Abstract
Introduction
Pansclerotic morphea is a poorly described form of morphea with little information on prevalence, demographics, and clinical features. Classification criteria for this subtype varies and the distinction from other forms of morphea such as extensive generalized morphea and pansclerotic morphea is not always clear. The purpose of this study was to clarify classification criteria for pansclerotic morphea by identifying its prevalence in the morphea in adults and children (MAC) cohort and describing its demographic and clinical features as compared with generalized morphea.
Methods
Patients who met predefined criteria for generalized and pansclerotic morphea were identified using a modified Laxer and Zulian classification system. Baseline demographic and clinical features of the patients were compiled and then analyzed for traits characteristic of pansclerotic morphea versus those of generalized morphea. 113 met criteria for inclusion – 13 pansclerotic and 100 generalized morphea type.
Results
Pansclerotic patients were more frequently male (46.2% vs. 6%, p<0.0001); had a shorter time to diagnosis (mean difference of 10.4 months [95% CI: 0.8-19.9 months], p=0.0332); higher rates of functional impairment (61.5% vs. 16%, p=0.0046); higher rates of deep involvement (61.5% vs. 17%, p=0.004); and higher average mRSS (mean difference of 10.8 points [95% CI: 5-16.6], p=0.0018), LoSDI (mean difference 28.3 [95% CI: 9-47.6], p=0.009), and PGA-D scores (mean difference 25.1 [95% CI: 0.3-50], p=0.048).
Conclusions
Our results suggest demographic and clinical features are sufficient to define the pansclerotic subtype as they represent a distinct clinical phenotype with a more rapidly progressive and severe course commonly accompanied by disability. Presence of features of the pansclerotic phenotype should alert practitioners to the possibility of significant morbidity and the need for early aggressive treatment.
Keywords: localized scleroderma, morphea, pansclerotic, sclerosis
Introduction
Morphea, also known as localized scleroderma, is an inflammatory skin disorder characterized by excessive collagen deposition in the skin, dermis, and/or subcutaneous tissues (1-5). Morphea causes permanent cosmetic and functional sequelae including hyper or hypopigmentary changes, tissue atrophy (both superficial and/or deep), or impaired joint mobility or deformity.
At this time, there is no widely accepted classification scheme for morphea. Several have been published, including those of Laxer and Zulian which includes 5 subtypes: circumscribed (superficial or deep), linear (superficial or deep), generalized, pansclerotic, or mixed (Table 1) (3). Another frequently cited alternate system by Peterson, et al also designates 5 types which include plaque, deep, linear, bullous, and generalized with “disabling pansclerotic morphea of children” noted as a subtype of deep morphea (4). Within these classification systems, the greatest variation is in the description of the pansclerotic subtype, particularly in the depth of tissue involved. Further, the distinction between extensive generalized morphea and pansclerotic morphea is not always clear due to the lesion distribution in pansclerotic morphea overlapping with the description of multiple body site involvement in generalized morphea. Some such as Tuffanelli, et al. categorize pansclerotic morphea as a subset of generalized morphea (6).
Table 1.
Preliminary proposed classification of juvenile localized scleroderma
| Main group | Subtype | Description |
|---|---|---|
| (1) Circumscribed morphoea | (a) Superficial | Oval or round circumscribed areas of induration limited to epidermis and dermis, often with altered pigmentation and violaceous, erythematous halo (lilac ring). They can be single or multiple |
| (b) Deep | Oval or round circumscribed deep induration of the skin involving subcutaneous tissue extending to fascia and may involve underlying muscle. The lesions can be single or multiple. Sometimes the primary site of involvement is in the subcutaneous tissue without involvement of the skin | |
| (2) Linear scleroderma | (a) Trunk/limbs | Linear induration involving dermis, subcutaneous tissue and, sometimes, muscle and underlying bone and affecting the limbs and the trunk |
| (b) Head | En coup de sabre (ECDS). Linear induration that affects the face and the scalp and sometimes involves muscle and underlying bone. Parry Romberg or progressive hemifacial atrophy loss of tissue on one side of the face that may involve dermis, subcutaneous tissue, muscle and bone. The skin is mobile | |
| (3) Generalized morphoea | Induration of the skin starting as individual plaques (four or more and larger than 3 cm) that become confluent and involve at least two out of seven anatomic sites (head-neck, right upper extremity, left upper extremity, right lower extremity, left lower extremity, anterior trunk, posterior trunk) | |
| (4) Pansclerotic morphoea* | Circumferential involvement of limb(s) affecting the skin, subcutaneous tissue, muscle and bone. The lesion may also involve other areas of the body without internal organs involvement | |
| (5) Mixed morphoea | Combination of two or more of the previous subtypes. The order of the concomitant subtypes, specified in brackets, will follow their predominant representation in the individual patient [i.e. mixed morphoea (linear-circumscribed)] |
MAC registry classification based on the following clinical description: Circumferential involvement of majority of body surface areas with sparing of fingers and toes; affecting the dermis and frequently subcutaneous tissue, muscle, and/or bone; no internal organ involvement
R Laxer, F Zulian. Localized scleroderma. Current Opinions in Rheumatology. 2006.18:606-613.
