Abstract
Neurological injury resulting from cardiac surgery has a range of manifestations from focal neurological deficit to encephalopathy or coma. As the safety of drug-eluting stents comes into question, more patients will likely undergo coronary artery bypass graft surgery. These projections, along with the growing proportions of elderly patients and those with comorbidities, portend the potential for rising rates of perioperative neurological complications. The risk for neurological injury may be determined by the type of procedure, by patient-specific characteristics, and by the extent of cerebral embolization and hypoperfusion during and after surgery. Changes in surgical techniques, including the use of off-pump surgery, have not decreased rates of brain injury from cardiac surgery. When appropriate, modern neuroimaging techniques should be used in postoperative patients to confirm diagnosis, to provide information on potential etiology, to direct appropriate therapy, and to help in prognostication. Management of postoperative medications and early use of rehabilitation services is a recommended strategy to optimize the recovery for individuals with neurological injury after cardiac surgery.
Keywords: Cardiopulmonary bypass, stroke, postoperative complications
Postoperative neurological injury is an important source of patient morbidity and mortality after cardiac surgery. Patients who experience strokes in the first 30 days after cardiac surgery have a mortality rate upwards of 20%, compared with 2 to 4% for patients without stroke.1 Stroke is only one form of neurological injury after cardiac surgery; other manifestations include encephalopathy or delirium and coma. In combination with other forms of brain injury, it is estimated that between 6 and 28% of patients experience some type of perioperative neurological injury.2,3 With the increasing use of magnetic resonance imaging (MRI) with diffusion-weighted imaging (DWI), it is likely that this estimate will increase.
Overall, the risk for mortality from cardiac surgery has remained low despite rising numbers of elderly patients and patients at risk for poor outcome. These observations, however, place increasing emphasis on perioperative complications as a source of patient outcome.4 The patients who are most likely to undergo cardiac surgery are the same individuals who are most likely to experience neurological complications resulting from surgery.
Over the last decade, cardiac surgery had become a less frequently used means of treatment for coronary artery disease due to a variety of reasons, including increasing use of percutaneous coronary artery interventions (PCI) with drug-eluting stents. In fact, PCI with stent implantation was used in over 80% of coronary revascularization cases in 2005.5 Nonetheless, the pendulum is likely to swing back toward coronary artery bypass graft (CABG) surgery as new concerns arise regarding late thrombosis of drug-coated stents.6 Further evidence supporting the theory that the number of CABG procedures may increase in coming years is found in an observational study comparing over 7,000 patients who underwent CABG surgery with almost 10,000 individuals who received PCI drug-eluting stents. Hannan and colleagues reported hazard ratios for death or myocardial infarction of 0.71 and 0.75 for two- and three-vessel disease, respectively, demonstrating decreased mortality in those individuals undergoing CABG surgery.7
Thus, neurologists are increasingly likely to be called to evaluate patients who are about to undergo or who have recently undergone cardiac surgery. The reasons for consultation range from preoperative risk estimation to postoperative evaluation of a new focal neurological deficit, seizures, slowness to emerge from general anesthesia, delirium, or coma. Understanding the likely etiology of a patient’s condition requires knowledge of the types of neurological injuries that can occur to cardiac surgical patients, as well as knowledge about risks associated with particular surgery types.
TYPES OF CARDIAC SURGERY AND ASSOCIATED NEUROLOGICAL INJURY
Coronary Artery Bypass Graft Surgery
Individuals undergoing coronary artery bypass graft (CABG) surgery are the most frequently studied group of cardiac surgery patients. Between 0.8 and 5.2% of individuals undergoing CABG surgery have clinically evident stroke postoperatively.8,9 The range in prevalence depends on the patients studied, the way they are studied (for instance, retrospective vs prospective studies), and the depth of neurological examination. In our experience, the rate of clinically evident stroke rises in patients undergoing CABG surgery in combination with other procedures, such as valvular surgery.10 In a German population, 1.7% of patients having isolated CABG had neurological complications after surgery, whereas 3.3% of patients undergoing CABG with valve surgery developed stroke. The number increased further (to 6.7%) in patients undergoing multiple valve surgery.11 Patients who require repeat cardiac surgical procedures are also at increased risk for adverse outcomes, including stroke.12
The frequency of brain ischemia is certainly much higher when patients are examined with brain imaging. Small case series where MRI with DWI has been performed in patients after CABG surgery (regardless of neurological symptoms) report that between 18 to 26% of low-risk patients13,14 and 45 to 62% of high-risk patients15,16 have acute perioperative brain ischemia. Many of these lesions are quite small, multifocal, and might not be associated with classic focal neurological deficits. The long-term importance of perioperative brain ischemia has not been extensively studied, but may contribute to eventual cognitive decline.
Aortic Surgery
Rates of postoperative clinical stroke in patients undergoing surgery to the aortic arch range from less than 5%17 to over 10%.18 Surgery to repair aneurysms of the ascending aorta and aortic arch are often performed with the use of hypothermic circulatory arrest, which was initially developed in an attempt to provide neurological protection. This procedure is still commonly used for many aortic surgeries, although the development of selective cerebral perfusion is increasingly employed to avert the need for circulatory arrest in selected patients.
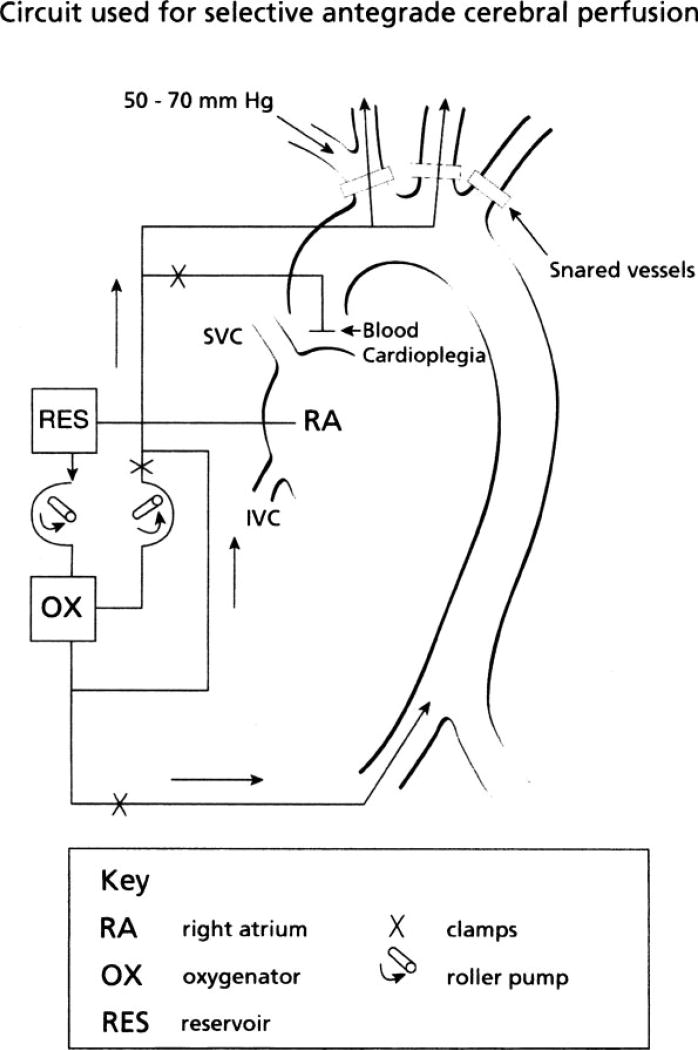
Retrograde cerebral perfusion, allowing flow to the brain via the superior vena cava during deep hypothermia, was developed as a means to protect the brain during surgery on the aortic arch; its use, however, has not been shown to reduce the frequency of adverse neurological outcomes and may actually worsen cerebral edema.19 A more frequently used technique is selective antegrade cerebral perfusion, a means of lessening the risk for neurological injury for selected patients undergoing extensive aortic aneurysm repairs.20 This technique usually consists of cannulation of the innominate or left carotid artery, with clamping or snaring of the left subclavian artery to avoid a steal phenomenon.19 Fig. 1 is an example of the circuit used for this technique. Outcomes with this technique have been promising in observational series,21,22 although concern has been raised that this technique may only perfuse one side of the brain in the absence of an intact circle of Willis. Urbanski and colleagues determined that the use of computed tomography angiography (CTA) to identify completeness of the circle of Willis prior to surgery did not correlate with functional tests of cerebral cross-perfusion during aortic surgery with selective unilateral cerebral perfusion.22 These authors, therefore, did not recommend the standard use of preoperative CTA for this purpose. The best management to allow for optimal cerebral perfusion during aortic arch surgery for patients with known intracranial stenosis is still not clear.
Figure 1.
Diagram of the circuit used for selective antegrade cerebral perfusion. (Adapted from Harrington et al.109)
Other specific potential neurological complications also must be considered in patients undergoing aortic surgery repair. For patients who have had dissection of the ascending aorta, the dissection can extend upwards into the neck vessels leading to stroke. The use of carotid stenting (without protection devices) has been described in a few case reports,23,24 but definitive evidence is lacking as to the most appropriate treatment for these patients.
