Abstract
AIMS AND OBJECTIVES:
Spectrum of hyperglycemia in pregnancy includes gestational diabetes mellitus (GDM), mild hyperglycemia, and overt diabetes. Many authors have worked on morphological changes of the placenta in diabetes, but few studies have correlated histopathological changes with vascular endothelial growth factor (VEGF) immunoexpression. The aim of this study was to detect different histopathological changes in various groups of diabetic placentas and to correlate with VEGF immunoexpression.
MATERIALS AND METHODS:
Pregnant women were screened for diabetes. They were subsequently divided into normoglycemic (12 cases), GDM (33 cases), mild hyperglycemic (13 cases), and overt diabetes (18 cases). Placentas collected were subjected to histopathological examination. VEGF expressions were studied by immunohistochemistry.
RESULTS:
Overt diabetic placenta displayed villous immaturity (44.4%), villous edema (38.9%), chorangiosis (61.1%), fibrinoid substance deposition (38.9%), and Hofbauer cell hyperplasia in 44.4% cases. GDM placentas displayed villous immaturity (45.5%), villous edema (45.5%), chorangiosis (42.4%), and fibrinoid substance deposition in 75.6% cases. Mild hyperglycemic placentas displayed villous immaturity (38.5%), chorangiosis (61.5%), and fibrinoid substance deposition in 61.5% cases. VEGF immunoexpression in GDM placentas was absent in all placental components except syncytiotrophoblast. VEGF expression in overt diabetic placentas was increased in syncytiotrophoblast and capillary endothelium compared to normoglycemic placentas. Mild hyperglycemic placentas expressed similar VEGF expression in all components when compared to normoglycemic controls. However, it displayed weak expression in vessel endothelium.
CONCLUSION:
Histopathological changes in diabetic placentas might be a consequence of altered or abnormal VEGF expression in diabetic placentas. Pathogenesis and VEGF expression in GDM placentas are significantly different from overt diabetic placentas.
Key words: Gestational diabetic placentas, histopathology, vascular endothelial growth factor immunoexpression
Introduction
The placenta is responsible for correct synchronization and integration of signals from the fetus and the mother in an effort to match fetal demand with maternal nutrient supply.[1] Maintenance of pregnancy requires both vasculogenesis and angiogenesis. Vasculogenesis differs from angiogenesis by the fact that the former means de novo synthesis of new blood vessels while angiogenesis means the formation of blood vessels from preexisting ones.[2,3] Vasculogenesis is characterized by in situ differentiation of hemangiogenic stem cells derived from the pluripotent mesenchyme, followed by proliferation of angioblastic cells which give rise to precursor cells. This is followed by angiogenesis.[3,4] Extensive vascular remodeling and stabilization of the vascular bed occur in second half of gestation.[5] The process of angiogenesis involves proliferation, migration, and maturation of both maternal and fetal endothelial cells.[6]
Vascular endothelial growth factor (VEGF) promotes neovascularization and angiogenesis.[7] Vasodilation in response to VEGF is mediated by the production of nitric oxide and prostaglandin I2 through stimulation of endothelial flt-1 and Flk-1/KDR receptors.[8] VEGF also induces proteinase expression of monocyte and endothelial cell chemotaxis[9] and is responsible for maintenance of vascular endothelium.[10] VEGF-A promotes angiogenesis, induces the growth of vascular endothelial cells, reduces apoptosis, and increases permeability.[11] Hypoxia is a potent stimulus for the expression of VEGF-A mRNA.[12] Other stimulating factors of VEGF are fibroblast growth factor, transforming growth factors (TGF-α and TGF-β), keratinocyte growth factor, insulin-like growth factor 1 and platelet-derived growth factor and inflammatory cytokines such as interleukin (IL)-1α and IL-6.[13] Vascular disorders can alter placental function and compromise pregnancy outcomes.[14] Diabetes leads to atherosclerosis that may impair uteroplacental blood flow, creates excessive syncytial knots, and accelerates perivillous fibrin deposition.[15] Proliferation of capillaries can be explained by stimulation of endothelial cells by VEGF through VEGFR-2 as a result of low oxygen level.[16] Villous degeneration without angiogenesis is generally associated with severe hypoxia despite stimulation by VEGF.[17]
Materials and Methods
The study was undertaken in a tertiary care hospital in the department of pathology with collaboration from department of obstetrics. The selected pregnant women signed an informed consent. Pregnant nonsmoking women between 18 and 40 years and with term placenta in their gestational period of 37–42 weeks were included in the study. While pregnant women with preeclampsia, eclampsia, and those in whom glucose tolerance test could not be done were not included in the study.