The largest case series to date describes 14 children with pansclerotic morphea characterized by extensive body surface area (BSA) involvement, often circumferential in nature, and deep tissue involvement (7). Lesions were noted to spare only the fingers and toes. A number of case reports, predominantly involving children, detail similar clinical findings, however the definition and frequency of “deep tissue involvement” was ambiguous and inconsistent (8-10). Further, the relative frequency among morphea patients, demographic features, clinical features, and response to treatment of pansclerotic morphea remains unknown, especially among adults.
The MAC (Morphea in Adults and Children) cohort is designed to assess the clinical, demographic, and autoimmune features of carefully phenotyped morphea patients. As a prospective cohort study, it is ideally situated to report the prevalence and clinical characteristics of patients with pansclerotic morphea including the nature and frequency of “deep involvement.”
We have observed several patients with morphea who have skin lesions consistent with the clinical description for pansclerotic morphea. However, none have demonstrable bony involvement. These observations along with the ambiguity in the literature led us to hypothesize that despite meeting criteria for generalized morphea based on number of body sites involved, pansclerotic morphea is clinically distinct. Thus we identified patients with pansclerotic morphea within the MAC cohort using a modified Laxer and Zulian classification scheme (based on consistent clinical features and free from the requirement of deep involvement) and described the demographic and clinical features of this subtype to better distinguish it from generalized morphea.
Methods
This is a cross-sectional study of patients in the MAC cohort meeting predefined criteria for pansclerotic morphea and generalized morphea.
Patients
All study patients were drawn from the Morphea in Adults and Children (MAC) cohort – an ongoing prospective registry established in 2007 at the University of Texas Southwestern Medical Center to better understand the demographic, clinical, and autoimmune features of morphea. The registry enrolls patients 3 years of age or older demonstrating clinical and/or histological features of morphea. Participants were recruited from within the University of Texas Southwestern Medical Center system, encompassing 2 dedicated pediatric care facilities, a county hospital, and a faculty-based practice. Additionally, patients were enrolled through regional and national referrals from private practitioners (both pediatric and adult rheumatologists and dermatologists) in an attempt to enroll patients of widely varied disease severity, socioeconomic, and demographic backgrounds.
All patients enrolled in the registry were examined by a single provider with expertise in morphea (HJ), assigned subtypes modeled after a modified version of the Laxer and Zulian classification system as noted in Table 1, and given clinical scores. Pansclerotic morphea was defined as the presence of a cutaneous distribution pattern consistent with prior reports of near total BSA involvement with the characteristic sparing of the fingers and toes. Additional criteria of deep involvement beyond the dermis was not factored into the categorization of patients due to the significant variation in both definition and lack of consistent reporting of deep involvement per prior reports (2, 3, 7, 10-13). Registry participants completed a comprehensive health questionnaire in addition to the collection of blood and/or skin biopsy samples for immunologic and genetic studies. Patient-reported findings were confirmed by interview, physical examination, and/or requisition of their medical records. Inclusion criteria for the present study consisted of any patient having 1 or more registry visits with the designation of either pansclerotic or generalized morphea. Clinical data and biological samples were obtained from patients at each annual follow-up study visit when available for longitudinal analysis.
Variables of Interest
Clinical and demographic information was extracted from case report forms (designed specifically for the MAC cohort) administered at the initial enrollment visit. Clinical measures used to assess disease severity included an assessment of functional impairment (defined as presence of limited range of motion, contractures, and/or joint deformity due to direct morphea involvement as determined by physical examination by a single provider [HJ]) and deep involvement beyond the dermis (determined by clinicopathological diagnosis and/or MRI imaging). Depth of involvement was assessed primarily by the location of inflammatory cell infiltrate and depth of sclerosis in conjunction with other signs such as thickening or homogenization of collagen bundles as well as compression or loss of appendegeal structures. MRI was used to assess for subcutaneous septal thickening, fascial thickening, perifascial enhancement, myositis, or enhancement of the bone to aid in lesion depth determination. Any reported systemic symptoms or concomitant disorders were verified by history, direct examination, or review of medical records. Systemic symptoms reported in the present study were established by referral to appropriate providers for evaluation and treatment.