Isolated Valve Surgery
Patients undergoing valve replacement, without concomitant bypass grafting, have been less frequently studied. At our institution, in recent years, clinical stroke rates in patients getting isolated valve repair or replacement range from 2 to 5%, with rates of other types of neurological injury, including encephalopathy and seizures, ranging from 8 to 20%. Cook and colleagues found new acute infarctions on DWI MRI in 41% of patients undergoing isolated aortic or mitral valve replacement, with 35% having persistent neurocognitive deficits at 4 to 6 weeks.25 In a sample of 42 patients who underwent MRI before and after surgery, all of whom had valve surgery, but only seven of whom also had CABG, Barber et al reported new clinical strokes in 5% of patients after surgery, but new postoperative DWI lesions in 43% of patients.26
PREOPERATIVE NEUROLOGICAL CONSULTATION FOR CARDIAC SURGERY PATIENTS
The consulting neurologist may be called upon for preoperative evaluation or treatment in patients, with or without history of cerebrovascular disease, scheduled for cardiac surgery. Assessment of carotid disease with duplex has a proven role in preoperative assessment of patients undergoing cardiac surgery.27 It has been proposed, however, that it is adequate to screen only those patients who are over 65 years of age, with a carotid bruit or with known cerebrovascular disease, and that such a practice would not worsen postoperative neurological outcomes.28
Although a detailed discussion of the controversy regarding the appropriate treatment of carotid disease in the setting of planned cardiac surgery is beyond the focus of this article, a brief discussion is warranted. The formal American College of Cardiology/American Heart Association (ACC/AHA) guidelines, updated in 2004, recommend, based on level C evidence (primarily from case series or extrapolated from observational studies), that carotid endarterectomy is “probably recommended before CABG or concomitant to CABG” in patients with high-grade asymptomatic disease (over 80%) or with symptomatic carotid stenosis. Further, they recommended carotid screening in patients over age 65 and those with left main coronary stenosis, peripheral vascular disease, history of smoking, history of transient ischemic attack (TIA) or stroke, or carotid bruit on examination (also level C evidence).29 The more recent use of cardiac stenting has added further uncertainty to the issue; observational evidence supports the safety and efficacy of a staged procedure consisting of carotid stenting prior to cardiac surgery for patients with asymptomatic carotid stenosis over 80%.30 It is appropriate to proceed with either surgical or endovascular repair of symptomatic carotid disease for patients scheduled to undergo cardiac surgery, but which of these is preferable, what to do with patients with asymptomatic disease, and when to do the procedures is still quite controversial. The approach chosen further depends on the experience of the surgical and interventional teams and on institutional outcomes. Some centers with low perioperative stroke rates contend that carotid endarterectomy is indicated in selected patients based on those indications recommended for patients not undergoing cardiac surgery. This practice is based on the theory that carotid surgery may/may not influence the perioperative stroke rate, but it improves long-term outcomes regardless of surgery.
Patients with cardiovascular disease of sufficient severity to require CABG surgery can also have intracranial vascular disease. In a Japanese study, Takeuchi and colleagues performed preoperative angiography in 100 high-risk patients, and found significant vascular stenosis or aneurysm in only seven of these patients.31 In another Japanese study, as many as 18.7% of patients scheduled for cardiac surgery had intracranial stenosis, with 33% having at least 50% extracranial carotid stenosis.32 However, intracranial stenosis is much more common in Japan than in the United States. There is no equivalent evidence supporting the role of preoperative vascular imaging, beyond carotid duplex, in American populations. This is particularly the case because the appropriate treatment, if intracranial stenosis were to be found, is not known. Known presence of such disease, though, might influence perioperative management (e.g., higher blood pressure during cardiopulmonary bypass).
Patients with a prior history of stroke who are on aspirin therapy should be maintained on aspirin, assuming no major concerns for hemorrhage. In fact, the use of aspirin for the 5 days preceding surgery, regardless of prior cerebrovascular history, is considered safe and is even associated with decreased in-hospital mortality.33 In addition, it is recommended to wait, ideally, 4 weeks between an ischemic stroke and elective CABG surgery, given the need for heparinization during surgery among other factors.29
Preoperative neurological evaluation of patients undergoing cardiac surgery may also involve risk assessment. Specific factors leading to increased risk for postoperative stroke or other brain injury are discussed below.
TYPES OF NEUROLOGICAL INJURY AFTER CARDIAC SURGERY
Stroke
PREOPERATIVE PREDICTORS OF RISK
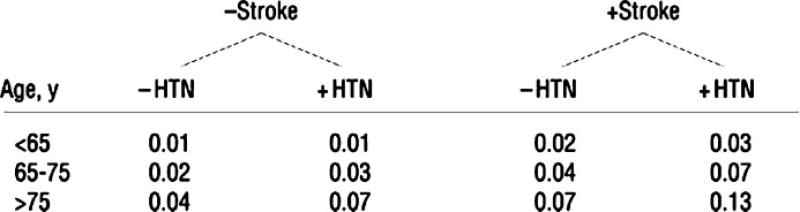
Patient preoperative characteristics are important in determining who is at risk for a postoperative stroke. In studies of predictors of postoperative stroke for patients undergoing CABG, hypertension, diabetes, and past stroke have been important indicators of stroke risk.34 Age is one of the most important predictors of brain injury. The risk for perioperative stroke is 4.6 times higher for individuals 65 to 75 years old and 5.2 times higher for patients over 75 years of age, compared with those under 65 years.1 Fig. 2 shows one schema used to predict probability of stroke in patients undergoing CABG surgery, based on patient preoperative characteristics.
Figure 2.
Probability of postoperative stroke. To determine a patient’s risk of developing a stroke, ask the following questions and then proceed to the correct part of the flowchart: Has the patient had a previous stroke, yes or no; does the patient have a diagnosis of hypertension (HTN), yes or no. Next, determine the patient’s age and proceed to the correct row of age groupings to see the probability for that patient. (Adapted from McKhann et al.34)
In addition, women appear to have a higher risk of postoperative cerebral ischemia, as well as higher postoperative mortality, even after adjustment for other markers of disease severity.35,36 Aortic atherosclerotic disease has also been identified as a risk factor for postoperative stroke and neurological dysfunction, both in autopsy studies37 and in observational epidemiologic studies.3,35 In addition, extensive atheromatous disease of the aorta has been linked to transcranial Doppler-detected cerebral microemboli.38
Other authors have identified preoperative internal carotid stenosis39,40 as a predictor of postoperative stroke, particularly when the stenosis has been symptomatic.41,42 Intracranial stenosis, in a Korean series,43 has also been reported as a predictor of postoperative stroke and of borderzone strokes, in particular. These sources of stenosis could also lead to artery-to-artery embolization, in addition to focal hypoperfusion (these mechanisms are further described below).
Genetic predisposition may help determine which patients are likely to develop stroke after cardiac surgery. Grocott and colleagues reported that certain alleles of the C-reactive protein and interleukin-6 polymorphisms were associated with a threefold increased rate of postoperative stroke.44 In addition, Tardiff et al described an association between presence of an Apolipoprotein E ε4 allele and cognitive dysfunction at 6 weeks postoperatively45; however, other authors have failed to find this association.46
Table 1 summarizes some of the factors most frequently identified in the literature as predictors of postoperative stroke. This information is not only important for individual decisions about surgery, but for planning of future research in which treatments can be aimed at individuals with the greatest predicted risk of brain injury.
Table 1.
Commonly Identified Preoperative Risk Factors for Postcardiac Surgery Stroke
| Preoperative Risk Factors |
|---|
| Hypertension |
| Diabetes |
| Prior stroke |
| Increasing age |
| Female gender |
| Symptomatic carotid stenosis |
| Aortic atheromatous disease |
| Peripheral vascular disease |
DIAGNOSIS
The approach to the diagnosis of a potential stroke after cardiac surgery should be similar to the approach to the diagnosis of acute stroke in any other setting. Leary and Caplan have emphasized that multimodal MRI, including DWI, perfusion-weighted MRI, and gradient-echo imaging, be used for patients with concern for postcardiac surgery ischemia.47 MRI with DWI is more sensitive than CT scanning in general for detecting new ischemic injury; this is also true in the post-CABG surgery population. This is particularly important because many patients with stroke after CABG surgery have multiple small embolic or watershed-territory infarcts that are less easily detected by CT scanning.48
The primary limitations to obtaining brain MRI scanning, beyond the standard contraindications in clinical practice, include the existence of temporary pacing wires, which remain in place for the first few days postoperatively, or hemodynamic instability precluding transport to the radiology suite. In most instances, MRI scanning can be performed in the first few postoperative days. If it is not immediately available, however, it is often still useful and acute infarction will remain bright on DWI MRI for up to 7 to 10 days.