Seventy-six placentas were collected immediately after delivery. Placentas were fixed in 10% formalin. Based on patient's history along with clinical examinations, pregnant women with no risk factors for diabetes underwent glucose challenge test at 24–28 weeks of gestation. Women with risk factors for diabetes underwent glucose challenge test at their first visit for antenatal care. A plasma glucose level above 130–140 mg/dL/1st hour was considered abnormal and necessitates a second test, the 3-h oral glucose tolerance test (OGTT). Interpretation of the OGTT was based on the Carpenter/Coustan conversion method.[18] Pregnant women were classified into four groups based on responses to the 100 g OGTT according to American Diabetes Association criteria and in respect to their glucose profile defined using Gillmer's threshold values as follows:[19,20,21] (a) normoglycemic – women with normal OGTT and glucose profile; (b) gestational diabetes mellitus (GDM) – women with abnormal OGTT and normal glucose profile; (c) mildly hyperglycemic – normal OGTT and altered glucose profile; and (d) overt or clinical diabetes type 1 and type 2 – women with pregestational abnormal OGTT. Normal values for glucose profiles were fixed at <100 g/dl. Glucose levels were determined using glucose oxidase method.
Collected placentas were subjected to the gross examinations. Two consultant pathologists examined the histopathological sections from all the placentas. Both pathologists were blinded about the diagnosis of diabetes in patients. Definitions of the placental lesions used were – lymphohistiocytic villitis was diagnosed by the presence of lymphocytic and macrophage infiltration in the villous stroma.[22] Ischemia of the villi was defined by Tenney-Parker changes, i.e., increased syncytial knots and placental infarcts. Villous fibrinoid necrosis was considered moderate if only a few foci of fibrinoid substances were seen in a small number of low power (×10) fields, and severe when some villi with fibrinoid necrosis were found in most low power fields examined. Villous immaturity was defined as when there was a decreased formation of terminal villi and a relatively increased presence of immature intermediate villi. Immature intermediate villi were defined by the presence of large stroma and loose reticular channels containing Hofbauer cells.[23] Chorangiosis was defined as the occurrence of 10 or more villi with 10 or more capillaries in 10 or more low power microscopic fields (×10).[24] Hydropic villi were diagnosed when large terminal villi were present with edematous fluid and villous macrophages. Fetal vessel thrombosis was diagnosed when a large fetal stem villous vessel was partially or completely occluded by a thrombus. Avascular villi were diagnosed when a group of at least five fibrotic avascular villi without inflammation were seen.
Immunohistochemistry
Paraffin blocks made from specimen were collected and the sections were used for hematoxylin and eosin staining and for immunohistochemical (IHC) examinations. IHC was performed using mouse monoclonal antibodies for VEGF (Monoclonal Mouse Anti-Human VEGF, DAKO, Clone VG1 code number M7273). Polymer labeling method (polymer chain two-step indirect technique) was used. For negative control, Dako Mouse IgG1, code No. X0931, diluted to the same concentration as the primary antibody was used. IHC was done according to standard protocols. Interpretation of immunostaining was analyzed according to a subjective evaluation of the intensity of reaction: (a) no expression (0 point), (b) weak expression (1 point), (c) moderate expression (1.5 points), and (d) strong expression (2 points). Data were represented as means or in percentage. Categorical data were compared using Fischer's exact test. Two-tailed P values were calculated using Fischer's exact test. Results with P < 0.05 were considered statistically significant.