The Dermatology Life Quality Index (DLQI) was used to assess morphea impact on life quality. Standardized clinical scoring systems included both the Localized Scleroderma Cutaneous Assessment Tool (LoSCAT) and the modified Rodnan Skin Score (mRSS) (14, 15). The mRSS was concurrently utilized because the LoSCAT was not yet available at the inception of the cohort study and was thus continued to provide continuity for patients enrolled prior to the implementation of the LoSCAT. The LoSCAT assesses activity and damage via the components of Localized Scleroderma Skin Activity Index (LoSAI), Physician Global Assessment of Disease Activity (PGA-A), Localized Scleroderma Skin Damage Index (LoSDI), and Physician Global Assessment of Disease Activity (PGA-A). These activity and damage indices have been validated for use in morphea and have shown substantial to excellent inter- and intra-rater reliability, with the damage components showing little variation in stable patients over a 3-month time period (15-17).
Autoantibody testing for anti-nuclear antibodies (ANA) and anti-histone antibodies (18) were performed with sera isolated from whole blood. ANA for all subjects were performed in a single laboratory by a single investigator (FA) and were determined using indirect immunofluorescence (IIF) on HEp-2 cells (Antibodies Inc., Davis, CA) using previously published methods (19). Titers ≥1:80 were considered positive and IIF patterns of either speckled, centromere, nucleolar, homogenous, or mitochondrial were designated by FA. AHA testing was determined in a single laboratory (HJ) using enzyme-linked immunosorbent assay (ELISA) kits (Orgentec Diangostika, Mainz, Germany) according to the manufacturer’s directions, with levels >40 U/ml considered positive as determined by the manufacturer.
Additional data collected on pansclerotic patients included systemic immuno-suppressive treatments administered after enrollment in the registry as well as any occurrence of systemic symptoms and related complications.
Statistics
Means or medians along with the standard deviation were calculated for continuous variables. Total count and percentages were calculated for categorical variables. We compared demographic and clinical features of generalized patients with and without the pansclerotic subtype using the Student t test for continues variables and a two-tailed Fisher exact test for categorical variables. P values less than or equal to 0.05 were considered significant. Statistical analysis was performed on GraphPad Prism software, version 5.04.
Results
Study Patients
Of the 360 patients enrolled in the MAC cohort between September 2007 and August 2012, 113 were identified with having either pansclerotic or generalized morphea at enrollment with 13 patients meeting predefined criteria for the pansclerotic type and 100 for the generalized type.
Baseline patient demographics and clinical characteristics for all patients are summarized in Table 2 with further reporting broken down by subtypes. Patients overall had a mean age at enrollment of 53 and were predominantly Caucasian and female.
Table 2.
Study patient characteristics
| All Patients | Pansclerotic | Generalized | P-value | |
|---|---|---|---|---|
| Total no. of patients | 113 | 13 | 100 | |
| Age, Yrs, mean (SD) | 53 (17) | 54 (21) | 53 (16) | 0.848 |
| Age Onset, Yrs, mean (SD) | 49 (19) | 53 (22) | 48 (18) | 0.447 |
| Time to Diagnosis, Mo, median (SD) | 9 (29) | 6 (13) | 10 (30) | 0.0332 |
| Gender, No. (%) | < 0.0001 | |||
| Male | 12 (10.6%) | 6 (46.2%) | 6 (6%) | |
| Female | 101 (89.4%) | 7 (53.8%) | 94 (94%) | |
| Race, n (%) | ||||
| Caucasian | 86 (76.1%) | 7 (53.8%) | 79 (79%) | 0.145 |
| Latino | 16 (14.2%) | 4 (30.8%) | 12 (12%) | 0.087 |
| African American | 6 (5.3%) | 2 (15.4%) | 4 (4%) | 0.141 |
| Asian | 3 (2.7%) | 0 (0%) | 3 (3%) | 1.000 |
| Other | 2 (1.8%) | 0 (0%) | 2 (2%) | 1.000 |
| Functional Impairment, No. (%) | 24 (21.2%) | 8 (61.5%) | 16 (16%) | 0.005 |
| Deep Involvement, No. (%) | 25 (22.1%) | 8 (61.5%) | 17 (17%) | 0.004 |
| No. of Treatments, No. (SD) | 2.4 (2) | 2.8 (2) | 2.3 (2) | 0.175 |
| MRSS, mean (SD) | 8 (6) | 18 (9) | 7 (4) | 0.002 |
| DLQI, mean (SD) | 7 (6) | 12 (8) | 6 (6) | 0.056 |
| LoSAI, mean (SD) | 31 (27) | 54 (40) | 27 (22) | 0.065 |
| LoSDI, mean (SD) | 24 (19) | 48 (26) | 20 (14) | 0.009 |