STROKE TYPES
Most strokes occurring as a result of cardiac surgery are either embolic-appearing in distribution (in multiple vascular territories) or in watershed territories between major cerebral arteries.48,49 Stroke in this setting is almost exclusively ischemic; intracerebral hemorrhage is rare,47 occurring in less than 1% of most patient populations and only up to 1 to 2% in patients undergoing valvular surgery.50
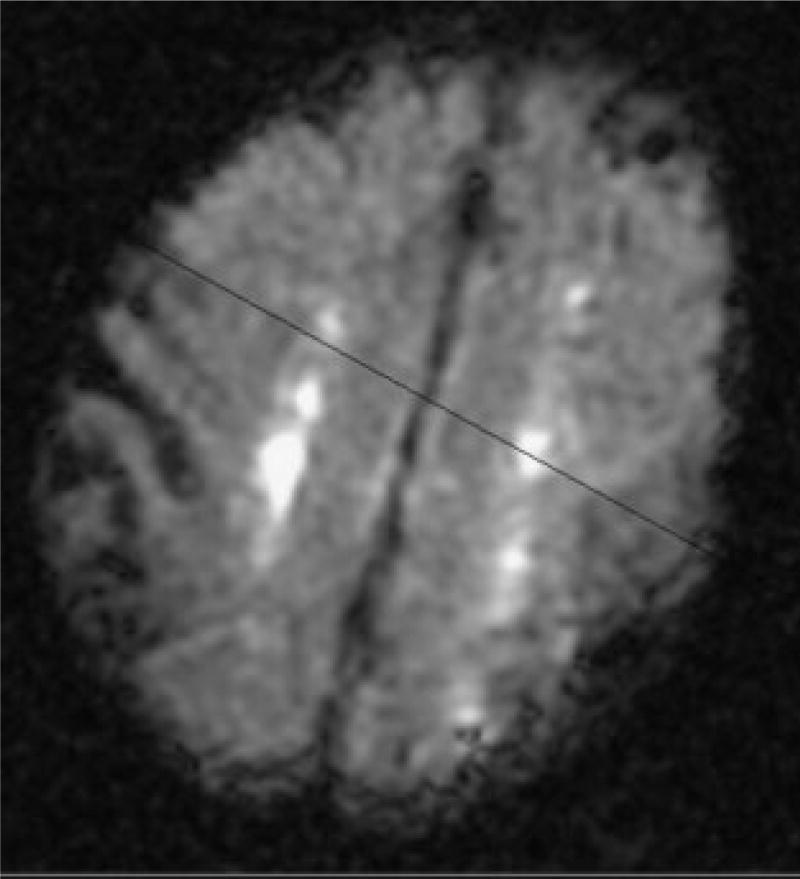
We have found that imaging of stroke may not only be helpful in the initial diagnosis, but may help in determining prognosis of patients with this complication. We reported that patients who had acute bilateral watershed infarcts by DWI MRI, when compared with patients with other acute infarct types, were 6 times as likely to go to inpatient rehabilitation, 12 times as likely to go to chronic nursing facility, and 19 times as likely to die in the hospital as to go home.48 In this population, 8 of 10 deaths were in patients with bilateral watershed strokes (compared with other stroke types).48 Fig. 3 displays an example of bilateral watershed strokes, as diagnosed using DWI MRI.
Figure 3.
Diffusion-weighted magnetic resonance imaging scan demonstrating bilateral watershed infarcts.
Infarct pattern is likely a representation of the mechanism of neurological injury; patients with bilateral watershed infarcts may have worse short-term outcomes because of potential hypoperfusion injury during surgery,48 which could cause other end-organ injury as well. Brain ischemic injury in the setting of hypoperfusion could be exacerbated by intraoperative anemia,51,52 which is a clear predictor of postoperative adverse outcomes including stroke. Karkouti et al described that each 1% drop in nadir hematocrit increased the odds of postoperative stroke by 10%.53 In a more recent study, the same authors have reported that the amount of anemia that a patient tolerates is dependent on his or her preoperative hemoglobin, with the highest risk for poor outcomes in patients with the maximum decrease in hemoglobin from baseline.54
Encephalopathy
Encephalopathy after cardiac surgery is often multifactorial in etiology and results from a combination of factors similar to those predictive of stroke 34 (and perhaps from subclinical stroke itself). Other contributing factors include medications (e.g., benzodiazepines) and metabolic derangements. Postcardiac surgery encephalopathy was initially described as a combination of alteration in level of consciousness and confusion55; more recently, it is used as a term to describe anything from slowness to emerge from general anesthesia to prolonged stupor.47 Between 18 and 28% of patients undergoing cardiac surgery become encephalopathic at some point in their postoperative course,2,8 although the encephalopathy is usually transient. However, this early encephalopathy may still predict poor long-term outcome; our institutional experience suggests out-of-hospital mortality may be linked to encephalopathy or other neurological injury in the early postoperative period.
The question as to whether encephalopathy, or even early cognitive dysfunction, represents otherwise subclinical stroke is controversial. In a study by Wityk et al, seven of eight patients with postoperative encephalopathy also had acute infarcts on MRI DWI, but not all patients with acute infarcts had encephalopathy or any other detectable neurological signs or symptoms.56
Treatment of encephalopathy can affect a patient’s outcome almost as much as the encephalopathy itself. In general, sedating and psychoactive medications should be avoided in elderly patients, as these medications may worsen delirium. Haloperidol, in particular, is not only associated with potential risk of prolonged QTc interval and risk of torsades de pointes,57 but it prolongs recovery from stroke and other neurological injuries58 and can remain in the body for weeks,59 and should thus be avoided in this setting. If a patient is extremely agitated and requires some form of psychoactive medication, use of a newer neuroleptic, such as quetiapine, olanzapine,60 or risperidone,61 may be considered.
Alpha-2 agonists have been studied recently as possibly beneficial in the treatment of postoperative delirium. These medications have been shown to decrease ischemia after cardiac surgery and may reduce postoperative mortality.62 In addition, they can be used for sedation and may actually prevent postoperative delirium. Dexmedetomidine, in particular, is an α-2 agonist that has proven to be an effective and safe sedative agent when compared with propofol in patients undergoing CABG,63 and has even shown reduced rates of delirium when compared with lorazepam in a randomized, controlled trial of critically ill patients.64 Based on animal data,65,66 dexmedetomidine has even been studied as a potential neuroprotectant, but results in human patients undergoing CABG have not been promising.63 In addition, the quality of the sedation brought about by dexmedetomidine may be preferable to other sedating agents; patients on the medication have been described as “tranquil,” in a sleep-like state but easy to rouse, without accompanying respiratory depression.67
Cognitive Dysfunction
There appears to be an early postoperative period when many patients who have undergone surgery with cardiopulmonary bypass have cognitive dysfunction, with particular problems with memory.68,69 Whether the process of undergoing major surgery with accompanying anesthesia is a risk factor for cognitive dysfunction, even for noncardiac surgery, is unclear. Although some authors have reported that patients undergoing noncardiac surgery are not generally at risk for cognitive dysfunction,68,70 Monk recently reported rates of cognitive dysfunction (at the time of hospital discharge) between 30 and 41%, depending on the age group, with persistent cognitive dysfunction at 3 months in elderly patients, beyond that seen in age-matched controls.71 There have been suggestions, however, that early post-CABG dysfunction may be due to cardiopulmonary bypass itself or to pathophysiologic perturbations resulting from bypass, such as inflammation or microembolism. However, patients undergoing cardiopulmonary bypass have much higher rates of other vascular disease, making it more likely that they not only have more preoperative small vessel ischemic disease and more preoperative strokes, but also increasing the odds of new postoperative infarction that could affect cognitive performance. It is difficult to estimate the extent of this dysfunction because this is highly dependent on how dysfunction is defined,72 and the timing of testing. Further, many studies have not included a baseline assessment of cognitive performance, which makes any analysis of cognitive decline virtually impossible.73 In addition, there is now evidence that this early cognitive dysfunction unique to the cardiac surgery milieu may not last beyond 6 weeks postoperatively.
Longstanding cognitive complaints had been described in patients undergoing cardiac surgery70 in as many as 42% of individuals at 5 years,74,75 but reports of this extent of dysfunction were in studies without a clear control group. In more recent studies, cognitive function was followed over a period of 6 years after surgery and compared with patients with coronary artery disease and equivalent systemic vascular disease (and thus presumably equivalent cerebrovascular disease), but who have been treated without surgery. The finding that the rates of cognitive decline after surgery are similar between medically and surgically treated patients with coronary artery disease suggests that the long-term decline previously reported in patients undergoing coronary bypass surgery is most likely due to underlying cerebrovascular disease, and is not unique to the surgery itself.76 Decline in both groups with coronary artery disease appears steeper than equivalent decline in another group of controls without systemic vascular (and presumably cerebrovascular) disease.77
The association between early cognitive dysfunction and new acute brain infarction detected with brain imaging is unclear. Multiple authors have failed to find an association between new postoperative infarction and early cognitive dysfunction,14,16,25 whereas others have suggested that subtle cognitive changes do correspond to new infarction as detected by MRI DWI.78 These findings might have resulted from the use of cognitive testing measures that do not assess brain areas involved with infarction. With increasing use of MRI DWI, more information may become available to help answer this question. In addition, the cumulative effect of multiple cerebral vascular lesions, such as those that can occur during cardiac surgery, is still not entirely understood.