Results
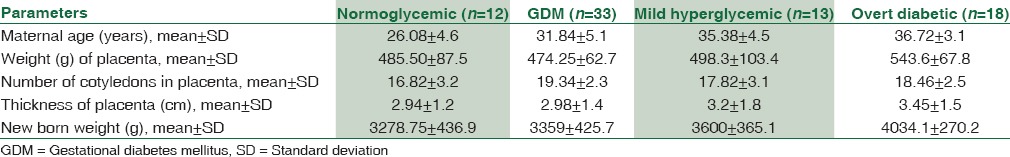
Out of total 76 placentas collected, 12 placentas were from normoglycemic women, 33 placentas from women with GDM, 13 from women with mild hyperglycemia, and rest 18 placentas from women with overt diabetes. Comparisons of placental parameters between different groups of pregnant women classified according to glycemic status are shown in Table 1.
Table 1.
Comparisons of maternal age, placental parameters, and newborn weight between normoglycemic, gestational diabetes mellitus, mild hyperglycemic, and overt diabetic women
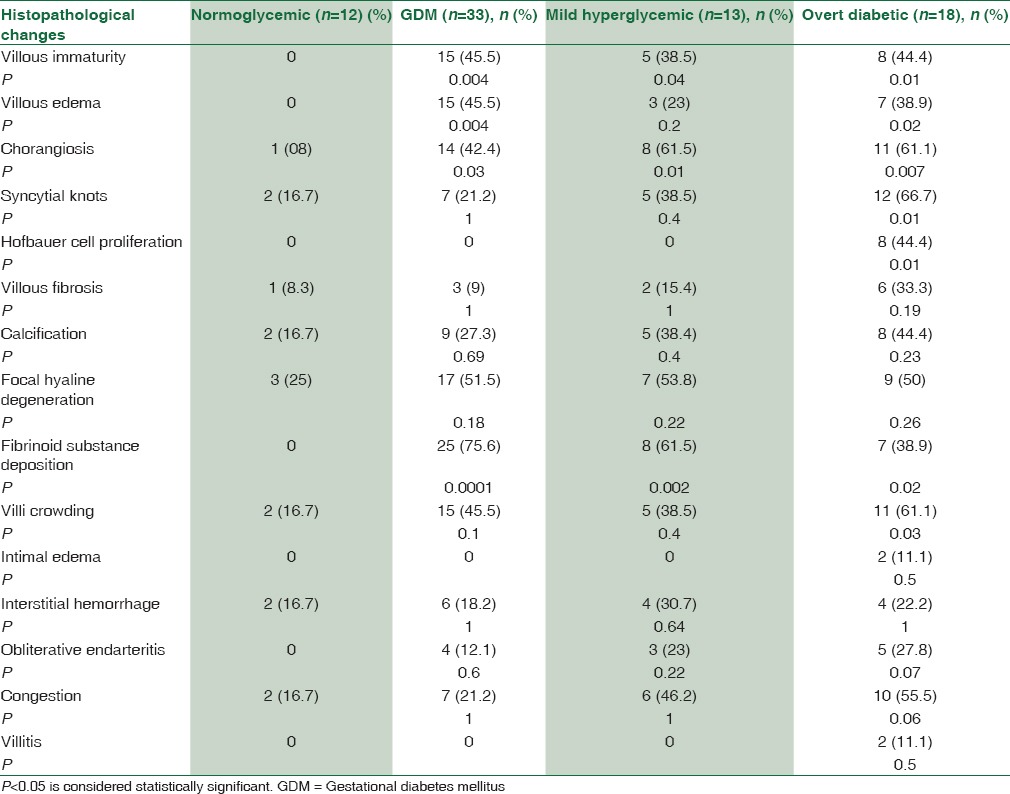
Microscopical examinations of placentas obtained from normoglycemic women showed the presence of chorionic villi which emerged from the chorionic plate. Terminal villi showed intervillous spaces filled with maternal blood and contained a mesenchymal core. The villi were surrounded by a trophoblastic layer, which included multinucleated syncytiotrophoblast and few generative cytotrophoblast. In few of the villi (2 cases), the nuclei of syncytiotrophoblast were grouped together to form syncytial knots. Cytotrophoblast was rare and appeared pale on staining. Histopathology of the placenta from mild hyperglycemic women revealed immature villi (5 cases) [Figure 1a], accumulation of fibrinoid material (8 cases), increased number of syncytial knots (5 cases), and the presence of chorangiosis (8 cases). Villous degenerative changes were present. Congestion of blood vessels (6 cases) and interstitial hemorrhage (4 cases) were also noted. Histopathology of the placenta in GDM showed the presence of villous immaturity [Figure 1b], chorangiosis, increased syncytial knots, villous edema, villous fibrosis, calcification, focal hyaline degenerations, and fibrinoid substance deposition. Histopathology of placenta in pregestational overt diabetes mellitus revealed the presence of villous immaturity [Figure 1c and d], chorangiosis, syncytial knots [Figure 1c and d], Hofbauer cell hyperplasia, villous edema, villous fibrosis, calcification, focal hyaline degeneration, and deposition of fibrinoid substances [Figure 1c and d]. Villitis was also found in few placentas (2 cases). Comparison of different histopathological findings in placentas from different groups of pregnant women based on glycemic status is given in Table 2.