| PGA-A, mean (SD) | 54 (29) | 64 (30) | 53 (29) | 0.308 |
| PGA-D, mean (SD) | 31 (23) | 52 (34) | 27 (19) | 0.048 |
Abbreviations: Dermatology Life Quality Index (DLQI), Localized Scleroderma Skin Activity Index (LoSAI), Localized Scleroderma Skin Damage Index (LoSDI), Modified Rodnan Skin Score (mRSS), Physician Global Assessment of Disease Activity (PGA-A), Physician Global Assessment of Disease Activity (PGA-A), standard deviation (SD)
Clinical characteristics of overall study patients
Caucasian (76.1%) females (89.4%) comprised the majority of patients in the group overall. Only 12 (10.6%) had lesion onset ≤ 18 years of age. 21.2% had functional impairment and 22.1% had evidence of deep involvement. LoSCAT scores for generalized morphea patients were as follows – means for the LoSDI, PGA-D, LoSAI, and PGA-A were 23.9, 30.7, 30.6, and 54.3 respectively. A mean number of 2.4 prior and/or current treatments were reported. Topical corticosteroids were the most frequently utilized therapy at enrollment with 69.9% reporting use, followed by phototherapy and methotrexate at 24.8% and 23% respectively. ANA testing was available for 59 patients, 18 of which had positive ANA titers. A speckled pattern predominated in 72.2% (13/18) of cases. Similarly, sera from 91 patients underwent AHA testing, 7 of which were positive. The most common concomitant autoimmune disorders among all generalized morphea patients were rheumatoid arthritis (n=6), genital lichen sclerosus et atrophicus (n=5), and psoriasis (n=4). None of the patients had sclerodactyly, Raynaud’s phenomenon, or nail-fold capillary changes consistent with systemic sclerosis. Skin biopsies were performed on nearly all study patients (12/13 pansclerotic patients and 92/100 generalized patients), but few had deep incisional biopsies (3/13 pansclerotic patients, 1/100 generalized patients) or MRI studies (4/13 pansclerotic patients, 1/100 generalized patients) performed at initial presentation.
The pansclerotic subtype
Pansclerotic morphea comprised 3.6% of all morphea patients in the MAC cohort. The characteristics of patients with the pansclerotic subtype compared to generalized morphea patients are presented in Table 2. Compared to generalized morphea patients, pansclerotic patients were more predominantly male (46.2% vs. 6%, p<0.0001), had shorter time to diagnosis (median time of 6 vs. 10 months, p<0.0001), higher rates of functional impairment (61.5% vs. 16%, p=0.0046), higher rates of deep involvement as defined by skin biopsy (61.5% vs. 17%, p=0.004), higher average mRSS (mean of 17.8 vs. 7, p=0.0017), higher LoSDI score (mean of 48 vs 19.7, p=0.009), and higher PGA-D score (mean of 52 vs 26.9, p=0.048). No significant differences were found in visit age, onset age, race, number of treatments received, DLQI score, or disease activity scores. The most common treatment modalities reported by patients with pansclerotic morphea were systemic corticosteroids, methotrexate, and topical steroids at rates of 61.5%, 53.8%, and 38.5%.
Autoantibody testing results were similar between both groups - ANA were present in 28.6% (2/7) in the pansclerotic group vs. 30.8% (16/52) in the generalized group, AHA were correspondingly present in 10% (1/10) and 7.4% (6/81) of pansclerotic and generalized patients. Treatments prescribed at baseline and other concomitant systemic features such as dysphagia and/or restrictive pulmonary defects for the pansclerotic patients are reported in Table 3. Patients with restrictive defect on PFT had follow up CT scans with none demonstrating parenchymal lung disease. The restrictive defect was attributed to circumferential involvement of the chest in all cases. Evaluation of patients with dysphagia revealed an absence of esophageal dysmotility. Rather, all defects were secondary to extensive sclerotic neck lesions or abdominal sclerosis. Those with hand edema had circumferential full thickness sclerosis of the forearm, in one case causing necrosis of the fingertips and autoamputation in the absence of direct involvement of morphea of the fingertips. Evaluation by hand surgery and vascular surgery revealed absence of intrinsic vascular disease, instead implicating the symptoms as a result of compartment syndrome due to extensive sclerosis of the forearm. 2 patients had squamous cell carcinoma (SCC) and basal cell carcinoma (BCC).
Table 3.
