples from farmworker family residences in North Carolina and Virginia. Environ Health Perspect. 2004;112:382–387. doi: 10.1289/ehp.6554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fenske RA, Lu C, Negrete M, Galvin K. Breaking the take home pesticide exposure pathway for agricultural families: workplace predictors of residential contamination. Am J Ind Med. 2013;56:1063–1071. doi: 10.1002/ajim.22225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arcury TA, Lu C, Chen H, Quandt SA. Pesticides present in migrant farmworker housing in North Carolina. Am J Ind Med. 2014;57:312–322. doi: 10.1002/ajim.22232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Androutsopoulos VP, Hernandez AF, Liesivuori J, Tsatsakis AM. A mechanistic overview of health associated effects of low levels of organochlorine and organophosphorous pesticides. Toxicology. 2013;307:89–94. doi: 10.1016/j.tox.2012.09.011. [DOI] [PubMed] [Google Scholar]
- 5.Kim KH, Kabir E, Jahan SA. Exposure to pesticides and the associated human health effects. Sci Total Environ. 2016 doi: 10.1016/j.scitotenv.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 6.Grandjean P, Landrigan PJ. Developmental neurotoxicity of industrial chemicals. Lancet. 2006;368:2167–2178. doi: 10.1016/S0140-6736(06)69665-7. [DOI] [PubMed] [Google Scholar]
- 7.Quandt SA, Preisser JS, Arcury TA. Mobility Patterns of Migrant Farmworkers in North Carolina: Implications for Occupational Health Research and Policy. Hum Organ. 2002;61:21–29. doi: 10.17730/humo.61.1.7ndbyxnqd56vatd7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McCauley LA, Anger WK, Keifer M, Langley R, Robson MG, Rohlman D. Studying health outcomes in farmworker populations exposed to pesticides. Environ Health Perspect. 2006;114:953–960. doi: 10.1289/ehp.8526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kisby GE, Muniz JF, Scherer J, Lasarev MR, Koshy M, Kow YW, McCauley L. Oxidative stress and DNA damage in agricultural workers. J Agromed. 2009;14:206–214. doi: 10.1080/10599240902824042. [DOI] [PubMed] [Google Scholar]
- 10.Ojha A, Srivastava N. In vitro studies on organophosphate pesticides induced oxidative DNA damage in rat lymphocytes. Mutat Res-Genet Toxicol Environ Mutag. 2014;761:10–17. doi: 10.1016/j.mrgentox.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 11.Fishel ML, Vasko MR, Kelley MR. DNA repair in neurons: so if they don’t divide what’s to repair? Mutat Res. 2007;614:24–36. doi: 10.1016/j.mrfmmm.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 12.Kulkarni A, Wilson DM. The involvement of DNA-damage and -repair defects in neurological dysfunction. Am J Hum Genet. 2008;82:539–566. doi: 10.1016/j.ajhg.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shiwaku H, Okazawa H. Impaired DNA damage repair as a common feature of neurodegenerative diseases and psychiatric disorders. Curr Mol Med. 2015;15:119–128. doi: 10.2174/1566524015666150303002556. [DOI] [PubMed] [Google Scholar]
- 14.Jones RR, Barone-Adesi F, Koutros S, Lerro CC, Blair A, Lubin J, Heltshe SL, Hoppin JA, Alavanja MC, Beane Freeman LE. Incidence of solid tumours among pesticide applicators exposed to the organophosphate insecticide diazinon in the Agricultural Health Study: an updated analysis. Occup Environ Med. 2015;72:496–503. doi: 10.1136/oemed-2014-102728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baris D, Waddell R, Beane Freeman LE, Schwenn M, Colt JS, Ayotte JD, Ward MH, Nuckols J, Schned A, Jackson B, Clerkin C, Rothman N, Moore LE, Taylor A, Robinson G, Hosain GM, Armenti KR, McCoy R, Samanic C, Hoover RN, Fraumeni JF, Jr, Johnson A, Karagas MR, Silverman DT. Elevated Bladder Cancer in Northern New England: The Role of Drinking Water and Arsenic. J Natl Cancer Inst. 2016;108 doi: 10.1093/jnci/djw099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koutros S, Silverman DT, Alavanja MC, Andreotti G, Lerro CC, Heltshe S, Lynch CF, Sandler DP, Blair A, Beane Freeman LE. Occupational exposure to pesticides and bladder cancer risk. Int J Epidemiol. 