Figure 1.
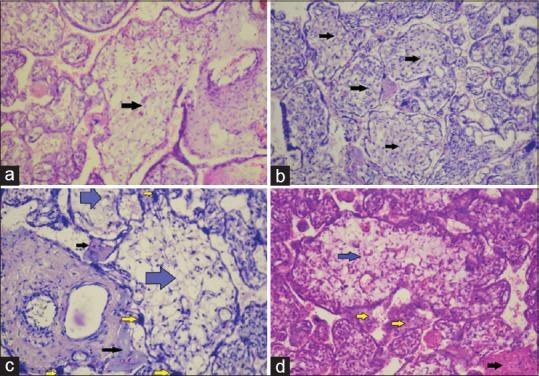
Villous immaturity. (a) Villous immaturity in mild hyperglycemic placenta (marked by arrow) (H and E, ×400). (b) Villous immaturity in gestational diabetic placenta (marked by arrows) (H and E, ×400). (c and d) Villous immaturity in overt diabetic placenta (marked by blue arrows), syncytial knots (marked by yellow arrows) and fibrinoid substance deposition (marked by black arrows) (H and E, ×400)
Table 2.
Number and percentage of various histopathological changes in different groups of women classified according to glycemic status
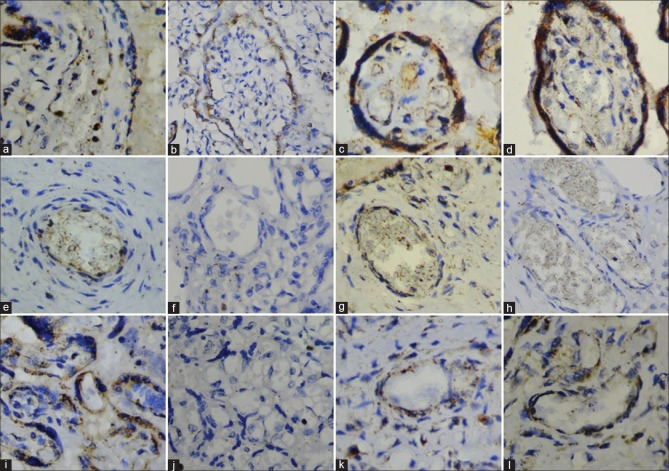
VEGF immunoexpression in normal placenta revealed syncytiotrophoblast [Figure 2a] and cytotrophoblast with moderate cytoplasmic staining [Figure 3a]. Moderate VEGF expression was also seen in villi mesenchymal cells [Figure 3f] and vessel endothelium [Figure 2g]. Strong intensity VEGF expression was seen in endothelial cells of the capillaries [Figure 2k].
Figure 2.