Prescribed treatments and systemic features of the pansclerotic morphea patients
| Patient | Treatment | Systemic Features |
|---|---|---|
| 1 | MTX, UVA-1 | |
| 2 | MTX | |
| 3 | UVA-1 | |
| 4 | prednisone, UVA-1 | edema of hands and fingers, dysphagia, restrictive-pattern PFT, chronic ulcers, fingertip necrosis, flexural fissures |
| 5 | MTX, prednisone, UVA-1 | edema of hands and fingers |
| 6 | MTX, prednisone | restrictive-pattern PFT, SCC*, BCC, flexural fissures |
| 7 | MTX, prednisone | restrictive-pattern PFT (oxygen dependent), ITP, dysphagia, SCC*, BCC, flexural fissures |
| 8 | MTX, prednisone | |
| 9 | MTX, prednisone | |
| 10 | MTX, prednisone | |
| 11 | MTX, prednisone | |
| 12 | MTX, prednisone | |
| 13 | None |
history of SCC prior to initial development of morphea lesions
Abbreviations: basal cell cancer (BCC), idiopathic thrombocytopenic purpura (ITP), methotrexate (MTX), pulmonary function tests (PFT), squamous cell cancer (SCC), ultraviolet light A-1 (UVA-1)
In addition, the traditional evolution of lesions among the pansclerotic patients was described as beginning on the trunk with subsequent rapid centrifugal spread. Abrupt cut off at the metacarpophalangeal (MCP) or metatarsophalangeal (MTP) joints were observed on examination of this series of patients (Figure 1). In contrast generalized morphea patients developed individual lesions that gradually proliferated over time and coalesced.
Figure 1.
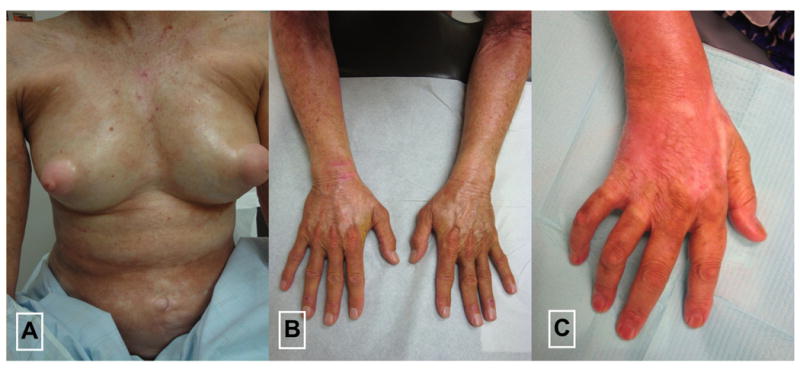
Pansclerotic morphea showing extensive body surface area involvement (A) with distinct lesion features such as abrupt cut off of at the metacarpophalangeal (MCP) joints (B, C)
Longitudinal data for 4 pansclerotic patients was available through the 3rd annual follow-up. The dynamic changes in the mean DLQI, mRSS, LoSAI, LoSDI, PGA-A, and PGA-D among these 4 patients is presented in Figure 2, which revealed gradual decrease in disease activity over time, but persistent damage related to the initial skin lesions.
Figure 2.
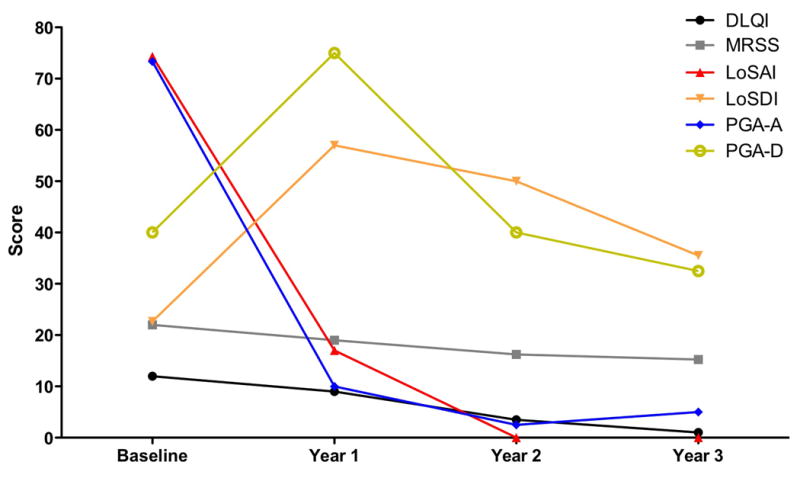
Longitudinal analysis of average clinical score measures of 4 pansclerotic patients with follow-up through year 3
Discussion
This cross-sectional assessment of patients with generalized morphea in the MAC cohort characterized demographic and clinical features of patients to identify unique attributes within this group. Our results indicate that pansclerotic morphea represents 3.6% of morphea cases in our cohort and is characterized by a more rapidly progressive and severe phenotype than generalized morphea with features distinct from systemic sclerosis. While prevalence in this cohort may not be representative of the overall morphea population, the results nonetheless support the clinical utility of identifying pansclerotic patients as a unique phenotype for purposes of evaluation and treatment.
The results of the present study confirm prior observations in which patients with pansclerotic morphea have a more severe clinical course with a higher relative frequency of males as compared with other morphea patients (7-13). The unique distribution of skin lesions in our case series was also consistent with prior reports of centrifugal spread encompassing near total body involvement except for the digits. Reports in the literature also rarely note early lesions initially mimimicking other morphea types such as linear morphea (7). Nonetheless, the pattern observed in our cohort and as most frequently reported in the literature is distinct from both the cutaneous manifestations reported for systemic sclerosis which typically begins with acrosclerosis and generalized morphea which tends to begin with individual plaques. A high frequency of deep tissue involvement, which has historically been noted as one of the defining features, was also observed in this cohort of pansclerotic patients even though it was not a defining factor.