Seizure
Fewer than 0.5% of patients undergoing surgery with cardiopulmonary bypass develop postoperative seizures.3,9 In our single institutional experience, seizures are more common among patients who have undergone aortic surgery involving the innominate and left carotid arteries, as compared with other types of cardiac surgery. The latter procedures often require deep hypothermic circulatory arrest or periods of low cardiopulmonary bypass flow. In the assessment of a patient with postoperative seizures, attention must be paid to typical agents that lower seizure threshold, including some analgesic and anesthetic medications as well as antiarrhythmic agents such as lidocaine. In addition, withdrawal either from alcohol or from medications such as benzodiazepines can contribute to postoperative seizures, as can metabolic derangements or infection. Perioperative injury can also occur that leads to seizures, including hypoxic-ischemic injury or embolic damage.
Peripheral Nerve injury
Neurological consultation may be requested for patients in the cardiac surgical intensive care unit (ICU) who are noted to have a focal motor or sensory weakness in a peripheral nerve distribution. Lederman et al described new peripheral nerve injuries (most commonly brachial plexopathies) in 13% of 421 patients undergoing CABG.79 It was felt that most of these injuries were due to chest wall retraction used during harvesting of the left internal mammary artery. In addition, the saphenous nerve can be injured by saphenous vein harvesting, which leads to numbness and discomfort in the distribution of this superficial sensory nerve.59
MECHANISMS OF CEREBRAL INJURY
Micro- and Macroembolization
There are many sources of micro- and macroembolism during cardiac surgery, including atherosclerotic debris, particulate matter such as fat or surgical debris, and air. Early clinical studies suggested a relationship between microembolic signals detected with transcranial Doppler monitoring of the middle cerebral arteries and neurocognitive dysfunction. Nonetheless, the evidence now suggests that it is the quality and not the quantity of embolism that is important for brain injury. Recent attention has focused on small lipid emboli found on histological examination at autopsy from patients after cardiac surgery. These lipid staining emboli are commonly referred to as small capillary arteriolar dilations or SCADs. These can be found in virtually all patients dying after cardiac surgery, with the quantity declining the longer death occurred after surgery. SCADs can be reproduced in canine models of cardiopulmonary bypass. Based on such animals studies, the source of SCADs is believed to be primarily lipid emboli originating from the pericardial aspirate returned to the cardiopulmonary bypass circuit unfiltered.80,81 These SCADs are likely to have some clinical significance,81,82 but are unlikely to account for all postsurgical neurological complications. In many institutions, cardiotomy suction blood is first processed with a cell saver device before it is returned to the bypass circuit, thus removing a large quantity of fat globules. There is some indirect evidence supporting this practice,83 but it is not clearly associated with improvement in outcomes.
Larger emboli can also come from the heart, either during or after surgery, from the aortic atheroma, or from large vessels in the neck or head. The extent of aortic atherosclerosis has been associated with postoperative stroke and early cognitive dysfunction after surgery.84 The use of epi-aortic ultrasound allows for improved visualization of aortic plaque and allows surgeons to either change placement of an aortic clamp or use a no-touch technique, to avoid disrupting an aortic plaque and causing cerebral embolization. The use of epi-aortic ultrasound has been associated with reduced postoperative stroke in observational series,22,85 and is the standard of care for intraoperative management in many centers. Another major source of macroemboli is atrial fibrillation; over 30% of individuals undergoing CABG develop this arrhythmia postoperatively, which can lead to mural thrombus formation.86 Endocarditis must also be considered in patients who have undergone valve replacement and have symptoms suggestive of stroke or encephalopathy. This condition can lead to mycotic aneurysms, septic emboli, or to meningoencephalitis.
Hypoperfusion
Dramatic fluctuations in blood pressure during surgery can increase an individual’s likelihood of postoperative neurological injury. Tufo and colleagues found that a drop in systolic blood pressure to 50 mm Hg or below for at least 10 minutes increased the risk of postcardiac surgery neurological complications fourfold.87 However, it is less clear whether more modest changes in blood pressure put most individuals at risk. In general, the brain can tolerate cerebral perfusion pressures (equal to mean arterial pressure [MAP] minus the intracranial pressure [ICP]) above 50 mm Hg.23,24 However, this may not apply in individuals with significant intracranial or extracranial stenosis or with preoperative chronic hypertension, as this may lead to a shift in their autoregulatory curve to the right. We have reported that a decrease in MAP from a patient’s baseline may increase odds of postoperative watershed strokes, as compared with other stroke types.48 Pulse pressure, as a marker of vascular stiffness, has also been identified as a predictor of stroke after cardiac surgery, as has decreasing ejection fraction,88 which may be another indicator of hypoperfusion to the brain.
Caplan and Hennerici have hypothesized that embolization and hypoperfusion work synergistically to cause neurological injury. He has proposed that hypoperfusion causes decreased washout of emboli, leading to injury in the watershed regions of the brain.89 There is no clear evidence supporting this hypothesis, although in the previously described randomized study of high versus low MAP, 66.7% of patients with grade V atheroma of the aorta who were in the low MAP group had stroke, compared with 20% of patients with an equivalent amount of atheroma in the high MAP group.90 These findings, although nonsignificant in this study, lend support to the possibility that both hypoperfusion and embolization are required to cause postoperative neurological injury.
CHANGES IN CARDIAC SURGERY TECHNIQUES
Several techniques or treatments have been developed in recent years in an attempt to improve neurological and other outcomes following cardiac surgery. As stated previously, these have not led to population-wide decreases in procedure-associated mortality and morbidity, most likely because the population undergoing surgery is older, sicker, and has more comorbidities.
Most of the technologies developed for cardiac surgery patients have been aimed at decreasing the extent of embolization to the brain. The use of epi-aortic ultrasound, as described above, is one technology that may allow for changes in operative management that could reduce cerebral embolization. Various arterial filters have been tested in an attempt to reduce distal embolization, with promising results, but unclear direct evidence for reduction in stroke and other neurological complications.91–93
Other evidence for changes in surgical technique includes a neuroprotective benefit using mild hypothermia, 94 or even use of higher MAPs to avoid hypoperfusion. The role of hypothermia has been controversial, with a Cochrane review failing to find definitive support for the routine use of hypothermia during CABG.95 This is likely to be because most patients who are exposed to hypothermic conditions are subsequently rewarmed to such a degree that they actually experience cerebral hyperthermia.96 Nathan and colleagues cooled patients to 32°C, with randomization to rewarming up to 37 degrees (standard) or 34 degrees, and found that patients maintained at lower temperatures had less cognitive decline postoperatively,97 with similar results when patients were followed for 5 years.98
Gold and colleagues published one clinical trial in which patients were randomized to “high” MAP (80 to 100 mm Hg) versus lower MAP (50 to 60 mm Hg) during cardiopulmonary bypass. These investigators found improved combined myocardial and neurological outcomes for the high versus the low MAP group.99 There was a trend for a reduced rate of stroke in the high compared with the low MAP group, but the study did not have sufficient power to adequately address this question. The optimal blood pressure during cardiopulmonary bypass in patients undergoing cardiac surgery is not known; it may be important to incorporate a patient’s baseline blood pressure in decisions about intraoperative management,48 but the exact relationship between blood pressure during surgery and postoperative neurological complications is still unclear. Additionally, although there is an association between anemia during surgery and poor outcomes,51,54 there is no evidence supporting routine transfusion of patients having cardiac surgery.100
Aprotinin had been used for over a decade as an antifibrinolytic therapy to reduce perioperative blood loss among patients undergoing CABG surgery. However, Mangano and colleagues found that in 4374 patients undergoing surgical revascularization, aprotinin was associated with a markedly increased risk of postoperative stroke or encephalopathy, in addition to increased risk of other end-organ damage.101 Aprotinin (marketed as Trasylol®, Bayer Pharmaceuticals, West Haven, CT) has since been removed from the market. Two recently published cohorts confirmed an association between aprotinin use and increased short-term and long-term mortality.102,103 Currently, there does not appear to be a link between other antifibrinolytic drugs (i.e., epsilon aminocaproic acid and tranexamic acid) and perioperative stroke. These drugs have been less intensely studied than aprotinin and much of the data comes from retrospective analyses, low-powered prospective trials, and meta-analyses.