Vascular endothelial growth factor expression. (a) Syncytiotrophoblast of normal placenta. (b) Syncytiotrophoblast of gestational diabetic placenta. (c) Syncytiotrophoblast of mild hyperglycemic placenta. (d) Syncytiotrophoblast of overt diabetic placenta. (e) Vessel endothelium of mild hyperglycemic placenta. (f) Vessel endothelium of gestational diabetes mellitus placentas. (g) Vessel endothelium of normal placenta. (h) Vessel endothelium of overt diabetic placenta placentas. (i) Capillary endothelium of mild hyperglycemic placenta. (j) Capillary endothelium of gestational diabetes mellitus placentas. (k) Capillary endothelium of normal placenta. (l) Capillary endothelium of overt diabetic placenta
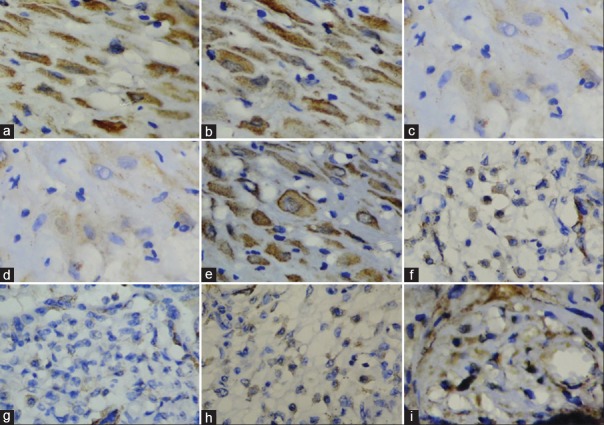
Figure 3.
Vascular endothelial growth factor expression. (a) Cytotrophoblast of normal placenta. (b) Cytotrophoblast of mild hyperglycemic placenta. (c and d) Cytotrophoblast of gestational diabetic placenta. (e) Cytotrophoblast of overt diabetic placenta. (f) Mesenchymal cells of normal placenta. (g) Mesenchymal cells of gestational diabetes mellitus placentas. (h) Mesenchymal cells of mild hyperglycemic placenta. (i) Mesenchymal cells of overt diabetic placenta
Syncytiotrophoblast [Figure 2c], cytotrophoblast [Figure 3b], and mesenchymal cells [Figure 3h] in placentas of mildly hyperglycemic women displayed moderate cytoplasmic VEGF expression. Capillary endothelium displayed strong expression [Figure 2i]. Weak expression of VEGF was seen in vessel endothelium [Figure 2e] and vascular smooth muscle cells.
In placentas of gestational diabetic women, syncytiotrophoblast displayed moderate cytoplasmic VEGF expression [Figure 2b]. Cytotrophoblast displayed weak VEGF expression [Figures 3c and 3d]. No other placental components expressed VEGF. [Figures 2f, 2j and 3g].
VEGF expression in the placenta of overt diabetic women revealed syncytiotrophoblast with strong VEGF expression [Figure 2d]. Cytotrophoblast [Figure 3e] and mesenchymal cells [Figure 3i] displayed moderate VEGF expression. Capillary endothelium displayed strong VEGF reactivity [Figure 2l], while vessel endothelium displayed no VEGF expression [Figure 2h]. Weak VEGF staining was noted in vessel smooth muscle cells.
Discussion
Various authors described different histopathological changes in the placentas of diabetic women. Fox found no villous immaturity in diabetic placentas.[25] Other histopathological changes observed by Fox in diabetic placentas were consistent with our study. Asmussen observed villous immaturity, increased vascularization in the diabetic placenta.[26] Björk et al. in their study on diabetic placentas found increase in hypovascular villi, syncytial knots, and immature villi.[27] Boyd et al. reported increased volume of parenchymal tissue but decreased volume of nonparenchymal tissue in the diabetic placenta.[28] In contrary, we found both significant increase in parenchymal tissue and nonparenchymal tissue in pregestational diabetic placenta. Jauniaux and Burton reported increased number of capillaries in the terminal villi and increased volume of trophoblasts.[29] These findings were consistent with our studies. Nelson et al. found that diabetic placentas had a significant increase in the intervillous volume but villous, nonparenchymal, trophoblast, and capillary volumes did not differ from placentas of normoglycemic placenta.[30] Our findings in histopathological changes in GDM collaborated well with findings of other studies.[31,32,33] In our study, we observed VEGF expression in all components of normoglycemic placentas. Similar staining patterns were seen in other study.[34] VEGF expression in villi mesenchymal cells and Hofbauer cells of overt diabetic placentas were also noted by other authors.[35] Cetinkaya et al. found that VEGF expression increased in diabetic placentas in comparison to normal placentas.[36] Barreiro et al. investigated the expression of VEGF in GDM placentas and clinical overt diabetic placentas. They found VEGF expression mainly in the syncytiotrophoblastic layer of the placental villi in normoglycemic placentas. They reported that GDM placentas displayed the strongest VEGF expression while clinically overt diabetic placentas displayed strongly reduced VEGF expression.[37] This is in sharp contrast to the study done by Pietro et al.[34] and our study. Differences between the study results of Barreiro et al. and our study may be due to better sensitivity and specificity of the monoclonal VEGF primary antibody used in our study and smaller numbers of GDM placentas (n = 3) studied by Barreiro et al. in comparison to our study (GDM, n = 33).