Prior reports indicate patients with pansclerotic morphea are at increased for squamous cell carcinoma. We observed 2 patients in the pansclerotic group who had SCC, but both were over 60 years of age with fair skin, and both already had a history of SCC preceding the onset of morphea. This picture is further complicated by the use of immunosuppressive agents in both cases, which are known to also increase the risk of squamous cell carcinoma (20). The literature suggests a more delayed onset of SCC in the range of 7-12 years after morphea development (11, 21) that is not yet captured by the shorter follow-up times currently on record for our patients. Therefore, conclusions regarding risk of SCC are difficult.
One novel observation in our cohort is the adult-predominant composition whereas most reported cases are of childhood-onset disease (1, 4, 7-10, 22). The age distribution of patients in the study is not the result of preferential enrollment in the MAC cohort, which is composed of 97 children and 263 adults. One explanation for this discrepancy could be attributed to a publication bias since these reports are not the result of a prospective study of morphea overall, but are rather a retrospective report of only the most severe cases which are most likely to be published in the literature. Children with pansclerotic morphea would be expected to have the most severe symptoms and complications from impaired growth due to the rapid growth taking place in this age group. Alternatively, pansclerotic morphea in children may entail a different clinical course and/or more severe disease pattern than that in adults.
While our patients had clinical findings similar to systemic sclerosis, these were secondary to cutaneous manifestations of morphea rather than direct internal organ involvement. Sclerodactyly-like changes were caused by edema of the hands and fingers due to circumferential sclerosis of the upper extremities. Severe circumferential sclerosis of the forearm causing vascular compromise of the fingers led to digital necrosis as was previously suggested in report by Maragh, et al (11). Restrictive pulmonary function test patterns were attributed to external restrictive mobility of the rib cage as follow-up CT scans did not reveal pulmonary parenchymal changes. Dysphagia only occurred in the setting of severe circumferential sclerosis of the neck without evidence of esophageal dysmotility. Further, characteristic findings of systemic sclerosis were absent (including sclerodactyly, Raynaud’s phenomenon, and nail-fold capillary changes) (23). In addition, the distribution pattern for systemic sclerosis has a tendency to spread proximally from the distal extremities, whereas pansclerotic lesions more frequently show the reverse pattern with proximal lesions spreading distally.
Longitudinal analysis was performed for 4 patients with sufficient follow-up. These results showed high measures for activity with high LoSAI and PGA-A scores that quickly resolved after initiation of treatment, accompanied by a concomitant rise in the damage measures, LoSDI and PGA-D, from baseline. These results seem to suggest some patients respond to aggressive treatment. On the other hand, one of 13 patients in this report passed away secondary to complications stemming from her morphea 3 years after disease onset despite aggressive treatment (Table 3). This compares to 4 deaths out of the remaining 343 patients in the whole registry (all were unrelated to morphea or its treatment). This confirms prior reports of poor long term survival (less than 10 years). However, future analysis of the cohort with longer follow-up will be needed to confirm these initial observations.
Historical descriptions of pansclerotic morphea have consistently defined it by a unique general distribution pattern with associated deep involvement (1-13, 22). Unfortunately, they have not been consistent in how depth of involvement is either defined (ranging from deep dermal to bone) or determined (2, 3, 7, 10-13). We removed this criteria due to ambiguity and instead solely assigned the pansclerotic subtype based on the clinical criteria of rapid evolution of near total body surface area involvement typically spreading centrifugally with sparing the fingers and toes. To earn the designation of deep involvement, we required either histopathologic or MRI evidence of involvement below the dermis. While skin biopsies were performed on the nearly all study patients, few were of the deep incisional type (due to problems with wound healing) and only a very small minority had MRI imaging performed. Thus sampling error is possible as these methods could miss or may not identify fascial, muscle or bone lesions. This may partially account for the lower rate of deep tissue involvement in our study compared to previous ones. However, our results suggest that patients with pansclerotic morphea are clinically distinct in terms of demographics, disease course, and morbidity. Therefore, the confirmation of deep tissue involvement, while commonly present, is not necessary for diagnosis. In fact, it may unnecessarily relegate patients with severe disease into the generalized or other subtype that is frequently treated less aggressively (24). It is important to highlight though that deep involvement of tissue below the dermis is a prominent feature in pansclerotic morphea contributing to the high rate of functional disability in this subtype.