Off-pump Surgery
Performing CABG surgery without the use of the cardiopulmonary bypass, or “off-pump” surgery, has been hypothesized to decrease the risk for brain injury by reducing cerebral embolization and the inflammatory response to this procedure. During off-pump CABG surgery, however, the patient is exposed to marked systemic hypotension and raised central venous pressure; thus, reduced cerebral perfusion pressure due to displacement of the heart for surgical exposure. In one study of 550 patients undergoing off-pump CABG surgery, ~15% had reductions in cerebral oxygenation, measured using cerebral oximetry and EEG.104
Observational studies have suggested that postoperative stroke and other neurological injury rates are modestly lower in individuals undergoing off-pump CABG surgery.105,106 Randomized trials, however, have failed to show a benefit in stroke reduction or in long-term cognitive dysfunction, even up to 5 years after surgery. The Octopus study group published their results of 281 low-risk CABG patients who were randomized to off-pump versus on-pump surgery, and found similar cognitive performance, even with relatively conservative definitions of cognitive decline, in both groups.107 A meta-analysis of 37 randomized trials found nonsignificant reductions in stroke (OR 0.68, 95% CI 0.33 to 1.40) with the use of off-pump CABG, with equivalent 30-day mortality compared with the conventional CABG group (OR 1.02 for off-pump CABG, 95% CI 0.58 to 1.80).108 Nonetheless, these trials to date have not included an adequate number of patients at high risk for neurological complications. Whether such patients, including those with advanced atherosclerosis of the ascending aorta, might benefit the most from off-pump surgery has not been adequately studied.
CONCLUSIONS
Neurological injury after cardiac surgery is not infrequent, despite recent advances in surgical technique. MRI techniques, particularly using diffusion-weighted imaging, should be used when possible in patients with neurological symptoms as patients may have multiple acute infarcts and, therefore, may not present with typical unilateral hemispheric symptoms. A consultation on a patient with encephalopathy or stroke in the cardiac surgical intensive care unit (ICU) should focus on any information about the extent of atheromatous disease (such as that detected by epi-aortic ultrasound), any noted postoperative atrial fibrillation, any major drops in blood pressure leading to sustained hypotension, and any use of sedating or psychoactive medications in the early postoperative period. Postsurgical management should include avoidance of further hypotension as well as avoidance of hyperglycemia and hyperthermia. In addition, in patients with neurological injury, early intervention with physical, occupational, and speech and swallowing therapists is critical for optimal recovery.
Acknowledgments
This work was supported by an American Heart Association Scientist Development Grant (R.F.G.), Johns Hopkins Clinician Scientist Award (R.F.G.), the Dana Foundation (G.M.M.), and the NIH (RO1- NS035610) (G.M.M.).
References
- 1.McKhann GM, Goldsborough MA, Borowicz LM, et al. Predictors of stroke risk in coronary artery bypass patients. Ann Thorac Surg. 1997;63:516–521. doi: 10.1016/s0003-4975(97)83384-x. [DOI] [PubMed] [Google Scholar]
- 2.Kornfeld DS, Heller SS, Frank KA, Edie RN, Barsa J. Delirium after coronary artery bypass surgery. J Thorac Cardiovasc Surg. 1978;76(1):93–96. [PubMed] [Google Scholar]
- 3.Roach GW, Kanchuger M, Mangano CM, et al. Adverse cerebral outcomes after coronary bypass surgery. N Engl J Med. 1996;335(25):1857–1863. doi: 10.1056/NEJM199612193352501. [DOI] [PubMed] [Google Scholar]
- 4.Ferguson TB, Hammill BG, Peterson ED, DeLong ER, Grover FL. A decade of change–Risk profiles and outcomes for isolated coronary artery bypass grafting procedures, 1990–1999: A report from the STS National Database Committee and the Duke Clinical Research Institute. Ann Thorac Surg. 2002;73:480–490. doi: 10.1016/s0003-4975(01)03339-2. [DOI] [PubMed] [Google Scholar]
- 5.Jeremias A, Kirtane A. Balancing efficacy and safety of drug-eluting stents in patients undergoing percutaneous coronary intervention. Ann Intern Med. 2008;148(3):234–238. doi: 10.7326/0003-4819-148-3-200802050-00199. [DOI] [PubMed] [Google Scholar]
- 6.Stettler C, Wandel S, Allemann S, et al. Outcomes associated with drug-eluting and bare-metal stents: a collaborative network meta-analysis. Lancet. 2007;370(9591):937–948. doi: 10.1016/S0140-6736(07)61444-5. [DOI] [PubMed] [Google Scholar]
- 7.Hannan EL, Wu C, Walford G, et al. Drug-eluting stent vs. coronary-artery bypass grafting in multivessel coronary disease. N Engl J Med. 2008;358:331–341. doi: 10.1056/NEJMoa071804. [DOI] [PubMed] [Google Scholar]
- 8.Breuer AC, Furlan AJ, Hanson MR, et al. Central nervous system complications of coronary artery bypass graft surgery: Prospective analysis of 421 patients. Stroke. 1983;14(5):682–687. doi: 10.1161/01.str.14.5.682. [DOI] [PubMed] [Google Scholar]
- 9.Coffey CE, Massey EW, Roberts KB, Curtis S, Jones RH, Pryor DB. Natural history of cerebral complications of coronary artery bypass graft surgery. Neurology. 1983;33:1416–1421. doi: 10.1212/wnl.33.11.1416. [DOI] [PubMed] [Google Scholar]
- 10.McKhann GM, Grega MA, Borowicz LM, Jr, Baumgartner WA, Selnes OA. Stroke and encephalopathy after cardiac surgery. An update. Stroke. 2006;37:562–571. doi: 10.1161/01.STR.0000199032.78782.6c. [DOI] [PubMed] [Google Scholar]
- 11.Boeken U, Litmathe J, Feindt P, Gams E. Neurological complications after cardiac surgery: Risk factors and correlation to the surgical procedure. Thorac Cardiovasc Surg. 2005;53:33–36. doi: 10.1055/s-2004-830426. [DOI] [PubMed] [Google Scholar]
- 12.Roselli EE, Pettersson GB, Blackstone EH, et al. Adverse events during reoperative cardiac surgery: Frequency, characterization, and rescue. J Thorac Cardiovasc Surg. 2008;135(2):316–323. doi: 10.1016/j.jtcvs.2007.08.060. [DOI] [PubMed] [Google Scholar]
- 13.Floyd TF, Shah PN, Price CC, et al. Clinically silent cerebral ischemic events after cardiac surgery: their incidence, regional vascular occurrence, and procedural dependence. Ann Thorac Surg. 2006;81(6):2160–2166. doi: 10.1016/j.athoracsur.2006.01.080. [DOI] [PubMed] [Google Scholar]
- 14.Bendszus M, Reents W, Franke D, et al. Brain damage after coronary artery bypass grafting. Arch Neurol. 2002;59:1090–1095. doi: 10.1001/archneur.59.7.1090. [DOI] [PubMed] [Google Scholar]
- 15.Djaiani G, Fedorko L, Borger M, et al. Mild to moderate atheromatous disease of the thoracic aorta and new ischemic brain lesions after conventional coronary artery bypass graft surgery. Stroke. 2004;35(9):e356–358. doi: 10.1161/01.STR.0000138783.63858.62. [DOI] [PubMed] [Google Scholar]
- 16.Knipp SC, Matatko N, Wilhelm H, et al. Evaluation of brain injury after coronary artery bypass grafting: A prospective study using neuropsychological assessment and diffusion-weighted magnetic resonance imaging. Eur J Cardiothorac Surg. 2004;25:791–800. doi: 10.1016/j.ejcts.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 17.Czerny M, Fleck T, Zimpfer D, et al. Risk factors of mortality and permanent neurologic injury in patients undergoing ascending aortic and arch repair. J Thorac Cardiovasc Surg. 2003;126(5):1296–1301. doi: 10.1016/s0022-5223(03)01046-8. [DOI] [PubMed] [Google Scholar]
- 18.Ergin MA, Galla JD, Lansman L, Quintana C, Bodian C, Griepp RB. Hypothermic circulatory arrest in operations on the thoracic aorta. Determinants of operative mortality and neurologic outcome. J Thorac Cardiovasc Surg. 1994;107(3):788–797. [PubMed] [Google Scholar]