Conclusion
Villous immaturity, chorangiosis, and fibrinoid substance deposition were statistically significant histopathological changes common to GDM, mild hyperglycemic, and overt diabetic placentas. VEGF expression in GDM placentas displayed most deviation from normoglycemic control placentas. VEGF expression in mild hyperglycemic placentas was almost similar to normoglycemic placentas. VEGF expressions in most components of clinically overt diabetic placentas were exaggeration of their normal counterparts. We concluded that changes in VEGF expression might contribute to various histopathological changes in diabetic placentas. VEGF expression pattern is altered in GDM, while VEGF expression in overt diabetic placenta is an exaggeration of the normal.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Kaufmann P, Mayhew TM, Charnock-Jones DS. Aspects of human fetoplacental vasculogenesis and angiogenesis. II. Changes during normal pregnancy. Placenta. 2004;25:114–26. doi: 10.1016/j.placenta.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 2.Helske S, Vuorela P, Carpén O, Hornig C, Weich H, Halmesmäki E. Expression of vascular endothelial growth factor receptors 1, 2 and 3 in placentas from normal and complicated pregnancies. Mol Hum Reprod. 2001;7:205–10. doi: 10.1093/molehr/7.2.205. [DOI] [PubMed] [Google Scholar]
- 3.Demir R, Kayisli UA, Cayli S, Huppertz B. Sequential steps during vasculogenesis and angiogenesis in the very early human placenta. Placenta. 2006;27:535–9. doi: 10.1016/j.placenta.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 4.Demir R, Kayisli UA, Seval Y, Celik-Ozenci C, Korgun ET, Demir-Weusten AY, et al. Sequential expression of VEGF and its receptors in human placental villi during very early pregnancy: Differences between placental vasculogenesis and angiogenesis. Placenta. 2004;25:560–72. doi: 10.1016/j.placenta.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 5.Mayhew TM. Fetoplacental angiogenesis during gestation is biphasic, longitudinal and occurs by proliferation and remodelling of vascular endothelial cells. Placenta. 2002;23:742–50. doi: 10.1016/s0143-4004(02)90865-9. [DOI] [PubMed] [Google Scholar]
- 6.Kingdom J, Huppertz B, Seaward G, Kaufmann P. Development of the placental villous tree and its consequences for fetal growth. Eur J Obstet Gynecol Reprod Biol. 2000;92:35–43. doi: 10.1016/s0301-2115(00)00423-1. [DOI] [PubMed] [Google Scholar]
- 7.Vaisman N, Gospodarowicz D, Neufeld G. Characterization of the receptors for vascular endothelial growth factor. J Biol Chem. 1990;265:19461–6. [PubMed] [Google Scholar]
- 8.He H, Venema VJ, Gu X, Venema RC, Marrero MB, Caldwell RB. Vascular endothelial growth factor signals endothelial cell production of nitric oxide and prostacyclin through flk-1/KDR activation of c-Src. J Biol Chem. 1999;274:25130–5. doi: 10.1074/jbc.274.35.25130. [DOI] [PubMed] [Google Scholar]
- 9.Koch AE, Harlow LA, Haines GK, Amento EP, Unemori EN, Wong WL, et al. Vascular endothelial growth factor. A cytokine modulating endothelial function in rheumatoid arthritis. J Immunol. 1994;152:4149–56. [PubMed] [Google Scholar]