Limitations of our study include those inherent to cross-sectional studies, potential selection bias, and the limited numbers of subjects available for analysis. Limitations due to limited numbers are inherent in studying a rare disease which restricts the types of analysis that can be performed. Selection bias of our registry patients may be evidenced by a adult-predominant age distribution seen in our series compared to the children-predominant prior reports. This may reflect the adult-focused practice at the study site. Additionally, as a tertiary referral center, our morphea registry cohort may not be an accurate reflection of the overall general morphea population due to an overrepresentation of the most severe types seen at this center. This factor may help explain the higher rates of some of the more severe types of morphea noted in our registry.
The practical clinical implications for identifying patients with the pansclerotic subtype based on these unique features pertain to the severe and rapidly progressing nature of this particular variant and warrants distinction from of generalized morphea and other morphea subtypes. When pansclerotic morphea is either present or clinically suspected, providers should be especially vigilant – patients should be closely followed with a low threshold for initiating aggressive treatment with systemic immunosuppressives and referrals to relevant specialists for management of the secondary systemic symptoms common in this subtype.
Acknowledgments
We would like to acknowledge Frank Arnett for his contribution in conducting autoantibody testing for anti-nuclear antibodies.
Funding/Support: Research for this manuscript was supported in part by NIH Grant No. K23AR056303-4.
This work was conducted with support from UT-STAR, NIH/NCRR/NCATS Grant Number UL1RR024982. The content is solely the responsibility of the authors and does not necessarily represent the official views of UT-STAR, The University of Texas Southwestern Medical Center at Dallas and its affiliated academic and health care centers, the National Center for Research Resources, or the National Institutes of Health.
Abbreviations
- ANA
Antinuclear Antibody
- AHA
Antihistone Antibody
- BCC
Basal Cell Carcinoma
- BSA
Body Surface Area
- DLQI
Dermatology Life Quality Index
- ELISA
Enzyme-Linked Immunosorbent Assay
- IIF
Indirect Immuno-Fluorescence
- LoSCAT
Localized Scleroderma Cutaneous Assessment Tool
- LoSAI
Localized Scleroderma Skin Activity Index
- LoSDI
Localized Scleroderma Skin Damage Index
- MCP
Metacarpophalangeal
- mRSS
Rodnan Skin Score
- MTP
Metatarsophalangeal
- MAC cohort
Morphea in Adults and Children cohort
- PFT
Pulmonary Function Test
- PGA-A
Physician Global Assessment of Disease Activity
- PGA-D
Physician Global Assessment of Disease Damage
- RNP
Ribonucleoprotein
- SCC
Squamous Cell Carcinoma
- SD
Standard Deviation
- Sm
Smith
Footnotes
Disclosures: None
References
- 1.Zulian F, Athreya BH, Laxer R, Nelson AM, Feitosa de Oliveira SK, Punaro MG, et al. Juvenile localized scleroderma: clinical and epidemiological features in 750 children. An international study Rheumatology (Oxford) 2006;45(5):614–20. doi: 10.1093/rheumatology/kei251. [DOI] [PubMed] [Google Scholar]
- 2.Fett N, Werth VP. Update on morphea: part I. Epidemiology, clinical presentation, and pathogenesis. Journal of the American Academy of Dermatology. 2011;64(2):217–28. doi: 10.1016/j.jaad.2010.05.045. quiz 29-30. [DOI] [PubMed] [Google Scholar]
- 3.Laxer RM, Zulian F. Localized scleroderma. Current opinion in rheumatology. 2006;18(6):606–13. doi: 10.1097/01.bor.0000245727.40630.c3. [DOI] [PubMed] [Google Scholar]
- 4.Peterson LS, Nelson AM, Su WP. Classification of morphea (localized scleroderma) Mayo Clinic proceedings Mayo Clinic. 1995;70(11):1068–76. doi: 10.4065/70.11.1068. [DOI] [PubMed] [Google Scholar]
- 5.Zulian F. Scleroderma in children. Pediatric clinics of North America. 2005;52(2):521–45. vii. doi: 10.1016/j.pcl.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 6.Tuffanelli DL. Localized scleroderma. Seminars in cutaneous medicine and surgery. 1998;17(1):27–33. doi: 10.1016/s1085-5629(98)80059-x. [DOI] [PubMed] [Google Scholar]
- 7.Diaz-Perez JL, Connolly SM, Winkelmann RK. Disabling pansclerotic morphea of children. Archives of dermatology. 1980;116(2):169–73. [PubMed] [Google Scholar]