2016;45:792–805. doi: 10.1093/ije/dyv195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alavanja MC, Hofmann JN, Lynch CF, Hines CJ, Barry KH, Barker J, Buckman DW, Thomas K, Sandler DP, Hoppin JA, Koutros S, Andreotti G, Lubin JH, Blair A, Beane Freeman LE. Non-hodgkin lymphoma risk and insecticide, fungicide and fumigant use in the agricultural health study. PLoS One. 2014;9:e109332. doi: 10.1371/journal.pone.0109332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schinasi L, Leon ME. Non-Hodgkin lymphoma and occupational exposure to agricultural pesticide chemical groups and active ingredients: a systematic review and meta-analysis. Int J Environ Res Public Health. 2014;11:4449–4527. doi: 10.3390/ijerph110404449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baris D, Silverman DT, Brown LM, Swanson GM, Hayes RB, Schwartz AG, Liff JM, Schoenberg JB, Pottern LM, Greenberg RS, Stewart PA. Occupation, pesticide exposure and risk of multiple myeloma. Scand J Work Environ Health. 2004;30:215–222. doi: 10.5271/sjweh.782. [DOI] [PubMed] [Google Scholar]
- 20.Jones RR, Yu CL, Nuckols JR, Cerhan JR, Airola M, Ross JA, Robien K, Ward MH. Farm residence and lymphohematopoietic cancers in the Iowa Women’s Health Study. Environ Res. 2014;133:353–361. doi: 10.1016/j.envres.2014.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cha ES, Hwang SS, Lee WJ. Childhood leukemia mortality and farming exposure in South Korea: A national population-based birth cohort study. Cancer Epidemiol. 2014;38:401–407. doi: 10.1016/j.canep.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 22.Chen M, Chang CH, Tao L, Lu C. Residential Exposure to Pesticide During Childhood and Childhood Cancers: A Meta-Analysis. Pediatrics. 2015;136:719–729. doi: 10.1542/peds.2015-0006. [DOI] [PubMed] [Google Scholar]
- 23.Ma X, Buffler PA, Gunier RB, Dahl G, Smith MT, Reinier K, Reynolds P. Critical windows of exposure to household pesticides and risk of childhood leukemia. Environ Health Perspect. 2002;110:955–960. doi: 10.1289/ehp.02110955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van Maele-Fabry G, Hoet P, Lison D. Parental occupational exposure to pesticides as risk factor for brain tumors in children and young adults: a systematic review and meta-analysis. Environ Int. 2013;56:19–31. doi: 10.1016/j.envint.2013.02.011. [DOI] [PubMed] [Google Scholar]
- 25.Gho CG, Braun JE, Tilli CM, Neumann HA, Ramaekers FC. Human follicular stem cells: their presence in plucked hair and follicular cell culture. Br J Dermatol. 2004;150:860–868. doi: 10.1111/j.1365-2133.2004.05862.x. [DOI] [PubMed] [Google Scholar]
- 26.Cotsarelis G. Gene expression profiling gets to the root of human hair follicle stem cells. J Clin Invest. 2006;116:19–22. doi: 10.1172/JCI27490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Redon CE, Nakamura AJ, Zhang YW, Ji JJ, Bonner WM, Kinders RJ, Parchment RE, Doroshow JH, Pommier Y. Histone gammaH2AX and poly(ADP-ribose) as clinical pharmacodynamic biomarkers. Clin Cancer Res. 2010;16:4532–4542. doi: 10.1158/1078-0432.CCR-10-0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fong PC, Boss DS, Yap TA, Tutt A, Wu P, Mergui-Roelvink M, Mortimer P, Swaisland H, Lau A, O’Connor MJ, Ashworth A, Carmichael J, Kaye SB, Schellens JH, de Bono JS. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med. 2009;361:123–134. doi: 10.1056/NEJMoa0900212. [DOI] [PubMed] [Google Scholar]
- 29.Tepe Cam S, Seyhan N. DNA damage in hair root cells as a biomarker for gamma ray exposure. Mutat Res. 2013;756:201–205. doi: 10.1016/j.mrgentox.2013.06.011. [DOI] [PubMed] [Google Scholar]