- 10.Jakeman LB, Winer J, Bennett GL, Altar CA, Ferrara N. Binding sites for vascular endothelial growth factor are localized on endothelial cells in adult rat tissues. J Clin Invest. 1992;89:244–53. doi: 10.1172/JCI115568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou Y, Bellingard V, Feng KT, McMaster M, Fisher SJ. Human cytotrophoblasts promote endothelial survival and vascular remodeling through secretion of Ang2, PlGF, and VEGF-C. Dev Biol. 2003;263:114–25. doi: 10.1016/s0012-1606(03)00449-4. [DOI] [PubMed] [Google Scholar]
- 12.Taylor CM, Stevens H, Anthony FW, Wheeler T. Influence of hypoxia on vascular endothelial growth factor and chorionic gonadotrophin production in the trophoblast-derived cell lines: JEG, JAr and BeWo. Placenta. 1997;18:451–8. doi: 10.1016/s0143-4004(97)80047-1. [DOI] [PubMed] [Google Scholar]
- 13.Neufeld G, Cohen T, Gengrinovitch S, Poltorak Z. Vascular endothelial growth factor (VEGF) and its receptors. FASEB J. 1999;13:9–22. [PubMed] [Google Scholar]
- 14.Calderon IM, Damasceno DC, Amorin RL, Costa RA, Brasil MA, Rudge MV. Morphometric study of placental villi and vessels in women with mild hyperglycemia or gestational or overt diabetes. Diabetes Res Clin Pract. 2007;78:65–71. doi: 10.1016/j.diabres.2007.01.023. [DOI] [PubMed] [Google Scholar]
- 15.Eriksson UJ, Borg LA, Forsberg H, Styrud J. Diabetic embryopathy. Studies with animal and in vitro models. Diabetes. 1991;40(Suppl 2):94–8. doi: 10.2337/diab.40.2.s94. [DOI] [PubMed] [Google Scholar]
- 16.Zhao B, Cai J, Boulton M. Expression of placenta growth factor is regulated by both VEGF and hyperglycaemia via VEGFR-2. Microvasc Res. 2004;68:239–46. doi: 10.1016/j.mvr.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 17.Kumazaki K, Nakayama M, Suehara N, Wada Y. Expression of vascular endothelial growth factor, placental growth factor, and their receptors Flt-1 and KDR in human placenta under pathologic conditions. Hum Pathol. 2002;33:1069–77. doi: 10.1053/hupa.2002.129420. [DOI] [PubMed] [Google Scholar]
- 18.Carpenter MW, Coustan DR. Criteria for screening tests for gestational diabetes. Am J Obstet Gynecol. 1982;144:768–73. doi: 10.1016/0002-9378(82)90349-0. [DOI] [PubMed] [Google Scholar]
- 19.Rudge MV, Peraçoli JC, Berezowski AT, Calderon IM, Brasil MA. The oral glucose tolerance test is a poor predictor of hyperglycemia during pregnancy. Braz J Med Biol Res. 1990;23:1079–89. [PubMed] [Google Scholar]
- 20.American Diabetes Association. Gestational diabetes mellitus. Diabetes Care. 2004;27(Suppl 1):S88–90. doi: 10.2337/diacare.27.2007.s88. [DOI] [PubMed] [Google Scholar]
- 21.Gillmer MD, Beard RW, Brooke FM, Oakley NW. Carbohydrate metabolism in pregnancy. Part I. Diurnal plasma glucose profile in normal and diabetic women. Br Med J. 1975;3:399–402. doi: 10.1136/bmj.3.5980.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Knox WF, Fox H. Villitis of unknown aetiology: Its incidence and significance in placentae from a British population. Placenta. 1984;5:395–402. doi: 10.1016/s0143-4004(84)80019-3. [DOI] [PubMed] [Google Scholar]
- 23.Gheorman L, Plesea IE, Gheorman V. Histopathological considerations of placenta in pregnancy with diabetes. Rom J Morphol Embryol. 2012;53:329–36. [PubMed] [Google Scholar]
- 24.Altshuler G. Chorangiosis. An important placental sign of neonatal morbidity and mortality. Arch Pathol Lab Med. 1984;108:71–4. [PubMed] [Google Scholar]