- 19.Harrington DK, Fragomeni F, Bonser RS. Cerebral perfusion. Ann Thorac Surg. 2007;83(2):S799–S804. doi: 10.1016/j.athoracsur.2006.11.018. [DOI] [PubMed] [Google Scholar]
- 20.Bakhtiary F, Dogan S, Zierer A, et al. Antegrade cerebral perfusion for acute type A aortic dissection in 120 consecutive patients. Ann Thorac Surg. 2008;85(2):465–469. doi: 10.1016/j.athoracsur.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 21.Kazui T, Washiyama N, Muhammed BAH, Terada H, Yamashita K, Takinami M. Improved results of atherosclerotic arch aneurysm operations with a refined technique. J Thorac Cardiovasc Surg. 2001;121:491–499. doi: 10.1067/mtc.2001.112469. [DOI] [PubMed] [Google Scholar]
- 22.Di Eusanio M, Schepens M, Morshuis WJ, et al. Brain protection using antegrade selective cerebral perfusion: a multicenter study. Ann Thorac Surg. 2003;76(4):1181–1189. doi: 10.1016/s0003-4975(03)00824-5. [DOI] [PubMed] [Google Scholar]
- 23.Johnsson P, Algotsson L, Ryding E, Stahl E, Messeter K. Cardiopulmonary perfusion and cerebral blood flow in bilateral carotid artery disease. Ann Thorac Surg. 1991;51:579–584. doi: 10.1016/0003-4975(91)90315-h. [DOI] [PubMed] [Google Scholar]
- 24.Lazar HL, Menzoian JO. Coronary artery bypass grafting in patients with cerebrovascular disease. Ann Thorac Surg. 1998;66:968–974. doi: 10.1016/s0003-4975(98)00687-0. [DOI] [PubMed] [Google Scholar]
- 25.Cook DJ, Huston TMR, III, Brown RD, Zehr KJ, Sundt TM., III Postcardiac surgical cognitive impairment in the aged using diffusion-weighted magnetic resonance imaging. Ann Thorac Surg. 2007;83(4):1389–1395. doi: 10.1016/j.athoracsur.2006.11.089. [DOI] [PubMed] [Google Scholar]
- 26.Barber PA, Hach S, Tippett LJ, Ross L, Merry AF, Milsom P. Cerebral ischemic lesions on diffusion-weighted imaging are associated with neurocognitive decline after cardiac surgery. Stroke. 2008;39:1427–1433. doi: 10.1161/STROKEAHA.107.502989. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 27.Brener BJ, Brief DK, Alpert J, et al. A four-year experience with preoperative noninvasive carotid evaluation of two thousand twenty-six patients undergoing cardiac surgery. J Vasc Surg. 1984;1(2):326–338. [PubMed] [Google Scholar]
- 28.Durand DJ, Perler BA, Roseborough GS, et al. Mandatory versus selective preoperative carotid screening: a retrospective analysis. Ann Thorac Surg. 2004;78(1):159–166. doi: 10.1016/j.athoracsur.2004.02.024. [DOI] [PubMed] [Google Scholar]
- 29.Ferris A, Robertson RM, Fabunmi R, Mosca L. American Heart Association and American Stroke Association national survey of stroke risk awareness among women. Circulation. 2005;111:1321–1326. doi: 10.1161/01.CIR.0000157745.46344.A1. [DOI] [PubMed] [Google Scholar]
- 30.Van der Heyden J, Suttorp MJ, Bal ET, et al. Staged carotid angioplasty and stenting followed by cardiac surgery in patients with severe asymptomatic carotid artery stenosis. Circulation. 2007;116:2036–2042. doi: 10.1161/CIRCULATIONAHA.106.658625. [DOI] [PubMed] [Google Scholar]
- 31.Takeuchi K, Maida K, Yoshida S, et al. Preoperative cerebrovascular screening before cardiovascular surgery in a high risk area of cerebrovascular events in Japan. J Cardiovasc Surg (Torino) 2000;41(6):911–914. [PubMed] [Google Scholar]
- 32.Nakamura Y, Kawachi K, Imagawa H, et al. The prevalence and severity of cerebrovascular disease in patients undergoing cardiovascular surgery. Ann Thorac Cardiovasc Surg. 2004;10(2):81–84. [PubMed] [Google Scholar]
- 33.Bybee KA, Powell BD, Valeti U, et al. Preoperative aspirin therapy is associated with improved postoperative outcomes in patients undergoing coronary artery bypass grafting. Circulation. 2005;112(9, Suppl):I286–I292. doi: 10.1161/CIRCULATIONAHA.104.522805. [DOI] [PubMed] [Google Scholar]
- 34.McKhann GM, Grega MA, Borowicz LM, et al. Encephalopathy and stroke after coronary artery bypass grafting: incidence, consequences, and prediction. Arch Neurol. 2002;59:1422–1428. doi: 10.1001/archneur.59.9.1422. [DOI] [PubMed] [Google Scholar]
- 35.Hogue CW, Murphy SF, Schechtman KB, Davila-Roman VG. Risk factors for early or delayed stroke after cardiac surgery. Circulation. 1999;100(6):642–647. doi: 10.1161/01.cir.100.6.642. [DOI] [PubMed] [Google Scholar]
- 36.Hogue CW, Barzilai B, Pieper KS, et al. Sex differences in neurological outcomes and mortality after cardiac surgery. Circulation. 2001;103:2133–2137. doi: 10.1161/01.cir.103.17.2133. [DOI] [PubMed] [Google Scholar]
- 37.Blauth CI, Cosgrove DM, Webb BW, et al. Atheroembolism from the ascending aorta. An emerging problem in cardiac surgery. J Thorac Cardiovasc Surg. 1992;103(6):1104–1111. [PubMed] [Google Scholar]
- 38.Mackensen GB, Ti LK, Phillips-Bute BG, Mathew JP, Newman MF, Grocott HP. Cerebral embolization during cardiac surgery: impact of aortic atheroma burden. Br J Anaesth. 2003;91:656–661. doi: 10.1093/bja/aeg234. [DOI] [PubMed] [Google Scholar]
- 39.Furlan AJ, Cracium AR. Risk of stroke during coronary artery bypass graft surgery in patients with internal carotid artery disease documented by angiography. Stroke. 1985;16:797–799. doi: 10.1161/01.str.16.5.797. [DOI] [PubMed] [Google Scholar]
- 40.Schwartz LB, Bridgman AH, Kieffer RW, et al. Asymptomatic carotid artery stenosis and stroke in patients undergoing cardiopulmonary bypass. J Vasc Surg. 1995;21:146–153. doi: 10.1016/s0741-5214(95)70253-9. [DOI] [PubMed] [Google Scholar]
- 41.D’Agostino RS, Svensson LG, Neumann DJ, Balkhy HH, Williamson WA, Shahian DM. Screening carotid ultrasonography and risk factors for stroke in coronary artery surgery patients. Ann Thorac Surg. 1996;62:1714–1723. doi: 10.1016/s0003-4975(96)00885-5. [DOI] [PubMed] [Google Scholar]
- 42.Gerraty RP, Gates PC, Doyle JC. Carotid stenosis and perioperative stroke risk in symptomatic and asymptomatic patients undergoing vascular or coronary surgery. Stroke. 1993;24:1115–1118. doi: 10.1161/01.str.24.8.1115. [DOI] [PubMed] [Google Scholar]
- 43.Yoon BW, Bae HJ, Kang DW, et al. Intracranial cerebral artery disease as a risk factor for central nervous system complications of coronary artery bypass graft surgery. Stroke. 2001;32:94–99. doi: 10.1161/01.str.32.1.94. [DOI] [PubMed] [Google Scholar]
- 44.Grocott HP, White WD, Morris RW, et al. Genetic polymorphisms and the risk of stroke after cardiac surgery. Stroke. 2005;36(9):1854–1858. doi: 10.1161/01.STR.0000177482.23478.dc. [DOI] [PubMed] [Google Scholar]
- 45.Tardiff BE, Newman MF, Saunders AM, et al. Preliminary report of a genetic basis for cognitive decline after cardiac operations. The Neurologic Outcome Research Group of the Duke Heart Center. Ann Thorac Surg. 1997;64(3):715–720. doi: 10.1016/s0003-4975(97)00757-1. [DOI] [PubMed] [Google Scholar]
- 46.Steed L, Kong R, Stygall J, et al. The role of apolipoprotein E in cognitive decline after cardiac operation. Ann Thorac Surg. 2001;71(3):823–826. doi: 10.1016/s0003-4975(00)02511-x. [DOI] [PubMed] [Google Scholar]
- 47.Leary MC, Caplan LR. Technology insight: brain MRI and cardiac surgery –detection of postoperative brain ischemia. Nat Clin Pract Cardiovasc Med. 2007 doi: 10.1038/ncpcardio0915. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 48.Gottesman RF, Sherman PM, Grega MA, et al. Watershed strokes after cardiac surgery: Diagnosis, etiology, and outcome. Stroke. 2006;37:2306–2311. doi: 10.1161/01.STR.0000236024.68020.3a. [DOI] [PubMed] [Google Scholar]
- 49.Likosky DS, Marrin CAS, Caplan LR, et al. Determination of etiologic mechanisms of strokes secondary to coronary artery bypass graft surgery. Stroke. 2003;34:2830–2834. doi: 10.1161/01.STR.0000098650.12386.B3. [DOI] [PubMed] [Google Scholar]