- 30.Bekker-Jensen S, Lukas C, Kitagawa R, Melander F, Kastan MB, Bartek J, Lukas J. Spatial organization of the mammalian genome surveillance machinery in response to DNA strand breaks. J Cell Biol. 2006;173:195–206. doi: 10.1083/jcb.200510130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schultz LB, Chehab NH, Malikzay A, Halazonetis TD. p53 binding protein 1 (53BP1) is an early participant in the cellular response to DNA double-strand breaks. J Cell Biol. 2000;151:1381–1390. doi: 10.1083/jcb.151.7.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vidi PA, Liu J, Salles D, Jayaraman S, Dorfman G, Gray M, Abad P, Moghe PV, Irudayaraj JM, Wiesmuller L, Lelievre SA. NuMA promotes homologous recombination repair by regulating the accumulation of the ISWI ATPase SNF2h at DNA breaks. Nucleic Acids Res. 2014;42:6365–6379. doi: 10.1093/nar/gku296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anderson KA, Seck D, Hobbie KA, Traore AN, McCartney MA, Ndaye A, Forsberg ND, Haigh TA, Sower GJ. Passive sampling devices enable capacity building and characterization of bioavailable pesticide along the Niger, Senegal and Bani Rivers of Africa, Philos. Trans R Soc Lond Ser B: Biol Sci. 2014;369:20130110. doi: 10.1098/rstb.2013.0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Donald CE, Scott RP, Blaustein KL, Halbleib ML, Sarr M, Jepson PC, Anderson KA. Silicone wristbands detect individuals’ pesticide exposures in West Africa. R Soc Open Sci. 2016;3 doi: 10.1098/rsos.160433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.A.C.o. Radiology. Practice Parameters, ACR–SPR Practice Parameter for Imaging Pregnant or Potentially Pregnant Adolescents and Women with Ionizing Radiations. 2013. [Google Scholar]
- 36.Camidge DR, Randall KR, Foster JR, Sadler CJ, Wright JA, Soames AR, Laud PJ, Smith PD, Hughes AM. Plucked human hair as a tissue in which to assess pharmacodynamic end points during drug development studies. Br J Cancer. 2005;92:1837–1841. doi: 10.1038/sj.bjc.6602558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arcury TA, Grzywacz JG, Isom S, Whalley LE, Vallejos QM, Chen H, Galvan L, Barr DB, Quandt SA. Seasonal variation in the measurement of urinary pesticide metabolites among Latino farmworkers in eastern North Carolina. Int J Occup Environ Health. 2009;15:339–350. doi: 10.1179/oeh.2009.15.4.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arcury TA, Grzywacz JG, Talton JW, Chen H, Vallejos QM, Galvan L, Barr DB, Quandt SA. Repeated pesticide exposure among North Carolina migrant and seasonal farmworkers. Am J Ind Med. 2010;53:802–813. doi: 10.1002/ajim.20856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Raymer JH, Studabaker WB, Gardner M, Talton J, Quandt SA, Chen H, Michael LC, McCombs M, Arcury TA. Pesticide exposures to migrant farmworkers in Eastern NC: detection of metabolites in farmworker urine associated with housing violations and camp characteristics. Am J Ind Med. 2014;57:323–337. doi: 10.1002/ajim.22284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arcury TA, Laurienti PJ, Chen H, Howard TD, Barr DB, Mora DC, Summers P, Quandt SA. Organophosphate Pesticide Urinary Metabolites Among Latino Immigrants: North Carolina Farmworkers and Non-farmworkers Compared. J Occup Environ Med. 2016;58:1079–1086. doi: 10.1097/JOM.0000000000000875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Griffith W, Curl CL, Fenske RA, Lu CA, Vigoren EM, Faustman EM. Organophosphate pesticide metabolite levels in pre-school children in an agricultural community: within- and between-child variability in a longitudinal study. Environ Res. 2011;111:751–756. doi: 10.1016/j.envres.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lu C, Kedan G, Fisker-Andersen J, Kissel JC, Fenske RA. Multipathway organophosphorus pesticide exposures of preschool children living in agricultural and nonagricultural communities. Environ Res. 2004;96:283–289. doi: 10.1016/j.envres.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 43.Arcury TA, Grzywacz JG, Davis SW, Barr DB, Quandt SA. Organophosphorus pesticide urinary metabolite levels of children in farmworker households in eastern North Carolina. Am J Ind Med. 2006;49:751–760. doi: 10.1002/ajim.20354. [DOI] [PubMed] [Google Scholar]