- 25.Fox H. Pathology of the placenta in maternal diabetes mellitus. Obstet Gynecol. 1969;34:792–8. [PubMed] [Google Scholar]
- 26.Asmussen I. Ultrastructure of the villi and fetal capillaries of the placentas delivered by non-smoking diabetic women (White group D) Acta Pathol Microbiol Immunol Scand A. 1982;90:95–101. doi: 10.1111/j.1699-0463.1982.tb00069_90a.x. [DOI] [PubMed] [Google Scholar]
- 27.Björk O, Persson B. Placental changes in relation to the degree of metabolic control in diabetes mellitus. Placenta. 1982;3:367–78. doi: 10.1016/s0143-4004(82)80030-1. [DOI] [PubMed] [Google Scholar]
- 28.Boyd PA, Scott A, Keeling JW. Quantitative structural studies on placentas from pregnancies complicated by diabetes mellitus. Br J Obstet Gynaecol. 1986;93:31–5. doi: 10.1111/j.1471-0528.1986.tb07809.x. [DOI] [PubMed] [Google Scholar]
- 29.Jauniaux E, Burton GJ. Villous histomorphometry and placental bed biopsy investigation in type I diabetic pregnancies. Placenta. 2006;27:468–74. doi: 10.1016/j.placenta.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 30.Nelson SM, Coan PM, Burton GJ, Lindsay RS. Placental structure in type 1 diabetes: Relation to fetal insulin, leptin, and IGF-I. Diabetes. 2009;58:2634–41. doi: 10.2337/db09-0739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Daskalakis G, Marinopoulos S, Krielesi V, Papapanagiotou A, Papantoniou N, Mesogitis S, et al. Placental pathology in women with gestational diabetes. Acta Obstet Gynecol Scand. 2008;87:403–7. doi: 10.1080/00016340801908783. [DOI] [PubMed] [Google Scholar]
- 32.Madazli R, Tuten A, Calay Z, Uzun H, Uludag S, Ocak V. The incidence of placental abnormalities, maternal and cord plasma malondialdehyde and vascular endothelial growth factor levels in women with gestational diabetes mellitus and nondiabetic controls. Gynecol Obstet Invest. 2008;65:227–32. doi: 10.1159/000113045. [DOI] [PubMed] [Google Scholar]
- 33.Rudge MV, Lima CP, Damasceno DC, Sinzato YK, Napoli G, Rudge CV, et al. Histopathological placental lesions in mild gestational hyperglycemic and diabetic women. Diabetol Metab Syndr. 2011;3:19. doi: 10.1186/1758-5996-3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pietro L, Daher S, Rudge MV, Calderon IM, Damasceno DC, Sinzato YK, et al. Vascular endothelial growth factor (VEGF) and VEGF-receptor expression in placenta of hyperglycemic pregnant women. Placenta. 2010;31:770–80. doi: 10.1016/j.placenta.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 35.Vuorela P, Halmesmäki E. Vascular endothelial growth factor, its receptors, and the tie receptors in the placental bed of women with preeclampsia, diabetes, and intrauterine growth retardation. Am J Perinatol. 2006;23:255–63. doi: 10.1055/s-2006-939562. [DOI] [PubMed] [Google Scholar]
- 36.Cetinkaya B, Ozmen A, Unek G, Bulbul G, Korgun ET. Expressions of vegf, vegfr1, vegfr2 and akt in pregestational diabetic human placentas. Placenta. 2014;35:31. [Google Scholar]
- 37.Barreiro EG, Pietro L, Mattar R, Damasceno DC, Calderon IM, Rudge MV, et al. Vascular endothelial growth factor (VEGF) in placentas from hyperglycemic pregnant women: Pilot study. J Reprod immunol. 2007;75:17. [Google Scholar]