- 50.Borger MA, Ivanov J, Weisel RD, et al. Decreasing incidence of stroke during valvular surgery. Circulation. 1998;98:II137–II143. [PubMed] [Google Scholar]
- 51.Karkouti K, Wijeysundera DN, Beattie WS. Risk associated with preoperative anemia in cardiac surgery: a multicenter cohort study. Circulation. 2008;117(4):478–484. doi: 10.1161/CIRCULATIONAHA.107.718353. [DOI] [PubMed] [Google Scholar]
- 52.Habib RH, Zacharias A, Schwann TA, Riordan CJ, Durham SJ, Shah A. Adverse effects of low hematocrit during cardiopulmonary bypass in the adult: Should current practice be changed? J Thorac Cardiovasc Surg. 2003;125:1438–1450. doi: 10.1016/s0022-5223(02)73291-1. [DOI] [PubMed] [Google Scholar]
- 53.Karkouti K, Djaiani G, Borger MA, et al. Low hematocrit during cardiopulmonary bypass is associated with increased risk of perioperative stroke in cardiac surgery. Ann Thorac Surg. 2005;80:1381–1387. doi: 10.1016/j.athoracsur.2005.03.137. [DOI] [PubMed] [Google Scholar]
- 54.Karkouti K, Wijeysundera DN, Yau TM, McCluskey SA, van Rensburg A, Beattie WS. The influence of baseline hemoglobin concentration on tolerance of anemia in cardiac surgery. Transfusion. 2008;48(4):666–672. doi: 10.1111/j.1537-2995.2007.01590.x. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 55.Gilman S. Cerebral disorders after open-heart operations. N Engl J Med. 1965;272:489–498. doi: 10.1056/NEJM196503112721001. [DOI] [PubMed] [Google Scholar]
- 56.Wityk RJ, Goldsborough MA, Hillis AE, et al. Diffusion-and perfusion-weighted brain magnetic resonance imaging in patients with neurologic complications after cardiac surgery. Arch Neurol. 2001;58:571–576. doi: 10.1001/archneur.58.4.571. [DOI] [PubMed] [Google Scholar]
- 57.Glassman AH, Bigger JT., Jr Antipsychotic drugs: prolonged QTc interval, torsade de pointes, and sudden death. Am J Psychiatry. 2001;158(11):1774–1782. doi: 10.1176/appi.ajp.158.11.1774. [DOI] [PubMed] [Google Scholar]
- 58.Feeney DM, Gonzalez A, Law WA. Amphetamine, haloperidol and experience interact to affect the rate of recovery after motor cortex injury. Science. 1982;217:855–857. doi: 10.1126/science.7100929. [DOI] [PubMed] [Google Scholar]
- 59.Llinas R, Barbut D, Caplan LR. Neurologic complications of cardiac surgery. Prog Cardiovasc Dis. 2000;43(2):101–112. doi: 10.1053/pcad.2000.9030. [DOI] [PubMed] [Google Scholar]
- 60.Rea RS, Battistone S, Fong JJ, Devlin JW. Atypical antipsychotics versus haloperidol for treatment of delirium in acutely ill patients. Pharmacotherapy. 2007;27(4):588–594. doi: 10.1592/phco.27.4.588. [DOI] [PubMed] [Google Scholar]
- 61.Prakanrattana U, Prapaitrakool S. Efficacy of risperidone for prevention of postoperative delirium in cardiac surgery. Anaesth Intensive Care. 2007;35(5):714–719. doi: 10.1177/0310057X0703500509. [DOI] [PubMed] [Google Scholar]
- 62.Wijeysundera DN, Naik JS, Beattie WS. Alpha-2 adrenergic agonists to prevent perioperative cardiovascular complications: A meta-analysis. Am J Med. 2003;114:742–752. doi: 10.1016/s0002-9343(03)00165-7. [DOI] [PubMed] [Google Scholar]
- 63.Herr DL, Sum-Ping ST, England M. ICU sedation after coronary artery bypass graft surgery: dexmedetomidine-based versus propofol-based regimens. J Cardiothorac Vasc Anesth. 2003;17(5):576–584. doi: 10.1016/s1053-0770(03)00200-3. [DOI] [PubMed] [Google Scholar]
- 64.Pandharipande PP, Pun BT, Herr DL, et al. Effect of sedation with dexmedetomidine vs lorazepam on acute brain dysfunction in mechanically ventilated patients: the MENDS randomized controlled trial. JAMA. 2007;298(22):2644–2653. doi: 10.1001/jama.298.22.2644. [DOI] [PubMed] [Google Scholar]
- 65.Hoffman WE, Kochs E, Werner C, Thomas C, Albrecht RF. Dexmedetomidine improves neurologic outcome from complete ischemia in the rat. Reversal by the alpha 2-adrenergic antagonist atipamezole. Anesthesiology. 1991;75(2):328–332. doi: 10.1097/00000542-199108000-00022. [DOI] [PubMed] [Google Scholar]
- 66.Cosar M, Eser O, Fidan H, et al. The neuroprotective effect of dexmedetomidine in the hippocampus of rabbits after subarachnoid hemorrhage. Surg Neurol. 2008 doi: 10.1016/j.surneu.2007.08.020. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 67.Aantaa R, Jalonen J. Perioperative use of alpha-2-adrenoceptor agonists and the cardiac patient. Eur J Anaesthesiol. 2006;23:361–372. doi: 10.1017/S0265021506000378. [DOI] [PubMed] [Google Scholar]
- 68.Shaw PJ, Bates D, Cartlidge NE, et al. Neurologic and neuropsychological morbidity following major surgery: comparison of coronary artery bypass and peripheral vascular surgery. Stroke. 1987;18(4):700–707. doi: 10.1161/01.str.18.4.700. [DOI] [PubMed] [Google Scholar]
- 69.Seines OA, Grega MA, Borowicz LM, Royall RM, McKhann GM, Baumgartner WA. Cognitive changes with coronary artery disease: a prospective study of coronary artery bypass graft patients and nonsurgical controls. Ann Thorac Surg. 2003;75:1377–1386. doi: 10.1016/s0003-4975(03)00021-3. [DOI] [PubMed] [Google Scholar]
- 70.Fearn SJ, Pole R, Wesnes K, Faragher EB, Hooper TL, McCollum CN. Cerebral injury during cardiopulmonary bypass: emboli impair memory. J Thorac Cardiovasc Surg. 2001;121:1150–1160. doi: 10.1067/mtc.2001.114099. [DOI] [PubMed] [Google Scholar]
- 71.Monk TG, Weldon BC, Garvan CW, et al. Predictors of cognitive dysfunction after major noncardiac surgery. Anesthesiology. 2008;108(1):18–30. doi: 10.1097/01.anes.0000296071.19434.1e. [DOI] [PubMed] [Google Scholar]
- 72.Selnes OA, Pham L, Zeger S, McKhann GM. Defining cognitive change after CABG: decline versus normal variability. Ann Thorac Surg. 2006;82(2):388–390. doi: 10.1016/j.athoracsur.2006.02.060. [DOI] [PubMed] [Google Scholar]
- 73.Selnes OA, Zeger SL. Coronary artery bypass grafting baseline cognitive assessment: essential not optional. Ann Thorac Surg. 2007;83:374–376. doi: 10.1016/j.athoracsur.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 74.Sotaniemi KA, Mononen H, Hokkanen TE. Long-term cerebral outcome after open-heart surgery. A five-year neuropsychological follow-up study. Stroke. 1986;17(3):410–416. doi: 10.1161/01.str.17.3.410. [DOI] [PubMed] [Google Scholar]
- 75.Newman MF, Kirchner JL, Phillips-Bute B, et al. Longitudinal assessment of neurocognitive function after coronary-artery bypass surgery. N Engl J Med. 2001;344:395–402. doi: 10.1056/NEJM200102083440601. [DOI] [PubMed] [Google Scholar]
- 76.Selnes OA, Grega MA, Bailey MM, et al. Neurocognitive outcomes 3 years after coronary artery bypass graft surgery: a controlled study. Ann Thorac Surg. 2007;84(6):1885–1896. doi: 10.1016/j.athoracsur.2007.06.054. [DOI] [PubMed] [Google Scholar]
- 77.Selines OA, Grega MA, Bailey MM, Zeger S, Pham L, McKhann GM. A controlled study of cognitive outcomes after coronary artery bypass surgery (abstract) Ann Neurol. 2007;62(suppl 11):S21. [Google Scholar]
- 78.Restrepo L, Wityk RJ, Grega MA, et al. Diffusion- and perfusion-weighted magnetic resonance imaging of the brain before and after coronary artery bypass grafting surgery. Stroke. 2002;33(12):2909–2915. doi: 10.1161/01.str.0000040408.75704.15. [DOI] [PubMed] [Google Scholar]
- 79.Lederman RJ, Breuer AC, Hanson MR, et al. Peripheral nervous system complications of coronary artery bypass graft surgery. Ann Neurol. 1982;12:297–301. doi: 10.1002/ana.410120315. [DOI] [PubMed] [Google Scholar]
- 80.Brooker RF, Brown WR, Moody DM, et al. Cardiotomy suction: a major source of brain lipid emboli during cardiopulmonary bypass. Ann Thorac Surg. 1998;65:1651–1655. doi: 10.1016/s0003-4975(98)00289-6. [DOI] [PubMed] [Google Scholar]