- 44.Hohl SD, Gonzalez C, Carosso E, Ibarra G, Thompson B. “I did it for us and I would do it again”: perspectives of rural latinos on providing biospecimens for research. Am J Public Health. 2014;104:911–916. doi: 10.2105/AJPH.2013.301726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.O’Connell SG, Kincl LD, Anderson KA. Silicone wristbands as personal passive samplers. Environ Sci Technol. 2014;48:3327–3335. doi: 10.1021/es405022f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kile ML, Scott RP, O’Connell SG, Lipscomb S, MacDonald M, McClelland M, Anderson KA. Using silicone wristbands to evaluate preschool children’s exposure to flame retardants. Environ Res. 2016;147:365–372. doi: 10.1016/j.envres.2016.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hammel SC, Hoffman K, Webster TF, Anderson KA, Stapleton HM. Measuring Personal Exposure to Organophosphate Flame Retardants Using Silicone Wristbands and Hand Wipes. Environ Sci Technol. 2016;50:4483–4491. doi: 10.1021/acs.est.6b00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bennett GW, Ballee DL, Hall RC, Fahey JE, Butts WL, Osmun JV. Persistence and distribution of chlordane and dieldrin applied as termiticides. Bull Environ Contam Toxicol. 1974;11:64–69. doi: 10.1007/BF01685030. [DOI] [PubMed] [Google Scholar]
- 49.Muniz JF, McCauley L, Scherer J, Lasarev M, Koshy M, Kow YW, Nazar-Stewart V, Kisby GE. Biomarkers of oxidative stress and DNA damage in agricultural workers: a pilot study. Toxicol Appl Pharmacol. 2008;227:97–107. doi: 10.1016/j.taap.2007.10.027. [DOI] [PubMed] [Google Scholar]
- 50.McCauley LA, Lasarev M, Muniz J, Nazar Stewart V, Kisby G. Analysis of pesticide exposure and DNA damage in immigrant farmworkers. J Agromed. 2008;13:237–246. doi: 10.1080/10599240802473817. [DOI] [PubMed] [Google Scholar]
- 51.Atherton KM, Williams FM, Egea Gonzalez FJ, Glass R, Rushton S, Blain PG, Mutch E. DNA damage in horticultural farmers: a pilot study showing an association with organophosphate pesticide exposure. Biomarkers. 2009;14:443–451. doi: 10.3109/13547500903137265. [DOI] [PubMed] [Google Scholar]
- 52.Koureas M, Tsakalof A, Tsatsakis A, Hadjichristodoulou C. Systematic review of biomonitoring studies to determine the association between exposure to organophosphorus and pyrethroid insecticides and human health outcomes. Toxicol Lett. 2012;210:155–168. doi: 10.1016/j.toxlet.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 53.How V, Hashim Z, Ismail P, Md Said S, Omar D, Bahri Mohd Tamrin S. Exploring cancer development in adulthood: cholinesterase depression and genotoxic effect from chronic exposure to organophosphate pesticides among rural farm children. J Agromed. 2014;19:35–43. doi: 10.1080/1059924X.2013.866917. [DOI] [PubMed] [Google Scholar]
- 54.Sutris JM, How V, Sumeri SA, Muhammad M, Sardi D, Mohd Mokhtar MT, Muhammad H, Ghazi HF, Isa ZM. Genotoxicity following Organophosphate Pesticides Exposure among Orang Asli Children Living in an Agricultural Island in Kuala Langat, Selangor, Malaysia. Int J Occup Environ Med. 2016;7:42–51. doi: 10.15171/ijoem.2016.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ciccia A, Elledge SJ. The DNA damage response: making it safe to play with knives. Mol Cell. 2010;40:179–204. doi: 10.1016/j.molcel.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hoeijmakers JH. Genome maintenance mechanisms for preventing cancer. Nature. 2001;411:366–374. doi: 10.1038/35077232. [DOI] [PubMed] [Google Scholar]
- 57.Vidi PA, Leary JF, Lelievre SA. Building risk-on-a-chip models to improve breast cancer risk assessment and prevention. Integr Biol (Camb) 2013;5:1110–1118. doi: 10.1039/c3ib40053k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Biro FM, Deardorff J. Identifying opportunities for cancer prevention during preadolescence and adolescence: puberty as a window of susceptibility. J Adolesc Health. 2013;52:S15–20. doi: 10.1016/j.jadohealth.2012.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.