- 8.Doede T, Wollina U, Hindermann W, Schier F, Bondartschuk M. Pansclerotic morphea in childhood: a case report. Pediatric surgery international. 2003;19(5):406–8. doi: 10.1007/s00383-003-1020-7. [DOI] [PubMed] [Google Scholar]
- 9.Wollina U, Looks A, Uhlemann C, Wollina K. Pansclerotic morphea of childhood-follow-up over 6 years. Pediatric dermatology. 1999;16(3):245–7. doi: 10.1046/j.1525-1470.1999.00153.x. [DOI] [PubMed] [Google Scholar]
- 10.Tekin NS, Altinyazar HC, Tekin IO, Keskin SI, Kucukoglu R, Onsun N. Disabling pansclerotic morphoea: a case report. International journal of clinical practice. 2010;64(1):99–101. doi: 10.1111/j.1742-1241.2006.01039.x. [DOI] [PubMed] [Google Scholar]
- 11.Maragh SH, Davis MD, Bruce AJ, Nelson AM. Disabling pansclerotic morphea: clinical presentation in two adults. Journal of the American Academy of Dermatology. 2005;53(2 Suppl 1):S115–9. doi: 10.1016/j.jaad.2004.10.881. [DOI] [PubMed] [Google Scholar]
- 12.Sherber NS, Boin F, Hummers LK, Wigley FM. The “tank top sign”: a unique pattern of skin fibrosis seen in pansclerotic morphea. Annals of the rheumatic diseases. 2009;68(9):1511–2. doi: 10.1136/ard.2008.102723. [DOI] [PubMed] [Google Scholar]
- 13.Song P, Gocke C, Wigley FM, Boin F. Resolution of pansclerotic morphea after treatment with antithymocyte globulin. Nature reviews Rheumatology. 2009;5(9):513–6. doi: 10.1038/nrrheum.2009.159. [DOI] [PubMed] [Google Scholar]
- 14.Furst DE, Clements PJ, Steen VD, Medsger TA, Jr, Masi AT, D’Angelo WA, et al. The modified Rodnan skin score is an accurate reflection of skin biopsy thickness in systemic sclerosis. The Journal of rheumatology. 1998;25(1):84–8. [PubMed] [Google Scholar]
- 15.Arkachaisri T, Vilaiyuk S, Torok KS, Medsger TA., Jr Development and initial validation of the localized scleroderma skin damage index and physician global assessment of disease damage: a proof-of-concept study. Rheumatology (Oxford) 2010;49(2):373–81. doi: 10.1093/rheumatology/kep361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arkachaisri T, Pino S. Localized scleroderma severity index and global assessments: a pilot study of outcome instruments. The Journal of rheumatology. 2008;35(4):650–7. [PubMed] [Google Scholar]
- 17.Arkachaisri T, Vilaiyuk S, Li S, O’Neil KM, Pope E, Higgins GC, et al. The localized scleroderma skin severity index and physician global assessment of disease activity: a work in progress toward development of localized scleroderma outcome measures. The Journal of rheumatology. 2009;36(12):2819–29. doi: 10.3899/jrheum.081284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rook AH, Freundlich B, Jegasothy BV, Perez MI, Barr WG, Jimenez SA, et al. Treatment of systemic sclerosis with extracorporeal photochemotherapy. Results of a multicenter trial. Arch Dermatol. 1992;128(3):337–46. [PubMed] [Google Scholar]
- 19.Arora-Singh RK, Assassi S, del Junco DJ, Arnett FC, Perry M, Irfan U, et al. Autoimmune diseases and autoantibodies in the first degree relatives of patients with systemic sclerosis. Journal of autoimmunity. 2010;35(1):52–7. doi: 10.1016/j.jaut.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rangwala S, Tsai KY. Roles of the immune system in skin cancer. The British journal of dermatology. 2011;165(5):953–65. doi: 10.1111/j.1365-2133.2011.10507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wollina U, Buslau M, Heinig B, Petrov I, Unger E, Kyriopoulou E, et al. Disabling pansclerotic morphea of childhood poses a high risk of chronic ulceration of the skin and squamous cell carcinoma. The international journal of lower extremity wounds. 2007;6(4):291–8. doi: 10.1177/1534734607308731. [DOI] [PubMed] [Google Scholar]
- 22.Wollina U, Buslau M, Petrov I, Pramatarov K. Disabling pansclerotic morphea of childhood. Expert Review of Deramtology. 2007;2(6):775–84. [Google Scholar]
- 23.Matucci-Cerinic M, Allanore Y, Czirjak L, Tyndall A, Muller-Ladner U, Denton C, et al. The challenge of early systemic sclerosis for the EULAR Scleroderma Trial and Research group (EUSTAR) community. It is time to cut the Gordian knot and develop a prevention or rescue strategy. Annals of the rheumatic diseases. 2009;68(9):1377–80. doi: 10.1136/ard.2008.106302. [DOI] [PubMed] [Google Scholar]
- 24.Johnson W, Jacobe H. Morphea in adults and children cohort II: patients with morphea experience delay in diagnosis and large variation in treatment. Journal of the American Academy of Dermatology. 2012;67(5):881–9. doi: 10.1016/j.jaad.2012.01.011. [DOI] [PubMed] [Google Scholar]