- 81.Moody DM, Brown WR, Challa VR, Stump DA, Reboussin DM, Legault C. Brain microemboli associated with cardiopulmonary bypass: a histologic and magnetic resonance imaging study. Ann Thorac Surg. 1995;59:1304–1307. doi: 10.1016/0003-4975(95)00057-r. [DOI] [PubMed] [Google Scholar]
- 82.Clark RE, Brillman J, Davis DA, Lovell MR, Price TRP, Magovern GJ. Microemboli during coronary artery bypass grafting. J Thorac Cardiovasc Surg. 1995;109:249–258. doi: 10.1016/S0022-5223(95)70386-1. [DOI] [PubMed] [Google Scholar]
- 83.Carrier M, Denault A, Lavoie J, Perrault LP. Randomized controlled trial of pericardial blood processing with a cell-saving device on neurologic markers in elderly patients undergoing coronary artery bypass graft surgery. Ann Thorac Surg. 2006;82:51–56. doi: 10.1016/j.athoracsur.2006.02.077. [DOI] [PubMed] [Google Scholar]
- 84.Goto T, Baba T, Matsumaya K, Honma K, Ura M, Koshiji T. Aortic atherosclerosis and postoperative neurological dysfunction in elderly coronary surgical patients. Ann Thorac Surg. 2003;75:1912–1918. doi: 10.1016/s0003-4975(03)00029-8. [DOI] [PubMed] [Google Scholar]
- 85.Suojaranta-Ylinen RT, Roine RO, Vento AE, Niskanen MM, Salmenpera MT. Improved neurologic outcome after implementing evidence-based guidelines for cardiac surgery. J Cardiothorac Vasc Anesth. 2007;21(4):529–534. doi: 10.1053/j.jvca.2006.12.019. [DOI] [PubMed] [Google Scholar]
- 86.Mathew JP, Fontes ML, Tudor IC, et al. A multicenter risk index for atrial fibrillation after cardiac surgery. JAMA. 2004;291:1720–1729. doi: 10.1001/jama.291.14.1720. [DOI] [PubMed] [Google Scholar]
- 87.Tufo HM, Oltfeld AM, Shekelle R. Central nervous system dysfunction following open-heart surgery. JAMA. 1970;212:1333–1340. [PubMed] [Google Scholar]
- 88.Benjo A, Thompson RE, Fine D, et al. Pulse pressure is an age-independent predictor of stroke development after cardiac surgery. Hypertension. 2007;50:630–635. doi: 10.1161/HYPERTENSIONAHA.107.095513. [DOI] [PubMed] [Google Scholar]
- 89.Caplan LR, Hennerici M. Impaired clearance of emboli (washout) is an important link between hypoperfusion, embolism, and ischemic stroke. Arch Neurol. 1998;55:1475–1482. doi: 10.1001/archneur.55.11.1475. [DOI] [PubMed] [Google Scholar]
- 90.Hartman GS, Yao FSF, Bruefach M, III, et al. Severity of aortic atheromatous disease diagnosed by transesophageal echocardiography predicts stroke and other outcomes associated with coronary artery surgery: a prospective study. Anesth Analg. 1996;83:701–708. doi: 10.1097/00000539-199610000-00007. [DOI] [PubMed] [Google Scholar]
- 91.Banbury MK, Kouchoukos NT, Allen KB, et al. Emboli capture using the Embol-X intraaortic filter in cardiac surgery: a multicentered randomized trial of 1,289 patients. Ann Thorac Surg. 2003;76:508–515. doi: 10.1016/s0003-4975(03)00530-7. [DOI] [PubMed] [Google Scholar]
- 92.Blauth CI. Macroemboli and microemboli during cardiopulmonary bypass. Ann Thorac Surg. 1995;59:1300–1303. doi: 10.1016/0003-4975(95)00105-t. [DOI] [PubMed] [Google Scholar]
- 93.Schmitz C, Weinreich S, White J, et al. Can particulate extraction from the ascending aorta reduce neurologic injury in cardiac surgery? J Thorac Cardiovasc Surg. 2003;126:1829–1836. doi: 10.1016/s0022-5223(03)01285-6. [DOI] [PubMed] [Google Scholar]
- 94.Nathan HJ, Wells GA, Munson JL, Wozny D. Neuroprotective effect of mild hypothermia in patients undergoing coronary artery surgery with cardiopulmonary bypass. Circulation. 2001;104(12 Suppl 1):85–91. doi: 10.1161/hc37t1.094710. [DOI] [PubMed] [Google Scholar]
- 95.Rees K, Beranek-Stanley M, Burke M, Ebrahim S. Hypothermia to reduce neurological damage following coronary artery bypass surgery. Cochrane Database Syst Rev. 2001;1 doi: 10.1002/14651858.CD002138. CD002138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Grocott HP, Mackenson GB, Grigore AM, et al. Postoperative hypothermia is associated with cognitive dysfunction after coronary artery bypass graft surgery. Stroke. 2002;33(2):537–541. doi: 10.1161/hs0202.102600. [DOI] [PubMed] [Google Scholar]
- 97.Nathan HJ, Wells GA, Munson JL, Wozny D. Neuroprotective effect of mild hypothermia in patients undergoing coronary artery surgery with cardiopulmonary bypass: a randomized trial. Circulation. 2001;104(12 Suppl 1):85–91. doi: 10.1161/hc37t1.094710. [DOI] [PubMed] [Google Scholar]
- 98.Nathan HJ, Rodriguez R, Wozny D, et al. Neuroprotective effect of mild hypothermia in patients undergoing coronary artery surgery with cardiopulmonary bypass: five-year follow-up of a randomized trial. J Thorac Cardiovasc Surg. 2007;133:1206–1211. doi: 10.1016/j.jtcvs.2006.09.112. [DOI] [PubMed] [Google Scholar]
- 99.Gold JP, Charlson ME, Williams-Russo P, et al. Improvements of outcomes after coronary artery bypass: a randomized trial comparing intraoperative high versus low mean arterial pressure. J Thorac Cardiovasc Surg. 1995;110:1302–1314. doi: 10.1016/S0022-5223(95)70053-6. [DOI] [PubMed] [Google Scholar]
- 100.Murphy GJ, Reeves BC, Rogers CA, Rizvi SI, Culliford L, Angelini GD. Increased mortality, postoperative morbidity, and cost after red blood cell transfusion in patients having cardiac surgery. Circulation. 2007;116(22):2544–2552. doi: 10.1161/CIRCULATIONAHA.107.698977. [DOI] [PubMed] [Google Scholar]
- 101.Mangano DT, Tudor IC, Dietzel C. The risk associated with aprotinin in cardiac surgery. N Engl J Med. 2006;354(4):353–365. doi: 10.1056/NEJMoa051379. [DOI] [PubMed] [Google Scholar]
- 102.Schneeweiss S, Seeger JD, Landon J, Walker AM. Aprotinin during coronary-artery bypass grafting and risk of death. N Engl J Med. 2008;358:771–783. doi: 10.1056/NEJMoa0707571. [DOI] [PubMed] [Google Scholar]
- 103.Shaw AD, Stafford-Smith M, White WD, et al. The effect of aprotinin on outcome after coronary-artery bypass grafting. N Engl J Med. 2008;358:784–793. doi: 10.1056/NEJMoa0707768. [DOI] [PubMed] [Google Scholar]
- 104.Novitzky D, Boswell BB. Total myocardial revascularization without cardiopulmonary bypass utilizing computer-processed monitoring to assess cerebral perfusion. Heart Surg Forum. 2000;3(3):198–202. [PubMed] [Google Scholar]
- 105.Biancari F, Mosorin M, Rasinaho E, et al. Postoperative stroke after off-pump versus on-pump coronary artery bypass surgery. J Thorac Cardiovasc Surg. 2007;133(1):169–173. doi: 10.1016/j.jtcvs.2006.06.052. [DOI] [PubMed] [Google Scholar]
- 106.Zangrillo A, Crescenzi G, Landoni G, et al. Off-pump coronary artery bypass grafting reduces postoperative neurologic complications. J Cardiothorac Vasc Anesth. 2005;19(2):193–196. doi: 10.1053/j.jvca.2005.01.030. [DOI] [PubMed] [Google Scholar]
- 107.van Dijk D, Spoor M, Hijman R, et al. Cognitive and cardiac outcomes 5 years after off-pump vs on-pump coronary artery bypass graft surgery. JAMA. 2007;297(7):701–708. doi: 10.1001/jama.297.7.701. [DOI] [PubMed] [Google Scholar]
- 108.Cheng DC, Bainbridge D, Martin JE, Novick RJ. Evidence-based Perioperative Clinical Outcomes Research Group. Does off-pump coronary artery bypass reduce mortality, morbidity, and resource utilization when compared with conventional coronary artery bypass? A meta-analysis of randomized trials Anesthesiology. 2005;102(1):188–203. doi: 10.1097/00000542-200501000-00028. [DOI] [PubMed] [Google Scholar]
- 109.Harrington DK, Walker AS, Kaukuntla H, et al. Selective antegrade cerebral perfusion attenuates brain metabolic deficit in aortic arch surgery: a prospective randomized trial. Circulation. 2004;110:231–236. doi: 10.1161/01.CIR.0000138945.78346.9c. [DOI] [PubMed] [Google Scholar]